In Silico Analysis of MiRNA Regulatory Networks to Identify Potential Biomarkers for the Clinical Course of Viral Infections
Abstract
1. Introduction
2. Results
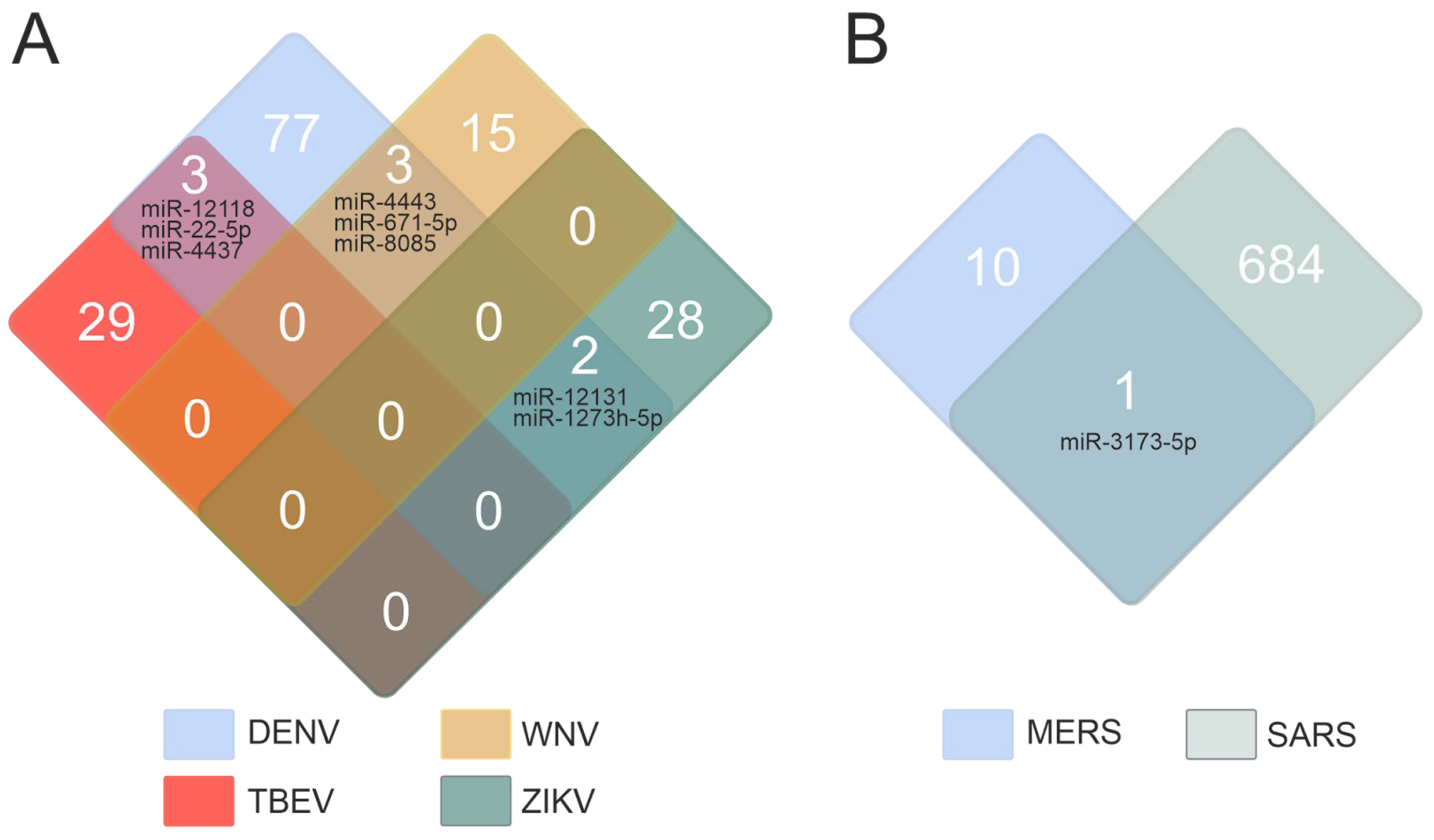
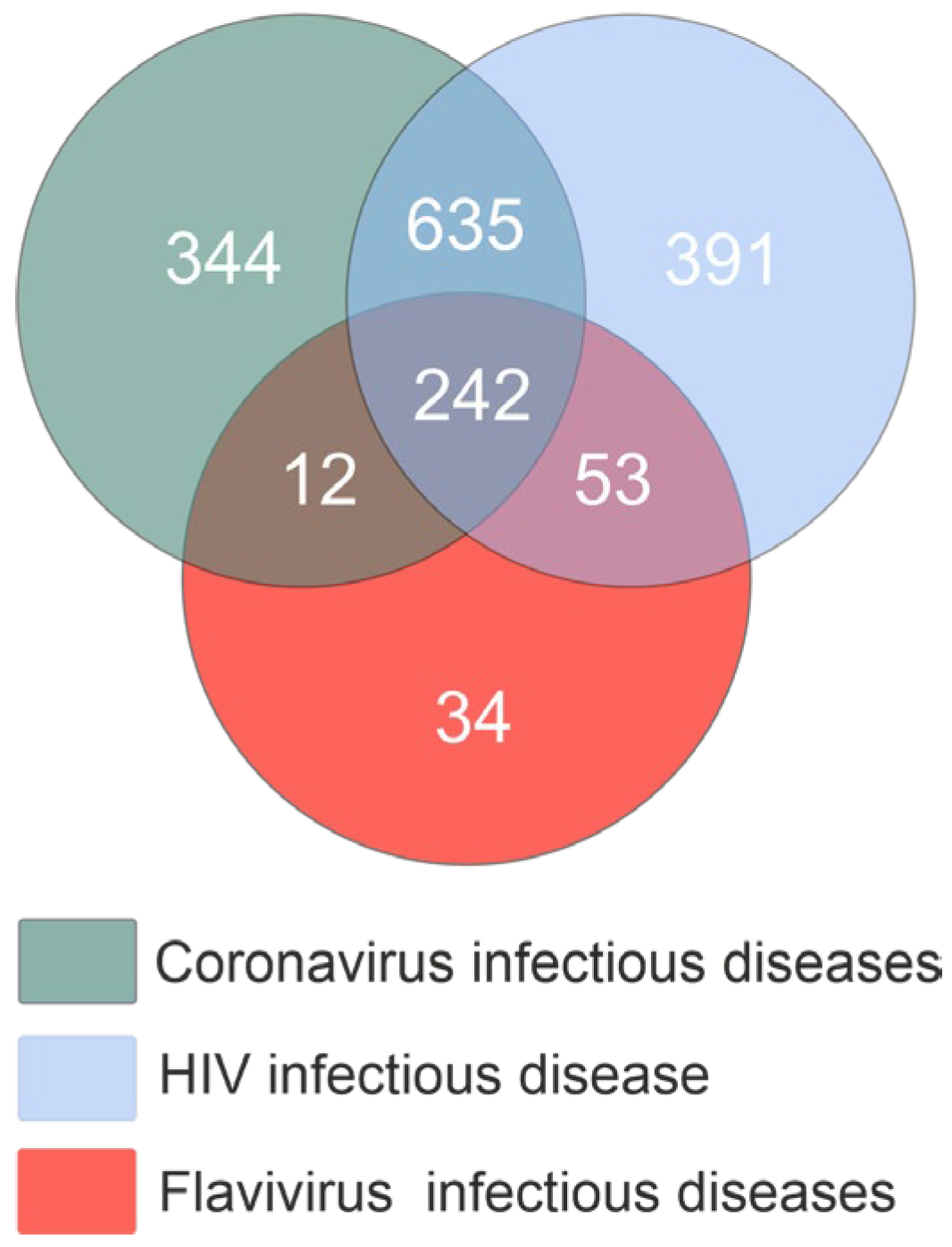
2.1. miRNAs Associated with Viral Infections
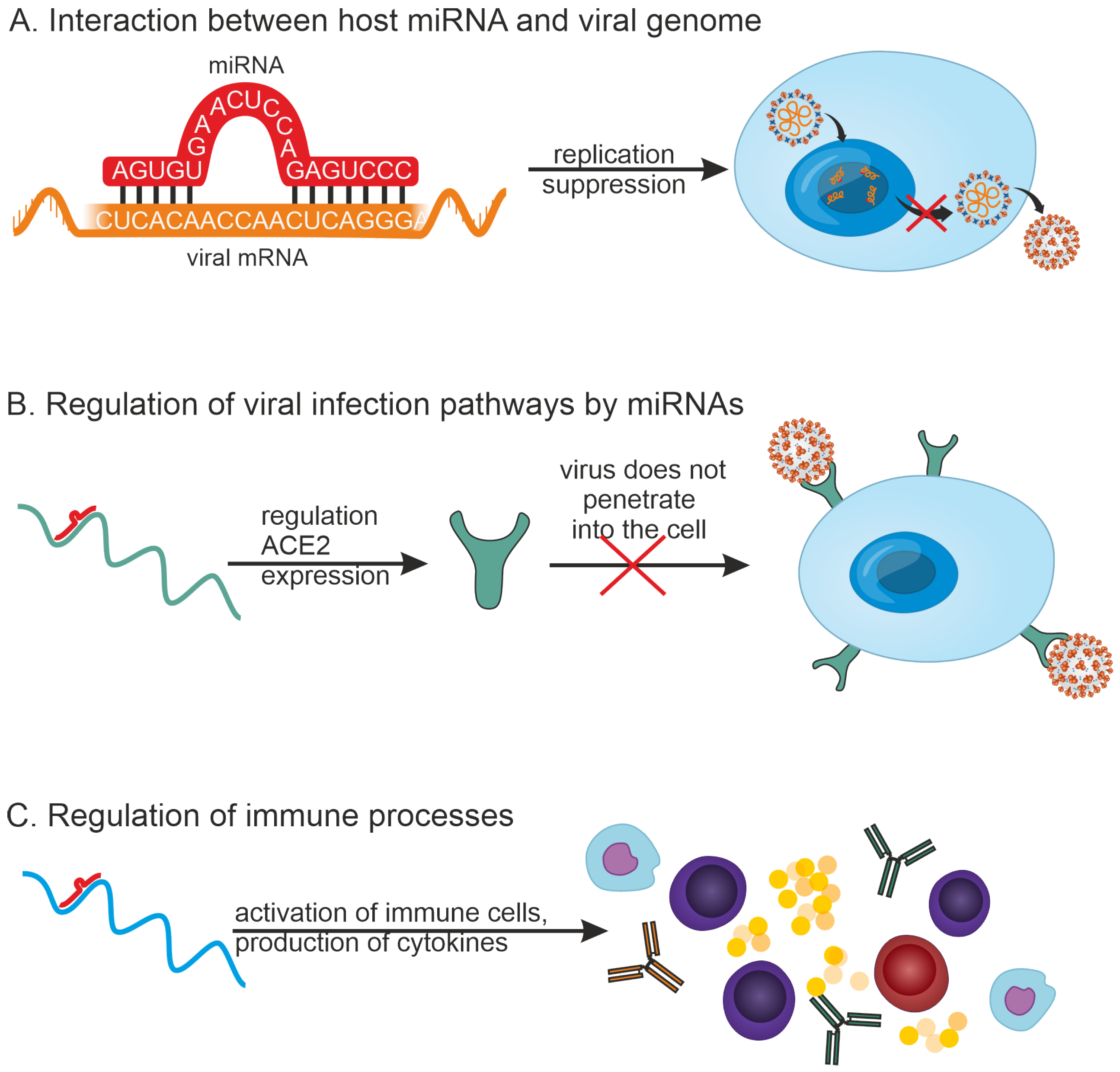
2.2. Characterization of miRNAs in the Context of Different Viral Infections
- (1)
- MiRNAs can inhibit viral replication by directly binding to the viral RNA genome. For example, 873 miRNAs that can target the SARS-CoV-2 genome have been detected by in silico methods [27].
- (2)
- MiRNAs can participate in regulating infection pathways. For example, it has been shown that a number of miRNAs can regulate the expression of ACE2, a cell membrane-associated protein that is required for SARS-CoV-2 virus entry into the host cell. miR-125b directly targets the 3′-UTR of ACE2 mRNA, thereby regulating its expression [28].
