Aggregation Characteristics of Tau Phosphorylated by Various Kinases as Observed by Quantum Dot Fluorescence Imaging
Abstract
1. Introduction
2. Results
2.1. Confirmation of Phosphorylation at Major Tau Phosphorylation Sites by Kinases
2.2. Effect of Tau Phosphorylation Buffer on Tau Aggregation
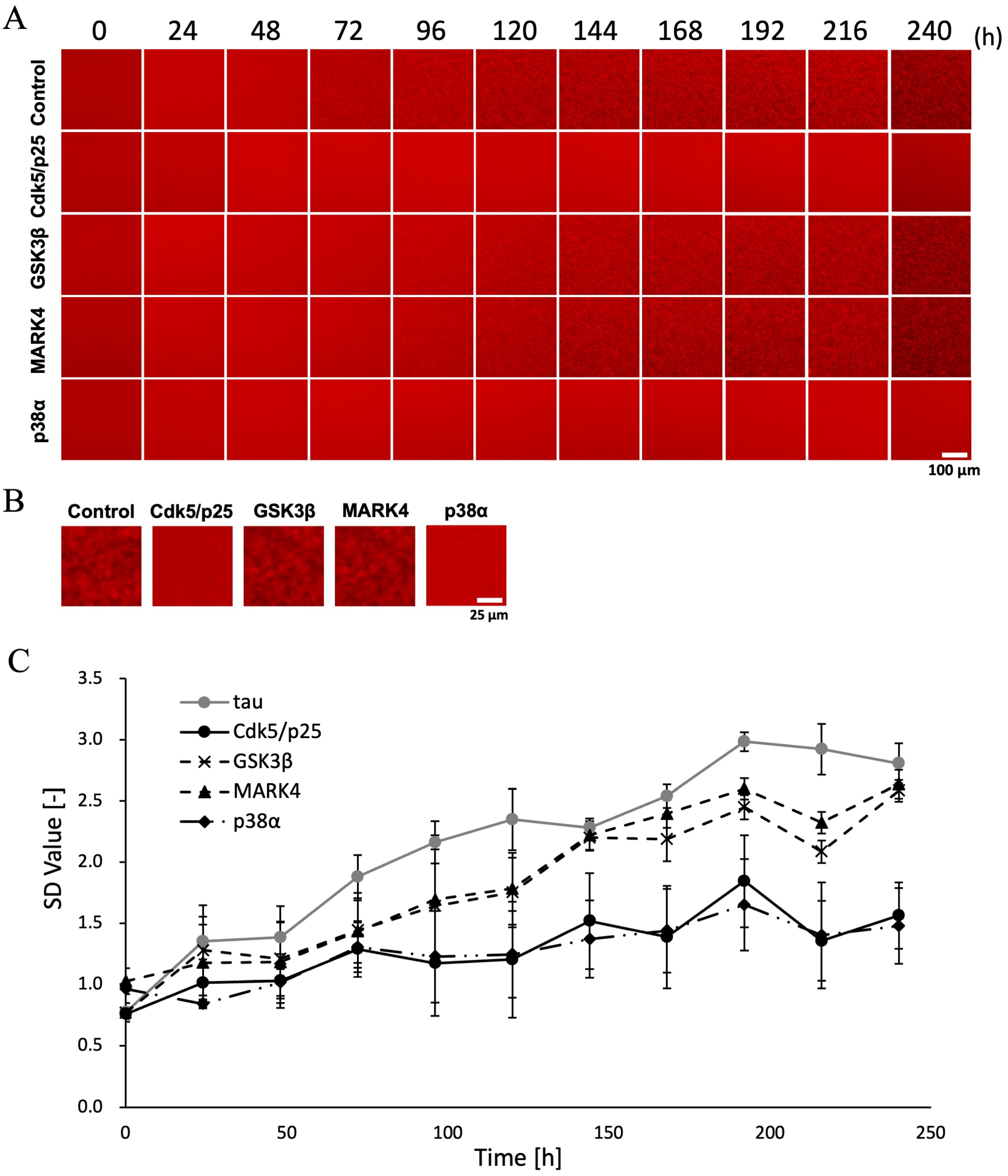
2.3. Differences in Aggregate Formation Due to Kinase Variation
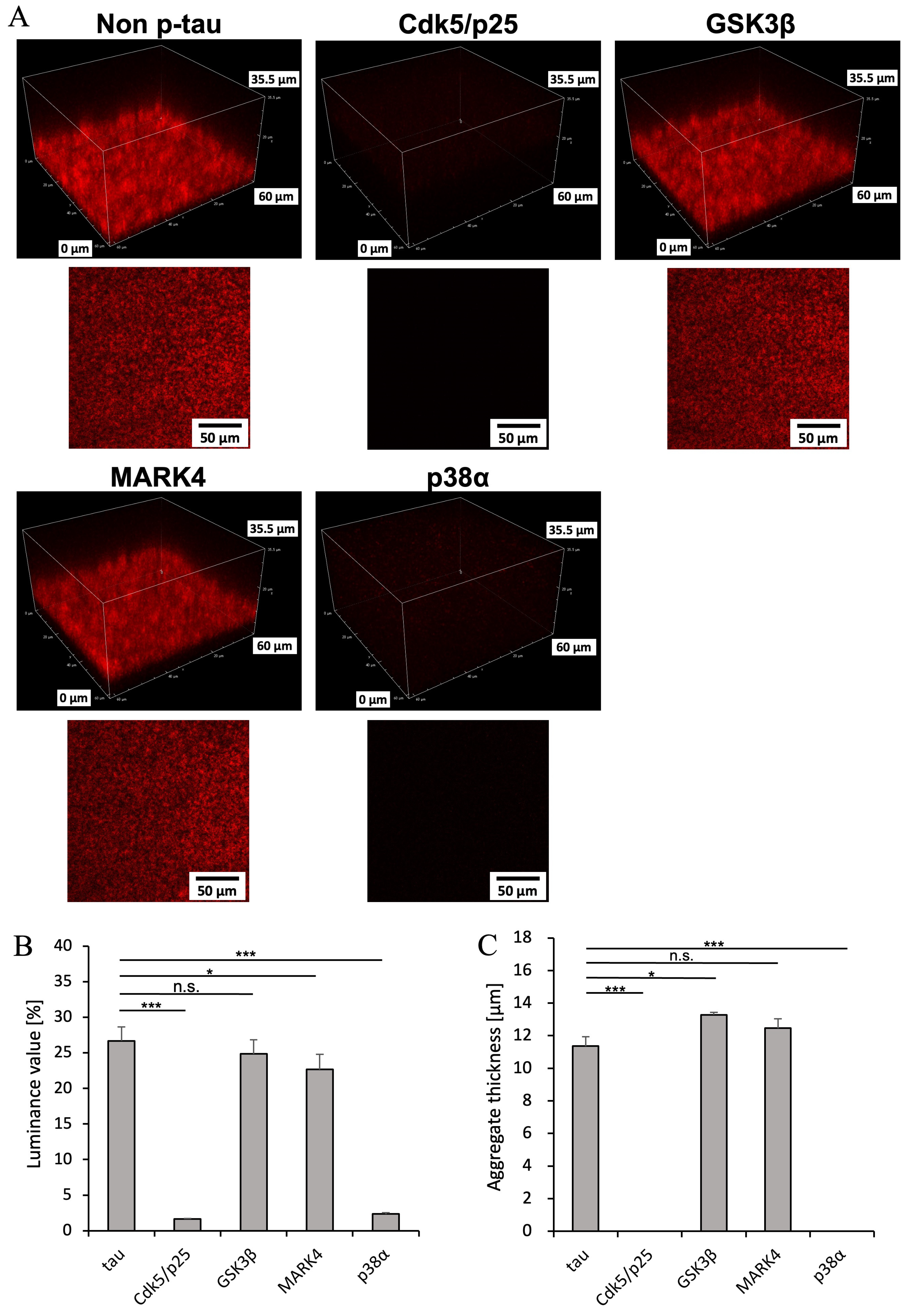
2.4. Differences in Aggregate Shape Due to Kinase Variation
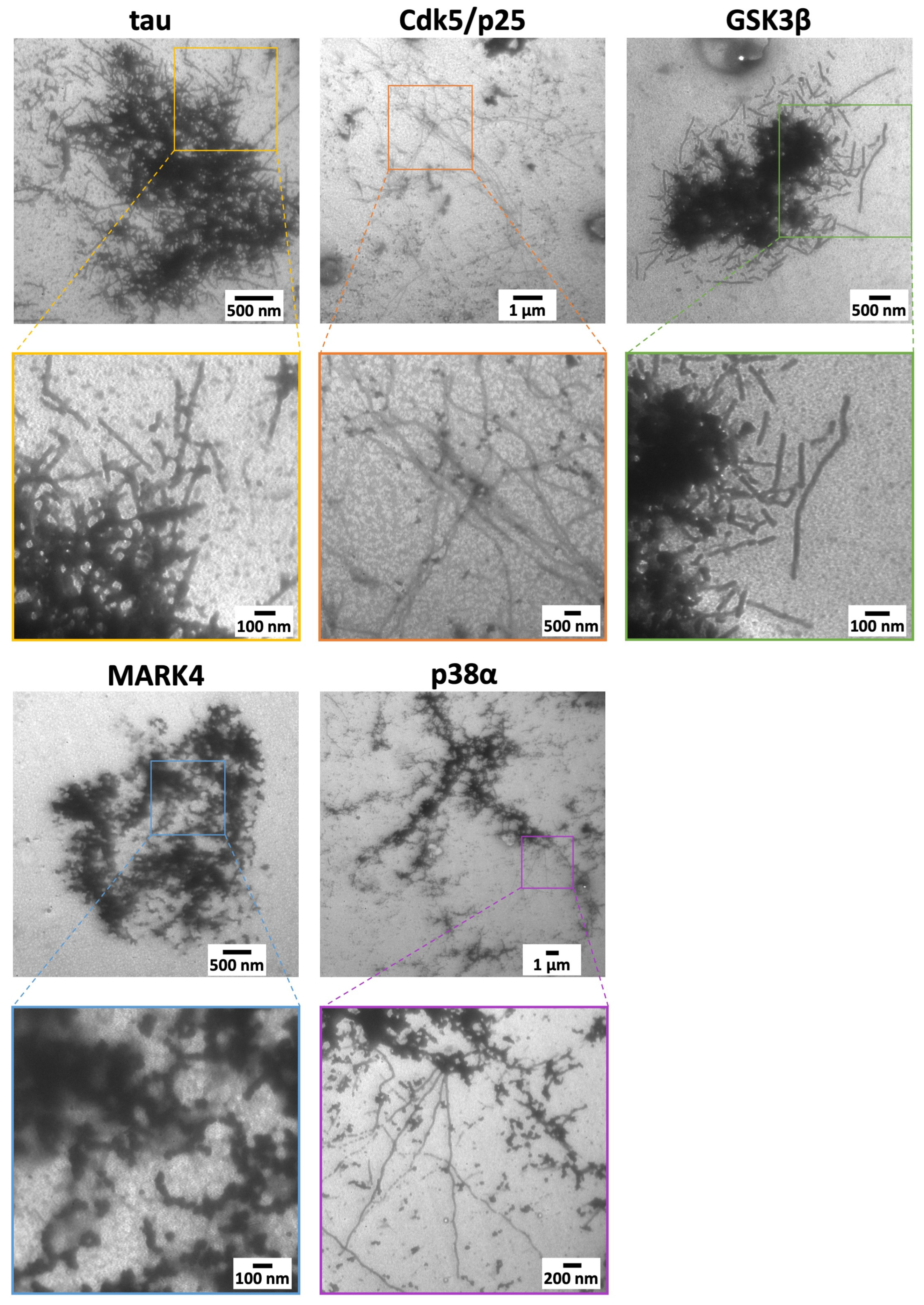
2.5. Differences in Tau Filament Structure Due to Kinase Activity
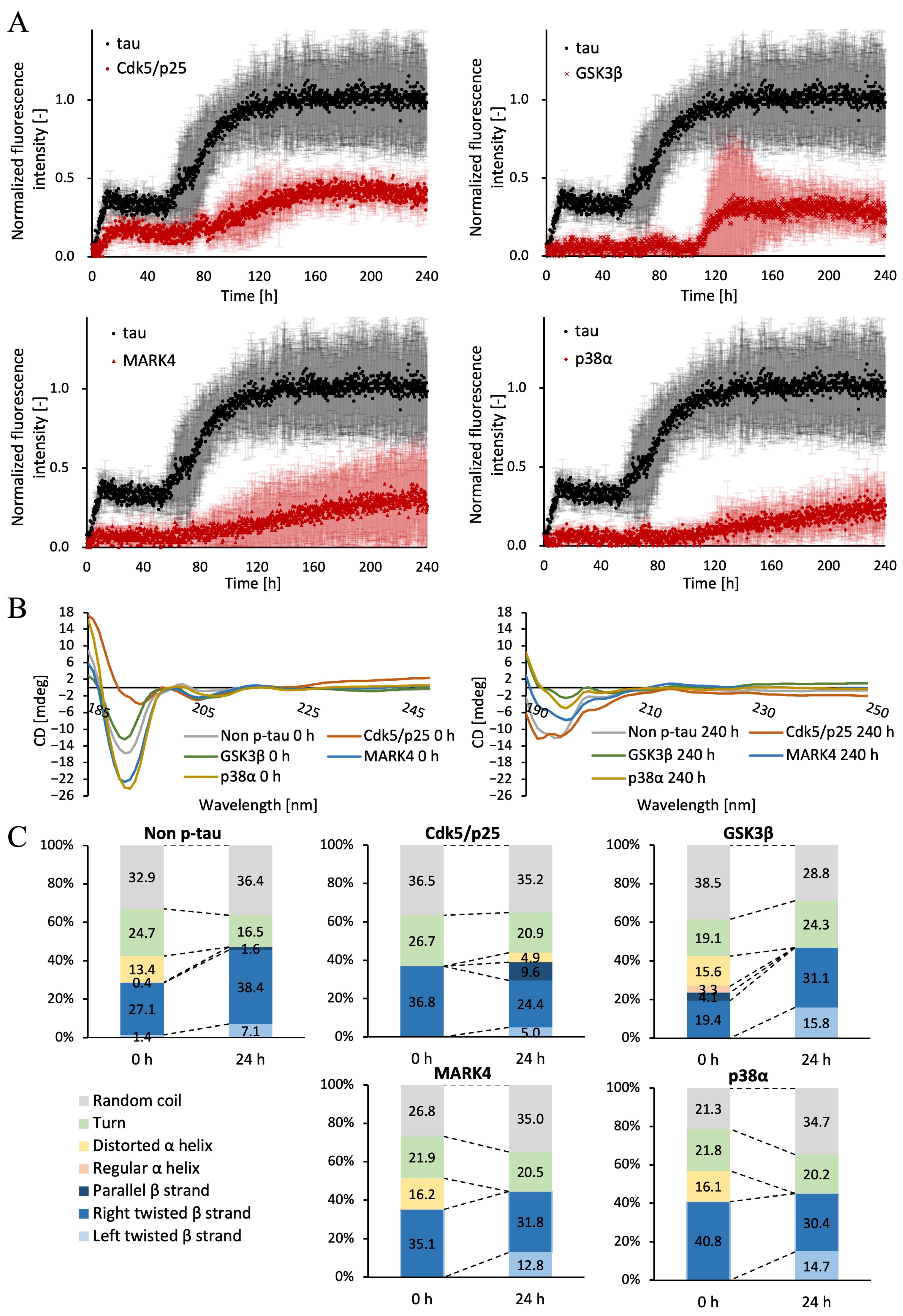
2.6. Differences in β-Sheet Structures Within Tau Filaments Due to Kinase Variations
3. Discussion
4. Materials and Methods
4.1. Purification of Mouse MBD Tau
4.2. Phosphorylation of Mouse MBD Tau
4.3. Phos-Tag SDS-PAGE Analysis
4.4. Western Blot
4.5. Observation of Mouse MBD Tau Aggregates Using Quantum Dots
4.6. Transmission Electron Microscope Observation
4.7. Thioflavin T Fluorescence Experiment
4.8. Circular Dichroism Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s Disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2016 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent Developments in Clinical Biomarkers and Therapies Targeting Tau Phosphorylation in Alzheimer’s Disease and Other Tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.W.; Stoothoff, W.H. Tau Phosphorylation in Neuronal Cell Function and Dysfunction. J. Cell Sci. 2004, 117, 5721–5729. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e13. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal Hyperphosphorylation of Tau: Sites, Regulation, and Molecular Mechanism of Neurofibrillary Degeneration. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S123–S139. [Google Scholar] [CrossRef]
