Pleiotropic Mucosal Innate Immune Memory in the Gastrointestinal Tract
Abstract
1. Introduction
2. Mechanisms Underlying Innate Immune Memory (Trained Immunity)
3. Emerging Roles for Innate Immune Cells in Trained Immunity
3.1. Neutrophils and Trained Granulopoiesis
3.2. Monocytes and Macrophages in Trained Immunity
3.3. Dendritic Cells (DCs) and Trained Antigen Presentation
3.4. Natural Killer (NK) Cells and Cytokine-Driven Innate Memory
3.5. Innate Lymphoid Cells (ILCs) and Tissue-Adapted Training
3.6. Integrative Perspective: Conserved Logic of Innate Training Across Cell Types
4. Overview of Mucosal Innate Immunity in the GI Tract
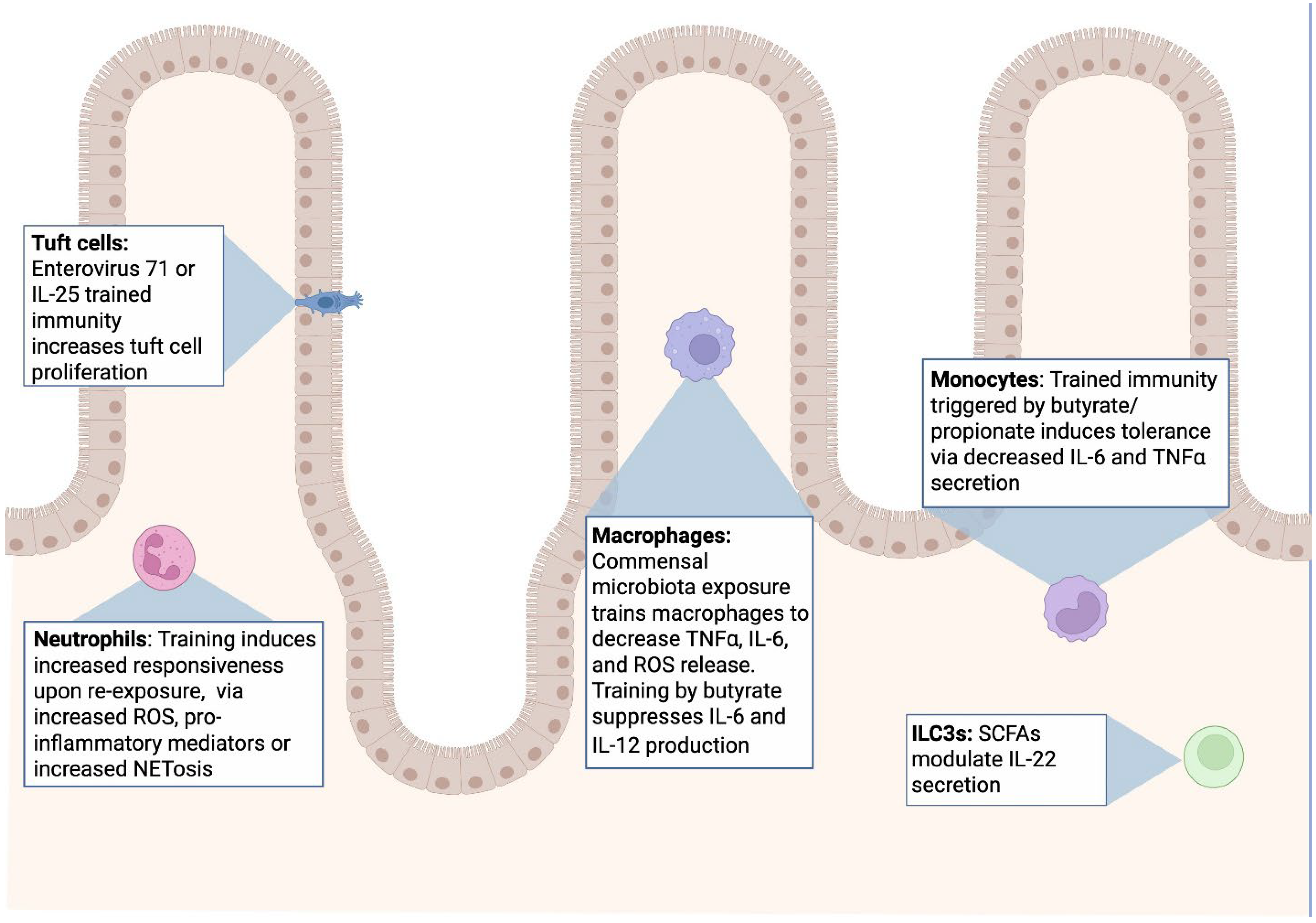
5. Specifics of Innate Immune Memory in the Gastrointestinal Tract
5.1. Microbiota Induced Trained Immunity
5.2. Short Chain Fatty Acids
6. Innate Immune Memory and GI-Related Diseases
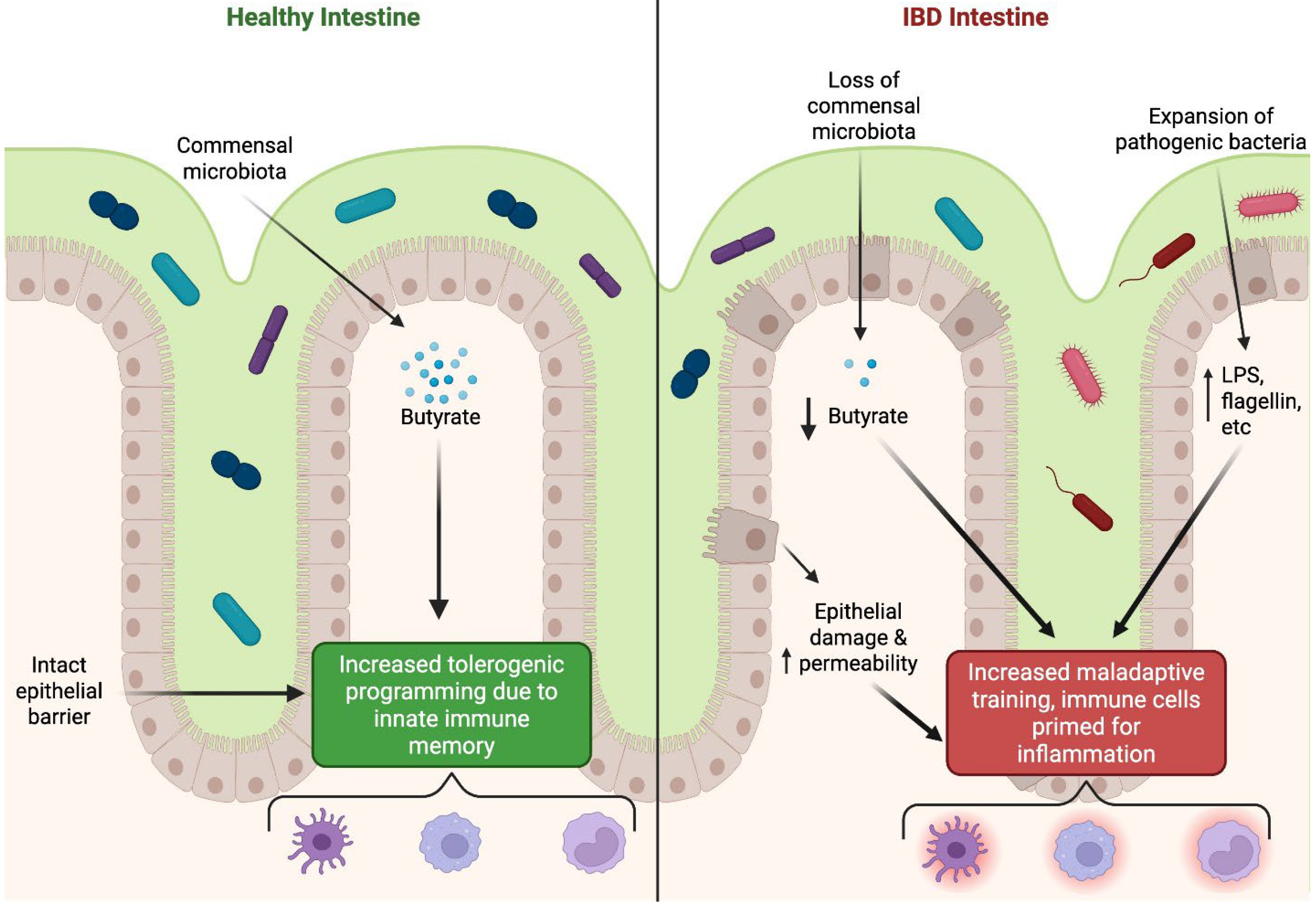
6.1. Inflammatory Bowel Diseases: Crohn’s Disease and Ulcerative Colitis
6.2. Irritable Bowel Syndrome (IBS) and Disorders of Gut–Brain Interactions (DGBI)
6.3. Infections and Mucosal Pathogens
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Lowry, C.A. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008, 29, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; van der Meer, J.W. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Caldwell, B.A.; Li, L. Epigenetic regulation of innate immune dynamics during inflammation. J. Leukoc. Biol. 2024, 115, 589–606. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Khan, N.; Novakovic, B.; Kaufmann, E.; Jansen, T.; van Crevel, R.; Divangahi, M.; Netea, M.G. beta-Glucan Induces Protective Trained Immunity against Mycobacterium tuberculosis Infection: A Key Role for IL-1. Cell Rep. 2020, 31, 107634. [Google Scholar] [CrossRef] [PubMed]
- Vierboom, M.P.M.; Dijkman, K.; Sombroek, C.C.; Hofman, S.O.; Boot, C.; Vervenne, R.A.W.; Haanstra, K.G.; van der Sande, M.; van Emst, L.; Dominguez-Andres, J.; et al. Stronger induction of trained immunity by mucosal BCG or MTBVAC vaccination compared to standard intradermal vaccination. Cell Rep. Med. 2021, 2, 100185. [Google Scholar] [CrossRef] [PubMed]
- Watts, E.R.; Howden, A.J.M.; Morrison, T.; Sadiku, P.; Hukelmann, J.; von Kriegsheim, A.; Ghesquiere, B.; Murphy, F.; Mirchandani, A.S.; Humphries, D.C.; et al. Hypoxia drives murine neutrophil protein scavenging to maintain central carbon metabolism. J. Clin. Investig. 2021, 131, e134073. [Google Scholar] [CrossRef]
- López-Collazo, E.; del Fresno, C. Endotoxin tolerance and trained immunity: Breaking down immunological memory barriers. Front. Immunol. 2024, 15, 1393283. [Google Scholar] [CrossRef]
- Novakovic, B.; Habibi, E.; Wang, S.Y.; Arts, R.J.W.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef]