- (3)
- MiRNAs can play a role in regulating the innate and adaptive immune systems.
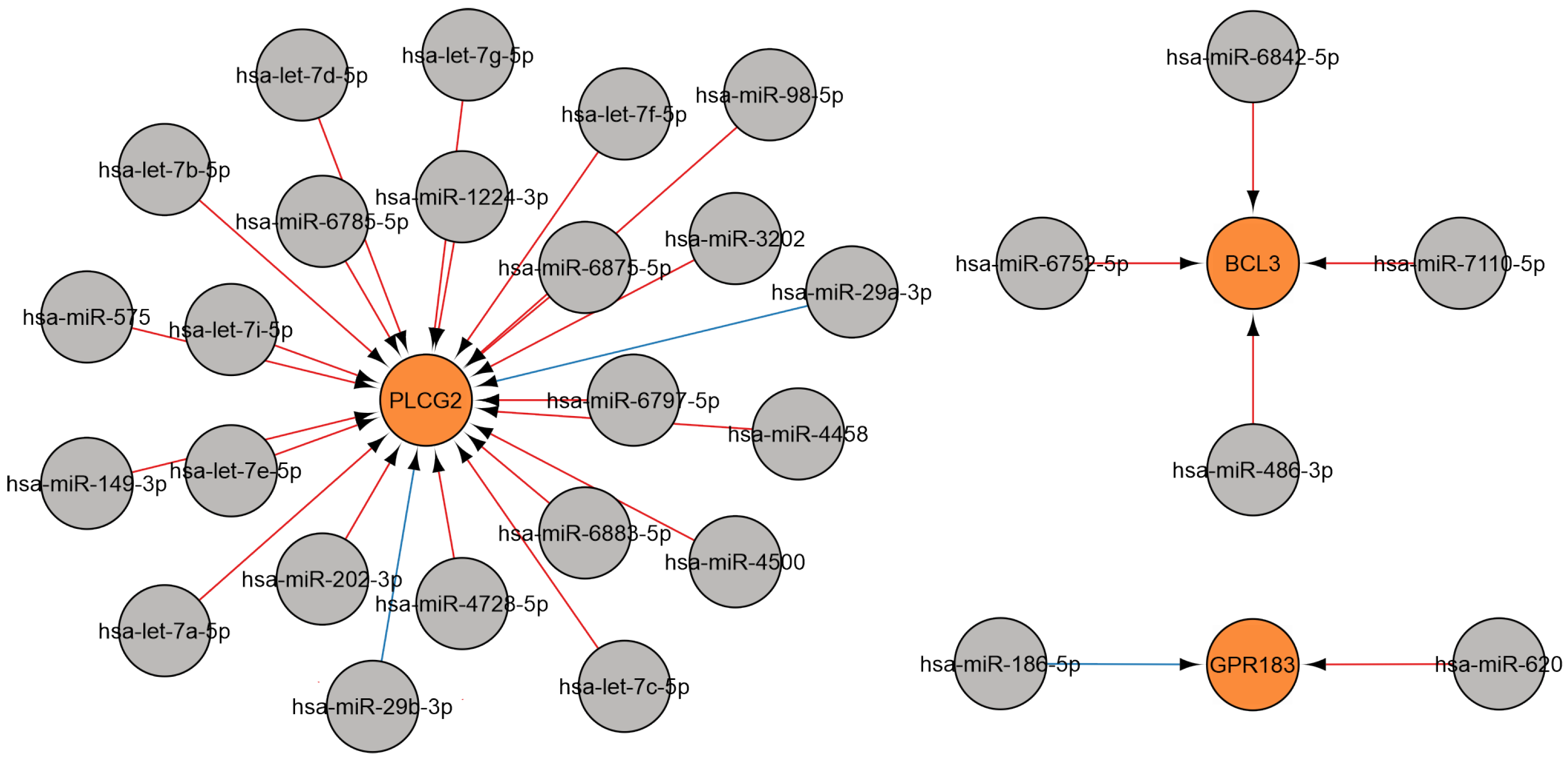
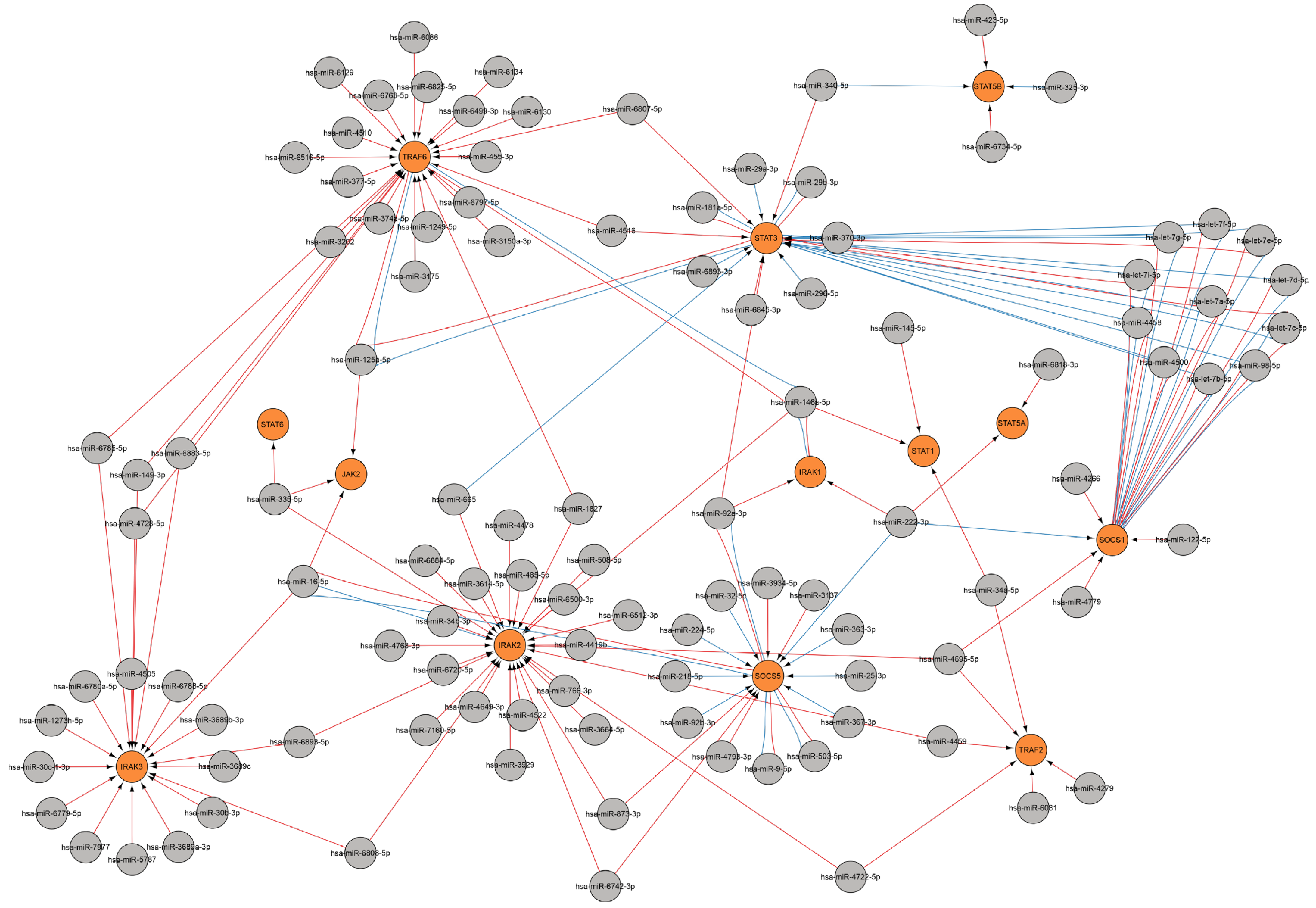
2.3. Analysis of miRNA-Gene Interactions and Gene Ontology
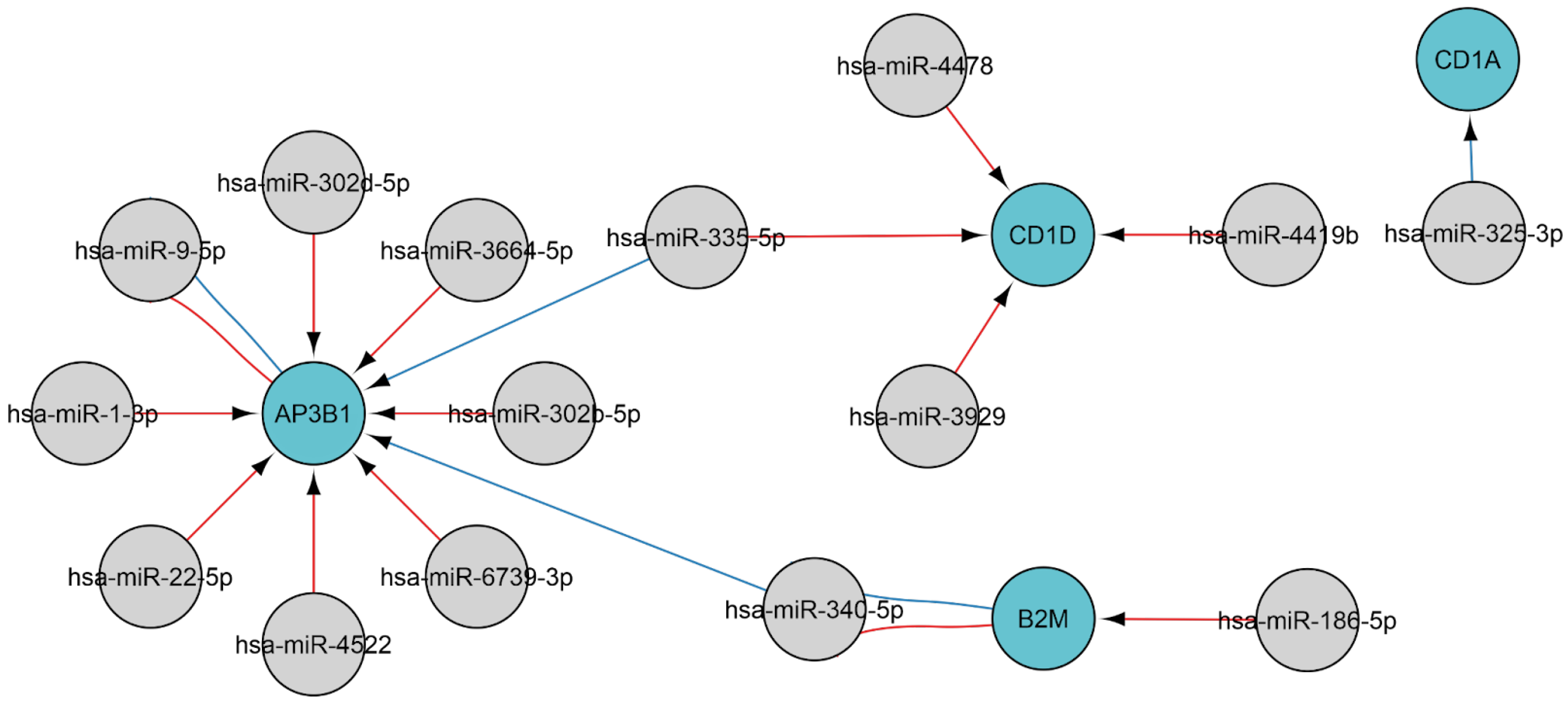
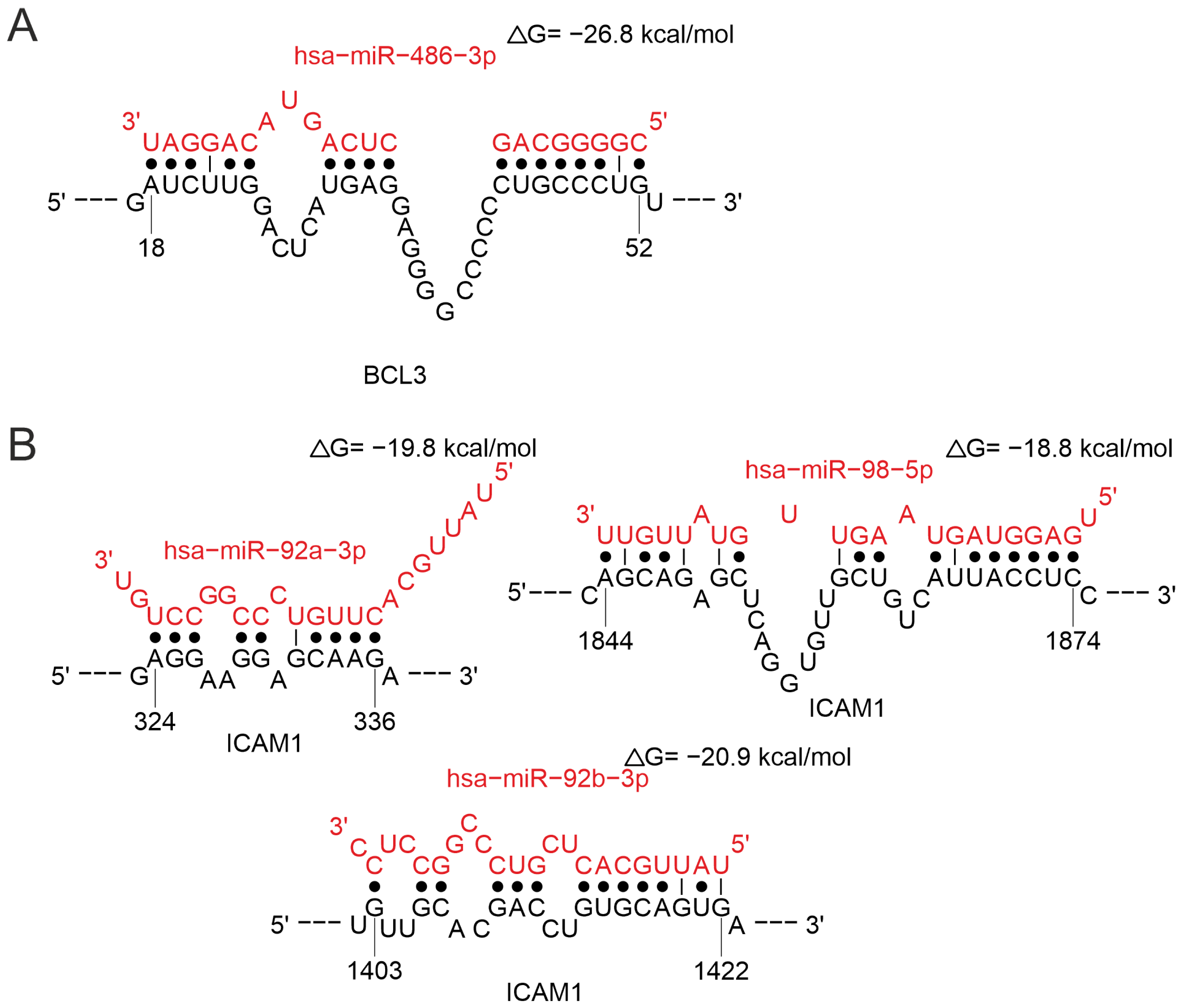
2.3.1. MiRNAs Associated with the B-Cell Response
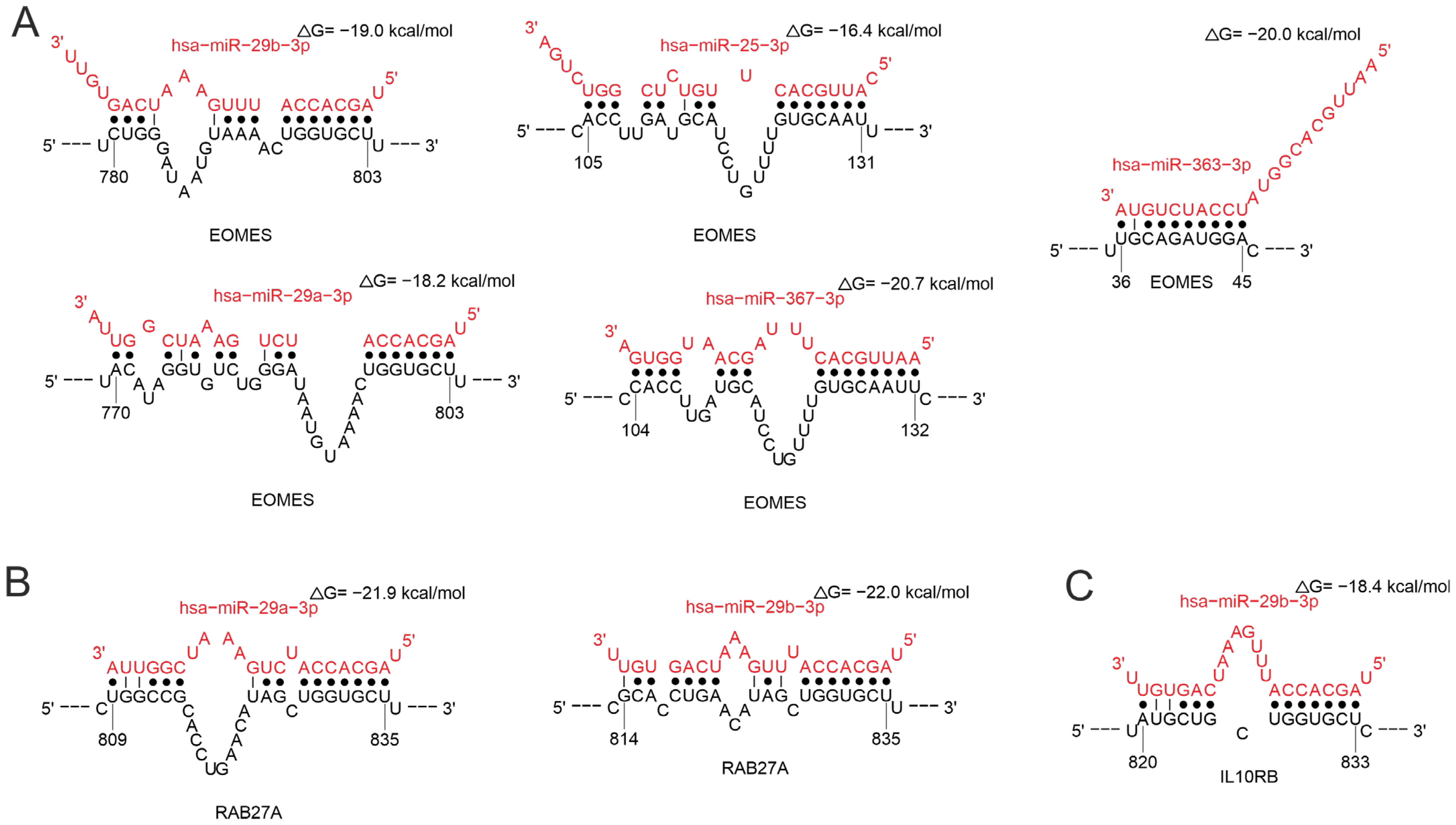
2.3.2. MiRNAs Associated with T-Cell Response
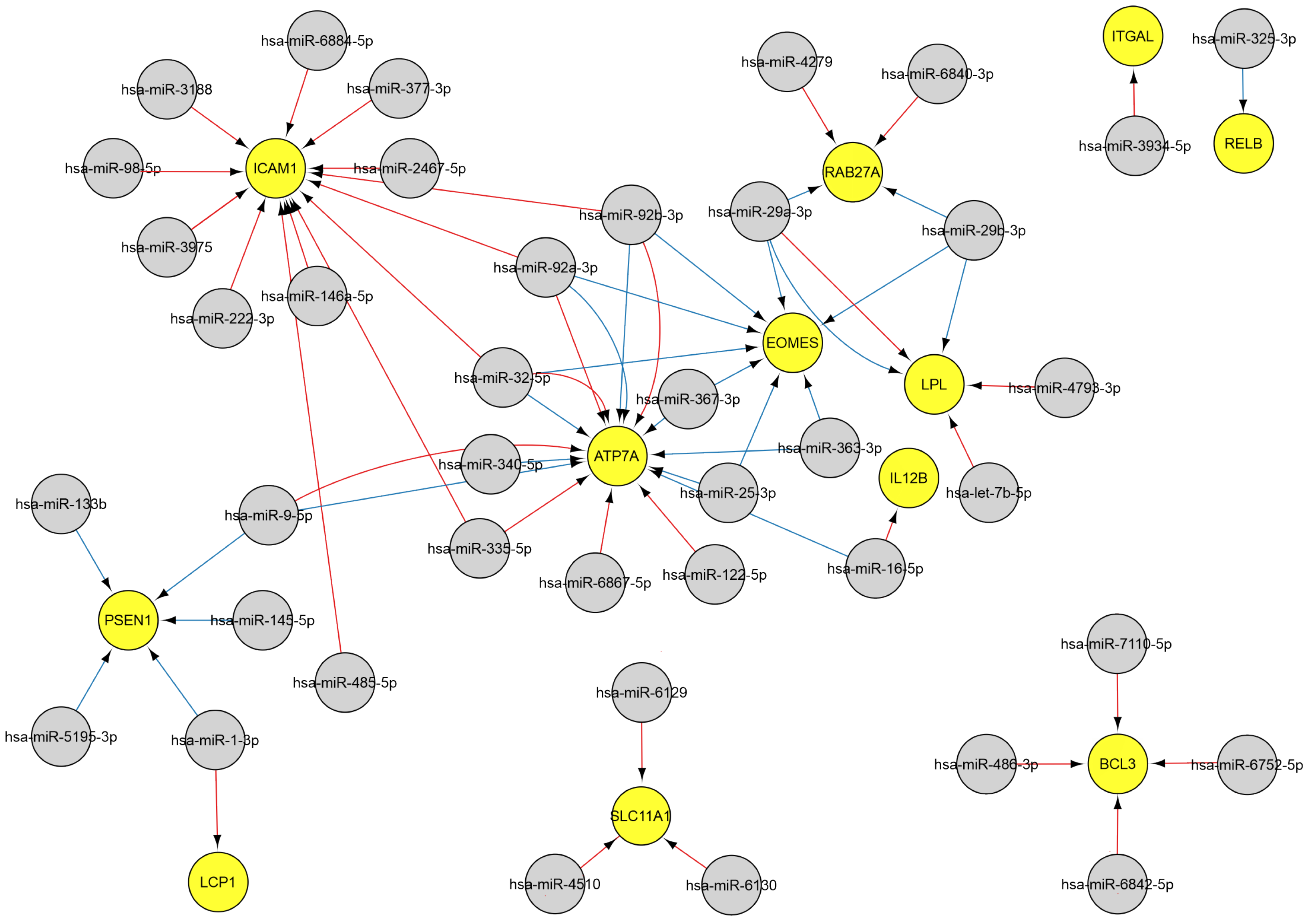
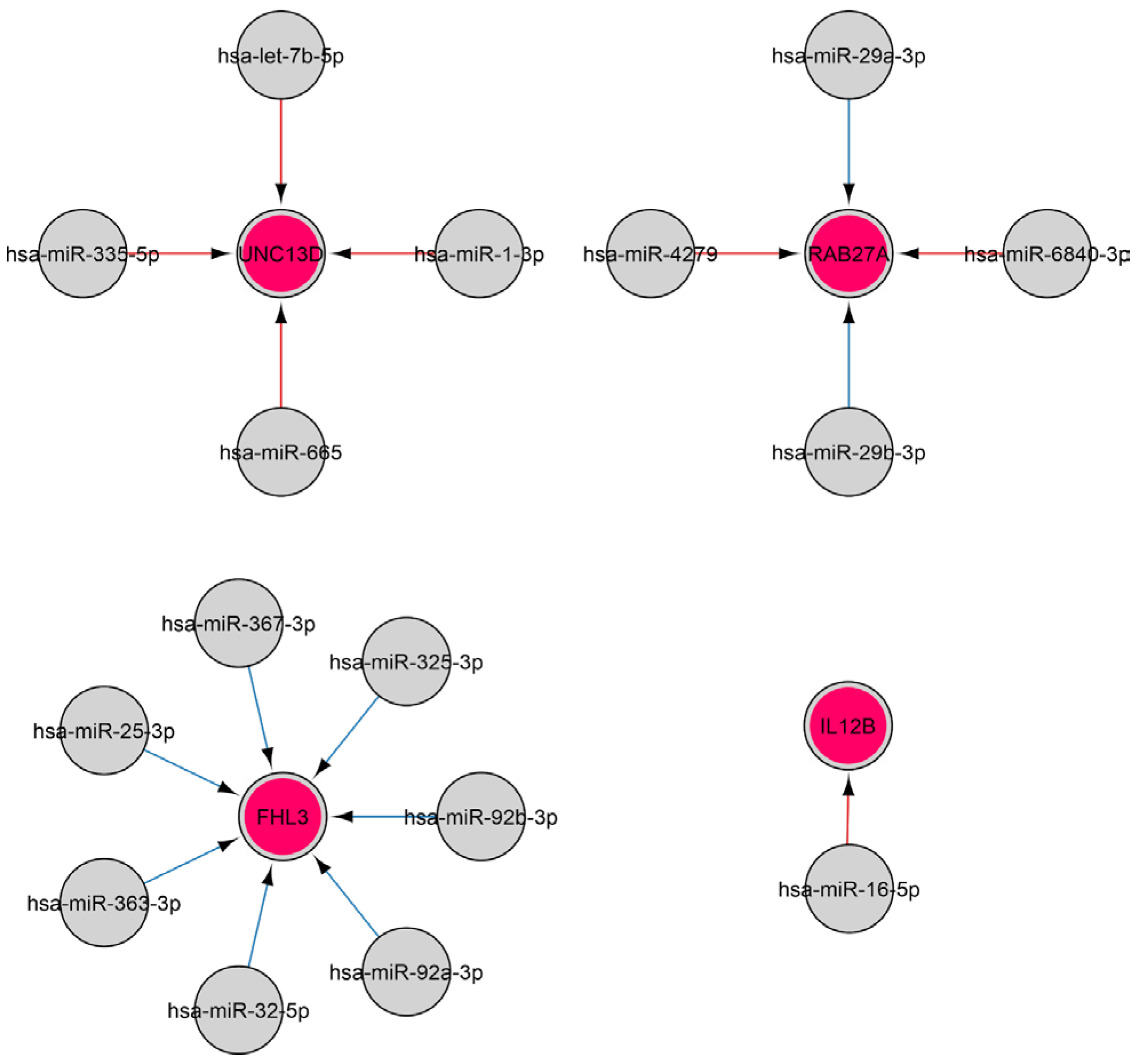
2.3.3. MiRNAs Associated with the Natural Killer Cell Response
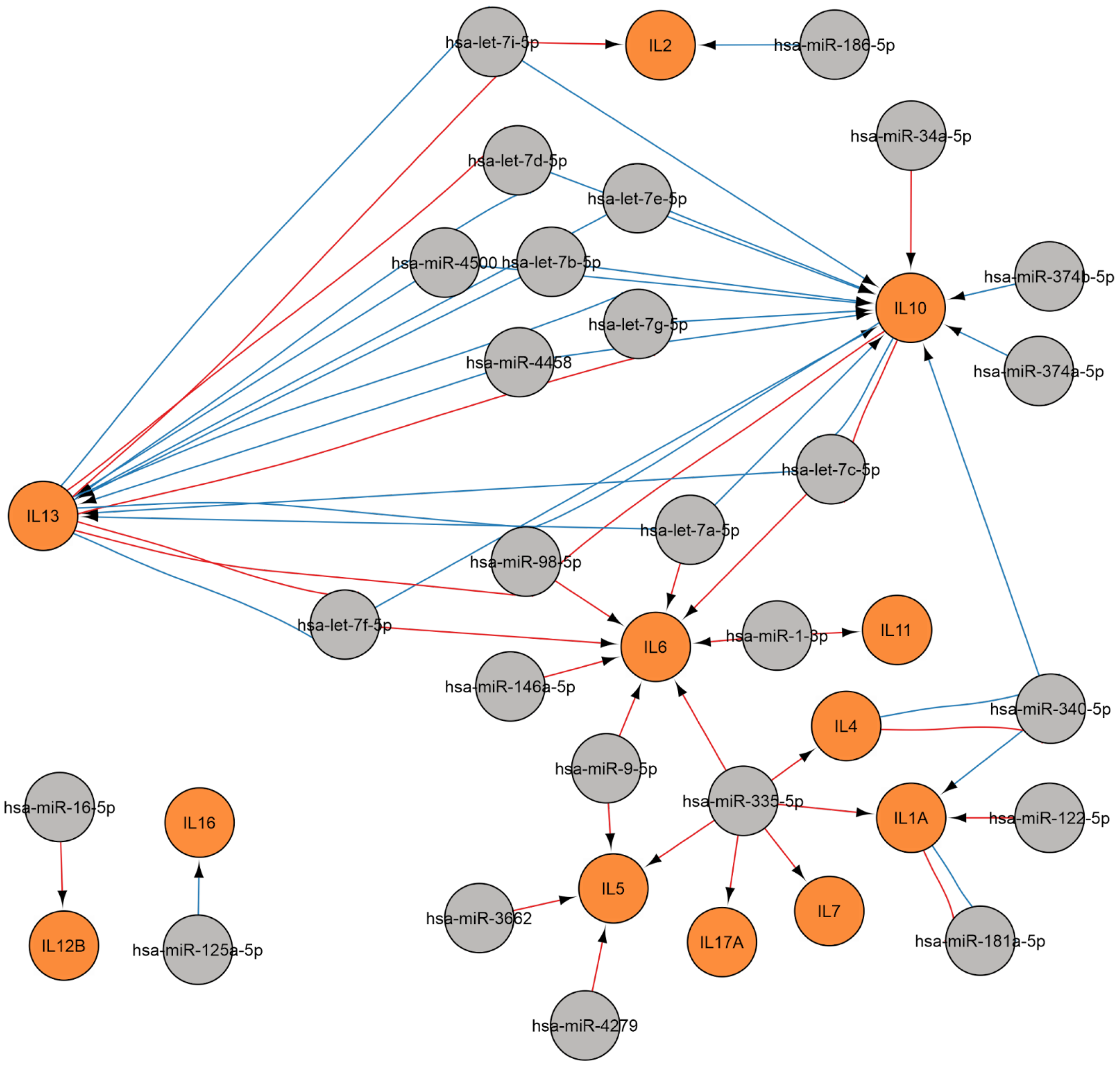
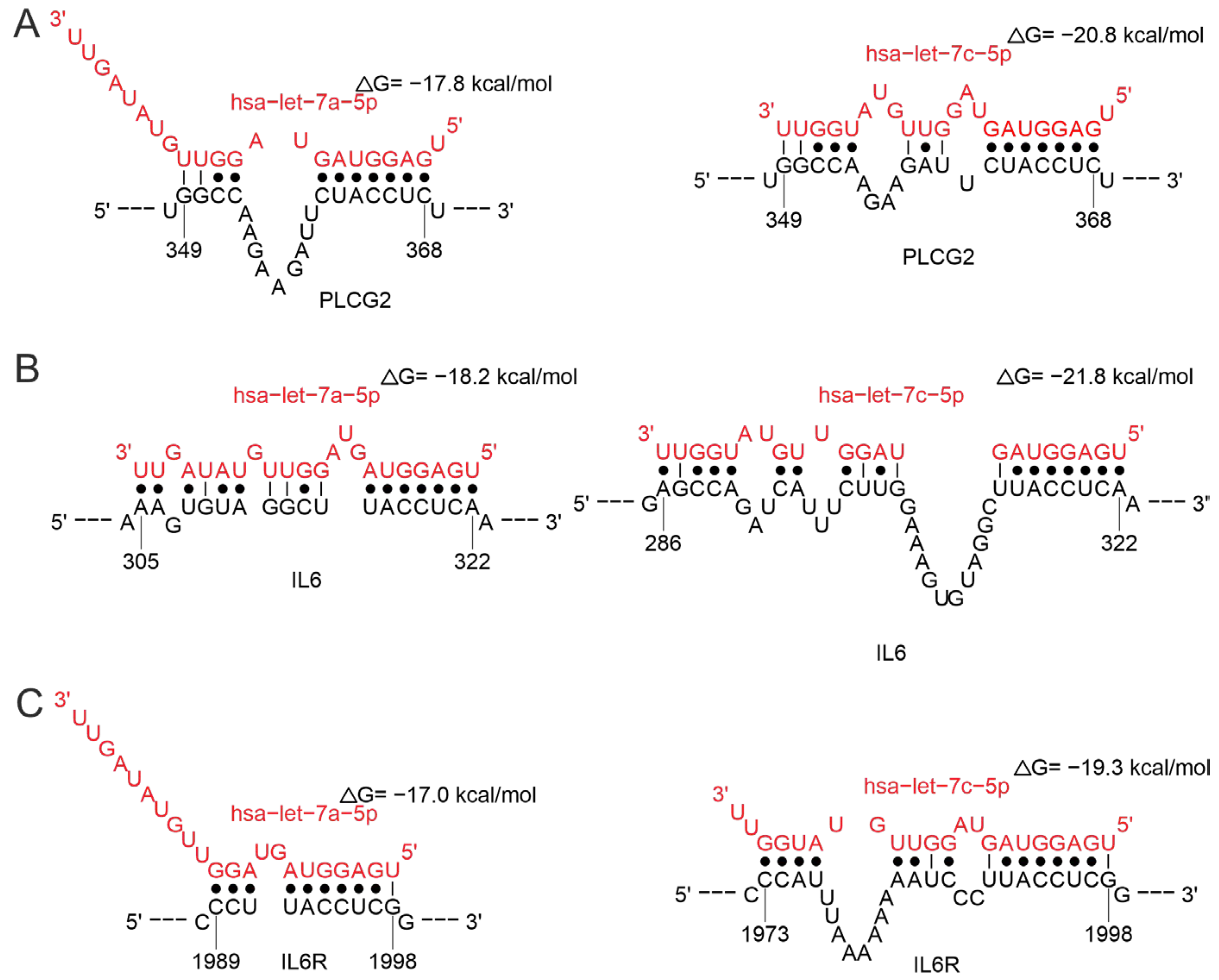
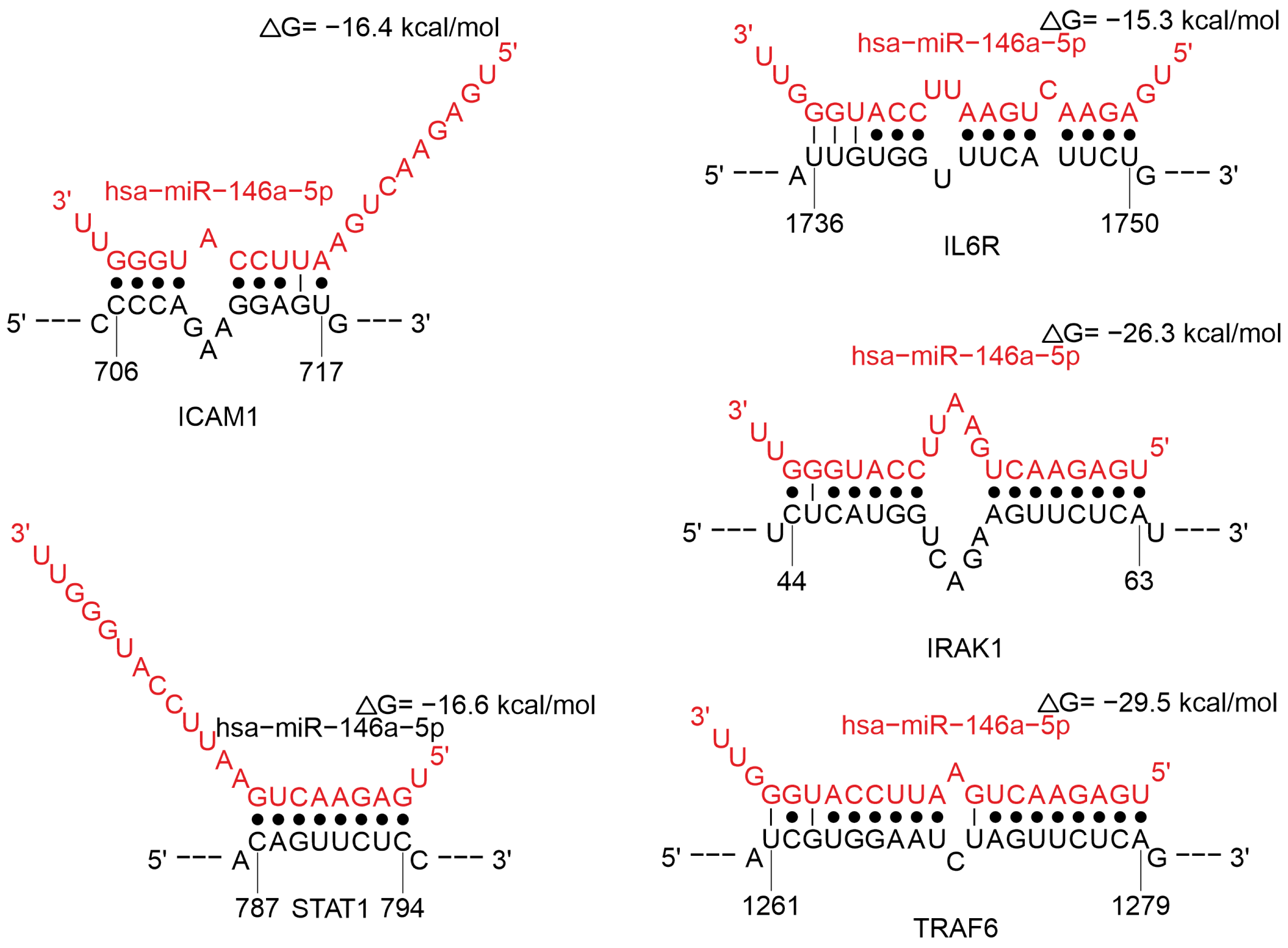
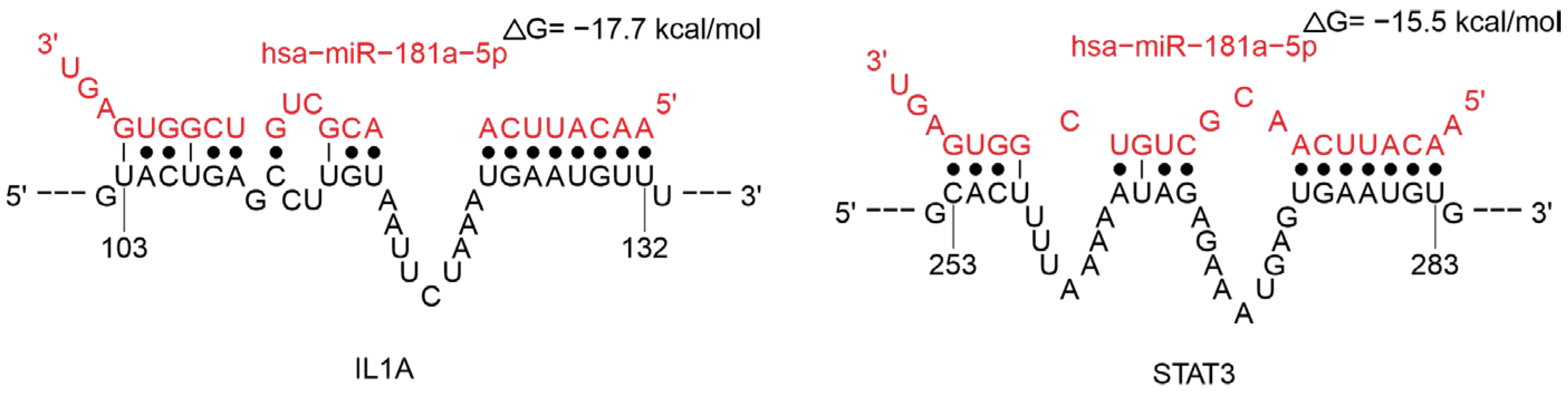
2.3.4. MiRNAs Regulating the Cytokine Response
Regulation of Cytokine Gene
Regulation of Cytokine Receptor Gene
Regulation of Cytokine Signaling Pathways
3. Discussion
Limitations and Prospects of the Study
- Clinical Correlation: Analysis of the relationship between the expression levels of the selected miRNAs and clinical parameters, including symptom severity, viral load, and disease outcome.
- Development of a Diagnostic Panel: Based on the confirmed data—the creation and testing of a diagnostic system or biomarker panel to assess the status of the immune response and predict the course of the disease.
4. Materials and Methods
4.1. Analysis of miRNAs Associated with Viral Infections
- for flavivirus infections: DOID:0050175#tick-borne encephalitis, DOID:12205#dengue disease, DOID:2366#West Nile fever, DOID:0060478#Zika fever;
- for coronavirus infections: DOID:0080599#Coronavirus infectious disease, DOID: 0080642#Middle East respiratory syndrome, DOID:2945#Severe acute respiratory syndrome (given the absence of data on other coronavirus infections within the miRWalk database, we deem the analysis to be exhaustive);
- for HIV infection: DOID:526#human immunodeficiency virus infection disease.
4.2. Target Predictions of the miRNAs Analyzed
4.3. Prediction of miRNA Binding Sites on Target mRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | Clusters of elements rich in adenine and uridine |
| DENV | Dengue virus |
| GO | Gene ontology |
| MHC | Major histocompatibility complex |
| TBEV | Tick-borne encephalitis virus |
| TLR | Toll-like receptors |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- Cherry, J.D.; Krogstad, P. SARS: The First Pandemic of the 21st Century. Pediatr. Res. 2004, 56, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.; Estrada Lobato, E.; Paez, D.; Langs, G.; Prosch, H. Artificial Intelligence in Respiratory Pandemics—Ready for Disease X? A Scoping Review. Eur. Radiol. 2024, 35, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, S.; Luthra, P.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Patel, J.; Lamothe, F.; Fredericks, A.C.; Tripathi, S.; Zhu, T.; Pintado-Silva, J.; et al. Dengue Virus NS2B Protein Targets CGAS for Degradation and Prevents Mitochondrial DNA Sensing during Infection. Nat. Microbiol. 2017, 2, 17037. [Google Scholar] [CrossRef]
- Ashour, J.; Laurent-Rolle, M.; Shi, P.-Y.; García-Sastre, A. NS5 of Dengue Virus Mediates STAT2 Binding and Degradation. J. Virol. 2009, 83, 5408–5418. [Google Scholar] [CrossRef]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika Virus Inhibits Type-I Interferon Production and Downstream Signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Fanunza, E.; Carletti, F.; Quartu, M.; Grandi, N.; Ermellino, L.; Milia, J.; Corona, A.; Capobianchi, M.R.; Ippolito, G.; Tramontano, E. Zika Virus NS2A Inhibits Interferon Signaling by Degradation of STAT1 and STAT2. Virulence 2021, 12, 1580–1596. [Google Scholar] [CrossRef]
- Molinero, M.; Perez-Pons, M.; González, J.; Barbé, F.; de Gonzalo-Calvo, D. Decoding Viral and Host MicroRNA Signatures in Airway-Derived Biosamples: Insights for Biomarker Discovery in Viral Respiratory Infections. Biomed. Pharmacother. 2024, 177, 116984. [Google Scholar] [CrossRef]
- Fayyad-Kazan, M. MicroRNAs in SARS-CoV-2 Infection: Emerging Modulators of Inflammation, Pathogenesis, and Therapeutic Potential. Inflammopharmacology 2025, 33, 4895–4910. [Google Scholar] [CrossRef]
- Paval, N.-E.; Căliman-Sturdza, O.A.; Lobiuc, A.; Dimian, M.; Sirbu, I.-O.; Covasa, M. MicroRNAs in Long COVID: Roles, Diagnostic Biomarker Potential and Detection. Hum. Genom. 2025, 19, 90. [Google Scholar] [CrossRef]
- Tahamtan, A.; Inchley, C.S.; Marzban, M.; Tavakoli-Yaraki, M.; Teymoori-Rad, M.; Nakstad, B.; Salimi, V. The Role of MicroRNAs in Respiratory Viral Infection: Friend or Foe? Rev. Med. Virol. 2016, 26, 389–407. [Google Scholar] [CrossRef]
- Paul, S.; Bravo Vázquez, L.A.; Reyes-Pérez, P.R.; Estrada-Meza, C.; Aponte Alburquerque, R.A.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Chakraborty, S.; Srivastava, A. The Role of MicroRNAs in Solving COVID-19 Puzzle from Infection to Therapeutics: A Mini-Review. Virus Res. 2022, 308, 198631. [Google Scholar] [CrossRef]
- Rezaei, T.; Amini, M.; Hashemi, Z.S.; Mansoori, B.; Rezaei, S.; Karami, H.; Mosafer, J.; Mokhtarzadeh, A.; Baradaran, B. MicroRNA-181 Serves as a Dual-Role Regulator in the Development of Human Cancers. Free Radic. Biol. Med. 2020, 152, 432–454. [Google Scholar] [CrossRef]