- Laurent, C.; Buée, L.; Blum, D. Tau and Neuroinflammation: What Impact for Alzheimer’s Disease and Tauopathies? Biomed. J. 2018, 41, 21–33. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xia, L.; Zhou, Z.; Qiu, Y.; Zhao, C.; Yin, X.; Qian, W. Tau Acts in Concert with Kinase/Phosphatase Underlying Synaptic Dysfunction. Front. Aging Neurosci. 2022, 14, 908881. [Google Scholar] [CrossRef]
- Flaherty, D.B.; Soria, J.P.; Tomasiewicz, H.G.; Wood, J.G. Phosphorylation of Human Tau Protein by Microtubule-Associated Kinases: GSK3β and Cdk5 Are Key Participants. J. Neurosci. Res. 2000, 62, 463–472. [Google Scholar] [CrossRef]
- Basheer, N.; Smolek, T.; Hassan, I.; Liu, F.; Iqbal, K.; Zilka, N.; Novak, P. Does Modulation of Tau Hyperphosphorylation Represent a Reasonable Therapeutic Strategy for Alzheimer’s Disease? From Preclinical Studies to the Clinical Trials. Mol. Psychiatry 2023, 28, 2197–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-X.; Iqbal, K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Curr. Med. Chem. 2008, 15, 2321–2328. [Google Scholar] [CrossRef]
- Pao, P.-C.; Seo, J.; Lee, A.; Kritskiy, O.; Patnaik, D.; Penney, J.; Raju, R.M.; Geigenmuller, U.; Silva, M.C.; Lucente, D.E.; et al. A Cdk5-Derived Peptide Inhibits Cdk5/P25 Activity and Improves Neurodegenerative Phenotypes. Proc. Natl. Acad. Sci. USA 2023, 120, e2217864120. [Google Scholar] [CrossRef]
- Pao, P.-C.; Tsai, L.-H. Three Decades of Cdk5. J. Biomed. Sci. 2021, 28, 79. [Google Scholar] [CrossRef]