- De Laval, B.; Maurizio, J.; Kandalla, P.K.; Brisou, G.; Simonnet, L.; Huber, C.; Gimenez, G.; Matcovitch-Natan, O.; Reinhardt, S.; David, E.; et al. C/EBPbeta-Dependent Epigenetic Memory Induces Trained Immunity in Hematopoietic Stem Cells. Cell Stem Cell 2020, 26, 793. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yu, X.; Saha, G.; Kalafati, L.; Ioannidis, C.; Mitroulis, I.; Netea, M.G.; Chavakis, T.; Hajishengallis, G. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 2022, 185, 1709–1727.e1718. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Netea, M.G.; Chavakis, T. Trained immunity in chronic inflammatory diseases and cancer. Nat. Rev. Immunol. 2025, 25, 497–514. [Google Scholar] [CrossRef]
- Kalafati, L.; Hatzioannou, A.; Hajishengallis, G.; Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 2023, 314, 142–157. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.M.; Curtis, V.F.; Lanis, J.M.; Alexeev, E.E.; Welch, N.; Goldberg, M.S.; Schaefer, R.E.M.; Gao, R.Y.; Chun, C.; Fennimore, B.; et al. Adaptation to inflammatory acidity through neutrophil-derived adenosine regulation of SLC26A3. Mucosal Immunol. 2020, 13, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.M.; Zhou, L.; Koch, S.D.; Welch, N.; Zakharov, D.; Callahan, R.; Steiner, C.A.; Gerich, M.E.; Onyiah, J.C.; Colgan, S.P. Chlorination of epithelial tight junction proteins by neutrophil myeloperoxidase promotes barrier dysfunction and mucosal inflammation. JCI Insight 2024, 9, e178525. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Campbell, E.L.; Kominsky, D.J. Hypoxia and Mucosal Inflammation. Annu. Rev. Pathol.-Mech. 2016, 11, 77–100. [Google Scholar] [CrossRef]
- Kinnebrew, M.A.; Pamer, E.G. Innate immune signaling in defense against intestinal microbes. Immunol. Rev. 2012, 245, 113–131. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity–basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef]
- Ziogas, A.; Bruno, M.; van der Meel, R.; Mulder, W.J.M.; Netea, M.G. Trained immunity: Target for prophylaxis and therapy. Cell Host Microbe 2023, 31, 1776–1791. [Google Scholar] [CrossRef]
- Gu, Z.R.; Chen, X.T.; Zhu, D.D.; Wu, S.T.; Yu, C.G. Histone deacetylase 1 and 3 inhibitors alleviate colon inflammation by inhibiting Th17 cell differentiation. J. Clin. Lab. Anal. 2022, 36, e24699. [Google Scholar] [CrossRef]
- Mennillo, E.; Kim, Y.J.; Lee, G.; Rusu, I.; Patel, R.K.; Dorman, L.C.; Flynn, E.; Li, S.; Bain, J.L.; Andersen, C.; et al. Single-cell and spatial multi-omics highlight effects of anti-integrin therapy across cellular compartments in ulcerative colitis. Nat. Commun. 2024, 15, 1493. [Google Scholar] [PubMed]