- Gao, X.; Qiao, Y.; Han, D.; Zhang, Y.; Ma, N. Enemy or Partner: Relationship between Intronic Micrornas and Their Host Genes. IUBMB Life 2012, 64, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Głobińska, A.; Pawełczyk, M.; Kowalski, M.L. MicroRNAs and the Immune Response to Respiratory Virus Infections. Expert Rev. Clin. Immunol. 2014, 10, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pato, A.; Virseda-Berdices, A.; Resino, S.; Ryan, P.; Martínez-González, O.; Pérez-García, F.; Martin-Vicente, M.; Valle-Millares, D.; Brochado-Kith, O.; Blancas, R.; et al. Plasma MiRNA Profile at COVID-19 Onset Predicts Severity Status and Mortality. Emerg. Microbes Infect. 2022, 11, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Giannella, A.; Riccetti, S.; Sinigaglia, A.; Piubelli, C.; Razzaboni, E.; Di Battista, P.; Agostini, M.; Dal Molin, E.; Manganelli, R.; Gobbi, F.; et al. Circulating MicroRNA Signatures Associated with Disease Severity and Outcome in COVID-19 Patients. Front. Immunol. 2022, 13, 968991. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. MiRWalk–Database: Prediction of Possible MiRNA Binding Sites by “Walking” the Genes of Three Genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. MiRWalk: An Online Resource for Prediction of MicroRNA Binding Sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Dweep, H.; Gretz, N.; Sticht, C. MiRWalk Database for MiRNA–Target Interactions. Methods Mol. Biol. 2014, 1182, 289–305. [Google Scholar]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates That Thousands of Human Genes Are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. MiRDB: An Online Database for Prediction of Functional MicroRNA Targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Papadopoulos, G.L.; Reczko, M.; Simossis, V.A.; Sethupathy, P.; Hatzigeorgiou, A.G. The Database of Experimentally Supported Targets: A Functional Update of TarBase. Nucleic Acids Res. 2009, 37, D155–D158. [Google Scholar] [CrossRef]
- Didiano, D.; Hobert, O. Molecular Architecture of a MiRNA-Regulated 3′ UTR. RNA 2008, 14, 1297–1317. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ajay, S.S.; Yook, J.I.; Kim, H.S.; Hong, S.H.; Kim, N.H.; Dhanasekaran, S.M.; Chinnaiyan, A.M.; Athey, B.D. New Class of MicroRNA Targets Containing Simultaneous 5′-UTR and 3′-UTR Interaction Sites. Genome Res. 2009, 19, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Guo, J.; Huang, Q.; Chen, X.; Li-Ling, J.; Li, Q.; Ma, F. Retained Introns Increase Putative MicroRNA Targets within 3′ UTRs of Human MRNA. FEBS Lett. 2007, 581, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar] [CrossRef]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis. 2020, 11, 509. [Google Scholar] [CrossRef]
- Widiasta, A.; Sribudiani, Y.; Nugrahapraja, H.; Hilmanto, D.; Sekarwana, N.; Rachmadi, D. Potential Role of ACE2-Related MicroRNAs in COVID-19-Associated Nephropathy. Non-Coding RNA Res. 2020, 5, 153–166. [Google Scholar] [CrossRef]
- Chou, C.-H.; Shrestha, S.; Yang, C.-D.; Chang, N.-W.; Lin, Y.-L.; Liao, K.-W.; Huang, W.-C.; Sun, T.-H.; Tu, S.-J.; Lee, W.-H.; et al. MiRTarBase Update 2018: A Resource for Experimentally Validated MicroRNA-Target Interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef]
- Chow, J.T.-S.; Salmena, L. Prediction and Analysis of SARS-CoV-2-Targeting MicroRNA in Human Lung Epithelium. Genes 2020, 11, 1002. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Lin, Y.-C.-D.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. MiRTarBase Update 2022: An Informative Resource for Experimentally Validated MiRNA–Target Interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The Biochemical Basis of MicroRNA Targeting Efficacy. Science 2019, 366, 6472. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective MicroRNA Target Sites in Mammalian MRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Kutmon, M.; Ehrhart, F.; Willighagen, E.L.; Evelo, C.T.; Coort, S.L. CyTargetLinker App Update: A Flexible Solution for Network Extension in Cytoscape. F1000Research 2018, 7, 743. [Google Scholar] [CrossRef]
- Kutmon, M.; Kelder, T.; Mandaviya, P.; Evelo, C.T.A.; Coort, S.L. CyTargetLinker: A Cytoscape App to Integrate Regulatory Interactions in Network Analysis. PLoS ONE 2013, 8, e82160. [Google Scholar] [CrossRef]
- Zheng, B.; Xi, Z.; Liu, R.; Yin, W.; Sui, Z.; Ren, B.; Miller, H.; Gong, Q.; Liu, C. The Function of MicroRNAs in B-Cell Development, Lymphoma, and Their Potential in Clinical Practice. Front. Immunol. 2018, 9, 936–946. [Google Scholar] [CrossRef]
- Borbet, T.C.; Hines, M.J.; Koralov, S.B. MicroRNA Regulation of B Cell Receptor Signaling. Immunol. Rev. 2021, 304, 111–125. [Google Scholar] [CrossRef]
- Brandl, A.; Daum, P.; Brenner, S.; Schulz, S.R.; Yap, D.Y.; Bösl, M.R.; Wittmann, J.; Schuh, W.; Jäck, H. The Microprocessor Component, DGCR8, Is Essential for Early B-cell Development in Mice. Eur. J. Immunol. 2016, 46, 2710–2718. [Google Scholar] [CrossRef]
- Coffre, M.; Benhamou, D.; Rieß, D.; Blumenberg, L.; Snetkova, V.; Hines, M.J.; Chakraborty, T.; Bajwa, S.; Jensen, K.; Chong, M.M.W.; et al. MiRNAs Are Essential for the Regulation of the PI3K/AKT/FOXO Pathway and Receptor Editing during B Cell Maturation. Cell Rep. 2016, 17, 2271–2285. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Regulation of Plasma-Cell Development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- van Nieuwenhuijze, A.; Dooley, J.; Humblet-Baron, S.; Sreenivasan, J.; Koenders, M.; Schlenner, S.M.; Linterman, M.; Liston, A. Defective Germinal Center B-Cell Response and Reduced Arthritic Pathology in MicroRNA-29a-Deficient Mice. Cell. Mol. Life Sci. 2017, 74, 2095–2106. [Google Scholar] [CrossRef]
