Determinants of Chain Selection and Staggering in Heterotrimeric Collagens: A Comprehensive Review of the Structural Data
Abstract
1. Background
2. The Molecular and Functional Diversity of the Collagen Superfamily
3. Heterotrimeric Procollagens: Analysis of the Sequence Similarities
Pairwise Sequence Comparisons
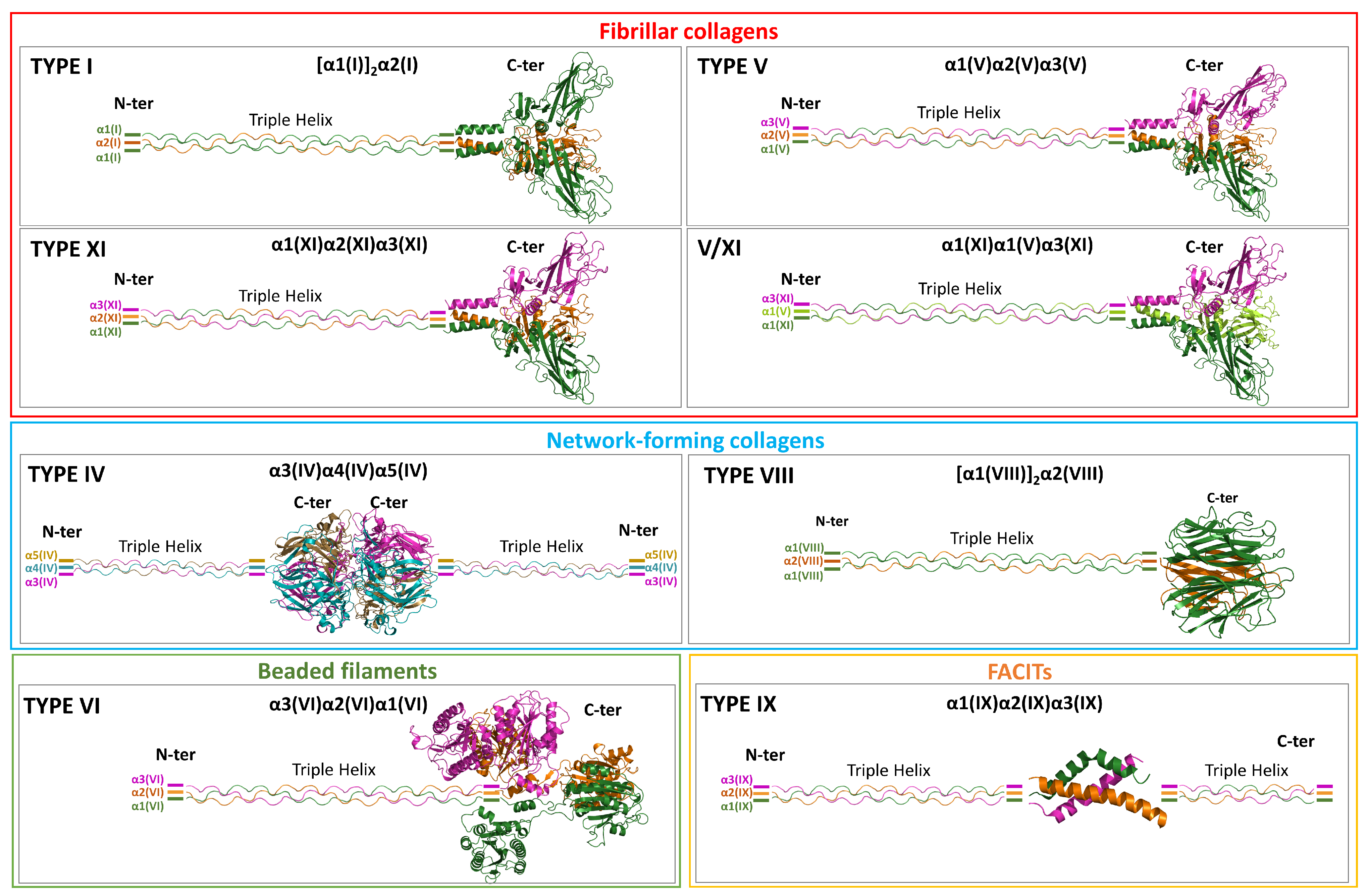
4. Heterotrimeric Procollagens: Structural Data
4.1. Survey of the PDB and AlphaFold Predictions
4.2. Heterotrimeric Fibrillar Collagens
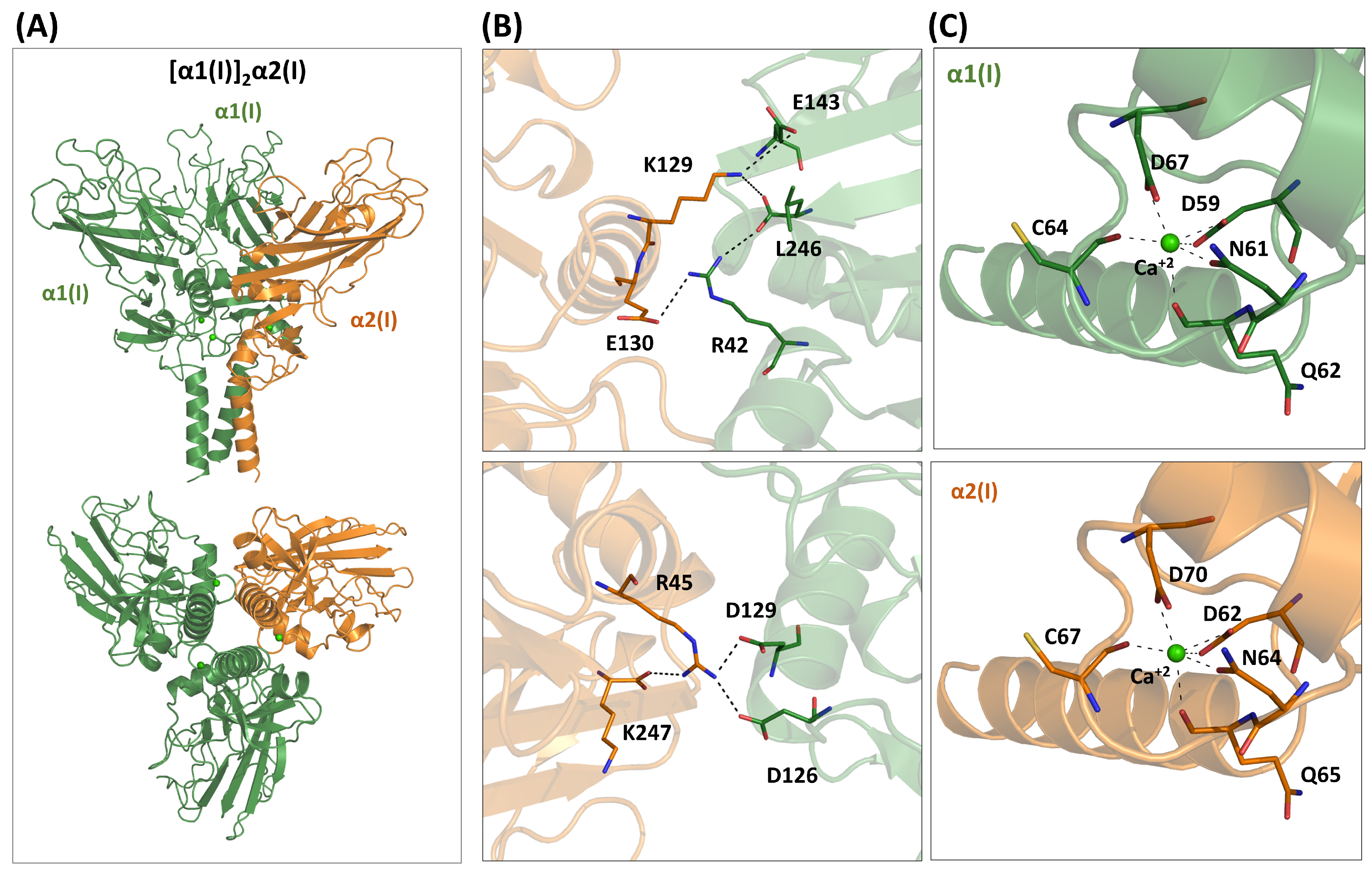
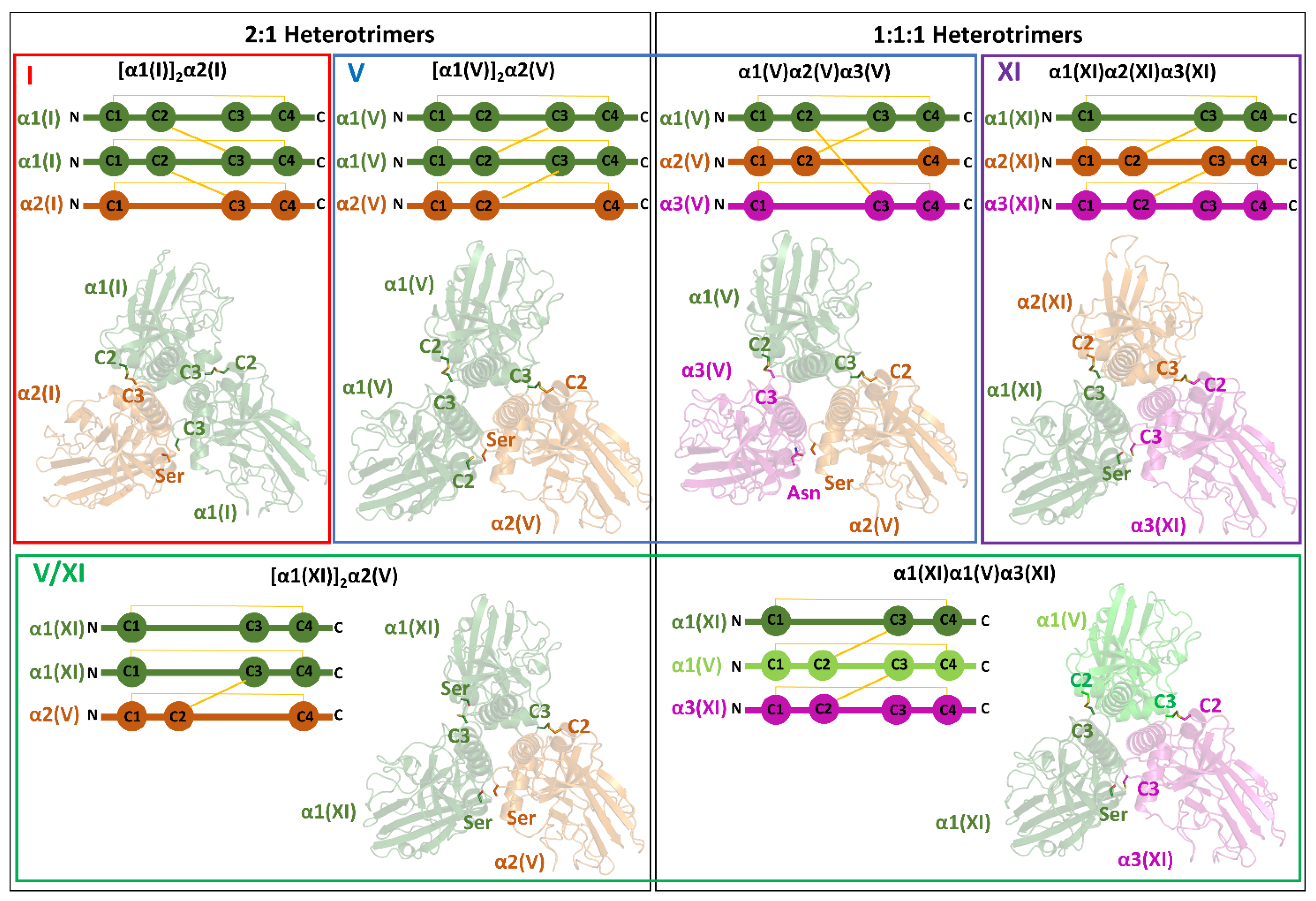
4.2.1. Type I
4.2.2. Type V
4.2.3. Type XI
4.2.4. Mixed Type V/XI
4.3. Heterotrimeric Network-Forming Collagens (Types IV and VIII)
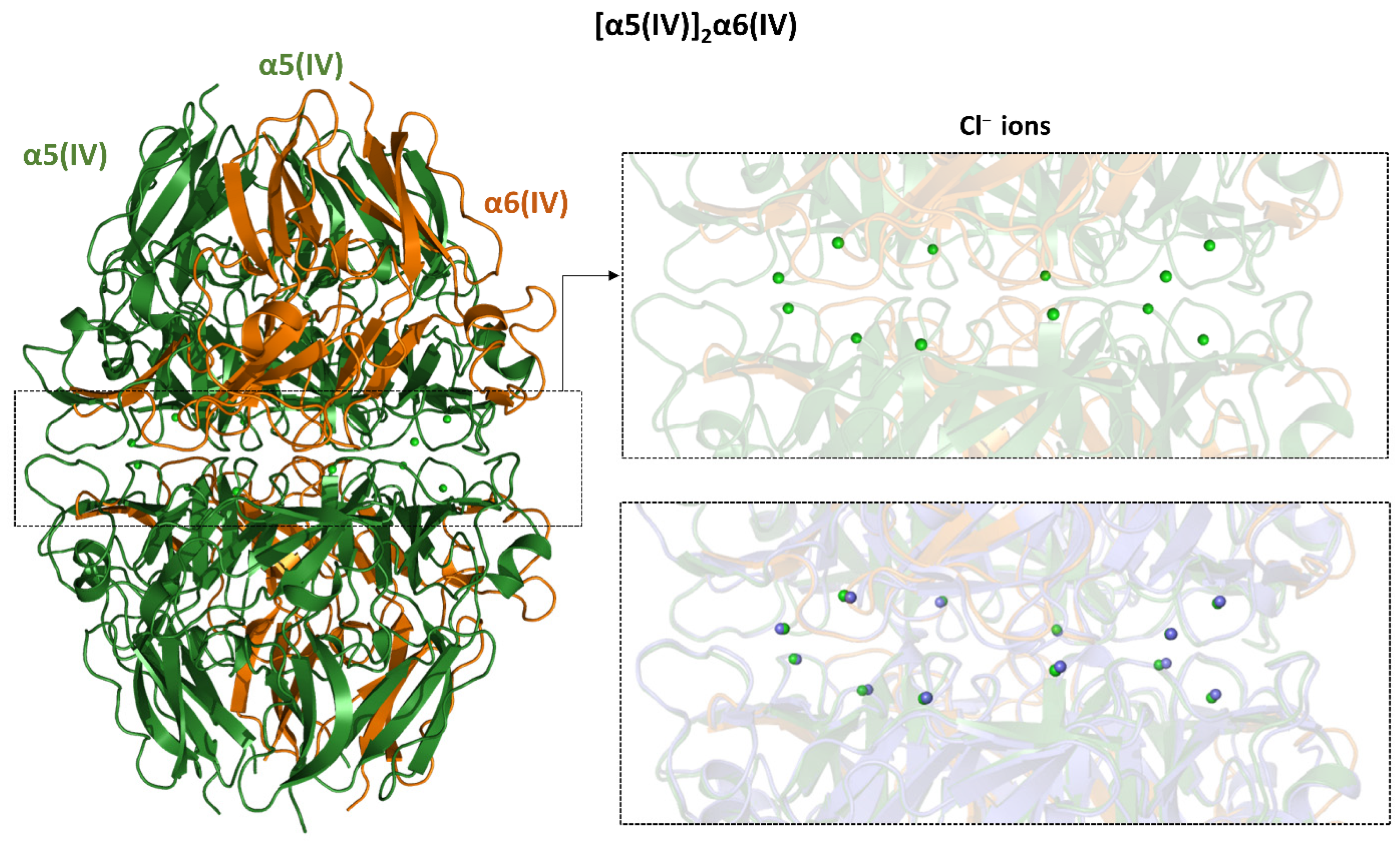
4.3.1. Type IV
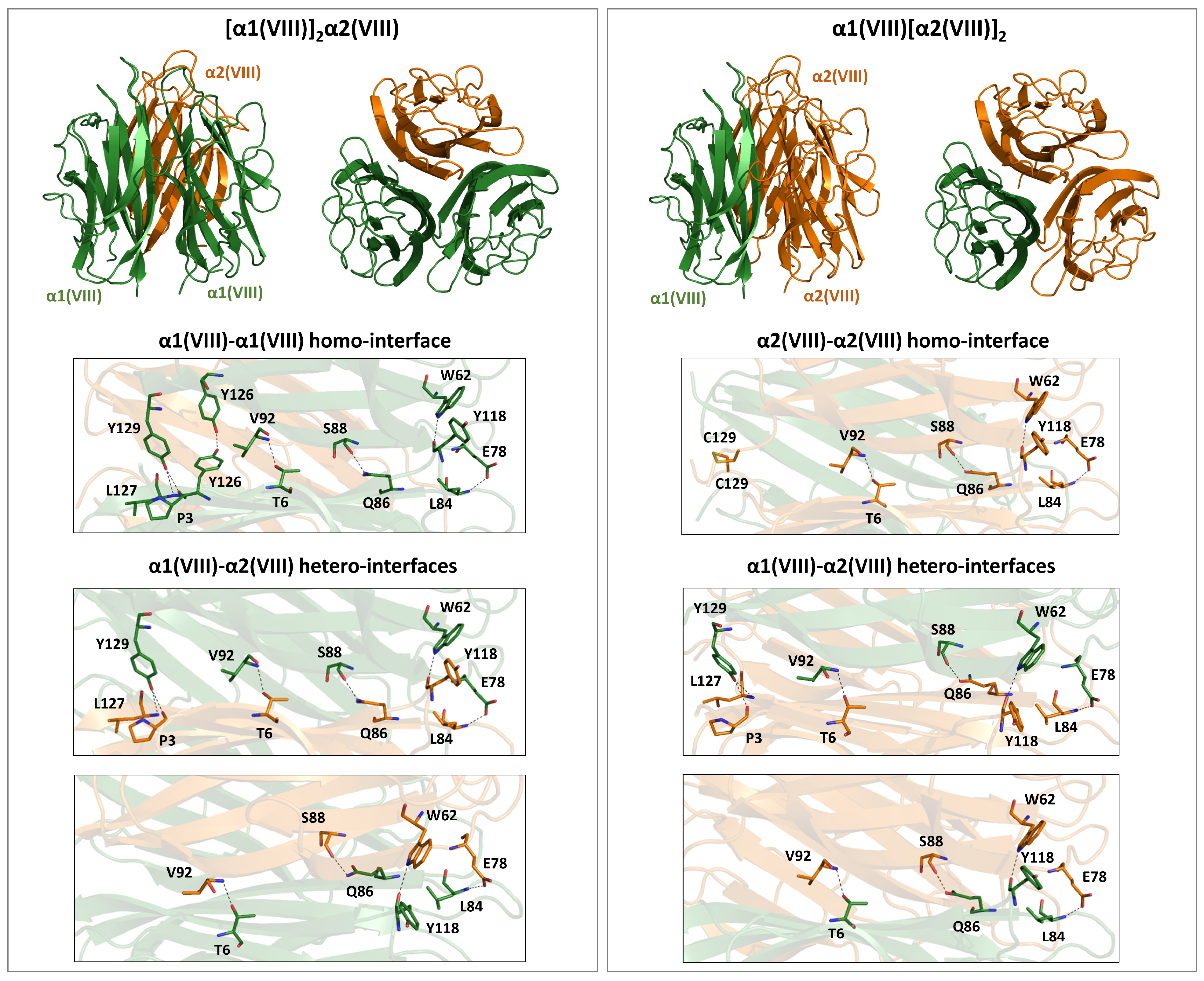
4.3.2. Type VIII
4.4. Heterotrimeric Beaded Filaments-Type VI
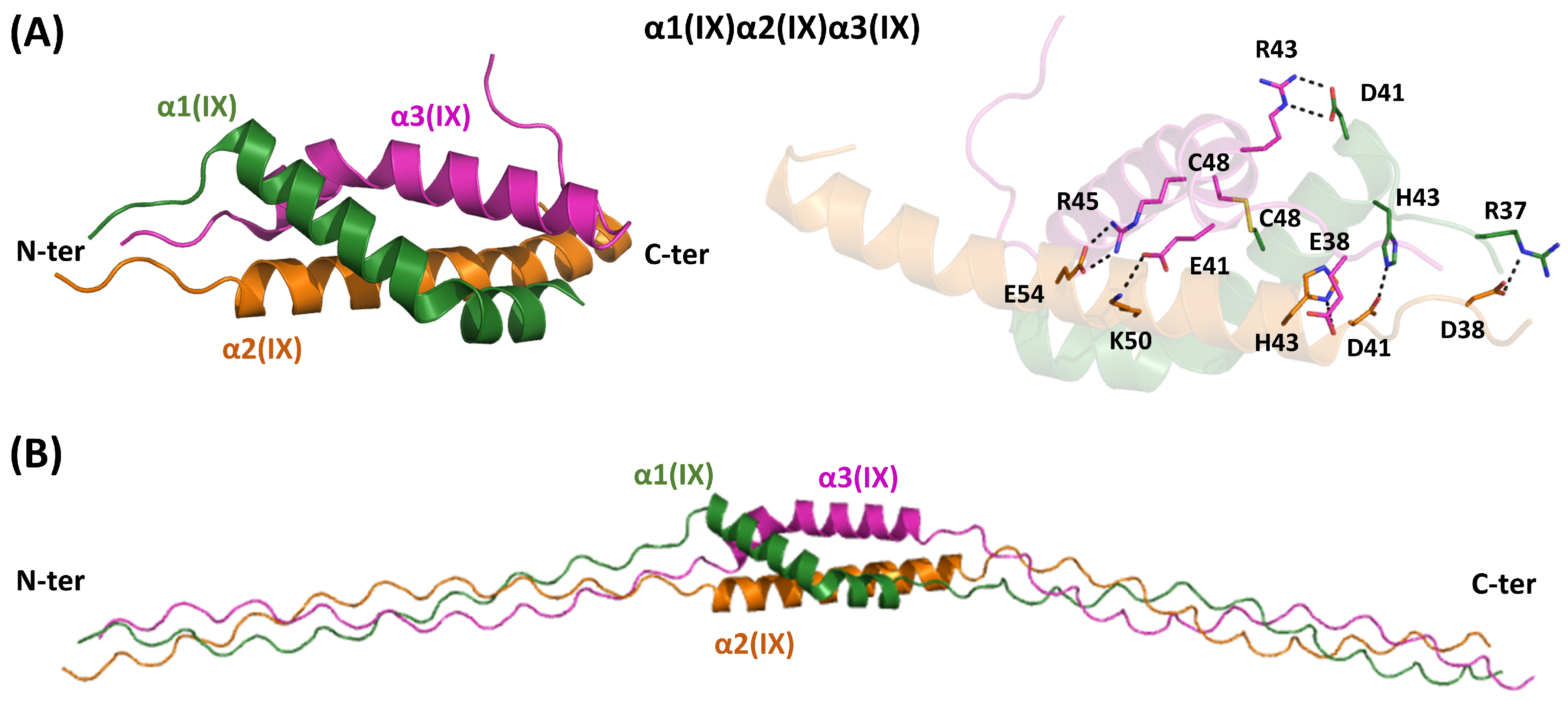
4.5. Heterotrimeric FACITs-Type IX
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulkarni, P.; Porter, L.; Chou, T.-F.; Chong, S.; Chiti, F.; Schafer, J.W.; Mohanty, A.; Ramisetty, S.; Onuchic, J.N.; Tuite, M.; et al. Evolving Concepts of the Protein Universe. iScience 2025, 28, 112012. [Google Scholar] [CrossRef]
- Richardson, J.S. The Anatomy and Taxonomy of Protein Structure. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 1981; Volume 34, pp. 167–339. ISBN 978-0-12-034234-1. [Google Scholar]
- Squire, J.M.; Parry, D.A.D. Fibrous Protein Structures: Hi erarchy, History and Heroes. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 1–33. ISBN 978-3-319-49672-6. [Google Scholar]
- Dunker, A.K.; Babu, M.M.; Barbar, E.; Blackledge, M.; Bondos, S.E.; Dosztányi, Z.; Dyson, H.J.; Forman-Kay, J.; Fuxreiter, M.; Gsponer, J.; et al. What’s in a Name? Why These Proteins Are Intrinsically Disordered: Why These Proteins Are IntrinsicallyDisordered. Intrinsically Disord. Proteins 2013, 1, e24157. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; Vitagliano, L. Polyproline and Triple Helix Motifs in Host-Pathogen Recognition. Curr. Protein Pept. Sci. 2012, 13, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Berisio, R.; De Simone, A.; Ruggiero, A.; Improta, R.; Vitagliano, L. Role of Side Chains in Collagen Triple Helix Stabilization and Partner Recognition. J. Pept. Sci. 2009, 15, 131–140. [Google Scholar] [CrossRef]
- Bella, J. Collagen Structure: New Tricks from a Very Old Dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular Structure of the Collagen Triple Helix. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 70, pp. 301–339. ISBN 978-0-12-034270-9. [Google Scholar]
