G4 Oligonucleotide-Based Chaperones of Heterogeneous Nuclear Ribonucleoprotein A1
Abstract
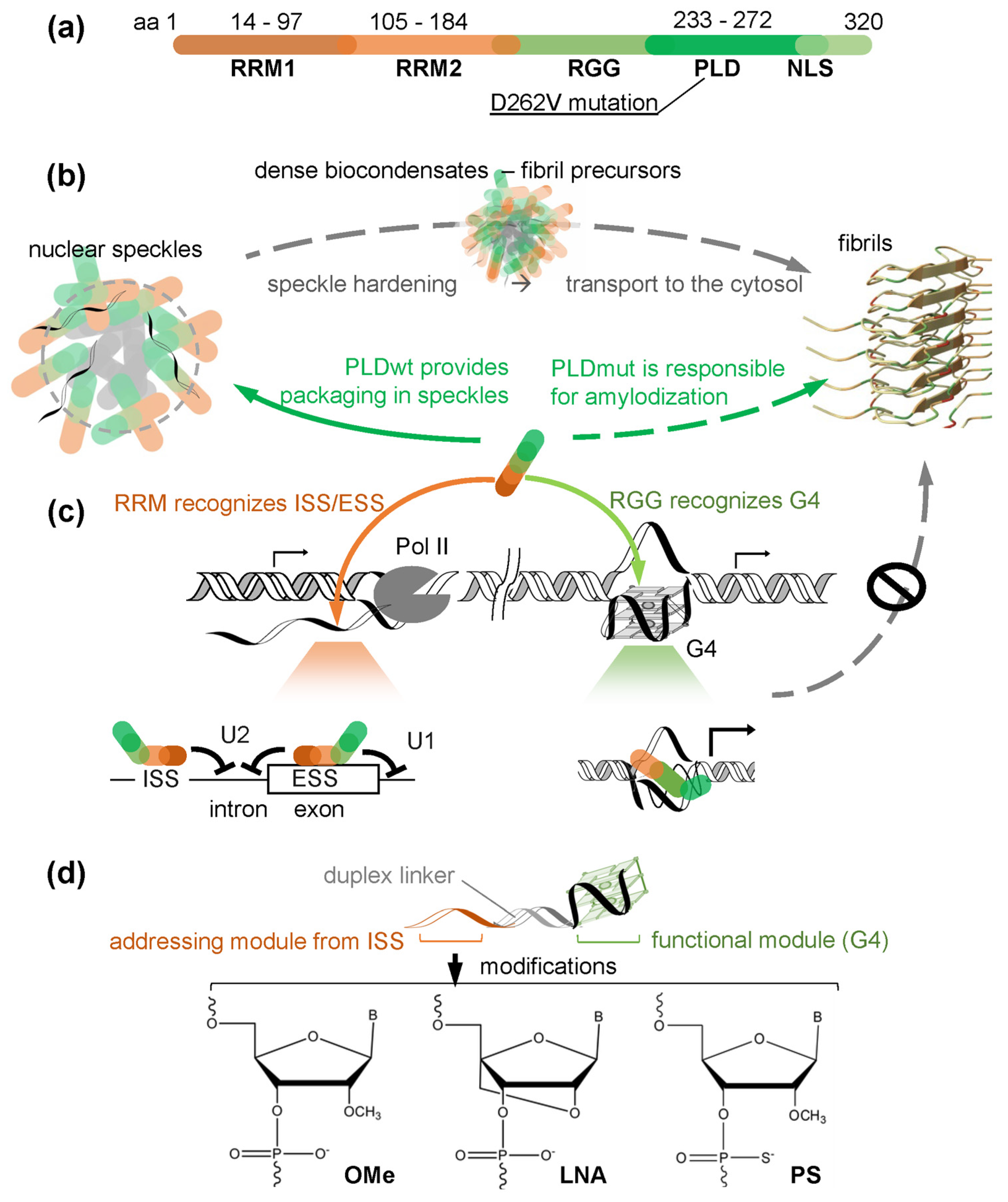
1. Introduction
2. Results
2.1. Chaperone Design
2.2. Analysis of Chaperon Secondary Structures and Affinity to hnRNP A1
- Oligonucleotides chap-LNA and chap-OMe, which contain RNA-mimicking backbone modifications in the addressing module, retain G4 folding in the functional module and exhibit the same affinity for the target protein as chap-RNA.
