Molecular Signatures of Dendritic Cell Activation upon TNF Stimulation: A Multi-Omics Study in Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
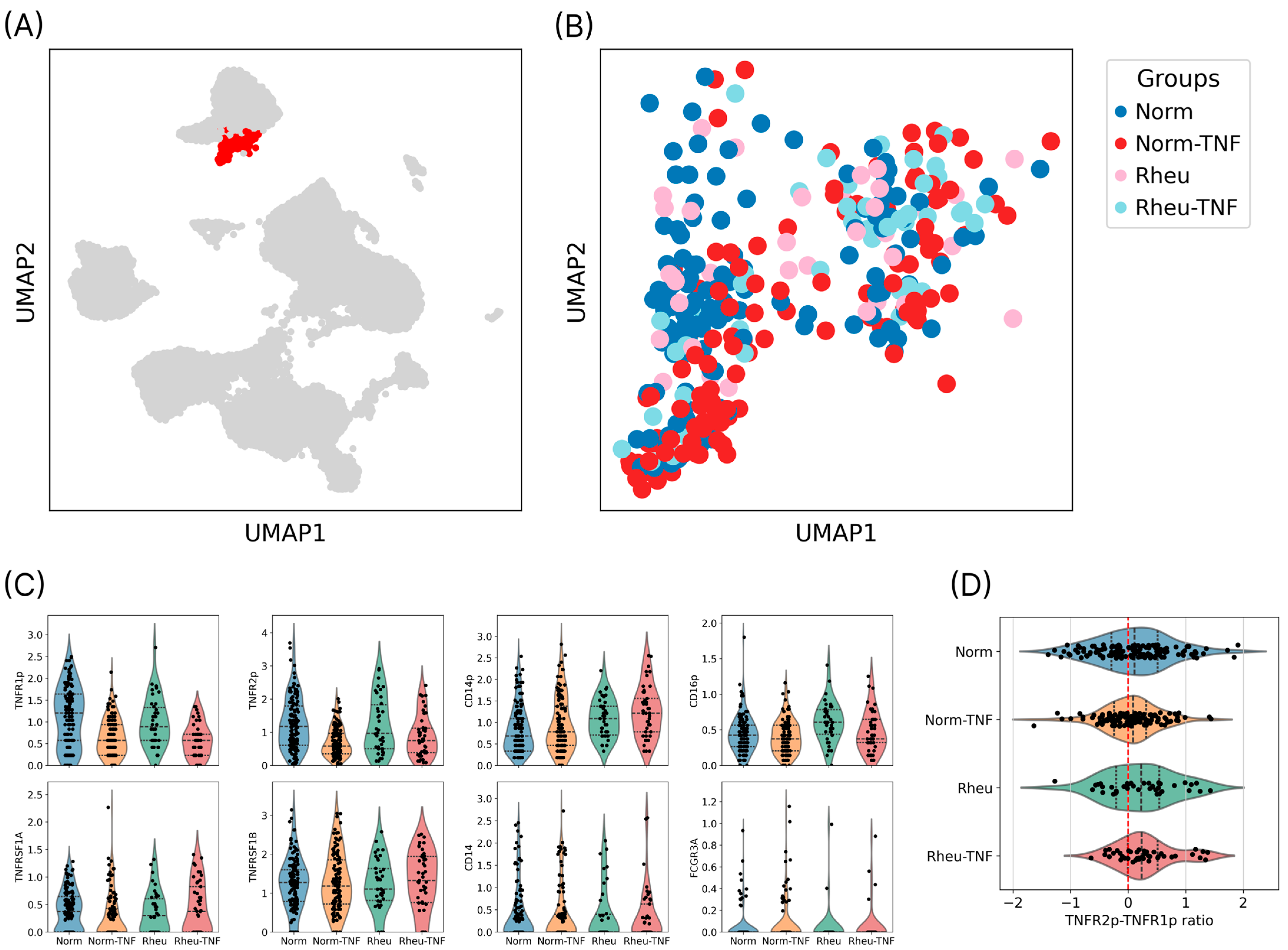
2.1. Identification and Annotation of the Dendritic Cell Population
2.2. Expression of TNF Receptors on the Surface of Dendritic Cells
2.3. TNFR1/TNFR2 Ratio and Receptor Profile Characteristics
2.4. Differential Gene Expression in Dendritic Cells Before and After TNF Stimulation
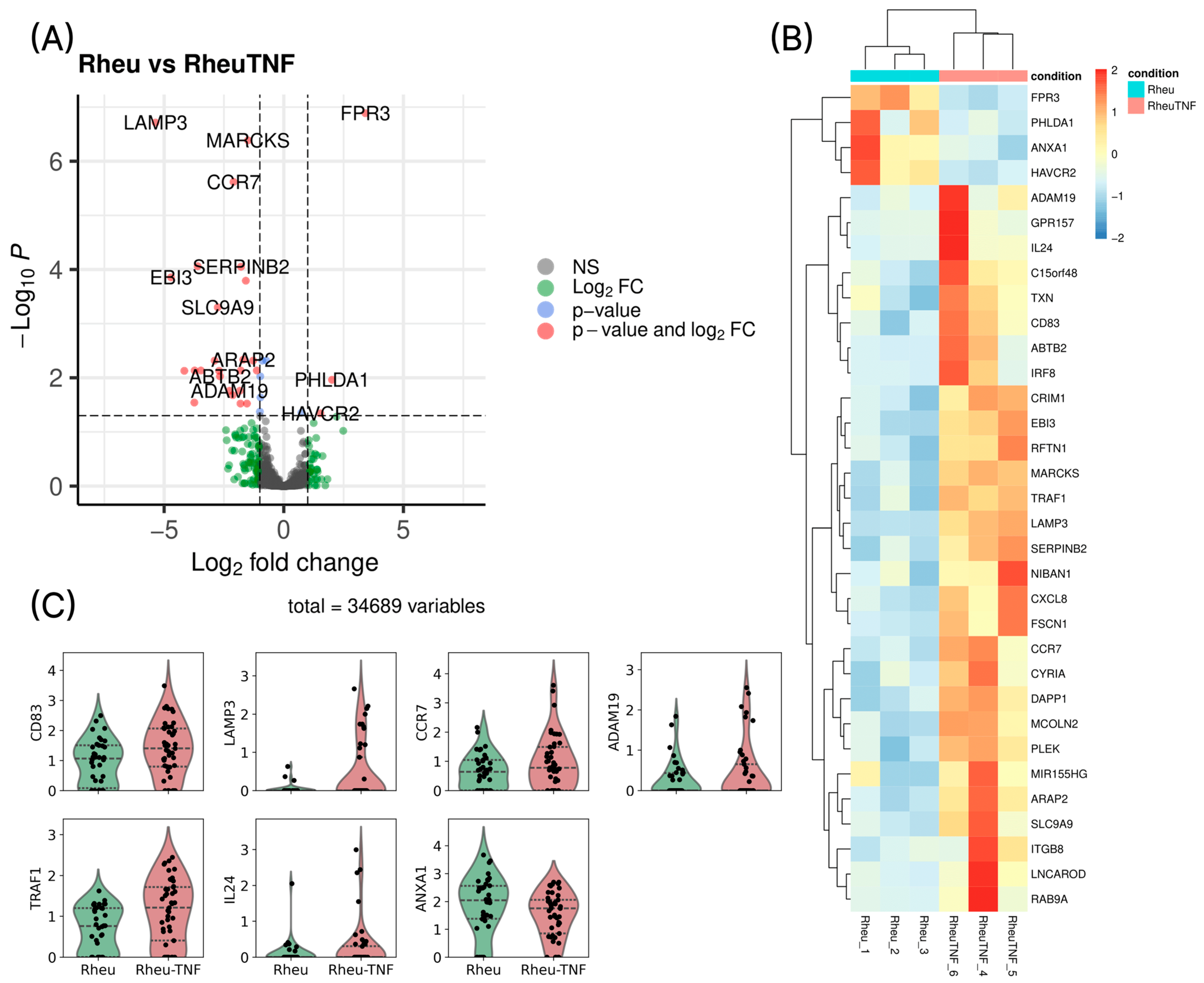
3. Discussion
4. Materials and Methods
4.1. Cell Preparation
4.2. Sample Tag and AbSeq Labeling
4.3. Library Preparation and Sequencing
4.4. Data Preprocessing
4.5. Differential Gene Expression Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DC | Dendritic cell |
| RA | Rheumatoid arthritis |
| TNF | Tumor necrosis factor alpha |
| WNN | Weighted nearest neighbor |
| UMAP | Uniform Manifold Approximation and Projection |
| PCA | Principal component analysis |
| HVGs | Highly variable genes |
| CLR | Centered log-ratio |
| PBMCs | Peripheral blood mononuclear cells |
References
- Lutzky, V.; Hannawi, S.; Thomas, R. Cells of the synovium in rheumatoid arthritis. Dendritic cells. Arthritis Res. Ther. 2007, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Suwa, Y.; Nagafuchi, Y.; Yamada, S.; Fujio, K. The role of dendritic cells and their immunometabolism in rheumatoid arthritis. Front. Immunol. 2023, 14, 1161148. [Google Scholar] [CrossRef] [PubMed]
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27. [Google Scholar] [CrossRef]
- Krueger, G.; Callis, K. Potential of tumor necrosis factor inhibitors in psoriasis and psoriatic arthritis. Arch. Dermatol. 2004, 140, 218–225. [Google Scholar] [CrossRef]
- Paleolog, E. The therapeutic potential of TNF-alpha blockade in rheumatoid arthritis. Expert Opin. Investig. Drugs 2003, 12, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Certolizumab pegol: A review of its use in patients with axial spondyloarthritis or psoriatic arthritis. Drugs 2014, 74, 999–1016. [Google Scholar] [CrossRef]
- Samarpita, S.; Rasool, M. Majoon chobchini reinstates PDL-1 expression and blocks dendritic cell–T helper 17 pathogenic axis in rheumatoid arthritis animal model. Cytokine 2023, 163, 156136. [Google Scholar] [CrossRef]
- Falaleeva, S.A.; Kurilin, V.V.; Shkaruba, N.S.; Alshevskaya, A.A.; Sennikov, S.V. Role subpopulation of dendritic cells in the pathogenesis of rheumatoid arthritis. Immunologiya 2014, 35, 291–294. [Google Scholar]