- Okuyama, K.; Xu, X.; Iguchi, M.; Noguchi, K. Revision of Collagen Molecular Structure. Biopolymers 2006, 84, 181–191. [Google Scholar] [CrossRef]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar Structure of Type I Collagen in Situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H.M. Crystal and Molecular Structure of a Collagen-Like Peptide at 1.9 Å Resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef]
- Kramer, R.Z.; Vitagliano, L.; Bella, J.; Berisio, R.; Mazzarella, L.; Brodsky, B.; Zagari, A.; Berman, H.M. X-Ray Crystallographic Determination of a Collagen-like Peptide with the Repeating Sequence (Pro-Pro-Gly). J. Mol. Biol. 1998, 280, 623–638. [Google Scholar] [CrossRef]
- Boudko, S.P.; Engel, J.; Okuyama, K.; Mizuno, K.; Bächinger, H.P.; Schumacher, M.A. Crystal Structure of Human Type III Collagen Gly991–Gly1032 Cystine Knot-Containing Peptide Shows Both 7/2 and 10/3 Triple Helical Symmetries. J. Biol. Chem. 2008, 283, 32580–32589. [Google Scholar] [CrossRef]
- Berisio, R.; Vitagliano, L.; Mazzarella, L.; Zagari, A. Crystal Structure of the Collagen Triple Helix Model [(Pro-Pro-Gly)10]3. Protein Sci. 2002, 11, 262–270. [Google Scholar] [CrossRef]
- Walker, D.R.; Hulgan, S.A.H.; Peterson, C.M.; Li, I.-C.; Gonzalez, K.J.; Hartgerink, J.D. Predicting the Stability of Homotrimeric and Heterotrimeric Collagen Helices. Nat. Chem. 2021, 13, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Fallas, J.A.; O’Leary, L.E.R.; Hartgerink, J.D. Synthetic Collagen Mimics: Self-Assembly of Homotrimers, Heterotrimers and Higher Order Structures. Chem. Soc. Rev. 2010, 39, 3510. [Google Scholar] [CrossRef] [PubMed]
- Jalan, A.A.; Demeler, B.; Hartgerink, J.D. Hydroxyproline-Free Single Composition ABC Collagen Heterotrimer. J. Am. Chem. Soc. 2013, 135, 6014–6017. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.C.; Walker, D.R.; Hulgan, S.A.H.; Pogostin, B.H.; Swain, J.W.R.; Miller, M.D.; Xu, W.; Duella, R.; Misiura, M.; Wang, X.; et al. Heterotrimeric Collagen Helix with High Specificity of Assembly Results in a Rapid Rate of Folding. Nat. Chem. 2024, 16, 1698–1704. [Google Scholar] [CrossRef]
- Bächinger, H.P.; Boudko, S.P. Mysteries of the Collagen Triple Helix. Matrix Biol. 2025, 137, 12–18. [Google Scholar] [CrossRef]
- Holmgren, S.K.; Taylor, K.M.; Bretscher, L.E.; Raines, R.T. Code for Collagen’s Stability Deciphered. Nature 1998, 392, 666–667. [Google Scholar] [CrossRef]