- de Yébenes, V.G.; Belver, L.; Pisano, D.G.; González, S.; Villasante, A.; Croce, C.; He, L.; Ramiro, A.R. MiR-181b Negatively Regulates Activation-Induced Cytidine Deaminase in B Cells. J. Exp. Med. 2008, 205, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Kuchen, S.; Resch, W.; Yamane, A.; Kuo, N.; Li, Z.; Chakraborty, T.; Wei, L.; Laurence, A.; Yasuda, T.; Peng, S.; et al. Regulation of MicroRNA Expression and Abundance during Lymphopoiesis. Immunity 2010, 32, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T Cells in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding Roles for CD4+ T Cells in Immunity to Viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Y.; Lin, C. Heterogeneity and Subtypes of CD4+ Regulatory T Cells: Implications for Tumor Therapy. Front. Immunol. 2024, 14, 1291796. [Google Scholar] [CrossRef]
- Atreya, I.; Schimanski, C.C.; Becker, C.; Wirtz, S.; Dornhoff, H.; Schnurer, E.; Berger, M.R.; Galle, P.R.; Herr, W.; Neurath, M.F. The T-Box Transcription Factor Eomesodermin Controls CD8 T Cell Activity and Lymph Node Metastasis in Human Colorectal Cancer. Gut 2007, 56, 1572–1578. [Google Scholar] [CrossRef]
- Wells, A.C.; Daniels, K.A.; Angelou, C.C.; Fagerberg, E.; Burnside, A.S.; Markstein, M.; Alfandari, D.; Welsh, R.M.; Pobezinskaya, E.L.; Pobezinsky, L.A. Modulation of Let-7 MiRNAs Controls the Differentiation of Effector CD8 T Cells. eLife 2017, 6, e26398. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Di Pietro, P.; Abate, A.C.; Izzo, C.; Toni, A.L.; Rusciano, M.R.; Folliero, V.; Dell’Annunziata, F.; Granata, G.; Visco, V.; Motta, B.M.; et al. Plasma MiR-1-3p Levels Predict Severity in Hospitalized COVID-19 Patients. Br. J. Pharmacol. 2025, 182, 451–467. [Google Scholar] [CrossRef]
- Letafati, A.; Ardekani, O.S.; Naderisemiromi, M.; Norouzi, M.; Shafiei, M.; Nik, S.; Mozhgani, S.-H. Unraveling the Dynamic Mechanisms of Natural Killer Cells in Viral Infections: Insights and Implications. Virol. J. 2024, 21, 18. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Sun, Q.; Yan, J.; Huang, J.; Zhu, S.; Yu, J. Identification of MicroRNA Transcriptome Involved in Human Natural Killer Cell Activation. Immunol. Lett. 2012, 143, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Wylie, T.; Germino, E.; Leong, J.W.; Magrini, V.J.; Koul, S.; Keppel, C.R.; Schneider, S.E.; Koboldt, D.C.; Sullivan, R.P.; et al. Next-Generation Sequencing Identifies the Natural Killer Cell MicroRNA Transcriptome. Genome Res. 2010, 20, 1590–1604. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.L.; Scoville, S.D.; Chen, L.; McConnell, K.; Mao, H.C.; Ahmed, E.H.; Zorko, N.; Harvey, S.; Cole, J.; Zhang, X.; et al. MicroRNA-29b Mediates Altered Innate Immune Development in Acute Leukemia. J. Clin. Investig. 2016, 126, 4404–4416. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.F.; Thomas, M.F.; Hu, J.K.; Yang, Z.; Babiarz, J.E.; Allen, C.D.C.; Matloubian, M.; Blelloch, R.; Ansel, K.M. MicroRNA-29 Regulates T-Box Transcription Factors and Interferon-γ Production in Helper T Cells. Immunity 2011, 35, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huang, Y.; Pang, L.; Gu, W.; Wang, N.; Hu, J.; Cui, X.; Zhang, J.; Zhao, J.; Liu, C.; et al. Prognostic Value of the Micro RNA—29 Family in Multiple Human Cancers: A Meta—Analysis and Systematic Review. Clin. Exp. Pharmacol. Physiol. 2017, 44, 441–454. [Google Scholar] [CrossRef]
- Asirvatham, A.J.; Gregorie, C.J.; Hu, Z.; Magner, W.J.; Tomasi, T.B. MicroRNA Targets in Immune Genes and the Dicer/Argonaute and ARE Machinery Components. Mol. Immunol. 2008, 45, 1995–2006. [Google Scholar] [CrossRef]
- Asirvatham, A.J.; Magner, W.J.; Tomasi, T.B. MiRNA Regulation of Cytokine Genes. Cytokine 2009, 45, 58–69. [Google Scholar] [CrossRef]
- Anderson, P. Post-Transcriptional Control of Cytokine Production. Nat. Immunol. 2008, 9, 353–359. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Rodríguez-Bayona, B.; Ramos-Amaya, A.; López-Blanco, R.; Campos-Caro, A.; Brieva, J.A. STAT-3 Activation by Differential Cytokines Is Critical for Human In Vivo–Generated Plasma Cell Survival and Ig Secretion. J. Immunol. 2013, 191, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the Context of an Inflammatory Cytokine Milieu Supports De Novo Differentiation of IL-17-Producing T Cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal Developmental Pathways for the Generation of Pathogenic Effector TH17 and Regulatory T Cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Menon, M.; Blair, P.A.; Isenberg, D.A.; Mauri, C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity 2016, 44, 683–697. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Amoozgar, B.; Bangolo, A.; Mohamed, A.; Mansour, C.; Elias, D.; Cho, C.; Reddy, S. JAK2 Inhibitors and Emerging Therapies in Graft-Versus-Host Disease: Current Perspectives and Future Directions. Biomedicines 2025, 13, 1527. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Su, Y.-H.; Fang, S.-S.; Huang, T.-N.; Qiu, Y.; Jou, Y.-S.; Shih, H.; Kung, H.-J.; Chen, R.-H. Etk, a Btk Family Tyrosine Kinase, Mediates Cellular Transformation by Linking Src to STAT3 Activation. Mol. Cell. Biol. 2000, 20, 2043–2054. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sekine, Y.; Kashima, K.; Kubota, A.; Sato, N.; Aoki, N.; Matsuda, T. The Nuclear Isoform of Protein-Tyrosine Phosphatase TC-PTP Regulates Interleukin-6-Mediated Signaling Pathway through STAT3 Dephosphorylation. Biochem. Biophys. Res. Commun. 2002, 297, 811–817. [Google Scholar] [CrossRef]
