How Genes Meet Diet in LCHAD Deficiency: Nutrigenomics of Fatty Acid Oxidation Disorder
Abstract
1. Introduction
2. Human Mitochondrial β-Oxidation Systems
3. The Genetic Architecture of LCHAD Deficiency
3.1. The HADHA Gene
| Variant | Amino Acid Change | Consequence | Frequency | References |
|---|---|---|---|---|
| c.180+3A>G rs781222705 | — | SNV, splice region | 1:19,800 | [22] |
| c.914T>A rs137852774 | p.Ile305Asn (I305N) | SNV, missense | 1:37,800 | [23] |
| c.919-2A>G rs200017313 | — | SNV, splice acceptor | 1:24,900 | [24] |
| c.1132C>T rs137852770 | p.Gln378Ter (Q378*) | SNV, nonsense | 1:66,200 | [25] |
| c.1528G>C rs137852769 | p.Glu510Gln (E510Q) | SNV, missense | 1:720 | [26] |
| c.1678C>T rs137852771 | p.Arg560Ter (R560*) | SNV, nonsense | 1:42,200 | [27] |
| c.1793_1794del rs769580842 | p.His598fs (H598fs) | Deletion, frameshift | 1:24,881 | [28] |
| c.1981_1999del rs749848370 | p.Leu661fs (Y639fs) | Deletion, frameshift | 1:40,400 | [29] |
| c.2026C>T rs771028541 | p.Arg676Cys (R676C) | SNV, missense | 1:52,938 | [30] |
| c.2225_2228dup rs868816467 | p.Phe744fs (F744fs) | Duplication, frameshift | 1:66,200 | [31] |
3.2. Pathogenic Variant c.1528G>C
| Country or Region | Carrier Frequency | References |
|---|---|---|
| Czechia | 1:145 | [37] |
| Denmark | 1:172 | [38] |
| Estonia | 1:173 | [39] |
| Finland, Northern | 1:365 | [40] |
| Finland, Western | 1:132 | [40] |
| Finland, Southern | 1:164 | [40] |
| Finland, Eastern | 1:193 | [40] |
| Germany | 1:243 | [41] |
| Poland, Kashubian | 1:57 | [32] |
| Poland, Pomeranian | 1:75 | [32] |
| Poland, rest of country | 1:187 | [32] |
| The Netherlands | 1:680 | [42] |
| Ukraine | 1:288 | [43] |
| United Kingdom | 1:927 | [17] |
| Worldwide | 1:720 | [17] |
4. Multisystem Health Complications in LCHAD Deficiency
5. Nutritional Strategies to Bypass the Enzymatic Block in LCHAD Deficiency
5.1. Dietary Restriction of Long-Chain Triglycerides
5.2. Replacing Long-Chain Triglycerides with Medium-Chain Triglycerides
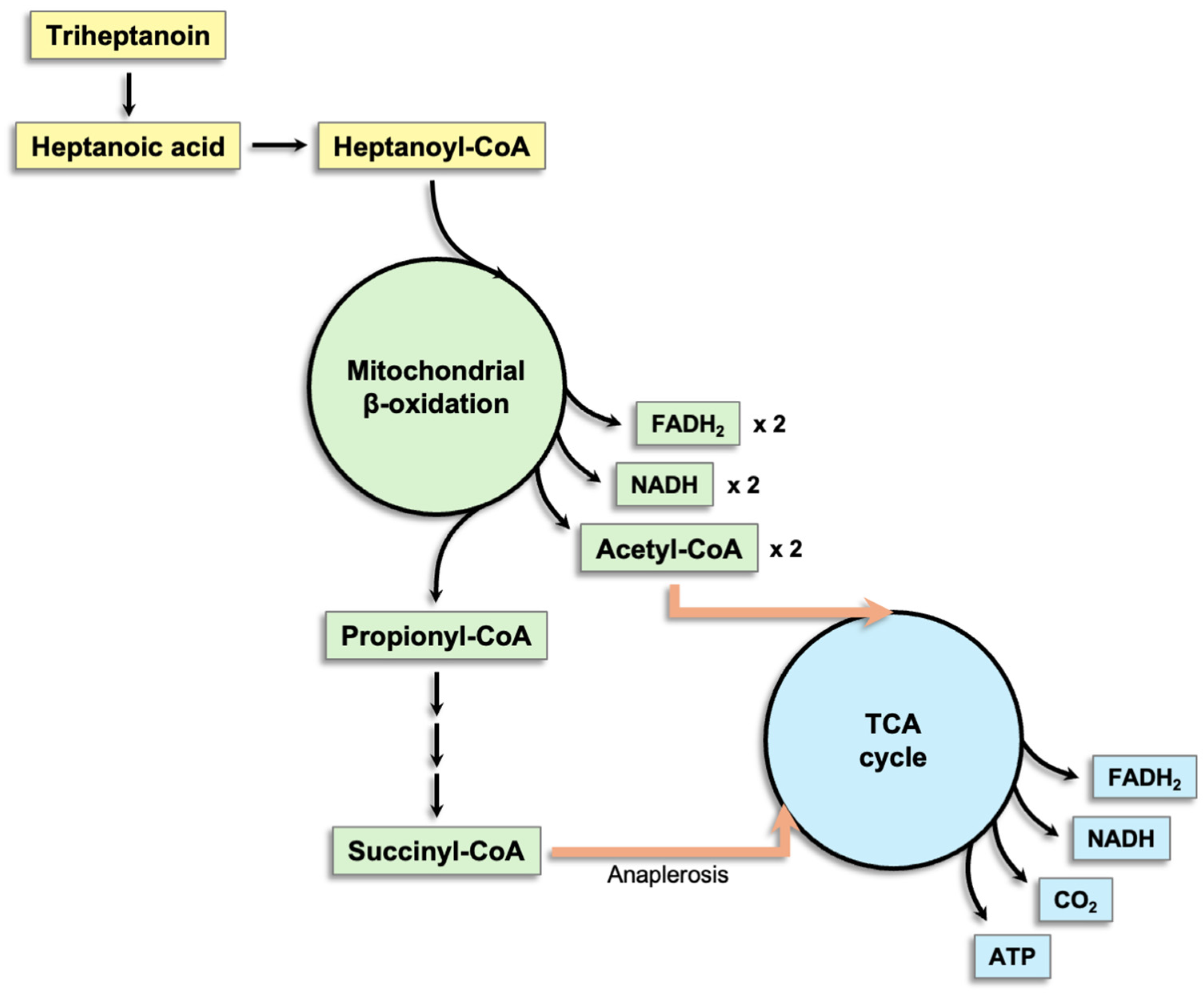
5.3. Triheptanoin
5.4. Carbohydrates
5.5. Treatment Response Monitoring