- Berisio, R.; Granata, V.; Vitagliano, L.; Zagari, A. Imino Acids and Collagen Triple Helix Stability: Characterization of Collagen-like Polypeptides Containing Hyp-Hyp-Gly Sequence Repeats. J. Am. Chem. Soc. 2004, 126, 11402–11403. [Google Scholar] [CrossRef]
- Boudko, S.P.; Engel, J.; Bächinger, H.P. The Crucial Role of Trimerization Domains in Collagen Folding. Int. J. Biochem. Cell Biol. 2012, 44, 21–32. [Google Scholar] [CrossRef]
- Khoshnoodi, J.; Cartailler, J.-P.; Alvares, K.; Veis, A.; Hudson, B.G. Molecular Recognition in the Assembly of Collagens: Terminal Noncollagenous Domains Are Key Recognition Modules in the Formation of Triple Helical Protomers. J. Biol. Chem. 2006, 281, 38117–38121. [Google Scholar] [CrossRef]
- Al-Shaer, A.; Forde, N.R. Decoding Collagen’s Thermally Induced Unfolding and Refolding Pathways. Proc. Natl. Acad. Sci. USA 2025, 122, e2420308122. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Shefelbine, S.J.; Buehler, M.J. Structural and Mechanical Differences between Collagen Homo- and Heterotrimers: Relevance for the Molecular Origin of Brittle Bone Disease. Biophys. J. 2012, 102, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.-N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural Basis of Homo- and Heterotrimerization of Collagen I. Nat. Commun. 2017, 8, 14671. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Naba, A. Mechanisms of Assembly and Remodelling of the Extracellular Matrix. Nat. Rev. Mol. Cell Biol. 2024, 25, 865–885. [Google Scholar] [CrossRef]
- Linden, T.A.; King, N. Widespread Distribution of Collagens and Collagen-Associated Domains in Eukaryotes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Garrone, R. Collagen, a Common Thread in Extracellular Matrix Evolution. Proc. Indian Acad. Sci. (Chem. Sci.) 1999, 111, 51–56. [Google Scholar] [CrossRef]
- Gay, S.; Miller, E.J. Overview: What Is Collagen, What Is Not. Ultrastruct. Pathol. 1983, 4, 365–377. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers, 3rd ed.; Karsdal, M.A., Ed.; AP Academic Press, an imprint of Elsevier: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2024; ISBN 978-0-443-15617-5. [Google Scholar]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The Triple Helix of Collagens—An Ancient Protein Structure That Enabled Animal Multicellularity and Tissue Evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J.S. Fibrillar Collagens. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 457–490. ISBN 978-3-319-49672-6. [Google Scholar]