- Lebre, M.C.; Tak, P.P. Dendritic cells in rheumatoid arthritis: Which subset should be used as a tool to induce tolerance? Hum. Immunol. 2009, 70, 321–324. [Google Scholar] [CrossRef]
- Kavousanaki, M.; Makrigiannakis, A.; Boumpas, D.; Verginis, P. Novel role of plasmacytoid dendritic cells in humans: Induction of interleukin-10-producing Treg cells in patients with rheumatoid arthritis responding to therapy. Arthritis Rheum. 2010, 62, 53–63. [Google Scholar] [CrossRef]
- Ning, B.; Wei, J.; Zhang, A.; Gong, W.; Fu, J.; Jia, T.; You-Yang, S. Antigen-specific tolerogenic dendritic cells ameliorate the severity of murine collagen-induced arthritis. PLoS ONE 2015, 10, e0131152. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.Y.; Huang, Y.S.; Lin, H.H.; Luo, S.F.; McCann, F.; McNamee, K.; Clanchy, F.; Williams, R. TNFR signalling and its clinical implications. Cytokine 2018, 101, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.M. Tumor necrosis factor receptor pre-ligand assembly domain is an important therapeutic target in inflammatory arthritis. BioDrugs 2007, 21, 23–29. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, A.W.; Barrera, P.; Smeets, R.L.; van Riel, P.L.C.M.; van den Berg, W.B.; Radstake, T.R.D.J. Inhibition of TNF alpha during maturation of dendritic cells results in the development of semi-mature cells. Ann. Rheum. Dis. 2005, 64, 408–414. [Google Scholar] [CrossRef]
- Ye, L.L.; Wei, X.S.; Zhang, M.; Niu, Y.R.; Zhou, Q. The significance of tumor necrosis factor receptor type II in CD8+ regulatory T cells and CD8+ effector T cells. Front. Immunol. 2018, 9, 583. [Google Scholar] [CrossRef]
- Zakharova, M.; Ziegler, H.K. Paradoxical anti-inflammatory actions of TNF-alpha via TNF receptor 1 in macrophages and dendritic cells. J. Immunol. 2005, 175, 5024–5033. [Google Scholar] [CrossRef]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Alshevskaya, A.; Lopatnikova, J.; Zhukova, J.; Chumasova, O.; Shkaruba, N.; Sizikov, A.; Evsegneeva, I.; Gladkikh, V.; Karaulov, A.; Sennikov, S.V. Co-expression profile of TNF membrane-bound receptors type 1 and 2 in rheumatoid arthritis on immunocompetent cells subsets. Int. J. Mol. Sci. 2019, 21, 288. [Google Scholar] [CrossRef]
