Exploring the Relevance of S100A8 and S100A9 Proteins in Preeclampsia: A Narrative Review
Abstract
1. Introduction
2. Data Search
3. General Functions of S100A8, S100A9, or Calprotectin
4. Preeclampsia Physiopathology and Molecular Biology
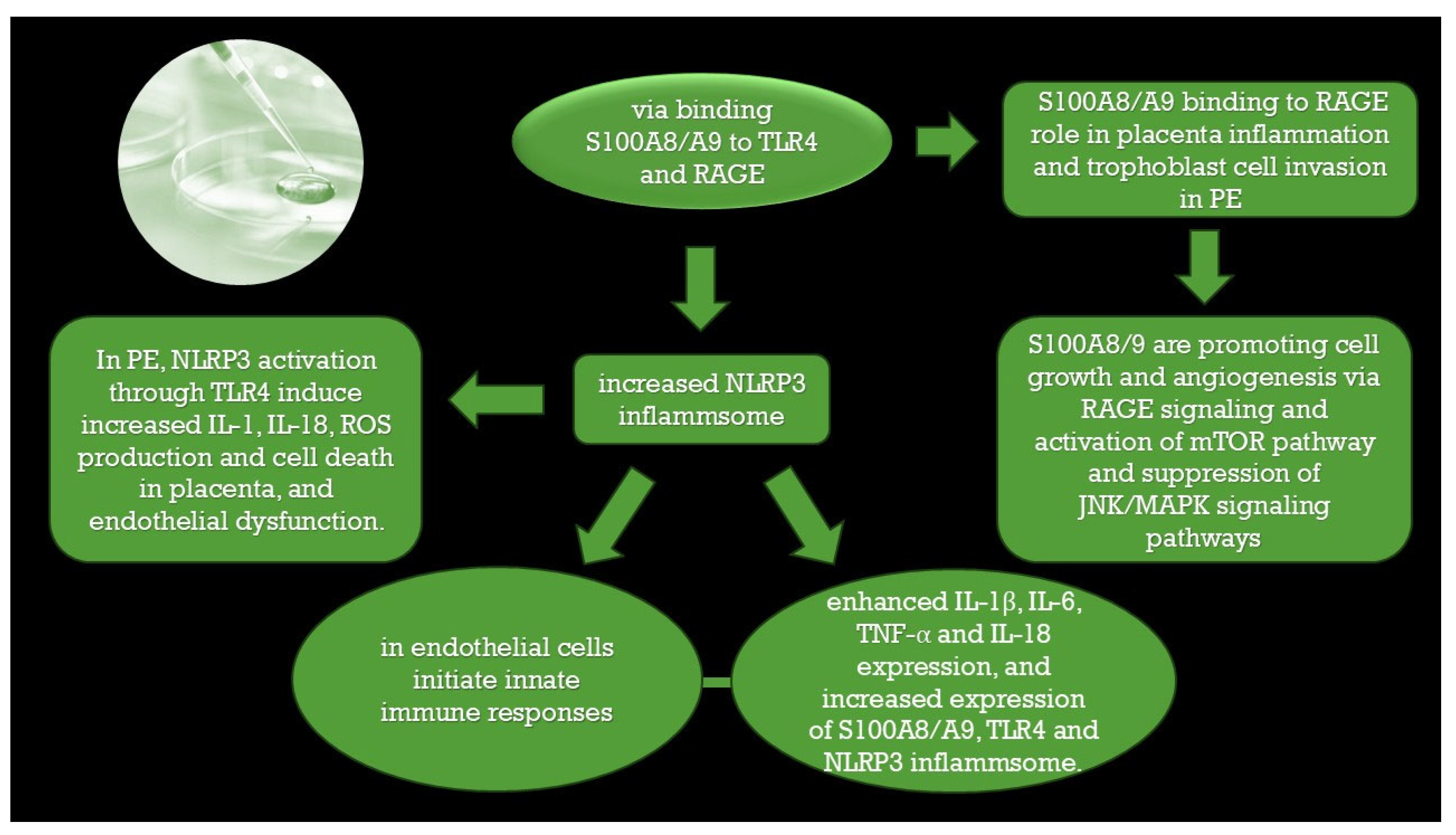
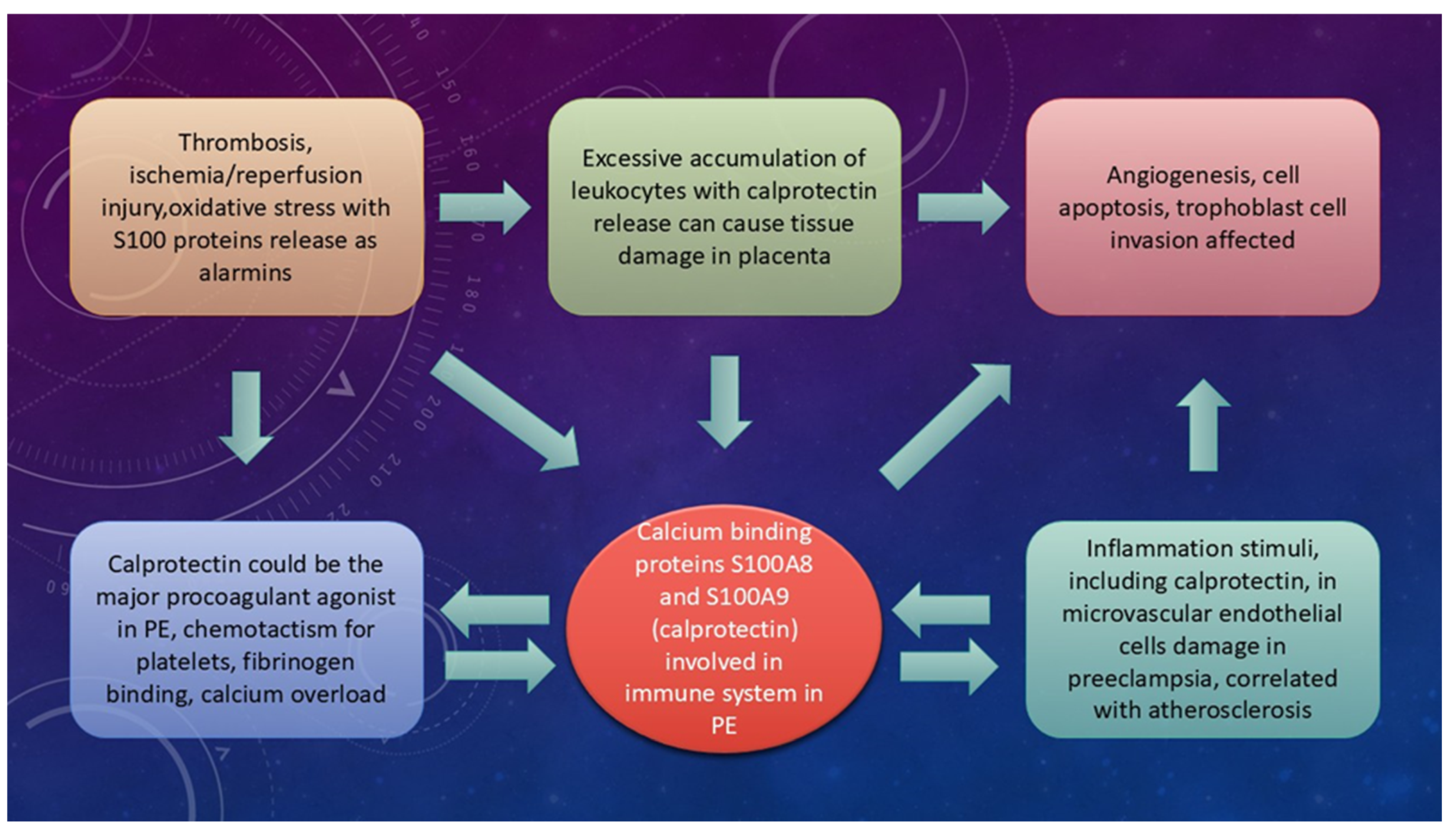
4.1. Trophoblast Invasion, Uterine Spiral Artery Remodeling, and the Potential Role of S100A8, S100A9, and Calprotectin
4.2. Potential Clinical Significance of S100A8, S100A9, or Calprotectin in PE
4.3. Different Pathologies as a Risk Factor for PE and the Association of S100A8 and S100A9
5. The Clinical Relevance of S100A8/A9 in Intrauterine Growth Restriction (IUGR)
6. Relevance in Future Therapy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| HELLP | syndrome hemolysis, elevated liver enzymes, low platelet count |
| IUGR | intrauterine growth restriction |
| ROS | reactive oxygen species |
| sFlt1 | soluble FMS-like tyrosine kinase 1 |
| s Eng | soluble endoglin |
| PAPP-A | pregnancy associated placental protein- A |
| PlGF | placental growth factor |
| TLR4 | toll-like receptor 4 |
| RAGE | the receptor for advanced glycation end products |
| DAMPs | danger-associated molecular patterns |
| SLE | systemic lupus erythematosus |
| NADPH | nicotinamide adenine dinucleotide phosphate oxidase |
| EMT | epithelial–mesenchymal transition |
| TAM | tumor-associated macrophage |
| NLRP3 | inflammasome NOD-, LRR- and pyrin domain-containing protein 3 inflammasome |
| RNS | reactive nitrogen species |
| MMPs | matrix metalloproteinases |
| DNA | deoxyribonucleic acid |
| HMGB1 | mobility group box 1 |
| ATP | adenosine triphosphate |
| HSP70 | heat shock protein 70 |
| IL | interleukin |
| TNF | tumor necrosis factor |
| ACOG | American College of Obstetricians and Gynecologists |
| BMI | body mass index |
| aPL | antiphospholipid antibodies |
| CVD | cardio-vascular disease |
| TTP | thrombotic thrombocytopenic purpura |
| CM-HUS | Complement-mediated hemolytic uremic syndrome |
| vWF | Ultra-large von Willebrand factor multimeric glycoproteins |
| NIPT | Non-invasive prenatal testing |
| APOs | adverse pregnancy outcomes |
| HUVECs | human umbilical vein endothelial cells |
References
- de Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.-C.; Dufour, A.; Agbani, E.O. Proteomics and Metabolomics Profiling of Platelets and Plasma Mediators of Thrombo-Inflammation in Gestational Hypertension and Preeclampsia. Cells 2022, 11, 1256. [Google Scholar] [CrossRef]
- Jurewicz, E.; Filipek, A. Ca2+- binding proteins of the S100 family in preeclampsia. Placenta 2022, 127, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mitranovici, M.-I.; Chiorean, D.M.; Moraru, R.; Moraru, L.; Caravia, L.; Tiron, A.T.; Craina, M.; Cotoi, O.S. Understanding the Pathophysiology of Preeclampsia: Exploring the Role of Antiphospholipid Anti-bodies and Future Directions. J. Clin. Med. 2024, 13, 2668. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, B.; Amylidi-Mohr, S.K.; Surbek, D.; Raio, L. First Trimester Screening For Preeclampsia–A Systematic Review. Hypertens. Pregnancy 2019, 39, 1–11. [Google Scholar] [CrossRef]
- Lai, J.; Syngelaki, A.; Nicolaides, K.H.; von Dadelszen, P.; Magee, L.A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 2021, 224, 518.e1–518.e11. [Google Scholar] [CrossRef]
- Quaresima, P.; Saccone, G.; Morelli, M.; Interlandi, F.; Votino, C.; Zuccalà, V.; Di Carlo, C.; Zullo, F.; Venturella, R. Stillbirth, potentially preventable cases: An Italian retrospective study. Ital. J. Gynaecol. Obstet. 2022, 34, 89. [Google Scholar] [CrossRef]
- Kornacki, J.; Olejniczak, O.; Sibiak, R.; Gutaj, P.; Wender-Ożegowska, E. Pathophysiology of Pre-Eclampsia—Two Theories of the Development of the Disease. Int. J. Mol. Sci. 2023, 25, 307. [Google Scholar] [CrossRef]
- Malone, S.L.; Yahya, R.H.; Kane, S.C. Reviewing Accuracy of First Trimester Screening for Preeclampsia Using Maternal Factors and Biomarkers. Int. J. Women’s Health 2022, 14, 1371–1384. [Google Scholar] [CrossRef]
- Mitranovici, M.I.; Chiorean, D.M.; Oală, I.E.; Petre, I.; Cotoi, O.S. Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania. Reports 2022, 5, 27. [Google Scholar] [CrossRef]
