Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Non-Cytotoxic Concentrations of CA, 3-CQA, and 5-CQA to the Mouse P815 Mast Cells
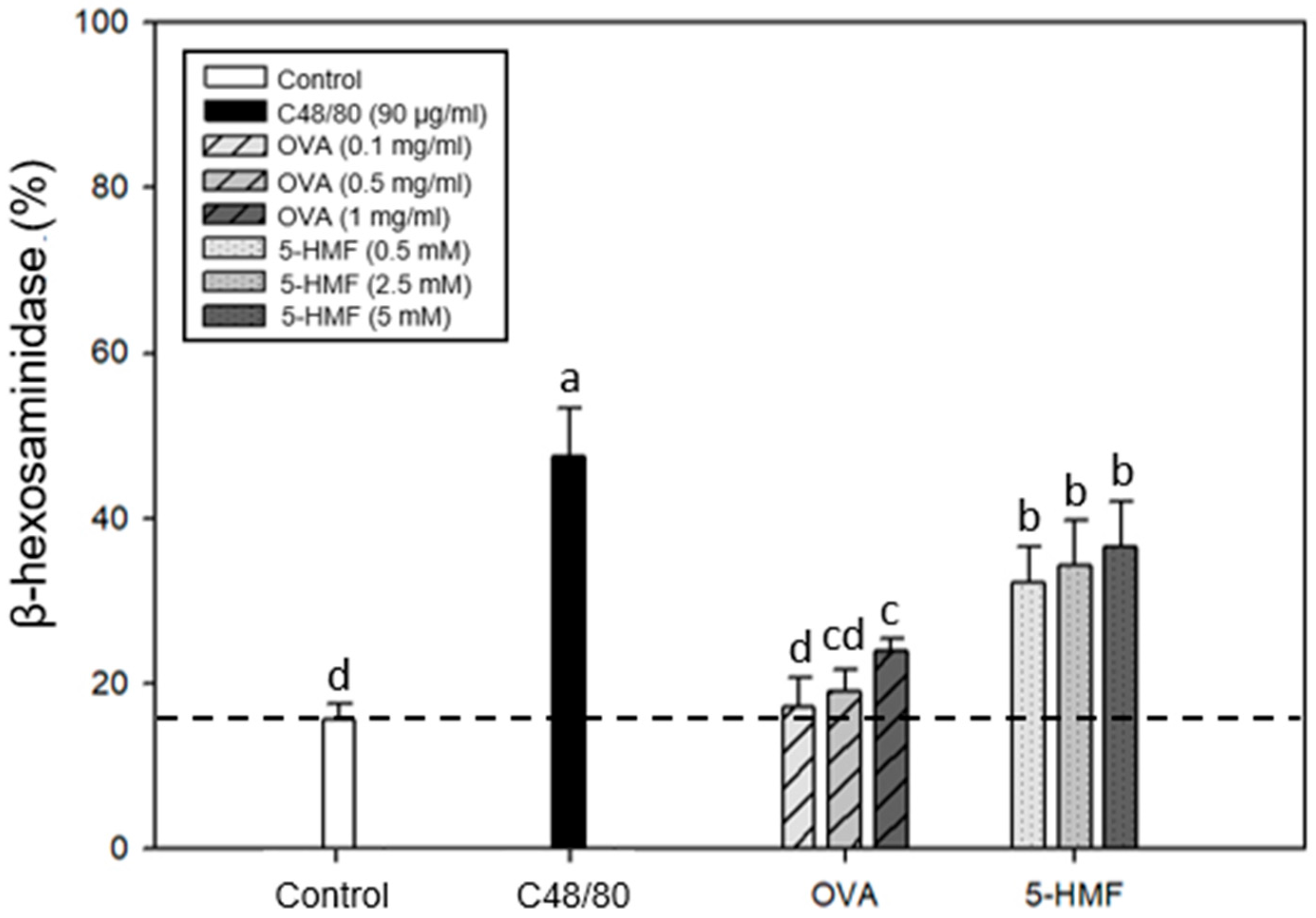
2.2. Degranulation Effects of C48/80, Ovalbumin (OVA), and 5-HMF on β-Hexosaminidase Release from Mouse P815 Mast Cells
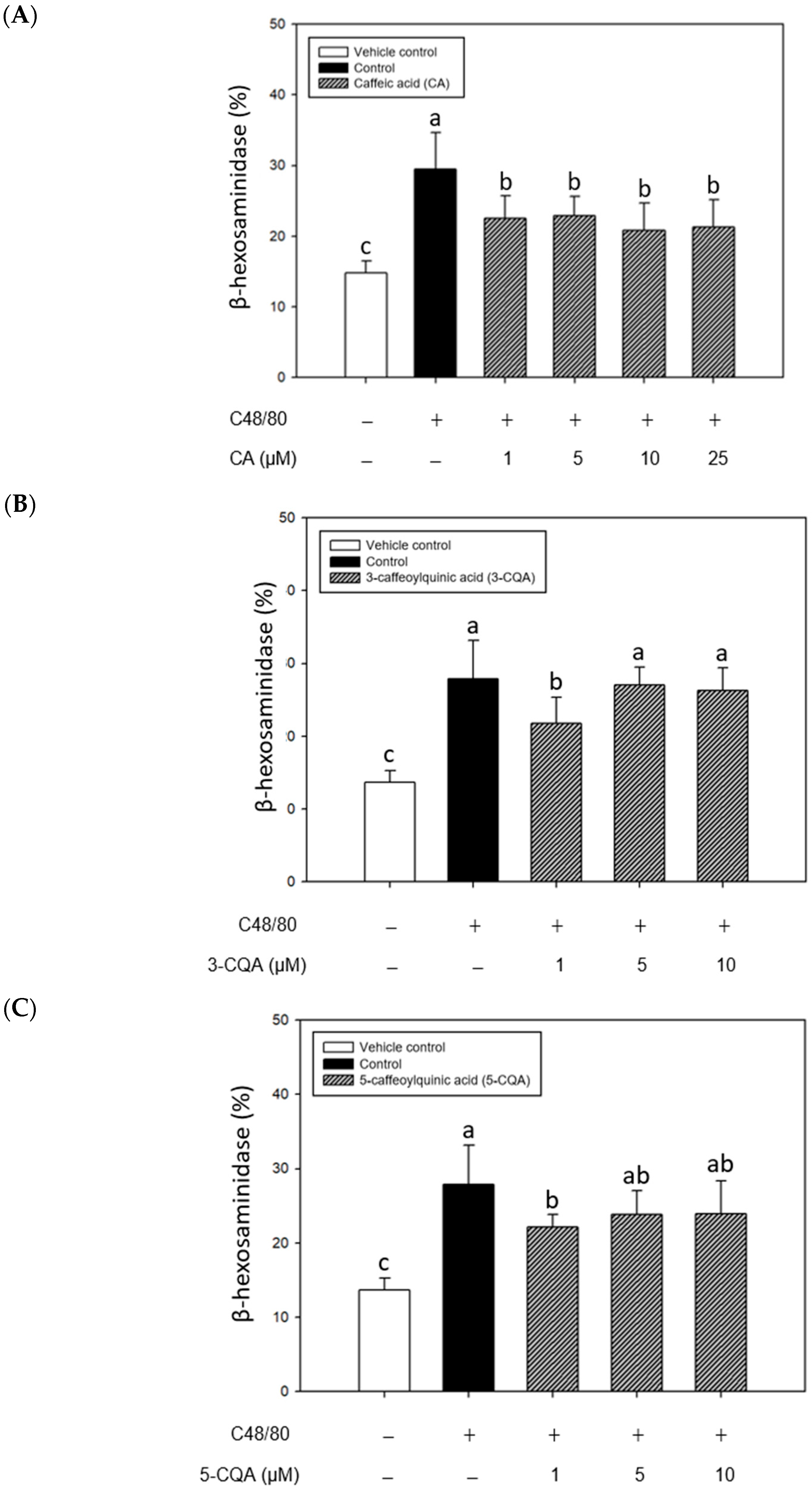
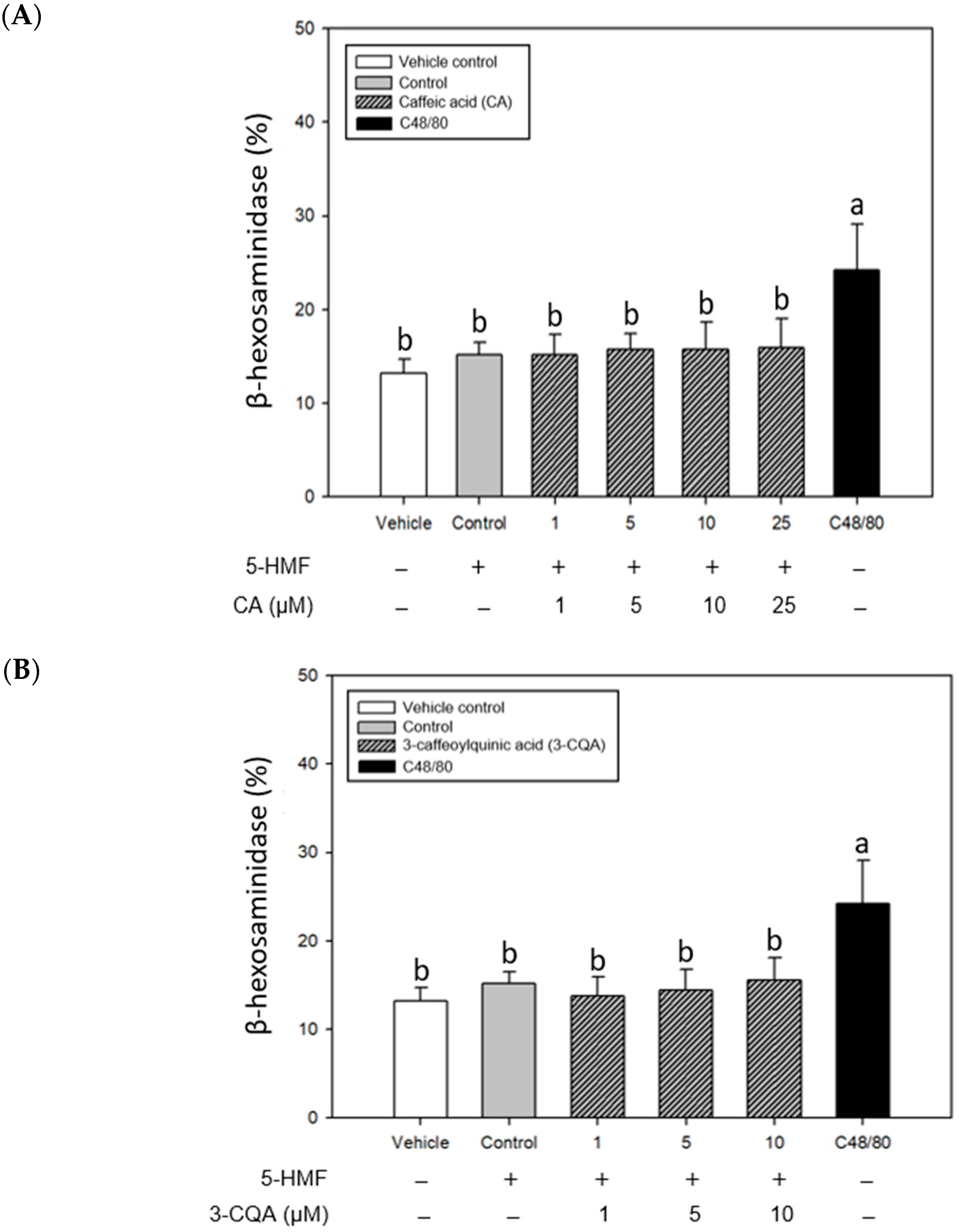
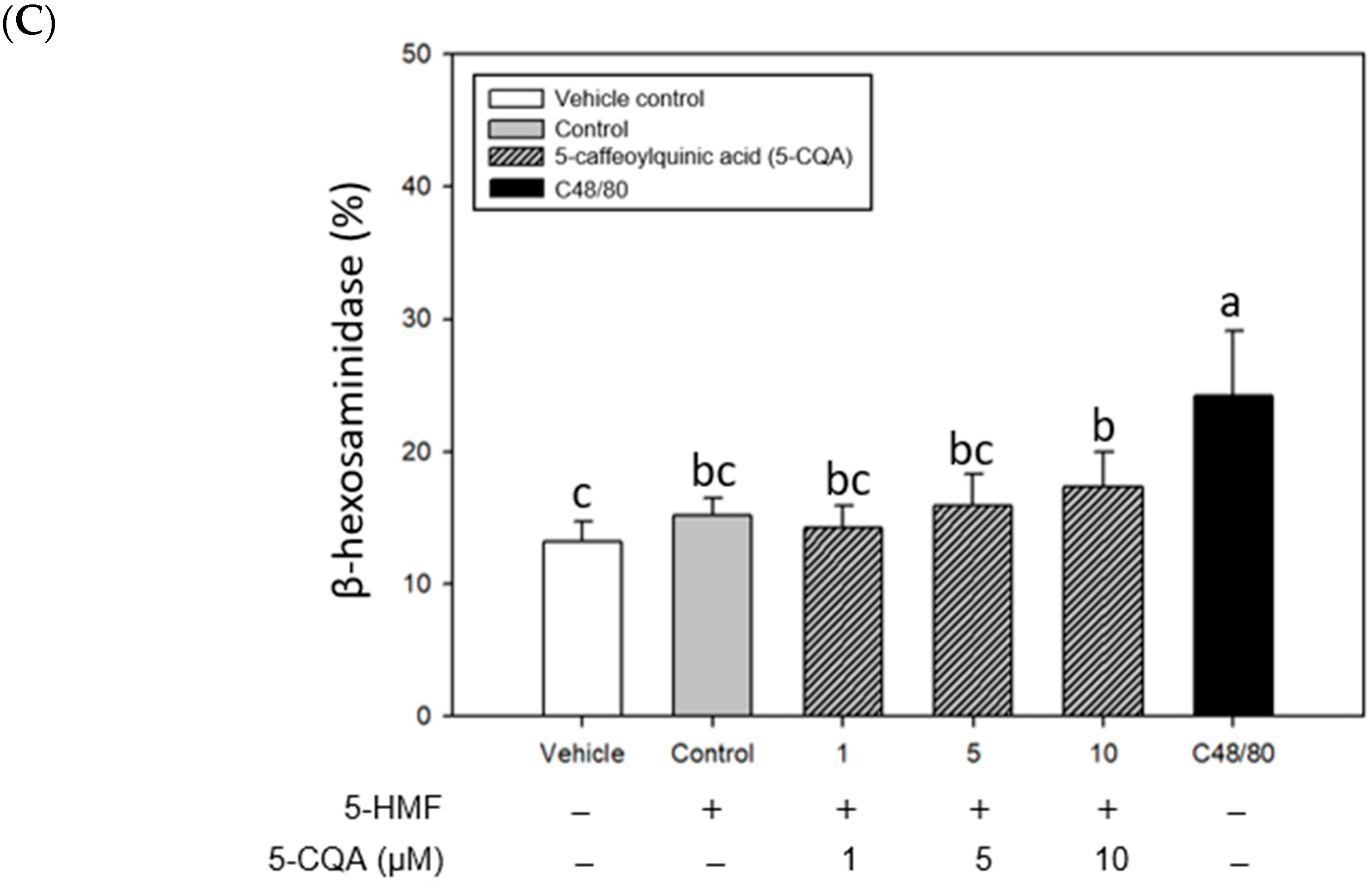
2.3. Inhibitory Effects of CA, 3-CQA, and 5-CQA Treatments on β-Hexosaminidase Release from Mouse P815 Mast Cells Stimulated with C48/80 or 5-HMF
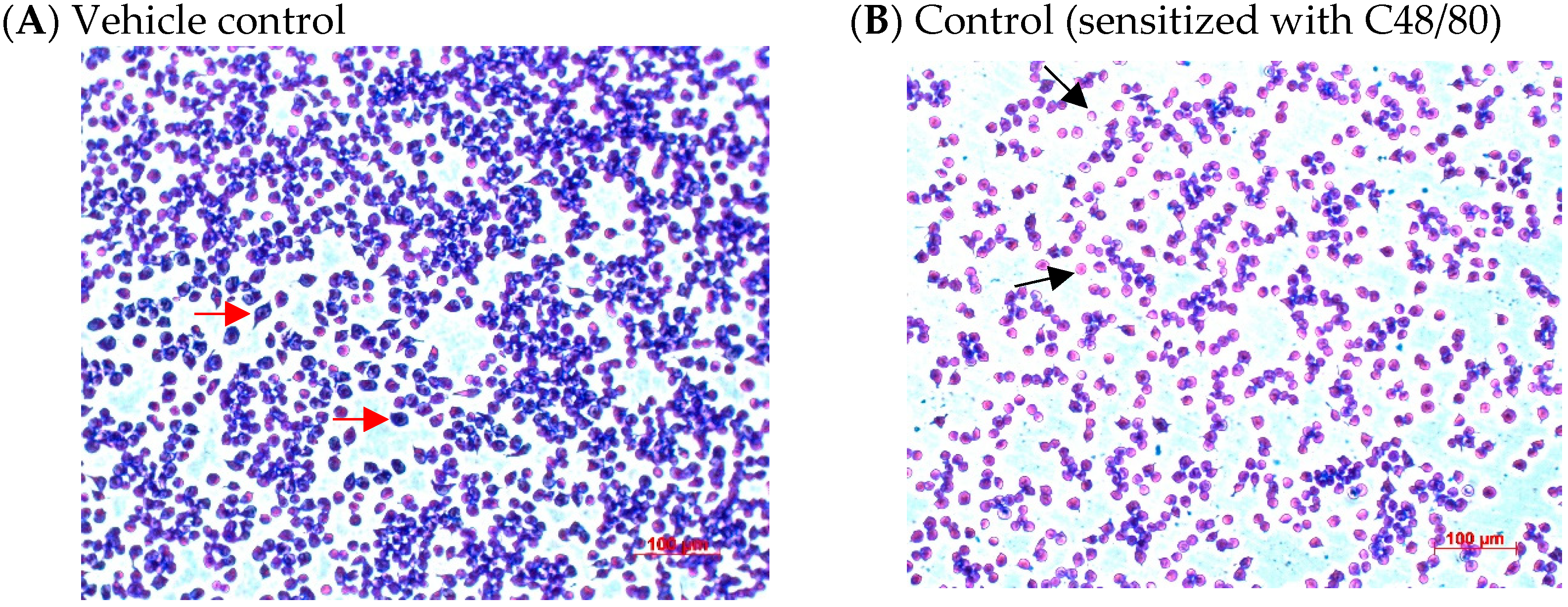
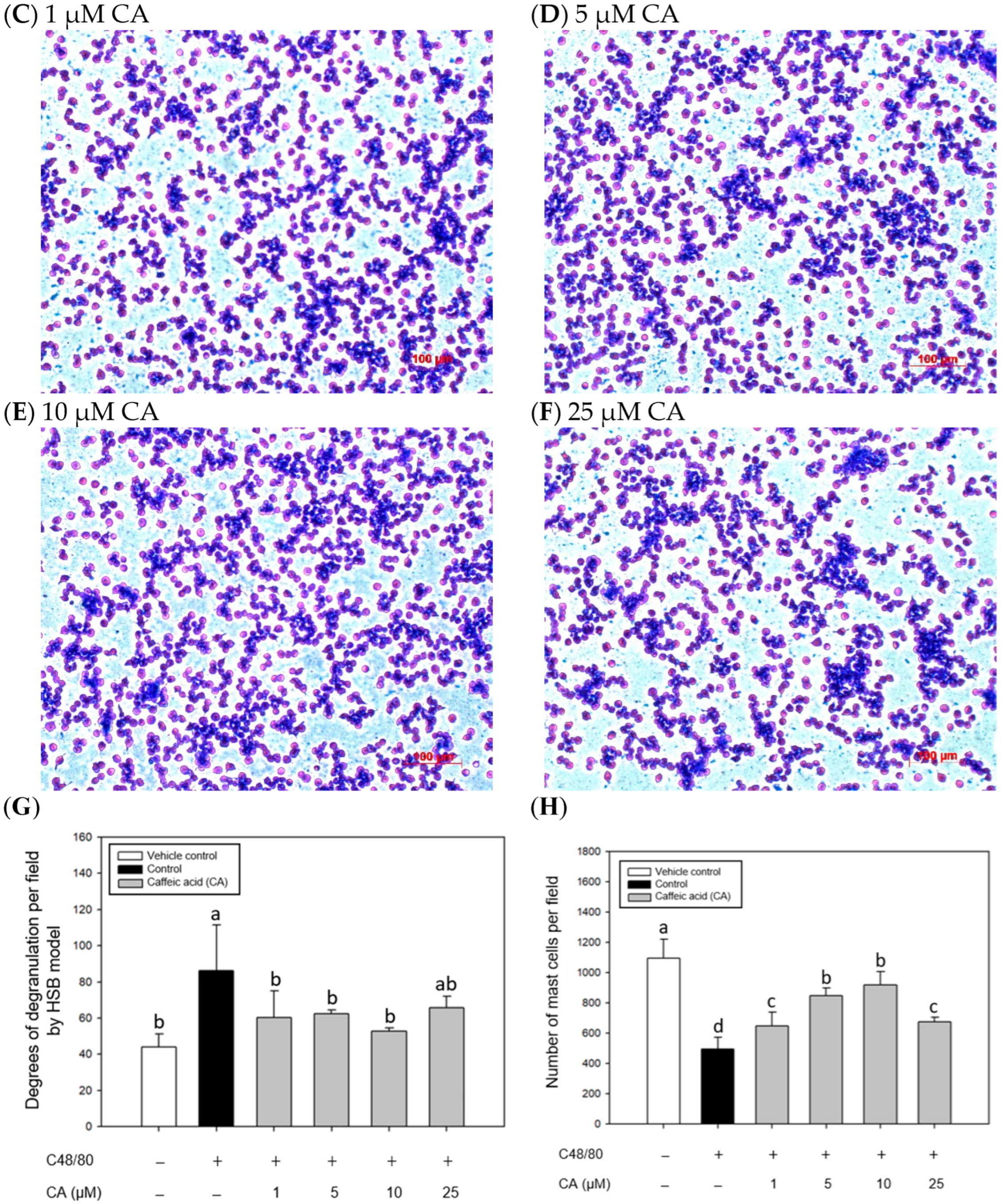
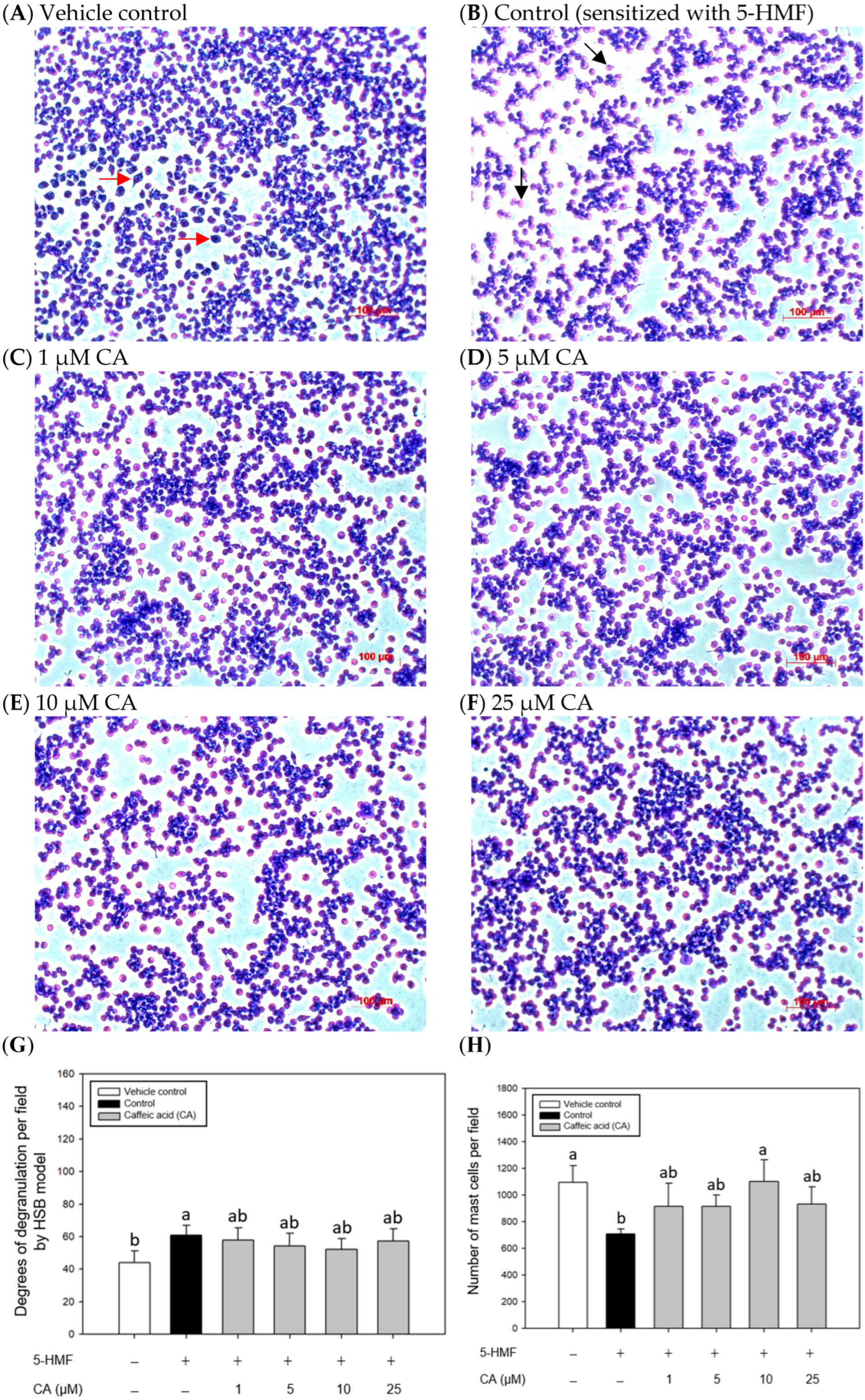
2.4. Effects of CA Treatments on the Appearance and Morphology of Mouse P815 Mast Cells Stimulated with C48/80 or 5-HMF