- Hou, T.; Ray, S.; Lee, C.; Brasier, A.R. The STAT3 NH2-Terminal Domain Stabilizes Enhanceosome Assembly by Interacting with the P300 Bromodomain. J. Biol. Chem. 2008, 283, 30725–30734. [Google Scholar] [CrossRef]
- Saxena, N.K.; Vertino, P.M.; Anania, F.A.; Sharma, D. Leptin-Induced Growth Stimulation of Breast Cancer Cells Involves Recruitment of Histone Acetyltransferases and Mediator Complex to CYCLIN D1 Promoter via Activation of Stat3. J. Biol. Chem. 2007, 282, 13316–13325. [Google Scholar] [CrossRef]
- Cheung, K.L.; Zhang, F.; Jaganathan, A.; Sharma, R.; Zhang, Q.; Konuma, T.; Shen, T.; Lee, J.-Y.; Ren, C.; Chen, C.-H.; et al. Distinct Roles of Brd2 and Brd4 in Potentiating the Transcriptional Program for Th17 Cell Differentiation. Mol. Cell 2017, 65, 1068–1080.e5. [Google Scholar] [CrossRef]
- Ma, L.; Huang, C.; Wang, X.-J.; Xin, D.E.; Wang, L.; Zou, Q.C.; Zhang, Y.S.; Tan, M.; Wang, Y.; Zhao, T.C.; et al. Lysyl Oxidase 3 Is a Dual-Specificity Enzyme Involved in STAT3 Deacetylation and Deacetylimination Modulation. Mol. Cell 2017, 65, 296–309. [Google Scholar] [CrossRef]
- Thaventhiran, J.E.D.; Lango Allen, H.; Burren, O.S.; Rae, W.; Greene, D.; Staples, E.; Zhang, Z.; Farmery, J.H.R.; Simeoni, I.; Rivers, E.; et al. Whole-Genome Sequencing of a Sporadic Primary Immunodeficiency Cohort. Nature 2020, 583, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Castro, C.N.; Tusseau, M.; Stolzenberg, M.-C.; Mazerolles, F.; Aladjidi, N.; Armstrong, M.; Ashrafian, H.; Cutcutache, I.; Ebetsberger-Dachs, G.; et al. Early-Onset Autoimmunity Associated with SOCS1 Haploinsufficiency. Nat. Commun. 2020, 11, 5341. [Google Scholar] [CrossRef] [PubMed]
- Swaidani, S.; Liu, C.; Zhao, J.; Bulek, K.; Li, X. TRAF Regulation of IL-17 Cytokine Signaling. Front. Immunol. 2019, 10, 1293. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-C.; Shahinian, A.; Speiser, D.; Kraunus, J.; Billia, F.; Wakeham, A.; de la Pompa, J.L.; Ferrick, D.; Hum, B.; Iscove, N.; et al. Early Lethality, Functional NF-ΚB Activation, and Increased Sensitivity to TNF-Induced Cell Death in TRAF2-Deficient Mice. Immunity 1997, 7, 715–725. [Google Scholar] [CrossRef]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA MiR-146a-5p Inhibits the Inflammatory Response and Injury of Airway Epithelial Cells via Targeting TNF Receptor-Associated Factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Arcipowski, K.M.; Stunz, L.L.; Bishop, G.A. TRAF6 Is a Critical Regulator of LMP1 Functions in Vivo. Int. Immunol. 2014, 26, 149–158. [Google Scholar] [CrossRef]
- Pan, J.; Du, M.; Cao, Z.; Zhang, C.; Hao, Y.; Zhu, J.; He, H. MiR-146a-5p Attenuates IL-1β-induced IL-6 and IL-1β Expression in a Cementoblast-derived Cell Line. Oral Dis. 2020, 26, 1308–1317. [Google Scholar] [CrossRef]
- Soytürk, H.; Önal, C.; Kılıç, Ü.; Türkoğlu, Ş.A.; Ayaz, E. The Effect of the HMGB1/RAGE/TLR4/NF-κB Signalling Pathway in Patients with Idiopathic Epilepsy and Its Relationship with Toxoplasmosis. J. Cell. Mol. Med. 2024, 28, e18542. [Google Scholar] [CrossRef]
- Amali, A.A.; Paramasivam, K.; Ravikumar, S.; Tan, Z.; Loong, S.S.E.; Chong, K.H.C.; Ang, A.; Zhu, J.; Rao, S.S.; Chai, L.Y.A. Severe Invasive Infections Linked to IRAK2 Immune Variants. Int. J. Infect. Dis. 2024, 148, 107245. [Google Scholar] [CrossRef] [PubMed]
- García, L.F. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front. Immunol. 2020, 11, 1441. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.M.; Sedykh, S.E.; Nevinsky, G.A. SARS-CoV-2 Infection as a Risk Factor for the Development of Autoimmune Pathology. Mol. Med. 2022, 20, 3–10. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N. The Mechanics of MiRNA-Mediated Gene Silencing: A Look under the Hood of MiRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Belver, L.; Papavasiliou, F.N.; Ramiro, A.R. MicroRNA Control of Lymphocyte Differentiation and Function. Curr. Opin. Immunol. 2011, 23, 368–373. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Mitchell, M.I.; Ben-Dov, I.Z.; Ye, K.; Liu, C.; Shi, M.; Sadoughi, A.; Shah, C.; Siddiqui, T.; Okorozo, A.; Gutierrez, M.; et al. Exhaled Breath Condensate Contains Extracellular Vesicles (EVs) That Carry MiRNA Cargos of Lung Tissue Origin That Can Be Selectively Purified and Analyzed. J. Extracell. Vesicles 2024, 13, e12440. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Nikitin, A.O.; Nevinsky, G.A. Circulating MiRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways. Non-Coding RNA 2024, 10, 48. [Google Scholar] [CrossRef]
- Liu, C.; Mallick, B.; Long, D.; Rennie, W.A.; Wolenc, A.; Carmack, C.S.; Ding, Y. CLIP-Based Prediction of Mammalian MicroRNA Binding Sites. Nucleic Acids Res. 2013, 41, e138. [Google Scholar] [CrossRef]
- Long, D.; Lee, R.; Williams, P.; Chan, C.Y.; Ambros, V.; Ding, Y. Potent Effect of Target Structure on MicroRNA Function. Nat. Struct. Mol. Biol. 2007, 14, 287–294. [Google Scholar] [CrossRef]
