Pro-Inflammatory and Anti-Inflammatory Interleukins in Periodontitis: Molecular Roles, Immune Crosstalk, and Therapeutic Perspectives
Abstract
1. Introduction
Literature Search Strategy
2. Overview of Immune Responses in Periodontitis
3. Pro-Inflammatory Interleukins in Periodontitis
4. Anti-Inflammatory Interleukins and Resolution
5. Dual and Context-Dependent Roles
6. Genetic and Epigenetic Regulation
7. Systemic Effects and Comorbidities
8. Diagnostic and Therapeutic Implications
9. Scope and Limitations of This Review
10. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Nibali, L.; D’Aiuto, F.; Griffiths, G.; Patel, K.; Suvan, J.; Tonetti, M.S. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: A case–control study. J. Clin. Periodontol. 2007, 34, 931–937. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Parkar, M.; Andreou, G.; Suvan, J.; Brett, P.M.; Ready, D.; Tonetti, M.S. Periodontitis and systemic inflammation: Control of the local infection is associated with a reduction in serum inflammatory markers. J. Dent. Res. 2004, 83, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [PubMed]
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Belstrøm, D.; Eick, S.; Gursoy, U.K.; Johansson, A.; Könönen, E. Periodontal microbiology and microbial etiology of periodontal diseases: Historical concepts and contemporary perspectives. Periodontol. 2000 2023. [Google Scholar] [CrossRef]
- Werner, N.; Frasheri, I.; Heck, K.; Ern, C.; Heym, R.; Bumm, C.V.; Folwaczny, M. The association between periodontal microbial biomarkers and primary therapy outcome. Clin. Oral Investig. 2024, 28, 523. [Google Scholar] [CrossRef]
- Fragkioudakis, I.; Riggio, M.P.; Apatzidou, D.A. Understanding the microbial components of periodontal diseases and periodontal treatment-induced microbiological shifts. J. Med. Microbiol. 2021, 70, 001247. [Google Scholar] [CrossRef]
- Pirih, F.Q.; Monajemzadeh, S.; Singh, N.; Sinacola, R.S.; Shin, J.M.; Chen, T.; Fenno, J.C.; Kamarajan, P.; Rickard, A.H.; Travan, S.; et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol. 2000 2021, 87, 50–75. [Google Scholar] [CrossRef]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central regulatory role of cytokines in periodontitis and targeting options. Curr. Med. Chem. 2021, 28, 3032–3058. [Google Scholar] [CrossRef]
- Omran, T.Z.; Jasmi, F.S.O.A.; Obaid, K.M.; Ghareeb, A.K.R.; Alsailawi, H.A.; Mudhafar, M. The interleukin gene landscape: Understanding its influence on inflammatory mechanisms in apical periodontitis. Mol. Biol. Rep. 2025, 52, 365. [Google Scholar] [CrossRef]
- Santos, R.T.N.d.; Lima, L.P.O.d.; Muniz, M.T.C.; Álvares, P.R.; Silveira, M.M.F.d.; Sobral, A.P.V. Genetic polymorphism of interleukins 6 and 17 correlated with apical periodontitis: A Cross-sectional study. Braz. Dent. J. 2023, 34, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Daria, P.; Igor, B.; Oleh, K.; Victor, R. Characteristics of HIF-1A and HSP70 MRNA expression, level, and interleukins in experimental chronic generalized periodontitis. MicroRNA 2024, 13, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The role of interleukin 6 in periodontitis and its complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Azuma Presse, M.M.; Furusho, H.; Stashenko, P.; Sasaki, H. Revisiting the role of IL-1 signaling in the development of apical periodontitis. Front. Dent. Med. 2022, 3, 985558. [Google Scholar] [CrossRef]
- Irwin, C.; Myrillas, T. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998, 4, 43–47. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nakajima, T.; Gemmell, E.; Polak, B.; Seymour, G.J.; Hara, K. IL-4-and IL-6-producing cells in human periodontal disease tissue. J. Oral Pathol. Med. 1994, 23, 347–353. [Google Scholar] [CrossRef]
- Reis, C.; Da Costa, A.V.; Guimarães, J.T.; Tuna, D.; Braga, A.C.; Pacheco, J.J.; Arosa, F.A.; Salazar, F.; Cardoso, E.M. Clinical improvement following therapy for periodontitis: Association with a decrease in IL-1 and IL-6. Exp. Ther. Med. 2014, 8, 323–327. [Google Scholar] [CrossRef]
- Colucci, S.; Mori, G.; Brunetti, G.; Coricciati, M.; Pignataro, P.; Oranger, A.; Cirulli, N.; Mastrangelo, F.; Grassi, F.; Grano, M. Interleukin-7 production by B lymphocytes affects the T cell-dependent osteoclast formation in an in vitro model derived from human periodontitis patients. Int. J. Immunopathol. Pharmacol. 2005, 18, 13–19. [Google Scholar]