- Moerings, B.G.J.; de Graaff, P.; Furber, M.; Witkamp, R.F.; Debets, R.; Mes, J.J.; van Bergenhenegouwen, J.; Govers, C. Continuous Exposure to Non-Soluble beta-Glucans Induces Trained Immunity in M-CSF-Differentiated Macrophages. Front. Immunol. 2021, 12, 672796. [Google Scholar]
- Sadeghi, A.; Biglari, M.; Nasseri Moghaddam, S. Post-infectious Irritable Bowel Syndrome: A Narrative Review. Middle East. J. Dig. Dis. 2019, 11, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Moorlag, S.J.; Dominguez-Andres, J.; Bulut, O.; Kilic, G.; Liu, Z.; van Crevel, R.; Xu, C.J.; Joosten, L.A.; Netea, M.G.; et al. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes. J. Clin. Investig. 2022, 132, e147719. [Google Scholar]
- Fanucchi, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Barroso de Figueiredo, A.M.; Teodoro Silva, M.V.; Cirovic, B.; de Bree, L.C.J.; Damen, M.; Moorlag, S.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. beta-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: A Crucial Role for IL-32. Cell Rep. 2019, 28, 2659–2672.e2656. [Google Scholar] [CrossRef]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin. Exp. Rheumatol. 2016, 34, S12–S16. [Google Scholar]
- Chang, P.V.; Hao, L.M.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Jia, H.L.; He, X.L.; Jiang, T.F.; Kong, F.Z. Roles of Bile Acid-Activated Receptors in Monocytes-Macrophages and Dendritic Cells. Cells 2025, 14, 920. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Porbahaie, M.; Hummel, A.; Saouadogo, H.; Coelho, R.M.L.; Savelkoul, H.F.J.; Teodorowicz, M.; van Neerven, R.J.J. Short-chain fatty acids inhibit the activation of T lymphocytes and myeloid cells and induce innate immune tolerance. Benef. Microbes 2023, 14, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Schütz, B.; Krause, F.F.; Taudte, R.V.; Zaiss, M.M.; Luu, M.; Visekruna, A. Modulation of Host Immunity by Microbiome-Derived Indole-3-Propionic Acid and Other Bacterial Metabolites. Eur. J. Immunol. 2025, 55, e202451594. [Google Scholar] [CrossRef] [PubMed]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Yip, W.; Hughes, M.R.; Li, Y.C.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- Adrover, J.M.; del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossío, I.; et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 2020, 183, 771–785.e712. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.M.; Chen, L.S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Natoli, G.; Cassatella, M.A.; Tamassia, N. Epigenetic regulation of neutrophil development and function. Semin. Immunol. 2016, 28, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Tso, G.H.W.; Tan, A.S.M.; Hor, P.X.; Bohme, J.; Teng, K.W.W.; Newell, E.W.; Singhal, A.; Pavelka, N. Gut-Evolved Candida albicans Induces Metabolic Changes in Neutrophils. Front. Cell Infect. Microbiol. 