- Kimura, T.; Ishiguro, K.; Hisanaga, S.-I. Physiological and Pathological Phosphorylation of Tau by Cdk5. Front. Mol. Neurosci. 2014, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Biernat, J.; Wu, Y.-Z.; Timm, T.; Zheng-Fischhöfer, Q.; Mandelkow, E.; Meijer, L.; Mandelkow, E.-M. Protein Kinase MARK/PAR-1 Is Required for Neurite Outgrowth and Establishment of Neuronal Polarity. Mol. Biol. Cell 2002, 13, 4013–4028. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.Y.; Knowles, R.B.; Schneider, A.; Drewes, G.; Mandelkow, E.M.; Hyman, B.T. Microtubule-Affinity Regulating Kinase (MARK) Is Tightly Associated with Neurofibrillary Tangles in Alzheimer Brain: A Fluorescence Resonance Energy Transfer Study. J. Neuropathol. Exp. Neurol. 2000, 59, 966–971. [Google Scholar] [CrossRef]
- Samra, S.; Sharma, M.; Vaseghi-Shanjani, M.; Del Bel, K.L.; Byres, L.; Lin, S.; Dalmann, J.; Salman, A.; Mwenifumbo, J.; Modi, B.P.; et al. Gain-of-Function MARK4 Variant Associates with Pediatric Neurodevelopmental Disorder and Dysmorphism. HGG Adv. 2024, 5, 100259. [Google Scholar] [CrossRef] [PubMed]
- Sultanakhmetov, G.; Limlingan, S.J.M.; Fukuchi, A.; Tsuda, K.; Suzuki, H.; Kato, I.; Saito, T.; Weitemier, A.Z.; Ando, K. Mark4 Ablation Attenuates Pathological Phenotypes in a Mouse Model of Tauopathy. Brain Commun. 2024, 6, fcae136. [Google Scholar] [CrossRef]
- Woodgett, J.R. Molecular Cloning and Expression of Glycogen Synthase Kinase-3/Factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ávila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef]
- Germann, U.A.; Alam, J.J. P38α MAPK Signaling-A Robust Therapeutic Target for Rab5-Mediated Neurodegenerative Disease. Int. J. Mol. Sci. 2020, 21, 5485. [Google Scholar] [CrossRef] [PubMed]
