Beyond Hematologic Malignancies: Colorectal Cancer as a Solid Tumor Manifestation of Inherited Bone Marrow Failure Syndromes
Abstract
1. Introduction
2. Overview of IBMFS
2.1. Fanconi Anemia
2.2. Dyskeratosis Congenita
2.3. Diamond–Blackfan Anemia Syndrome
2.4. Shwachman–Diamond Syndrome
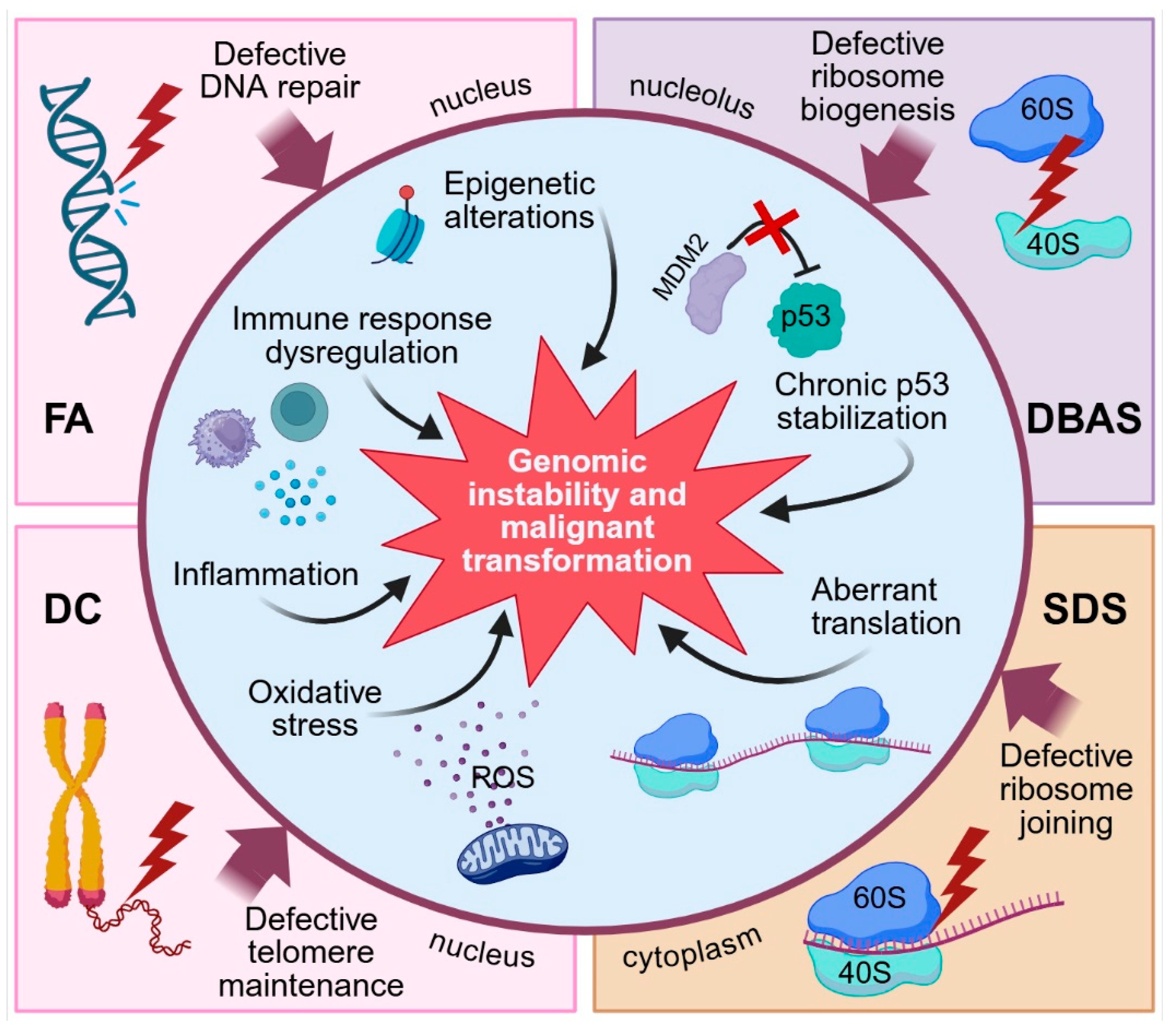
3. Molecular Mechanisms Underlying Colorectal Carcinogenesis in IBMFS
3.1. DNA Repair Defects and Genomic Instability
3.2. p53 Pathway Activation and Cellular Stress Response
3.3. Immune Dysfunction and Inflammation
3.4. Oxidative Stress
3.5. Other Mechanisms Possibly Involved in Colorectal Carcinogenesis
4. Clinical Implications
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| CRC | Colorectal cancer |
| DC | Dyskeratosis congenita |
| DDR | DNA damage repair |
| DBAS | Diamond–Blackfan anemia syndrome |
| FA | Fanconi anemia |
| HR | Homologous recombination |
| HSCT | Hematopoietic stem cell transplantation |
| IBMFS | Inherited bone marrow failure syndrome |
| ICL | Interstrand crosslink |
| MDS | Myelodysplastic syndrome |
| ROS | Reactive oxygen species |
| RP | Ribosomal protein |
| SDS | Shwachman–Diamond syndrome |
References
- Park, M. Overview of Inherited Bone Marrow Failure Syndromes. Blood Res. 2022, 57, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wegman-Ostrosky, T.; Savage, S.A. The Genomics of Inherited Bone Marrow Failure: From Mechanism to the Clinic. Br. J. Haematol. 2017, 177, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Crisà, E.; Boggione, P.; Nicolosi, M.; Mahmoud, A.M.; Essa, W.A.; Awikeh, B.; Aspesi, A.; Andorno, A.; Boldorini, R.; Dianzani, I.; et al. Genetic Predisposition to Myelodysplastic Syndromes: A Challenge for Adult Hematologists. Int. J. Mol. Sci. 2021, 22, 2525. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M. Genomic Testing for Germline Predisposition to Hematologic Malignancies. Blood Res. 2024, 59, 12. [Google Scholar] [CrossRef]
- Willig, T.N.; Niemeyer, C.M.; Leblanc, T.; Tiemann, C.; Robert, A.; Budde, J.; Lambiliotte, A.; Kohne, E.; Souillet, G.; Eber, S.; et al. Identification of New Prognosis Factors from the Clinical and Epidemiologic Analysis of a Registry of 229 Diamond-Blackfan Anemia Patients. Pediatr. Res. 1999, 46, 553–561. [Google Scholar] [CrossRef]
- Wlodarski, M.W.; Vlachos, A.; Farrar, J.E.; Da Costa, L.M.; Kattamis, A.; Dianzani, I.; Belendez, C.; Unal, S.; Tamary, H.; Pasauliene, R.; et al. Diagnosis, Treatment, and Surveillance of Diamond-Blackfan Anaemia Syndrome: International Consensus Statement. Lancet Haematol. 2024, 11, e368–e382. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, E.; Klaassen, R.; Fernandez, C.V.; Yanofsky, R.; Shereck, E.; Champagne, J.; Silva, M.; Lipton, J.H.; Brossard, J.; Michon, B.; et al. Genetic Analysis of Inherited Bone Marrow Failure Syndromes from One Prospective, Comprehensive and Population-Based Cohort and Identification of Novel Mutations. J. Med. Genet. 2011, 48, 618–628. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mendez, K.J.W.; Cole, S.; Gadalla, S.M.; Alter, B.P.; Savage, S.A.; Giri, N. Genotype-phenotype Associations in Individuals with Diamond Blackfan Anaemia. EJHaem 2024, 5, 1117–1124. [Google Scholar] [CrossRef]
- Vlachos, A.; Rosenberg, P.S.; Atsidaftos, E.; Alter, B.P.; Lipton, J.M. Incidence of Neoplasia in Diamond Blackfan Anemia: A Report from the Diamond Blackfan Anemia Registry. Blood 2012, 119, 3815–3819. [Google Scholar] [CrossRef]
- Altintas, B.; Giri, N.; McReynolds, L.J.; Best, A.; Alter, B.P. Genotype-Phenotype and Outcome Associations in Patients with Fanconi Anemia: The National Cancer Institute Cohort. Haematologica 2023, 108, 69–82. [Google Scholar] [CrossRef]
- Aspesi, A.; Ellis, S.R. Rare Ribosomopathies: Insights into Mechanisms of Cancer. Nat. Rev. Cancer 2019, 19, 228–238. [Google Scholar] [CrossRef]
- De Keersmaecker, K.; Sulima, S.O.; Dinman, J.D. Ribosomopathies and the Paradox of Cellular Hypo- to Hyperproliferation. Blood 2015, 125, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.L.H.; Sanders, M.A.; Patel, K.; Dietrich, R.; Noonan, R.J.; Lach, F.P.; White, R.R.; Goldfarb, A.; Hadi, K.; Edwards, M.M.; et al. Genomic Signature of Fanconi Anaemia DNA Repair Pathway Deficiency in Cancer. Nature 2022, 612, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Roka, K.; Solomou, E.; Kattamis, A.; Stiakaki, E. Telomere Biology Disorders: From Dyskeratosis Congenita and Beyond. Postgrad. Med. J. 2024, 100, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Gianni, P.; Matenoglou, E.; Geropoulos, G.; Agrawal, N.; Adnani, H.; Zafeiropoulos, S.; Miyara, S.J.; Guevara, S.; Mumford, J.M.; Molmenti, E.P.; et al. The Fanconi Anemia Pathway and Breast Cancer: A Comprehensive Review of Clinical Data. Clin. Breast Cancer 2022, 22, 10–25. [Google Scholar] [CrossRef]
- Alter, B.P.; Giri, N.; Savage, S.A.; Peters, J.A.; Loud, J.T.; Leathwood, L.; Carr, A.G.; Greene, M.H.; Rosenberg, P.S. Malignancies and Survival Patterns in the National Cancer Institute Inherited Bone Marrow Failure Syndromes Cohort Study. Br. J. Haematol. 2010, 150, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in the National Cancer Institute Inherited Bone Marrow Failure Syndrome Cohort after Fifteen Years of Follow-Up. Haematologica 2017, 103, 30–39. [Google Scholar] [CrossRef]
- McReynolds, L.J.; Biswas, K.; Giri, N.; Sharan, S.K.; Alter, B.P. Genotype-Cancer Association in Patients with Fanconi Anemia Due to Pathogenic Variants in FANCD1 (BRCA2) or FANCN (PALB2). Cancer Genet. 2021, 258–259, 101–109. [Google Scholar] [CrossRef]
- Namikawa, T.; Tanaka, T.; Utsunomiya, M.; Yokota, K.; Munekage, M.; Maeda, H.; Kitagawa, H.; Kurioka, Y.; Satake, H.; Kobayashi, M.; et al. Gastric Cancer with Fanconi Anemia in Adolescent and Young Adult Patient Diagnosed by Comprehensive Genome Profiling Using Next-Generation Sequencing. Clin. J. Gastroenterol. 2024, 17, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.S.; Greene, M.H.; Alter, B.P. Cancer Incidence in Persons with Fanconi Anemia. Blood 2003, 101, 822–826. [Google Scholar] [CrossRef]