- Singh, P.; Ali, S.A. Multifunctional Role of S100 Protein Family in the Immune System: An Update. Cells 2022, 11, 2274. [Google Scholar] [CrossRef]
- Chen, F.; He, Z.; Wang, C.; Si, J.; Chen, Z.; Guo, Y. Advances in the study of S100A9 in cardiovascular diseases. Cell Prolif. 2024, 57, e13636. [Google Scholar] [CrossRef] [PubMed]
- Cotoi, O.S.; Dunér, P.; Ko, N.; Hedblad, B.; Nilsson, J.; Björkbacka, H.; Schiopu, A. Plasma S100A8/A9 Correlates With Blood Neutrophil Counts, Traditional Risk Factors, and Cardiovascular Disease in Middle-Aged Healthy Individuals. Arter. Thromb. Vasc. Biol. 2014, 34, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the Crossroads between Innate Immunity, Traditional Risk Factors, and Cardiovascular Disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Vogl, T.; Voskort, M.; Lueken, A.; Kupas, V.; Nacken, W.; Klenner, L.; Kuhn, A.; Foell, D.; Sorokin, L.; et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010, 16, 713–717. [Google Scholar] [CrossRef]
- Källberg, E.; Vogl, T.; Liberg, D.; Olsson, A.; Björk, P.; Wikström, P.; Bergh, A.; Roth, J.; Ivars, F.; Leanderson, T. S100A9 Interaction with TLR4 Promotes Tumor Growth. PLoS ONE 2012, 7, e34207. [Google Scholar] [CrossRef]
- Holzinger, D.; Frosch, M.; Kastrup, A.; Prince, F.H.M.; Otten, M.H.; A Van Suijlekom-Smit, L.W.; Cate, R.T.; Hoppenreijs, E.P.A.H.; Hansmann, S.; Moncrieffe, H.; et al. The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann. Rheum. Dis. 2012, 71, 974–980. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; Zoelen, M.A.D.v.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Aslam, A. Comparison of Serum Calprotectin in Preeclamptic and Normotensive Pregnant Females. PJMHS Pak. J. Med. Health Sci. 2020, 14, 1. [Google Scholar]
- Viemann, D.; Strey, A.; Janning, A.; Jurk, K.; Klimmek, K.; Vogl, T.; Hirono, K.; Ichida, F.; Foell, D.; Kehrel, B.; et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005, 105, 2955–2962. [Google Scholar] [CrossRef]
- Bouma, G.; Lam-Tse, W.K.; Wierenga-Wolf, A.F.; Drexhage, H.A.; Versnel, M.A. Increased Serum Levels of MRP-8/14 in Type 1 Diabetes Induce an Increased Expression of CD11b and an Enhanced Adhesion of Circulating Monocytes to Fibronectin. Diabetes 2004, 53, 1979–1986. [Google Scholar] [CrossRef]
- Ryckman, C.; Vandal, K.; Rouleau, P.; Talbot, M.; Tessier, P.A. Proinflammatory Activities of S100: Proteins S100A8, S100A9, and S100A8/A9 Induce Neutrophil Chemotaxis and Adhesion. J. Immunol. 2003, 170, 3233–3242. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 26, 5. [Google Scholar] [CrossRef]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003, 60, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, G.; Papareddy, P.; Andersson, H.; Mulholland, M.; Bhongir, R.; Ljungcrantz, I.; Engelbertsen, D.; Björkbacka, H.; Nilsson, J.; Manea, A.; et al. Therapeutic S100A8/A9 blockade inhibits myocardial and systemic inflammation and mitigates sepsis-induced myocardial dysfunction. Crit. Care 2023, 27, 374. [Google Scholar] [CrossRef] [PubMed]
- Mares, R.G.; Suica, V.I.; Uyy, E.; Boteanu, R.M.; Ivan, L.; Cocuz, I.G.; Sabau, A.H.; Yadav, V.; Szabo, I.A.; Cotoi, O.S.; et al. Short-term S100A8/A9 Blockade Promotes Cardiac Neovascularization after Myocardial Infarction. J. Cardiovasc. Transl. Res. 2024, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Ji, X.; Yan, L.; Lian, S.; Chen, Z.; Luo, Y. Roles of S100A8, S100A9 and S100A12 in infection, inflammation and immunity. Immunology 2023, 171, 365–376. [Google Scholar] [CrossRef]
- Averill, M.M.; Barnhart, S.; Becker, L.; Li, X.; Heinecke, J.W.; LeBoeuf, R.C.; Hamerman, J.A.; Sorg, C.; Kerkhoff, C.; Bornfeldt, K.E. S100A9 Differentially Modifies Phenotypic States of Neutrophils, Macrophages, and Dendritic Cells. Circulation 2011, 123, 1216–1226. [Google Scholar] [CrossRef]
- Yao, D.; Brownlee, M. Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of the Receptor for Advanced Glycation End Products (RAGE) and RAGE Ligands. Diabetes 2009, 59, 249–255. [Google Scholar] [CrossRef]
- Yen, T.; Harrison, C.A.; Devery, J.M.; Leong, S.; Iismaa, S.E.; Yoshimura, T.; Geczy, C.L. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood 1997, 90, 4812–4821. [Google Scholar] [CrossRef]