- Ashton, M.P.; Eugster, A.; Dietz, S.; Loebel, D.; Lindner, A.; Kuehn, D.; Taranko, A.E.; Heschel, B.; Gavrisan, A.; Ziegler, A.-G.; et al. Association of dendritic cell signatures with autoimmune inflammation revealed by single-cell profiling. Arthritis Rheumatol. 2019, 71, 817–828. [Google Scholar] [CrossRef]
- Figueiredo, M.L. Applications of single-cell RNA sequencing in rheumatoid arthritis. Front. Immunol. 2024, 15, 1491318. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, X.; Zhang, Y.; Yang, F.; Su, H.; Zhang, H.; Li, Z.; Zhang, W.; Sun, W. Interactions between LAMP3+ dendritic cells and T-cell subpopulations promote immune evasion in papillary thyroid carcinoma. J. Immunother. Cancer 2024, 12, e008983. [Google Scholar] [CrossRef] [PubMed]
- Grosche, L.; Knippertz, I.; König, C.; Royzman, D.; Wild, A.B.; Zinser, E.; Sticht, H.; Muller, Y.A.; Steinkasserer, A.; Lechmann, M. The CD83 molecule—An important immune checkpoint. Front. Immunol. 2020, 11, 721. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.; Chong, W. Interleukin-24 immunobiology and its roles in inflammatory diseases. Int. J. Mol. Sci. 2022, 23, 627. [Google Scholar] [CrossRef]
- Edilova, M.I.; Abdul-Sater, A.A.; Watts, T.H. TRAF1 signaling in human health and disease. Front. Immunol. 2018, 9, 2969. [Google Scholar] [CrossRef]
- Tang, Y.; Aleithan, F.; Madahar, S.S.; Mirzaesmaeili, A.; Saran, S.; Tang, J.; Zangiabadi, S.; Inman, R.; Sweeney, G.; Abdul-Sater, A.A. Selective disruption of Traf1/cIAP2 interaction attenuates inflammatory responses and rheumatoid arthritis. J. Autoimmun. 2025, 152, 103377. [Google Scholar] [CrossRef]
- Huggins, A.; Paschalidis, N.; Flower, R.J.; Perretti, M.; D’Acquisto, F. Annexin-1-deficient dendritic cells acquire a mature phenotype during differentiation. FASEB J. 2009, 23, 985–996. [Google Scholar] [CrossRef]
- Pieper, D.; Schirmer, S.; Prechtel, A.T.; Kehlenbach, R.H.; Hauber, J.; Chemnitz, J. Functional characterization of the HuR:CD83 mRNA interaction. PLoS ONE 2011, 6, e23290. [Google Scholar] [CrossRef]
- Kim, C.H.; Nagata, K.; Butcher, E.C. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J. Immunol. 2003, 171, 152–158. [Google Scholar] [CrossRef]
- Bourque, J.; Hawiger, D. Immunomodulatory bonds of the partnership between dendritic cells and T cells. Crit. Rev. Immunol. 2018, 38, 379–401. [Google Scholar] [CrossRef]