2.5. Effects of CA Treatments on Th2 (IL-4) and Pro-Inflammatory (IL-6) Cytokines Secretion by Mouse P815 Mast Cells Stimulated with C48/80 or 5-HMF
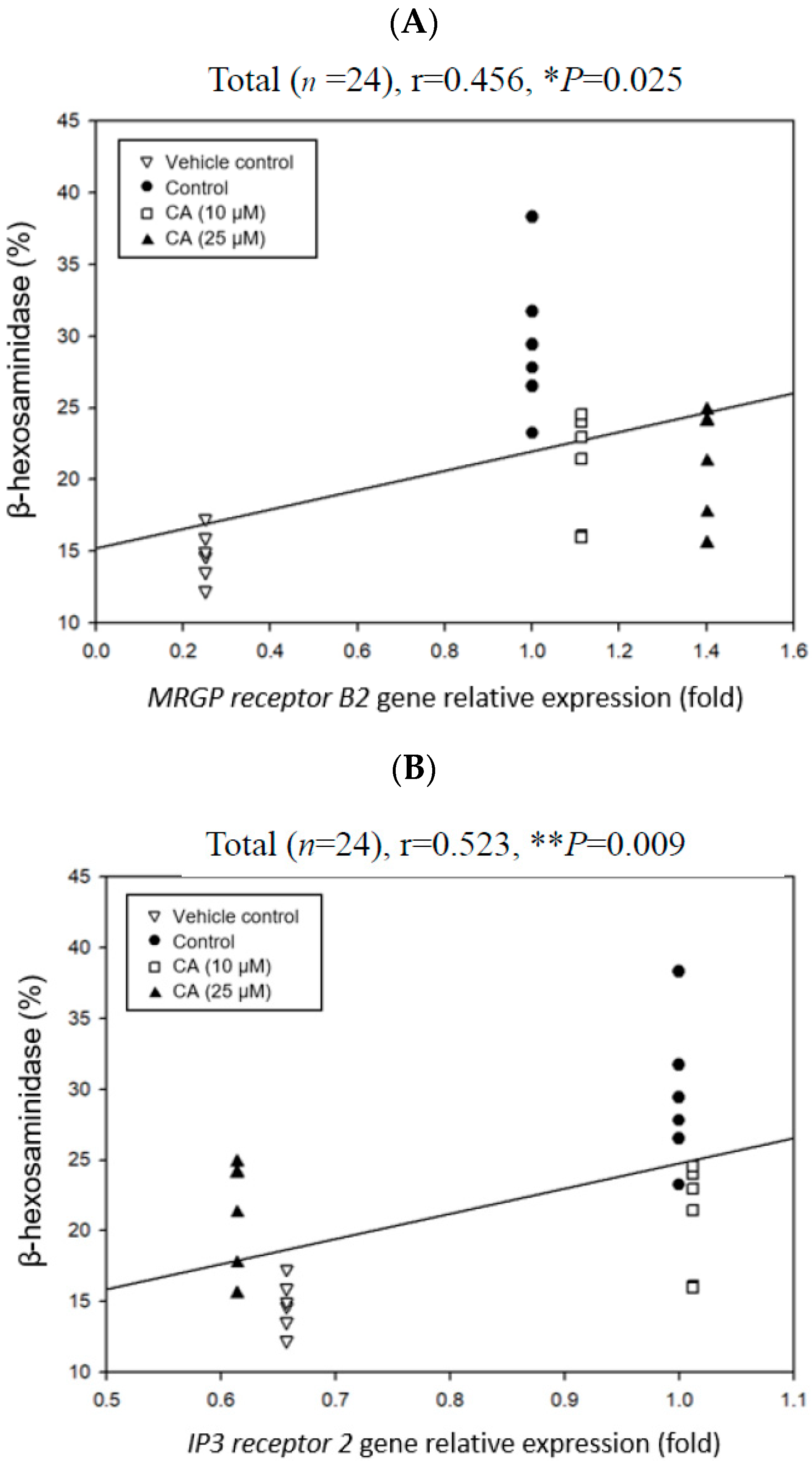
2.6. Effects of CA Treatments on the Relative Expression Folds of MRGP Receptor B2 and IP3 Receptor 2 Genes in C48/80-Stimulated Mouse P815 Mast Cells
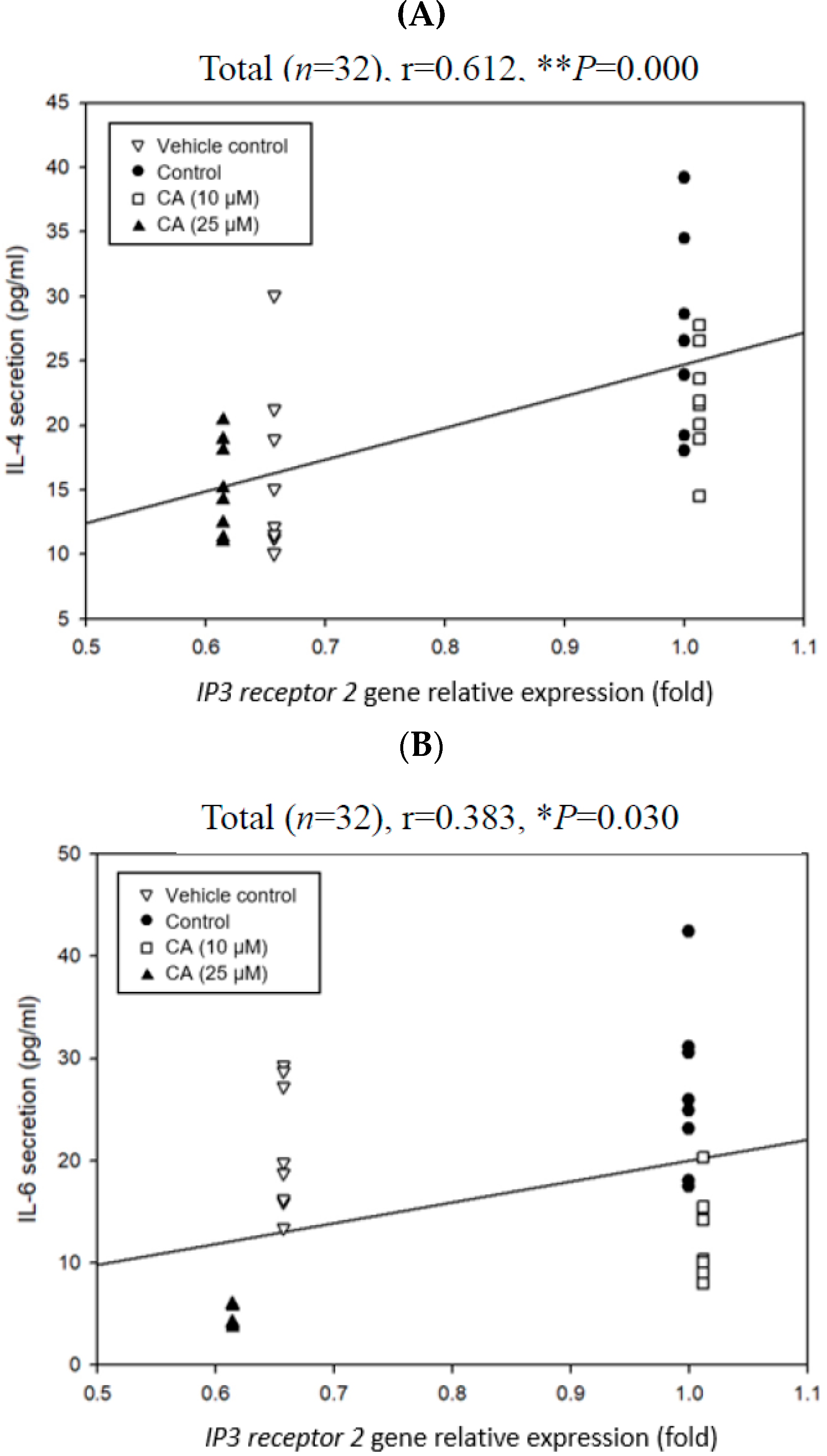
2.7. Associations Among Degranulation Degrees, Relative Gene Expression Folds, and Cytokine Scretions by Mast Cells
3. Materials and Methods
3.1. Preparation of CA, 3-CQA, and 5-CQA Preparation
3.2. Culture of the Mouse P815 Mast Cell Line
3.2.1. Source of Mouse P815 Mast Cell Line
3.2.2. Cell Thawing, Subculturing, and Cryopreservation
3.3. Effects of Treatments with CA, 3-CQA, and 5-CQA on the Viability of Mouse P815 Mast Cells
3.4. Effects of C48/80, Ovalbumin (OVA), and 5-HMF on β-Hexosaminidase Release from Mouse P815 Mast Cells