2021, 11, 743735. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Nayer, B.; Singh, S.K.; Alshoubaki, Y.K.; Yuan, E.; Park, A.J.; Maruyama, K.; Akira, S.; Martino, M.M. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 2024, 628, 604–611. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Ryu, S.; Lim, M.; Kim, J.; Kim, H.Y. Versatile roles of innate lymphoid cells at the mucosal barrier: From homeostasis to pathological inflammation. Exp. Mol. Med. 2023, 55, 1845–1857. [Google Scholar] [CrossRef]
- Dominguez-Andres, J.; Netea, M.G. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 2019, 105, 329–338. [Google Scholar] [CrossRef]
- Velikova, T.; El Kaouri, I.; Bakopoulou, K.; Gulinac, M.; Naydenova, K.; Dimitrov, M.; Peruhova, M.; Lazova, S. Mucosal Immunity and Trained Innate Immunity of the Gut. Gastroenterol. Insights 2024, 15, 661–675. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Wu, J.; Zhang, F.; Lyu, R.; You, Q.; Qian, Y.J.; Cai, Y.R.; Tian, X.Y.; Tao, H.J.; He, Y.T.; et al. Trained immunity of intestinal tuft cells during infancy enhances host defense against enteroviral infections in mice. EMBO Mol. Med. 2024, 16, 2516–2538. [Google Scholar] [PubMed]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e317. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharm. Ther. 2008, 27, 104–119. [Google Scholar]
- Cao, S.; Nguyen, K.M.; Ma, K.; Du, X.; Liu, X.; Ulezko Antonova, A.; Rood, R.P.; Gremida, A.; Chen, C.H.; Gutierrez, A.; et al. Mucosal Single-Cell Profiling of Crohn’s-Like Disease of the Pouch Reveals Unique Pathogenesis and Therapeutic Targets. Gastroenterology 2024, 167, 1399–1414.e1392. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Kokkinou, E.; Soini, T.; Pandey, R.V.; van Acker, A.; Theorell, J.; Czarnewski, P.; Kvedaraite, E.; Vandamme, N.; Lourda, M.; Sorini, C.; et al. The single-cell transcriptional landscape of innate and adaptive lymphocytes in pediatric-onset colitis. Cell Rep. Med. 2023, 4, 101038. [Google Scholar] [CrossRef]
- Knight, H.R.; Kim, M.; Kannan, N.; Taylor, H.; Main, H.; Azcue, E.; Esser-Kahn, A. Bioengineering approaches to trained immunity: Physiologic targets and therapeutic strategies. eLife 2025, 14, e106339. [Google Scholar] [CrossRef]
- Martin-Cruz, L.; Benito-Villalvilla, C.; Angelina, A.; Subiza, J.L.; Palomares, O. Trained immunity-based vaccines for infections and allergic diseases. J. Allergy Clin. Immunol. 2024, 154, 1085–1094. [Google Scholar] [CrossRef]
- Gudino, V.; Bartolome-Casado, R.; Salas, A. Single-cell omics in inflammatory bowel disease: Recent insights and future clinical applications. Gut 2025, 74, 1335–1345. [Google Scholar] [CrossRef]
