Ultrastructural and Immunohistochemical Study on the Nephrotoxicity Following Intravitreal Administration of the Antifungal Agents Voriconazole and Micafungin in New Zealand White Rabbits
Abstract
1. Introduction
1.1. Fungal Endophthalmitis (FE)
1.2. Voriconazole and Micafungin: Pharmacology and Toxicity Concerns
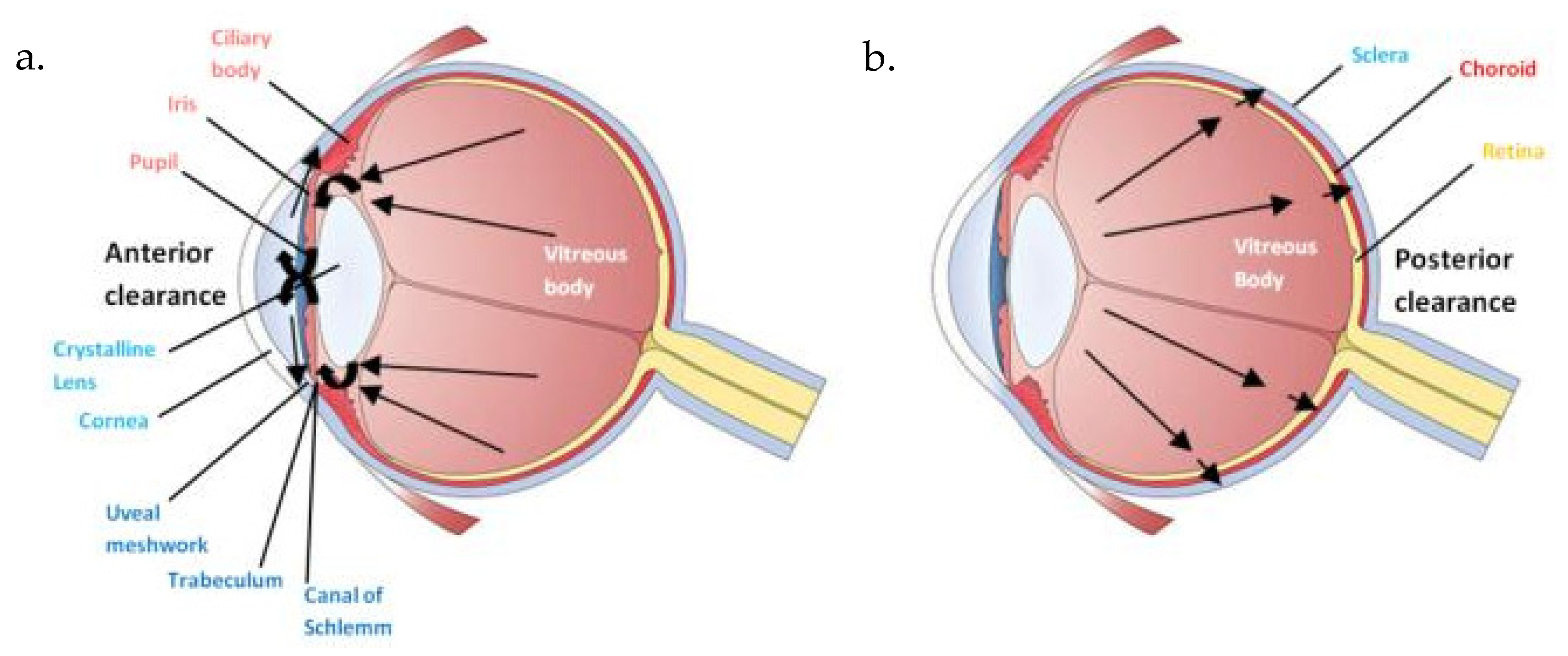
1.3. Intravitreal Administration: A Local Route of Administration?