- Dutertre, C.A.; Becht, E.; Irac, S.E.; Khalilnezhad, A.; Narang, V.; Khalilnezhad, S.; Ng, P.Y.; van den Hoogen, L.L.; Leong, J.Y.; Lee, B.; et al. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity 2019, 51, 573–589.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Li, Y.; Qi, L.; Si, W.; Bo, Y.; Chen, X.; Ye, Z.; Fan, H.; Liu, B.; et al. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell 2024, 42, 1268–1285.e7. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.A.; Angerer, P.; Theis, F.J. SCANPY: Large-scale single-cell gene expression data analysis. Genome Biol. 2018, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Wolock, S.L.; Lopez, R.; Klein, A.M. Scrublet: Computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 2019, 8, 281–291.e9. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.-r.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., III; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshevskaya, A.; Suleimanov, S.; Sheveleva, E.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Lopatnikova, J.; Zhukova, J.; Shkaruba, N.; Sivitskaya, N.; et al. Molecular Signatures of Dendritic Cell Activation upon TNF Stimulation: A Multi-Omics Study in Rheumatoid Arthritis. Int. J. Mol. Sci. 2025, 26, 6071. https://doi.org/10.3390/ijms26136071
Alshevskaya A, Suleimanov S, Sheveleva E, Perik-Zavodskii R, Perik-Zavodskaia O, Alrhmoun S, Lopatnikova J, Zhukova J, Shkaruba N, Sivitskaya N, et al. Molecular Signatures of Dendritic Cell Activation upon TNF Stimulation: A Multi-Omics Study in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2025; 26(13):6071. https://doi.org/10.3390/ijms26136071
Chicago/Turabian StyleAlshevskaya, Alina, Shakir Suleimanov, Elizaveta Sheveleva, Roman Perik-Zavodskii, Olga Perik-Zavodskaia, Saleh Alrhmoun, Julia Lopatnikova, Julia Zhukova, Nadezhda Shkaruba, Natalia Sivitskaya, and et al. 2025. "Molecular Signatures of Dendritic Cell Activation upon TNF Stimulation: A Multi-Omics Study in Rheumatoid Arthritis" International Journal of Molecular Sciences 26, no. 13: 6071. https://doi.org/10.3390/ijms26136071
APA StyleAlshevskaya, A., Suleimanov, S., Sheveleva, E., Perik-Zavodskii, R., Perik-Zavodskaia, O., Alrhmoun, S., Lopatnikova, J., Zhukova, J., Shkaruba, N., Sivitskaya, N., Sizikov, A., Golikova, E., & Sennikov, S. (2025). Molecular Signatures of Dendritic Cell Activation upon TNF Stimulation: A Multi-Omics Study in Rheumatoid Arthritis. International Journal of Molecular Sciences, 26(13), 6071. https://doi.org/10.3390/ijms26136071