- Liu, C.; Rennie, W.A.; Mallick, B.; Kanoria, S.; Long, D.; Wolenc, A.; Carmack, C.S.; Ding, Y. MicroRNA Binding Sites in C. Elegans 3′ UTRs. RNA Biol. 2014, 11, 693–701. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Cairns, M.J. Identifying MiRNAs, Targets and Functions. Brief. Bioinform. 2014, 15, 1–19. [Google Scholar] [CrossRef]
- Letafati, A.; Najafi, S.; Mottahedi, M.; Karimzadeh, M.; Shahini, A.; Garousi, S.; Abbasi-Kolli, M.; Sadri Nahand, J.; Tamehri Zadeh, S.S.; Hamblin, M.R.; et al. MicroRNA Let-7 and Viral Infections: Focus on Mechanisms of Action. Cell. Mol. Biol. Lett. 2022, 27, 14. [Google Scholar] [CrossRef] [PubMed]
- Leontyeva, M.E.; Bogdanova, D.V.; Moiseeva, A.A.; Burlakov, V.I.; Nesterenko, Z.A.; Merkushov, A.Y.; Kan, N.Y.; Khoreva, A.L.; Rodina, Y.A.; Shvets, O.A.; et al. A Case Report of Autoinflammation and PLCy2-Associated Antibody Deficiency and Immune Dysregulation. Pediatr. Hematol. Immunopathol. 2022, 21, 163–168. [Google Scholar] [CrossRef]
- Baysac, K.; Sun, G.; Nakano, H.; Schmitz, E.G.; Cruz, A.C.; Fisher, C.; Bailey, A.C.; Abbott, J.K.; Aldave Becerra, J.C.; Allenspach, E.J.; et al. PLCG2-Associated Immune Dysregulation (PLAID) Comprises Broad and Distinct Clinical Presentations Related to Functional Classes of Genetic Variants. J. Allergy Clin. Immunol. 2024, 153, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S. Recent Findings Regarding Let-7 in Immunity. Cancer Lett. 2018, 434, 130–131. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, L.; Sun, H.-X.; Zhang, Z.; Xu, J.; Xu, Y.; Lin, Y.; Zhu, A.; Luo, Y.; et al. Plasma Cell-Free RNA Characteristics in COVID-19 Patients. Genome Res. 2022, 32, 228–241. [Google Scholar] [CrossRef]
- Saulle, I.; Garziano, M.; Cappelletti, G.; Limanaqi, F.; Strizzi, S.; Vanetti, C.; Lo Caputo, S.; Poliseno, M.; Santantonio, T.A.; Clerici, M.; et al. Salivary MiRNA Profiles in COVID-19 Patients with Different Disease Severities. Int. J. Mol. Sci. 2023, 24, 10992. [Google Scholar] [CrossRef]
- Devadoss, D.; Acharya, A.; Manevski, M.; Houserova, D.; Cioffi, M.D.; Pandey, K.; Nair, M.; Chapagain, P.; Mirsaeidi, M.; Borchert, G.M.; et al. Immunomodulatory LncRNA on Antisense Strand of ICAM-1 Augments SARS-CoV-2 Infection-Associated Airway Mucoinflammatory Phenotype. iScience 2022, 25, 104685. [Google Scholar] [CrossRef]
- Farr, R.J.; Rootes, C.L.; Rowntree, L.C.; Nguyen, T.H.O.; Hensen, L.; Kedzierski, L.; Cheng, A.C.; Kedzierska, K.; Au, G.G.; Marsh, G.A.; et al. Altered MicroRNA Expression in COVID-19 Patients Enables Identification of SARS-CoV-2 Infection. PLoS Pathog. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Ramphan, S.; Chumchanchira, C.; Sornjai, W.; Chailangkarn, T.; Jongkaewwattana, A.; Assavalapsakul, W.; Smith, D.R. Strain Variation Can Significantly Modulate the MiRNA Response to Zika Virus Infection. Int. J. Mol. Sci. 2023, 24, 16216. [Google Scholar] [CrossRef]
- Stanciu, L.; Djukanovic, R. The Role of ICAM-1 on T-Cells in the Pathogenesis of Asthma. Eur. Respir. J. 1998, 11, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Bezbradica, J.S.; Schroder, K. TRAF6 Is a Nexus for TLR-STAT1 Crosstalk. Immunol. Cell Biol. 2014, 92, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Muroi, M.; Tanamoto, K. TRAF6 Distinctively Mediates MyD88- and IRAK-1-Induced Activation of NF-ΚB. J. Leukoc. Biol. 2008, 83, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Rom, I.; Passiatore, G.; Pacifici, M.; Radhakrishnan, S.; Del Valle, L.; Piña-Oviedo, S.; Khalili, K.; Eletto, D.; Peruzzi, F. CCL8/MCP-2 Is a Target for Mir-146a in HIV-1-infected Human Microglial Cells. FASEB J. 2010, 24, 2292–2300. [Google Scholar] [CrossRef]
- Hou, J.; Wang, P.; Lin, L.; Liu, X.; Ma, F.; An, H.; Wang, Z.; Cao, X. MicroRNA-146a Feedback Inhibits RIG-I-Dependent Type I IFN Production in Macrophages by Targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009, 183, 2150–2158. [Google Scholar] [CrossRef]
- Motsch, N.; Pfuhl, T.; Mrazek, J.; Barth, S.; Grässer, F.A. Epstein-Barr Virus-Encoded Latent Membrane Protein 1 (LMP1) Induces the Expression of the Cellular MicroRNA MiR-146a. RNA Biol. 2007, 4, 131–137. [Google Scholar] [CrossRef]
- Wu, S.; He, L.; Li, Y.; Wang, T.; Feng, L.; Jiang, L.; Zhang, P.; Huang, X. MiR-146a Facilitates Replication of Dengue Virus by Dampening Interferon Induction by Targeting TRAF6. J. Infect. 2013, 67, 329–341. [Google Scholar] [CrossRef]
- Gaytán-Pacheco, N.; Ibáñez-Salazar, A.; Herrera-Van Oostdam, A.S.; Oropeza-Valdez, J.J.; Magaña-Aquino, M.; Adrián López, J.; Monárrez-Espino, J.; López-Hernández, Y. MiR-146a, MiR-221, and MiR-155 Are Involved in Inflammatory Immune Response in Severe COVID-19 Patients. Diagnostics 2022, 13, 133. [Google Scholar] [CrossRef]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 Represses Endothelial Activation by Inhibiting Pro-inflammatory Pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef]
- Ramkaran, P.; Khan, S.; Phulukdaree, A.; Moodley, D.; Chuturgoon, A.A. MiR-146a Polymorphism Influences Levels of MiR-146a, IRAK-1, and TRAF-6 in Young Patients with Coronary Artery Disease. Cell Biochem. Biophys. 2014, 68, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-ΚB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Jahromi, S.S.; Aslani, M.; Mirshafiey, A. A Comprehensive Review on MiR-146a Molecular Mechanisms in a Wide Spectrum of Immune and Non-Immune Inflammatory Diseases. Immunol. Lett. 2020, 227, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Svegliati Baroni, S.; Pavani, M.; Moretti, M.; et al. Decreased Serum Levels of the Inflammaging Marker MiR-146a Are Associated with Clinical Non-Response to Tocilizumab in COVID-19 Patients. Mech. Ageing Dev. 2021, 193, 111413. [Google Scholar] [CrossRef]