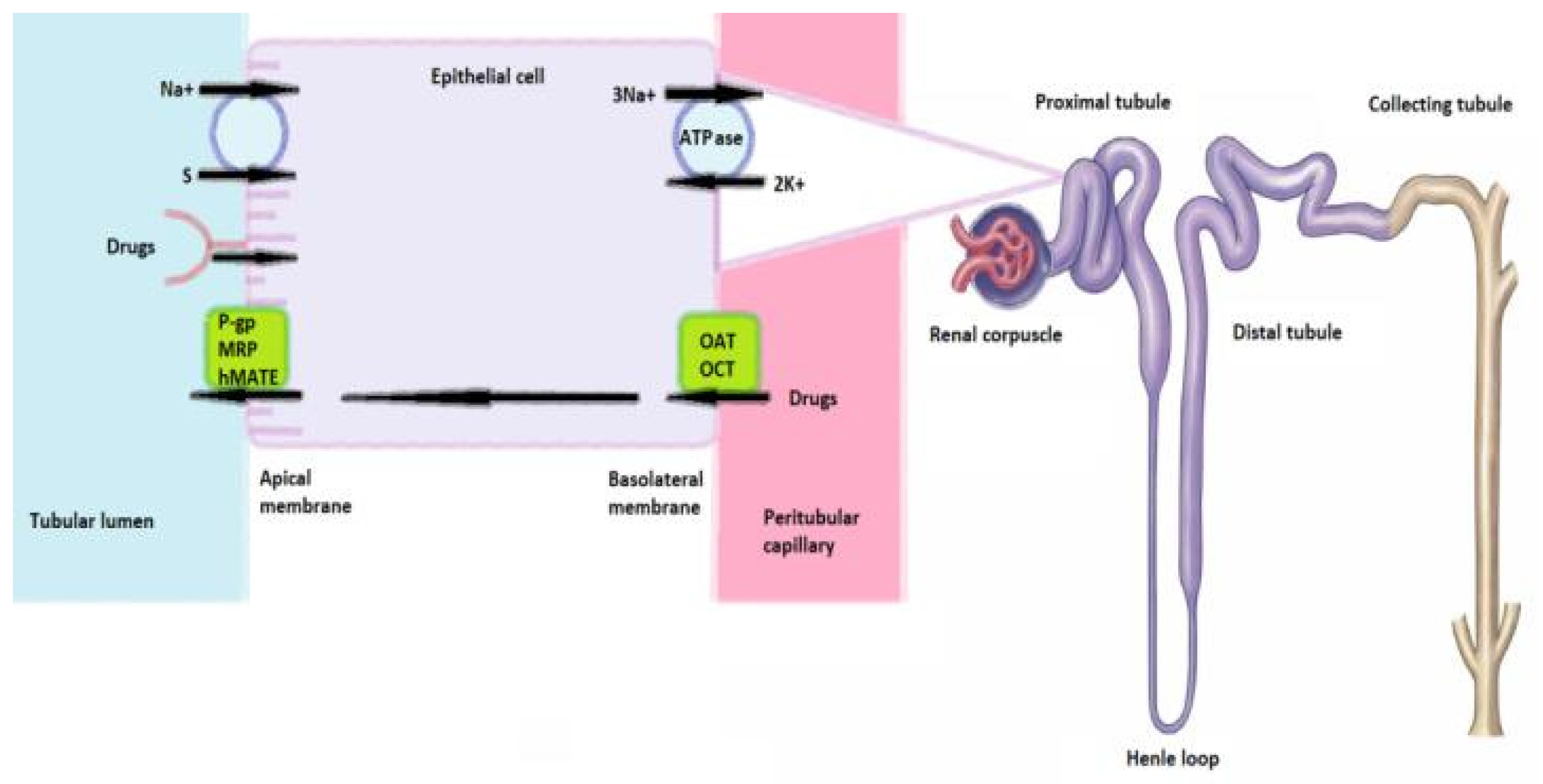
1.4. Renal Vulnerability
1.5. Immunohistochemical Markers
1.6. Aim of This Study
2. Results
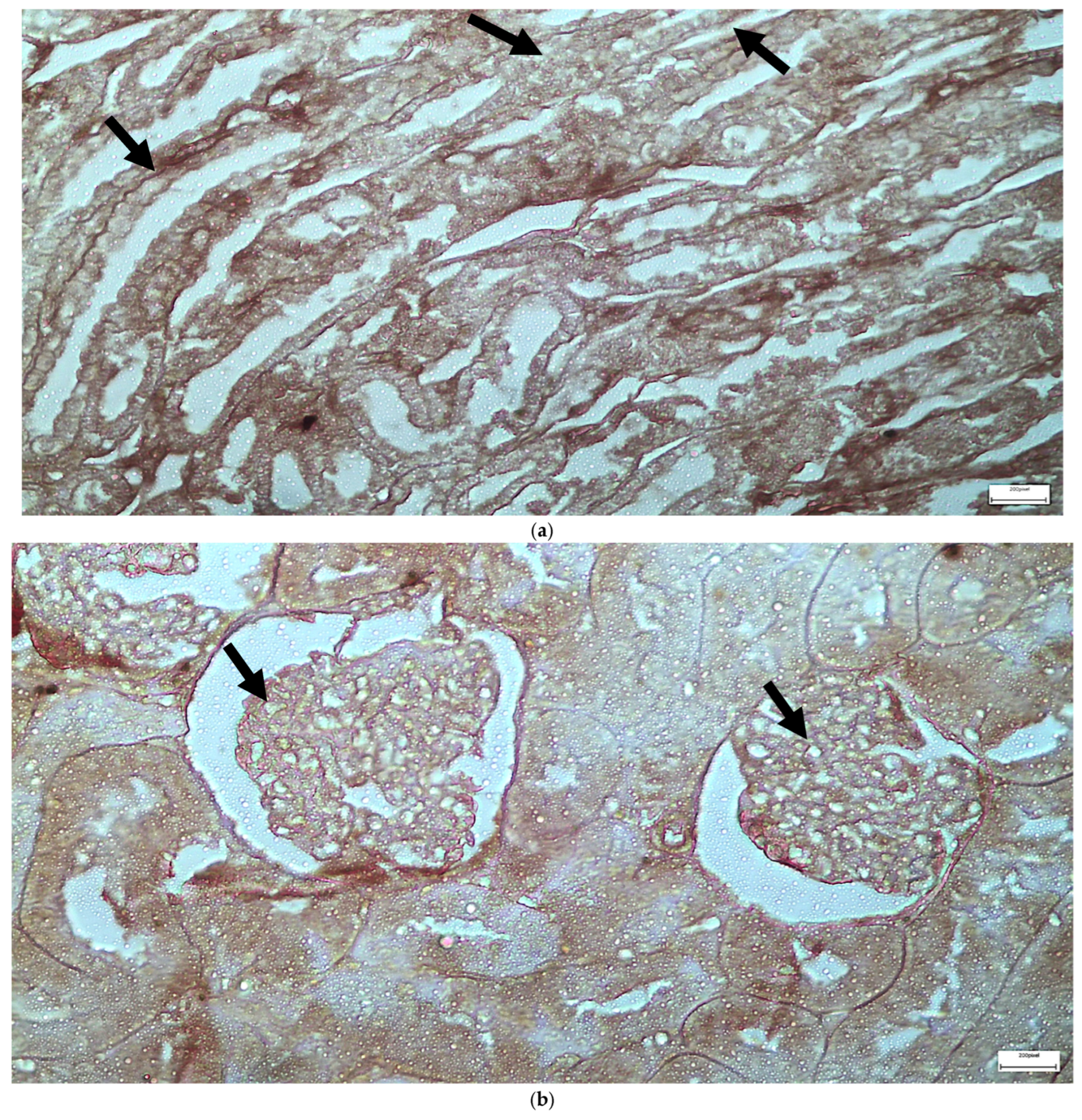
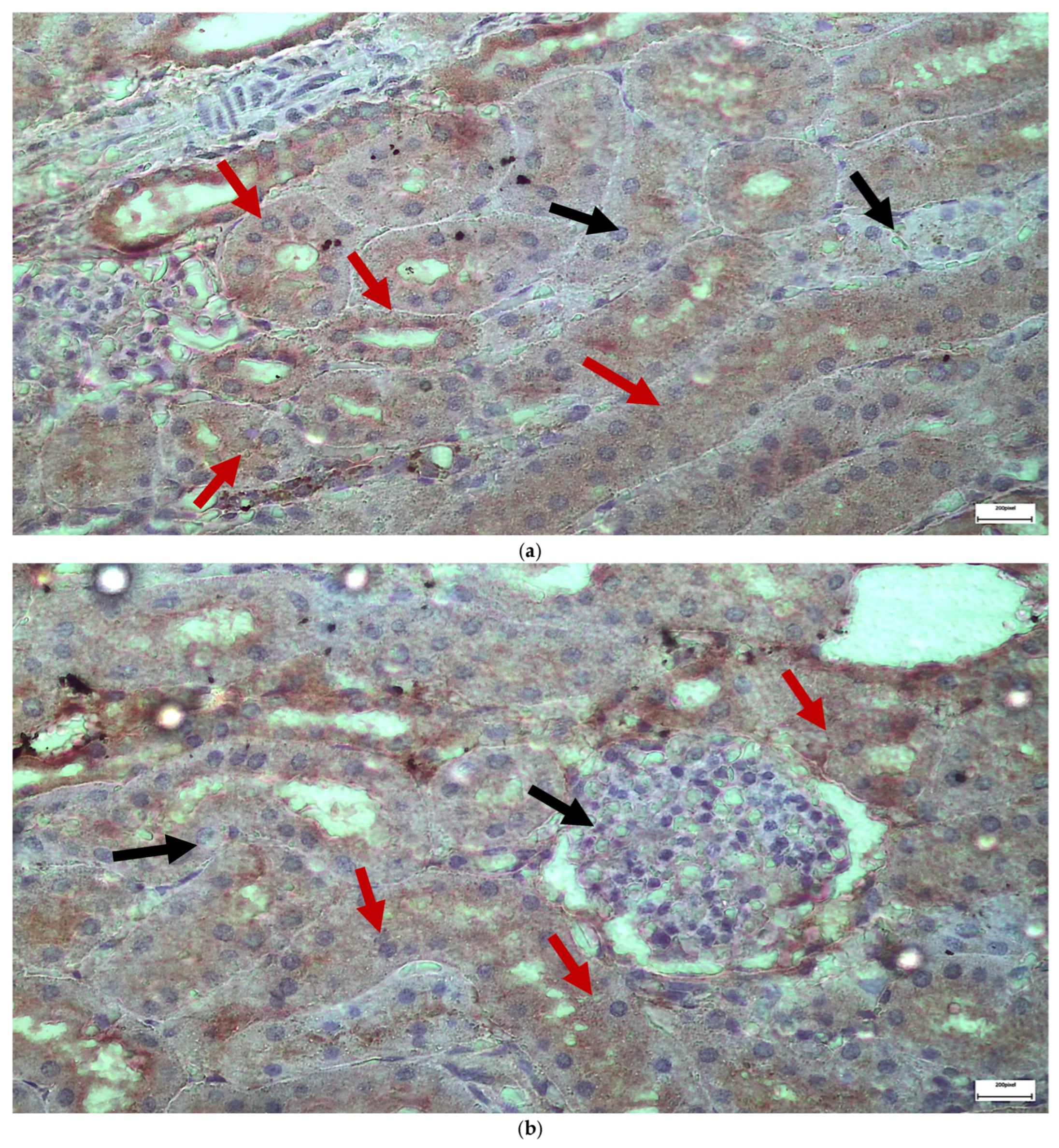
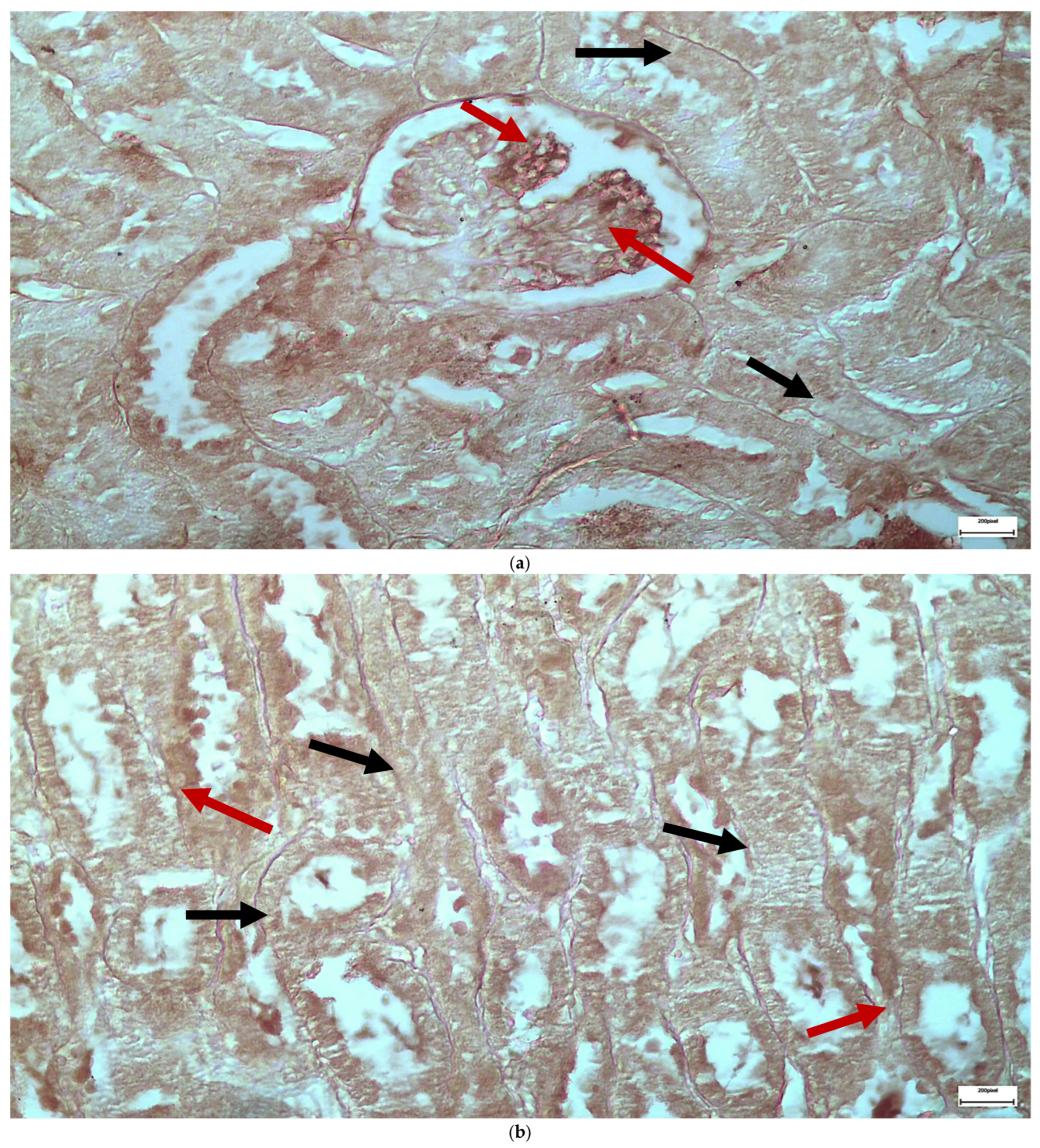
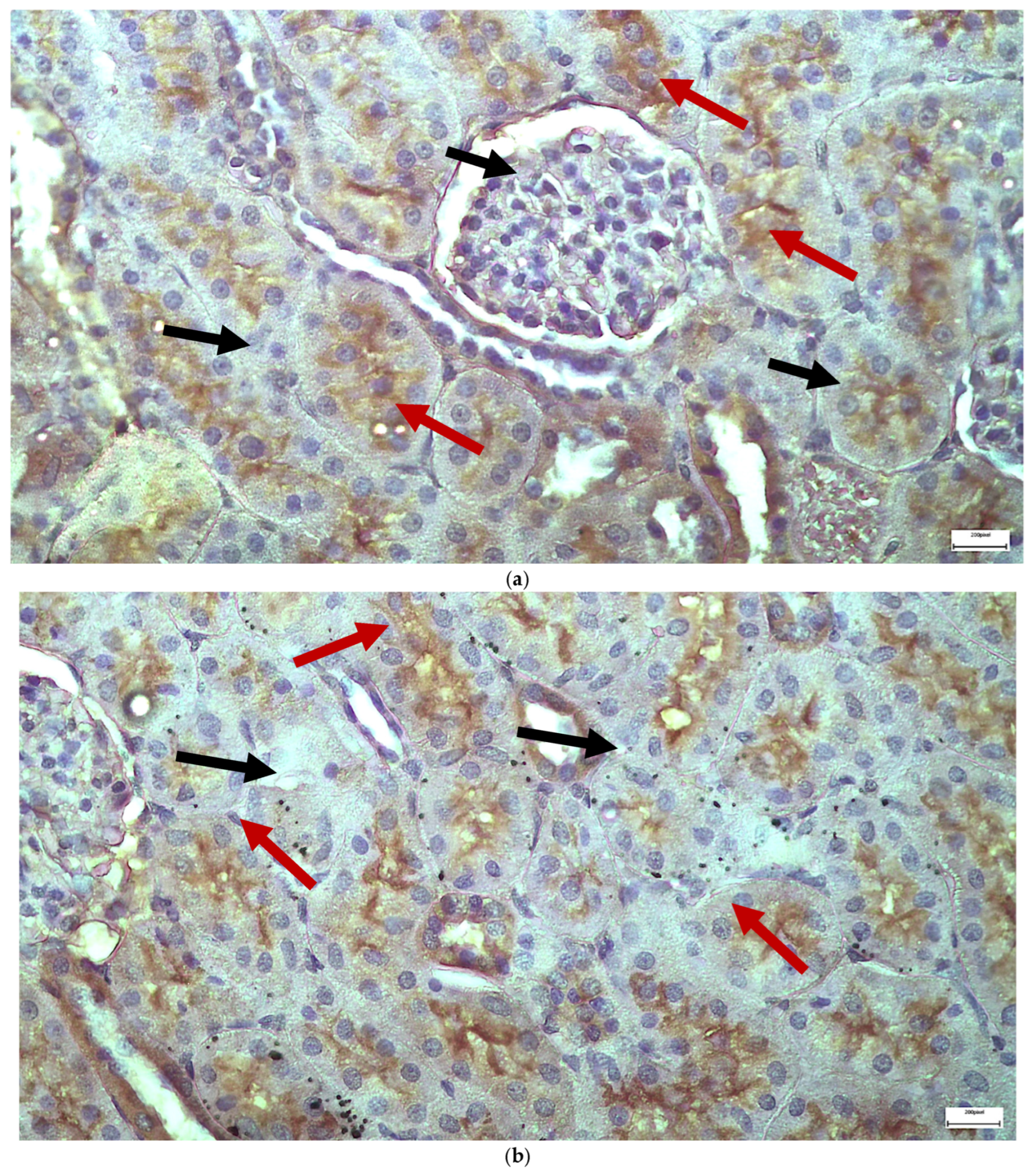
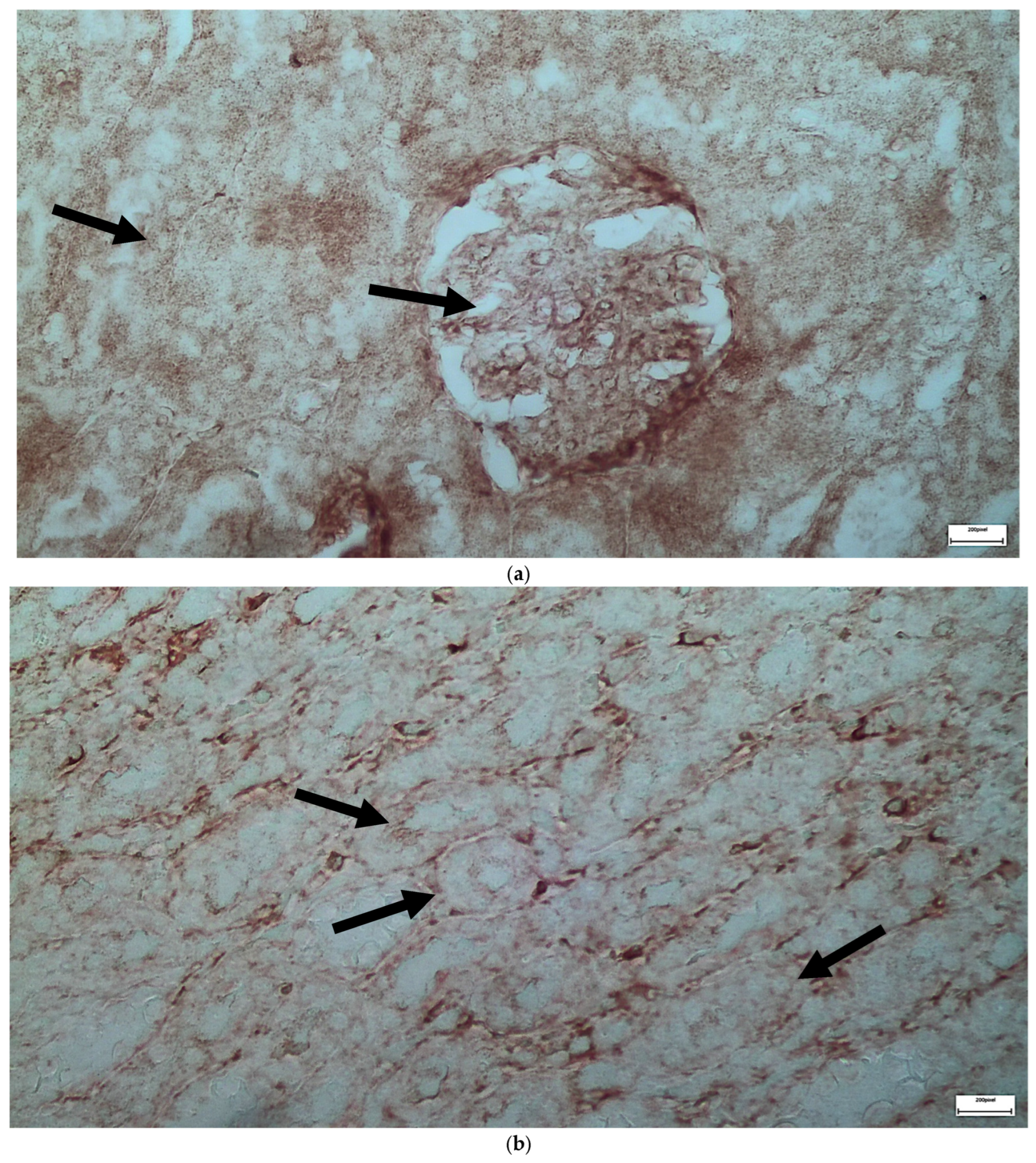
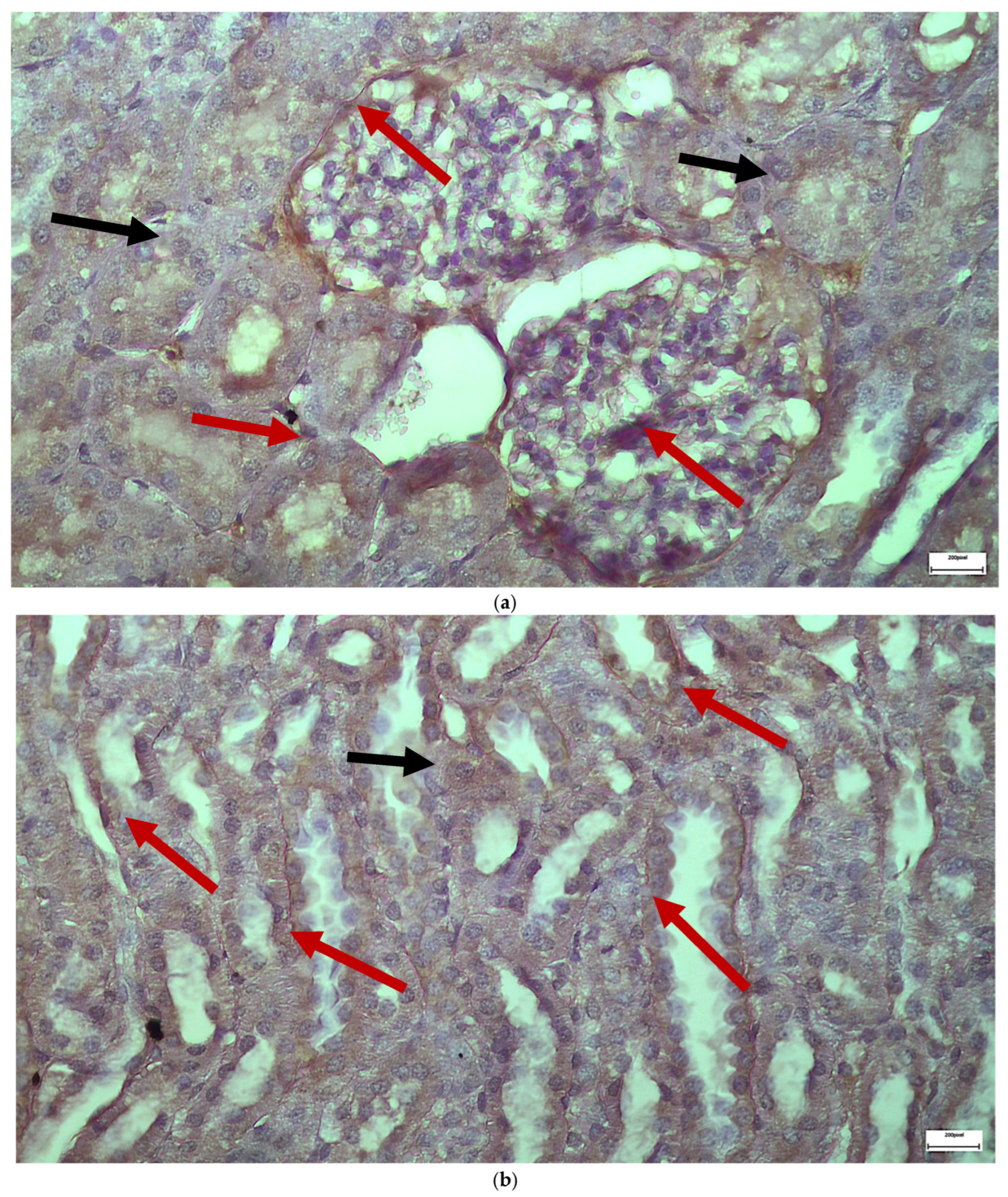