- Kutler, D.I.; Singh, B.; Satagopan, J.; Batish, S.D.; Berwick, M.; Giampietro, P.F.; Hanenberg, H.; Auerbach, A.D. A 20-Year Perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003, 101, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.; Che, R.; Zhang, J.; Ma, C.; Fei, P. Fanconi Anemia Signaling and Cancer. Trends Cancer 2017, 3, 840–856. [Google Scholar] [CrossRef]
- Tummala, H.; Walne, A.M.A.J.; Badat, M.; Patel, M.; Walne, A.M.A.J.; Alnajar, J.; Chow, C.C.; Albursan, I.; Frost, J.M.; Ballard, D.; et al. The Evolving Genetic Landscape of Telomere Biology Disorder Dyskeratosis Congenita. EMBO Mol. Med. 2024, 16, 2560–2582. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A.; Niewisch, M.R. Dyskeratosis Congenita and Related Telomere Biology Disorders. In GeneReviews®; University of Washington: Seattle, WA, USA, 2023. [Google Scholar]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in Dyskeratosis Congenita. Blood 2009, 113, 6549–6557. [Google Scholar] [CrossRef]
- Liu, Y.; Karlsson, S. Perspectives of Current Understanding and Therapeutics of Diamond-Blackfan Anemia. Leukemia 2023, 38, 1–9, Erratum in Leukemia 2024, 38, 228. https://doi.org/10.1038/s41375-023-02101-w. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.M.; Molmenti, C.L.S.; Hussain, M.; Desai, P.; Florento, M.; Atsidaftos, E.; Vlachos, A. Colorectal Cancer Screening and Surveillance Strategy for Patients with Diamond Blackfan Anemia: Preliminary Recommendations from the Diamond Blackfan Anemia Registry. Pediatr. Blood Cancer 2021, 68, e28984. [Google Scholar] [CrossRef]
- Lipton, J.M.; Molmenti, C.L.S.; Desai, P.; Lipton, A.; Ellis, S.R.; Vlachos, A. Early Onset Colorectal Cancer: An Emerging Cancer Risk in Patients with Diamond Blackfan Anemia. Genes 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Quarello, P.; Garelli, E.; Carando, A.; Cillario, R.; Brusco, A.; Giorgio, E.; Ferrante, D.; Corti, P.; Zecca, M.; Luciani, M.; et al. A 20-Year Long Term Experience of the Italian Diamond-Blackfan Anaemia Registry: RPS and RPL Genes, Different Faces of the Same Disease? Br. J. Haematol. 2020, 190, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Oyarbide, U.; Cipolli, M.; Bezzerri, V.; Corey, S.J. Shwachman-Diamond Syndromes: Clinical, Genetic, and Biochemical Insights from the Rare Variants. Haematologica 2023, 108, 2594–2605. [Google Scholar] [CrossRef]
- Thompson, A.S.; Giri, N.; Gianferante, D.M.; Jones, K.; Savage, S.A.; Alter, B.P.; McReynolds, L.J. Shwachman Diamond Syndrome: Narrow Genotypic Spectrum and Variable Clinical Features. Pediatr. Res. 2022, 92, 1671–1680. [Google Scholar] [CrossRef]
- Bou Mitri, F.; Beaupain, B.; Flejou, J.F.; Patient, M.; Okhremchuck, I.; Blaise, D.; Izadifar-Legrand, F.; Martignoles, J.A.; Delhommeau, F.; Bellanne-Chantelot, C.; et al. Shwachman-Diamond Syndrome and Solid Tumors: Three New Patients from the French Registry for Severe Chronic Neutropenia and Literature Review. Pediatr. Blood Cancer 2021, 68, e29071. [Google Scholar] [CrossRef]
- Singh, S.A.; Vlachos, A.; Morgenstern, N.J.; Ouansafi, I.; Ip, W.; Rommens, J.M.; Durie, P.; Shimamura, A.; Lipton, J.M. Breast Cancer in a Case of Shwachman Diamond Syndrome. Pediatr. Blood Cancer 2012, 59, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Dhanraj, S.; Manji, A.; Pinto, D.; Scherer, S.W.; Favre, H.; Loh, M.L.; Chetty, R.; Wei, A.C.; Dror, Y. Molecular Characteristics of a Pancreatic Adenocarcinoma Associated with Shwachman-Diamond Syndrome. Pediatr. Blood Cancer 2013, 60, 754–760. [Google Scholar] [CrossRef]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; Mcguffog, L.; et al. Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, X.; Tian, M.; Zeng, N.; Bai, Z.; Deng, W.; Zhao, Y.; Guo, J.; Yang, Y.; Zhang, Z.; et al. BRCA Genes as Candidates for Colorectal Cancer Genetic Testing Panel: Systematic Review and Meta-Analysis. BMC Cancer 2023, 23, 807. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Sun, D.; Gao, Y.; Jiang, Y.; Zhong, M.; Zhao, G.; Chen, J.; Wang, Z.; Liu, Q.; Hong, J.; et al. Germline Mutations in a DNA Repair Pathway Are Associated with Familial Colorectal Cancer. JCI Insight 2021, 6, e148931. [Google Scholar] [CrossRef] [PubMed]
- AlDubayan, S.H.; Giannakis, M.; Moore, N.D.; Han, G.C.; Reardon, B.; Hamada, T.; Mu, X.J.; Nishihara, R.; Qian, Z.; Liu, L.; et al. Inherited DNA-Repair Defects in Colorectal Cancer. Am. J. Hum. Genet. 2018, 102, 401–414. [Google Scholar] [CrossRef]
- Susswein, L.R.; Marshall, M.L.; Nusbaum, R.; Vogel Postula, K.J.; Weissman, S.M.; Yackowski, L.; Vaccari, E.M.; Bissonnette, J.; Booker, J.K.; Cremona, M.L.; et al. Pathogenic and Likely Pathogenic Variant Prevalence among the First 10,000 Patients Referred for next-Generation Cancer Panel Testing. Genet. Med. 2016, 18, 823–832, Erratum in Genet. Med. 2016, 18, 531–532. https://doi.org/10.1038/gim.2016.21. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kaderi Kibria, K.M.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Krishnan, K. Bone Marrow Failure. In StatPearls [Internet]; StatPearls: Petersburg, FL, USA, 2023. [Google Scholar]
- Gutierrez-Rodrigues, F.; Patel, B.A.; Groarke, E.M. When to Consider Inherited Marrow Failure Syndromes in Adults. Hematol. Am. Soc. Hematol. Educ. Progr. 2023, 2023, 548–555. [Google Scholar] [CrossRef]
- Vlachos, A.; Klein, G.W.; Lipton, J.M. The Diamond Blackfan Anemia Registry: Tool for Investigating the Epidemiology and Biology of Diamond-Blackfan Anemia. J. Pediatr. Hematol. Oncol. 2001, 23, 377–382. [Google Scholar] [CrossRef]
- Kolinjivadi, A.M.; Crismani, W.; Ngeow, J. Emerging Functions of Fanconi Anemia Genes in Replication Fork Protection Pathways. Hum. Mol. Genet. 2020, 29, R158–R164. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.D.; Noguchi, E. Fanconi Anemia: Current Insights Regarding Epidemiology, Cancer, and DNA Repair. Hum. Genet. 2022, 141, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ramanagoudr-Bhojappa, R.; van Twest, S.; Rosti, R.O.; Murphy, V.; Tan, W.; Donovan, F.X.; Lach, F.P.; Kimble, D.C.; Jiang, C.S.; et al. Association of Clinical Severity with FANCB Variant Type in Fanconi Anemia. Blood 2020, 135, 1588–1602. [Google Scholar] [CrossRef]