- Oligonucleotides chap-tLNA and chap-tOMe, which contain RNA-mimicking backbone modifications throughout the chain, have reduced affinity for the target protein, likely due to loss of the G4 structure and changes in its geometry, respectively.
- Oligonucleotides chap-PS, chap-tPS, cntr-noISS have low affinity for the target protein, likely due to intramolecular folding.
2.3. Analysis of Chaperone Activity
- Chap-LNA and chap-OMe, the chaperones with the highest affinity for hnRNP A1, efficiently inhibited condensate formation and amyloidization according to fluorescence microscopy and ThT assays.
- PS-modified chaperones inhibited amyloidization, according to ThT assays, but were inferior to other modified chaperones in terms of their effects on the condensates according to fluorescence microscopy assays.
- AFM assays confirmed the inhibitory effect of the leading chaperone, chap-LNA, on amyloidization.
3. Discussion
4. Materials and Methods
4.1. Oligonucleotides
4.2. Proteins and Peptides
4.3. Absorption and Circular Dichroism (CD) Spectroscopy
4.4. Fluorimetry
4.5. Microscale Thermophoresis (MST)
4.6. Fluorescence Microscopy
4.7. Atomic Force Microscopy (AFM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| hnRNP | Heterogeneous nuclear ribonucleoprotein |
| RRM | RNA-recognizing motif |
| EPR | Endoplasmic reticulum |
| LCR | Low-complexity region |
| PLD | Prion-like domain |
| ISS | Intronic splicing silencer |
| CD | Circular dichroism |
| MST | Microscale thermophoresis |
| AFM | Atomic force microscopy |
| ThT | Thioflavin T |
| PEG | Polyethylene glycol |
References
- Hartl, F.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Parenti, G.; Andria, G.; Valenzano, K.J. Pharmacological Chaperone Therapy: Preclinical Development, Clinical Translation, and Prospects for the Treatment of Lysosomal Storage Disorders. Mol. Ther. 2015, 23, 1138–1148. [Google Scholar] [CrossRef]
- Suzuki, Y. Chaperone therapy for molecular pathology in lysosomal diseases. Brain Dev. 2021, 43, 45–54. [Google Scholar] [CrossRef]
- Suzuki, Y. Chaperone therapy: Stabilization and enhancement of endogenous and exogenous lysosomal enzymes. Brain Dev. 2025, 47, 104298. [Google Scholar] [CrossRef]
- Bayer, T.A. Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur. Neuropsychopharmacol. 2015, 25, 713–724. [Google Scholar] [CrossRef]
- Wankhede, N.L.; Kale, M.B.; Upaganlawar, A.B.; Taksande, B.G.; Umekar, M.J.; Behl, T.; Abdellatif, A.A.H.; Bhaskaran, P.M.; Dachani, S.R.; Sehgal, A.; et al. Involvement of molecular chaperone in protein-misfolding brain diseases. Biomed. Pharmacother. 2022, 147, 112647. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sahu, M.; Srivastava, D.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Post-translational modifications: Regulators of neurodegenerative proteinopathies. Ageing Res. Rev. 2021, 68, 101336. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef]
- Macario, A.J.; Conway de Macario, E. Chaperonopathies by defect, excess, or mistake. Ann. N. Y. Acad. Sci. 2007, 1113, 178–191. [Google Scholar] [CrossRef]
- Vendruscolo, M.; Fuxreiter, M. Protein condensation diseases: Therapeutic opportunities. Nat. Commun. 2022, 13, 5550. [Google Scholar] [CrossRef] [PubMed]
- Fonte, V.; Kipp, D.R.; Yerg, J.; Merin, D.; Forrestal, M.; Wagner, E.; Roberts, C.M.; Link, C.D. Suppression of in Vivo β-Amyloid Peptide Toxicity by Overexpression of the HSP-16.2 Small Chaperone Protein. J. Biol. Chem. 2008, 283, 784–791. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev. Mol. Med. 2007, 9, 1–18. [Google Scholar] [CrossRef]