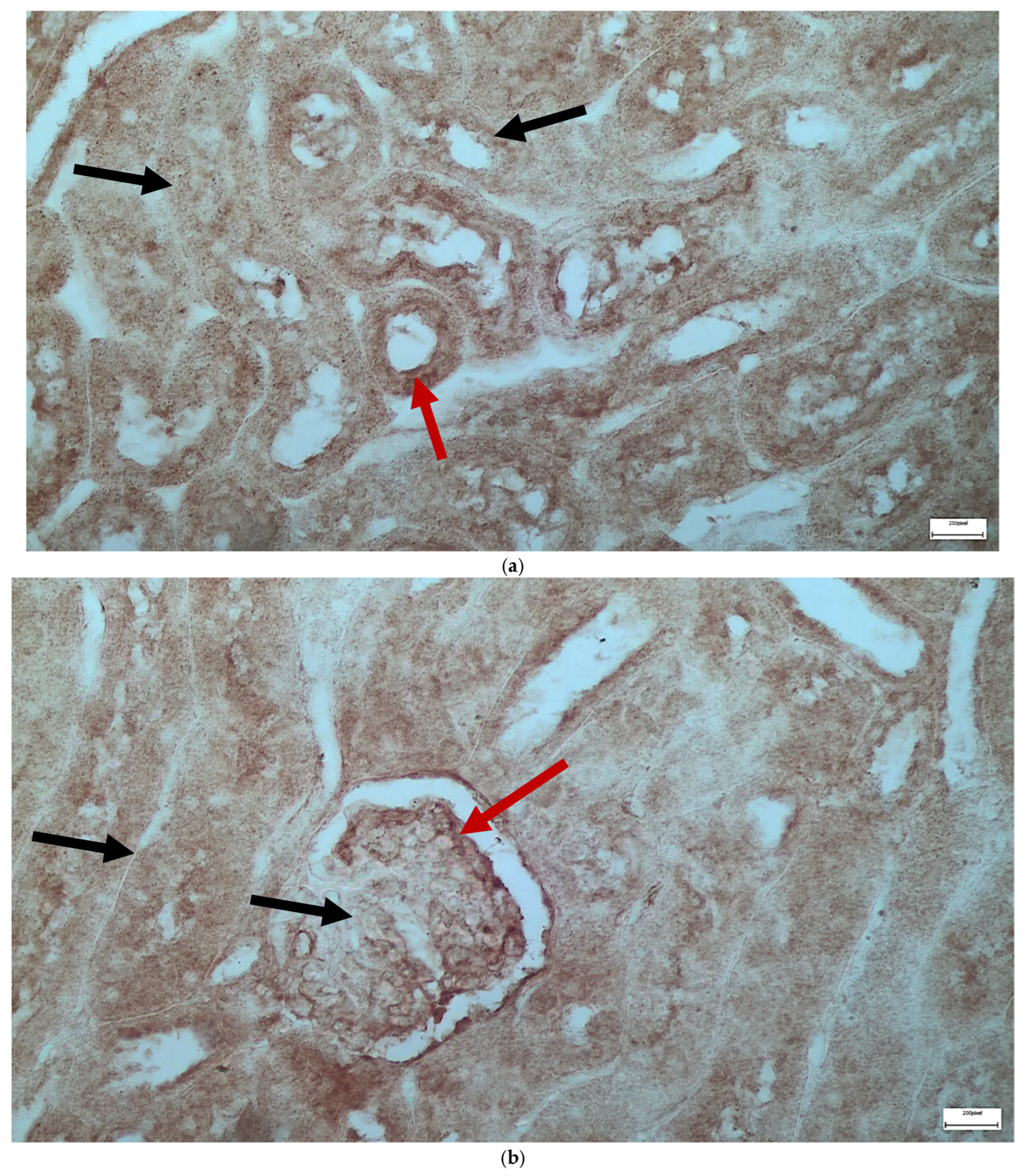
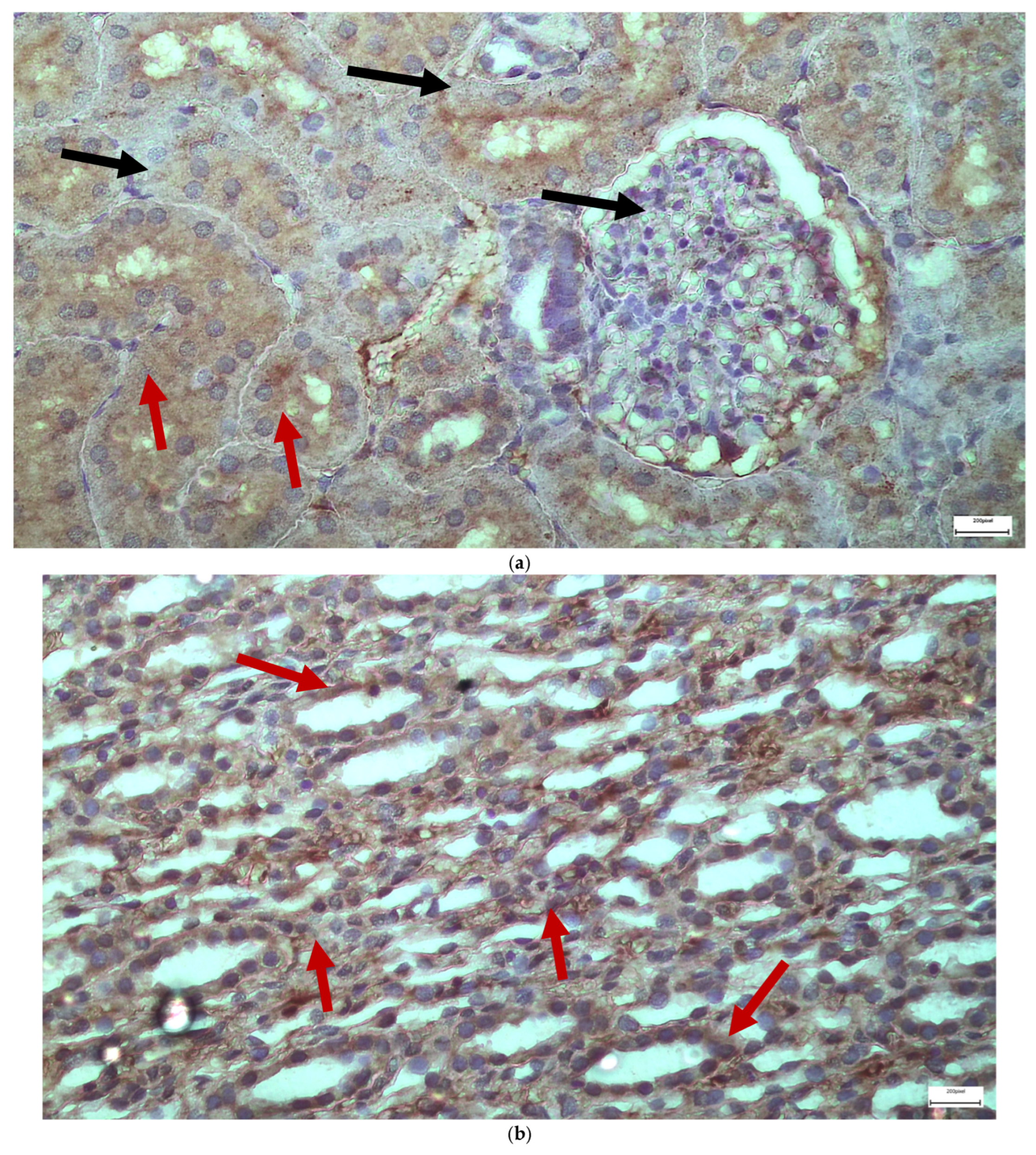
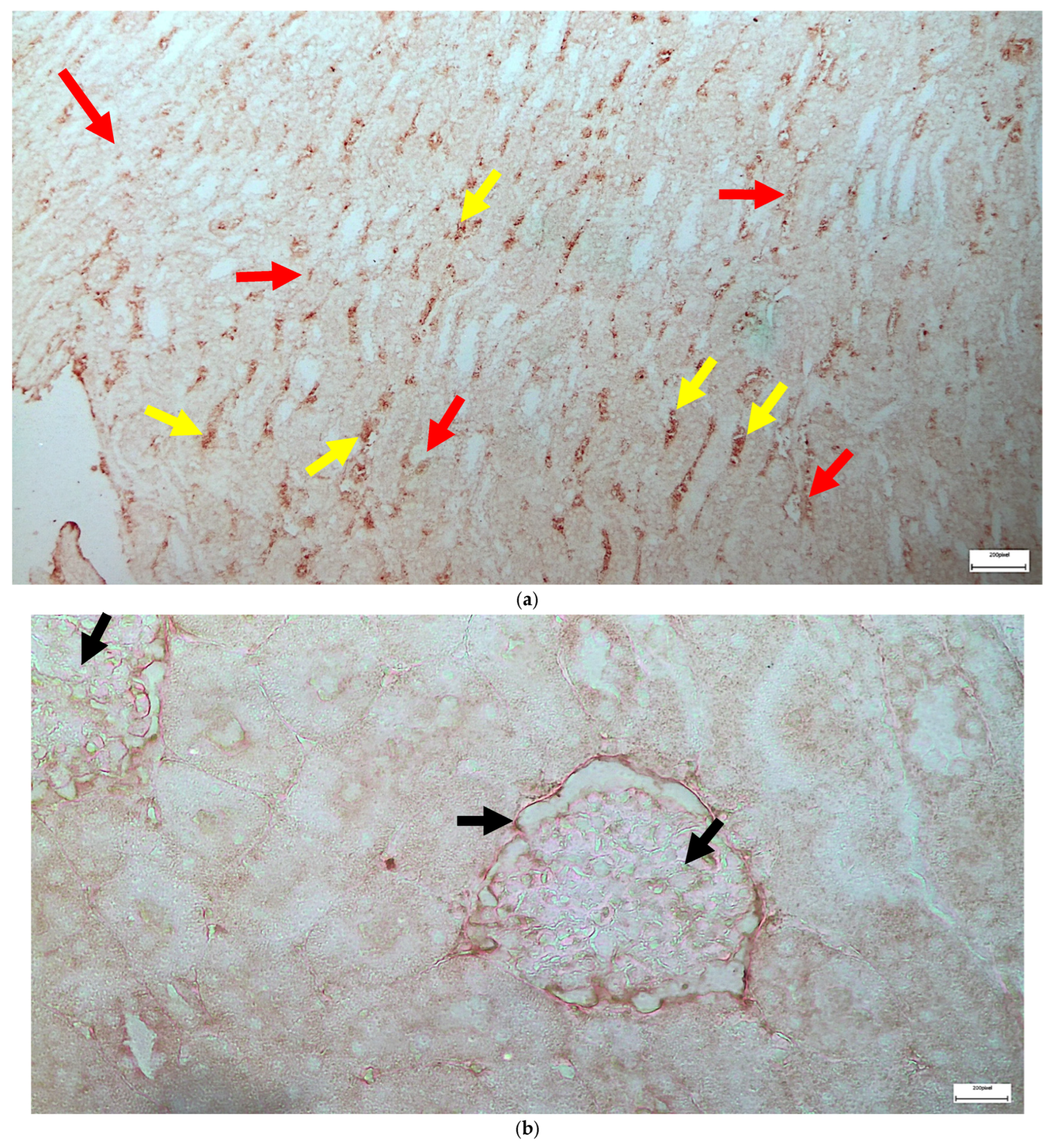
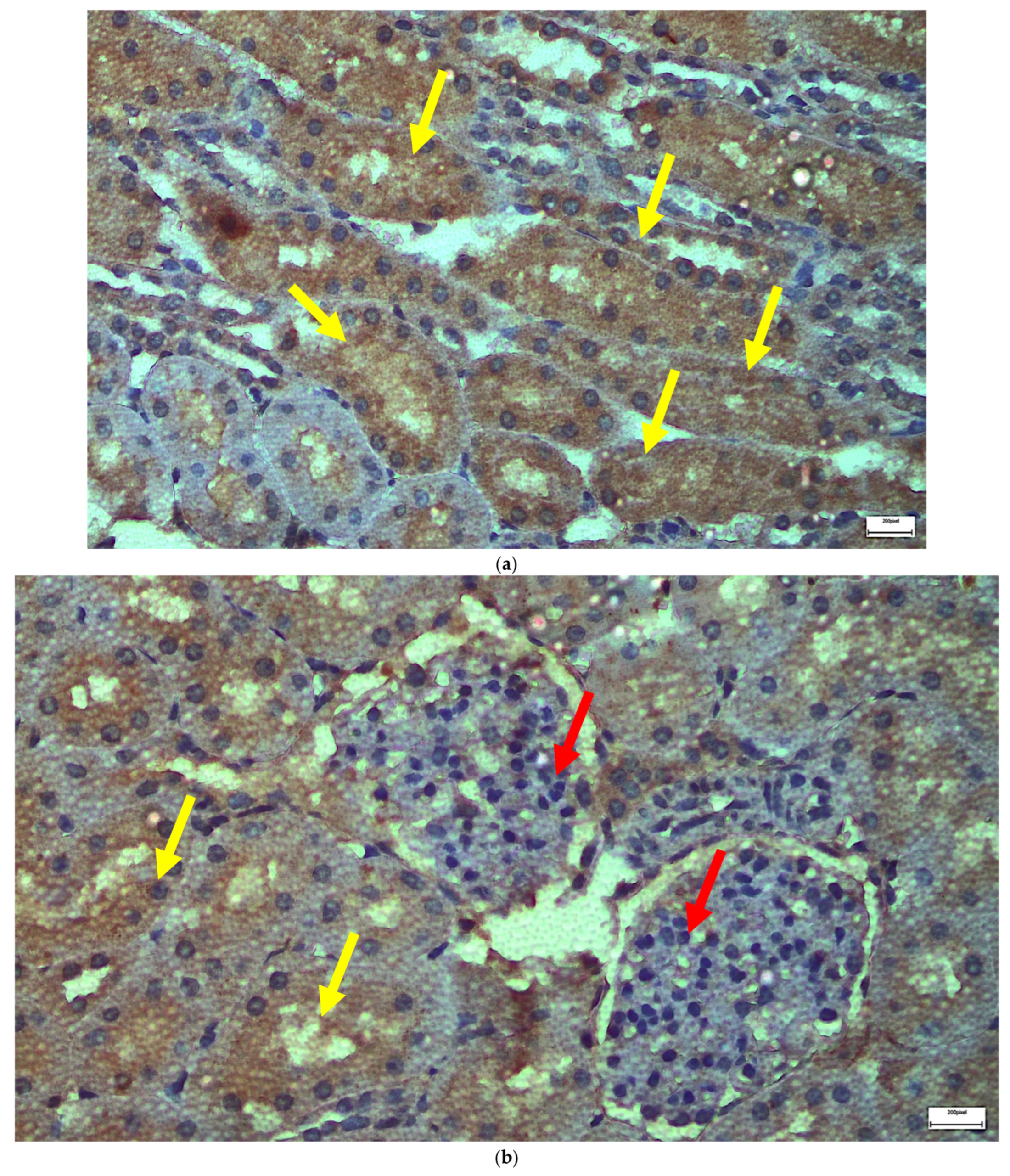
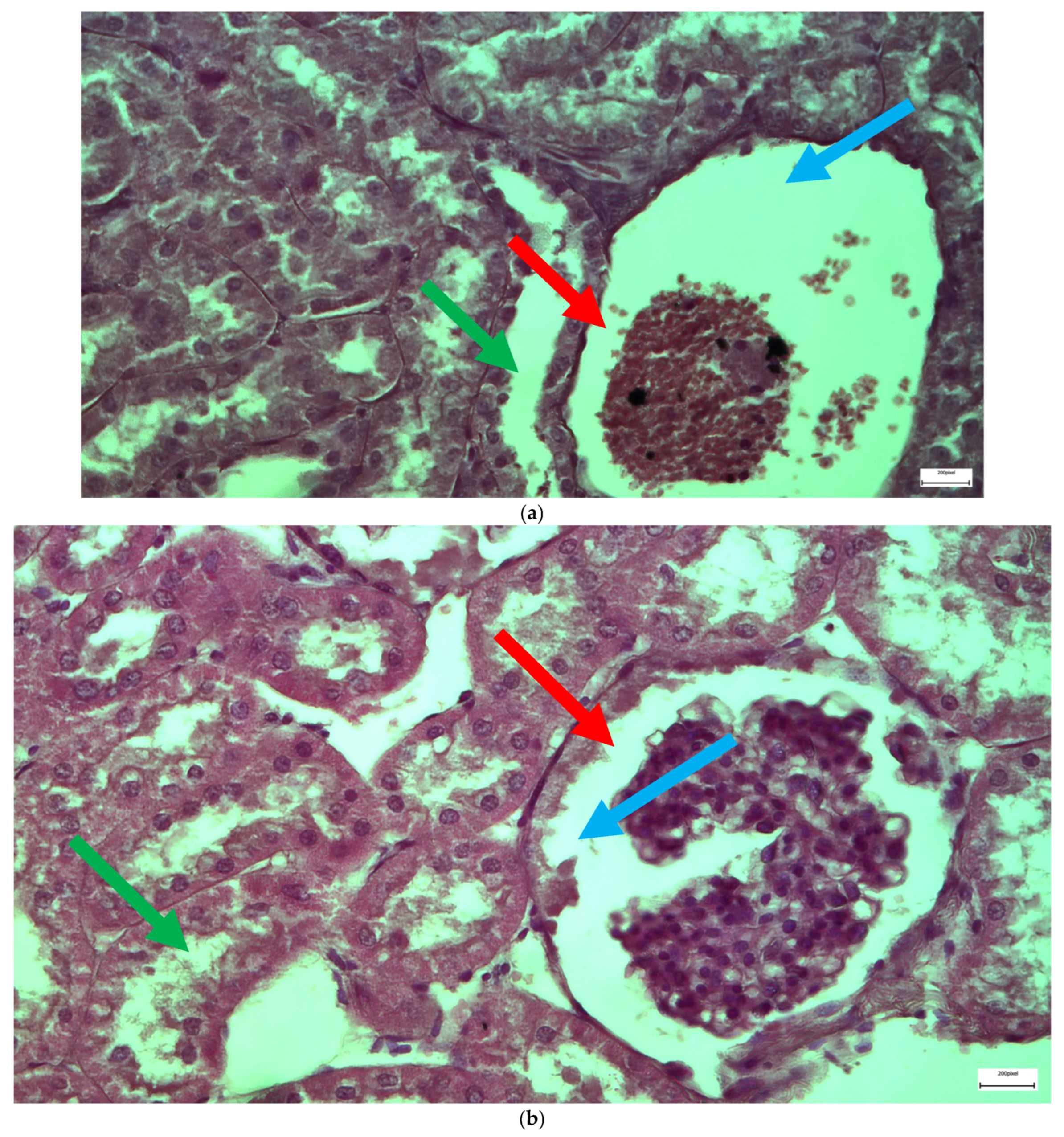
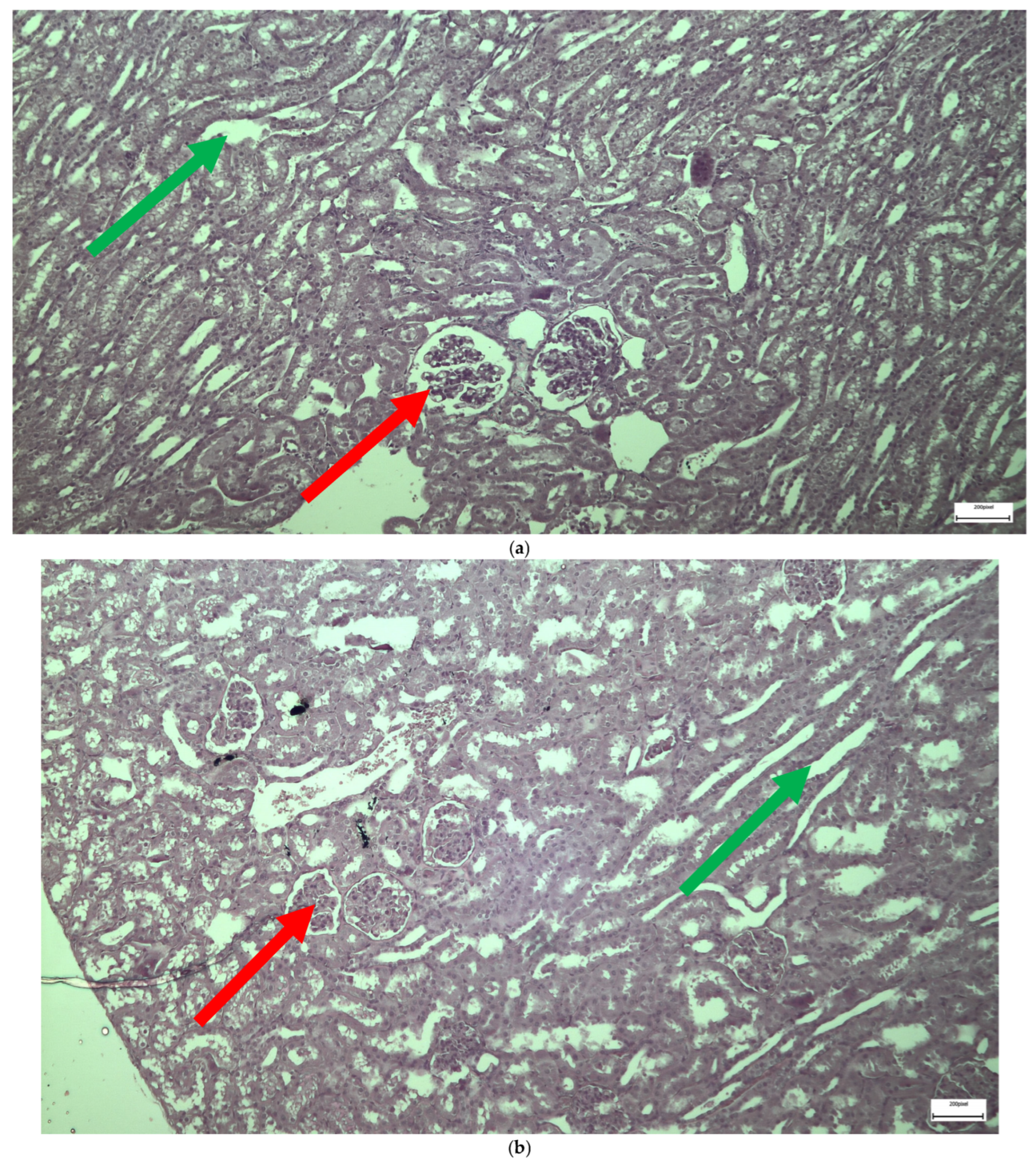
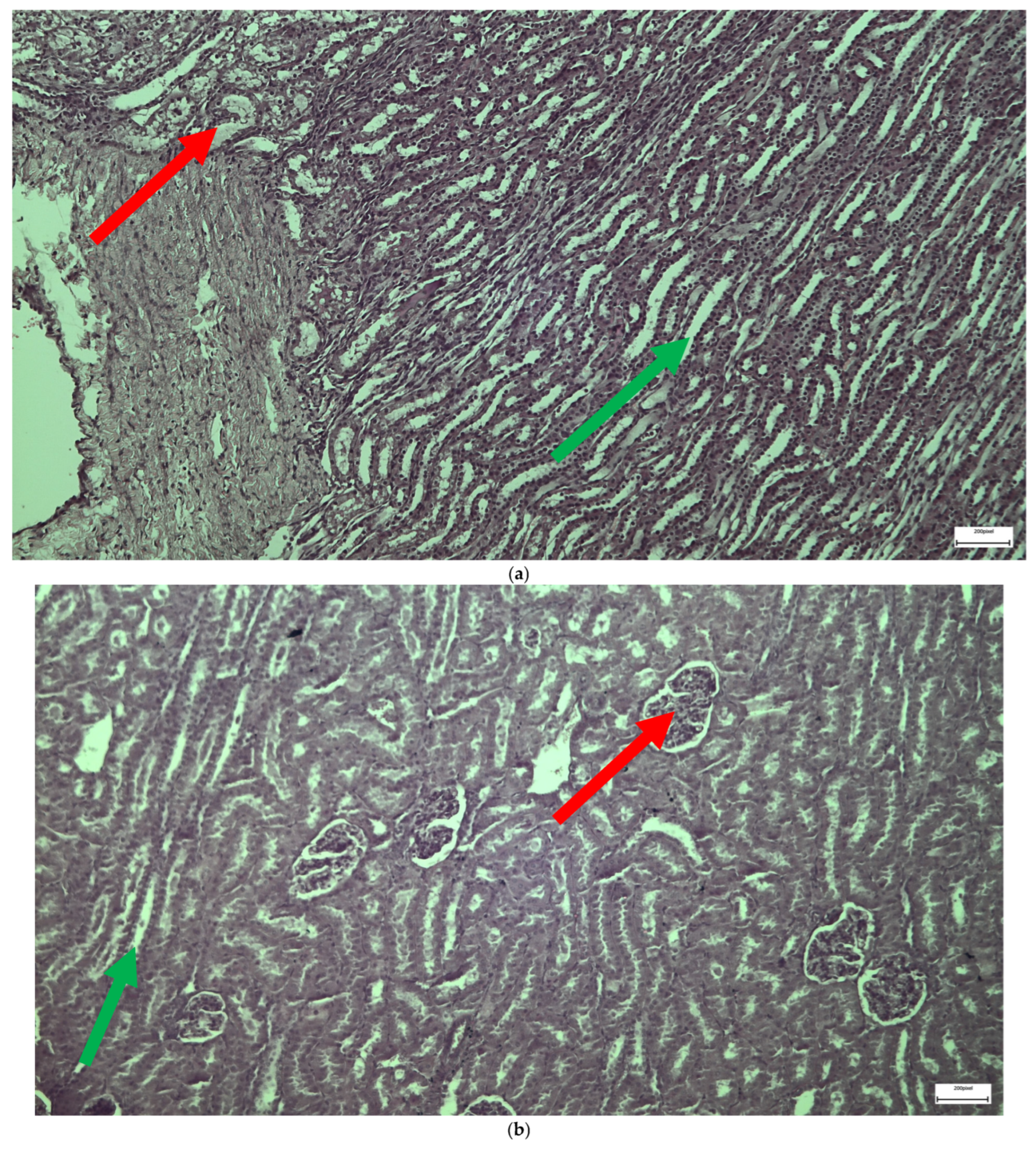
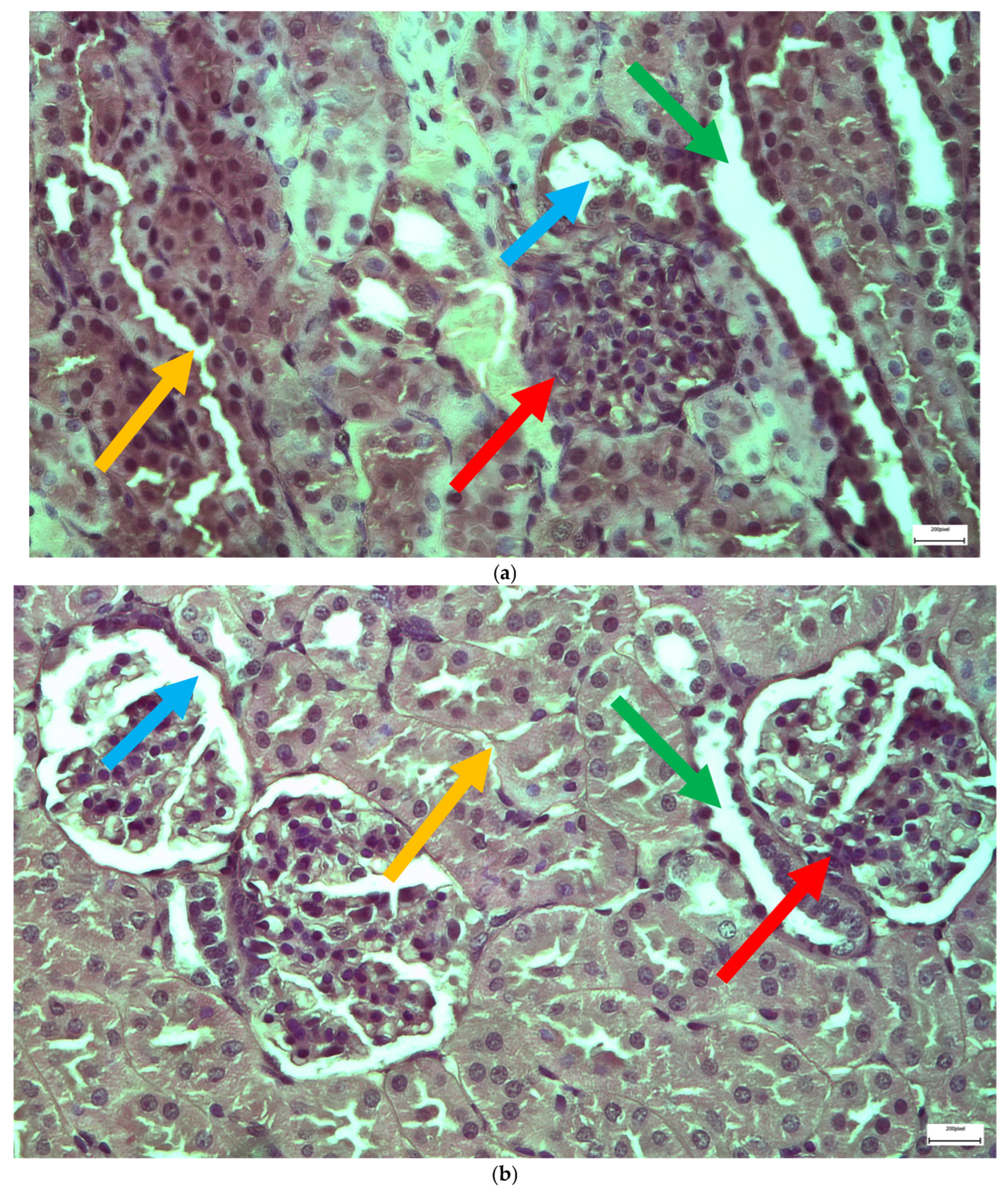
2.1. Light Microscopy-Immunohistochemistry Results
2.2. Statistical Analysis