6. Nutrigenomic Signals Elicited by Dietary Treatment in LCHAD Deficiency
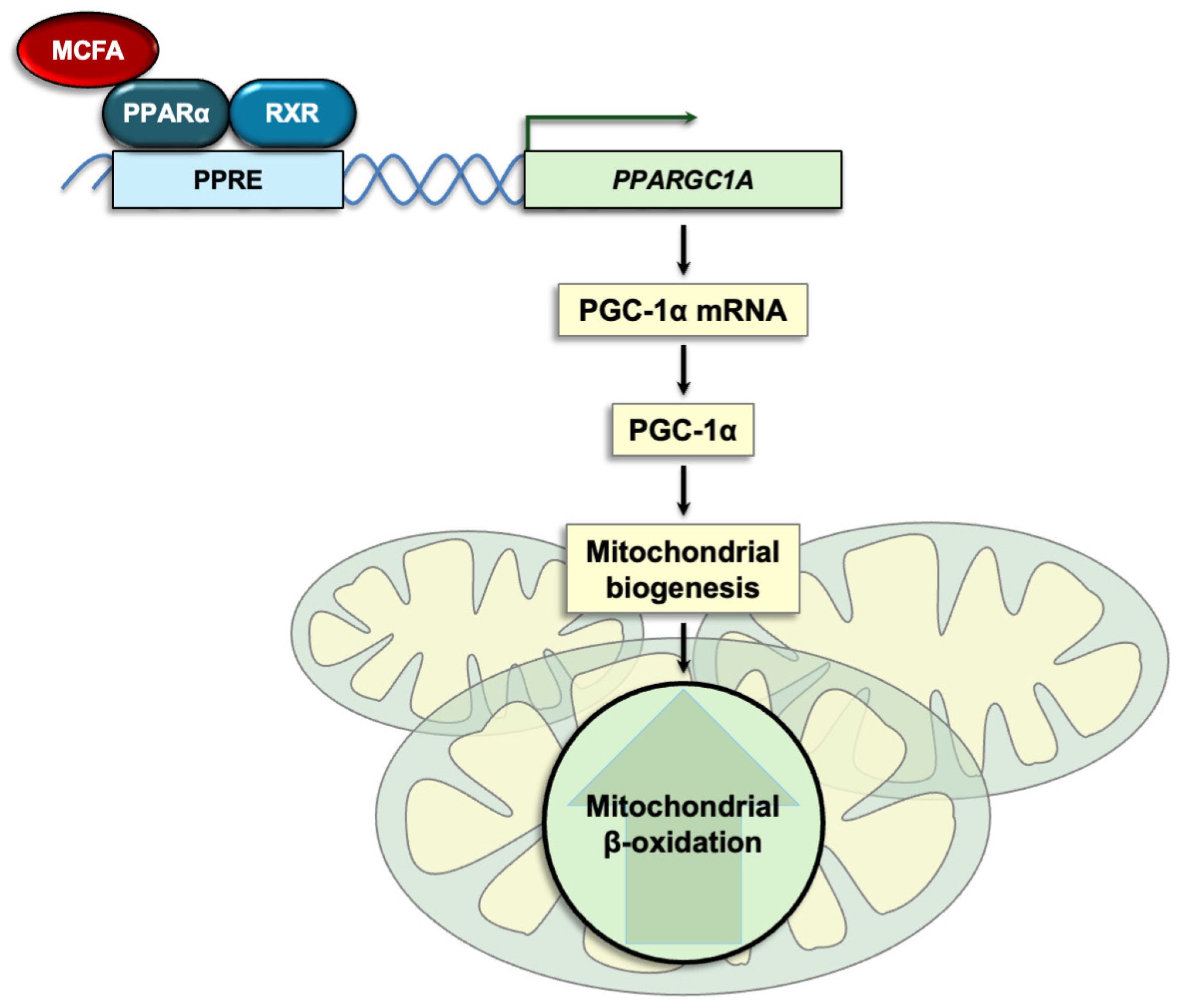
6.1. MCT-Derived Fatty Acids Are Ligands for Nuclear Receptors
6.2. MCFA Effects on PPARs and Their Target Genes Are Cell- and Tissue-Specific
6.3. MCFAs Increase FGF21 and Activate AMPK
7. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coughlin, C.R. Fatty Acid Oxidation Disorders. In Nutrition Management of Inherited Metabolic Diseases; Bernstein, L.E., Rohr, F., Van Calcar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 309–323. ISBN 978-3-030-94509-1. [Google Scholar]
- den Boer, M.E.J.; Wanders, R.J.A.; Morris, A.A.M.; IJlst, L.; Heymans, H.S.A.; Wijburg, F.A. Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency: Clinical Presentation and Follow-up of 50 Patients. Pediatrics 2002, 109, 99–104. [Google Scholar] [CrossRef]
- Sharma, S.; McKenzie, M. The Pathogenesis of Very Long-Chain Acyl-CoA Dehydrogenase Deficiency. Biomolecules 2025, 15, 416. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zou, D.; Chen, G.; Wan, T.; Zhang, M.; Cao, X. Cloning and Functional Characterization of ACAD-9, a Novel Member of Human Acyl-CoA Dehydrogenase Family. Biochem. Biophys. Res. Commun. 2002, 297, 1033–1042. [Google Scholar] [CrossRef]
- Nouws, J.; Te Brinke, H.; Nijtmans, L.G.; Houten, S.M. ACAD9, a Complex I Assembly Factor with a Moonlighting Function in Fatty Acid Oxidation Deficiencies. Hum. Mol. Genet. 2014, 23, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Giguet-Valard, A.G.; Ait-El-Mkadem Saadi, S.; Duclos, S.; Lacombe, D.; Bellance, R.; Bellance, N. A Late-Onset and Mild Phenotype of Mitochondrial Complex I Deficiency Due to a Novel Reported Variant Within the ACAD9 Gene. Int. J. Mol. Sci. 2025, 26, 7128. [Google Scholar] [CrossRef] [PubMed]
- Gene HADHB–Hydroxyacyl-CoA Dehydrogenase Trifunctional Multienzyme Complex Subunit Beta (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/HADHB/human (accessed on 21 July 2025).
- Gene HADHA–Hydroxyacyl-CoA Dehydrogenase Trifunctional Multienzyme Complex Subunit Alpha (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/HADHA/human (accessed on 21 July 2025).