- Detka, J.; Płachtij, N.; Strzelec, M.; Manik, A.; Sałat, K. P38α Mitogen-Activated Protein Kinase-An Emerging Drug Target for the Treatment of Alzheimer’s Disease. Molecules 2024, 29, 4354. [Google Scholar] [CrossRef] [PubMed]
- Stefanoska, K.; Gajwani, M.; Tan, A.R.P.; Ahel, H.I.; Asih, P.R.; Volkerling, A.; Poljak, A.; Ittner, A. Alzheimer’s Disease: Ablating Single Master Site Abolishes Tau Hyperphosphorylation. Sci. Adv. 2022, 8, eabl8809. [Google Scholar] [CrossRef]
- Chakraborty, P.; Rivière, G.; Liu, S.; de Opakua, A.I.; Dervişoğlu, R.; Hebestreit, A.; Andreas, L.B.; Vorberg, I.M.; Zweckstetter, M. Co-Factor-Free Aggregation of Tau into Seeding-Competent RNA-Sequestering Amyloid Fibrils. Nat. Commun. 2021, 12, 4231. [Google Scholar] [CrossRef]
- Lövestam, S.; Koh, F.A.; van Knippenberg, B.; Kotecha, A.; Murzin, A.G.; Goedert, M.; Scheres, S.H.W. Assembly of Recombinant Tau into Filaments Identical to Those of Alzheimer’s Disease and Chronic Traumatic Encephalopathy. eLife 2022, 11, e76494. [Google Scholar] [CrossRef]
- Sasaki, R.; Tainaka, R.; Ando, Y.; Hashi, Y.; Deepak, H.V.; Suga, Y.; Murai, Y.; Anetai, M.; Monde, K.; Ohta, K.; et al. An Automated Microliter-Scale High-Throughput Screening System (MSHTS) for Real-Time Monitoring of Protein Aggregation Using Quantum-Dot Nanoprobes. Sci. Rep. 2019, 9, 2587. [Google Scholar] [CrossRef]
- Tokuraku, K.; Marquardt, M.; Ikezu, T. Real-Time Imaging and Quantification of Amyloid-Beta Peptide Aggregates by Novel Quantum-Dot Nanoprobes. PLoS ONE 2009, 4, e8492. [Google Scholar] [CrossRef]