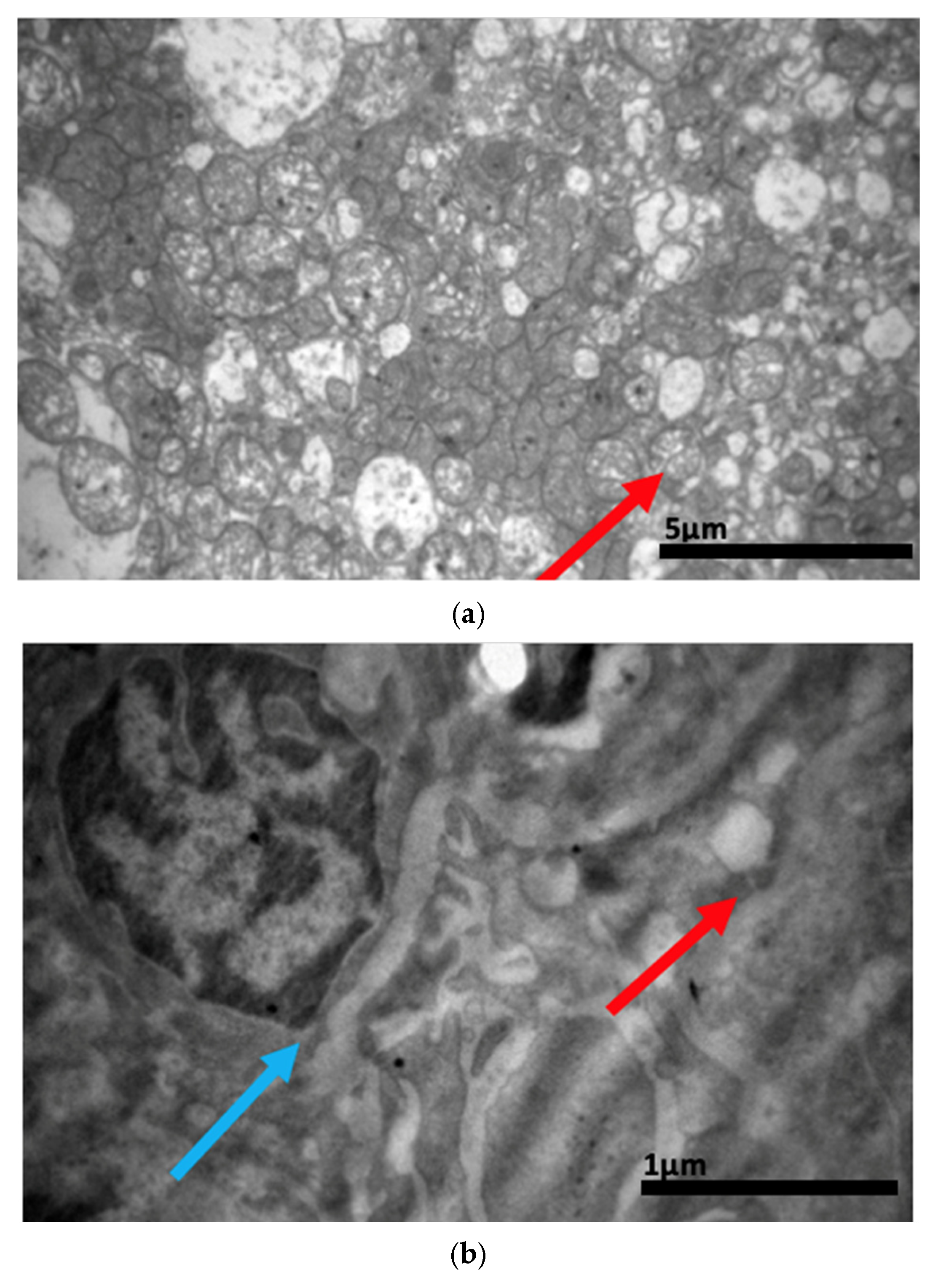
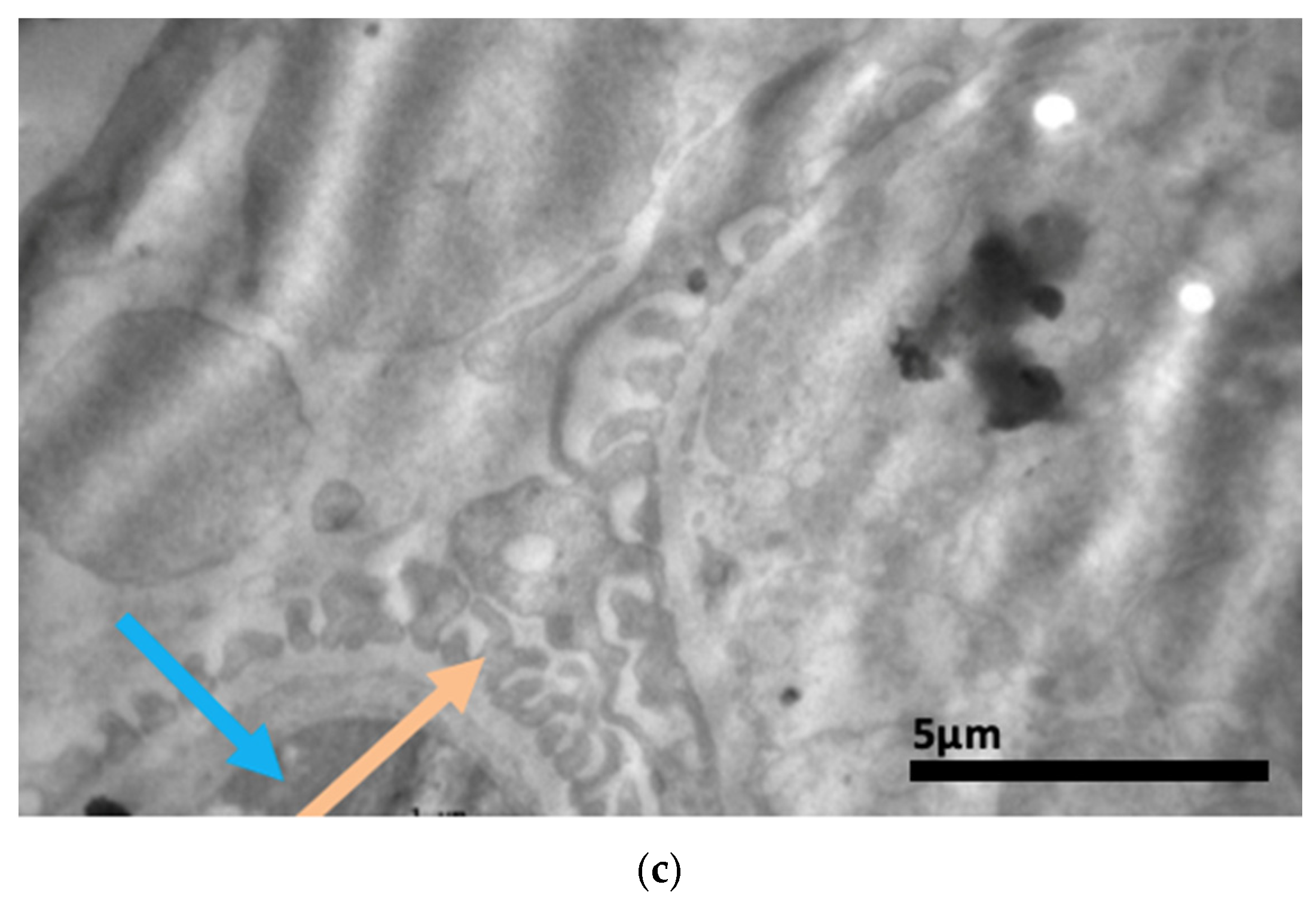
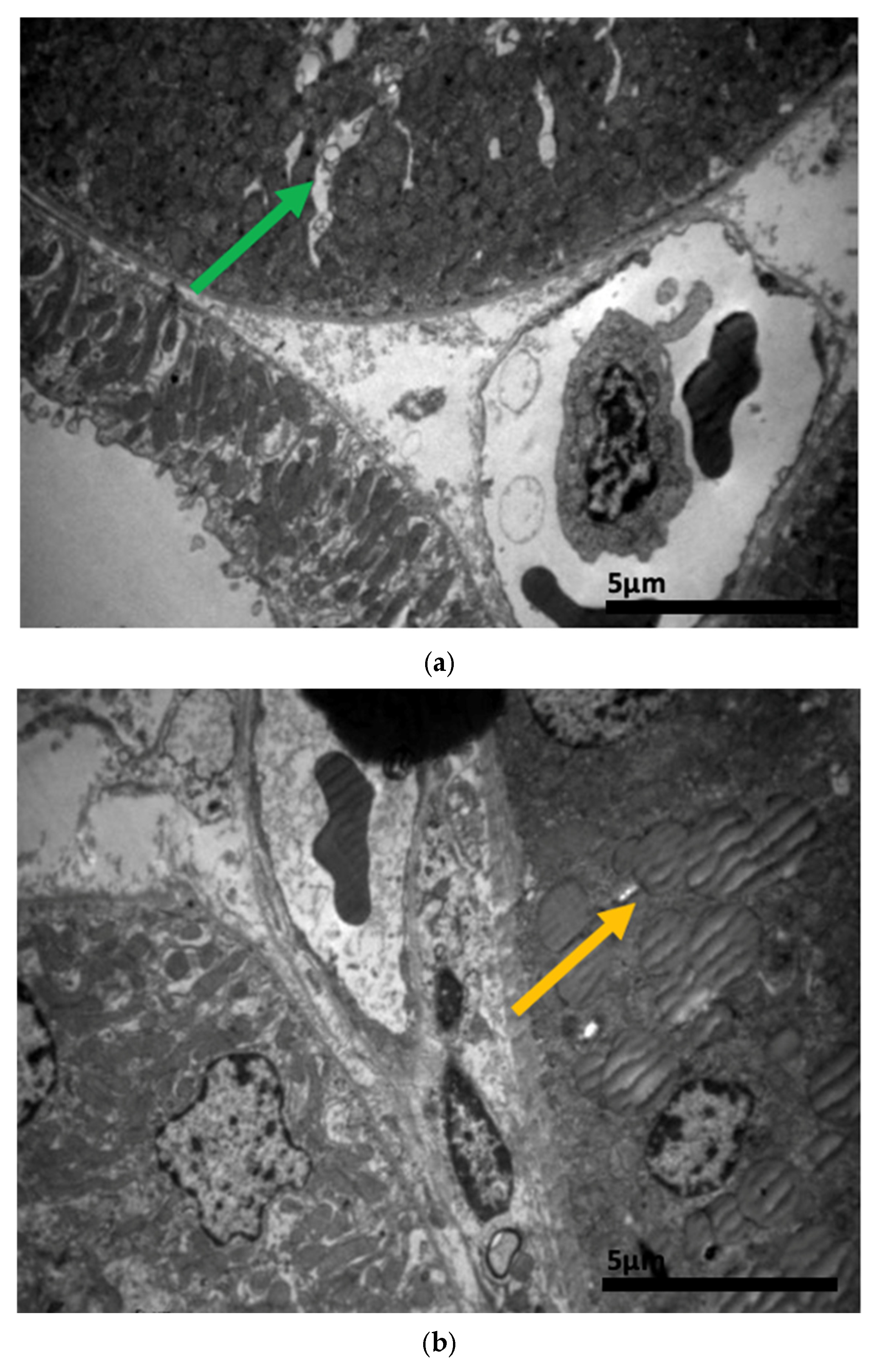
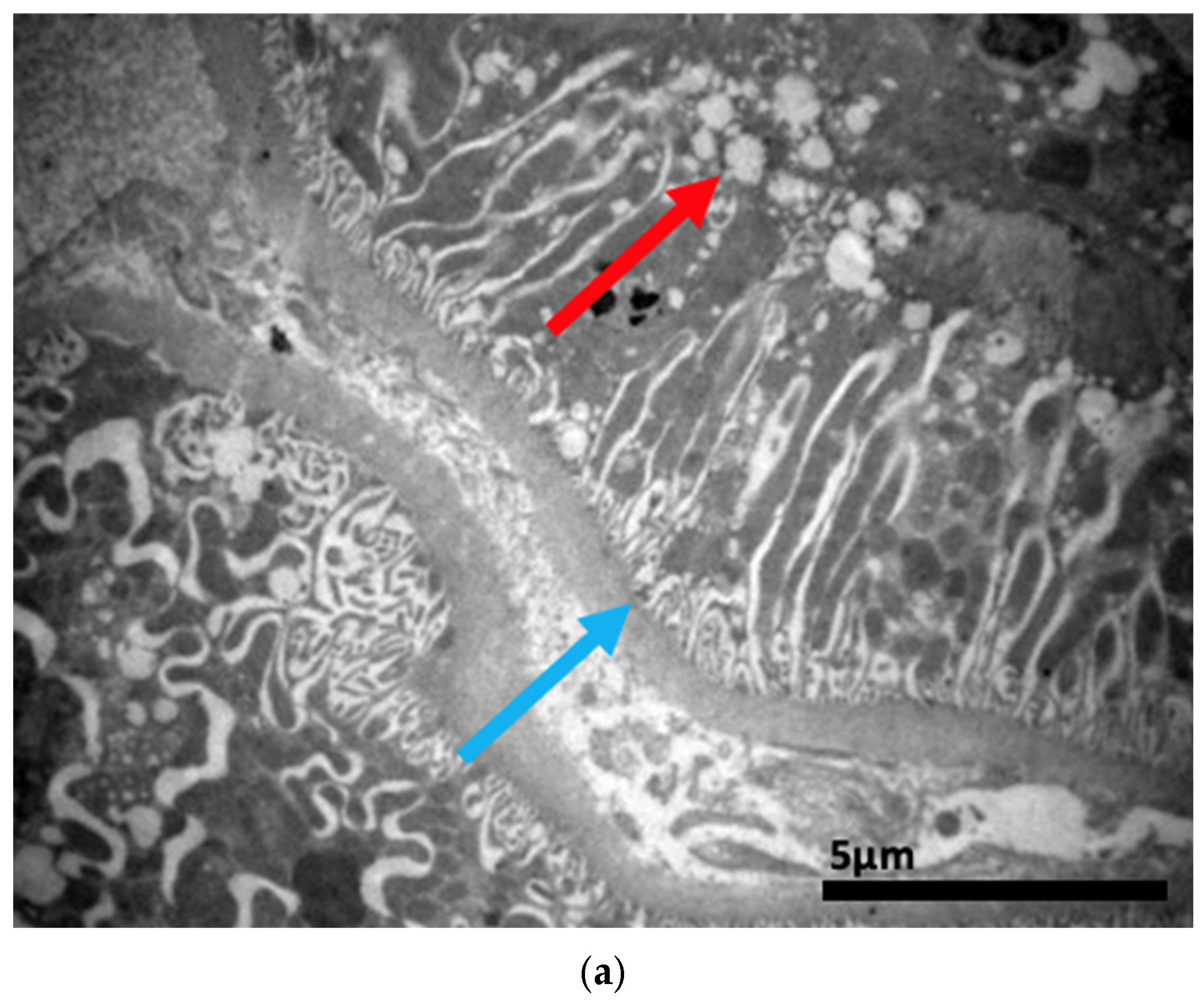
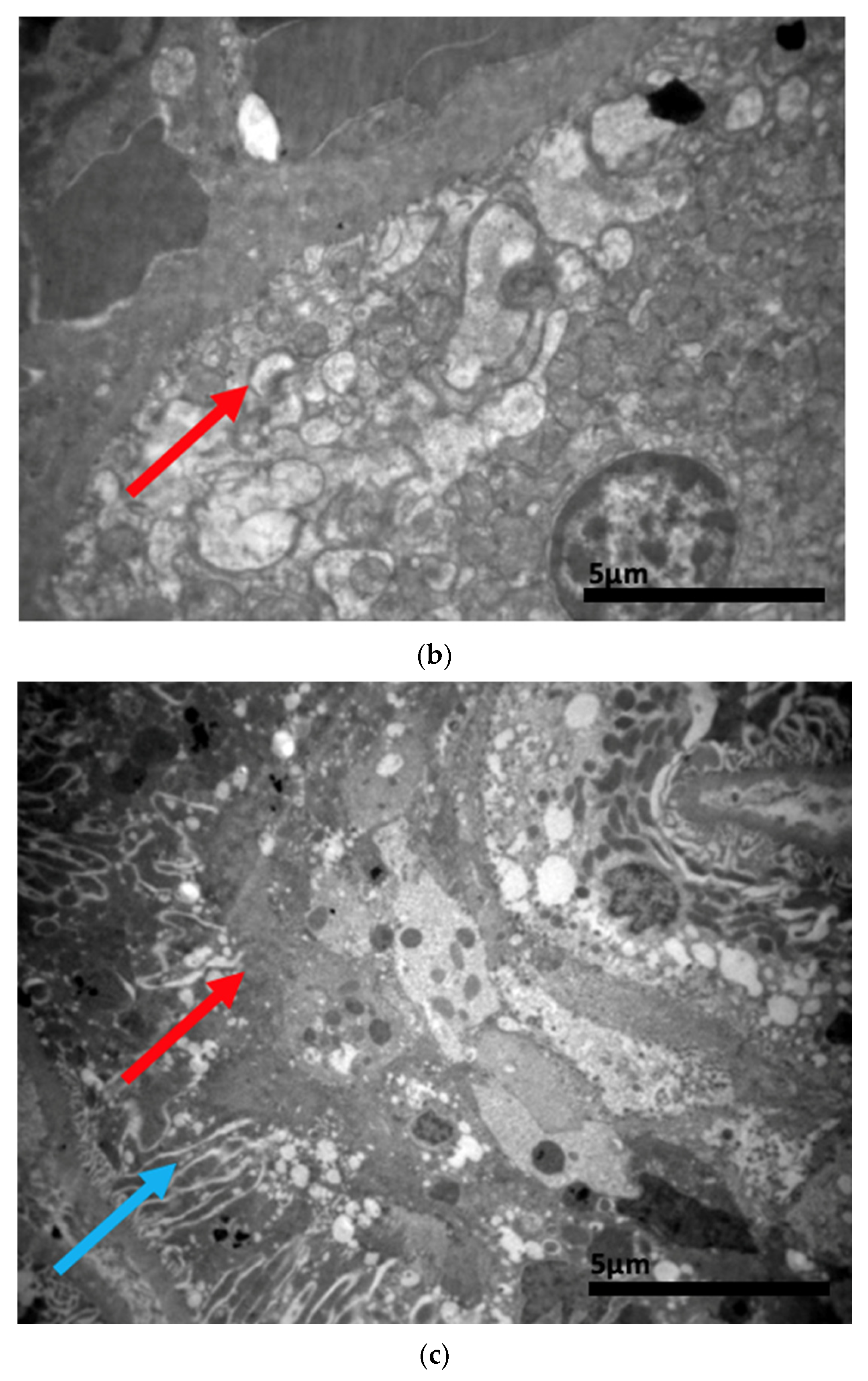
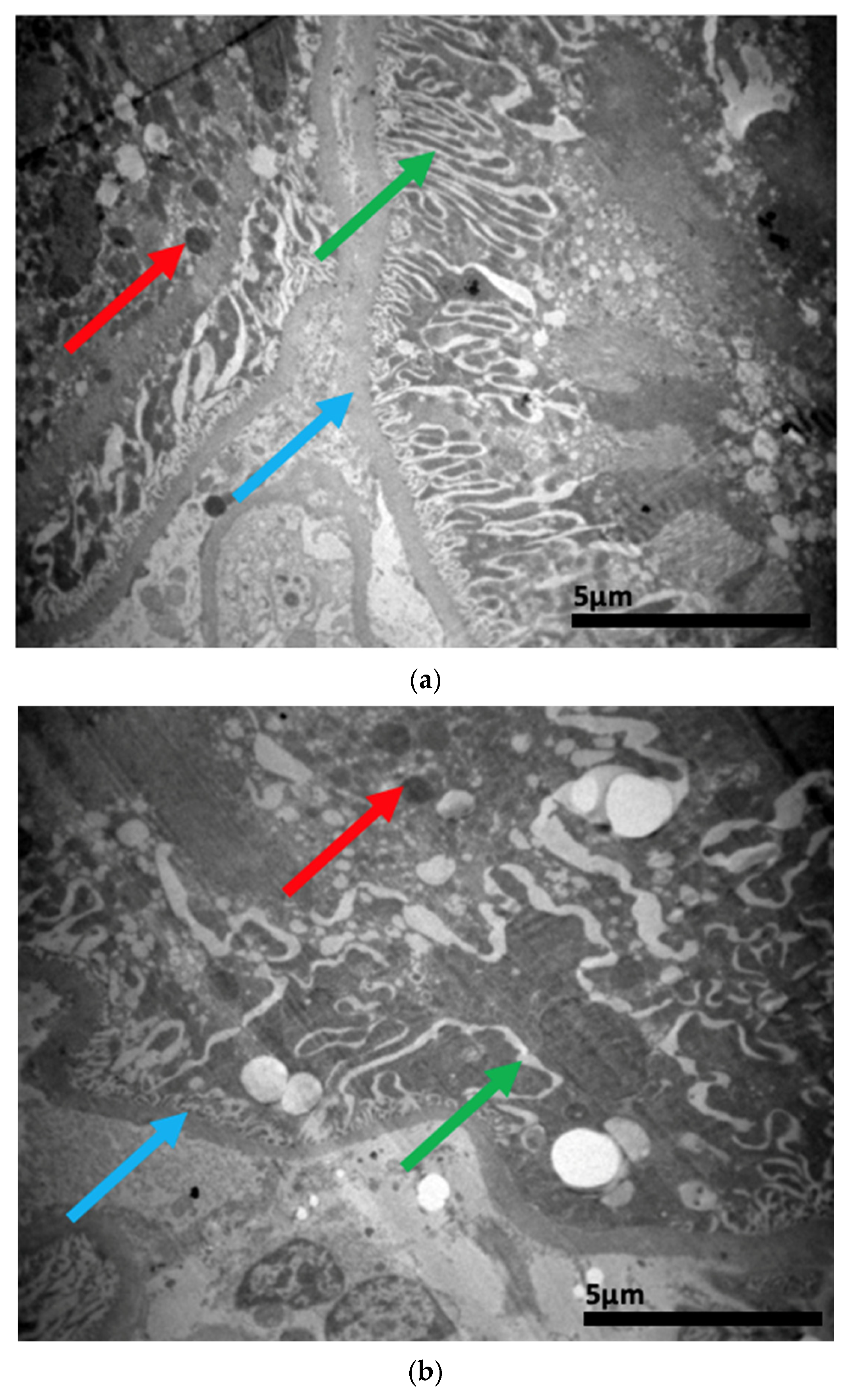
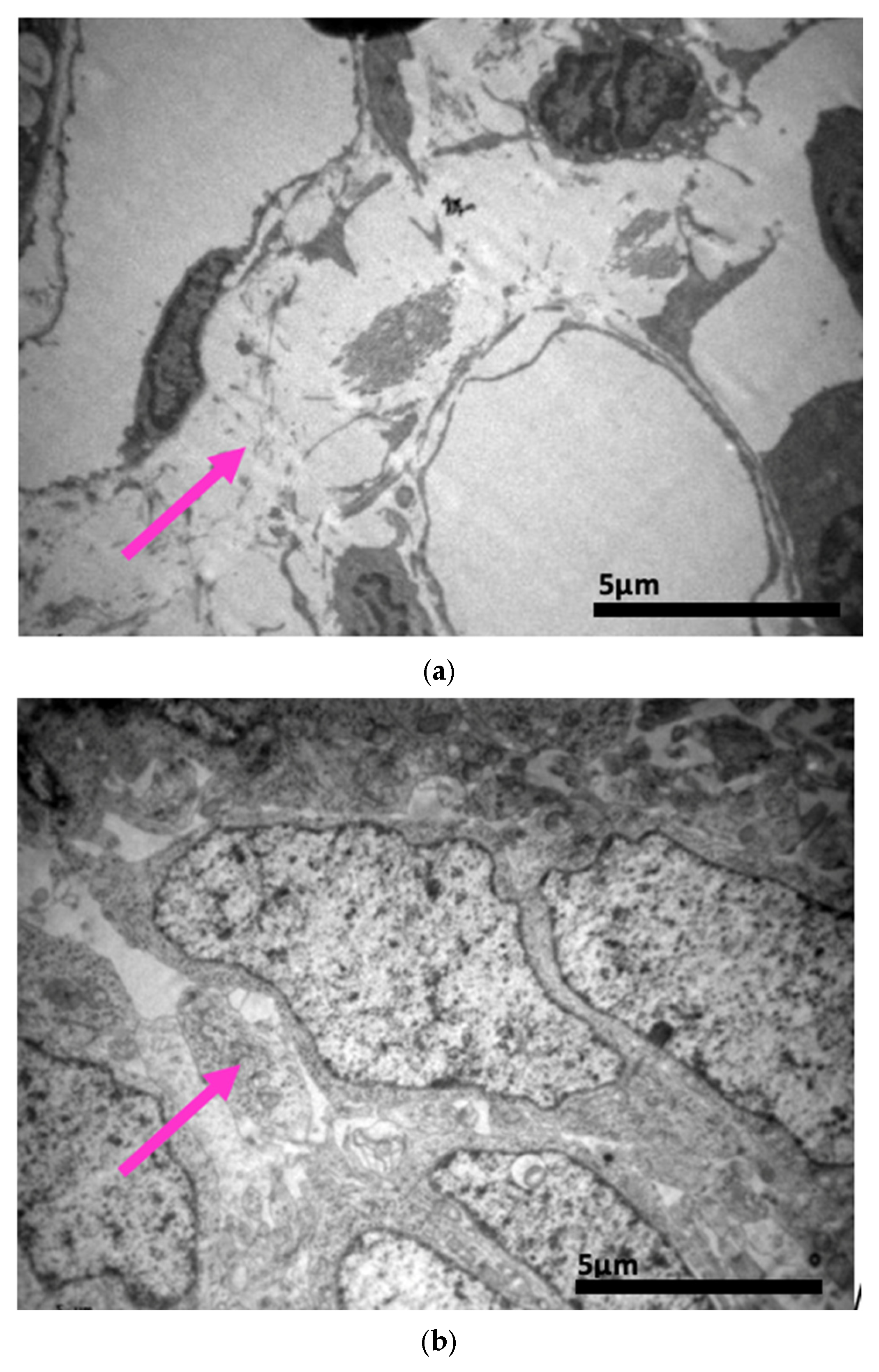
2.3. Electron Microscopy Results
3. Discussion
4. Materials and Methods
4.1. Selected Animals
4.2. Forming Groups and Subgroups
4.3. Experimental Protocol
4.4. Sample Preparation Prior to Optical Microscopy
4.5. Sample Preparation Prior to Immunohistochemistry
4.6. Statistical Analysis
4.7. Sample Preparation Prior to Electron Microscopy
4.8. Study Advantages-Uniqueness
4.9. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endophthalmitis—EyeWiki. Available online: https://eyewiki.org/Endophthalmitis (accessed on 27 October 2024).