- Trubiani, O.; Isgro, A.; Zini, N.; Antonucci, I.; Aiuti, F.; Di Primio, R.; Nanci, A.; Caputi, S.; Paganelli, R. Functional interleukin-7/interleukin-7Rα, and SDF-1α/CXCR4 are expressed by human periodontal ligament derived mesenchymal stem cells. J. Cell. Physiol. 2008, 214, 706–713. [Google Scholar] [CrossRef]
- Kaymaz, K.; Beikler, T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Girnary, M.; Wang, L.; Jiao, Y.; Zeng, E.; Mercer, K.; Zhang, J.; Marchesan, J.T.; Yu, N.; Moss, K.; et al. IL-10 dampens an IL-17–mediated periodontitis-associated inflammatory network. J. Immunol. 2020, 204, 2177–2191. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, T.; Moutsopoulos, N.M. Osteoimmunology in periodontitis; a paradigm for Th17/IL-17 inflammatory bone loss. Bone 2022, 163, 116500. [Google Scholar] [CrossRef]
- Wang, F.; Guan, M.; Wei, L.; Yan, H. IL-18 promotes the secretion of matrix metalloproteinases in human periodontal ligament fibroblasts by activating NF-κB signaling. Mol. Med. Rep. 2019, 19, 703–710. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, P.; Zamora-Perez, A.; Fuentes-Lerma, M.; Robles-Gómez, C.; Mariaud-Schmidt, R.; Guerrero-Velázquez, C. IL-12 and IL-18 levels in serum and gingival tissue in aggressive and chronic periodontitis. Oral Dis. 2011, 17, 522–529. [Google Scholar] [CrossRef]
- Johnson, R.; Serio, F. Interleukin-18 concentrations and the pathogenesis of periodontal disease. J. Periodontol. 2005, 76, 785–790. [Google Scholar] [CrossRef]
- Shahbeik, S.; Taleghani, F.; Sattari, M.; Mohammadi, M.M.; Moravej, M. Evaluation of NLRP3 and IL-18 levels after periodontal therapy. Iran. J. Allergy Asthma Immunol. 2021, 20, 764–770. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, N.D.; Bey, A.; Khan, S. Salivary TNF-alpha: A potential marker of periodontal destruction. J. Indian Soc. Periodontol. 2014, 18, 306–310. [Google Scholar] [CrossRef]
- Varghese, S.S.; Thomas, H.; Jayakumar, N.; Sankari, M.; Lakshmanan, R. Estimation of salivary tumor necrosis factor-alpha in chronic and aggressive periodontitis patients. Contemp. Clin. Dent. 2015, 6, S152–S156. [Google Scholar] [CrossRef]
- Yun, F.; Firkova, E.I.; Xun, H.; Jun-Qi, L. Effects of surgical periodontal therapy on serum levels of TNF-alpha in patients with chronic periodontitis. Folia Med. 2007, 49, 37–40. [Google Scholar]
- Gomes, F.I.F.; Aragão, M.G.B.; Barbosa, F.C.B.; Bezerra, M.M.; Pinto, V.d.P.T.; Chaves, H.V. Inflammatory cytokines interleukin-1β and tumour necrosis factor-α-novel biomarkers for the detection of periodontal diseases: A literature review. J. Oral Maxillofac. Res. 2016, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.K.; Jung, M.; Kim, S.H.; Lee, S.R.; Park, K.H.; Kim, D.H.; Kim, H.H.; Park, Y.G. Assessment of IL-6, IL-8 and TNF-α levels in the gingival tissue of patients with periodontitis. Exp. Ther. Med. 2013, 6, 847–851. [Google Scholar] [CrossRef]
- Beklen, A.; Ainola, M.; Hukkanen, M.; Gürgan, C.; Sorsa, T.; Konttinen, Y.T. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J. Dent. Res. 2007, 86, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Gorgun, E.P.; Korkmaz, E.M.; Yüce, H.B.; Poyraz, O. The effects of IL-10 gene polymorphism on serum, and gingival crevicular fluid levels of IL-6 and IL-10 in chronic periodontitis. J. Appl. Oral Sci. 2018, 26, e20170232. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, C.; Cortinhas, A.; Morinha, F.; Leitão, J.; Viegas, C.A.; Bastos, E. Association of the IL-10 polymorphisms and periodontitis: A meta-analysis. Mol. Biol. Rep. 2012, 39, 9319–9329. [Google Scholar] [CrossRef]
- Lappin, D.; MacLeod, C.; Kerr, A.; Mitchell, T.; Kinane, D. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin. Exp. Immunol. 2001, 123, 294–300. [Google Scholar] [CrossRef]
- Bastos, M.; Lima, J.; Vieira, P.; Mestnik, M.; Faveri, M.; Duarte, P. TNF-α and IL-4 levels in generalized aggressive periodontitis subjects. Oral Dis. 2009, 15, 82–87. [Google Scholar] [CrossRef]
- Teixeira, J.F.L.; Henning, P.; Magalhaes, F.A.C.; Coletto-Nunes, G.; Floriano-Marcelino, T.; Westerlund, A.; Movérare-Skrtic, S.; Oliveira, G.J.; Lerner, U.H.; Souza, P.P.C. Osteoprotective effect by interleukin-4 (IL-4) on lipoprotein-induced periodontitis. Cytokine 2023, 172, 156399. [Google Scholar] [CrossRef]
- Shaker, O.G.; Ghallab, N.A. IL-17 and IL-11 GCF levels in aggressive and chronic periodontitis patients: Relation to PCR bacterial detection. Mediat. Inflamm. 2012, 2012, 174764. [Google Scholar] [CrossRef]
- Yetkin Ay, Z.; Sütçü, R.; Uskun, E.; Bozkurt, F.; Berker, E. The impact of the IL-11: IL-17 ratio on the chronic periodontitis pathogenesis: A preliminary report. Oral Dis. 2009, 15, 93–99. [Google Scholar] [CrossRef]