- Gronau, L.; Duecker, R.P.; Jerkic, S.-P.; Eickmeier, O.; Trischler, J.; Chiocchetti, A.G.; Blumchen, K.; Zielen, S.; Schubert, R. Dual Role of MicroRNA-146a in Experimental Inflammation in Human Pulmonary Epithelial and Immune Cells and Expression in Inflammatory Lung Diseases. Int. J. Mol. Sci. 2024, 25, 7686. [Google Scholar] [CrossRef]
- Bautista-Becerril, B.; Nava-Quiroz, K.J.; Muñoz-Soria, E.; Camarena, Á.; Fricke-Galindo, I.; Buendia-Roldan, I.; Pérez-Rubio, G.; Chavez-Galán, L.; Pérez-Torres, K.; Téllez-Quijada, F.; et al. High Expression Levels of MiR-21-5p in Younger Hospitalized COVID-19 Patients Are Associated with Mortality and Critical Disease. Int. J. Mol. Sci. 2023, 24, 10112. [Google Scholar] [CrossRef]
- Wang, P.; Qu, X.; Zhou, X.; Shen, Y.; Ji, H.; Fu, Z.; Deng, J.; Lu, P.; Yu, W.; Lu, H.; et al. Two Cellular MicroRNAs, MiR-196b and MiR-1290, Contribute to HIV-1 Latency. Virology 2015, 486, 228–238. [Google Scholar] [CrossRef]
- Ho, B.-C.; Yu, I.-S.; Lu, L.-F.; Rudensky, A.; Chen, H.-Y.; Tsai, C.-W.; Chang, Y.-L.; Wu, C.-T.; Chang, L.-Y.; Shih, S.-R.; et al. Inhibition of MiR-146a Prevents Enterovirus-Induced Death by Restoring the Production of Type I Interferon. Nat. Commun. 2014, 5, 3344. [Google Scholar] [CrossRef]
- Sharma, N.; Verma, R.; Kumawat, K.L.; Basu, A.; Singh, S.K. MiR-146a Suppresses Cellular Immune Response during Japanese Encephalitis Virus JaOArS982 Strain Infection in Human Microglial Cells. J. Neuroinflamm. 2015, 12, 30. [Google Scholar] [CrossRef]
- Salem, S.; Lotfy, R.; Eltaweel, N.; Elbadry, M. Association of Plasma MicroRNAs with COVID-19 Severity and Outcome. J. Genet. Eng. Biotechnol. 2024, 22, 100433. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The Noncoding and Coding Transcriptional Landscape of the Peripheral Immune Response in Patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef]
- Aslani, M.; Mortazavi-Jahromi, S.S.; Mirshafiey, A. Cytokine Storm in the Pathophysiology of COVID-19: Possible Functional Disturbances of MiRNAs. Int. Immunopharmacol. 2021, 101, 108172. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, S.; Tonacci, A. AntagomiRs: A Novel Therapeutic Strategy for Challenging COVID-19 Cytokine Storm. Cytokine Growth Factor Rev. 2021, 58, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jin, J.; Ye, Z.; Jadhav, R.R.; Gustafson, C.E.; Hu, B.; Cao, W.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Histone Deficiency and Accelerated Replication Stress in T Cell Aging. J. Clin. Investig. 2021, 131, e143632. [Google Scholar] [CrossRef] [PubMed]
- Knarr, M.; Avelar, R.A.; Sekhar, S.C.; Kwiatkowski, L.J.; Dziubinski, M.L.; McAnulty, J.; Skala, S.; Avril, S.; Drapkin, R.; DiFeo, A. MiR-181a Initiates and Perpetuates Oncogenic Transformation through the Regulation of Innate Immune Signaling. Nat. Commun. 2020, 11, 3231. [Google Scholar] [CrossRef]
- Dejean, A.S.; Joulia, E.; Walzer, T. The Role of Eomes in Human CD4 T Cell Differentiation: A Question of Context. Eur. J. Immunol. 2019, 49, 38–41. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Hao, J.; Ni, L.; Dong, C. High Levels of Eomes Promote Exhaustion of Anti-Tumor CD8+ T Cells. Front. Immunol. 2018, 9, 2981. [Google Scholar] [CrossRef]
- Llaó-Cid, L.; Roessner, P.M.; Chapaprieta, V.; Öztürk, S.; Roider, T.; Bordas, M.; Izcue, A.; Colomer, D.; Dietrich, S.; Stilgenbauer, S.; et al. EOMES Is Essential for Antitumor Activity of CD8+ T Cells in Chronic Lymphocytic Leukemia. Leukemia 2021, 35, 3152–3162. [Google Scholar] [CrossRef]
- Emerson, D.A.; Rolig, A.S.; Redmond, W.L. Enhancing the Generation of Eomeshi CD8+ T Cells Augments the Efficacy of OX40- and CTLA-4–Targeted Immunotherapy. Cancer Immunol. Res. 2021, 9, 430–440. [Google Scholar] [CrossRef]
- Medlyn, M.J.; Maeder, E.; Bradley, C.; Phatarpekar, P.; Ham, H.; Billadeau, D.D. MADD Regulates Natural Killer Cell Degranulation through Rab27a Activation. J. Cell Sci. 2024, 137, jcs261582. [Google Scholar] [CrossRef]
- Liu, D.; Meckel, T.; Long, E.O. Distinct Role of Rab27a in Granule Movement at the Plasma Membrane and in the Cytosol of NK Cells. PLoS ONE 2010, 5, e12870. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S.; Sankaranarayanan, R.; Petrović, N. Clinically Informative Micrornas for SARS-CoV-2 Infection. Epigenomics 2023, 15, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Keikha, R.; Hashemi-Shahri, S.M.; Jebali, A. The Relative Expression of MiR-31, MiR-29, MiR-126, and MiR-17 and Their MRNA Targets in the Serum of COVID-19 Patients with Different Grades during Hospitalization. Eur. J. Med. Res. 2021, 26, 75. [Google Scholar] [CrossRef]
- Centa, A.; Fonseca, A.S.; Da Silva Ferreira, S.G.; Azevedo, M.L.V.; De Paula, C.B.V.; Nagashima, S.; MacHado-Souz, C.; Dos Santos Miggiolaro, A.F.R.; Baena, C.P.; De Noronha, L.; et al. Deregulated MiRNA Expression Is Associated with Endothelial Dysfunction in Post-Mortem Lung Biopsies of COVID-19 Patients. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L405–L412. [Google Scholar] [CrossRef]