- Tokuraku, K.; Matsushima, K.; Matui, T.; Nakagawa, H.; Katsuki, M.; Majima, R.; Kotani, S. The Number of Repeat Sequences in Microtubule-Associated Protein 4 Affects the Microtubule Surface Properties. J. Biol. Chem. 2003, 278, 29609–29618. [Google Scholar] [CrossRef] [PubMed]
- Tokuraku, K.; Noguchi, T.Q.P.; Nishie, M.; Matsushima, K.; Kotani, S. An Isoform of Microtubule-Associated Protein 4 Inhibits Kinesin-Driven Microtubule Gliding. J. Biochem. 2007, 141, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Doki, C.; Nishida, K.; Saito, S.; Shiga, M.; Ogara, H.; Kuramoto, A.; Kuragano, M.; Nozumi, M.; Igarashi, M.; Nakagawa, H.; et al. Microtubule Elongation along Actin Filaments Induced by Microtubule-Associated Protein 4 Contributes to the Formation of Cellular Protrusions. J. Biochem. 2020, 168, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, E.; Kinoshita-Kikuta, E.; Koike, T. Separation and Detection of Large Phosphoproteins Using Phos-Tag SDS-PAGE. Nat. Protoc. 2009, 4, 1513–1521. [Google Scholar] [CrossRef]
- Ishigaki, Y.; Tanaka, H.; Akama, H.; Ogara, T.; Uwai, K.; Tokuraku, K. A Microliter-Scale High-Throughput Screening System with Quantum-Dot Nanoprobes for Amyloid-β Aggregation Inhibitors. PLoS ONE 2013, 8, e72992. [Google Scholar] [CrossRef]
- Lin, X.; Galaqin, N.; Tainaka, R.; Shimamori, K.; Kuragano, M.; Noguchi, T.Q.P.; Tokuraku, K. Real-Time 3D Imaging and Inhibition Analysis of Various Amyloid Aggregations Using Quantum Dots. Int. J. Mol. Sci. 2020, 21, 1978. [Google Scholar] [CrossRef]
- Tokuraku, K.; Ikezu, T. Bio-Nanoimaging Protein Misfolding and Aggregation; Uversky, V.N., Lyubchenko, Y.L., Eds.; Elsevier: London, UK, 2014; pp. 121–131. ISBN 9780123944313. [Google Scholar]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein Inhibits Heparin-Induced Tau Aggregation by Initializing Non-Toxic Tau Oligomer Formation. Cell Commun. Signal. 2021, 19, 16. [Google Scholar] [CrossRef]