3.5. Effects of Treatments with CA, 3-CQA, and 5-CQA on β-Hexosaminidase Release from Mouse P815 Mast Cells Stimulated by C48/80 or 5-HMF
3.6. Effects of CA Treatments on the Appearance and Morphology Change in the Mouse P815 Mast Cell Line Stimulated with C48/80 or 5-HMF
3.7. Effects of CA Treatments on Cytokine Secretions by Mouse P815 Mast Cells Stimulated with C48/80 or 5-HMF
3.8. Effects of CA Treatments on the Expression of Degranulation-Related Pathway Genes in Mouse P815 Mast Cells Stimulated with C48/80
3.8.1. Cell Culture for Total RNA Extraction
3.8.2. Synthesis of First-Strand Complementary DNA (cDNA) from the Extracted Total RNA
3.8.3. Determination of the Relative Expression Amount of Degranulation-Related Genes, MRGP Receptor B2, and IP3 Receptor 2 Using Real-Time qPCR
3.8.4. Data Calculations and Expressions
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C48/80 | compound 48/80 |
| CA | caffeic acid |
| CAPE | caffeic acid phenethyl ester |
| cDNA | complementary DNA |
| CM | Culture medium |
| COX-2 | clcyooxygenase-2 |
| CQA | caffeoylquinic acid |
| 3-CQA | 3-O-caffeoylquinic acid |
| 5-CQA | 5-O-caffeoylquinic acid |
| DAG | diacylglycerol |
| DMSO | dimethyl sulfoxide |
| DTT | dithiothreitol |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| ER | endoplasmic reticulum |
| FcεR I | high-affinity IgE receptors |
| GPC | G-protein-coupled receptor |
| 5-HMF | 5-hydroxymethyl-2-furaldehyde |
| IC50 | half maximal inhibitory concentration |
| IL | interleukin |
| IP3 receptor | inositol-1,4,5-trisphosphate receptor |
| IP3 receptor 2 | inositol-1,4,5-trisphosphate receptor 2 |
| MAPKs | mitogen-activated protein kinases |
| MRGP receptor B2 | Mas-related G protein-coupled receptor, member B2 |