- Ameziane, N.; May, P.; Haitjema, A.; Van De Vrugt, H.J.; Van Rossum-Fikkert, S.E.; Ristic, D.; Williams, G.J.; Balk, J.; Rockx, D.; Li, H.; et al. A Novel Fanconi Anaemia Subtype Associated with a Dominant-Negative Mutation in RAD51. Nat. Commun. 2015, 6, 8829. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, W.; Zhang, K.; Li, C.; Mu, X.; Chu, Y.; Gai, Z.; Wei, H. Clinical and Genetic Features of Fanconi Anemia Associated with a Variant of FANCA Gene: Case Report and Literature Review. Medicine 2024, 103, e39358. [Google Scholar] [CrossRef] [PubMed]
- Farkas, G.; Székely, G.; Goda, V.; Kállay, K.M.; Kocsis, Z.S.; Szakszon, K.; Benyó, G.; Erdélyi, D.; Liptai, Z.; Csordás, K.; et al. Chromosomal Breakage Tests in the Differential Diagnosis of Fanconi Anemia and Aplastic Anemia. Eur. J. Haematol. 2023, 111, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.R.; Meyer, S. Fanconi Anaemia, Childhood Cancer and the BRCA Genes. Genes 2021, 12, 1520. [Google Scholar] [CrossRef] [PubMed]
- Beddok, A.; Velleuer, E.; Sicre de Fontbrune, F.; Brakenhoff, R.H.; Dalle, J.H.; Dufour, C.; Faivre, S.; Genet, C.; Klijanienko, J.; Krieg, C.; et al. Strategies for Early Detection and Detailed Characterization of Oral Lesions and Head and Neck Squamous Cell Carcinoma in Fanconi Anemia Patients. Cancer Lett. 2025, 617, 217529. [Google Scholar] [CrossRef]
- Rosenberg, P.S.; Alter, B.P.; Ebell, W. Cancer Risks in Fanconi Anemia: Findings from the German Fanconi Anemia Registry. Haematologica 2008, 93, 511–517. [Google Scholar] [CrossRef]
- Lee, R.H.; Kang, H.; Yom, S.S.; Smogorzewska, A.; Johnson, D.E.; Grandis, J.R. Treatment of Fanconi Anemia–Associated Head and Neck Cancer: Opportunities to Improve Outcomes. Clin. Cancer Res. 2021, 27, 5168–5187. [Google Scholar] [CrossRef]
- de Pablo García-Cuenca, A.; Rofín-Fontanet, P.; Lorente-Guerrero, J.; Balmaña-Gelpí, J.; Carrasco-López, E.; Temprana-Salvador, J.; Granado-Carrasco, R.; Braña-García, I.; Vaquero-Martínez, P.; Pujol-Pina, R.; et al. Head and Neck Cancer in Fanconi Anemia: Report of 11 Cases and a Systematic Review. Cancers 2025, 17, 349. [Google Scholar] [CrossRef] [PubMed]
- Masserot-Lureau, C.; Adoui, N.; Degos, F.; de Bazelaire, C.; Soulier, J.; Chevret, S.; Socié, G.; Leblanc, T. Incidence of Liver Abnormalities in Fanconi Anemia Patients. Am. J. Hematol. 2012, 87, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Bogliolo, M.; Surrallés, J. Fanconi Anemia: A Model Disease for Studies on Human Genetics and Advanced Therapeutics. Curr. Opin. Genet. Dev. 2015, 33, 32–40. [Google Scholar] [CrossRef]
- Bogliolo, M.; Bluteau, D.; Lespinasse, J.; Pujol, R.; Vasquez, N.; D’Enghien, C.D.; Stoppa-Lyonnet, D.; Leblanc, T.; Soulier, J.; Surrallés, J. Biallelic Truncating FANCM Mutations Cause Early-Onset Cancer but Not Fanconi Anemia. Genet. Med. 2018, 20, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Catucci, I.; Osorio, A.; Arver, B.; Neidhardt, G.; Bogliolo, M.; Zanardi, F.; Riboni, M.; Minardi, S.; Pujol, R.; Azzollini, J.; et al. Individuals with FANCM Biallelic Mutations Do Not Develop Fanconi Anemia, but Show Risk for Breast Cancer, Chemotherapy Toxicity and May Display Chromosome Fragility. Genet. Med. 2018, 20, 452–457. [Google Scholar] [CrossRef]
- Parsa, F.G.; Nobili, S.; Karimpour, M.; Aghdaei, H.A.; Nazemalhosseini-Mojarad, E.; Mini, E. Fanconi Anemia Pathway in Colorectal Cancer: A Novel Opportunity for Diagnosis, Prognosis and Therapy. J. Pers. Med. 2022, 12, 396. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Altintas, B.; Haley, J.S.; Kim, J.; Ramos, M.; Carey, D.J.; Stewart, D.R.; McReynolds, L.J. Most Fanconi Anemia Heterozygotes Are Not at Increased Cancer Risk: A Genome-First DiscovEHR Cohort Population Study. Genet. Med. 2024, 26, 101042. [Google Scholar] [CrossRef]
- McReynolds, L.J.; Giri, N.; Leathwood, L.; Risch, M.O.; Carr, A.G.; Alter, B.P. Risk of Cancer in Heterozygous Relatives of Patients with Fanconi Anemia. Genet. Med. 2022, 24, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Seal, S.; Thompson, D.; Kelly, P.; Renwick, A.; Elliott, A.; Reid, S.; Spanova, K.; Barfoot, R.; Chagtai, T.; et al. PALB2, Which Encodes a BRCA2-Interacting Protein, Is a Breast Cancer Susceptibility Gene. Nat. Genet. 2007, 39, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Jurado, C.; Franch-Expósito, S.; Muñoz, J.; Ocaña, T.; Carballal, S.; López-Cerón, M.; Cuatrecasas, M.; Vila-Casadesús, M.; Lozano, J.J.; Serra, E.; et al. The Fanconi Anemia DNA Damage Repair Pathway in the Spotlight for Germline Predisposition to Colorectal Cancer. Eur. J. Hum. Genet. 2016, 24, 1501–1505. [Google Scholar] [CrossRef]
- Cannon-Albright, L.A.; Teerlink, C.C.; Stevens, J.; Snow, A.K.; Thompson, B.A.; Bell, R.; Nguyen, K.N.; Sargent, N.R.; Kohlmann, W.K.; Neklason, D.W.; et al. FANCM C5791C>T Stopgain Mutation (Rs144567652) Is a Familial Colorectal Cancer Risk Factor. Mol. Genet. Genom. Med. 2020, 8, e1532. [Google Scholar] [CrossRef]
- Norris, K.; Walne, A.J.; Ponsford, M.J.; Cleal, K.; Grimstead, J.W.; Ellison, A.; Alnajar, J.; Dokal, I.; Vulliamy, T.; Baird, D.M. High-Throughput STELA Provides a Rapid Test for the Diagnosis of Telomere Biology Disorders. Hum. Genet. 2021, 140, 945–955. [Google Scholar] [CrossRef]
- Liao, P.; Yan, B.; Wang, C.; Lei, P. Telomeres: Dysfunction, Maintenance, Aging and Cancer. Aging Dis. 2023, 15, 2595–2631. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Gomes, W.R.; Calado, R.T. Recent Advances in Understanding Telomere Diseases. Fac. Rev. 2022, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Tummala, H.; Walne, A.; Dokal, I. The Biology and Management of Dyskeratosis Congenita and Related Disorders of Telomeres. Expert Rev. Hematol. 2022, 15, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A. Telomere Length and Cancer Risk: Finding Goldilocks. Biogerontology 2024, 25, 265–278. [Google Scholar] [CrossRef]