- DiChiara, A.S.; Li, R.C.; Suen, P.H.; Hosseini, A.S.; Taylor, R.J.; Weickhardt, A.F.; Malhotra, D.; McCaslin, D.R.; Shoulders, M.D. A Cysteine-Based Molecular Code Informs Collagen C-Propeptide Assembly. Nat. Commun. 2018, 9, 4206. [Google Scholar] [CrossRef]
- Yammine, K.M.; Li, R.C.; Borgula, I.M.; Mirda Abularach, S.; DiChiara, A.S.; Raines, R.T.; Shoulders, M.D. An Outcome-Defining Role for the Triple-Helical Domain in Regulating Collagen-I Assembly. Proc. Natl. Acad. Sci. USA 2024, 121, e2412948121. [Google Scholar] [CrossRef]
- Malcor, J.-D.; Ferruz, N.; Romero-Romero, S.; Dhingra, S.; Sagar, V.; Jalan, A.A. Deciphering the Folding Code of Collagens. Nat. Commun. 2025, 16, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ayad, S.; Weiss, J.B. A New Look at Vitreous-Humour Collagen. Biochem. J. 1984, 218, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Weis, M.A.; Kim, L.S.; Carter, B.G.; Eyre, D.R. Differences in Chain Usage and Cross-Linking Specificities of Cartilage Type V/XI Collagen Isoforms with Age and Tissue. J. Biol. Chem. 2009, 284, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Mayne, R.; Burgeson, R.E. Structure and Function of Collagen Types; Biology of extracellular matrix; Academic Press: Orlando, FL, USA, 1987; ISBN 978-0-12-481280-2. [Google Scholar]
- Hoffman, G.G.; Branam, A.M.; Huang, G.; Pelegri, F.; Cole, W.G.; Wenstrup, R.M.; Greenspan, D.S. Characterization of the Six Zebrafish Clade B Fibrillar Procollagen Genes, with Evidence for Evolutionarily Conserved Alternative Splicing within the pro-A1(V) C-Propeptide. Matrix Biol. 2010, 29, 261–275. [Google Scholar] [CrossRef]
- Berman, H.M.; Burley, S.K. Protein Data Bank (PDB): Fifty-Three Years Young and Having a Transformative Impact on Science and Society. Quart. Rev. Biophys. 2025, 58, e9. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Henriksen, K.; Karsdal, M.A. Type I Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–11. ISBN 978-0-12-809847-9. [Google Scholar]
- Bourhis, J.-M.; Mariano, N.; Zhao, Y.; Harlos, K.; Exposito, J.-Y.; Jones, E.Y.; Moali, C.; Aghajari, N.; Hulmes, D.J.S. Structural Basis of Fibrillar Collagen Trimerization and Related Genetic Disorders. Nat. Struct. Mol. Biol. 2012, 19, 1031–1036. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Roles of the Procollagen C-Propeptides in Health and Disease. Essays Biochem. 2019, 63, 313–323. [Google Scholar] [CrossRef]