- Spagnolli, G.; Massignan, T.; Astolfi, A.; Biggi, S.; Rigoli, M.; Brunelli, P.; Libergoli, M.; Ianeselli, A.; Orioli, S.; Boldrini, A.; et al. Pharmacological inactivation of the prion protein by targeting a folding intermediate. Commun. Biol. 2021, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Henderson, B.J.; Lester, H.A.; Richards, C.I. Pharmacological chaperoning of nAChRs: A therapeutic target for Parkinson’s disease. Pharmacol. Res. 2014, 83, 20–29. [Google Scholar] [CrossRef]
- Han, T.-U.; Sam, R.; Sidransky, E. Small Molecule Chaperones for the Treatment of Gaucher Disease and GBA1-Associated Parkinson Disease. Front. Cell Dev. Biol. 2020, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Kopytova, A.E.; Rychkov, G.N.; Cheblokov, A.A.; Grigor’eva, E.V.; Nikolaev, M.A.; Yarkova, E.S.; Sorogina, D.A.; Ibatullin, F.M.; Baydakova, G.V.; Izyumchenko, A.D.; et al. Potential Binding Sites of Pharmacological Chaperone NCGC00241607 on Mutant β-Glucocerebrosidase and Its Efficacy on Patient-Derived Cell Cultures in Gaucher and Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 9105. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-G.; Chiu, J.; Ramanjulu, M.; Blass, B.E.; Praticò, D. A pharmacological chaperone improves memory by reducing Aβ and tau neuropathology in a mouse model with plaques and tangles. Mol. Neurodegener. 2020, 15, 1. [Google Scholar] [CrossRef]
- Li, J.-G.; Blass, B.E.; Praticò, D. Beneficial Effect of a Small Pharmacologic Chaperone on the Established Alzheimer’s Disease Phenotype. J. Alzheimer’s Dis. 2023, 91, 463–469. [Google Scholar] [CrossRef]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef]
- Alberti, S.; Halfmann, R.; King, O.; Kapila, A.; Lindquist, S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009, 137, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Farag, M.; Borcherds, W.M.; Peran, I.; Martin, E.W.; Pappu, R.V.; Mittag, T. Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains. Nat. Chem. 2022, 14, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pardo, J.; Ventura, S. Cryo-EM structures of functional and pathological amyloid ribonucleoprotein assemblies. Trends Biochem. Sci. 2024, 49, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 2018, 174, 688–699.e16. [Google Scholar] [CrossRef]
- Willbold, D.; Strodel, B.; Schröder, G.F.; Hoyer, W.; Heise, H. Amyloid-type Protein Aggregation and Prion-like Properties of Amyloids. Chem. Rev. 2021, 121, 8285–8307. [Google Scholar] [CrossRef]
- Zacco, E.; Graña-Montes, R.; Martin, S.R.; de Groot, N.S.; Alfano, C.; Tartaglia, G.G.; Pastore, A. RNA as a key factor in driving or preventing self-assembly of the TAR DNA-binding protein 43. J. Mol. Biol. 2019, 431, 1671–1688. [Google Scholar] [CrossRef]
- Hamad, N.; Yoneda, R.; So, M.; Kurokawa, R.; Nagata, T.; Katahira, M. Non-coding RNA suppresses FUS aggregation caused by mechanistic shear stress on pipetting in a sequence-dependent manner. Sci. Rep. 2021, 11, 9523. [Google Scholar] [CrossRef]
- Chong, P.A.; Vernon, R.M.; Forman-Kay, J.D. RGG/RG Motif Regions in RNA Binding and Phase Separation. J. Mol. Biol. 2018, 430, 4650–4665. [Google Scholar] [CrossRef]
- Brázda, V.; Červeň, J.; Bartas, M.; Mikysková, N.; Coufal, J.; Pečinka, P. The Amino Acid Composition of Quadruplex Binding Proteins Reveals a Shared Motif and Predicts New Potential Quadruplex Interactors. Molecules 2018, 23, 2341. [Google Scholar] [CrossRef]