- Li, S.; Zou, Y.; McMasters, A.; Chen, F.; Yan, J. Trained immunity: A new player in cancer immunotherapy. eLife 2025, 14, e104920. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, H.; Jiang, G.; Fang, L.; Hao, J. Knowledge mapping of trained immunity/innate immune memory: Insights from two decades of studies. Hum. Vaccin. Immunother. 2024, 20, 2415823. [Google Scholar] [CrossRef] [PubMed]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained immunity: General and emerging concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef]
- Bleriot, C.; Dunsmore, G.; Alonso-Curbelo, D.; Ginhoux, F. A temporal perspective for tumor-associated macrophage identities and functions. Cancer Cell 2024, 42, 747–758. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, G.; Fan, X.Y.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, A.; Shah, K.; Bernier-Latmani, J.; Köller, Y.; Coakley, G.; Moyat, M.; Hamelin, R.; Armand, F.; Wong, N.C.; Ramay, H.; et al. Small intestinal resident eosinophils maintain gut homeostasis following microbial colonization. Immunity 2022, 55, 1250–1267.e12. [Google Scholar] [CrossRef]
- Arnold, I.C.; Artola-Borán, M.; Tallón de Lara, P.; Kyburz, A.; Taube, C.; Ottemann, K.; van den Broek, M.; Yousefi, S.; Simon, H.U.; Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J. Exp. Med. 2018, 215, 2055–2072. [Google Scholar] [CrossRef]
- Spencer, L.A.; Weller, P.F. Eosinophils and Th2 immunity: Contemporary insights. Immunol. Cell Biol. 2010, 88, 250–256. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Ahrens, R.; Osterfeld, H.; Gurish, M.F.; Han, X.N.; Åbrink, M.; Finkelman, F.D.; Pejler, G.; Hogan, S.P. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 22381–22386. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Pellon, A.; Barriales, D.; Peña-Cearra, A.; Castelo-Careaga, J.; Palacios, A.; Lopez, N.; Atondo, E.; Pascual-Itoiz, M.A.; Martín-Ruiz, I.; Sampedro, L.; et al. The commensal bacterium imprints innate memory-like responses in mononuclear phagocytes. Gut Microbes 2021, 13, 1939598. [Google Scholar] [CrossRef]
- Peña-Cearra, A.; Palacios, A.; Pellon, A.; Castelo, J.; Pasco, S.T.; Seoane, I.; Barriales, D.; Martin, J.E.; Pascual-Itoiz, M.A.; Gonzalez-Lopez, M.; et al. Akkermansia muciniphila-induced trained immune phenotype increases bacterial intracellular survival and attenuates inflammation. Commun. Biol. 2024, 7, 192. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, W.D.; Yu, D.; Forman, B.M.; Huang, W. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef]