- Balasco, N.; Esposito, L.; Vitagliano, L. Structural Biology in the AlphaFold Era: How Far Is Artificial Intelligence from Deciphering the Protein Folding Code? Biomolecules 2025, 15, 674. [Google Scholar] [CrossRef]
- Weil, D.; Bernard, M.; Gargano, S.; Ramirez, F. The pro A2(V) Collagen Gene Is Evolutionarily Related to the Major Fibrillar-Forming Collagens. Nucl. Acids Res. 1987, 15, 181–198. [Google Scholar] [CrossRef]
- Mak, K.M.; Png, C.Y.M.; Lee, D.J. Type V Collagen in Health, Disease, and Fibrosis. Anat. Rec. 2016, 299, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Karsdal, M.A. Type V Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2024; pp. 55–60. ISBN 978-0-443-15617-5. [Google Scholar]
- Luo, Y.Y.; Szlarski, P.M.; Kehlet, S.N.; Karsdal, M.A. Type XI Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 99–106. ISBN 978-0-12-817068-7. [Google Scholar]
- Knupp, C.; Squire, J.M. Molecular Packing in Network-Forming Collagens. In Advances in Protein Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; Volume 70, pp. 375–403. ISBN 978-0-12-034270-9. [Google Scholar]
- Sand, J.M.B.; Genovese, F.; Gudmann, N.S.; Karsdal, M.A. Type IV Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–49. ISBN 978-0-12-817068-7. [Google Scholar]
- Ishikawa, Y.; Lennon, R.; Forneris, F.; Myllyharju, J.; Salo, A.M. Collagen IV Biosynthesis: Intracellular Choreography of Post-Translational Modifications. Matrix Biol. 2025, 140, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Than, M.E.; Henrich, S.; Huber, R.; Ries, A.; Mann, K.; Kühn, K.; Timpl, R.; Bourenkov, G.P.; Bartunik, H.D.; Bode, W. The 1.9-Å Crystal Structure of the Noncollagenous (NC1) Domain of Human Placenta Collagen IV Shows Stabilization via a Novel Type of Covalent Met-Lys Cross-Link. Proc. Natl. Acad. Sci. USA 2002, 99, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, M.; Meiyappan, M.; Todd, P.; Hudson, B.G. Crystal Structure of NC1 Domains. J. Biol. Chem. 2002, 277, 31142–31153. [Google Scholar] [CrossRef]
- Vanacore, R.M.; Shanmugasundararaj, S.; Friedman, D.B.; Bondar, O.; Hudson, B.G.; Sundaramoorthy, M. The A1.A2 Network of Collagen IV. J. Biol. Chem. 2004, 279, 44723–44730. [Google Scholar] [CrossRef]
- Casino, P.; Gozalbo-Rovira, R.; Rodríguez-Díaz, J.; Banerjee, S.; Boutaud, A.; Rubio, V.; Hudson, B.G.; Saus, J.; Cervera, J.; Marina, A. Structures of Collagen IV Globular Domains: Insight into Associated Pathologies, Folding and Network Assembly. IUCrJ 2018, 5, 765–779, Erratum in IUCrJ 2020, 7, 777. https://doi.org/10.1107/S2052252520007216. [Google Scholar] [CrossRef]