- Adlhart, M.; Hoffmann, D.; Polyansky, A.A.; Žagrović, B. Coding relationship links RNA G-quadruplexes and protein RGG motifs in RNA-binding protein autoregulation. Proc. Natl. Acad. Sci. USA 2025, 122, e2413721122. [Google Scholar] [CrossRef]
- Louka, A.; Zacco, E.; Temussi, P.A.; Tartaglia, G.G.; Pastore, A. RNA as the stone guest of protein aggregation. Nucleic Acids Res. 2020, 48, 11880–11889. [Google Scholar] [CrossRef]
- Sanchez de Groot, N.; Armaos, A.; Graña-Montes, R.; Alriquet, M.; Calloni, G.; Vabulas, R.M.; Tartaglia, G.G. RNA structure drives interaction with proteins. Nat. Commun. 2019, 10, 3246. [Google Scholar] [CrossRef]
- Rengifo-Gonzalez, J.C.; El Hage, K.; Clément, M.-J.; Steiner, E.; Joshi, V.; Craveur, P.; Durand, D.; Pastré, D.; Bouhss, A. The cooperative binding of TDP-43 to GU-rich RNA repeats antagonizes TDP-43 aggregation. eLife 2021, 10, e67605. [Google Scholar] [CrossRef]
- Begeman, A.; Son, A.; Litberg, T.J.; Wroblewski, T.H.; Gehring, T.; Huizar Cabral, V.; Bourne, J.; Xuan, Z.; Horowitz, S. G-Quadruplexes act as sequence-dependent protein chaperones. EMBO Rep. 2020, 21, e49735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Das, T.; Chao, T.F.; Trinh, V.; Carmen-Orozco, R.P.; Ling, J.P.; Kalab, P.; Hayes, L.R. Multivalent GU-rich oligonucleotides sequester TDP-43 in the nucleus by inducing high molecular weight RNP complexes. iScience 2024, 27, 110109. [Google Scholar] [CrossRef]
- Schwalbe, H.; Burkhart, I. G-quadruplexes and their unexpected ability to fold proteins. Proc. Natl. Acad. Sci. USA 2025, 122, e2501246122. [Google Scholar] [CrossRef] [PubMed]
- Vedekhina, T.; Svetlova, J.; Pavlova, I.; Barinov, N.; Alieva, S.; Malakhova, E.; Rubtsov, P.; Shtork, A.; Klinov, D.; Varizhuk, A. Cross-Effects in Folding and Phase Transitions of hnRNP A1 and C9Orf72 RNA G4 In Vitro. Molecules 2024, 29, 4369. [Google Scholar] [CrossRef] [PubMed]
- Linsenmeier, M.; Faltova, L.; Morelli, C.; Capasso Palmiero, U.; Seiffert, C.; Küffner, A.M.; Pinotsi, D.; Zhou, J.; Mezzenga, R.; Arosio, P. The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils. Nat. Chem. 2023, 15, 1340–1349. [Google Scholar] [CrossRef]
- Mukherjee, S.; Poudyal, M.; Dave, K.; Kadu, P.; Maji, S.K. Protein misfolding and amyloid nucleation through liquid–liquid phase separation. Chem. Soc. Rev. 2024, 53, 4976–5013. [Google Scholar] [CrossRef]
- Purice, M.D.; Taylor, J.P. Linking hnRNP Function to ALS and FTD Pathology. Front. Neurosci. 2018, 12, 326. [Google Scholar] [CrossRef]
- Bampton, A.; Gittings, L.M.; Fratta, P.; Lashley, T.; Gatt, A. The role of hnRNPs in frontotemporal dementia and amyotrophic lateral sclerosis. Acta Neuropathol. 2020, 140, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé-Nafría, A.; García-Pardo, J.; Ventura, S. Mutations in human prion-like domains: Pathogenic but not always amyloidogenic. Prion 2024, 18, 28–39. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.C.; Wang, Y.-D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef]