- Rahimi, F.; Hsu, K.; Endoh, Y.; Geczy, C.L. FGF-2, IL-1β and TGF-β regulate fibroblast expression of S100A8. FEBS J. 2005, 272, 2811–2827. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Geczy, C.L.; Tedla, N.; Finlay-Jones, J.J.; Hart, P.H. S100A8 Induction in Keratinocytes by Ultraviolet A Irradiation Is Dependent on Reactive Oxygen Intermediates. J. Investig. Dermatol. 2003, 121, 1168–1174. [Google Scholar] [CrossRef]
- Goyette, J.; Geczy, C.L. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids 2010, 41, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Grajales, C.; Barraclough, M.L.; Diaz-Martinez, J.P.; Su, J.; Bingham, K.; Kakvan, M.; Kretzmann, R.P.; Tartaglia, M.C.; Ruttan, L.; Choi, M.Y.; et al. Serum S100A8/A9 and MMP-9 levels are elevated in systemic lupus erythematosus patients with cognitive impairment. Front. Immunol. 2024, 14, 1326751. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Ikemoto, M.; Okada, K. Intraperitoneal Administration of S100A8 Ameliorates Experimental Acute Colitis in Rats. Biology 2024, 13, 916. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Gao, Y.; Wang, Q.; Chi, J.; Zhu, Z.; Diao, Q.; Li, X.; Wang, Z.; Qu, M.; Shi, Y. Preliminary clinical analysis and pathway study of S100A8 as a biomarker for the diagnosis of acute deep vein thrombosis. Sci. Rep. 2024, 14, 13298. [Google Scholar] [CrossRef]
- Maliqueo, M.; Echiburú, B.; Crisosto, N. Sex Steroids Modulate Uterine-Placental Vasculature: Implications for Obstetrics and Neonatal Outcomes. Front. Physiol. 2016, 7, 152. [Google Scholar] [CrossRef]
- Fang, S.; Du, S.; Luo, X.; Qing, X.; Wang, L.; Ban, Y.; Song, G.; Yang, Y.; Wei, W. The role of the S100A8/S100A9 in gastric tumor progression. Sci. Rep. 2024, 14, 23574. [Google Scholar] [CrossRef]
- Sui, Q.; Hu, Z.; Liang, J.; Lu, T.; Bian, Y.; Jin, X.; Li, M.; Huang, Y.; Yang, H.; Wang, Q.; et al. Targeting TAM-secreted S100A9 effectively enhances the tumor-suppressive effect of metformin in treating lung adenocarcinoma. Cancer Lett. 2023, 581, 216497. [Google Scholar] [CrossRef]
- Stenhouse, C.; Halloran, K.M.; Newton, M.G.; Gaddy, D.; Suva, L.J.; Bazer, F.W. Novel mineral regulatory pathways in ovine pregnancy: II. Calcium-binding proteins, calcium transporters, and vitamin D signaling. Biol. Reprod. 2021, 105, 232–243. [Google Scholar] [CrossRef]
- Velicky, P.; Knöfler, M.; Pollheimer, J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adhes. Migr. 2016, 10, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Prameswari, H.S.; Effendi, C.A.; Khalid, A.F.; Irianti, S.; Fatati, I.; Akbar, M.R. Relationship between serum soluble suppression of tumorigenicity (ST) 2 and global longitudinal strain in early onset preeclampsia. BMC Cardiovasc. Disord. 2024, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y.; Cai, X.; Diao, L.; Yang, C.; Yang, J. Crosstalk Between Trophoblast and Macrophage at the Maternal-Fetal Interface: Current Status and Future Perspectives. Front. Immunol. 2021, 12, 758281. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Johnsen, G.M.; Dechend, R.; Redman, C.W. Preeclampsia and uteroplacental acute atherosis: Immune and inflammatory factors. J. Reprod. Immunol. 2014, 101–102, 120–126. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef]
- Galaviz-Hernandez, C.; Sosa-Macias, M.; Teran, E.; Garcia-Ortiz, J.E.; Lazalde-Ramos, B.P. Paternal Determinants in Preeclampsia. Front. Physiol. 2019, 9, 1870. [Google Scholar] [CrossRef]
- Fei, H.; Lu, X.; Shi, Z.; Liu, X.; Yang, C.; Zhu, X.; Lin, Y.; Jiang, Z.; Wang, J.; Huang, D.; et al. Deciphering the preeclamp-sia-specific immune microenvironment and the role of pro-inflammatory macrophages at the maternal–fetal interface. eLife 2025, 13, RP100002. [Google Scholar] [CrossRef]
- Baker, T.M.; Nakashige, T.G.; Nolan, E.M.; Neidig, M.L. Magnetic circular dichroism studies of iron(ii) binding to human calprotectin. Chem. Sci. 2016, 8, 1369–1377. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Giambanco, I. S100A6 protein: Functional roles. Cell. Mol. Life Sci. 2017, 74, 2749–2760. [Google Scholar] [CrossRef]