- Strang, K.H.; Sorrentino, Z.A.; Riffe, C.J.; Gorion, K.-M.M.; Vijayaraghavan, N.; Golde, T.E.; Giasson, B.I. Phosphorylation of Serine 305 in Tau Inhibits Aggregation. Neurosci. Lett. 2019, 692, 187–192. [Google Scholar] [CrossRef]
- Trinczek, B.; Brajenovic, M.; Ebneth, A.; Drewes, G. MARK4 Is a Novel Microtubule-Associated Proteins/Microtubule Affinity-Regulating Kinase That Binds to the Cellular Microtubule Network and to Centrosomes. J. Biol. Chem. 2004, 279, 5915–5923. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ibáñez de Opakua, A.; Purslow, J.A.; Fromm, S.A.; Chatterjee, D.; Zachrdla, M.; Zhuang, S.; Puri, S.; Wolozin, B.; Zweckstetter, M. GSK3β Phosphorylation Catalyzes the Aggregation of Tau into Alzheimer’s Disease-like Filaments. Proc. Natl. Acad. Sci. USA 2024, 121, e2414176121. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Yang, Y.; Murzin, A.G.; Falcon, B.; Kotecha, A.; van Beers, M.; Tarutani, A.; Kametani, F.; Garringer, H.J.; et al. Structure-Based Classification of Tauopathies. Nature 2021, 598, 359–363. [Google Scholar] [CrossRef]
- Scheres, S.H.; Zhang, W.; Falcon, B.; Goedert, M. Cryo-EM Structures of Tau Filaments. Curr. Opin. Struct. Biol. 2020, 64, 17–25. [Google Scholar] [CrossRef]
- Jin, N.; Yin, X.; Yu, D.; Cao, M.; Gong, C.-X.; Iqbal, K.; Ding, F.; Gu, X.; Liu, F. Truncation and Activation of GSK-3β by Calpain I: A Molecular Mechanism Links to Tau Hyperphosphorylation in Alzheimer’s Disease. Sci. Rep. 2015, 5, 8187. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Involvement of Aberrant Glycosylation in Phosphorylation of Tau by cdk5 and GSK-3β. FEBS Lett. 2002, 530, 209–214. [Google Scholar] [CrossRef]