| MRGP receptor B3 | Mas-related G protein-coupled receptor, member B3 |
| MRGP receptor X2 | Mas-related G protein-coupled receptor, member X2 |
| MTT | 3-(4,5-dimethylthiazol-2-diphenyl)-2,5-tetrazolium bromide |
| NF-κB | nuclear factor-κB |
| ORAI | store-operated calcium entry channel |
| OVA | ovalbumin |
| PBS | phosphate-buffered saline |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| PLCγ | phospholipase Cγ |
| P-NAG | p-nitrophenyl-N-acetyl-β-D-glucosaminide |
| qPCR | quantitative polymerase chain reaction |
| SP | substance P |
| STIM1 | stromal interaction molecule 1 |
| TB | Toluidine Blue |
| Th2 | type 2 helper T cells |
| TLR | Toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| TRPC | transient receptor potential canonical |
References
- LoVerde, D.; Iweala, O.I.; Eginli, A.; Krishnaswamy, G. Anaphylaxis. Chest 2018, 153, 528–543. [Google Scholar] [CrossRef]
- Mayorga, C.; Muñoz-Cano, R.; Rodríguez-Nogales, A.; Fernandez-Santamaría, R.; Fernandez, T.D. New insights of biomarkers in IgE and non-IgE-mediated drug hypersensitivity. Curr. Treat. Options Allerg. 2019, 6, 42–55. [Google Scholar] [CrossRef]
- Cianferoni, A. Non-IgE-mediated anaphylaxis. J. Allergy Clin. Immunol. 2021, 147, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.T.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms governing anaphylaxis: Inflammatory cells, mediators, endothelial gap junctions and beyond. Int. J. Mol. Sci. 2021, 22, 7785. [Google Scholar] [CrossRef]
- Kim, H.S.; Kawakami, Y.; Kasakura, K.; Kawakami, T. Recent advances in mast cell activation and regulation. F1000Research 2020, 9, 196. [Google Scholar] [CrossRef]