- Tometten, M.; Beier, F.; Kirschner, M.; Schumacher, Y.; Walter, J.; Vieri, M.; Kricheldorf, K.; Röth, A.; Platzbecker, U.; Radsak, M.; et al. Late-Onset Telomere Biology Disorders in Adults: Clinical Insights and Treatment Outcomes from a Retrospective Registry Cohort. Blood Adv. 2025, 9, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hiyoshi, Y.; Ono, A.; Ouchi, M.; Kosumi, K.; Eto, K.; Ida, S.; Iwatsuki, M.; Baba, Y.; Miyamoto, Y.; et al. Locally Advanced Rectal Cancer in a Young Adult Affected with Dyskeratosis Congenita (Zinsser–Cole–Engman Syndrome): A Case Report. Surg. Case Rep. 2024, 10, 206. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamamoto, G.; Fujiyoshi, K.; Akagi, Y.; Kakuta, M.; Nishimura, Y.; Akagi, K. Development of Metachronous Rectal Cancers in a Young Man with Dyskeratosis Congenita: A Case Report. J. Med. Case Rep. 2019, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.; Leblanc, T.; Mohandas, N. Diamond-Blackfan Anemia. Blood 2020, 136, 1262–1273. [Google Scholar] [CrossRef]
- Iskander, D.; Roy, N.B.A.; Payne, E.; Drasar, E.; Hennessy, K.; Harrington, Y.; Christodoulidou, C.; Karadimitris, A.; Batkin, L.; de la Fuente, J. Diamond-Blackfan Anemia in Adults: In Pursuit of a Common Approach for a Rare Disease. Blood Rev. 2023, 61, 101097. [Google Scholar] [CrossRef] [PubMed]
- Flygare, J.; Aspesi, A.; Bailey, J.C.; Miyake, K.; Caffrey, J.M.; Karlsson, S.; Ellis, S.R. Human RPS19, the Gene Mutated in Diamond-Blackfan Anemia, Encodes a Ribosomal Protein Required for the Maturation of 40S Ribosomal Subunits. Blood 2007, 109, 980–986. [Google Scholar] [CrossRef]
- Lindström, M.S.; Bartek, J.; Maya-Mendoza, A. P53 at the Crossroad of DNA Replication and Ribosome Biogenesis Stress Pathways. Cell Death Differ. 2022, 29, 972–982. [Google Scholar] [CrossRef]
- Bhoopalan, S.V.; Yen, J.S.; Mayuranathan, T.; Mayberry, K.D.; Yao, Y.; Lillo Osuna, M.A.; Jang, Y.; Liyanage, J.S.S.; Blanc, L.; Ellis, S.R.; et al. An RPS19-Edited Model for Diamond-Blackfan Anemia Reveals TP53-Dependent Impairment of Hematopoietic Stem Cell Activity. JCI Insight 2023, 8, e161810. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Keel, S.B.; Shimamura, A.; Liu, L.; Gerds, A.T.; Li, H.Y.; Wood, B.L.; Scott, B.L.; Abkowitz, J.L. Delayed Globin Synthesis Leads to Excess Heme and the Macrocytic Anemia of Diamond Blackfan Anemia and Del(5q) Myelodysplastic Syndrome. Sci. Transl. Med. 2016, 8, 338ra67. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.T.; Yan, X.; Meng, C.; Lausted, C.; Tian, Q.; Abkowitz, J.L. Single-Cell Analysis of Erythropoiesis in Rpl11 Haploinsufficient Mice Reveals Insight into the Pathogenesis of Diamond–Blackfan Anemia. Exp. Hematol. 2021, 97, 66–78.e6. [Google Scholar] [CrossRef]
- Vlachos, A.; Rosenberg, P.S.; Atsidaftos, E.; Kang, J.; Onel, K.; Sharaf, R.N.; Alter, B.P.; Lipton, J.M. Increased Risk of Colon Cancer and Osteogenic Sarcoma in Diamond Blackfan Anemia. Blood 2018, 132, 2205–2208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lieberman, D.; Anderson, J.C.; Burke, C.A.; Dominitz, J.A.; Kaltenbach, T.; Robertson, D.J.; Shaukat, A.; Syngal, S.; Rex, D.K. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 158, 1131–1153.e5. [Google Scholar] [CrossRef]
- Nieminen, T.T.; O’Donohue, M.F.; Wu, Y.; Lohi, H.; Scherer, S.W.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.P.; et al. Germline Mutation of RPS20, Encoding a Ribosomal Protein, Causes Predisposition to Hereditary Nonpolyposis Colorectal Carcinoma without DNA Mismatch Repair Deficiency. Gastroenterology 2014, 147, 595–598. [Google Scholar] [CrossRef]
- Broderick, P.; Dobbins, S.E.; Chubb, D.; Kinnersley, B.; Dunlop, M.G.; Tomlinson, I.; Houlston, R.S. Validation of Recently Proposed Colorectal Cancer Susceptibility Gene Variants in an Analysis of Families and Patients—A Systematic Review. Gastroenterology 2017, 152, 75–77.e4. [Google Scholar] [CrossRef]
- Thompson, B.A.; Snow, A.K.; Koptiuch, C.; Kohlmann, W.K.; Mooney, R.; Johnson, S.; Huff, C.D.; Yu, Y.; Teerlink, C.C.; Feng, B.J.; et al. A Novel Ribosomal Protein S20 Variant in a Family with Unexplained Colorectal Cancer and Polyposis. Clin. Genet. 2020, 97, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Amiot, J.; Gubeljak, L.; Fontaine, A.; Smith, D.; Mortemousque, I.; Parodi, N.; Mauillon, J.; Kasper, E.; Baert-Desurmont, S.; Tinat, J.; et al. New RPS20 Gene Variant in Colorectal Cancer Diagnosis: Insight from a Large Series of Patients. Fam. Cancer 2025, 24, 22. [Google Scholar] [CrossRef]
- Djursby, M.; Madsen, M.B.; Frederiksen, J.H.; Berchtold, L.A.; Therkildsen, C.; Willemoe, G.L.; Hasselby, J.P.; Wikman, F.; Okkels, H.; Skytte, A.B.; et al. New Pathogenic Germline Variants in Very Early Onset and Familial Colorectal Cancer Patients. Front. Genet. 2020, 11, 566266. [Google Scholar] [CrossRef]
- Bhar, S.; Zhou, F.; Reineke, L.C.; Morris, D.K.; Khincha, P.P.; Giri, N.; Mirabello, L.; Bergstrom, K.; Lemon, L.D.; Williams, C.L.; et al. Expansion of Germline RPS20 Mutation Phenotype to Include Diamond–Blackfan Anemia. Hum. Mutat. 2020, 41, 1918–1930. [Google Scholar] [CrossRef]
- Han, X.; Lu, S.; Gu, C.; Bian, Z.; Xie, X.; Qiao, X. Clinical Features, Epidemiology, and Treatment of Shwachman-Diamond Syndrome: A Systematic Review. BMC Pediatr. 2023, 23, 503. [Google Scholar] [CrossRef]
- Hamabata, T.; Umeda, K.; Kouzuki, K.; Tanaka, T.; Daifu, T.; Nodomi, S.; Saida, S.; Kato, I.; Baba, S.; Hiramatsu, H.; et al. Pluripotent Stem Cell Model of Shwachman–Diamond Syndrome Reveals Apoptotic Predisposition of Hemoangiogenic Progenitors. Sci. Rep. 2020, 10, 14859, Erratum in Sci. Rep. 2021, 11, 2107. https://doi.org/10.1038/s41598-021-81066-1. [Google Scholar] [CrossRef] [PubMed]
- Oyarbide, U.; Bezzerri, V.; Staton, M.; Boni, C.; Shah, A.; Cipolli, M.; Calo, E.; Corey, S.J. Reduced EIF6 Dosage Attenuates TP53 Activation in Models of Shwachman-Diamond Syndrome. J. Clin. Investig. 2025, 135, e187778. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.R.; Shimamura, A. Predisposition to Myeloid Malignancies in Shwachman-Diamond Syndrome: Biological Insights and Clinical Advances. Blood 2022, 141, 1513–1523. [Google Scholar] [CrossRef]
- Furutani, E.; Liu, S.; Galvin, A.; Steltz, S.; Malsch, M.M.; Loveless, S.K.; Mount, L.; Larson, J.H.; Queenan, K.; Bertuch, A.A.; et al. Hematologic Complications with Age in Shwachman-Diamond Syndrome. Blood Adv. 2022, 6, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Donadieu, J.; Fenneteau, O.; Beaupain, B.; Beaufils, S.; Bellanger, F.; Mahlaoui, N.; Lambilliotte, A.; Aladjidi, N.; Bertrand, Y.; Mialou, V.; et al. Classification of and Risk Factors for Hematologic Complications in a French National Cohort of 102 Patients with Shwachman-Diamond Syndrome. Haematologica 2012, 97, 1312–1319. [Google Scholar] [CrossRef]