- Ly, V.; Sallam, A. Fungal Endophthalmitis; StatPearls: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559257/ (accessed on 27 October 2024).
- Na, S.K.; Park, K.J.; Kim, H.J.; Lee, S.C. Hematogenous Endophthalmitis in a Patient with Candidemia. Korean J. Intern. Med. 1997, 12, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.G.; Flynn, H.W.; Davis, J.L. Exogenous Endophthalmitis. Intraocular Inflamm. 2016, 1437–1446. [Google Scholar] [CrossRef]
- Sheu, S.-J. Endophthalmitis. Korean J. Ophthalmol. 2017, 31, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.E.; Fisher, B.T. Candidiasis. Pediatr. Transpl. Oncol. Infect. Dis. 2021, 195–205.e3. [Google Scholar] [CrossRef]
- Das, T.; Agarwal, M.; Anand, A.R.; Behera, U.C.; Bhende, M.; Das, A.V.; Dasgupta, D.; Dave, V.P.; Gandhi, J.; Gunasekaran, R.; et al. Fungal Endophthalmitis. Ophthalmol. Retin. 2022, 6, 243–251. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, J.; Zhang, J.; Chen, N.; Gao, Y.; Xie, L.-X. Risk factors, treatment strategies, and outcomes of endophthalmitis associated with severe fungal keratitis. Retina 2019, 39, 1076–1082. [Google Scholar] [CrossRef]
- Urtti, A. Pharmacokinetics of retinal drug delivery. Acta Ophthalmol. 2019, 97, 343. [Google Scholar] [CrossRef]
- Patil, A.; Majumdar, S. Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharmacol. 2017, 69, 1635–1660. [Google Scholar] [CrossRef]
- Hata, T.; Furukawa, Y.; Konosu, T.; Oida, S. Structural Study of Triazole Antifungals. II. Crystal and Molecular Structures of the Two Diastereoisomers (4R*,5R*)- and (4S*,5R*)-5-(2,4-Difluorophenyl)-4-methyl-5-[(1H-1,2,4-triazol-1-yl)methyl]-3-[4-(trifluoromethyl)benzoyl]oxazolidine. Bull. Chem. Soc. Jpn. 1991, 64, 2877–2881. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Johnson, L.B.; Kauffman, C.A. Voriconazole: A New Triazole Antifungal Agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar] [CrossRef]
- Maertens, J. History of the development of azole derivatives. Clin. Microbiol. Infect. 2004, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, D.; Oniga, O.; Moldovan, C.; Ionuț, I.; Marc, G.; Stana, A.; Pele, R.; Duma, M.; Tiperciuc, B. An Insight into Rational Drug Design: The Development of In-House Azole Compounds with Antimicrobial Activity. Antibiotics 2024, 13, 763. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New Antifungal Agents with Azole Moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Haas, A. Voriconazole: A Broad Spectrum Triazole for the Treatment of Serious and Invasive Fungal Infections. Futur. Microbiol. 2006, 1, 365–385. [Google Scholar] [CrossRef]
- Salavert, M.; Jarque, I.; Zaragoza, R.; Gobernado, M.; Pemán, J.; Cantón, E.; Romá, E.; Viudes, Á. Voriconazole in the management of nosocomial invasive fungal infections. Ther. Clin. Risk Manag. 2006, 2, 129–157. [Google Scholar] [CrossRef]
- Beran, K.; Abrahamsson, B.; Charoo, N.; Cristofoletti, R.; Holm, R.; Kambayashi, A.; Langguth, P.; Parr, A.; Polli, J.E.; Shah, V.P.; et al. Biowaiver monographs for immediate-release solid oral dosage forms: Voriconazole. J. Pharm. Sci. 2024, 114, 660–680. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Profile of Voriconazole. Clin. Pharmacokinet. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- Mangal, N.; Hamadeh, I.S.; Arwood, M.J.; Cavallari, L.H.; Samant, T.S.; Klinker, K.P.; Bulitta, J.; Schmidt, S. Optimization of Voriconazole Therapy for the Treatment of Invasive Fungal Infections in Adults. Clin. Pharmacol. Ther. 2018, 104, 957–965. [Google Scholar] [CrossRef]
- Donnelly, J.; De Pauw, B. Voriconazole—A new therapeutic agent with an extended spectrum of antifungal activity. Clin. Microbiol. Infect. 2004, 10, 107–117. [Google Scholar] [CrossRef]
- Emami, S.; Tavangar, P.; Keighobadi, M. An overview of azoles targeting sterol 14α-demethylase for antileishmanial therapy. Eur. J. Med. Chem. 2017, 135, 241–259. [Google Scholar] [CrossRef]
- Han, G.; Liu, N.; Li, C.; Tu, J.; Li, Z.; Sheng, C. Discovery of Novel Fungal Lanosterol 14α-Demethylase (CYP51)/Histone Deacetylase Dual Inhibitors to Treat Azole-Resistant Candidiasis. J. Med. Chem. 2020, 63, 5341–5359. [Google Scholar] [CrossRef]
- Hargrove, T.Y.; Friggeri, L.; Wawrzak, Z.; Qi, A.; Hoekstra, W.J.; Schotzinger, R.J.; York, J.D.; Guengerich, F.P.; Lepesheva, G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017, 292, 6728–6743. [Google Scholar] [CrossRef] [PubMed]
- Klomp, F.; Wenzel, C.; Drozdzik, M.; Oswald, S. Drug–Drug Interactions Involving Intestinal and Hepatic CYP1A Enzymes. Pharmaceutics 2020, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, B.H.; Abdelaal, N.M.; Anwer, E.K.E.; Rashwan, A.A.; Hussein, M.A.; Ahmed, Y.F.; Khashana, R.; Hanna, M.M.; Abdelnaser, A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines 2024, 12, 1467. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Wang, Y.; Song, Y.; Cao, Y.; Jiang, Y.; Sun, Q.; Wu, Q. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives containing the 1,2,3-triazole group. RSC Adv. 2013, 3, 13486–13490. [Google Scholar] [CrossRef]
- Xiong, W.-H.; Brown, R.L.; Reed, B.; Burke, N.S.; Duvoisin, R.M.; Morgans, C.W. Voriconazole, an Antifungal Triazol That Causes Visual Side Effects, Is an Inhibitor of TRPM1 and TRPM3 Channels. Investig. Opthalmol. Vis. Sci. 2015, 56, 1367–1373. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef]
- Isern, J.A.; Carlucci, R.; Labadie, G.R.; Porta, E.O.J. Progress and Prospects of Triazoles in Advanced Therapies for Parasitic Diseases. Trop. Med. Infect. Dis. 2025, 10, 142. [Google Scholar] [CrossRef]
- Freifeld, A.; Proia, L.; Andes, D.; Baddour, L.M.; Blair, J.; Spellberg, B.; Arnold, S.; Lentnek, A.; Wheat, L.J. Voriconazole Use for Endemic Fungal Infections. Antimicrob. Agents Chemother. 2009, 53, 1648–1651. [Google Scholar] [CrossRef]
- Clary, R.T.; Deja, E.; Rittmann, B.; Bearman, G. Impact of Voriconazole Therapeutic Drug Monitoring on Adverse Effects and Clinical Outcomes: A Literature Review. Curr. Infect. Dis. Rep. 2025, 27, 6. [Google Scholar] [CrossRef]
- Lewis, R.E. Pharmacokinetic–pharmacodynamic optimization of triazole antifungal therapy. Curr. Opin. Infect. Dis. 2011, 24, S14–S29. [Google Scholar] [CrossRef]
- Yanni, S.B.; Annaert, P.P.; Augustijns, P.; Bridges, A.; Gao, Y.; Benjamin, D.K.; Thakker, D.R. Role of Flavin-Containing Monooxygenase in Oxidative Metabolism of Voriconazole by Human Liver Microsomes. Drug Metab. Dispos. 2008, 36, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Czyrski, A.; Resztak, M.; Świderski, P.; Brylak, J.; Główka, F.K. The Overview on the Pharmacokinetic and Pharmacodynamic Interactions of Triazoles. Pharmaceutics 2021, 13, 1961. [Google Scholar] [CrossRef] [PubMed]
- Carmo, A.; Rocha, M.; Pereirinha, P.; Tomé, R.; Costa, E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12, 884. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Liu, X.; Wang, D.; Hu, Y. Characteristics of voriconazole-induced visual disturbances and hallucinations: Case reports and literature review. Front. Pharmacol. 2024, 15, 1420046. [Google Scholar] [CrossRef]
- Kinoshita, J.; Iwata, N.; Ohba, M.; Kimotsuki, T.; Yasuda, M. Mechanism of Voriconazole-Induced Transient Visual Disturbance: Reversible Dysfunction of Retinal ON-Bipolar Cells in Monkeys. Investig. Opthalmol. Vis. Sci. 2011, 52, 5058–5063. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Xiang, Z.-J.; Yang, J.-H.; Wang, W.-J.; Xu, Z.-C.; Xiang, R.-L. Adverse Effects Associated With Currently Commonly Used Antifungal Agents: A Network Meta-Analysis and Systematic Review. Front. Pharmacol. 2021, 12, 697330. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Gkampeta, A.; Vousvouki, M.; Vasileiou, E.; Groll, A.H. Antifungal agents and the kidney: Pharmacokinetics, clinical nephrotoxicity, and interactions. Expert Opin. Drug Saf. 2021, 20, 1061–1074. [Google Scholar] [CrossRef]
- Somchit, N.; Chung, J.H.; Yaacob, A.; Ahmad, Z.; Zakaria, Z.A.; Kadir, A.A. Lack of hepato- and nephrotoxicity induced by antifungal drug voriconazole in laboratory rats. Drug Chem. Toxicol. 2012, 35, 304–309. [Google Scholar] [CrossRef]
- Burden, A.M.; Hausammann, L.; Ceschi, A.; Kupferschmidt, H.; Weiler, S. Observational cross-sectional case study of toxicities of antifungal drugs. J. Glob. Antimicrob. Resist. 2022, 29, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, N.V. Drug interactions of azole antifungals. J. Ski. Sex. Transm. Dis. 2021, 5, 50–54. [Google Scholar] [CrossRef]
- Lempers, V.J.; Martial, L.C.; Schreuder, M.F.; Blijlevens, N.M.; Burger, D.M.; Aarnoutse, R.E.; Brüggemann, R.J. Drug-interactions of azole antifungals with selected immunosuppressants in transplant patients: Strategies for optimal management in clinical practice. Curr. Opin. Pharmacol. 2015, 24, 38–44. [Google Scholar] [CrossRef]
- Brüggemann, R.J.M.; Alffenaar, J.-W.C.; Blijlevens, N.M.A.; Billaud, E.M.; Kosterink, J.G.W.; Verweij, P.E.; Burger, D.M. Pharmacokinetic drug interactions of azoles. Curr. Fungal Infect. Rep. 2008, 2, 20–27. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular mechanisms governing antifungal drug resistance. npj Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef]
- Paul, S.; Shaw, D.; Joshi, H.; Singh, S.; Chakrabarti, A.; Rudramurthy, S.M.; Ghosh, A.K.; Sturtevant, J. Mechanisms of azole antifungal resistance in clinical isolates of Candida tropicalis. PLoS ONE 2022, 17, e0269721. [Google Scholar] [CrossRef]
- Bustamante, B. Antifungal Drugs. In Current Progress in Medical Mycology; Mora-Montes, H.M., Lopes-Bezerra, L.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–89. [Google Scholar]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins-structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Pirri, G.; Giuliani, A.; Nicoletto, S.; Pizzuto, L.; Rinaldi, A. Lipopeptides as anti-infectives: A practical perspective. Open Life Sci. 2009, 4, 258–273. [Google Scholar] [CrossRef]
- James, K.D.; Laudeman, C.P.; Malkar, N.B.; Krishnan, R.; Polowy, K. Structure-Activity Relationships of a Series of Echinocandins and the Discovery of CD101, a Highly Stable and Soluble Echinocandin with Distinctive Pharmacokinetic Properties. Antimicrob. Agents Chemother. 2017, 61, e01541-16. [Google Scholar] [CrossRef]
- Grover, N.D. Echinocandins: A ray of hope in antifungal drug therapy. Indian J. Pharmacol. 2010, 42, 9–11. [Google Scholar] [CrossRef]
- Hüttel, W. Echinocandins: Structural diversity, biosynthesis, and development of antimycotics. Appl. Microbiol. Biotechnol. 2020, 105, 55–66. [Google Scholar] [CrossRef]
- Logviniuk, D.; Jaber, Q.Z.; Dobrovetsky, R.; Kozer, N.; Ksiezopolska, E.; Gabaldón, T.; Carmeli, S.; Fridman, M. Benzylic Dehydroxylation of Echinocandin Antifungal Drugs Restores Efficacy against Resistance Conferred by Mutated Glucan Synthase. J. Am. Chem. Soc. 2022, 144, 5965–5975. [Google Scholar] [CrossRef]
- Loh, B.S.; Ang, W.H. “Illuminating” Echinocandins’ Mechanism of Action. ACS Central Sci. 2020, 6, 1651–1653. [Google Scholar] [CrossRef]
- Garcia-Effron, G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. J. Fungi 2020, 6, 262. [Google Scholar] [CrossRef]
- Alsowaida, Y.S.; Alshoumr, B.; Alowais, S.A.; Bin Saleh, K.; Alshammari, A.; Alshurtan, K.; Wali, H.A. Effectiveness and safety of echinocandins combination therapy with the standard of care compared to the standard of care monotherapy for the treatment of invasive aspergillosis infection: A meta-analysis. Front. Pharmacol. 2024, 15, 1500529. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Melero, I.; García-Rodríguez, J. Antifúngicos de uso sistémico. Rev. Iberoam. Micol. 2021, 38, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Zhao, Y.; Perlin, D.S. Review of the Novel Echinocandin Antifungal Rezafungin: Animal Studies and Clinical Data. J. Fungi 2020, 6, 192. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lewis, R.E. The echinocandin antifungals: An overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 2003, 12, 1313–1333. [Google Scholar] [CrossRef]
- Swaminathan, S.; Kamat, S.; Pinto, N.A. Echinocandins: Their Role in the Management of Candida Biofilms. Indian J. Med. Microbiol. 2018, 36, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Ioannidou, M.; Pana, Z.-D.; Haidich, A.-B.; Antachopoulos, C.; Iosifidis, E.; Kolios, G.; Roilides, E. Efficacy and Safety of Echinocandins for the Treatment of Invasive Candidiasis in Children. Pediatr. Infect. Dis. J. 2019, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; Pascher, A.; Cointault, O.; Laterre, P.-F.; Cervera, C.; De Waele, J.J.; Cillo, U.; Langer, R.M.; Lugano, M.; Göran-Ericzon, B.; et al. Randomized Trial of Micafungin for the Prevention of Invasive Fungal Infection in High-Risk Liver Transplant Recipients. Clin. Infect. Dis. 2014, 60, 997–1006. [Google Scholar] [CrossRef]