- Jean-Philippe, J.; Paz, S.; Caputi, M. hnRNP A1: The Swiss Army Knife of Gene Expression. Int. J. Mol. Sci. 2013, 14, 18999–19024. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Katahira, M.; Tsuchiya, N.; Enokizono, Y.; Sugimura, T.; Nagao, M.; Nakagama, H. Unfolding of quadruplex structure in the G-rich strand of the minisatellite repeat by the binding protein UP1. Proc. Natl. Acad. Sci. USA 2002, 99, 12685–12690. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, J.; Lin, Y.; Huang, W. HnRNP A1 in RNA metabolism regulation and as a potential therapeutic target. Front. Pharmacol. 2022, 13, 986409. [Google Scholar] [CrossRef]
- Cogoi, S.; Rapozzi, V.; Cauci, S.; Xodo, L.E. Critical role of hnRNP A1 in activating KRAS transcription in pancreatic cancer cells: A molecular mechanism involving G4 DNA. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 1389–1398. [Google Scholar] [CrossRef]
- Nishikawa, T.; Kuwano, Y.; Takahara, Y.; Nishida, K.; Rokutan, K. HnRNPA1 interacts with G-quadruplex in the TRA2B promoter and stimulates its transcription in human colon cancer cells. Sci. Rep. 2019, 9, 10276. [Google Scholar] [CrossRef]
- Todd, A.K. Highly prevalent putative quadruplex sequence motifs in human DNA. Nucleic Acids Res. 2005, 33, 2901–2907. [Google Scholar] [CrossRef]
- Mayeda, A.; Krainer, A.R. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell 1992, 68, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Beusch, I.; Barraud, P.; Moursy, A.; Cléry, A.; Allain, F.H.-T. Tandem hnRNP A1 RNA recognition motifs act in concert to repress the splicing of survival motor neuron exon 7. eLife 2017, 6, e25736. [Google Scholar] [CrossRef]
- Vasilyev, N.; Polonskaia, A.; Darnell, J.C.; Darnell, R.B.; Patel, D.J.; Serganov, A. Crystal structure reveals specific recognition of a G-quadruplex RNA by a β-turn in the RGG motif of FMRP. Proc. Natl. Acad. Sci. USA 2015, 112, E5391–E5400. [Google Scholar] [CrossRef]
- Ding, J.; Hayashi, M.K.; Zhang, Y.; Manche, L.; Krainer, A.R.; Xu, R.M. Crystal structure of the two-RRM domain of hnRNP A1 (UP1) complexed with single-stranded telomeric DNA. Genes Dev. 1999, 13, 1102–1115. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, C.-C.; Tan, W. Nuclease Resistance of Telomere-like Oligonucleotides Monitored in Live Cells by Fluorescence Anisotropy Imaging. Anal. Chem. 2006, 78, 1478–1484. [Google Scholar] [CrossRef]
- Roxo, C.; Kotkowiak, W.; Pasternak, A. G4 Matters—The Influence of G-Quadruplex Structural Elements on the Antiproliferative Properties of G-Rich Oligonucleotides. Int. J. Mol. Sci. 2021, 22, 4941. [Google Scholar] [CrossRef]
- Kawai, G.; Yamamoto, Y.; Kamimura, T.; Masegi, T.; Sekine, M.; Hata, T.; Iimori, T.; Watanabe, T.; Miyazawa, T.; Yokoyama, S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2’-hydroxyl group. Biochemistry 1992, 31, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Obika, S.; Nanbu, D.; Hari, Y.; Andoh, J.; Morio, K.; Doi, T.; Imanishi, T. Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett. 1998, 39, 5401–5404. [Google Scholar] [CrossRef]
- Swayze, E.E.; Siwkowski, A.M.; Wancewicz, E.V.; Migawa, M.T.; Wyrzykiewicz, T.K.; Hung, G.; Monia, B.P.; Bennett, C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007, 35, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, A.E.; Plavec, J.; Persil, Ö.; Hud, N.V. Metal Ion Interactions with G-Quadruplex Structures. In Nucleic Acid–Metal Ion Interactions; The Royal Society of Chemistry: London, UK, 2008; pp. 118–153. [Google Scholar]
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef]
- Li, Z.; Lech, C.J.; Phan, A.T. Sugar-modified G-quadruplexes: Effects of LNA-, 2′F-RNA– and 2′F-ANA-guanosine chemistries on G-quadruplex structure and stability. Nucleic Acids Res. 2014, 42, 4068–4079. [Google Scholar] [CrossRef]