- Morecroft, R.; Logothetics, C.N.; Tarnawsky, S.P.; Davis, A.A. Chemotherapy-Induced Neutropenia Management in a Patient with Metastatic Breast Cancer and Shwachman-Diamond Syndrome (SDS): A Case Report. Transl. Breast Cancer Res. 2024, 5, 26. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Z.; Liu, J.; Wu, X.; Fan, X.; Pang, J.; Bao, H.; Yin, J.; Wu, X.; Shao, Y.; et al. Identification of Pathogenic Germline Variants in a Large Chinese Lung Cancer Cohort by Clinical Sequencing. Mol. Oncol. 2024, 18, 1301–1315. [Google Scholar] [CrossRef]
- Sculco, M.; La Vecchia, M.; Aspesi, A.; Pinton, G.; Clavenna, M.G.; Casalone, E.; Allione, A.; Grosso, F.; Libener, R.; Muzio, A.; et al. Malignant Pleural Mesothelioma: Germline Variants in DNA Repair Genes May Steer Tailored Treatment. Eur. J. Cancer 2022, 163, 44–54. [Google Scholar] [CrossRef]
- Alonso-Luna, O.; Mercado-Celis, G.E.; Melendez-Zajgla, J.; Barquera, R.; Zapata-Tarres, M.; Juárez-Villegas, L.E.; Mendoza-Caamal, E.C.; Rey-Helo, E.; Borges-Yañez, S.A. Germline Mutations in Pediatric Cancer Cohort with Mixed-Ancestry Mexicans. Mol. Genet. Genom. Med. 2024, 12, e2332. [Google Scholar] [CrossRef]
- García-De-teresa, B.; Rodríguez, A.; Frias, S. Chromosome Instability in Fanconi Anemia: From Breaks to Phenotypic Consequences. Genes 2020, 11, 1528. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Peralta, C.; Yaseen, A.; Pavletich, N.P. Structure of the FA Core Ubiquitin Ligase Closing the ID Clamp on DNA. Nat. Struct. Mol. Biol. 2021, 28, 300–309. [Google Scholar] [CrossRef]
- Renaudin, X.; Rosselli, F. The FANC/BRCA Pathway Releases Replication Blockades by Eliminating DNA Interstrand Cross-Links. Genes 2020, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Longerich, S.; Li, J.; Xiong, Y.; Sung, P.; Kupfer, G.M. Stress and DNA Repair Biology of the Fanconi Anemia Pathway. Blood 2014, 124, 2812–2819. [Google Scholar] [CrossRef]
- Sumpter, R.; Levine, B. Emerging Functions of the Fanconi Anemia Pathway at a Glance. J. Cell Sci. 2017, 130, 2657–2662. [Google Scholar] [CrossRef]
- Michl, J.; Zimmer, J.; Tarsounas, M. Interplay between Fanconi Anemia and Homologous Recombination Pathways in Genome Integrity. EMBO J. 2016, 35, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Hosea, R.; Hillary, S.; Naqvi, S.; Wu, S.; Kasim, V. The Two Sides of Chromosomal Instability: Drivers and Brakes in Cancer. Signal Transduct. Target. Ther. 2024, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Langevin, F.; Crossan, G.P.; Rosado, I.V.; Arends, M.J.; Patel, K.J. Fancd2 Counteracts the Toxic Effects of Naturally Produced Aldehydes in Mice. Nature 2011, 475, 53–58. [Google Scholar] [CrossRef]
- Pawlikowska, P.; Delestré, L.; Gregoricchio, S.; Oppezzo, A.; Esposito, M.; Diop, M.B.; Rosselli, F.; Guillouf, C. FANCA Deficiency Promotes Leukaemic Progression by Allowing the Emergence of Cells Carrying Oncogenic Driver Mutations. Oncogene 2023, 42, 2764–2775. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Lazzerini-Denchi, E.; Sfeir, A. Stop Pulling My Strings-What Telomeres Taught Us about the DNA Damage Response. Nat. Rev. Mol. Cell. Biol. 2016, 17, 364–378. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Wan, Y.; Gao, J.; Ma, Y.; Zhang, Y.; Tong, J.; Zhang, Y.; Liu, J.; Chang, L.; et al. Decoding the Pathogenesis of Diamond–Blackfan Anemia Using Single-Cell RNA-Seq. Cell Discov. 2022, 8, 41. [Google Scholar] [CrossRef]
- Austin, K.M.; Gupta, M.L.; Coats, S.A.; Tulpule, A.; Mostoslavsky, G.; Balazs, A.B.; Mulligan, R.C.; Daley, G.; Pellman, D.; Shimamura, A. Mitotic Spindle Destabilization and Genomic Instability in Shwachman-Diamond Syndrome. J. Clin. Investig. 2008, 118, 1511–1518. [Google Scholar] [CrossRef]
- Sera, Y.; Imanaka, T.; Iguchi, Y.; Yamaguchi, M. Mitotic Abnormalities and Spindle Assembly Checkpoint Inactivation in a Cell Model of Shwachman-Diamond Syndrome with Mutations in the Shwachman-Bodian-Diamond Syndrome Gene, 258+2T > C. Drug Discov. Ther. 2024, 18, 283–289. [Google Scholar] [CrossRef]
- Valli, R.; De Paoli, E.; Nacci, L.; Frattini, A.; Pasquali, F.; Maserati, E. Novel Recurrent Chromosome Anomalies in Shwachman–Diamond Syndrome. Pediatr. Blood Cancer 2017, 64, e26454. [Google Scholar] [CrossRef] [PubMed]
- Maserati, E.; Pressato, B.; Valli, R.; Minelli, A.; Sainati, L.; Patitucci, F.; Marletta, C.; Mastronuzzi, A.; Poli, F.; Lo Curto, F.; et al. The Route to Development of Myelodysplastic Syndrome/Acute Myeloid Leukaemia in Shwachman-Diamond Syndrome: The Role of Ageing, Karyotype Instability, and Acquired Chromosome Anomalies. Br. J. Haematol. 2009, 145, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Su, C.H.; Tarn, W.Y. P53 Activation in Genetic Disorders: Different Routes to the Same Destination. Int. J. Mol. Sci. 2021, 22, 9307. [Google Scholar] [CrossRef]
- Ceccaldi, R.; Parmar, K.; Mouly, E.; Delord, M.; Kim, J.M.; Regairaz, M.; Pla, M.; Vasquez, N.; Zhang, Q.S.; Pondarre, C.; et al. Bone Marrow Failure in Fanconi Anemia Is Triggered by an Exacerbated P53/P21 DNA Damage Response That Impairs Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 2012, 11, 36–49. [Google Scholar] [CrossRef]
- Dutt, S.; Narla, A.; Lin, K.; Mullally, A.; Abayasekara, N.; Megerdichian, C.; Wilson, F.H.; Currie, T.; Khanna-Gupta, A.; Berliner, N.; et al. Haploinsufficiency for Ribosomal Protein Genes Causes Selective Activation of P53 in Human Erythroid Progenitor Cells. Blood 2011, 117, 2567–2576. [Google Scholar] [CrossRef]
- Fok, W.C.; Niero, E.L.d.O.; Dege, C.; Brenner, K.A.; Sturgeon, C.M.; Batista, L.F.Z. P53 Mediates Failure of Human Definitive Hematopoiesis in Dyskeratosis Congenita. Stem Cell Rep. 2017, 9, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Marion, W.; Boettcher, S.; Ruiz-Torres, S.; da Rocha, E.L.; Lundin, V.; Morris, V.; Chou, S.; Zhao, A.M.; Kubaczka, C.; Aumais, O.; et al. An Induced Pluripotent Stem Cell Model of Fanconi Anemia Reveals Mechanisms of P53-Driven Progenitor Cell Differentiation. Blood Adv. 2020, 4, 4679–4692. [Google Scholar] [CrossRef]