- Pedchenko, V.; Bauer, R.; Pokidysheva, E.N.; Al-Shaer, A.; Forde, N.R.; Fidler, A.L.; Hudson, B.G.; Boudko, S.P. A Chloride Ring Is an Ancient Evolutionary Innovation Mediating the Assembly of the Collagen IV Scaffold of Basement Membranes. J. Biol. Chem. 2019, 294, 7968–7981. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.V.; Bauer, R.; Pokidysheva, E.N.; Boudko, S.P. Collagen IV Exploits a Cl- Step Gradient for Scaffold Assembly. In Protein Reviews; Atassi, M.Z., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 21, pp. 129–141. ISBN 978-3-030-67813-5. [Google Scholar]
- Cummings, C.F.; Pedchenko, V.; Brown, K.L.; Colon, S.; Rafi, M.; Jones-Paris, C.; Pokydeshava, E.; Liu, M.; Pastor-Pareja, J.C.; Stothers, C.; et al. Extracellular Chloride Signals Collagen IV Network Assembly during Basement Membrane Formation. J. Cell Biol. 2016, 213, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Boudko, S.P.; Bauer, R.; Chetyrkin, S.V.; Ivanov, S.; Smith, J.; Voziyan, P.A.; Hudson, B.G. Collagen IVα345 Dysfunction in Glomerular Basement Membrane Diseases. II. Crystal Structure of the A345 Hexamer. J. Biol. Chem. 2021, 296, 100591. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.U.B.; Karsdal, M.A. Type VIII Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 61–65. ISBN 978-0-12-809847-9. [Google Scholar]
- Illidge, C.; Kielty, C.; Shuttleworth, A. Type VIII Collagen: Heterotrimeric Chain Association. Int. J. Biochem. Cell Biol. 2001, 33, 521–529. [Google Scholar] [CrossRef]
- Kvansakul, M.; Bogin, O.; Hohenester, E.; Yayon, A. Crystal Structure of the Collagen A1(VIII) NC1 Trimer. Matrix Biol. 2003, 22, 145–152. [Google Scholar] [CrossRef]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a Glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef]
- Sun, S.; Karsdal, M.A. Type VI Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–55. ISBN 978-0-12-809847-9. [Google Scholar]
- Ball, S.; Bella, J.; Kielty, C.; Shuttleworth, A. Structural Basis of Type VI Collagen Dimer Formation. J. Biol. Chem. 2003, 278, 15326–15332. [Google Scholar] [CrossRef]
- Di Martino, A.; Cescon, M.; D’Agostino, C.; Schilardi, F.; Sabatelli, P.; Merlini, L.; Faldini, C. Collagen VI in the Musculoskeletal System. Int. J. Mol. Sci. 2023, 24, 5095. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Rich, C.; Zhou, F.H.; Hansen, U. Three Novel Collagen VI Chains, A4(VI), A5(VI), and A6(VI). J. Biol. Chem. 2008, 283, 20170–20180. [Google Scholar] [CrossRef]