- Mergny, J.-L. Thermal difference spectra: A specific signature for nucleic acid structures. Nucleic Acids Res. 2005, 33, e138. [Google Scholar] [CrossRef]
- Ooga, M.; Sahayasheela, V.J.; Hirose, Y.; Sasaki, D.; Hashiya, K.; Bando, T.; Sugiyama, H. A dual DNA-binding conjugate that selectively recognizes G-quadruplex structures. Chem. Commun. 2024, 60, 8744–8747. [Google Scholar] [CrossRef]
- Rachwal, P.A.; Fox, K.R. Quadruplex melting. Methods 2007, 43, 291–301. [Google Scholar] [CrossRef]
- Varizhuk, A.M.; Protopopova, A.D.; Tsvetkov, V.B.; Barinov, N.A.; Podgorsky, V.V.; Tankevich, M.V.; Vlasenok, M.A.; Severov, V.V.; Smirnov, I.P.; Dubrovin, E.V.; et al. Polymorphism of G4 associates: From stacks to wires via interlocks. Nucleic Acids Res. 2018, 46, 8978–8992. [Google Scholar] [CrossRef]
- Renaud de la Faverie, A.; Guédin, A.; Bedrat, A.; Yatsunyk, L.A.; Mergny, J.-L. Thioflavin T as a fluorescence light-up probe for G4 formation. Nucleic Acids Res. 2014, 42, e65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheludev, I.N.; Hagey, R.J.; Haslecker, R.; Hou, Y.J.; Kretsch, R.; Pintilie, G.D.; Rangan, R.; Kladwang, W.; Li, S.; et al. Cryo-EM and antisense targeting of the 28-kDa frameshift stimulation element from the SARS-CoV-2 RNA genome. Nat. Struct. Mol. Biol. 2021, 28, 747–754. [Google Scholar] [PubMed]
- Knizhnik, E.; Chumakov, S.; Svetlova, J.; Pavlova, I.; Khodarovich, Y.; Brylev, V.; Severov, V.; Alieva, R.; Kozlovskaya, L.; Andreev, D.; et al. Unwinding the SARS-CoV-2 Ribosomal Frameshifting Pseudoknot with LNA and G-Clamp-Modified Phosphorothioate Oligonucleotides Inhibits Viral Replication. Biomolecules 2023, 13, 1660. [Google Scholar] [CrossRef]
- Galganski, L.; Urbanek, M.O.; Krzyzosiak, W.J. Nuclear Speckles: Molecular Organization, Biological Function and Role in Disease. Nucleic Acids Res. 2017, 45, 10350–10368. [Google Scholar] [CrossRef] [PubMed]
- Shtork, A.S.; Pavlova, I.I.; Svetlova, J.I.; Iudin, M.S.; Grafskaia, E.N.; Manuvera, V.A.; Alieva, S.E.; Varizhuk, A.M.; Lazarev, V.N.; Vedekhina, T.S. Quantitative Analysis of Biomolecular Condensates on a Modified Support. Russ. J. Bioorg. Chem. 2025, 51, 273–284. [Google Scholar] [CrossRef]
- Law, J.O.; Jones, C.M.; Stevenson, T.; Williamson, T.A.; Turner, M.S.; Kusumaatmaja, H.; Grellscheid, S.N. A bending rigidity parameter for stress granule condensates. Sci. Adv. 2023, 9, eadg0432. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- Xu, S.; Li, Q.; Xiang, J.; Yang, Q.; Sun, H.; Guan, A.; Wang, L.; Liu, Y.; Yu, L.; Shi, Y.; et al. Thioflavin T as an efficient fluorescence sensor for selective recognition of RNA G-quadruplexes. Sci. Rep. 2016, 6, 24793. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y. HnRNPA1 Specifically Recognizes the Base of Nucleotide at the Loop of RNA G-Quadruplex. Molecules 2018, 23, 237. [Google Scholar] [CrossRef]
- Litberg, T.J.; Sannapureddi, R.K.R.; Huang, Z.; Son, A.; Sathyamoorthy, B.; Horowitz, S. Why are G-quadruplexes good at preventing protein aggregation? RNA Biol. 2023, 20, 495–509, Erratum in RNA Biol. 2024, 21, 1. [Google Scholar] [CrossRef]
- Pavlova, I.; Iudin, M.; Surdina, A.; Severov, V.; Varizhuk, A. G-Quadruplexes in Nuclear Biomolecular Condensates. Genes 2023, 14, 1076. [Google Scholar] [CrossRef]