- Choijilsuren, H.B.; Park, Y.; Jung, M. Mechanisms of Somatic Transformation in Inherited Bone Marrow Failure Syndromes. Hematolgy 2021, 2021, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.L.; Myers, K.C.; Bowman, J.; Gibson, C.J.; Camarda, N.D.; Furutani, E.; Muscato, G.M.; Klein, R.H.; Ballotti, K.; Liu, S.; et al. Distinct Genetic Pathways Define Pre-Malignant versus Compensatory Clonal Hematopoiesis in Shwachman-Diamond Syndrome. Nat. Commun. 2021, 12, 1334. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nihrane, A.; Aglipay, J.; Sironi, J.; Arkin, S.; Lipton, J.M.; Ouchi, T.; Liu, J.M. Upregulated ATM Gene Expression and Activated DNA Crosslink-Induced Damage Response Checkpoint in Fanconi Anemia: Implications for Carcinogenesis. Mol. Med. 2008, 14, 167–174. [Google Scholar] [CrossRef]
- Biller, M.; Kabir, S.; Boado, C.; Nipper, S.; Saffa, A.; Tal, A.; Allen, S.; Sasanuma, H.; Dréau, D.; Vaziri, C.; et al. REV7-P53 Interaction Inhibits ATM-Mediated DNA Damage Signaling. Cell Cycle 2024, 23, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Nedoszytko, B.; Kalinowski, L.; Repczynska, A.; Ciastek, B.; Haus, O. New Insights into the Fanconi Anemia Pathogenesis: A Crosstalk Between Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 11619. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, Y.; Guo, X.; Ramchandani, S.; Ma, J.; Shen, M.-F.; Garcia, D.A.; Deng, Y.; Multani, A.S.; You, M.J.; et al. Pot1b Deletion and Telomerase Haploinsufficiency in Mice Initiate an ATR-Dependent DNA Damage Response and Elicit Phenotypes Resembling Dyskeratosis Congenita. Mol. Cell. Biol. 2009, 29, 229–240. [Google Scholar] [CrossRef]
- Pereboeva, L.; Westin, E.; Patel, T.; Flaniken, I.; Lamb, L.; Klingelhutz, A.; Goldman, F. DNA Damage Responses and Oxidative Stress in Dyskeratosis Congenita. PLoS ONE 2013, 8, e76473. [Google Scholar] [CrossRef] [PubMed]
- Pereboeva, L.; Hubbard, M.; Goldman, F.D.; Westin, E.R. Robust DNA Damage Response and Elevated Reactive Oxygen Species in TINF2-Mutated Dyskeratosis Congenita Cells. PLoS ONE 2016, 11, e0148793. [Google Scholar] [CrossRef][Green Version]
- Hannan, K.M.; Soo, P.; Wong, M.S.; Lee, J.K.; Hein, N.; Poh, P.; Wysoke, K.D.; Williams, T.D.; Montellese, C.; Smith, L.K.; et al. Nuclear Stabilization of P53 Requires a Functional Nucleolar Surveillance Pathway. Cell Rep. 2022, 41, 111571. [Google Scholar] [CrossRef]
- Donati, G.; Peddigari, S.; Mercer, C.A.; Thomas, G. 5S Ribosomal RNA Is an Essential Component of a Nascent Ribosomal Precursor Complex That Regulates the Hdm2-P53 Checkpoint. Cell Rep. 2013, 4, 87–98. [Google Scholar] [CrossRef]
- Weinstein, H.N.W.; Hu, K.; Fish, L.; Chen, Y.A.; Allegakoen, P.; Pham, J.H.; Hui, K.S.F.; Chang, C.H.; Tutar, M.; Benitez-Rivera, L.; et al. RPL22 Is a Tumor Suppressor in MSI-High Cancers and a Splicing Regulator of MDM4. Cell Rep. 2024, 43, 114622. [Google Scholar] [CrossRef]
- Jansen, J.; Bohnsack, K.E.; Böhlken-Fascher, S.; Bohnsack, M.T.; Dobbelstein, M. The Ribosomal Protein L22 Binds the MDM4 Pre-MRNA and Promotes Exon Skipping to Activate P53 upon Nucleolar Stress. Cell Rep. 2024, 43, 114610. [Google Scholar] [CrossRef] [PubMed]
- Aspesi, A.; Monteleone, V.; Betti, M.; Actis, C.; Morleo, G.; Sculco, M.; Guarrera, S.; Wlodarski, M.W.; Ramenghi, U.; Santoro, C.; et al. Lymphoblastoid Cell Lines from Diamond Blackfan Anaemia Patients Exhibit a Full Ribosomal Stress Phenotype That Is Rescued by Gene Therapy. Sci. Rep. 2017, 7, 12010, Erratum in Sci. Rep. 2018, 8, 17227. https://doi.org/10.1038/s41598-018-35522-0. [Google Scholar] [CrossRef]
- Ellis, S.R. Nucleolar Stress in Diamond Blackfan Anemia Pathophysiology. Biochim. Biophys. Acta 2014, 1842, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Dobbelstein, M. MDM4 Exon Skipping upon Dysfunctional Ribosome Assembly. Trends Cell Biol. 2025, 35, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.S. The Central Role of Ribosomal Proteins in P53 Regulation. Cancers 2025, 17, 1597. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, H.; Stachelek, G.C.; Begum, A.; Davis, C.E.; Dorado, T.E.; Ernst, G.; Reinhold, W.C.; Ozbek, B.; Zheng, Q.; et al. Ribosomal RNA Transcription Regulates Splicing through Ribosomal Protein RPL22. Cell Chem. Biol. 2025, 32, 908–925.e9. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, J.; Chen, Y.; Wang, S.; Cao, M.; Lu, H.; Zhou, X. Dual Regulation of P53 by the Ribosome Maturation Factor SBDS. Cell Death Dis. 2020, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Sera, Y.; Yamamoto, S.; Mutou, A.; Koba, S.; Kurokawa, Y.; Imanaka, T.; Yamaguchi, M. SBDS Gene Mutation Increases ROS Production and Causes DNA Damage as Well as Oxidation of Mitochondrial Membranes in the Murine Myeloid Cell Line 32Dcl3. Biol. Pharm. Bull. 2024, 47, 1376–1382. [Google Scholar] [CrossRef]
- Frattini, A.; Bolamperti, S.; Valli, R.; Cipolli, M.; Pinto, R.M.; Bergami, E.; Frau, M.R.; Cesaro, S.; Signo, M.; Bezzerri, V.; et al. Enhanced P53 Levels Are Involved in the Reduced Mineralization Capacity of Osteoblasts Derived from Shwachman–Diamond Syndrome Subjects. Int. J. Mol. Sci. 2021, 22, 13331. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, C.; Das, B.; Yeger, H.; Dror, Y. SBDS-Deficiency Results in Deregulation of Reactive Oxygen Species Leading to Increased Cell Death and Decreased Cell Growth. Pediatr. Blood Cancer 2010, 55, 1138–1144. [Google Scholar] [CrossRef]
- Ravera, S.; Dufour, C.; Cesaro, S.; Bottega, R.; Faleschini, M.; Cuccarolo, P.; Corsolini, F.; Usai, C.; Columbaro, M.; Cipolli, M.; et al. Evaluation of Energy Metabolism and Calcium Homeostasis in Cells Affected by Shwachman-Diamond Syndrome. Sci. Rep. 2016, 6, 25441. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Sheng, J.; Wu, F.; Li, K.; Huang, R.; Wang, X.; Jiao, T.; Guan, X.; Lu, Y.; et al. P53-R273H Mutation Enhances Colorectal Cancer Stemness through Regulating Specific LncRNAs. J. Exp. Clin. Cancer Res. 2019, 38, 379. [Google Scholar] [CrossRef]