- Chakraborty, D.; Zenker, S.; Rossaint, J.; Hölscher, A.; Pohlen, M.; Zarbock, A.; Roth, J.; Vogl, T. Alarmin S100A8 Activates Alveolar Epithelial Cells in the Context of Acute Lung Injury in a TLR4-Dependent Manner. Front. Immunol. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Xu, Y.-D.; Yin, L.-M.; Wang, Y.; Wei, Y.; Yang, Y.-Q. S100A8 protein in inflammation. Acta Physiol. Sin. 2012, 64, 231–237. [Google Scholar]
- Oliva, K.; Barker, G.; E Rice, G.; Bailey, M.J.; Lappas, M. 2D-DIGE to identify proteins associated with gestational diabetes in omental adipose tissue. J. Endocrinol. 2013, 218, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Pușcașiu, L.; Mitranovici, M.-I.; Crișan, A.; Budianu, M.A.; Bănescu, C.; Chiorean, D.M.; Niculescu, R.; Sabău, A.-H.; Cocuz, I.-G.; et al. Oxidative-Stress Related Gene Polymorphism in Endometriosis-Associated Infertility. Medicina 2022, 58, 1105. [Google Scholar] [CrossRef]
- Gyselaers, W. Preeclampsia Is a Syndrome with a Cascade of Pathophysiologic Events. J. Clin. Med. 2020, 9, 2245. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Sundl, M.; Glasner, A.; Huppertz, B.; Moser, G. The trophoblast plug during early pregnancy: A deeper insight. Histochem. Cell Biol. 2016, 146, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Windsperger, K.; Pollheimer, J.; de Sousa Lopes, S.C.; Huppertz, B. Human trophoblast invasion: New and unex-pected routes and functions. Histochem. Cell Biol. 2018, 150, 361–370. [Google Scholar]
- Sung, D.C.; Chen, X.; Chen, M.; Yang, J.; Schultz, S.; Babu, A.; Xu, Y.; Gao, S.; Keller, T.S.; Mericko-Ishizuka, P.; et al. VE-cadherin enables trophoblast endovascular invasion and spiral artery remodeling during placental development. eLife 2022, 11, e77241. [Google Scholar] [CrossRef]
- Nair, R.; Khanna, A.; Singh, K. Role of inflammatory proteins S100A8 and S100A9 in pathophysiology of recurrent early pregnancy loss. Placenta 2013, 34, 824–827. [Google Scholar] [CrossRef]
- Berkane, N.; Liere, P.; Oudinet, J.-P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal–fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef]
- Vince, G.S.; Starkey, P.M.; Austgulen, R.; Kwiatkowski, D.; Redman, C.W.G. Interleukin-6, turnour necrosis factor and soluble turnour necrosis factor receptors in women with pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 1995, 102, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2016; Volume 77, pp. 361–431. [Google Scholar]
- Braekke, K.; Holthe, M.R.; Harsem, N.K.; Fagerhol, M.K.; Staff, A.C. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, W.; Huang, C.; Zhou, Y.; Wang, Q.; Tian, X.; Li, M.; Zhao, Y.; Zeng, X.; Zhao, J. Serum Calprotectin as a Potential Predictor of Microvascular Manifestations in Patients with Antiphospholipid Syndrome. Rheumatol. Ther. 2023, 10, 1769–1783. [Google Scholar] [CrossRef] [PubMed]
- Slavik, L.; Prochazkova, J.; Prochazka, M.; Simetka, O.; Hlusi, A.; Ulehlova, J. The Pathophysiology Of Endothelial Function In Pregnancy And The Usefulness Of Endothelial Markers. Biomed. Pap. 2011, 155, 333–337. [Google Scholar] [CrossRef]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.Y.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 2020, 218, e20200891. [Google Scholar] [CrossRef]
- Brien, M.-E.; Baker, B.; Duval, C.; Gaudreault, V.; Jones, R.L.; Girard, S. Alarmins at the maternal–fetal interface: Involvement of inflammation in placental dysfunction and pregnancy complications. Can. J. Physiol. Pharmacol. 2019, 97, 206–212. [Google Scholar] [CrossRef]
- Przybyl, L.; Haase, N.; Golic, M.; Rugor, J.; Solano, M.E.; Arck, P.C.; Herse, F. CD74-dysregulation of placental macrophage-trophoblastic interactions in preeclampsia. J. Reprod. Immunol. 2016, 100, 41–42. [Google Scholar] [CrossRef]
- Gomes, L.H.; Raftery, M.J.; Yan, W.X.; Goyette, J.D.; Thomas, P.S.; Geczy, C.L. S100A8 and S100A9—Oxidant scavengers in inflammation. Free. Radic. Biol. Med. 2013, 58, 170–186. [Google Scholar] [CrossRef]
- Louis, J.M.; Parchem, J.; Vaught, A.; Tesfalul, M.; Kendle, A.; Tsigas, E. Preeclampsia: A report and recommendations of the workshop of the Society for Maternal-Fetal Medicine and the Preeclampsia Foundation. Am. J. Obstet. Gynecol. 2022, 227, B2–B24. [Google Scholar] [CrossRef]