- Vieira Neto, E.; Wang, M.; Szuminsky, A.J.; Ferraro, L.; Koppes, E.; Wang, Y.; Van’t Land, C.; Mohsen, A.-W.; Zanatta, G.; El-Gharbawy, A.H.; et al. Mitochondrial Bioenergetics and Cardiolipin Remodeling Abnormalities in Mitochondrial Trifunctional Protein Deficiency. JCI Insight 2024, 9, e176887. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Izai, K.; Orii, T.; Hashimoto, T. Novel Fatty Acid Beta-Oxidation Enzymes in Rat Liver Mitochondria. II. Purification and Properties of Enoyl-Coenzyme A (CoA) Hydratase/3-Hydroxyacyl-CoA Dehydrogenase/3-Ketoacyl-CoA Thiolase Trifunctional Protein. J. Biol. Chem. 1992, 267, 1034–1041. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Ito, M.; Tanaka, K. Purification and Properties of Short Chain Acyl-CoA, Medium Chain Acyl-CoA, and Isovaleryl-CoA Dehydrogenases from Human Liver. J. Biol. Chem. 1987, 262, 7982–7989. [Google Scholar] [CrossRef]
- He, M.; Pei, Z.; Mohsen, A.-W.; Watkins, P.; Murdoch, G.; Van Veldhoven, P.P.; Ensenauer, R.; Vockley, J. Identification and Characterization of New Long Chain Acyl-CoA Dehydrogenases. Mol. Genet. Metab. 2011, 102, 418–429. [Google Scholar] [CrossRef]
- Kieweg, V.; Kräutle, F.G.; Nandy, A.; Engst, S.; Vock, P.; Abdel-Ghany, A.G.; Bross, P.; Gregersen, N.; Rasched, I.; Strauss, A.; et al. Biochemical Characterization of Purified, Human Recombinant Lys304-->Glu Medium-Chain Acyl-CoA Dehydrogenase Containing the Common Disease-Causing Mutation and Comparison with the Normal Enzyme. Eur. J. Biochem. 1997, 246, 548–556. [Google Scholar] [CrossRef]
- Odendaal, C.; Reijngoud, D.-J.; Bakker, B.M. How Lipid Transfer Proteins and the Mitochondrial Membrane Shape the Kinetics of β-Oxidation the Liver. Biochim. Biophys. Acta Bioenerg. 2025, 1866, 149519. [Google Scholar] [CrossRef]
- Henriques, B.J.; Katrine Jentoft Olsen, R.; Gomes, C.M.; Bross, P. Electron Transfer Flavoprotein and Its Role in Mitochondrial Energy Metabolism in Health and Disease. Gene 2021, 776, 145407. [Google Scholar] [CrossRef] [PubMed]
- Uniprot Trifunctional Enzyme Subunit Alpha, Mitochondrial. Available online: https://www.uniprot.org/uniprotkb/P40939/entry (accessed on 18 May 2025).
- ClinVar HADHA. Available online: https://www.ncbi.nlm.nih.gov/clinvar/?term=%22HADHA%22%5BGENE%5D (accessed on 23 May 2025).
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- Cassini, T.; Silverstein, S.; Behan, M.; Tifft, C.J.; Malicdan, M.C.; Adams, D.R.; Undiagnosed Diseases Network; Ahn, S.-Y.; Regier, D.S. Mitochondrial Trifunctional Protein Deficiency Caused by a Deep Intronic Deletion Leading to Aberrant Splicing. JIMD Rep. 2025, 66, e12459. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C.; Derks, T.G.J.; Adrian, K.; Al-Thihli, K.; Ballhausen, D.; Bidiuk, J.; Bordugo, A.; Boyer, M.; Bratkovic, D.; Brunner-Krainz, M.; et al. Efficacy and Safety of Empagliflozin in Glycogen Storage Disease Type Ib: Data from an International Questionnaire. Genet. Med. 2022, 24, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, C.M.; Prokisch, H. Digenic Inheritance in Rare Disorders and Mitochondrial Disease-Crossing the Frontier to a More Comprehensive Understanding of Etiology. Int. J. Mol. Sci. 2024, 25, 4602. [Google Scholar] [CrossRef] [PubMed]
- Rs781222705 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs781222705 (accessed on 20 August 2025).
- Rs137852774 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs137852774 (accessed on 20 August 2025).
- Rs200017313 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs200017313 (accessed on 20 August 2025).
- Rs137852770 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs137852770 (accessed on 20 August 2025).
- Rs137852769 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs137852769 (accessed on 20 August 2025).
- Rs137852771 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs137852771 (accessed on 20 August 2025).
- Rs769580842 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs769580842 (accessed on 20 August 2025).
- Rs749848370 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs749848370 (accessed on 20 August 2025).
- Rs771028541 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs771028541 (accessed on 20 August 2025).
- Rs868816467 RefSNP Report. Available online: https://www.ncbi.nlm.nih.gov/snp/rs868816467 (accessed on 20 August 2025).
- Nedoszytko, B.; Siemińska, A.; Strapagiel, D.; Dąbrowski, S.; Słomka, M.; Sobalska-Kwapis, M.; Marciniak, B.; Wierzba, J.; Skokowski, J.; Fijałkowski, M.; et al. High Prevalence of Carriers of Variant c.1528G>C of HADHA Gene Causing Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (LCHADD) in the Population of Adult Kashubians from North Poland. PLoS ONE 2017, 12, e0187365. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.L.; Pennesi, M.E.; Harding, C.O.; Weleber, R.G.; Gillingham, M.B. Observations Regarding Retinopathy in Mitochondrial Trifunctional Protein Deficiencies. Mol. Genet. Metab. 2012, 106, 18–24. [Google Scholar] [CrossRef]
- Bo, R.; Yamada, K.; Kobayashi, H.; Jamiyan, P.; Hasegawa, Y.; Taketani, T.; Fukuda, S.; Hata, I.; Niida, Y.; Shigematsu, Y.; et al. Clinical and Molecular Investigation of 14 Japanese Patients with Complete TFP Deficiency: A Comparison with Caucasian Cases. J. Hum. Genet. 2017, 62, 809–814. [Google Scholar] [CrossRef]
- Jankowski, M.; Daca-Roszak, P.; Obracht-Prondzyński, C.; Płoski, R.; Lipska-Ziętkiewicz, B.S.; Ziętkiewicz, E. Genetic Diversity in Kashubs: The Regional Increase in the Frequency of Several Disease-Causing Variants. J. Appl. Genet. 2022, 63, 691–701. [Google Scholar] [CrossRef]
- Alatibi, K.I.; Hagenbuchner, J.; Wehbe, Z.; Karall, D.; Ausserlechner, M.J.; Vockley, J.; Spiekerkoetter, U.; Grünert, S.C.; Tucci, S. Different Lipid Signature in Fibroblasts of Long-Chain Fatty Acid Oxidation Disorders. Cells 2021, 10, 1239. [Google Scholar] [CrossRef]
- Rücklová, K.; Hrubá, E.; Pavlíková, M.; Hanák, P.; Farolfi, M.; Chrastina, P.; Vlášková, H.; Kousal, B.; Smolka, V.; Foltenová, H.; et al. Impact of Newborn Screening and Early Dietary Management on Clinical Outcome of Patients with Long Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency and Medium Chain Acyl-CoA Dehydrogenase Deficiency—A Retrospective Nationwide Study. Nutrients 2021, 13, 2925. [Google Scholar] [CrossRef]