- Ruhnke, M.; Paiva, J.A.; Meersseman, W.; Pachl, J.; Grigoras, I.; Sganga, G.; Menichetti, F.; Montravers, P.; Auzinger, G.; Dimopoulos, G.; et al. Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected critically ill patients. Clin. Microbiol. Infect. 2012, 18, 680–687. [Google Scholar] [CrossRef]
- Mainas, E.; Apostolopoulou, O.; Siopi, M.; Apostolidi, S.; Neroutsos, E.; Mirfendereski, H.; Marchand, S.; Couet, W.; Dokoumetzidis, A.; Valsami, G.; et al. Comparative pharmacokinetics of the three echinocandins in ICU patients. J. Antimicrob. Chemother. 2020, 75, 2969–2976. [Google Scholar] [CrossRef]
- Albanell-Fernández, M. Echinocandins Pharmacokinetics: A Comprehensive Review of Micafungin, Caspofungin, Anidulafungin, and Rezafungin Population Pharmacokinetic Models and Dose Optimization in Special Populations. Clin. Pharmacokinet. 2024, 64, 27–52. [Google Scholar] [CrossRef]
- Yamasaki, K.; Sakurama, K.; Nishi, K.; Tsukigawa, K.; Seo, H.; Otagiri, M.; Taguchi, K. An in-vitro comparative study of the binding of caspofungin and micafungin to plasma proteins. J. Pharm. Pharmacol. 2021, 74, 88–93. [Google Scholar] [CrossRef]
- Al Musaimi, O. FDA-Approved Antibacterials and Echinocandins. Antibiotics 2025, 14, 166. [Google Scholar] [CrossRef]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Kofla, G.; Ruhnke, M. Pharmacology and metabolism of anidulafungin, caspofungin and micafungin in the treatment of invasive candidosis—Review of the literature. Eur. J. Med. Res. 2011, 16, 159–166. [Google Scholar] [CrossRef]
- Bormann, A.M.; Morrison, V.A. Review of the pharmacology and clinical studies of micafungin. Drug Des. Dev. Ther. 2009, 3, 295–302. [Google Scholar] [CrossRef]
- Aguilar-Zapata, D.; Petraitiene, R.; Petraitis, V. Echinocandins: The Expanding Antifungal Armamentarium. Clin. Infect. Dis. 2015, 61, S604–S611. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The Newest Class of Antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Bal, A. The echinocandins: Three useful choices or three too many? Int. J. Antimicrob. Agents 2010, 35, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wegner, B.; Baer, P.; Gauer, S.; Oremek, G.; Hauser, I.A.; Geiger, H. Caspofungin is less nephrotoxic than amphotericin B in vitro and predominantly damages distal renal tubular cells. Nephrol. Dial. Transplant. 2005, 20, 2071–2079. [Google Scholar] [CrossRef]
- Dermitzaki, N.; Balomenou, F.; Gialamprinou, D.; Giapros, V.; Rallis, D.; Baltogianni, M. Perspectives on the Use of Echinocandins in the Neonatal Intensive Care Unit. Antibiotics 2024, 13, 1209. [Google Scholar] [CrossRef]
- Rosanova, M.T.; Bes, D.; Aguilar, P.S.; Pompa, L.C.; Sberna, N.; Lede, R. Efficacy and safety of caspofungin in children: Systematic review and meta-analysis. Arch. Argent. Pediatr. 2016, 114, 305–312. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Carver, P.L.; Datta, K.; Galar, A.; Johnson, M.D.; Johnson, M.G.; Marty, F.M.; Nagel, J.; Najdzinowicz, M.; Saul, M.; et al. Short-term risk of liver and renal injury in hospitalized patients using micafungin: A multicentre cohort study. J. Antimicrob. Chemother. 2016, 71, 2938–2944. [Google Scholar] [CrossRef]
- Niwa, T.; Imagawa, Y.; Yamazaki, H. Drug Interactions between Nine Antifungal Agents and Drugs Metabolized by Human Cytochromes P450. Curr. Drug Metab. 2015, 15, 651–679. [Google Scholar] [CrossRef]
- Flanagan, S.; Walker, H.; Ong, V.; Sandison, T.; Anderson, M.Z. Absence of Clinically Meaningful Drug-Drug Interactions with Rezafungin: Outcome of Investigations. Microbiol. Spectr. 2023, 11, e0133923. [Google Scholar] [CrossRef]
- Gubbins, P.O.; Heldenbrand, S. Clinically relevant drug interactions of current antifungal agents. Mycoses 2010, 53, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.T.; Kritikos, A.; Li, J.; Khanna, N.; Goldenberger, D.; Garzoni, C.; Zehnder, C.; Boggian, K.; Neofytos, D.; Riat, A.; et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020, 48, 761–766. [Google Scholar] [CrossRef]
- Zajac, C.; Scott, N.E.; Kline, S.; Erayil, S.E.; Selmecki, A. Hotspot gene conversion between FKS1 and FKS2 in echinocandin resistant Candida glabrata serial isolates. npj Antimicrob. Resist. 2025, 3, 31. [Google Scholar] [CrossRef]
- Jones, R.N.; Castanheira, M.; Pfaller, M.A. Fixed-Ratio Combination Testing of an Echinocandin, Anidulafungin, and an Azole, Voriconazole, against 1467 Candida Species Isolates. Antimicrob. Agents Chemother. 2010, 54, 4041–4043. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Dias, C.S.; Anand, B.S.; Mitra, A.K. Effect of Mono- and Di-acylation on the Ocular Disposition of Ganciclovir: Physicochemical Properties, Ocular Bioreversion, and Antiviral Activity of Short Chain Ester Prodrugs. J. Pharm. Sci. 2002, 91, 660–668. [Google Scholar] [CrossRef]
- Doft, B.H.; Weiskopf, J.; Nilsson-Ehle, I.; Wingard, L.B. Amphotericin Clearance in Vitrectomized Versus Nonvitrectomized Eyes. Ophthalmology 1985, 92, 1601–1605. [Google Scholar] [CrossRef]
- Mishra, D.; Gade, S.; Glover, K.; Sheshala, R.; Singh, T.R.R. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr. Eye Res. 2022, 48, 208–218. [Google Scholar] [CrossRef]
- Moschovakou, D.; Ntoupa, S.-P.; Dona, A.; Athanaselis, S.; Spiliopoulou, C.; Nikolaou, P.; Papoutsis, I. Vitreous humor in the forensic toxicology of quetiapine and its metabolites. Forensic Toxicol. 2024, 42, 212–220. [Google Scholar] [CrossRef]
- Rimpelä, A.-K.; Kiiski, I.; Deng, F.; Kidron, H.; Urtti, A. Pharmacokinetic Simulations of Intravitreal Biologicals: Aspects of Drug Delivery to the Posterior and Anterior Segments. Pharmaceutics 2018, 11, 9. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferroni, M.; De Gaetano, F.; Cereda, M.G.; Boschetti, F. A drug delivery analysis of large molecules in ocular vitreous chamber: Dependency on saccadic movements after intravitreal injection. Med. Eng. Phys. 2020, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Normand, G.; Maker, M.; Penraat, J.; Kovach, K.; Ghosh, J.G.; Grosskreutz, C.; Chandra, S. Non-invasive molecular tracking method that measures ocular drug distribution in non-human primates. Commun. Biol. 2020, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.M.; Ruiz, E.A.C.; Ohr, M.P.; Swindle-Reilly, K.E.; Versypt, A.N.F. Computer modeling of bevacizumab drug distribution after intravitreal injection in rabbit and human eyes. J. Pharm. Sci. 2024, 114, 1164–1174. [Google Scholar] [CrossRef]
- Zehden, J.A.; Mortensen, X.M.; Reddy, A.; Zhang, A.Y. Systemic and Ocular Adverse Events with Intravitreal Anti-VEGF Therapy Used in the Treatment of Diabetic Retinopathy: A Review. Curr. Diabetes Rep. 2022, 22, 525–536. [Google Scholar] [CrossRef]
- Johari, M.K.; Askari, M.; Amini, A.; Yasemi, M. Acute systemic complications of intravitreal bevacizumab and triamcinolone injections—A comparative study. Folia Medica 2022, 64, 240–247. [Google Scholar] [CrossRef]
- George, B.; You, D.; Joy, M.S.; Aleksunes, L.M. Xenobiotic transporters and kidney injury. Adv. Drug Deliv. Rev. 2017, 116, 73–91. [Google Scholar] [CrossRef]
- Sakolish, C.; Tsai, H.-H.D.; Lin, H.-C.; Bajaj, P.; Villenave, R.; Ferguson, S.S.; Stanko, J.P.; Becker, R.A.; Hewitt, P.; Chiu, W.A.; et al. Comparative Analysis of Proximal Tubule Cell Sources for In Vitro Studies of Renal Proximal Tubule Toxicity. Biomedicines 2025, 13, 563. [Google Scholar] [CrossRef]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2021, 37, 973–982. [Google Scholar] [CrossRef]
- Hall, A.M.; de Seigneux, S. Metabolic mechanisms of acute proximal tubular injury. Pflugers Arch. 2022, 474, 813–827. [Google Scholar] [CrossRef]
- Degirmenci, C.; Palamar, M.; Aktug, H.; Yigittürk, G.; Veral, A.; Yagcı, A. The effect of topical voriconazole on conjunctiva in rats as revealed by histopathology and immunohistochemistry. J. Chemother. 2019, 31, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rajčáni, J.; Bánáti, F.; Szenthe, K.; Niller, H.H.; Minarovits, J.; Stipkovits, L.; Szathmary, S. Detection of Interleukin 6 (IL-6) antigen in the head and neck carcinoma cells. Clin. Med. Investig. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Sato, K.; Takeda, A.; Hasegawa, E.; Jo, Y.-J.; Arima, M.; Oshima, Y.; Ryoji, Y.; Nakazawa, T.; Yuzawa, M.; Nakashizuka, H.; et al. Interleukin-6 plays a crucial role in the development of subretinal fibrosis in a mouse model. Immunol. Med. 2018, 41, 23–29. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Yan, J.; Zhang, L.; Chen, G.; He, L.; Wang, Y.; Charonis, A.S. Effect of Interleukin 6 Deficiency on Renal Interstitial Fibrosis. PLoS ONE 2012, 7, e52415. [Google Scholar] [CrossRef]
- Farahani, M.; Niknam, Z.; Amirabad, L.M.; Amiri-Dashatan, N.; Koushki, M.; Nemati, M.; Pouya, F.D.; Rezaei-Tavirani, M.; Rasmi, Y.; Tayebi, L. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed. Pharmacother. 2021, 145, 112420. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Yu, H.; Zhang, Y.; Dai, Y.; Ning, C.; Tao, L.; Sun, H.; Kellems, R.E.; Blackburn, M.R.; et al. Interleukin 6 Underlies Angiotensin II–Induced Hypertension and Chronic Renal Damage. Hypertension 2012, 59, 136–144. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef]
- Gallucci, R.M.; Simeonova, P.P.; Matheson, J.M.; Kommineni, C.; Guriel, J.L.; Sugawara, T.; Luster, M.I. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000, 14, 2525–2531. [Google Scholar] [CrossRef]
- Tenbrock, L.; Wolf, J.; Boneva, S.; Schlecht, A.; Agostini, H.; Wieghofer, P.; Lange, C. Subretinal fibrosis in neovascular age-related macular degeneration: Current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022, 387, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Sgrignoli, M.R.; Silva, D.A.; Nascimento, F.F.; Sgrignoli, D.A.M.; Nai, G.A.; da Silva, M.G.; de Barros, M.A.; Bittencourt, M.K.W.; de Morais, B.P.; Dinallo, H.R.; et al. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFα in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem Cell Res. 2019, 39, 101525. [Google Scholar] [CrossRef]
- Marinho, M.; Monteiro, C.; Peiró, J.; Machado, G.; Oliveira-Júnior, I. TNF-α and IL-6 immunohistochemistry in rat renal tissue experimentaly infected with Leptospira interrogans serovar Canicola. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 533–540. [Google Scholar] [CrossRef][Green Version]
- Paule, B.; Belot, J.; Rudant, C.; Coulombel, C.; Abbou, C.C. The importance of IL-6 protein expression in primary human renal cell carcinoma: An immunohistochemical study: Figure 1. J. Clin. Pathol. 2000, 53, 388–390. [Google Scholar] [CrossRef]
- Dong, L.; Shi, X.H.; Li, Y.F.; Jiang, X.; Wang, Y.X.; Lan, Y.J.; Wu, H.T.; Jonas, J.B.; Bin Wei, W. Blockade of epidermal growth factor and its receptor and axial elongation in experimental myopia. FASEB J. 2020, 34, 13654–13670. [Google Scholar] [CrossRef]
- Kumar, S.; Purohit, P.; Dagar, S. A review: Status of genetic modulated nonsmall cell lung cancer targets and treatment (current updates in drugs for non-small cell lung cancer treatment). Asian J. Pharm. Clin. Res. 2018, 11, 40–55. [Google Scholar] [CrossRef]
- Frattini, M.; Saletti, P.; Molinari, F.; De Dosso, S. EGFR signaling in colorectal cancer: A clinical perspective. Gastrointest. Cancer Targets Ther. 2015, 5, 21–38. [Google Scholar] [CrossRef]
- Janani, B.; Vijayakumar, M.; Priya, K.; Kim, J.H.; Prabakaran, D.S.; Shahid, M.; Al-Ghamdi, S.; Alsaidan, M.; Bahakim, N.O.; Abdelzaher, M.H.; et al. EGFR-Based Targeted Therapy for Colorectal Cancer—Promises and Challenges. Vaccines 2022, 10, 499. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.-K.; Nagai, K.; Plieth, D.; Tan, M.; Lee, T.-C.; Threadgill, D.W.; Neilson, E.G.; Harris, R.C. EGFR Signaling Promotes TGFβ-Dependent Renal Fibrosis. J. Am. Soc. Nephrol. 2012, 23, 215–224. [Google Scholar] [CrossRef]
- Modjtahedi, H.; Khelwatty, S.A.; Kirk, R.S.; Seddon, A.M.; Essapen, S.; Del Vecchio, C.A.; Wong, A.J.; Eccles, S. Immunohistochemical discrimination of wild-type EGFR from EGFRvIII in fixed tumour specimens using anti-EGFR mAbs ICR9 and ICR10. Br. J. Cancer 2012, 106, 883–888. [Google Scholar] [CrossRef][Green Version]
- Atkins, D.; Reiffen, K.-A.; Tegtmeier, C.L.; Winther, H.; Bonato, M.S.; Störkel, S. Immunohistochemical Detection of EGFR in Paraffin-embedded Tumor Tissues. J. Histochem. Cytochem. 2004, 52, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Sweeney, W.E.; Wang, X.; Qian, Q.; Harris, P.C.; Frost, P.; Avner, E.D. EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int. 2003, 64, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, A.; Abbara, C.; Cellier, M.; Lagarce, L.; Babin, M.; Bourneau-Martin, D.; Briet, M. Renal Safety Profile of EGFR Targeted Therapies: A Study from VigiBase® the WHO Global Database of Individual Case Safety Reports. Cancers 2021, 13, 5907. [Google Scholar] [CrossRef]