- Yücel, Ö.Ö.; Berker, E.; Gariboğlu, S.; Otlu, H. Interleukin-11, interleukin-1β, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J. Clin. Periodontol. 2008, 35, 365–370. [Google Scholar] [CrossRef]
- Johnson, R.; Wood, N.; Serio, F. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J. Periodontol. 2004, 75, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W. Interleukin-6: A masterplayer in the cytokine network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Khan, A.A.; Dinner, A.R. Gene regulatory networks in the immune system. Trends Immunol. 2014, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.J.; Mahajan, V.S.; Trajman, L.C.; Irvine, D.J.; Lauffenburger, D.A.; Chen, J. Interleukin-7 receptor signaling network: An integrated systems perspective. Cell. Mol. Immunol. 2008, 5, 79–89. [Google Scholar] [CrossRef]
- Schmitz, M.L.; Weber, A.; Roxlau, T.; Gaestel, M.; Kracht, M. Signal integration, crosstalk mechanisms and networks in the function of inflammatory cytokines. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 2165–2175. [Google Scholar] [CrossRef]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef]
- Ghanbari, M.; Maragheh, S.M.; Aghazadeh, A.; Mehrjuyan, S.R.; Hussen, B.M.; Shadbad, M.A.; Dastmalchi, N.; Safaralizadeh, R. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int. Immunopharmacol. 2021, 96, 107765. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, low-grade inflammation and systemic health: A scoping review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Madianos, P.N. Low-grade inflammation in chronic infectious diseases: Paradigm of periodontal infections. Ann. N. Y. Acad. Sci. 2006, 1088, 251–264. [Google Scholar] [CrossRef]
- Van Dyke, T.E. The management of inflammation in periodontal disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.O.; Goncalves, D.; Figueredo, C.M.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Falcao, A.; Bullón, P. A review of the influence of periodontal treatment in systemic diseases. Periodontol. 2000 2019, 79, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Chang, H.D.; Radbruch, A. The pro-and anti-inflammatory potential of interleukin-12. Ann. N. Y. Acad. Sci. 2007, 1109, 40–46. [Google Scholar] [CrossRef]
- Saxton, R.A.; Tsutsumi, N.; Su, L.L.; Abhiraman, G.C.; Mohan, K.; Henneberg, L.T.; Aduri, N.G.; Gati, C.; Garcia, K.C. Structure-based decoupling of the pro-and anti-inflammatory functions of interleukin-10. Science 2021, 371, eabc8433. [Google Scholar] [CrossRef]
- Hajishengallis, G. Aging and its impact on innate immunity and inflammation: Implications for periodontitis. J. Oral Biosci. 2014, 56, 30–37. [Google Scholar] [CrossRef]
- Meyle, J.; Dommisch, H.; Groeger, S.; Giacaman, R.A.; Costalonga, M.; Herzberg, M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017, 44, 1215–1225. [Google Scholar] [CrossRef]
- Gankovskaya, L.; Khelminskaya, N.; Molchanova, E.; Svitich, O. Role of innate immunity factors in periodontitis pathogenesis. J. Microbiol. Epidemiol. Immunobiol. 2016, 93, 100–107. [Google Scholar] [CrossRef]
- El-Awady, A.R.; Elashiry, M.; Morandini, A.C.; Meghil, M.M.; Cutler, C.W. Dendritic cells a critical link to alveolar bone loss and systemic disease risk in periodontitis: Immunotherapeutic implications. Periodontol. 2000 2022, 89, 41–50. [Google Scholar] [CrossRef]
- Cutler, C.W.; Jotwani, R. Antigen-presentation and the role of dendritic cells in periodontitis. Periodontol. 2000 2004, 35, 135–157. [Google Scholar] [CrossRef]
- Wilensky, A.; Segev, H.; Mizraji, G.; Shaul, Y.; Capucha, T.; Shacham, M.; Hovav, A.H. Dendritic cells and their role in periodontal disease. Oral Dis. 2014, 20, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Lappin, D.F. Immune processes in periodontal disease: A review. Ann. Periodontol. 2002, 7, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.T.; Liu, J.; Seymour, G.J.; Faggion, C.M., Jr.; Cullinan, M.P. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontol. 2000 2016, 71, 22–51. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, B.; Monajemzadeh, S.; Silva, D.; Pirih, F.Q. Modulating the immune response in periodontitis. Front. Dent. Med. 2022, 3, 879131. [Google Scholar] [CrossRef]
- Berglundh, T.; Liljenberg, B.; Lindhe, J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J. Clin. Periodontol. 2002, 29, 705–709. [Google Scholar] [CrossRef]