- Lund, A.M.; Hougaard, D.M.; Simonsen, H.; Andresen, B.S.; Christensen, M.; Dunø, M.; Skogstrand, K.; Olsen, R.K.J.; Jensen, U.G.; Cohen, A.; et al. Biochemical Screening of 504,049 Newborns in Denmark, the Faroe Islands and Greenland--Experience and Development of a Routine Program for Expanded Newborn Screening. Mol. Genet. Metab. 2012, 107, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Joost, K.; Ounap, K.; Zordania, R.; Uudelepp, M.-L.; Olsen, R.K.; Kall, K.; Kilk, K.; Soomets, U.; Kahre, T. Prevalence of Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency in Estonia. JIMD Rep. 2012, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pastinen, T.; Perola, M.; Ignatius, J.; Sabatti, C.; Tainola, P.; Levander, M.; Syvänen, A.C.; Peltonen, L. Dissecting a Population Genome for Targeted Screening of Disease Mutations. Hum. Mol. Genet. 2001, 10, 2961–2972. [Google Scholar] [CrossRef]
- Klose, D.A.; Kölker, S.; Heinrich, B.; Prietsch, V.; Mayatepek, E.; von Kries, R.; Hoffmann, G.F. Incidence and Short-Term Outcome of Children with Symptomatic Presentation of Organic Acid and Fatty Acid Oxidation Disorders in Germany. Pediatrics 2002, 110, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- den Boer, M.E.; Ijlst, L.; Wijburg, F.A.; Oostheim, W.; van Werkhoven, M.A.; van Pampus, M.G.; Heymans, H.S.; Wanders, R.J. Heterozygosity for the Common LCHAD Mutation (1528g>C) Is Not a Major Cause of HELLP Syndrome and the Prevalence of the Mutation in the Dutch Population Is Low. Pediatr. Res. 2000, 48, 151–154. [Google Scholar] [CrossRef]
- Barvinska, O.; Olkhovych, N.; Gorovenko, N. High Prevalence of c.1528G>C Rearrangement in Patients with Long Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency from Ukraine. Cytol. Genet. 2018, 52, 198–203. [Google Scholar] [CrossRef]
- Merritt, J.L.; MacLeod, E.; Jurecka, A.; Hainline, B. Clinical Manifestations and Management of Fatty Acid Oxidation Disorders. Rev. Endocr. Metab. Disord. 2020, 21, 479–493. [Google Scholar] [CrossRef]
- Schwantje, M.; Fuchs, S.A.; de Boer, L.; Bosch, A.M.; Cuppen, I.; Dekkers, E.; Derks, T.G.J.; Ferdinandusse, S.; Ijlst, L.; Houtkooper, R.H.; et al. Genetic, Biochemical, and Clinical Spectrum of Patients with Mitochondrial Trifunctional Protein Deficiency Identified after the Introduction of Newborn Screening in the Netherlands. J. Inherit. Metab. Dis. 2022, 45, 804–818. [Google Scholar] [CrossRef]
- Torkar, A.D.; Klinc, A.; Remec, Z.I.; Rankovic, B.; Bartolj, K.; Bertok, S.; Colja, S.; Cuk, V.; Debeljak, M.; Kozjek, E.; et al. Sudden Death of a Four-Day-Old Newborn Due to Mitochondrial Trifunctional Protein/Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiencies and a Systematic Literature Review of Early Deaths of Neonates with Fatty Acid Oxidation Disorders. Int. J. Neonatal Screen. 2025, 11, 9. [Google Scholar] [CrossRef]
- Gillingham, M.B.; Choi, D.; Gregor, A.; Wongchaisuwat, N.; Black, D.; Scanga, H.L.; Nischal, K.K.; Sahel, J.-A.; Arnold, G.; Vockley, J.; et al. Early Diagnosis and Treatment by Newborn Screening (NBS) or Family History Is Associated with Improved Visual Outcomes for Long-Chain 3-hydroxyacylCoA Dehydrogenase Deficiency (LCHADD) Chorioretinopathy. J. Inherit. Metab. Dis. 2024, 47, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Sykut-Cegielska, J.; Gradowska, W.; Piekutowska-Abramczuk, D.; Andresen, B.S.; Olsen, R.K.J.; Ołtarzewski, M.; Pronicki, M.; Pajdowska, M.; Bogdańska, A.; Jabłońska, E.; et al. Urgent Metabolic Service Improves Survival in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase (LCHAD) Deficiency Detected by Symptomatic Identification and Pilot Newborn Screening. J. Inherit. Metab. Dis. 2011, 34, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Immonen, T.; Turanlahti, M.; Paganus, A.; Keskinen, P.; Tyni, T.; Lapatto, R. Earlier Diagnosis and Strict Diets Improve the Survival Rate and Clinical Course of Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. Acta Paediatr. Oslo Nor. 1992 2016, 105, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, D.; Gregor, A.; Sim, E.; Lau, A.; Black, D.; Scanga, H.L.; Linshinski, A.; Pennesi, M.E.; Sahel, J.-A.; et al. Plasma Metabolomics, Lipidomics, and Acylcarnitines Are Associated with Vision and Genotype but Not with Dietary Intake in Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (LCHADD). J. Inherit. Metab. Dis. 2025, 48, e70060. [Google Scholar] [CrossRef]
- Kwiatkowska, J.; Wierzba, J.; Karaszewska, A.; Kozlowski, D.; Sykut-Cegielska, J.; Stanko, A. Clinical Course and Cardiovascular Outcomes in Patients with the Long-Chain 3-Hydroxyacyl-Coenzyme A Dehydrogenase Deficiency. Cardiol. J. 2017, 24, 101–104. [Google Scholar] [CrossRef]
- Grünert, S.C.; Eckenweiler, M.; Haas, D.; Lindner, M.; Tsiakas, K.; Santer, R.; Tucci, S.; Spiekerkoetter, U. The Spectrum of Peripheral Neuropathy in Disorders of the Mitochondrial Trifunctional Protein. J. Inherit. Metab. Dis. 2021, 44, 893–902. [Google Scholar] [CrossRef]
- Castro Casal, N.; Olivier Pascual, N.; Arroyo Castillo, R. Pigmentary Chorioretinopathy Due to Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (LCHAD): A Case Report with Long-Term Follow-Up. Arch. Soc. Espanola Oftalmol. 2025, 100, 497–503. [Google Scholar] [CrossRef]
- Gillingham, M.B.; Weleber, R.G.; Neuringer, M.; Connor, W.E.; Mills, M.; van Calcar, S.; Ver Hoeve, J.; Wolff, J.; Harding, C.O. Effect of Optimal Dietary Therapy upon Visual Function in Children with Long-Chain 3-Hydroxyacyl CoA Dehydrogenase and Trifunctional Protein Deficiency. Mol. Genet. Metab. 2005, 86, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Connor, W.E.; Matern, D.; Rinaldo, P.; Burlingame, T.; Meeuws, K.; Harding, C.O. Optimal Dietary Therapy of Long-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency. Mol. Genet. Metab. 2003, 79, 114–123. [Google Scholar] [CrossRef]
- Yang, Z.; Yamada, J.; Zhao, Y.; Strauss, A.W.; Ibdah, J.A. Prospective Screening for Pediatric Mitochondrial Trifunctional Protein Defects in Pregnancies Complicated by Liver Disease. JAMA 2002, 288, 2163–2166. [Google Scholar] [CrossRef]