- Lucas, J.J.; Hernández, F.; Gómez-Ramos, P.; Morán, M.A.; Hen, R.; Avila, J. Decreased Nuclear β-Catenin, Tau Hyperphosphorylation and Neurodegeneration in GSK-3β Conditional Transgenic Mice. EMBO J. 2001, 20, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Biernat, J.; Gustke, N.; Drewes, G.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of Ser262 Strongly Reduces Binding of Tau to Microtubules: Distinction between PHF-like Immunoreactivity and Microtubule Binding. Neuron 1993, 11, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Saito, T.; Asada, A.; Shimizu, S.; Iijima, K.M.; Ando, K. Microtubule Affinity-Regulating Kinase 4 with an Alzheimer’s Disease-Related Mutation Promotes Tau Accumulation and Exacerbates Neurodegeneration. J. Biol. Chem. 2020, 295, 17138–17147. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]


| Kinase | Anti-Tau | AT8 | S262 | S396 |
|---|---|---|---|---|
| Non-p-tau | ○ | |||
| Cdk5/p25 | ○ | ○ | ○ | |
| GSK3β | ○ | ○ | ○ | |
| MARK4 | ○ | ○ | ||
| p38α | ○ | ○ | ○ |
| Kinase (n = 3) | Non-p-Tau | Cdk5/p25 | GSK3β | MARK4 | p38α |
|---|---|---|---|---|---|
|
Luminance value (density)
(standard deviation) | 11.36 μm (0.57 μm) | 0 μm | 13.28 μm (0.16 μm) | 11.36 μm (0.57 μm) | 0 μm |
|
Aggregates thickness (standard deviation) | 26.67% (1.96%) | 1.63% (0.07%) | 24.84% (2.00%) | 22.67% (2.14%) | 2.35% (0.17%) |
| Shape of aggregates | Mesh-like | Only fibers confirmed | A collection of fragments | Thick aggregates | Radial |