- Godwin, A.R.F.; Becker, M.H.; Dajani, R.; Snee, M.; Roseman, A.M.; Baldock, C. Collagen VI Microfibril Structure Reveals Mechanism for Molecular Assembly and Clustering of Inherited Pathogenic Mutations. Nat. Commun. 2025, 16, 7549. [Google Scholar] [CrossRef]
- He, Y.; Sardar, S.; Karsdal, M.A. Type IX Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–89. ISBN 978-0-12-817068-7. [Google Scholar]
- Boudko, S.P.; Bächinger, H.P. Structural Insight for Chain Selection and Stagger Control in Collagen. Sci. Rep. 2016, 6, 37831. [Google Scholar] [CrossRef]
- Sudha, G.; Naveenkumar, N.; Srinivasan, N. Evolutionary and Structural Analyses of Heterodimeric Proteins Composed of Subunits with Same Fold: Heterodimers with Subunits of Same Fold. Proteins 2015, 83, 1766–1786. [Google Scholar] [CrossRef]
- Balasco, N.; Esposito, L.; Smaldone, G.; Salvatore, M.; Vitagliano, L. A Comprehensive Analysis of the Structural Recognition between KCTD Proteins and Cullin 3. Int. J. Mol. Sci. 2024, 25, 1881. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Balasco, N.; Smaldone, G.; Berisio, R.; Ruggiero, A.; Vitagliano, L. AlphaFold-Predicted Structures of KCTD Proteins Unravel Previously Undetected Relationships among the Members of the Family. Biomolecules 2021, 11, 1862. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Balasco, N.; Vitagliano, L. Alphafold Predictions Provide Insights into the Structural Features of the Functional Oligomers of All Members of the KCTD Family. Int. J. Mol. Sci. 2022, 23, 13346. [Google Scholar] [CrossRef] [PubMed]
- Balasco, N.; Modjtahedi, N.; Monti, A.; Ruvo, M.; Vitagliano, L.; Doti, N. CHCHD4 Oxidoreductase Activity: A Comprehensive Analysis of the Molecular, Functional, and Structural Properties of Its Redox-Regulated Substrates. Molecules 2025, 30, 2117. [Google Scholar] [CrossRef]
- Islami, V.; Bittner, P.; Fiala, T.; Hentzen, N.B.; Zenobi, R.; Wennemers, H. Self-Sorting Collagen Heterotrimers. J. Am. Chem. Soc. 2024, 146, 1789–1793. [Google Scholar] [CrossRef]
- Kreutzberger, M.A.B.; Yu, L.T.; Bui, T.H.; Hancu, M.C.; Purdy, M.D.; Osinski, T.; Kasson, P.M.; Egelman, E.H.; Hartgerink, J.D. A Collagen Triple Helix without the Superhelical Twist. ACS Cent. Sci. 2025, 11, 331–345. [Google Scholar] [CrossRef]

| Collagen Family | Type | Chains | UniProtKB | Homotrimeric Assemblies | Heterotrimeric Assemblies | ||
|---|---|---|---|---|---|---|---|
| Stoichiometry | PDB ID (Resolution, Å) | Stoichiometry | PDB ID | ||||
| Fibrillar | I | α1(I) | P02452 | [α1(I)]3 | 5k31 (2.20), 7e7b (2.60), 7e7d (3.20) | [α1(I)]2α2(I) | - |