- Bursle, C.; Weintraub, R.; Ward, C.; Justo, R.; Cardinal, J.; Coman, D. Mitochondrial Trifunctional Protein Deficiency: Severe Cardiomyopathy and Cardiac Transplantation. JIMD Rep. 2018, 40, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, G.; Saini, A.; Gonzalez de Alba, C.; Gregor, A.; Harding, C.O.; Gillingham, M.B.; Vinocur, J.M. Cardiac Phenotype in Adolescents and Young Adults with Long-Chain 3-Hydroxyacyl CoA Dehydrogenase (LCHAD) Deficiency. Genet. Med. Off. J. Am. Coll. Med. Genet. 2024, 26, 101123. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Gramer, G.; Haege, G.; Fang-Hoffmann, J.; Schwab, K.O.; Tacke, U.; Trefz, F.K.; Mengel, E.; Wendel, U.; Leichsenring, M.; et al. Efficacy and Outcome of Expanded Newborn Screening for Metabolic Diseases--Report of 10 Years from South-West Germany. Orphanet J. Rare Dis. 2011, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Marsden, D.; Bedrosian, C.L.; Vockley, J. Impact of Newborn Screening on the Reported Incidence and Clinical Outcomes Associated with Medium- and Long-Chain Fatty Acid Oxidation Disorders. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 816–829. [Google Scholar] [CrossRef]
- Uusimaa, J.; Kettunen, J.; Varilo, T.; Järvelä, I.; Kallijärvi, J.; Kääriäinen, H.; Laine, M.; Lapatto, R.; Myllynen, P.; Niinikoski, H.; et al. The Finnish Genetic Heritage in 2022–From Diagnosis to Translational Research. Dis. Model. Mech. 2022, 15, dmm049490. [Google Scholar] [CrossRef]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 31 July 2025).
- Karbowska, J.; Kochan, Z. Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans. Int. J. Mol. Sci. 2025, 26, 4817. [Google Scholar] [CrossRef]
- Kochan, Z.; Szupryczynska, N.; Malgorzewicz, S.; Karbowska, J. Dietary Lipids and Dyslipidemia in Chronic Kidney Disease. Nutrients 2021, 13, 3138. [Google Scholar] [CrossRef]
- Vockley, J. Long-Chain Fatty Acid Oxidation Disorders and Current Management Strategies. Am. J. Manag. Care 2020, 26, S147–S154. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Treatment Recommendations in Long-Chain Fatty Acid Oxidation Defects: Consensus from a Workshop. J. Inherit. Metab. Dis. 2009, 32, 498–505. [Google Scholar] [CrossRef]
- Nutricia MCT Oil. Available online: https://www.nutricia.co.uk/hcp/pim-products/mct-oil.html (accessed on 24 May 2025).
- Watanabe, S.; Tsujino, S. Applications of Medium-Chain Triglycerides in Foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, A.S.; McLaughlin, K.L.; Buddo, K.A.; Ellis, J.M. Medium-Chain Fatty Acid Oxidation Is Independent of l-Carnitine in Liver and Kidney but Not in Heart and Skeletal Muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G287–G294. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, V.; Vandenberghe, C.; Lowry, C.-M.; Fortier, M.; Castellano, C.-A.; Wagner, R.; Cunnane, S.C. Plasma Ketone and Medium Chain Fatty Acid Response in Humans Consuming Different Medium Chain Triglycerides During a Metabolic Study Day. Front. Nutr. 2019, 6, 46. [Google Scholar] [CrossRef]
- Jones, P.M.; Butt, Y.; Messmer, B.; Boriak, R.; Bennett, M.J. Medium-Chain Fatty Acids Undergo Elongation before Beta-Oxidation in Fibroblasts. Biochem. Biophys. Res. Commun. 2006, 346, 193–197. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; van Harskamp, D.; Schierbeek, H.; Bleeker, J.C.; Crefcoeur, L.L.; Ferdinandusse, S.; van Goudoever, J.B.; Houtkooper, R.H.; IJlst, L.; Langeveld, M.; et al. Exploring the Metabolic Fate of Medium-Chain Triglycerides in Healthy Individuals Using a Stable Isotope Tracer. Clin. Nutr. 2021, 40, 1396–1404. [Google Scholar] [CrossRef]
- Roe, C.R.; Brunengraber, H. Anaplerotic Treatment of Long-Chain Fat Oxidation Disorders with Triheptanoin: Review of 15 Years Experience. Mol. Genet. Metab. 2015, 116, 260–268. [Google Scholar] [CrossRef]
- Roe, C.R.; Sweetman, L.; Roe, D.S.; David, F.; Brunengraber, H. Treatment of Cardiomyopathy and Rhabdomyolysis in Long-Chain Fat Oxidation Disorders Using an Anaplerotic Odd-Chain Triglyceride. J. Clin. Investig. 2002, 110, 259–269. [Google Scholar] [CrossRef]
- German, H.M.; Ciapaite, J.; Verhoeven-Duif, N.M.; Jans, J.J.M. Anaplerosis by Medium-Chain Fatty Acids through Complex Interplay with Glucose and Glutamine Metabolism. J. Biol. Chem. 2025, 301, 108307. [Google Scholar] [CrossRef]
- Borges, K.; Sonnewald, U. Triheptanoin—A Medium Chain Triglyceride with Odd Chain Fatty Acids: A New Anaplerotic Anticonvulsant Treatment? Epilepsy Res. 2012, 100, 239–244. [Google Scholar] [CrossRef]
- Porta, F.; Maiorana, A.; Gragnaniello, V.; Procopio, E.; Gasperini, S.; Taurisano, R.; Spada, M.; Dionisi-Vici, C.; Burlina, A. Triheptanoin in Patients with Long-Chain Fatty Acid Oxidation Disorders: Clinical Experience in Italy. Ital. J. Pediatr. 2024, 50, 204. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, M.B.; Heitner, S.B.; Martin, J.; Rose, S.; Goldstein, A.; El-Gharbawy, A.H.; Deward, S.; Lasarev, M.R.; Pollaro, J.; DeLany, J.P.; et al. Triheptanoin versus Trioctanoin for Long-Chain Fatty Acid Oxidation Disorders: A Double Blinded, Randomized Controlled Trial. J. Inherit. Metab. Dis. 2017, 40, 831–843. [Google Scholar] [CrossRef]
- Zöggeler, T.; Stock, K.; Jörg-Streller, M.; Spenger, J.; Konstantopoulou, V.; Hufgard-Leitner, M.; Scholl-Bürgi, S.; Karall, D. Long-Term Experience with Triheptanoin in 12 Austrian Patients with Long-Chain Fatty Acid Oxidation Disorders. Orphanet J. Rare Dis. 2021, 16, 28. [Google Scholar] [CrossRef]
- Kahraman, A.B.; Yildiz, Y.; Gokmen-Ozel, H.; Kadayifcilar, S.; Sivri, S. Successful Management of Rhabdomyolysis with Triheptanoin in a Child with Severe Long-Chain 3-Hydroxyacyl-Coenzyme A Dehydrogenase (LCHAD) Deficiency. Neuromuscul. Disord. 2023, 33, 315–318. [Google Scholar] [CrossRef]
- Guffon, N.; Mochel, F.; Schiff, M.; De Lonlay, P.; Douillard, C.; Vianey-Saban, C. Clinical Outcomes in a Series of 18 Patients with Long Chain Fatty Acids Oxidation Disorders Treated with Triheptanoin for a Median Duration of 22 Months. Mol. Genet. Metab. 2021, 132, 227–233. [Google Scholar] [CrossRef]
- Vockley, J.; Burton, B.K.; Berry, G.; Longo, N.; Phillips, J.; Sanchez-Valle, A.; Chapman, K.A.; Tanpaiboon, P.; Grunewald, S.; Murphy, E.; et al. Triheptanoin for the Treatment of Long-Chain Fatty Acid Oxidation Disorders: Final Results of an Open-Label, Long-Term Extension Study. J. Inherit. Metab. Dis. 2023, 46, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Medicines and Healthcare Products Regulatory Agency (MHRA) Early Access to Medicines Scheme—Treatment Protocol—Information for Healthcare Professionals: Dojolvi (Triheptanoin). Available online: https://assets.publishing.service.gov.uk/media/67f521a2563cc9c84bacc328/EAMS_Treatment_Protocol_for_Healthcare_Professionals_Dojolvi__Triheptanoin_.pdf (accessed on 12 June 2025).