- Vidal Ceballos, A.; Geissmann, A.; Favaro, D.C.; Deshpande, P.; Elbaum-Garfinkle, S. RNA guanine content and G-quadruplex structure tune the phase behavior and material properties of biomolecular condensates. Sci. Rep. 2025, 15, 9295. [Google Scholar] [CrossRef]
- Antariksa, N.F.; Di Antonio, M. The Emerging Roles of Multimolecular G-Quadruplexes in Transcriptional Regulation and Chromatin Organization. Acc. Chem. Res. 2024, 57, 3397–3406. [Google Scholar] [CrossRef]
- Raguseo, F.; Wang, Y.; Li, J.; Petrić Howe, M.; Balendra, R.; Huyghebaert, A.; Vadukul, D.M.; Tanase, D.A.; Maher, T.E.; Malouf, L.; et al. The ALS/FTD-related C9orf72 hexanucleotide repeat expansion forms RNA condensates through multimolecular G-quadruplexes. Nat. Commun. 2023, 14, 8272. [Google Scholar] [CrossRef] [PubMed]
- Iacoangeli, A.; Al Khleifat, A.; Jones, A.R.; Sproviero, W.; Shatunov, A.; Opie-Martin, S.; Morrison, K.E.; Shaw, P.J.; Shaw, C.E.; Fogh, I.; et al. C9orf72 intermediate expansions of 24–30 repeats are associated with ALS. Acta Neuropathol. Commun. 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Barnes, R.; Lin, Y.; Jeon, B.; Najafi, S.; Delaney, K.T.; Fredrickson, G.H.; Shea, J.-E.; Hwang, D.S.; Han, S. Dehydration entropy drives liquid-liquid phase separation by molecular crowding. Commun. Chem. 2020, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Klinov, D.; Dwir, B.; Kapon, E.; Borovok, N.; Molotsky, T.; Kotlyar, A. High-resolution atomic force microscopy of duplex and triplex DNA molecules. Nanotechnology 2007, 18, 225102. [Google Scholar] [CrossRef]


| Code | Sequence, 5′→3′ * | Kd_hnRNP A1, μM |
|---|---|---|
| chap-RNA | UCAGUUGCUGCGGGUGGGUGGGUGGGGCAGC | 6 ± 1 |
| chap-DNA | d(TCAGTTGCTGCGGGTGGGTGGGTGGGGCAGC) | 14 ± 3 |
| cntr-KRAS | d(TCAGTTGCTGCAGGGCGGTGTGGGAAGAGGGAAGAGGGGGAGGGCAGC) | 15 ± 3 |
| cntr-noISS | d(GCTGCGGGTGGGTGGGTGGGGCAGC) | >100 |
| chap-PS | d(TCAGTTGCTGC) †GGGTGGGTGGGTGGG(GCAGC) † | 96 ± 7 |
| chap-tPS | d(TCAGTTGCTGCGGGTGGGTGGGTGGGGCAGC) † | 86 ± 8 |
| chap-LNA | (TCAGTTGCTGC) ‡GGGTGGGTGGGTGGG(GCAGC) ‡ | 6 ± 5 |
| chap-tLNA | (TCAGTTGCTGCGGGTGGGTGGGTGGGGCAGC) ‡ | 14 ± 5 |
| chap-Ome | (TCAGTTGCTGC) #GGGTGGGTGGGTGGG(GCAGC) # | 6 ± 4 |
| chap-tOMe | (TCAGTTGCTGCGGGTGGGTGGGTGGGGCAGC) # | 30 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malakhova, E.; Svetlova, J.; Pavlova, I.; Alieva, S.; Severov, V.; Barinov, N.; Klinov, D.; Vedekhina, T.; Varizhuk, A. G4 Oligonucleotide-Based Chaperones of Heterogeneous Nuclear Ribonucleoprotein A1. Int. J. Mol. Sci. 2025, 26, 10104. https://doi.org/10.3390/ijms262010104
Malakhova E, Svetlova J, Pavlova I, Alieva S, Severov V, Barinov N, Klinov D, Vedekhina T, Varizhuk A. G4 Oligonucleotide-Based Chaperones of Heterogeneous Nuclear Ribonucleoprotein A1. International Journal of Molecular Sciences. 2025; 26(20):10104. https://doi.org/10.3390/ijms262010104
Chicago/Turabian StyleMalakhova, Elizaveta, Julia Svetlova, Iuliia Pavlova, Sabina Alieva, Vyacheslav Severov, Nikolay Barinov, Dmitry Klinov, Tatiana Vedekhina, and Anna Varizhuk. 2025. "G4 Oligonucleotide-Based Chaperones of Heterogeneous Nuclear Ribonucleoprotein A1" International Journal of Molecular Sciences 26, no. 20: 10104. https://doi.org/10.3390/ijms262010104
APA StyleMalakhova, E., Svetlova, J., Pavlova, I., Alieva, S., Severov, V., Barinov, N., Klinov, D., Vedekhina, T., & Varizhuk, A. (2025). G4 Oligonucleotide-Based Chaperones of Heterogeneous Nuclear Ribonucleoprotein A1. International Journal of Molecular Sciences, 26(20), 10104. https://doi.org/10.3390/ijms262010104