- Bhargavi, G.; Subbian, S. The causes and consequences of trained immunity in myeloid cells. Front. Immunol. 2024, 15, 1365127. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Jiménez, E.; Vázquez, A.; González, S.; Sacedón, R.; Fernández-Sevilla, L.M.; Varas, A.; Subiza, J.L.; Valencia, J.; Vicente, A. Mucosal Bacterial Immunotherapy Attenuates the Development of Experimental Colitis by Reducing Inflammation Through the Regulation of Myeloid Cells. Int. J. Mol. Sci. 2024, 25, 13629. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The Immune System in Irritable Bowel Syndrome. J. Neurogastroenterol. 2011, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Liebregts, T.; Adam, B.; Bredack, C.; Roth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Evans, M.; Chow, C.E.T.; Chan, L.S.; Leyer, G. Impact of a probiotic product on bowel habits and microbial profile in participants with functional constipation: A randomized controlled trial. J. Digest Dis. 2019, 20, 435–446. [Google Scholar] [CrossRef]
- Piche, T. Tight junctions and IBS—The link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroent. Motil. 2014, 26, 296–302. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Spiller, R.; Lam, C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J. Neurogastroenterol. 2012, 18, 258–268. [Google Scholar] [CrossRef]
- Dore, M.P.; Pes, G.M. Trained Immunity and Trained Tolerance: The Case of Infection. Int. J. Mol. Sci. 2024, 25, 5856. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Serafini, N.; Jarade, A.; Surace, L.; Goncalves, P.; Sismeiro, O.; Varet, H.; Legendre, R.; Coppee, J.Y.; Disson, O.; Durum, S.K.; et al. Trained ILC3 responses promote intestinal defense. Science 2022, 375, 859–863. [Google Scholar] [CrossRef]
- Hu, T.Y.; Liu, C.H.; Lei, M.; Zeng, Q.M.; Li, L.; Tang, H.; Zhang, N.N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]

| Innate Cell Type | Shared Mechanistic Features | Distinct/Cell-Specific Mechanisms | Representative References |
|---|---|---|---|
| Common to all trained innate cells | Epigenetic remodeling: ↑ H3K4me3/H3K27ac; ↓ H3K9me3; enhanced chromatin accessibility at cytokine/metabolic loci; mTOR–HIF-1α axis linking glycolysis and lipid metabolism; accumulation of fumarate, succinate, acetyl-CoA reinforcing histone acetylation; interplay of metabolic intermediates and chromatin modifiers; balance between inflammatory “training” and tolerogenic “trained tolerance.” | — | [1,2,5,7,13,30,36] |
| Neutrophils | Share metabolic and redox-dependent pathways with monocytes. | Short-lived “trained granulopoiesis” (IL-1β/GM-CSF imprint HSPCs in bone marrow); MPO-dependent redox signaling modifies chromatin in neighboring cells; rapid glycolytic reprogramming; transient chromatin accessibility at oxidative/NETosis loci; reversible, progenitor-driven short-term memory (days). | [12,20,47,48,49,50,52,53] |