- FDA DOJOLVI (Triheptanoin) Oral Liquid Initial U.S. Approval 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213687s000lbl.pdf (accessed on 20 August 2025).
- Ruppert, P.M.M.; Kersten, S. Regulation of Adipose Tissue Metabolism During Fasting. Annu. Rev. Nutr. 2025, 45, 41–64. [Google Scholar] [CrossRef]
- Guilherme, A.; Rowland, L.A.; Wang, H.; Czech, M.P. The Adipocyte Supersystem of Insulin and cAMP Signaling. Trends Cell Biol. 2023, 33, 340–354. [Google Scholar] [CrossRef]
- Halldin, M.U.; Forslund, A.; von Döbeln, U.; Eklund, C.; Gustafsson, J. Increased Lipolysis in LCHAD Deficiency. J. Inherit. Metab. Dis. 2007, 30, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Ottenberger, A.; Gleich, F.; Maier, E.M.; Lindner, M.; Husain, R.A.; Palm, K.; Beblo, S.; Freisinger, P.; Santer, R.; et al. Neurological Outcome in Long-Chain Hydroxy Fatty Acid Oxidation Disorders. Ann. Clin. Transl. Neurol. 2024, 11, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Ferdinandusse, S.; Houtkooper, R.H.; Langeveld, M.; Nederveen, A.J.; Strijkers, G.J.; Visser, G.; Wanders, R.J.A.; Wijburg, F.A.; et al. Subclinical Effects of Long-Chain Fatty Acid β-Oxidation Deficiency on the Adult Heart: A Case-Control Magnetic Resonance Study. J. Inherit. Metab. Dis. 2020, 43, 969–980. [Google Scholar] [CrossRef]
- Van Calcar, S.C.; Sowa, M.; Rohr, F.; Beazer, J.; Setlock, T.; Weihe, T.U.; Pendyal, S.; Wallace, L.S.; Hansen, J.G.; Stembridge, A.; et al. Nutrition Management Guideline for Very-Long Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD): An Evidence- and Consensus-Based Approach. Mol. Genet. Metab. 2020, 131, 23–37. [Google Scholar] [CrossRef]
- Everard, E.; Laeremans, H.; Boemer, F.; Marie, S.; Vincent, M.-F.; Dewulf, J.P.; Debray, F.-G.; De Laet, C.; Nassogne, M.-C. Impact of Newborn Screening for Fatty Acid Oxidation Disorders on Neurological Outcome: A Belgian Retrospective and Multicentric Study. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2024, 49, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Shakerdi, L.A.; McNulty, J.; Gillman, B.; McCarthy, C.M.; Ivory, J.; Sheerin, A.; O’Byrne, J.J.; Donnelly, J.C.; Treacy, E.P. Management of Pregnancy in a Patient with Long-Chain 3-Hydroxyacyl CoA Dehydrogenase Deficiency. JIMD Rep. 2022, 63, 265–270. [Google Scholar] [CrossRef]
- Gillingham, M.B. Nutrition Studies in Long-Chain Fatty Acid Oxidation Disorders: Diet Composition and Monitoring. In Nutrition Management of Inherited Metabolic Diseases; Bernstein, L.E., Rohr, F., Helm, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 255–270. ISBN 978-3-319-14620-1. [Google Scholar]
- Burris, T.P.; De Vera, I.M.S.; Cote, I.; Flaveny, C.A.; Wanninayake, U.S.; Chatterjee, A.; Walker, J.K.; Steinauer, N.; Zhang, J.; Coons, L.A.; et al. International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily—Update 2023. Pharmacol. Rev. 2023, 75, 1233–1318. [Google Scholar] [CrossRef]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 8298. [Google Scholar] [CrossRef] [PubMed]
- Karbowska, J.; Kochan, Z.; Smoleński, R.T. Peroxisome Proliferator-Activated Receptor α Is Downregulated in the Failing Human Heart. Cell. Mol. Biol. Lett. 2003, 8, 49–53. [Google Scholar]
- Malapaka, R.R.V.; Khoo, S.; Zhang, J.; Choi, J.H.; Zhou, X.E.; Xu, Y.; Gong, Y.; Li, J.; Yong, E.-L.; Chalmers, M.J.; et al. Identification and Mechanism of 10-Carbon Fatty Acid as Modulating Ligand of Peroxisome Proliferator-Activated Receptors. J. Biol. Chem. 2012, 287, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Mihaylov, S.R.; Castelli, L.M.; Lin, Y.-H.; Gül, A.; Soni, N.; Hastings, C.; Flynn, H.R.; Păun, O.; Dickman, M.J.; Snijders, A.P.; et al. The Master Energy Homeostasis Regulator PGC-1α Exhibits an mRNA Nuclear Export Function. Nat. Commun. 2023, 14, 5496. [Google Scholar] [CrossRef]
- Nishida, R.; Nukaga, S.; Kawahara, I.; Miyagawa, Y.; Goto, K.; Nakashima, C.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; et al. Differential Effects of Three Medium-Chain Fatty Acids on Mitochondrial Quality Control and Skeletal Muscle Maturation. Antioxidants 2024, 13, 821. [Google Scholar] [CrossRef]
- Charlot, A.; Morel, L.; Bringolf, A.; Georg, I.; Charles, A.-L.; Goupilleau, F.; Geny, B.; Zoll, J. Octanoic Acid-Enrichment Diet Improves Endurance Capacity and Reprograms Mitochondrial Biogenesis in Skeletal Muscle of Mice. Nutrients 2022, 14, 2721. [Google Scholar] [CrossRef]