- Medara, N.; Lenzo, J.C.; Walsh, K.A.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Darby, I.B. Peripheral T helper cell profiles during management of periodontitis. J. Clin. Periodontol. 2021, 48, 77–91. [Google Scholar] [CrossRef]
- Dutzan, N.; Abusleme, L. T helper 17 cells as pathogenic drivers of periodontitis. In Oral Mucosal Immunity and Microbiome; Springer: Cham, Switzerland, 2019; pp. 107–117. [Google Scholar]
- Bi, C.S.; Sun, L.J.; Qu, H.L.; Chen, F.; Tian, B.M.; Chen, F.M. The relationship between T-helper cell polarization and the RANKL/OPG ratio in gingival tissues from chronic periodontitis patients. Clin. Exp. Dent. Res. 2019, 5, 377–388. [Google Scholar] [CrossRef]
- Deng, J.; Lu, C.; Zhao, Q.; Chen, K.; Ma, S.; Li, Z. The Th17/Treg cell balance: Crosstalk among the immune system, bone and microbes in periodontitis. J. Periodontal Res. 2022, 57, 246–255. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Jerez, A.; Vernal, R.; Monasterio, G.; Pandis, N.; Faggion, C.M., Jr. The therapeutic potential of regulatory T lymphocytes in periodontitis: A systematic review. J. Periodontal Res. 2019, 54, 207–217. [Google Scholar] [CrossRef]
- Zou, J.; Zeng, Z.; Xie, W.; Zeng, Z. Immunotherapy with regulatory T and B cells in periodontitis. Int. Immunopharmacol. 2022, 109, 108797. [Google Scholar] [CrossRef]
- Alvarez, C.; Rojas, C.; Rojas, L.; Cafferata, E.A.; Monasterio, G.; Vernal, R. Regulatory T lymphocytes in periodontitis: A translational view. Mediat. Inflamm. 2018, 2018, 7806912. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Romero-Castro, N.S.; Becerra-Ruiz, J.S.; Romero-Servin, S.; Heboyan, A. Increased of IL-18 levels are associated with periodontitis: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 981. [Google Scholar] [CrossRef]
- Peluzzo, A.M.; Autieri, M.V. Challenging the paradigm: Anti-inflammatory interleukins and angiogenesis. Cells 2022, 11, 587. [Google Scholar] [CrossRef]
- Cuneo, A.A.; Autieri, M.V. Expression and function of anti-inflammatory interleukins: The other side of the vascular response to injury. Curr. Vasc. Pharmacol. 2009, 7, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.P.; Diez, D.; Miranda-Saavedra, D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Briefings Funct. Genom. 2013, 12, 489–498. [Google Scholar]
- Ip, W.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar]
- Pretolani, M. Interleukin-10: An anti-inflammatory cytokine with therapeutic potential. Clin. Exp. Allergy 1999, 29, 1164. [Google Scholar] [CrossRef]
- Khalaf, H.; Lönn, J.; Bengtsson, T. Cytokines and chemokines are differentially expressed in patients with periodontitis: Possible role for TGF-β1 as a marker for disease progression. Cytokine 2014, 67, 29–35. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Ni, Q.; Wang, T.; Bao, C.; Geng, Y.; Lu, Y.; Cao, Y.; Li, Y.; Li, L.; et al. B-cell–derived TGF-β1 inhibits osteogenesis and contributes to bone loss in periodontitis. J. Dent. Res. 2023, 102, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Kini, V.; Mohanty, I.; Telang, G.; Vyas, N. Immunopathogenesis and distinct role of Th17 in periodontitis: A review. J. Oral Biosci. 2022, 64, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Javadi, M.; Borghaei, R.C. Interleukin-4 Suppresses IL-17–Induced Expression of Matrix Metalloproteinase-3 in Human Gingival Fibroblasts. J. Periodontol. 2004, 75, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E. IL-4 and IL-13: Regulators and effectors of wound repair. Annu. Rev. Immunol. 2023, 41, 229–254. [Google Scholar] [CrossRef]
- Ho, J.Y.; Yeo, B.S.; Yang, X.L.; Thirugnanam, T.; Hakeem, M.F.; Sahu, P.S.; Pulikkotil, S.J. Local and systemic expression profile of IL-10, IL-17, IL-27, IL-35, and IL-37 in periodontal diseases: A cross-sectional study. J. Contemp. Dent. Pract. 2021, 22, 73–79. [Google Scholar]
- Tang, Z.; Jin, L.; Yang, Y. The dual role of IL-17 in periodontitis regulating immunity and bone homeostasis. Front. Immunol. 2025, 16, 1578635. [Google Scholar] [CrossRef]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: A crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef]