- Ozeki, A.; Oogaki, Y.; Henmi, Y.; Karasawa, T.; Takahashi, M.; Takahashi, H.; Ohkuchi, A.; Shirasuna, K. Elevated S100A9 in preeclampsia induces soluble endoglin and IL-1β secretion and hypertension via the NLRP3 inflammasome. J. Hypertens. 2021, 40, 84–93. [Google Scholar] [CrossRef]
- Del Vecchio, G.; Li, Q.; Li, W.; Thamotharan, S.; Tosevska, A.; Morselli, M.; Sung, K.; Janzen, C.; Zhou, X.; Pellegrini, M.; et al. Cell-free DNA Methylation and Transcriptomic Signature Prediction of Pregnancies with Adverse Outcomes. Epigenetics 2020, 16, 642–661. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Wang, S.; Zhang, Z. Increased serum levels of high mobility group protein B1 and calprotectin in pre-eclampsia. Int. J. Gynecol. Obstet. 2018, 142, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, F.; Cao, G. Expression of S100 calcium-binding protein A8 in peripheral blood of patients with preeclampsia during pregnancy. Eur. J. Inflamm. 2019, 17. [Google Scholar] [CrossRef]
- Starodubtseva, N.L.; Tokareva, A.O.; Volochaeva, M.V.; Kononikhin, A.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Timofeeva, A.V.; Kukaev, E.N.; Tyutyunnik, V.L.; Kan, N.E.; et al. Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction. Int. J. Mol. Sci. 2023, 24, 16832. [Google Scholar] [CrossRef] [PubMed]
- Maroudias, G.; Vrachnis, D.; Fotiou, A.; Loukas, N.; Mantzou, A.; Pergialiotis, V.; Valsamakis, G.; Machairiotis, N.; Stavros, S.; Panagopoulos, P.; et al. Measurement of Calprotectin and PTH in the Amniotic Fluid of Early Second Trimester Pregnancies and Their Impact on Fetuses with Growth Disorders: Are Their Levels Related to Oxidative Stress? J. Clin. Med. 2024, 13, 855. [Google Scholar] [CrossRef]
- Holzinger, D.; Foell, D.; Kessel, C. The role of S100 proteins in the pathogenesis and monitoring of autoinflammatory diseases. Mol. Cell. Pediatr. 2018, 5, 7. [Google Scholar] [CrossRef]
- Sugulle, M.; Kvehaugen, A.S.; Brække, K.; Harsem, N.K.; Staff, A.C. Plasma calprotectin as inflammation marker in pregnancies complicated by diabetes mellitus and superimposed preeclampsia. Pregnancy Hypertens. 2011, 1, 137–142. [Google Scholar] [CrossRef]
- Brien, M.; Boufaied, I.; Bernard, N.; Forest, J.; Giguere, Y.; Girard, S. Specific inflammatory profile in each pregnancy complication: A comparative study. Am. J. Reprod. Immunol. 2020, 84, e13316. [Google Scholar] [CrossRef]
- Williamson, R.D.; McCarthy, F.P.; Kenny, L.C.; McCarthy, C.M. Activation of a TLR9 mediated innate immune response in preeclampsia. Sci. Rep. 2019, 9, 5920. [Google Scholar] [CrossRef]
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar]
- Kuo, Y.-L.; Chan, T.-F.; Wu, C.-Y.; Ker, C.-R.; Tu, H.-P. Preeclampsia-eclampsia and future cardiovascular risk among women in Taiwan. Taiwan. J. Obstet. Gynecol. 2018, 57, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, K.; Evangelou, E.; Papatheodorou, S.I. Genetic and non-genetic risk factors for pre-eclampsia: Umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet. Gynecol. 2018, 51, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, E.; E Medcalf, K.; Park, A.L.; Ray, J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef]
- Kosińska-Kaczyńska, K. Placental Syndromes—A New Paradigm in Perinatology. Int. J. Environ. Res. Public. Health 2022, 19, 7392. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef]
- Ehlermann, P.; Eggers, K.; Bierhaus, A.; Most, P.; Weichenhan, D.; Greten, J.; Nawroth, P.P.; A Katus, H.; Remppis, A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc. Diabetol. 2006, 5, 6. [Google Scholar] [CrossRef][Green Version]
- Robinson, M.J.; Tessier, P.; Poulsom, R.; Hogg, N. The S100 Family Heterodimer, MRP-8/14, Binds with High Affinity to Heparin and Heparan Sulfate Glycosaminoglycans on Endothelial Cells. J. Biol. Chem. 2002, 277, 3658–3665. [Google Scholar] [CrossRef]
- Vokalova, L.; van Breda, S.V.; Ye, X.L.; Huhn, E.A.; Than, N.G.; Hasler, P.; Lapaire, O.; Hoesli, I.; Rossi, S.W.; Hahn, S. Excessive Neutrophil Activity in Gestational Diabetes Mellitus: Could It Contribute to the Development of Preeclampsia? Front. Endocrinol. 2018, 9, 542. [Google Scholar] [CrossRef]
- Ramachandrarao, S.P.; Hamlin, A.A.; Awdishu, L.; Overcash, R.; Zhou, M.; Proudfoot, J.; Naviaux, R.K. Proteomic analyses of urine exosomes reveal new biomarkers of diabetes in pregnancy. Madridge J. Diabetes 2016, 1, 11. [Google Scholar] [CrossRef]