- Wang, B.; Fu, J.; Li, L.; Gong, D.; Wen, X.; Yu, P.; Zeng, Z. Medium-Chain Fatty Acid Reduces Lipid Accumulation by Regulating Expression of Lipid-Sensing Genes in Human Liver Cells with Steatosis. Int. J. Food Sci. Nutr. 2016, 67, 288–297. [Google Scholar] [CrossRef]
- Rial, S.A.; Ravaut, G.; Malaret, T.B.; Bergeron, K.-F.; Mounier, C. Hexanoic, Octanoic and Decanoic Acids Promote Basal and Insulin-Induced Phosphorylation of the Akt-mTOR Axis and a Balanced Lipid Metabolism in the HepG2 Hepatoma Cell Line. Molecules 2018, 23, 2315. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Baumgardner, J.N.; Sharma, N.; Vantrease, J.; Ferguson, M.; Tong, Y.; Wu, X.; Cleves, M.A.; Badger, T.M. Medium Chain Triglycerides Dose-Dependently Prevent Liver Pathology in a Rat Model of Non-Alcoholic Fatty Liver Disease. Exp. Biol. Med. 2013, 238, 151–162. [Google Scholar] [CrossRef]
- Wakabayashi, M.; Kamijo, Y.; Nakajima, T.; Tanaka, N.; Sugiyama, E.; Yangyang, T.; Kimura, T.; Aoyama, T. Fatty Acid Accumulation and Resulting PPARα Activation in Fibroblasts Due to Trifunctional Protein Deficiency. PPAR Res. 2012, 2012, 371691. [Google Scholar] [CrossRef]
- Cao, Y.; Araki, M.; Nakagawa, Y.; Deisen, L.; Lundsgaard, A.; Kanta, J.M.; Holm, S.; Johann, K.; Brings Jacobsen, J.C.; Jähnert, M.; et al. Dietary Medium-Chain Fatty Acids Reduce Hepatic Fat Accumulation via Activation of a CREBH-FGF21 Axis. Mol. Metab. 2024, 87, 101991. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Borlak, J. A Comparative Genomic Study across 396 Liver Biopsies Provides Deep Insight into FGF21 Mode of Action as a Therapeutic Agent in Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Transl. Med. 2025, 15, e70218. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARalpha and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.L.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast Growth Factor 21 Regulates Energy Metabolism by Activating the AMPK-SIRT1-PGC-1alpha Pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Huh, C.-S.; Choi, I.-D.; Jeong, J.-W.; Ku, H.-K.; Ra, J.-H.; Kim, T.-Y.; Kim, G.-B.; Sim, J.-H.; Ahn, Y.-T. The Anti-Diabetic Activity of Bifidobacterium Lactis HY8101 in Vitro and in Vivo. J. Appl. Microbiol. 2014, 117, 834–845. [Google Scholar] [CrossRef]
- Collins, Q.F.; Xiong, Y.; Lupo, E.G.; Liu, H.-Y.; Cao, W. P38 Mitogen-Activated Protein Kinase Mediates Free Fatty Acid-Induced Gluconeogenesis in Hepatocytes. J. Biol. Chem. 2006, 281, 24336–24344. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New Insights into Activation and Function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Korpela, M.; Isohanni, P.; Marjavaara, S.K.; Tyni, T.; Kiuru-Enari, S.; et al. FGF-21 as a Biomarker for Muscle-Manifesting Mitochondrial Respiratory Chain Deficiencies: A Diagnostic Study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.P.; Morgan, D.A.; Sullivan, A.I.; Fu, X.; Inigo-Vollmer, M.; Burgess, S.C.; Meyerholz, D.K.; Rahmouni, K.; Potthoff, M.J. FGF21 Reverses MASH through Coordinated Actions on the CNS and Liver. Cell Metab. 2025, 37, 1515–1529.e6. [Google Scholar] [CrossRef] [PubMed]
| Enzyme/ Activity | Gene | Substrates | Activity |
|---|---|---|---|
| Very-Long- and Long-Chain β-oxidation System | |||
| VLCAD | ACADVL | C12–C24 | very-long-chain acyl-CoA dehydrogenase, EC:1.3.8.9 |
| ACAD9 | ACAD9 | – | moonlighting protein, probably oxidating long-chain acyl-CoAs |
| LCAD | ACADL | C8–C18 | long-chain acyl-CoA dehydrogenase, EC:1.3.8.8 |
| MTP-LCEH | HADHA | C8–C24 | long-chain enoyl-CoA hydratase, EC:4.2.1.17 |
| MTP-MLCLAT | HADHA | C18 | monolysocardiolipin acyltransferase, EC:2.3.1 |
| MTP-LCHAD | HADHA | C6–C24 | long-chain 3-hydroxyacyl-CoA dehydrogenase, EC:1.1.1.211 |
| MTP-LCKAT | HADHB | C6–C24 | long-chain 3-ketoacyl-CoA thiolase, EC:2.3.1.155/EC:2.3.1.16 |
| Medium- and Short-Chain β-oxidation System | |||
| MCAD | ACADM | C6–C16 | medium-chain acyl-CoA dehydrogenase, EC:1.3.8.7 |
| SCAD | ACADS | C4–C6 | short-chain acyl-CoA dehydrogenase, EC:1.3.8.1 |
| ECHS1 | ECHS1 | C4–C16 | enoyl-CoA hydratase, EC:4.2.1.17/EC:5.3.3.8 |
| HADH | HADH | C4–C16 | hydroxyacyl-CoA dehydrogenase, EC:1.1.1.35 |
| KAT | ACAA2 | C4–C16 | 3-ketoacyl-CoA thiolase, EC:2.3.1.16 |
| Source | Fatty Acids | |||||
|---|---|---|---|---|---|---|
| <C14 | C14 | C16 | C18 | C20 | >C20 | |
| Cooking Oils and Spreads [per 100 g] (% total fat) | ||||||