- Mills, K.H. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Abbas, R.F.; Akram, H.M. Salivary IL-17 and IL-10 as potential diagnostic biomarkers of different stages of periodontitis in smoker and nonsmoker patients. Eur. J. Dent. 2024, 18, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.A.; de Oliveira Alves, R.; Nascimento, I.M.; Hidalgo, M.A.R.; Scarel-Caminaga, R.M.; Cristina Pigossi, S. Pro-and anti-inflammatory cytokines and osteoclastogenesis-related factors in peri-implant diseases: Systematic review and meta-analysis. BMC Oral Health 2023, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.O. Mechanisms involved in regulation of periodontal ligament cell production of pro-inflammatory cytokines: Implications in periodontitis. J. Periodontal Res. 2021, 56, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.S.; Heluy, S.L.; Cruz, D.F.; da Silva, H.D.P.; Feres, M.; Figueiredo, L.C.; Duarte, P.M. The ratios of pro-inflammatory to anti-inflammatory cytokines in the serum of chronic periodontitis patients with and without type 2 diabetes and/or smoking habit. Clin. Oral Investig. 2019, 23, 641–650. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Moore, B.B.; Zhang, S.; Xiao, P.; Decker, A.M.; Wang, H.L. IL-17: Balancing protective immunity and pathogenesis. J. Immunol. Res. 2023, 2023, 3360310. [Google Scholar] [CrossRef]
- Majumder, S.; McGeachy, M.J. IL-17 in the pathogenesis of disease: Good intentions gone awry. Annu. Rev. Immunol. 2021, 39, 537–556. [Google Scholar] [CrossRef]
- Abusleme, L. IL-17/Th17 Responses and Their Influence on Oral Microbial Communities. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2017. [Google Scholar]
- Margraf, A.; Perretti, M. Immune cell plasticity in inflammation: Insights into description and regulation of immune cell phenotypes. Cells 2022, 11, 1824. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology 2021, 163, 3–18. [Google Scholar] [CrossRef]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef]
- Alhendi, A.; Naser, S.A. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front. Immunol. 2023, 14, 1295230. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Lv, Z.; Xiong, L.; Zhang, H.; Yin, N.; Qi, H. The dual role of IL-27 in CD4+ T cells. Mol. Immunol. 2021, 138, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Hughes, F.J.; Taams, L.S. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J. Clin. Periodontol. 2014, 41, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Jin, Y.; Miao, Y.; Wang, Y.; Zhou, Y.; Lin, X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology 2020, 108, 350–357. [Google Scholar] [CrossRef]
- Rajendran, M.; Looney, S.; Singh, N.; Elashiry, M.; Meghil, M.M.; El-Awady, A.R.; Tawfik, O.; Susin, C.; Arce, R.M.; Cutler, C.W. Systemic antibiotic therapy reduces circulating inflammatory dendritic cells and Treg–Th17 plasticity in periodontitis. J. Immunol. 2019, 202, 2690–2699. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Morand, D.; Davideau, J.L.; Clauss, F.; Jessel, N.; Tenenbaum, H.; Huck, O. Cytokines during periodontal wound healing: Potential application for new therapeutic approach. Oral Dis. 2017, 23, 300–311. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Howells, G. Cytokine networks in destructive periodontal disease. Oral Dis. 1995, 1, 266–270. [Google Scholar] [CrossRef]
- Trindade, F.; Oppenheim, F.G.; Helmerhorst, E.J.; Amado, F.; Gomes, P.S.; Vitorino, R. Uncovering the molecular networks in periodontitis. Proteom.–Clin. Appl. 2014, 8, 748–761. [Google Scholar] [CrossRef]
- Larsson, L.; Castilho, R.M.; Giannobile, W.V. Epigenetics and its role in periodontal diseases: A state-of-the-art review. J. Periodontol. 2015, 86, 556–568. [Google Scholar] [CrossRef]
- Jurdziński, K.T.; Potempa, J.; Grabiec, A.M. Epigenetic regulation of inflammation in periodontitis: Cellular mechanisms and therapeutic potential. Clin. Epigenet. 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Fahimipour, F.; Tarran, R.; Kim, S.; Scarel-Caminaga, R.M.; Justice, A.; North, K. Epigenetic reprogramming in periodontal disease: Dynamic crosstalk with potential impact in oncogenesis. Periodontol. 2000 2020, 82, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; di Giovine, F.S. Genetic variations in cytokine expression: A risk factor for severity of adult periodontitis. Ann. Periodontol. 1998, 3, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Brodzikowska, A.; Górska, R.; Kowalski, J. Interleukin-1 genotype in periodontitis. Arch. Immunol. Ther. Exp. 2019, 67, 367–373. [Google Scholar] [CrossRef]
- López, N.J.; Jara, L.; Valenzuela, C.Y. Association of interleukin-1 polymorphisms with periodontal disease. J. Periodontol. 2005, 76, 234–243. [Google Scholar] [CrossRef]
- Gao, C.; Iles, M.; Larvin, H.; Bishop, D.T.; Bunce, D.; Ide, M.; Sun, F.; Pavitt, S.; Wu, J.; Kang, J. Genome-wide association studies on periodontitis: A systematic review. PLoS ONE 2024, 19, e0306983. [Google Scholar] [CrossRef]