| α2(I) | P08123 | - | - | ||||
| V | α1(V) | P20908 | [α1(V)]3 | - | [α1(V)]2α2(V) α1(V)α2(V)α3(V) | - | |
| α2(V) | P05997 | - | - | ||||
| α3(V) | P25940 | - | - | ||||
| XI | α1(XI) | P12107 | - | - | α1(XI)α2(XI)α3(XI) | - | |
| α2(XI) | P13942 | - | - | ||||
| α3(XI) # | P02458 | - | - | ||||
| V/XI | - | - | - | - | [α1(XI)]2α2(V) α1(XI)α1(V)α3(XI) | - | |
| Network-forming | IV | α1(IV) | P02462 | [α1(IV)]3 | 5nay (1.80) | [α1(IV)]2α2(IV) | 1li1 (1.90), 1m3d § (2.00), 1t60 § (1.50), 5nax (2.82), 6mpx (1.90) |
| α2(IV) | P08572 | [α2(IV)]3 | 5nb2 (2.50) | ||||
| α3(IV) | Q01955 | [α3(IV)]3 | 5nb0 (2.70) | α3(IV)α4(IV)α5(IV) | 6wku (1.76) | ||
| α4(IV) | P53420 | [α4(IV)]3 | 5nb1 (2.82) | ||||
| α5(IV) | P29400 | [α5(IV)]3 | 5naz (1.85) | ||||
| α6(IV) | Q14031 | - | - | [α5(IV)]2α6(IV) | - | ||
| VIII | α1(VIII) | P27658 | [α1(VIII)]3 | 1o91 * (1.90) | [α1(VIII)]2α2(VIII) α1(VIII)[α2(VIII)]2 | - | |
| α2(VIII) | P25067 | [α2(VIII)]3 | |||||
| Beaded filaments | VI | α1(VI) | P12109 | - | - | α3(VI)α2(VI)α1(VI) α5(VI)α2(VI)α1(VI) α6(VI)α2(VI)α1(VI) | - |
| α2(VI) | P12110 | - | - | ||||
| α3(VI) | P12111 | - | - | ||||
| α5(VI) | A8TX70 | - | - | ||||
| α6(VI) | A6NMZ7 | - | - | ||||
| FACITs | IX | α1(IX) | P20849 | - | - | α1(IX)α2(IX)α3(IX) | 5ctd (1.60), 5cti (1.90), 5cva (2.10), 5cvb (2.25) |
| α2(IX) | Q14055 | - | - | ||||
| α3(IX) | Q14050 | - | - | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitagliano, L.; Doti, N.; Balasco, N. Determinants of Chain Selection and Staggering in Heterotrimeric Collagens: A Comprehensive Review of the Structural Data. Int. J. Mol. Sci. 2025, 26, 10134. https://doi.org/10.3390/ijms262010134
Vitagliano L, Doti N, Balasco N. Determinants of Chain Selection and Staggering in Heterotrimeric Collagens: A Comprehensive Review of the Structural Data. International Journal of Molecular Sciences. 2025; 26(20):10134. https://doi.org/10.3390/ijms262010134
Chicago/Turabian StyleVitagliano, Luigi, Nunzianna Doti, and Nicole Balasco. 2025. "Determinants of Chain Selection and Staggering in Heterotrimeric Collagens: A Comprehensive Review of the Structural Data" International Journal of Molecular Sciences 26, no. 20: 10134. https://doi.org/10.3390/ijms262010134
APA StyleVitagliano, L., Doti, N., & Balasco, N. (2025). Determinants of Chain Selection and Staggering in Heterotrimeric Collagens: A Comprehensive Review of the Structural Data. International Journal of Molecular Sciences, 26(20), 10134. https://doi.org/10.3390/ijms262010134