- Ortega, F.J.; Sabater, M.; Moreno-Navarrete, J.M. Serum and urinary concentrations of calprotectin as markers of insulin re-sistance and type 2 diabetes. Eur. J. Endocrinol. 2012, 167, 569–578. [Google Scholar] [CrossRef]
- Tanaka, H.; Tenkumo, C.; Mori, N.; Kokame, K.; Fujimura, Y.; Hata, T. Case of maternal and fetal deaths due to severe congenital thrombotic thrombocytopenic purpura (U pshaw–S chulman syndrome) during pregnancy. J. Obstet. Gynaecol. Res. 2014, 40, 247–249. [Google Scholar] [CrossRef]
- Mitranovici, M.I.; Pușcașiu, L.; Oală, I.E.; Petre, I.; Craina, M.L.; Mager, A.R.; Vasile, K.; Chiorean, D.M.; Sabău, A.-H.; Turdean, S.G.; et al. A Race against the Clock: A Case Report and Literature Review Concerning the Importance of ADAMTS13 Testing in Diagnosis and Management of Thrombotic Thrombocytopenic Purpura during Pregnancy. Diagnostics 2022, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, Y.; Zhu, C.; Guo, L.; Wei, J.; Xu, B.; Song, Y.; Qin, H.; Li, X. Serum Mrp 8/14 as a Potential Biomarker for Predicting the Occurrence of Acute Respiratory Distress Syndrome Induced by Sepsis: A Retrospective Controlled Study. J. Inflamm. Res. 2024, 17, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L.; Hurd, K.; Chaturvedi, S.; Chow, L.; Nicholas, J.; Lee, A.; Young, D.; Goodyear, D.; Soucie, J.; Girard, L.; et al. Hypercoagulability and Inflammatory Markers in a Case of Congenital Thrombotic Thrombocytopenic Purpura Complicated by Fetal Demise. J. Clin. Med. 2022, 11, 7115. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Neave, L. Etiology and outcomes: Thrombotic microangiopathies in pregnancy. Res. Pract. Thromb. Haemost. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Karumanchi, S.A. Angiogenic Factors in Preeclampsia: From Diagnosis to Therapy. Hypertension 2016, 67, 1072–1079. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Yan, R.; Wang, C.; Wang, Y.; Zhang, C.; Liu, M.; Zhou, T.; Zhu, W.; Zhang, H.; et al. Increased Neutrophil Activation and Plasma DNA Levels in Patients with Pre-Eclampsia. Thromb. Haemost. 2018, 118, 2064–2073. [Google Scholar] [CrossRef]
- Agbani, E.O.; Hers, I.; Poole, A.W. Platelet procoagulant membrane dynamics: A key distinction between thrombosis and hemostasis? Blood Adv. 2023, 7, 1615–1619. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Zulu, M.Z.; Doratt, B.M.; Jankeel, A.; Tifrea, D.; Edwards, R.; Rincon, M.; Marshall, N.E.; Messaoudi, I. Single-cell RNA sequencing reveals immunological rewiring at the maternal-fetal interface following asymptomatic/mild SARS-CoV-2 infection. Cell Rep. 2022, 39, 110938. [Google Scholar] [CrossRef]
- Krishnan, U.; Goodall, A.H.; Bugert, P. Letter by Krishnan et al Regarding Article, “Platelet Expression Profiling and Clinical Validation of Myeloid-Related Protein-14 as a Novel Determinant of Cardiovascular Events”. Circulation 2007, 115, 186. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Dechend, R.; Pijnenborg, R. Learning from the placenta: Acute atherosis and vascular remodeling in preeclamp-sianovel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010, 56, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Wirstlein, P.; Wender-Ozegowska, E. Levels of syndecan-1 and hyaluronan in early- and late-onset preeclampsia. Pregnancy Hypertens. 2019, 18, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Vondra, S.; Höbler, A.L.; Lackner, A.I.; Raffetseder, J.; Mihalic, Z.N.; Vogel, A.; Pollheimer, J. The human placenta shapes the phenotype of decidual macrophages. Cell Rep. 2023, 42, 111977. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, Y.; Liu, X.; Tian, Y.; Zhao, X.; Li, Q.; Wang, X. NETs exacerbate placental inflammation and injury through high mobility group protein B1 during preeclampsia. Placenta 2025, 159, 131–139. [Google Scholar] [CrossRef]
- Stenhouse, C.; Halloran, K.M.; Moses, R.M.; Seo, H.; Gaddy, D.; A Johnson, G.; Wu, G.; Suva, L.J.; Bazer, F.W. Effects of progesterone and interferon tau on ovine endometrial phosphate, calcium, and vitamin D signaling. Biol. Reprod. 2022, 106, 888–899. [Google Scholar] [CrossRef]