- Munz, M.; Richter, G.M.; Loos, B.G.; Jepsen, S.; Divaris, K.; Offenbacher, S.; Teumer, A.; Holtfreter, B.; Kocher, T.; Bruckmann, C.; et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur. J. Hum. Genet. 2019, 27, 102–113. [Google Scholar] [CrossRef]
- Divaris, K.; Monda, K.L.; North, K.E.; Olshan, A.F.; Reynolds, L.M.; Hsueh, W.C.; Lange, E.M.; Moss, K.; Barros, S.P.; Weyant, R.J.; et al. Exploring the genetic basis of chronic periodontitis: A genome-wide association study. Hum. Mol. Genet. 2013, 22, 2312–2324. [Google Scholar] [CrossRef]
- Schulz, S.; Immel, U.D.; Just, L.; Schaller, H.G.; Gläser, C.; Reichert, S. Epigenetic characteristics in inflammatory candidate genes in aggressive periodontitis. Hum. Immunol. 2016, 77, 71–75. [Google Scholar] [CrossRef]
- Shaddox, L.; Mullersman, A.; Huang, H.; Wallet, S.; Langaee, T.; Aukhil, I. Epigenetic regulation of inflammation in localized aggressive periodontitis. Clin. Epigenet. 2017, 9, 94. [Google Scholar] [CrossRef]
- Wa Lee, Y.H.; Am Na, H.S.; Jeong, S.Y.; Jeong, S.H.E.; Park, H.R.Y.; Chung, J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell 2011, 35, 43–49. [Google Scholar] [CrossRef]
- Motedayyen, H.; Ghotloo, S.; Saffari, M.; Sattari, M.; Amid, R. Evaluation of microRNA-146a and its targets in gingival tissues of patients with chronic periodontitis. J. Periodontol. 2015, 86, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Letra, A.; Silva, R.M. MicroRNAs markedly expressed in apical periodontitis cooperatively regulate cytokines and growth factors promoting an anti-inflammatory response. J. Endod. 2023, 49, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G. MicroRNAs and immunity in periodontal health and disease. Int. J. Oral Sci. 2018, 10, 24. [Google Scholar] [CrossRef]
- D’aiuto, F.; Nibali, L.; Parkar, M.; Patel, K.; Suvan, J.; Donos, N. Oxidative stress, systemic inflammation, and severe periodontitis. J. Dent. Res. 2010, 89, 1241–1246. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28, e20190248. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Hasturk, H.; Kantarci, A.; Serhan, C.N.; Van Dyke, T. Atherosclerosis, periodontal disease, and treatment with resolvins. Curr. Atheroscler. Rep. 2017, 19, 57. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Bartold, P.M.; Lopez-Oliva, I. Periodontitis and rheumatoid arthritis: An update 2012–2017. Periodontol. 2000 2020, 83, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and rheumatoid arthritis: The same inflammatory mediators? Mediat. Inflamm. 2019, 2019, 6034546. [Google Scholar] [CrossRef]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of periodontitis and rheumatoid arthritis: Current evidence and potential biological interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef]
- González-Febles, J.; Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol. 2000 2021, 87, 181–203. [Google Scholar] [CrossRef]
- Bobetsis, Y.A.; Graziani, F.; Gürsoy, M.; Madianos, P.N. Periodontal disease and adverse pregnancy outcomes. Periodontol. 2000 2020, 83, 154–174. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Aizawa, S.; Hayakawa, S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. Gynaecol. Res. 2019, 45, 5–12. [Google Scholar] [CrossRef]
- Figuero, E.; Han, Y.W.; Furuichi, Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol. 2000 2020, 83, 175–188. [Google Scholar] [CrossRef]
- Markovics, A.; Rosenthal, K.S.; Mikecz, K.; Carambula, R.E.; Ciemielewski, J.C.; Zimmerman, D.H. Restoring the balance between pro-inflammatory and anti-inflammatory cytokines in the treatment of rheumatoid arthritis: New insights from animal models. Biomedicines 2021, 10, 44. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A cross-talk between diet and the oral microbiome: Balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Tan, A.; Gürbüz, N.; Özbalci, F.İ.; Koşkan, Ö.; Yetkin Ay, Z. Increase in serum and salivary neutrophil gelatinase-associated lipocalin levels with increased periodontal inflammation. J. Appl. Oral Sci. 2020, 28, e20200276. [Google Scholar] [CrossRef]
- Holmström, S.B.; Lira-Junior, R.; Zwicker, S.; Majster, M.; Gustafsson, A.; Åkerman, S.; Klinge, B.; Svensson, M.; Boström, E.A. MMP-12 and S100s in saliva reflect different aspects of periodontal inflammation. Cytokine 2019, 113, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Keller, T.; Noack, B.; Freitag, A.; Netuschil, L.; Hoffmann, T. Evaluation of a novel point-of-care test for active matrix metalloproteinase-8: Agreement between qualitative and quantitative measurements and relation to periodontal inflammation. J. Periodontal Res. 2017, 52, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Dolińska, E.; Wiśniewski, P.; Pietruska, M. Periodontal molecular diagnostics: State of knowledge and future prospects for clinical application. Int. J. Mol. Sci. 2024, 25, 12624. [Google Scholar] [CrossRef]