- Li, Q.; Lin, J.; Hao, G.; Xie, A.; Liu, S.; Tang, B. Nephrotoxicity of targeted therapy used to treat lung cancer. Front. Immunol. 2024, 15, 1369118. [Google Scholar] [CrossRef]
- Haider, A.A.; Gallagher, J.R.; Johnson, J.S.; Benevento, J.D. Intravitreal Voriconazole for the Treatment of Cryptococcus neoformans Endogenous Endophthalmitis. Ochsner J. 2020, 20, 319–322. [Google Scholar] [CrossRef]
- Danielescu, C.; Cantemir, A.; Chiselita, D. Successful treatment of fungal endophthalmitis using intravitreal caspofungin. Arq. Bras. Oftalmol. 2017, 80, 196–198. [Google Scholar] [CrossRef]
- Neofytos, D.; Lombardi, L.R.; Shields, R.K.; Ostrander, D.; Warren, L.; Nguyen, M.H.; Thompson, C.B.; Marr, K.A. Administration of Voriconazole in Patients With Renal Dysfunction. Clin. Infect. Dis. 2012, 54, 913–921. [Google Scholar] [CrossRef]
- Vena, A.; Bouza, E.; Bassetti, M.; Menichetti, F.; Merelli, M.; Grau, S.; Fortun, J.; Sánchez, M.I.; Aguado, J.M.; Merino, P.; et al. Comparison of the Safety and Tolerance Profile of Micafungin with that of Other Echinocandins and Azoles in Patients with Pre-existing Child-Pugh B or C Liver Disease: A Case-Control Retrospective Study. Infect. Dis. Ther. 2020, 9, 151–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruffini, A.; Casalucci, A.; Cara, C.; Ethier, C.R.; Repetto, R. Drug Distribution After Intravitreal Injection: A Mathematical Model. Investig. Opthalmology Vis. Sci. 2024, 65, 9. [Google Scholar] [CrossRef]
- Yetgin, C.; Demiröz, F.N.T.; Takka, S. Ocular Drug Delivery Routes: Diseases Overview and Advanced Administration Methods. FABAD J. Pharm. Sci. 2024, 49, 627–646. [Google Scholar] [CrossRef]
- Desideri, L.F.; Sim, P.Y.; Bernardi, E.; Paschon, K.; Roth, J.; Fung, A.T.; Ni Wu, X.; Chou, H.-D.; Henderson, R.; Tsui, E.; et al. Evidence-based guidelines for drug dosing in intravitreal injections in silicone oil-filled eyes: Pharmacokinetics, safety, and optimal dosage. Surv. Ophthalmol. 2024, 70, 96–105. [Google Scholar] [CrossRef]
- Lamirande, P.; Gaffney, E.A.; Gertz, M.; Maini, P.K.; Crawshaw, J.R.; Caruso, A. A First-Passage Model of Intravitreal Drug Delivery and Residence Time—Influence of Ocular Geometry, Individual Variability, and Injection Location. Investig. Opthalmology Vis. Sci. 2024, 65, 21. [Google Scholar] [CrossRef] [PubMed]
- Karachrysafi, S.; Sioga, A.; Komnenou, A.; Karamitsos, A.; Xioteli, M.; Dori, I.; Delis, G.; Kofidou, E.; Anastasiadou, P.; Sotiriou, S.; et al. Histological Effects of Intravitreal Injection of Antifungal Agents in New Zealand White Rabbits: An Electron Microscopic and Immunohistochemical Study. Pharmaceuticals 2020, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ríos, A.; Correa-Gallegos, E.Y.; Medina-Espinoza, J.M.; Navarro-Sanchez, A.A.; Olvera-Montaño, O.; Baiza-Durán, L.; Muñoz-Villegas, P. Validation of a preclinical dry eye model in New Zealand white rabbits during and following topical instillation of 1% ophthalmic atropine sulfate. Anim. Model. Exp. Med. 2022, 5, 266–273. [Google Scholar] [CrossRef]
- Shimizu, S.; Ochiai, Y.; Kamijima, K.; Takai, N.; Watanabe, S.; Aihara, M. Development and characterization of a chronic high intraocular pressure model in New Zealand white rabbits for glaucoma research. Exp. Eye Res. 2024, 245, 109973. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Li, X.; Zhang, M. Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization. Open Life Sci. 2021, 16, 1261–1267. [Google Scholar] [CrossRef]
- Valdez-Garcia, J.E.; Lozano-Ramirez, J.F.; Zavala, J. Adult white New Zealand rabbit as suitable model for corneal endothelial engineering. BMC Res. Notes 2015, 8, 28. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, P.; Sun, M.; Fu, W.; Cai, C. Comprehensive spectral libraries for various rabbit eye tissue proteomes. Sci. Data 2022, 9, 111. [Google Scholar] [CrossRef]
- Yamagiwa, Y.; Kurata, M.; Satoh, H. Histological Features of Postnatal Development of the Eye in White Rabbits. Toxicol. Pathol. 2020, 49, 419–437. [Google Scholar] [CrossRef]
- Yamashita, K.; Hatou, S.; Inagaki, E.; Higa, K.; Tsubota, K.; Shimmura, S. A Rabbit Corneal Endothelial Dysfunction Model Using Endothelial-Mesenchymal Transformed Cells. Sci. Rep. 2018, 8, 16868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kousholt, B.S.; Præstegaard, K.F.; Stone, J.C.; Thomsen, A.F.; Johansen, T.T.; Ritskes-Hoitinga, M.; Wegener, G. Reporting of 3Rs Approaches in Preclinical Animal Experimental Studies—A Nationwide Study. Animals 2023, 13, 3005. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Zambrano, E.; Flores, M.E.; Contreras, M.; Crispín, J.C.; Alemán, G.; Bravo, C.; Armenta, A.; Valdés, V.J.; Tovar, A.; et al. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev. Investig. Clin. 2021, 73, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, N.M.; Todorovska, M.M. FORMULA FOR THE WELL-BEING OF EXPERIMENTAL ANIMALS: 3R + 1R. Facta Univ. Series Med. Biol. 2017, 19, 018–022. [Google Scholar] [CrossRef]
- Louis-Maerten, E.; Perez, C.R.; Cajiga, R.M.; Persson, K.; Elger, B.S. Conceptual foundations for a clarified meaning of the 3Rs principles in animal experimentation. Anim. Welf. 2024, 33, e37. [Google Scholar] [CrossRef]
- Verderio, P.; Lecchi, M.; Ciniselli, C.M.; Shishmani, B.; Apolone, G.; Manenti, G. 3Rs Principle and Legislative Decrees to Achieve High Standard of Animal Research. Animals 2023, 13, 277. [Google Scholar] [CrossRef]
- Coşkun, N.C. Design of Animal Experiments in Pharmacological Research. Duzce Med. J. 2024, 26, 87–94. [Google Scholar] [CrossRef]
- Piper, S.K.; Zocholl, D.; Toelch, U.; Roehle, R.; Stroux, A.; Hoessler, J.; Zinke, A.; Konietschke, F. Statistical review of animal trials—A guideline. Biom. J. 2022, 65, e2200061. [Google Scholar] [CrossRef]
- Baiza-Durán, L.; Sánchez-Ríos, A.; González-Barón, J.; Olvera-Montaño, O.; Correa-Gallegos, E.; Navarro-Sánchez, A.; Muñoz-Villegas, P. Safety and tolerability evaluation after repeated intravitreal injections of a humanized anti-VEGF-A monoclonal antibody (PRO-169) versus ranibizumab in New Zealand white rabbits. Int. J. Retin. Vitr. 2020, 6, 32. [Google Scholar] [CrossRef]
- Tassew, N.G.; Laing, S.T.; Aaronson, J.; de Jong, I.; Schuetz, C.; Lorget, F. Tolerability Assessment of Formulation pH in New Zealand White Rabbits Following Intravitreal Administration. Toxicol. Pathol. 2020, 49, 605–609. [Google Scholar] [CrossRef]
- Starodubtseva, M.N.; Karachrysafi, S.; Shkliarava, N.M.; Chelnokova, I.A.; Kavvadas, D.; Papadopoulou, K.; Samara, P.; Papaliagkas, V.; Sioga, A.; Komnenou, A.; et al. The Effects of Intravitreal Administration of Antifungal Drugs on the Structure and Mechanical Properties Peripheral Blood Erythrocyte Surface in Rabbits. Int. J. Mol. Sci. 2022, 23, 10464. [Google Scholar] [CrossRef]
- Aguirre, S.A.; Gukasyan, H.J.; Younis, H.S.; Huang, W. Safety Assessment of Formulation Vehicles Following Intravitreal Administration in Rabbits. Pharm. Res. 2018, 35, 173. [Google Scholar] [CrossRef]
- Cortez, R.T.; Ramirez, G.; Collet, L.; Thakuria, P.; Giuliari, G.P. Intravitreous Bevacizumab Injection. Arch. Ophthalmol. 2010, 128, 884–887. [Google Scholar] [CrossRef][Green Version]
- Laing, S.T.; Tassew, N.; Tesar, D.; Wang, Y.; Crowell, S.R.; Gray, J.; Kwong, M.; Loyet, K.M.; Andaya, R.; Kusi, A.; et al. Retinal and Lens Degeneration in New Zealand White Rabbits Administered Intravitreal TSG-6 Link Domain-Rabbit FAb Fusion Proteins. Toxicol. Pathol. 2020, 49, 634–646. [Google Scholar] [CrossRef]
- Kansara, V.S.; Muya, L.W.; Ciulla, T.A. Evaluation of Long-Lasting Potential of Suprachoroidal Axitinib Suspension Via Ocular and Systemic Disposition in Rabbits. Transl. Vis. Sci. Technol. 2021, 10, 19. [Google Scholar] [CrossRef]
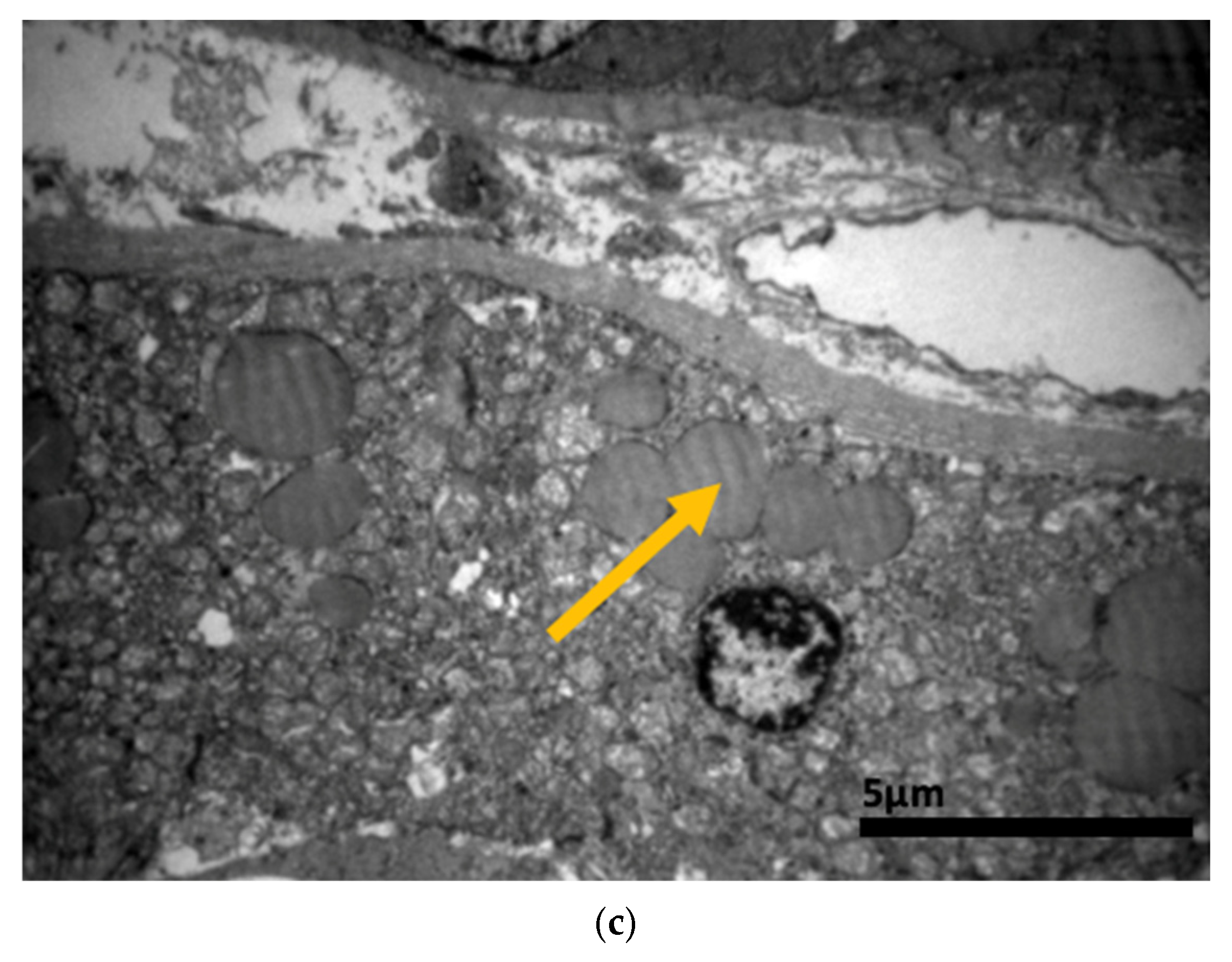
| Antifungal Agent | Structure | Metabolism |
|---|---|---|
| Voriconazole | 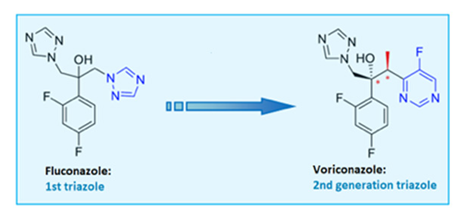 | 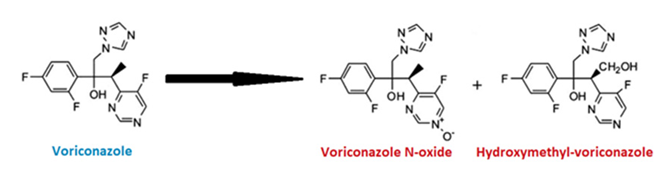 |
| Micafungin | 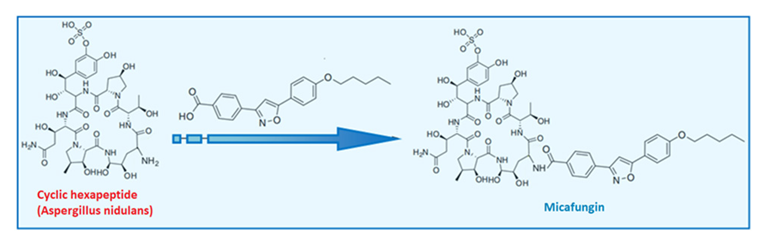 |  |
| Subgroup | Structure | Biomarker | Intensity | Positively Stained Cells | IRS |
|---|---|---|---|---|---|
| C | Renal tubules and corpuscles | IL-6 | 1 | 2 | 2 |
| C | Renal tubules and corpuscles | EGFR | 1 | 1 | 1 |
| V1 | Renal tubules | IL-6 | 2 | 4 | 8 |
| V1 | Renal corpuscles | IL-6 | 2 | 2 | 4 |
| V1 | Renal tubules | EGFR | 3 | 4 | 12 |
| V1 | Renal corpuscles | EGFR | 2 | 2 | 4 |
| V2 | Renal corpuscles | IL-6 | 2 | 1 | 2 |
| V2 | Renal tubules | IL-6 | 2 | 3 | 6 |
| V2 | Renal corpuscles | EGFR | 3 | 4 | 12 |
| V2 | Renal tubules | EGFR | 2 | 4 | 8 |
| M1 | Renal corpuscles | IL-6 | 2 | 3 | 6 |
| M1 | Renal tubules | IL-6 | 2 | 1 | 2 |
| M1 | Renal corpuscles | EGFR | 3 | 4 | 12 |
| M1 | Renal tubules | EGFR | 1 | 2 | 2 |
| M2 | Renal corpuscles | IL-6 | 3 | 3 | 9 |
| M2 | Renal tubules | IL-6 | 2 | 3 | 6 |
| M2 | Renal corpuscles | EGFR | 1 | 2 | 2 |
| M2 | Renal tubules | EGFR | 1 | 3 | 3 |
| VM | Renal corpuscles | IL-6 | 3 | 4 | 12 |
| VM | Renal tubules | IL-6 | 1 | 2 | 2 |
| VM | Renal corpuscles | EGFR | 3 | 3 | 9 |
| VM | Renal tubules | EGFR | 2 | 3 | 6 |
| Marker | Tissue | Control (C) | Voriconazole (V) | Micafungin (M) | Voriconazole + Micafungin (VM) | p-Value | Significant Comparisons (Dunn’s Post Hoc) |
|---|---|---|---|---|---|---|---|
| IL-6 | Renal Tubules | 2 (1–4) | 3 (1–5) | 3 (2–5) | 5 (3–6) | 0.048 | VM > C (p = 0.03) |
| Renal Corpuscles | 1 (0–3) | 2 (1–4) | 4 (2–5) | 5 (3–6) | 0.0089 | VM > C (p = 0.006), M > C (p = 0.049) | |
| EGFR | Renal Tubules | 3 (2–4) | 5 (3–6) | 4 (3–6) | 6 (5–7) | 0.0197 | VM > C (p = 0.012), V > C (p = 0.043) |
| Renal Corpuscles | 3 (2–4) | 4 (3–5) | 5 (4–6) | 6 (5–7) | 0.0247 | VM > C (p = 0.013), M > C (p = 0.038) |
| Animal Group | Medication Administrated | Dose Applied |
|---|---|---|
| Control group C | No medication administrated | - |
| Study Subgroup V1 | One (1) intravitreal injection of voriconazole solution | 40 μg/0.1 mL |
| Study Subgroup V2 | Two (2) intravitreal injections of voriconazole solution | 40 μg/0.1 mL (each injection) |
| Study Subgroup M1 | One (1) intravitreal injection of micafungin solution | 25 μg/0.1 mL |
| Study Subgroup M2 | Two (2) intravitreal injections of micafungin solution | 25 μg/0.1 mL (each injection) |
| Study group VM | One (1) intravitreal injection of voriconazole solution and one (1) intravitreal injection of micafungin simultaneously | 40 μg/0.1 mL (voriconazole) 25 μg/0.1 mL (micafungin) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karachrysafi, S.; Karakousis, V.-A.; Liatsos, A.; Kavvadas, D.; Ioannou, D.; Anastasiadou, P.; Kofidou, E.; Karampatakis, V.; Sioga, A.; Raikos, N.; et al. Ultrastructural and Immunohistochemical Study on the Nephrotoxicity Following Intravitreal Administration of the Antifungal Agents Voriconazole and Micafungin in New Zealand White Rabbits. Int. J. Mol. Sci. 2025, 26, 10129. https://doi.org/10.3390/ijms262010129
Karachrysafi S, Karakousis V-A, Liatsos A, Kavvadas D, Ioannou D, Anastasiadou P, Kofidou E, Karampatakis V, Sioga A, Raikos N, et al. Ultrastructural and Immunohistochemical Study on the Nephrotoxicity Following Intravitreal Administration of the Antifungal Agents Voriconazole and Micafungin in New Zealand White Rabbits. International Journal of Molecular Sciences. 2025; 26(20):10129. https://doi.org/10.3390/ijms262010129
Chicago/Turabian StyleKarachrysafi, Sofia, Vasileios-Alexandros Karakousis, Alexandros Liatsos, Dimitrios Kavvadas, Despoina Ioannou, Pinelopi Anastasiadou, Evangelia Kofidou, Vasileios Karampatakis, Antonia Sioga, Nikolaos Raikos, and et al. 2025. "Ultrastructural and Immunohistochemical Study on the Nephrotoxicity Following Intravitreal Administration of the Antifungal Agents Voriconazole and Micafungin in New Zealand White Rabbits" International Journal of Molecular Sciences 26, no. 20: 10129. https://doi.org/10.3390/ijms262010129
APA StyleKarachrysafi, S., Karakousis, V.-A., Liatsos, A., Kavvadas, D., Ioannou, D., Anastasiadou, P., Kofidou, E., Karampatakis, V., Sioga, A., Raikos, N., & Papamitsou, T. (2025). Ultrastructural and Immunohistochemical Study on the Nephrotoxicity Following Intravitreal Administration of the Antifungal Agents Voriconazole and Micafungin in New Zealand White Rabbits. International Journal of Molecular Sciences, 26(20), 10129. https://doi.org/10.3390/ijms262010129