- Alfian, I.; Chakraborty, A.; Yong, H.E.; Saini, S.; Lau, R.W.; Kalionis, B.; Murthi, P. The placental NLRP3 in-flammasome and its downstream targets, caspase-1 and interleukin-6, are increased in human fetal growth restriction: Im-plications for aberrant inflammation-induced trophoblast dysfunction. Cells 2022, 11, 1413. [Google Scholar] [CrossRef]
- Somu, M. Non-Invasive Biomarker Detection for Pregnancy Complications Using Cell-Free RNA. J. Stud. Res. 2023, 12. [Google Scholar] [CrossRef]
- Jencks, K.J.; Turgeon, D.K.; Stidham, R.W.; McClintock, S.D.; Allen, R.; Moraga, G.; Harber, I.; McNeely, M.M.; Varani, J.; Aslam, M.N. S1193 Improvement in Disease-Related Biomarkers with an Adjuvant Treatment for 180 Days: Results From an Interventional Trial for Patients with Ulcerative Colitis. Am. J. Gastroenterol. 2024, 119, S848. [Google Scholar] [CrossRef]
- Gao, Y.; Dasgupta, C.; Huang, L.; Song, R.; Zhang, Z.; Zhang, L. Multi-Omics Integration Reveals Short and Long-Term Effects of Gestational Hypoxia on the Heart Development. Cells 2019, 8, 1608. [Google Scholar] [CrossRef]
- Akçum, S.; Zülfikaroğlu, E.; Eserdağ, S.; Özcan, U. Plasma Calprotectin Levels in Preeclamptic Normotensive Pregnant and Nonpregnant Women. Gynecol. Obstet. Reprod. Med. 2010, 16, 65–69. [Google Scholar]
- Li, M.; Qian, L.; Yu, J.; Zou, Y. Interleukin-35 inhibits human umbilical vein endothelial cell injury induced by sera from pre-eclampsia patients by up-regulating S100A8 protein expression. Hypertens. Pregnancy 2020, 39, 126–138. [Google Scholar] [CrossRef]
- Björk, P.; Björk, A.; Vogl, T.; Stenström, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of Human S100A9 as a Novel Target for Treatment of Autoimmune Disease via Binding to Quinoline-3-Carboxamides. PLoS Biol. 2009, 7, e1000097–812. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Sturfelt, G.; Lood, C.; Rönnblom, L.; van Vollenhoven, R.F.; Axelsson, B.; Sparre, B.; Tuvesson, H.; Öhman, M.W.; Leanderson, T. Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: Studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Aberšek, N.; Tsiartas, P.; Jonsson, D.; Grankvist, A.; Barman, M.; Hallingström, M.; Kacerovsky, M.; Jacobsson, B. Calprotectin levels in amniotic fluid in relation to intra-amniotic inflammation and infection in women with preterm labor with intact membranes: A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 24–29. [Google Scholar] [CrossRef]
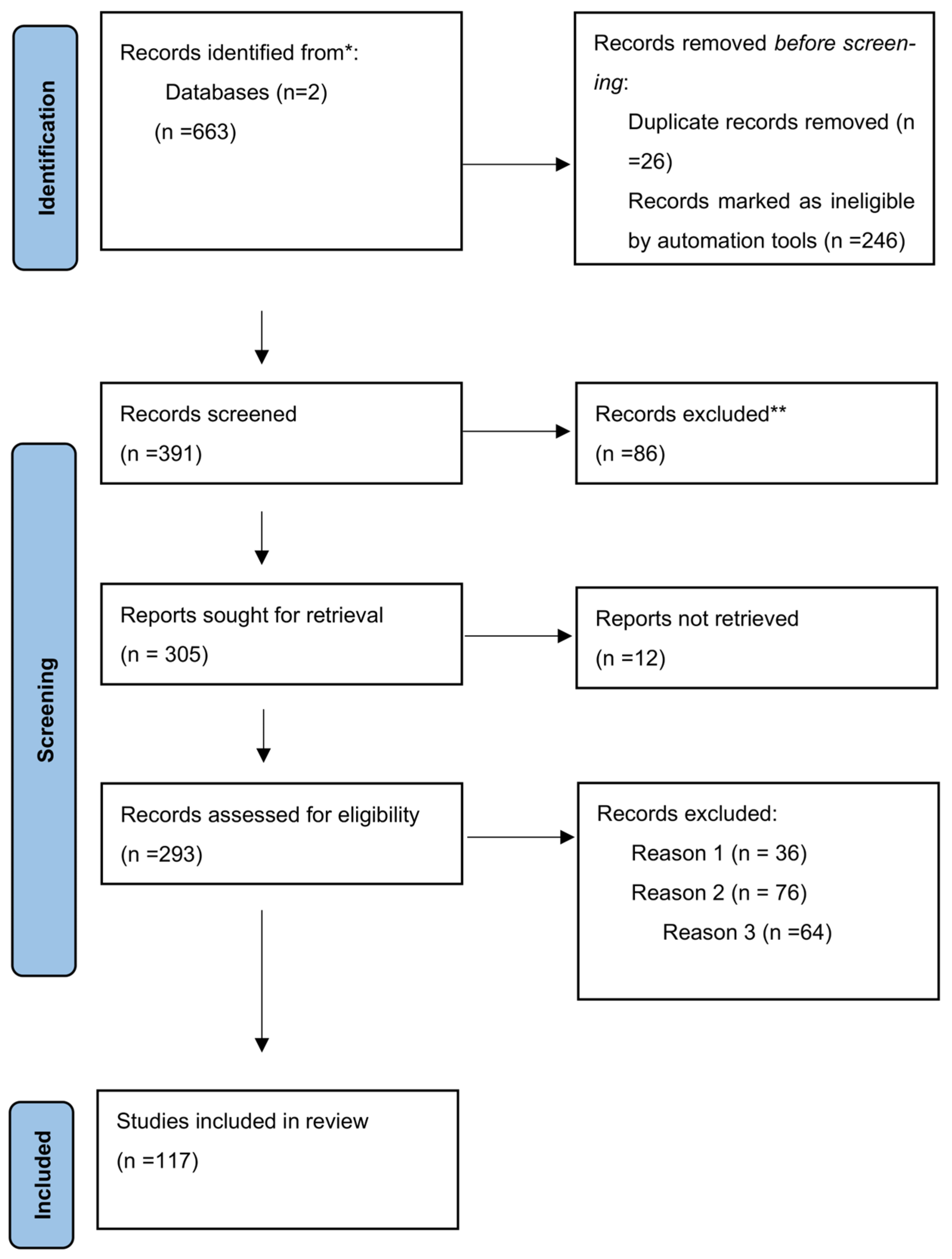
| Research | S100A8, S100A9, Heterodimer S100A8/S100A9 or Calprotectin | Preeclampsia | IUGR | Influence | Sample Size | Signifficance |
|---|---|---|---|---|---|---|
| de Almeida (2022) [1] | S100 as proteomics | yes | yes | PE in relation with cardio-vascular diseases; contribute to angiogenesis, apoptosis and proliferation | 17 | Small sample size, not signifficant |
| Jurewicz, Ewelina (2022) [2] | S100 | yes | Including IUGR | Inflammation | Review | Detailed |
| Singh, Parul (2022) [10] | S100 | yes | yes | Affecting Immune system | Review | Few elements regarding calprotectin |
| Cotoi, Ovidiu (2014) [12] | S100A8, S100A9, heterodimer S100A8/S100A9 or calprotectin | yes | NA | PE in relation to cardio-vascular diseases; correlates with atherosclerosis, including in placenta, in ischemia/reperfusion injury | 664 | Large sample but related to cardio-vascular disease, few details about PE |
| ASLAM, ALIYA (2020) [18] | calprotectin | yes | NA | Serum calprotectin as inflammatory biomarker in PE | 24 | Article from a grey zone |
| Viemann D (2005) [19] | S100A8 | yes | NA | Inflammation of endothelial cells | There is no sample size declared | Inconsistent data |
| Stenhouse, Claire (2021) [40] | S100A8, S100A9, heterodimer S100A8/S100A9 or calprotectin | yes | NA | establishment of pregnancy and the regulation of fetal and placental growth, faecal calprotectin as biomarker | Animal study | Good quality |
| Zhao, Yuan (2023) [65] | calprotectin | Yes in antiphospholipid syndrome | NA | Serum calprotectin in microvascular manifestations | 466 | Relevant information |
| Maliqueo, M (2016) [37] | S100A9 | yes | NA | trophoblast invasion during early pregnancy, artery remodelling | review | Few information regarding calprotectin |