| Canola oil | 0 g | 0 g | 4.51 g (4.55%) | 92.33 g (93.1%) | 1.32 g (1.33%) | 0.33 g (0.33%) |
| Corn oil | 0 g | 0.03 g (0.03%) | 10.9 g (11.6%) | 82.8 g (87.5%) | 0.25 g (0.26%) | 0.21 g (0.22%) |
| Olive oil | 0 g | 0 g | 12.6 g (13.0%) | 83.8 g (86.2%) | 0.31 g (0.32%) | 0.13 g (0.13%) |
| Soybean oil | 0 g | 0.10 g (0.10%) | 9.82 g (10.2%) | 85.1 g (88.6%) | 0.19 g (0.20%) | 0.48 g (0.50%) |
| Sunflower oil | 0.01 g | 0.05 g (0.05%) | 4.60 g (4.94%) | 86.8 g (93.2%) | 0.26 g (0.28%) | 1.15 g (1.23%) |
| Butter | 11.6 g (14.4%) | 7.44 g (9.29%) | 23.5 g (29.4%) | 37.0 g (46.2%) | 0.10 g (0.12%) | 0 g |
| Lard | 0.30 g (0.32%) | 4.00 g (4.21%) | 65.0 g (68.4%) | 25.7 g (27.1%) | 0 g | 0 g |
| Fatty and Processed Meats [per 100 g] (% total fat) | ||||||
| Pork, shoulder | 0.03 g (0.19%) | 0.22 g (1.38%) | 4.39 g (27.5%) | 11.1 g (69.5%) | 0.23 g (1.45%) | 0 g |
| Salami, pork | 0 g | 0.52 g (1.66%) | 8.86 g (28.3%) | 21.8 g (69.6%) | 0.16 g (0.51%) | 0 g |
| High-Fat Dairy [per 100 g] (% total fat) | ||||||
| Cheese, Gouda | 4.19 g (16.8%) | 3.04 g (12.2%) | 7.74 g (31.0%) | 9.97 g (40.0%) | 0 g | 0 g |
| Cheese, cream | 3.54 g (11.2%) | 3.63 g (11.5%) | 10.1 g (31.7%) | 14.1 g (44.4%) | 0.17 g (0.52%) | 0.05 g (0.16%) |
| Snacks and Baked Goods [per 100 g] (% total fat) | ||||||
| Muffins, blueberry | 0 g | 0 g | 1.91 g (12.0%) | 13.8 g (86.3%) | 0.13 g (0.80%) | 0.08 g (0.50%) |
| Snacks, potato sticks | 0 g | 0.27 g (0.82%) | 8.08 g (24.6%) | 24.6 g (74.7%) | 0.03 g (0.09%) | 0 g |
| Nuts and Nut Butter [per 100 g] (% total fat) | ||||||
| Walnuts | 0 g | 0 g | 4.40 g (7.07%) | 57.6 g (92.6%) | 0.13 g (0.22%) | 0 g |
| Peanuts | 0 g | 0.03 g (0.05%) | 5.16 g (11.13%) | 40.50 g (87.39%) | 0.66 g (1.43%) | 0 g |
| Peanut butter | 0.02 g (0.05%) | 0.05 g (0.11%) | 5.50 g (12.1%) | 39.3 g (86.2%) | 0.81 g (1.76%) | 0 g |
| Fatty Fish [per 100 g] (% total fat) | ||||||
| Herring | 0.02 g (0.20%) | 0.55 g (6.59%) | 1.97 g (23.5%) | 1.86 g (22.2%) | 1.49 g (17.7%) | 1.76 g (20.9%) |
| Mackerel | 0.02 g (0.14%) | 0.67 g (5.67%) | 2.85 g (24.0%) | 3.08 g (25.9%) | 2.12 g (17.9%) | 3.02 g (25.4%) |
| Salmon | 0 g | 0.56 g (5.33%) | 2.67 g (25.6%) | 4.28 g (41.0%) | 1.22 g (11.7%) | 1.50 g (14.4%) |
| Source | Caproic (Hexanoic) Acid C6 | Caprylic (Octanoic) Acid C8 | Capric (Decanoic) Acid C10 | Lauric (Dodecanoic) Acid C12 | Other Fatty Acids >C12 |
|---|---|---|---|---|---|
| Natural Dietary Sources [per 100 g] (% total fat) | |||||
| Coconut oil | 0.477 g (0.53%) | 6.80 g (7.51%) | 5.39 g (5.95%) | 41.8 g (46.2%) | 35.9 g (39.7%) |
| Palm kernel oil | 0.2 g (0.21%) | 3.3 g (3.49%) | 3.7 g (3.92%) | 47.0 g (49.7%) | 40.3 g (42.7%) |
| Milk, cow, whole | 0.054 g (1.97%) | 0.034 g (1.24%) | 0.084 g (3.07%) | 0.097 g (3.54%) | 2.40 g (87.6%) |
| Milk, goat | 0.09 g (2.65%) | 0.10 g (2.71%) | 0.26 g (7.33%) | 0.12 g (3.50%) | 2.89 g (81.3%) |
| Milk, sheep | 0.15 g (2.35%) | 0.14 g (2.24%) | 0.40 g (6.48%) | 0.24 g (3.87%) | 5.18 g (83.8%) |
| Milk, human | 0 g | 0 g | 0.063 g (1.59%) | 0.256 g (6.48%) | 3.63 g (93.2%) |
| Medical Foods [per 100 mL] (% total fat) | |||||
| MCT oil Nutricia (coconut and/or palm oil) | 0.44 g (0.50%) | 52.1 g (59.6%) | 34.6 g (39.6%) | 0.17 g (0.19%) | 0.09 g (0.10%) |
| Clinical Manifestations | Pathophysiology | Dietary Strategies |
|---|---|---|
| Hypoketotic hypoglycemia |
|
|
| Rhabdomyolysis (skeletal muscle myopathy) |
|
|
| Cardiomyopathy and arrhythmias |
|
|
| Hepatic dysfunction |
|
|
| Pigmentary retinopathy |
|
|
| Peripheral neuropathy |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, Z.; Karbowska, J. How Genes Meet Diet in LCHAD Deficiency: Nutrigenomics of Fatty Acid Oxidation Disorder. Int. J. Mol. Sci. 2025, 26, 10140. https://doi.org/10.3390/ijms262010140
Kochan Z, Karbowska J. How Genes Meet Diet in LCHAD Deficiency: Nutrigenomics of Fatty Acid Oxidation Disorder. International Journal of Molecular Sciences. 2025; 26(20):10140. https://doi.org/10.3390/ijms262010140
Chicago/Turabian StyleKochan, Zdzislaw, and Joanna Karbowska. 2025. "How Genes Meet Diet in LCHAD Deficiency: Nutrigenomics of Fatty Acid Oxidation Disorder" International Journal of Molecular Sciences 26, no. 20: 10140. https://doi.org/10.3390/ijms262010140
APA StyleKochan, Z., & Karbowska, J. (2025). How Genes Meet Diet in LCHAD Deficiency: Nutrigenomics of Fatty Acid Oxidation Disorder. International Journal of Molecular Sciences, 26(20), 10140. https://doi.org/10.3390/ijms262010140