- Wong, R.P.C.; Tsang, W.P.; Chau, P.Y.; Co, N.N.; Tsang, T.Y.; Kwok, T.T. P53-R273H Gains New Function in Induction of Drug Resistance through down-Regulation of Procaspase-3. Mol. Cancer Ther. 2007, 6, 1054–1061. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rozenfeld, N.; et al. Mutant P53 Prolongs NF-ΚB Activation and Promotes Chronic Inflammation and Inflammation-Associated Colorectal Cancer. Cancer Cell 2013, 23, 634–646, Erratum in Cancer Cell 2013, 24, 272. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Clonal Hematopoiesis, a Risk Condition for Developing Myeloid Neoplasia. Hemato 2025, 6, 10. [Google Scholar] [CrossRef]
- Patnaik, M.M. Clonal Hematopoiesis in Inherited Bone Marrow Failure Syndromes: Adaptive Origins With Maladaptive Consequences? Hematology 2023, 20, 1615–1622. [Google Scholar] [CrossRef]
- Neoman, N.; Kim, H.N.; Viduya, J.; Goyal, A.; Liu, Y.L.; Sakamoto, K.M. Inflammatory Pathways and the Bone Marrow Microenvironment in Inherited Bone Marrow Failure Syndromes. Stem Cells 2025, 43, sxaf021. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Bezzerri, V.; Corey, S.J. The Molecular and Genetic Mechanisms of Inherited Bone Marrow Failure Syndromes: The Role of Inflammatory Cytokines in Their Pathogenesis. Biomolecules 2023, 13, 1249. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Corcione, A.; Svahn, J.; Haupt, R.; Poggi, V.; Béka’ssy, A.N.; Scimè, R.; Pistorio, A.; Pistoia, V. TNF-α and IFN-γ Are Overexpressed in the Bone Marrow of Fanconi Anemia Patients and TNF-α Suppresses Erythropoiesis in Vitro. Blood 2003, 102, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Helbling-Leclerc, A.; Garcin, C.; Rosselli, F. Beyond DNA Repair and Chromosome Instability—Fanconi Anaemia as a Cellular Senescence-Associated Syndrome. Cell Death Differ. 2021, 28, 1159–1173. [Google Scholar] [CrossRef]
- Zhang, H.; Kozono, D.E.; O’Connor, K.W.; Vidal-Cardenas, S.; Rousseau, A.; Hamilton, A.; Moreau, L.; Gaudiano, E.F.; Greenberger, J.; Bagby, G.; et al. TGF-β Inhibition Rescues Hematopoietic Stem Cell Defects and Bone Marrow Failure in Fanconi Anemia. Cell Stem Cell 2016, 18, 668–681. [Google Scholar] [CrossRef]
- Rodríguez, A.; Yang, C.; Furutani, E.; García de Teresa, B.; Velázquez, M.; Filiatrault, J.; Sambel, L.A.; Phan, T.; Flores-Guzmán, P.; Sánchez, S.; et al. Inhibition of TGFβ1 and TGFβ3 Promotes Hematopoiesis in Fanconi Anemia. Exp. Hematol. 2020, 93, 70–84.e4. [Google Scholar] [CrossRef]
- Giri, N.; Alter, B.P.; Penrose, K.; Falk, R.T.; Pan, Y.; Savage, S.A.; Williams, M.; Kemp, T.J.; Pinto, L.A. Immune Status of Patients with Inherited Bone Marrow Failure Syndromes. Am. J. Hematol. 2015, 90, 702–708. [Google Scholar] [CrossRef]
- Myers, K.C.; Sauter, S.; Zhang, X.; Bleesing, J.J.; Davies, S.M.; Wells, S.I.; Mehta, P.A.; Kumar, A.; Marmer, D.; Marsh, R.; et al. Impaired Immune Function in Children and Adults with Fanconi Anemia. Pediatr. Blood Cancer 2017, 64, 26599. [Google Scholar] [CrossRef]
- Jyonouchi, S.; Forbes, L.; Ruchelli, E.; Sullivan, K.E. Dyskeratosis Congenita: A Combined Immunodeficiency with Broad Clinical Spectrum—A Single-Center Pediatric Experience. Pediatr. Allergy Immunol. 2011, 22, 313–319. [Google Scholar] [CrossRef]
- Knight, S.W.; Heiss, N.S.; Vulliamy, T.J.; Aalfs, C.M.; McMahon, C.; Richmond, P.; Jones, A.; Hennekam, R.C.M.; Poustka, A.; Mason, P.J.; et al. Unexplained Aplastic Anaemia, Immunodeficiency, and Cerebellar Hypoplasia (Hoyeraal-Hreidarsson Syndrome) Due to Mutations in the Dyskeratosis Congenita Gene, DKC1. Br. J. Haematol. 1999, 107, 335–339. [Google Scholar] [CrossRef]
- Iskander, D.; Roberts, I.; Rees, C.; Szydlo, R.; Alikian, M.; Neale, M.; Harrington, Y.; Kelleher, P.; Karadimitris, A.; de la Fuente, J. Impaired Cellular and Humoral Immunity Is a Feature of Diamond-Blackfan Anaemia; Experience of 107 Unselected Cases in the United Kingdom. Br. J. Haematol. 2019, 186, 321–326. [Google Scholar] [CrossRef]
- Ragab, I.; Makkeyah, S.; Hassan, N.; Botros, M.; Da Costa, L.; Aly, N.H. Immunodeficiency in Children with Diamond Blackfan and Diamond Blackfan like Anemia. Blood Cells Mol. Dis. 2025, 111, 102911. [Google Scholar] [CrossRef] [PubMed]
- Rochowski, A.; Sun, C.; Glogauer, M.; Alter, B.P. Neutrophil Functions in Patients with Inherited Bone Marrow Failure Syndromes. Pediatr. Blood Cancer 2010, 57, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.E.; Saadatpour, A.; Ruiz-Gutierrez, M.; Bolukbasi, O.V.; Jiang, L.; Thomas, D.D.; Young, S.; Hofmann, I.; Sieff, C.A.; Myers, K.C.; et al. TGF-β Signaling Underlies Hematopoietic Dysfunction and Bone Marrow Failure in Shwachman-Diamond Syndrome. J. Clin. Investig. 2019, 129, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, G.; D’Aversa, E.; Breveglieri, G.; Altieri, M.T.; Boni, C.; Pegoraro, A.; Finotti, A.; Gambari, R.; D’Amico, G.; Vella, A.; et al. Constitutive Systemic Inflammation in Shwachman-Diamond Syndrome. Mol. Med. 2025, 31, 81. [Google Scholar] [CrossRef] [PubMed]
- Kulanuwat, S.; Jungtrakoon, P.; Tangjittipokin, W.; Yenchitsomanus, P.T.; Plengvidhya, N. Fanconi Anemia Complementation Group C Protection against Oxidative Stress-Induced ß-Cell Apoptosis. Mol. Med. Rep. 2018, 18, 2485–2491. [Google Scholar] [CrossRef]
- Cappelli, E.; Bertola, N.; Bruno, S.; Degan, P.; Regis, S.; Corsolini, F.; Banelli, B.; Dufour, C.; Ravera, S. A Multidrug Approach to Modulate the Mitochondrial Metabolism Impairment and Relative Oxidative Stress in Fanconi Anemia Complementation Group A. Metabolites 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-Related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef]
- Doty, R.T.; Phelps, S.R.; Shadle, C.; Sanchez-Bonilla, M.; Keel, S.B.; Abkowitz, J.L. Coordinate Expression of Heme and Globin Is Essential for Effective Erythropoiesis. J. Clin. Investig. 2015, 125, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- Aspesi, A.; Pavesi, E.; Robotti, E.; Crescitelli, R.; Boria, I.; Avondo, F.; Moniz, H.; Da Costa, L.; Mohandas, N.; Roncaglia, P.; et al. Dissecting the Transcriptional Phenotype of Ribosomal Protein Deficiency: Implications for Diamond-Blackfan Anemia. Gene 2014, 545, 282–289. [Google Scholar] [CrossRef]
- Kapralova, K.; Jahoda, O.; Koralkova, P.; Gursky, J.; Lanikova, L.; Pospisilova, D.; Divoky, V.; Horvathova, M. Oxidative DNA Damage, Inflammatory Signature, and Altered Erythrocytes Properties in Diamond-Blackfan Anemia. Int. J. Mol. Sci. 2020, 21, 9652. [Google Scholar] [CrossRef] [PubMed]