- Moore, G. The Effect of Interproximal Home Regimens of Inflammatory Biomarkers in Periodontal Maintenance Patients. Master’s Thesis, University of Nebraska Medical Center, Omaha, NE, USA, 2022. [Google Scholar]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Church, L.D.; McDermott, M.F. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr. Opin. Mol. Ther. 2009, 11, 81–89. [Google Scholar] [PubMed]
- Alten, R.; Gomez-Reino, J.; Durez, P.; Beaulieu, A.; Sebba, A.; Krammer, G.; Preiss, R.; Arulmani, U.; Widmer, A.; Gitton, X.; et al. Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: Results of a 12-week, phase II, dose-finding study. BMC Musculoskelet. Disord. 2011, 12, 153. [Google Scholar] [CrossRef]
- Kavanaugh, A.F. Anti-tumor necrosis factor-α monoclonal antibody therapy for rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 1998, 24, 593–614. [Google Scholar] [CrossRef]
- Reynolds, M.A. Modifiable risk factors in periodontitis: At the intersection of aging and disease. Periodontol. 2000 2014, 64, 7–19. [Google Scholar] [CrossRef]
- Bartold, P.M. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontol. 2000 2018, 78, 7–11. [Google Scholar] [CrossRef]
- Sabine Elisabeth, G.; Zhou, Y.; Yong, J.; Wang, L.; Sabine, R.; Joerg, M. Systemic, Lifestyle and Environmental Modifying Factors in the Pathogenesis of Periodontitis. J. Periodontal Res. 2025. [Google Scholar] [CrossRef]
- Korte, D.L.; Kinney, J. Personalized medicine: An update of salivary biomarkers for periodontal diseases. Periodontol. 2000 2016, 70, 26–37. [Google Scholar] [CrossRef]
- Gundelly, M.; Pusuluri, S.V.; Koduganti, R.R.; Ambati, M.; Chiluveru, S.; Chandaka, M.; Mrunalini, G.; Pusuluri, S.; Sneha, C.; Chandaka, M. Precision Medicine in Periodontics: A Literature Review. Cureus 2024, 16, e68952. [Google Scholar] [CrossRef]
- Vomero, M.; Manganelli, V.; Barbati, C.; Colasanti, T.; Capozzi, A.; Finucci, A.; Spinelli, F.; Ceccarelli, F.; Perricone, C.; Truglia, S.; et al. Reduction of autophagy and increase in apoptosis correlates with a favorable clinical outcome in patients with rheumatoid arthritis treated with anti-TNF drugs. Arthritis Res. Ther. 2019, 21, 39. [Google Scholar] [CrossRef]
- Riitano, G.; Spinelli, F.; Manganelli, V.; Caissutti, D.; Capozzi, A.; Garufi, C.; Garofalo, T.; Misasi, R.; Sorice, M.; Conti, F.; et al. Wnt signaling as a translational target in rheumatoid and psoriatic arthritis. J. Transl. Med. 2025, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Tomás, I.; Arias-Bujanda, N.; Alonso-Sampedro, M.; Casares-de Cal, M.; Sánchez-Sellero, C.; Suárez-Quintanilla, D.; Balsa-Castro, C. Cytokine-based predictive models to estimate the probability of chronic periodontitis: Development of diagnostic nomograms. Sci. Rep. 2017, 7, 11580. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pintos, T.; Regueira-Iglesias, A.; Balsa-Castro, C.; Tomás, I. Update on the role of cytokines as oral biomarkers in the diagnosis of periodontitis. In Periodontitis: Advances in Experimental Research; Springer: Cham, Switzerland, 2022; pp. 283–302. [Google Scholar]
- Martínez-García, M.; Hernández-Lemus, E. The Molecular Comorbidity Network of Periodontal Disease. Int. J. Mol. Sci. 2024, 25, 10161. [Google Scholar] [CrossRef] [PubMed]
- Brozek, R.; Lorenz, A.; Dorocka-Bobkowska, B.; Kurpisz, M. Immunoinflammatory response in periodontal diseases. J. Physiol. Pharmacol. 2025, 76, 49–258. [Google Scholar]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]


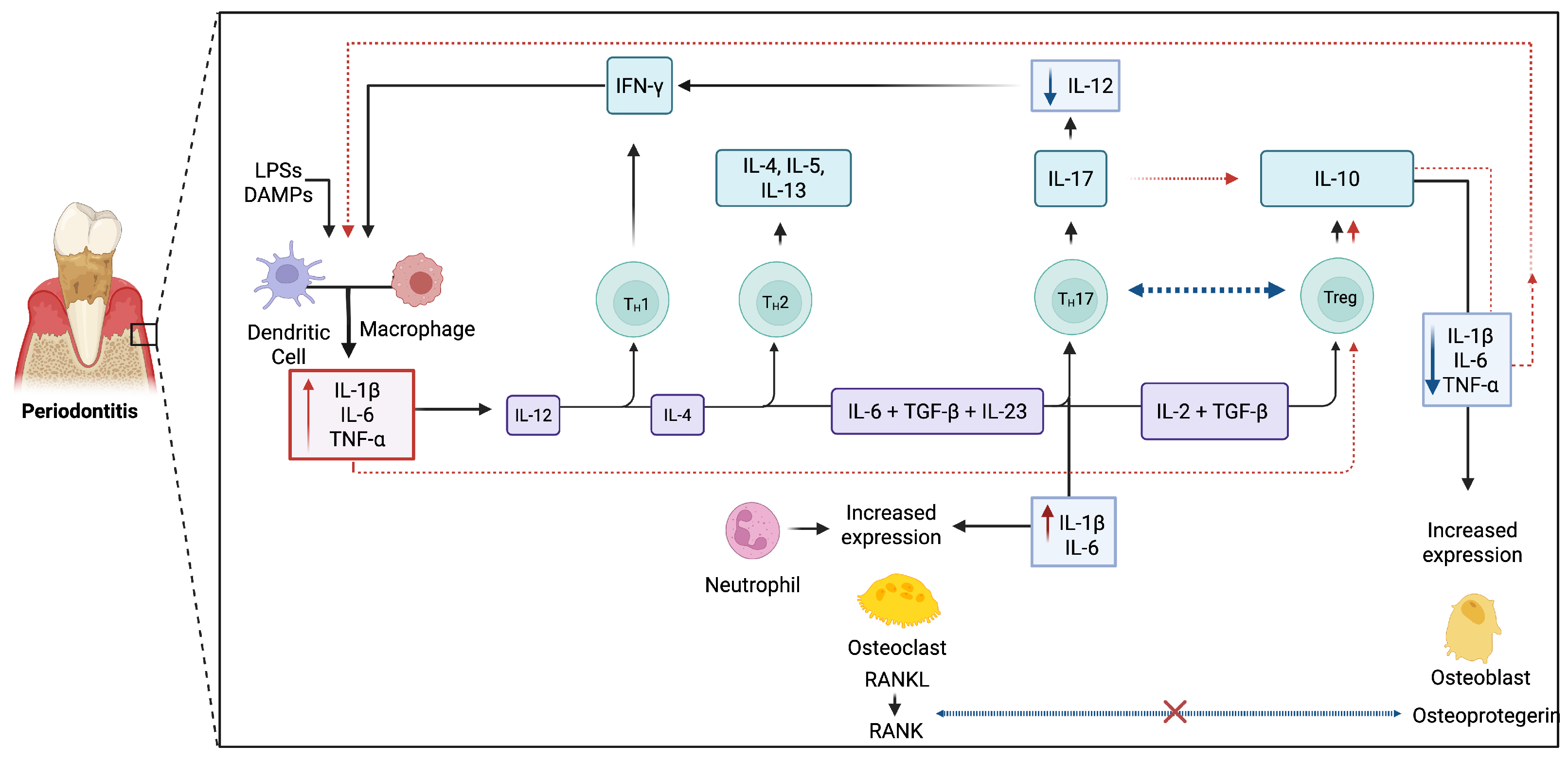

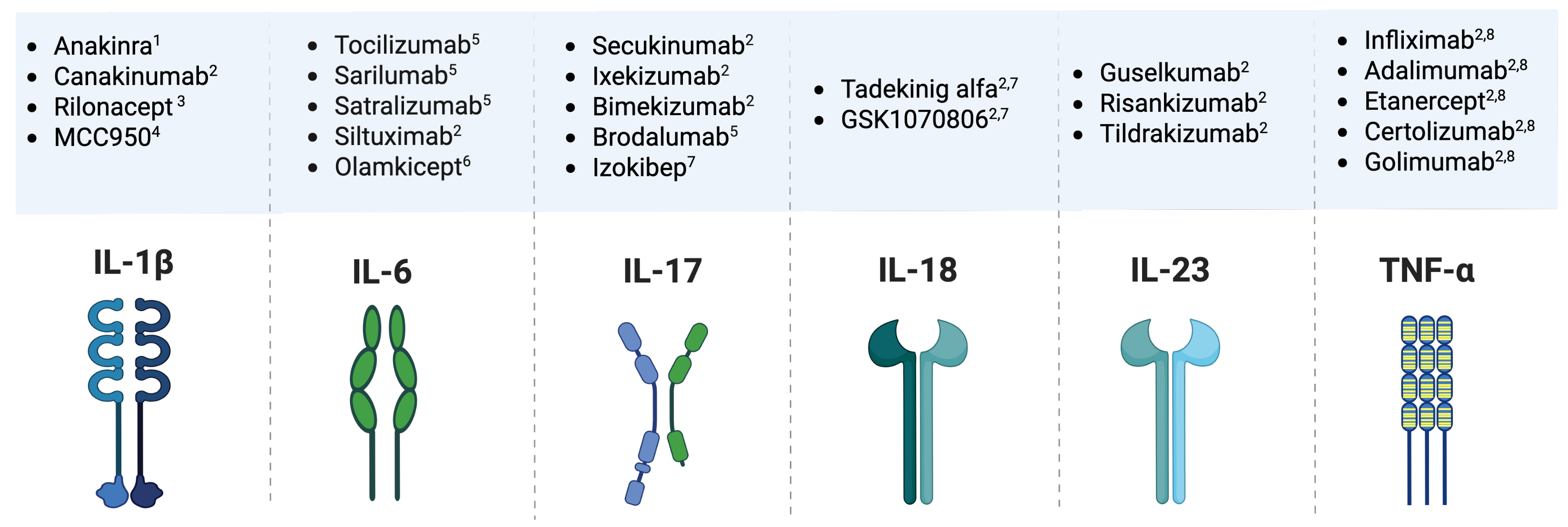
| IL | Source | Target | Effect | Relationship with Other Interleukins |
|---|---|---|---|---|
| IL-1 | Fb, GECs, DCs and M | Endo cells and Osteoclast precursors | Amplifies inflammation, induces MMPs and RANKL; enhances osteoclastogenesis and tissue destruction | Synergizes with TNF- and induces IL-8 |
| IL-6 | Fb, Endo cells, Epi cells and M | Hepatocytes, B cells, T cells and Osteoclasts | Stimulates acute-phase response and promotes osteoclast differentiation | Induced by IL-1 and TNF-; promotes IL-17 production |
| IL-8 | Fb, Epi cells and M | Neutrophils | Neutrophil recruitment and activation; sustains inflammation and collateral tissue damage | Induced by IL-1 and TNF-; promotes further IL-8 via neutrophil activation |