- Azouz, F.; Arora, K.; Krause, K.; Nerurkar, V.R.; Kumar, M. Integrated MicroRNA and MRNA Profiling in Zika Virus-Infected Neurons. Viruses 2019, 11, 162. [Google Scholar] [CrossRef]
- Adoro, S.; Cubillos-Ruiz, J.R.; Chen, X.; Deruaz, M.; Vrbanac, V.D.; Song, M.; Park, S.; Murooka, T.T.; Dudek, T.E.; Luster, A.D.; et al. IL-21 Induces Antiviral MicroRNA-29 in CD4 T Cells to Limit HIV-1 Infection. Nat. Commun. 2015, 6, 7562. [Google Scholar] [CrossRef]
- Ortega, P.A.S.; Saulle, I.; Mercurio, V.; Ibba, S.V.; Lori, E.M.; Fenizia, C.; Masetti, M.; Trabattoni, D.; Caputo, S.L.; Vichi, F.; et al. Interleukin 21 (IL-21)/MicroRNA-29 (MiR-29) Axis Is Associated with Natural Resistance to HIV-1 Infection. AIDS 2018, 32, 2453–2461. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, L.; Pan, M.; Huang, L.; Li, H.; Liu, M.; Niu, X.; Zhang, C.; Wang, H. Bcl-3 Regulates T Cell Function through Energy Metabolism. BMC Immunol. 2023, 24, 35. [Google Scholar] [CrossRef]
- Bautista-Becerril, B.; Pérez-Dimas, G.; Sommerhalder-Nava, P.C.; Hanono, A.; Martínez-Cisneros, J.A.; Zarate-Maldonado, B.; Muñoz-Soria, E.; Aquino-Gálvez, A.; Castillejos-López, M.; Juárez-Cisneros, A.; et al. MiRNAs, from Evolutionary Junk to Possible Prognostic Markers and Therapeutic Targets in COVID-19. Viruses 2021, 14, 41. [Google Scholar] [CrossRef]
- Farr, R.J.; Rootes, C.L.; Stenos, J.; Foo, C.H.; Cowled, C.; Stewart, C.R. Detection of SARS-CoV-2 Infection by MicroRNA Profiling of the Upper Respiratory Tract. PLoS ONE 2022, 17, e0265670. [Google Scholar] [CrossRef]
- Luo, Q.; Zhu, J.; Zhang, Q.; Xie, J.; Yi, C.; Li, T. MicroRNA-486-5p Promotes Acute Lung Injury via Inducing Inflammation and Apoptosis by Targeting OTUD7B. Inflammation 2020, 43, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Wuttge, D.M.; Carlsen, A.L.; Teku, G.; Steen, S.O.; Wildt, M.; Vihinen, M.; Hesselstrand, R.; Heegaard, N.H.H. Specific Autoantibody Profiles and Disease Subgroups Correlate with Circulating Micro-RNA in Systemic Sclerosis. Rheumatology 2015, 54, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Mallick, B.; Ghosh, Z.; Chakrabarti, J. MicroRNome Analysis Unravels the Molecular Basis of SARS Infection in Bronchoalveolar Stem Cells. PLoS ONE 2009, 4, e7837. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Chen, S.; Liu, L. The Landscape of MiRNA-MRNA Regulatory Network and Cellular Sources in Inflammatory Bowel Diseases: Insights from Text Mining and Single Cell RNA Sequencing Analysis. Front. Immunol. 2024, 15. [Google Scholar] [CrossRef]
- Kramer, M.F. Stem-Loop RT-qPCR for MiRNAs. Curr. Protoc. Mol. Biol. 2011, 95. [Google Scholar] [CrossRef]
- Chen, C. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Forero, D.A.; González-Giraldo, Y.; Castro-Vega, L.J.; Barreto, G.E. QPCR-Based Methods for Expression Analysis of MiRNAs. Biotechniques 2019, 67, 192–199. [Google Scholar] [CrossRef]
- He, X.-H.; Xiao, Y.-T.; Chen, W.-Y.; Wang, M.-J.; Wu, X.-D.; Mei, L.-Y.; Gao, K.-X.; Huang, Q.-C.; Huang, R.-Y.; Chen, X.-M. In Silico Analysis of Serum MiRNA Profiles in Seronegative and Seropositive Rheumatoid Arthritis Patients by Small RNA Sequencing. PeerJ 2023, 11, e15690. [Google Scholar] [CrossRef]
- Gautam, A.; Kumar, R.; Dimitrov, G.; Hoke, A.; Hammamieh, R.; Jett, M. Identification of Extracellular MiRNA in Archived Serum Samples by Next-Generation Sequencing from RNA Extracted Using Multiple Methods. Mol. Biol. Rep. 2016, 43, 1165–1178. [Google Scholar] [CrossRef]
- Sorensen, K. Individualized MiRNA Assay Panels Using Optically Encoded Beads. In Next-Generation MicroRNA Expression Profiling Technology; Humana Press: Totowa, NJ, USA, 2012; pp. 131–141. [Google Scholar]
- Garcia, O.; Saveanu, C.; Cline, M.; Fromont-Racine, M.; Jacquier, A.; Schwikowski, B.; Aittokallio, T. GOlorize: A Cytoscape Plug-in for Network Visualization with Gene Ontology-Based Layout and Coloring. Bioinformatics 2007, 23, 394–396. [Google Scholar] [CrossRef]
- Kanoria, S.; Rennie, W.; Liu, C.; Carmack, C.S.; Lu, J.; Ding, Y. STarMir Tools for Prediction of MicroRNA Binding Sites. Methods Mol. Biol. 2016, 1490, 73–82. [Google Scholar]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and Effective Prediction of MicroRNA/Target Duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikheeva, E.V.; Aulova, K.S.; Nevinsky, G.A.; Timofeeva, A.M. In Silico Analysis of MiRNA Regulatory Networks to Identify Potential Biomarkers for the Clinical Course of Viral Infections. Int. J. Mol. Sci. 2025, 26, 10100. https://doi.org/10.3390/ijms262010100
Mikheeva EV, Aulova KS, Nevinsky GA, Timofeeva AM. In Silico Analysis of MiRNA Regulatory Networks to Identify Potential Biomarkers for the Clinical Course of Viral Infections. International Journal of Molecular Sciences. 2025; 26(20):10100. https://doi.org/10.3390/ijms262010100
Chicago/Turabian StyleMikheeva, Elena V., Kseniya S. Aulova, Georgy A. Nevinsky, and Anna M. Timofeeva. 2025. "In Silico Analysis of MiRNA Regulatory Networks to Identify Potential Biomarkers for the Clinical Course of Viral Infections" International Journal of Molecular Sciences 26, no. 20: 10100. https://doi.org/10.3390/ijms262010100
APA StyleMikheeva, E. V., Aulova, K. S., Nevinsky, G. A., & Timofeeva, A. M. (2025). In Silico Analysis of MiRNA Regulatory Networks to Identify Potential Biomarkers for the Clinical Course of Viral Infections. International Journal of Molecular Sciences, 26(20), 10100. https://doi.org/10.3390/ijms262010100