- Kampen, K.R.; Sulima, S.O.; Vereecke, S.; de Keersmaecker, K. Hallmarks of Ribosomopathies. Nucleic Acids Res. 2021, 48, 1013–1028. [Google Scholar] [CrossRef]
- Jensen, L.T.; Phyu, T.; Jain, A.; Kaewwanna, C.; Jensen, A.N. Decreased Accumulation of Superoxide Dismutase 2 within Mitochondria in the Yeast Model of Shwachman-Diamond Syndrome. J. Cell. Biochem. 2019, 120, 13867–13880. [Google Scholar] [CrossRef]
- Richardson, C.; Yan, S.; Vestal, C.G. Oxidative Stress, Bone Marrow Failure, and Genome Instability in Hematopoietic Stem Cells. Int. J. Mol. Sci. 2015, 16, 2366–2385. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, R.K.; Munschauer, M.; Ulirsch, J.C.; Fiorini, C.; Ludwig, L.S.; McFarland, S.K.; Abdulhay, N.J.; Specht, H.; Keshishian, H.; Mani, D.R.; et al. Ribosome Levels Selectively Regulate Translation and Lineage Commitment in Human Hematopoiesis. Cell 2018, 173, 90–103.e19. [Google Scholar] [CrossRef]
- Horos, R.; Ijspeert, H.; Pospisilova, D.; Sendtner, R.; Andrieu-Soler, C.; Taskesen, E.; Nieradka, A.; Cmejla, R.; Sendtner, M.; Touw, I.P.; et al. Ribosomal Deficiencies in Diamond-Blackfan Anemia Impair Translation of Transcripts Essential for Differentiation of Murine and Human Erythroblasts. Blood 2012, 119, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.S.; Gazda, H.T.; Eng, J.C.; Eichhorn, S.W.; Thiru, P.; Ghazvinian, R.; George, T.I.; Gotlib, J.R.; Beggs, A.H.; Sieff, C.A.; et al. Altered Translation of GATA1 in Diamond-Blackfan Anemia. Nat. Med. 2014, 20, 748–753. [Google Scholar] [CrossRef]
- In, K.; Zaini, M.A.; Müller, C.; Warren, A.J.; Von Lindern, M.; Calkhoven, C.F. Shwachman-Bodian-Diamond Syndrome (SBDS) Protein Deficiency Impairs Translation Re-Initiation from C/EBPα and C/EBPβ MRNAs. Nucleic Acids Res. 2016, 44, 4134–4146. [Google Scholar] [CrossRef]
- Yoon, A.; Peng, G.; Brandenburg, Y.; Zollo, O.; Xu, W.; Rego, E.; Ruggero, D. Impaired Control of IRES-Mediated Translation in X-Linked Dyskeratosis Congenita. Science 2006, 312, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, D.; Pandolfi, P.P. Does the Ribosome Translate Cancer? Nat. Rev. Cancer 2003, 3, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Penzo, M.; Rocchi, L.; Brugiere, S.; Carnicelli, D.; Onofrillo, C.; Coute, Y.; Brigotti, M.; Montanaro, L. Human Ribosomes from Cells with Reduced Dyskerin Levels Are Intrinsically Altered in Translation. FASEB J. 2015, 29, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Belo, H.; Silva, G.; Cardoso, B.A.; Porto, B.; Minguillon, J.; Barbot, J.; Coutinho, J.; Casado, J.A.; Benedito, M.; Saturnino, H.; et al. Epigenetic Alterations in Fanconi Anaemia: Role in Pathophysiology and Therapeutic Potential. PLoS ONE 2015, 10, e0139740. [Google Scholar] [CrossRef] [PubMed]
- Velleuer, E.; Carlberg, C. Impact of Epigenetics on Complications of Fanconi Anemia: The Role of Vitamin d-Modulated Immunity. Nutrients 2020, 12, 1355. [Google Scholar] [CrossRef]
- Skouteris, N.; Papageorgiou, G. PARP Inhibitors in Colorectal Malignancies: A 2023 Update. Rev. Recent Clin. Trials 2023, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Wei, H.Z.; Deng, H.Y.; Xiao, G.H.; Zhang, Y.C. PARP in Colorectal Cancer: Molecular Mechanisms, Immunity, Clinical Trials, and Drug Combinations. Neoplasma 2023, 70, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
| Disease | Affected Genes | Associated Solid Tumors |
|---|---|---|
| Fanconi Anemia | FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, FANCM, FANCN, FANCO, FANCP, FANCQ, FANCR, FANCS, FANCT, FANCU, FANCV, FANCW [15] | Breast, ovarian, colorectal, lung, thyroid, head and neck, brain, bladder, kidney, vulval, vaginal, cervical, prostate, testicular, esophageal, gastric, pancreatic, liver, anal cancer; neuroblastoma, squamous cell carcinoma, basal cell carcinoma, melanoma, soft-tissue sarcoma, osteosarcoma [10,16,17,18,19,20,21,22]. |
| Dyskeratosis Congenita | DKC1, TERC, TERT, TINF2, NAF1, NHP2, NOP10, PARN, POT1, RTEL1, STN1, CTC1, WRAP53, POLA1, ACD, USB1, ZCCHC8, NPM1, MDM4, RPA1, DCLRE1B, TYMS-ENOSF1 [23,24] | Head and neck, colorectal, thyroid, liver, lung, esophageal, gastric, anal, pancreatic, cervical cancer; squamous cell carcinoma, basal cell carcinoma [16,17,25]. |
| Diamond–Blackfan Anemia Syndrome | RPS19, RPL5, RPS26, RPL11, RPL35a, RPS7, RPL8, RPL35, RPS10, RPS24, RPS17, RPL15, RPS20, RPS28, RPS29, RPS15a, RPS27, RPL9, RPL17, RPL18, RPL26, RPL27, RPL31. TSR2, GATA1, HEATR3 (non-RP genes) [26] | Colorectal cancer, osteosarcoma; liver, breast, lung, thyroid, kidney, testicular, uterine, cervical, vaginal, gastroesophageal, esophageal, gastric cancer; melanoma, squamous cell carcinoma, basal cell carcinoma, rhabdomyosarcoma, soft-tissue sarcoma [16,17,27,28,29]. |
| Shwachman–Diamond Syndrome | SBDS, EFL1, DNAJC21, SRP54 [30] | Ovarian, breast, pancreatic cancer; dermatofibrosarcoma, esophageal squamous cell carcinoma, peritoneal carcinoma [17,31,32,33,34]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagliano, S.; Potenza, M.; La Vecchia, M.; Ellis, S.R.; Dianzani, I.; Aspesi, A. Beyond Hematologic Malignancies: Colorectal Cancer as a Solid Tumor Manifestation of Inherited Bone Marrow Failure Syndromes. Int. J. Mol. Sci. 2025, 26, 10105. https://doi.org/10.3390/ijms262010105
Cagliano S, Potenza M, La Vecchia M, Ellis SR, Dianzani I, Aspesi A. Beyond Hematologic Malignancies: Colorectal Cancer as a Solid Tumor Manifestation of Inherited Bone Marrow Failure Syndromes. International Journal of Molecular Sciences. 2025; 26(20):10105. https://doi.org/10.3390/ijms262010105
Chicago/Turabian StyleCagliano, Sara, Marta Potenza, Marta La Vecchia, Steven R. Ellis, Irma Dianzani, and Anna Aspesi. 2025. "Beyond Hematologic Malignancies: Colorectal Cancer as a Solid Tumor Manifestation of Inherited Bone Marrow Failure Syndromes" International Journal of Molecular Sciences 26, no. 20: 10105. https://doi.org/10.3390/ijms262010105
APA StyleCagliano, S., Potenza, M., La Vecchia, M., Ellis, S. R., Dianzani, I., & Aspesi, A. (2025). Beyond Hematologic Malignancies: Colorectal Cancer as a Solid Tumor Manifestation of Inherited Bone Marrow Failure Syndromes. International Journal of Molecular Sciences, 26(20), 10105. https://doi.org/10.3390/ijms262010105