| Monocytes/Macrophages | Canonical model of trained immunity. | β-glucan/BCG activate Dectin-1–Syk–CARD9 → mTOR–HIF-1α; mevalonate and glutaminolysis pathways generate epigenetic cofactors; SCFAs induce tolerogenic “trained tolerance” via HDAC inhibition → ↑ IL-10, ↓ IL-6/TNF; distinct chromatin landscapes in intestinal macrophages shaped by microbiota. | [2,5,7,10,12,17,18,32,35,38,49,53,66,68,76,77] |
| Dendritic Cells | Share metabolic rewiring with monocytes. | β-glucan/peptidoglycan → persistent H3K4me3/H3K27ac at IL12, IL23, CD80/CD86; mTOR–HIF-1α-driven glycolysis/mevalonate metabolism; retinoic acid & TSLP induce tolerogenic DCs; CD103+ vs. CD11b+ DCs show distinct trained/tolerogenic chromatin states. | [10,36,57,60,63] |
| Natural Killer Cells | Epigenetic and metabolic circuits parallel macrophage training. | Cytokine-induced NK memory (IL-12/IL-15/IL-18) → stable accessibility at IFNG, PRF1, GZMB; mTORC1/c-Myc activation → glycolysis + mitochondrial biogenesis; trained by BCG, β-glucan, inosine, butyrate; persistent mucosal activation via IL-18/IL-12. | [13,62,66,68] |
| Innate Lymphoid Cells (ILCs) | Share epigenetic accessibility at cytokine and metabolic enhancers. | ILC2: repeated IL-33/TSLP → H3K27ac at IL5/IL13; ↑ FAO/glycolytic plasticity. ILC3: IL-1β/AhR ligands → H3K27ac at IL22/IL17F; switch to oxidative phosphorylation + FAO. ILC1: IL-12/IL-18 → long-lived IFN-γ-competent state; distinct tissue-adapted enhancer landscapes at mucosal surfaces. | [36,57,59,60,61,69,71,78] |
| Contextual modifiers (mucosal environment) | — | Microbial metabolites (SCFAs, indoles, bile acids) modulate trained vs. tolerant states through AHR, FXR, and TGR5; cytokine milieu (IL-10, IL-33, IL-25) and epithelial signals integrate metabolic context. | [57,62,79,80,81,82,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, R.H.; Colgan, S.P.; Cartwright, I.M. Pleiotropic Mucosal Innate Immune Memory in the Gastrointestinal Tract. Int. J. Mol. Sci. 2025, 26, 10093. https://doi.org/10.3390/ijms262010093
Cohen RH, Colgan SP, Cartwright IM. Pleiotropic Mucosal Innate Immune Memory in the Gastrointestinal Tract. International Journal of Molecular Sciences. 2025; 26(20):10093. https://doi.org/10.3390/ijms262010093
Chicago/Turabian StyleCohen, Rachel H., Sean P. Colgan, and Ian M. Cartwright. 2025. "Pleiotropic Mucosal Innate Immune Memory in the Gastrointestinal Tract" International Journal of Molecular Sciences 26, no. 20: 10093. https://doi.org/10.3390/ijms262010093
APA StyleCohen, R. H., Colgan, S. P., & Cartwright, I. M. (2025). Pleiotropic Mucosal Innate Immune Memory in the Gastrointestinal Tract. International Journal of Molecular Sciences, 26(20), 10093. https://doi.org/10.3390/ijms262010093