| Shape of fibers | Straight | Wave | Rigid torsional straight | Granular | Twisted wave |
| Increased fibers | 2-step increase | 2-step increase | Sharp rise in the middle | Gradual increase | Gradual increase |
| Increased β-sheet structure | Right-twisted β-strand | Left-twisted β-strand | Right-twisted β-strand | Right-twisted β-strand | Left-twisted β-strand |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, E.; Araya, K.; Nakamura, K.; Shimamori, K.; Uwai, K.; Kuragano, M.; Tokuraku, K. Aggregation Characteristics of Tau Phosphorylated by Various Kinases as Observed by Quantum Dot Fluorescence Imaging. Int. J. Mol. Sci. 2025, 26, 10122. https://doi.org/10.3390/ijms262010122
Ishibashi E, Araya K, Nakamura K, Shimamori K, Uwai K, Kuragano M, Tokuraku K. Aggregation Characteristics of Tau Phosphorylated by Various Kinases as Observed by Quantum Dot Fluorescence Imaging. International Journal of Molecular Sciences. 2025; 26(20):10122. https://doi.org/10.3390/ijms262010122
Chicago/Turabian StyleIshibashi, Eisuke, Koki Araya, Kota Nakamura, Keiya Shimamori, Koji Uwai, Masahiro Kuragano, and Kiyotaka Tokuraku. 2025. "Aggregation Characteristics of Tau Phosphorylated by Various Kinases as Observed by Quantum Dot Fluorescence Imaging" International Journal of Molecular Sciences 26, no. 20: 10122. https://doi.org/10.3390/ijms262010122
APA StyleIshibashi, E., Araya, K., Nakamura, K., Shimamori, K., Uwai, K., Kuragano, M., & Tokuraku, K. (2025). Aggregation Characteristics of Tau Phosphorylated by Various Kinases as Observed by Quantum Dot Fluorescence Imaging. International Journal of Molecular Sciences, 26(20), 10122. https://doi.org/10.3390/ijms262010122