| Lai, J. (2021) [5] | calprotectin | yes | NA | Serum level of calprotectin as biomarkers for PE | In vitro experiment | Relevant information |
| Xiaoyun Li (2019) [75] | S100A8 | yes | NA | Urinary S100A8 as biomarker in PE | 90 | Relevant information, good quality research |
| Braekke, Kristin (2005) [64] | Calprotectin | yes | NA | Serum calprotectin marker of inflammation in PE, but not in their offsprings | 69 | Relevant for our review |
| Brien, Marie-Eve (2019) [68] | S100A8, S100A9, heterodimer S100A8/S100A9 or calprotectin as DAMPs | yes | yes | trophoblast and Hofbauer cells, are involved in the placental response to DAMPs, with role in pregnancy complications including PE, IUGR, | review | Relevant information |
| Gomes L. H. (2013) [70] | S100A8 and S100A9 | yes | yes | as oxidant scavenging in inflammations | 56 | Few relevant information for PE |
| SP Ramachandrarao (2016) [91] | S100A9 | yes | yes | Diabetes Mellitus, urinary exosomes as biomarkers in association with PE | 28 | Small sample size |
| Robinson M. J (2002) [89] | S100A8/A9 | yes | yes | S100A8/A9 binds heparan sulphate proteoglycans, coagulation cascade | In vitro experiment | High quality study design |
| Ortega F. J (2012) [92] | calprotectina | yes | yes | Serum and urinary calprotectina related to inflammation in Diabetes Mellitus and PE | 298 | Few link to PE |
| Skeith, Leslie (2022) [96] | S100A8 and S100A9 | yes | NA | TTP with fetal demise, proteomics analysis identified a 6-fold to 7-fold overexpression of S100A8 and S100A9 | Case report | Can not be generalized |
| Sureshchandra S (2021) [101] | S100A9 | yes | NA | A causal role for S100A9 in thrombosis has been reported, with micro-aggregation and clumping | 15 | Small sample size with no control group identified |
| Li J (2018) [74] | S100A9 | yes | yes | Common in placental insufficiency | Special report | Clear information and recommendationes |
| Jencks (2024) [110] | calprotectina | yes | yes | Serum calprotectin as biomarker in colitis, determin IUGR | report | Few information |
| Maroudias, George (2024) [77] | calprotectin | yes | yes | Higher level of calprotectin in amniotic fluid in PE with IUGR, caused by oxidative stress | 64 | Relevant for our review |
| Li, Ming (2020) [113] | S100A8 | yes | yes | Upregulated by IL-35, in PE targeted treatment | 48 | Relevant information |
| Björk P. (2009) [114] | S100A9 as target | PE in autoimmune diseases such as SLE | yes | Targeted treatment with Quinoline-3-carboxamides | In vitro experiment | Relevant information |
| Bengtsson A. A (2012) [115] | S100A9 as target | PE in autoimmune diseases such as SLE | yes | Targeted treatment with Quinoline-3-carboxamides | Animal model | Relevant information |
| Ackum(2010) [112] | Serum Calprotectin | PE | yes | Biomarker | Small sample size | Low relevance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitranovici, M.-I.; Caravia, L.; Oală, I.E.; Tiron, A.T.; Buicu, C.-F.; Dumitrascu-Biris, D.; Munteanu, M.; Ivan, V.; Apostol, A.; Petre, I.; et al. Exploring the Relevance of S100A8 and S100A9 Proteins in Preeclampsia: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 10118. https://doi.org/10.3390/ijms262010118
Mitranovici M-I, Caravia L, Oală IE, Tiron AT, Buicu C-F, Dumitrascu-Biris D, Munteanu M, Ivan V, Apostol A, Petre I, et al. Exploring the Relevance of S100A8 and S100A9 Proteins in Preeclampsia: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(20):10118. https://doi.org/10.3390/ijms262010118
Chicago/Turabian StyleMitranovici, Melinda-Ildiko, Laura Caravia, Ioan Emilian Oală, Andreea Taisia Tiron, Corneliu-Florin Buicu, Dan Dumitrascu-Biris, Mihai Munteanu, Viviana Ivan, Adrian Apostol, Ion Petre, and et al. 2025. "Exploring the Relevance of S100A8 and S100A9 Proteins in Preeclampsia: A Narrative Review" International Journal of Molecular Sciences 26, no. 20: 10118. https://doi.org/10.3390/ijms262010118
APA StyleMitranovici, M.-I., Caravia, L., Oală, I. E., Tiron, A. T., Buicu, C.-F., Dumitrascu-Biris, D., Munteanu, M., Ivan, V., Apostol, A., Petre, I., & Pușcașiu, L. (2025). Exploring the Relevance of S100A8 and S100A9 Proteins in Preeclampsia: A Narrative Review. International Journal of Molecular Sciences, 26(20), 10118. https://doi.org/10.3390/ijms262010118