| IL-12 | DCs and M | Naïve T cells and NK cells | Promotes Th1 differentiation and IFN- production | Works with IL-18 to enhance IFN-; antagonizes IL-4 effects |
| IL-17 | Th17 cells | Fb, Epi cells, Osteoblasts and M | Stimulates neutrophil recruitment and induces pro-inflammatory cytokines and MMPs | Induced by IL-6 and IL-23; promotes IL-8 and TNF- |
| IL-18 | DCs and M | T cells and NK cells | Enhances IFN- production, promotes Th1 responses and contributes to tissue damage | Works with IL-12 to promote Th1; synergizes with IL-1 and counter-regulated by IL-10 |
| IL-23 | DCs and M | Th17 cells | Stabilizes and expands Th17 responses that drive bone resorption | Upstream of IL-17A/F; induced by TLR/IL-1 signals |
| IL-33 | Fb, Epi cells and Endo cells | Th2 cells and Mast cells | Acts as alarmin, triggers type-2 cytokine production and contributes to tissue inflammation and repair | Induces IL-4, IL-5 and IL-13; interacts with Treg cells |
| IL | Source | Target | Effect | Relationship with Other Interleukins |
|---|---|---|---|---|
| IL-4 | Th2 cells and Mast cells | Naïve T cells and M | Promotes humoral/Th2 responses and suppresses excessive macrophage activation and MMP production | Induced by IL-33; antagonizes IL-12 and IFN- |
| IL-10 | Treg cells and M | M, DCs and T cells | Suppresses pro-inflammatory cytokine production and antigen presentation, limiting tissue damage | Inhibits IL-1, IL-6, and TNF- |
| IL-11 | Fb and Epi cells | M | Downregulates TNF- and MMPs, protects connective tissue, and modulates mucosal immunity | Antagonizes IL-1 and TNF-; acts synergistically with IL-10 |
| IL-13 | Th2 cells and Mast cells | M | Induces M2-like macrophage polarization and may limit excessive tissue destruction | Induced by IL-4 and IL-33; antagonizes IL-12 |
| IL-27 | DCs and M | Naïve T cells | Suppresses Th17 differentiation, induces IL-10, and promotes a balance between inflammation and tissue healing | Antagonizes IL-6 and IL-17 pathways |
| Cytokine | Matrix (Periodontitis vs. Control) | Assay/Platform | Effect Size (Fold-Change) | Diagnostic Metrics (AUC, Cut-Off When Available) | Pre-Analytical Notes |
|---|---|---|---|---|---|
| IL-1 | GCF (site-specific) [32]; Saliva [155] | ELISA, Helsinki, Finland, multiplex bead immunoassay | ∼3–5× higher in active sites vs. healthy [32]; decreases after therapy [19] | Tomás et al., 2017 nomogram including IL-1 yielded AUC ≈ 0.82 for chronic periodontitis [159] | Standardize collection (isolated vs. pooled GCF), avoid saliva stimulation; store at −80 °C |
| IL-6 | GCF, saliva, serum [17,35] | ELISA or Luminex®, Austin, TX, USA multiplex | ∼2–4× higher in periodontitis vs. controls [17]; reduction parallels clinical improvement [19] | Combined with IL-1 improved discrimination (AUC ≈ 0.80–0.83) [159] | Circadian variation; influenced by smoking/diabetes; matrix choice critical |
| IL-17 | GCF [42]; Saliva (smokers vs. nonsmokers) [94] | ELISA, high-sensitivity multiplex | Detected in 60–80% of diseased vs. <20% of healthy sites [32]; salivary IL-17 rises with disease stage and smoking [92] | Mohammed et al., 2024: IL-17 + IL-10 panel AUC ≈ 0.78 for stage III–IV vs. controls [92] | Freeze–thaw sensitive; single vs. pooled GCF alters values; adjust for smoking status |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-García, M.; Hernández-Lemus, E. Pro-Inflammatory and Anti-Inflammatory Interleukins in Periodontitis: Molecular Roles, Immune Crosstalk, and Therapeutic Perspectives. Int. J. Mol. Sci. 2025, 26, 10094. https://doi.org/10.3390/ijms262010094
Martínez-García M, Hernández-Lemus E. Pro-Inflammatory and Anti-Inflammatory Interleukins in Periodontitis: Molecular Roles, Immune Crosstalk, and Therapeutic Perspectives. International Journal of Molecular Sciences. 2025; 26(20):10094. https://doi.org/10.3390/ijms262010094
Chicago/Turabian StyleMartínez-García, Mireya, and Enrique Hernández-Lemus. 2025. "Pro-Inflammatory and Anti-Inflammatory Interleukins in Periodontitis: Molecular Roles, Immune Crosstalk, and Therapeutic Perspectives" International Journal of Molecular Sciences 26, no. 20: 10094. https://doi.org/10.3390/ijms262010094
APA StyleMartínez-García, M., & Hernández-Lemus, E. (2025). Pro-Inflammatory and Anti-Inflammatory Interleukins in Periodontitis: Molecular Roles, Immune Crosstalk, and Therapeutic Perspectives. International Journal of Molecular Sciences, 26(20), 10094. https://doi.org/10.3390/ijms262010094