- Syed, M.; Kammala, A.K.; Callahan, B.; Oskeritzian, C.A.; Subramanian, H. Lactic acid suppresses MRGPRX2 mediated mast cell responses. Cell. Immunol. 2021, 368, 104422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Che, D.; Zeng, Y.; Wu, Y.; Qin, Q.; Wang, N. Baicalin induces Mrgprb2-dependent pseudo-allergy in mice. Immunol. Lett. 2020, 226, 55–61. [Google Scholar] [CrossRef]
- Mi, Y.N.; Ping, N.N.; Cao, Y.X. Ligands and signaling of Mas-related G protein-coupled receptor-X2 in mast cell activation. Rev. Physiol. Biochem. Pharmacol. 2021, 179, 139–188. [Google Scholar] [PubMed]
- Zhang, Y.; Xue, Z.; Hu, S.; Bai, H.; Wang, J.; Wang, N. Chrysin inhibits pseudo-allergic reaction by suppressing mitochondrial STAT3 activation via MAS-related GPR family member X2. J. Agric. Food Chem. 2021, 69, 6569–6577. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, Y.; Zong, Y.; Zhang, J.; Zhu, C.; Yang, Y.; Tang, Z. PLC-IP3-ORAI pathway participates in the activation of the MRGPRB2 receptor in mouse peritoneal mast cells. Immunol. Lett. 2022, 248, 37–44. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, W.S.; Jung, W.K.; Qian, Z.J.; Lee, D.S.; Choi, J.S.; Lee, D.Y.; Park, S.G.; Seo, S.K.; Kim, H.J.; et al. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm. Biol. 2014, 52, 926–932. [Google Scholar] [CrossRef]
- Nader, M.A. Caffeic acid phenethyl ester attenuates IgE-induced immediate allergic reaction. Inflammopharmacology 2013, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Lee, D.Y.; Seo, S.K.; Lee, S.W.; Kim, S.K.; Jung, W.Y.; Kang, M.S.; Choi, Y.H.; Yea, S.S.; Choi, I.; et al. Evaluation of anti-allergic properties of caffeic acid phenethyl ester in a murine model of systemic anaphylaxis. Toxicol. Appl. Pharmacol. 2008, 226, 22–29. [Google Scholar] [CrossRef]
- McLeod, J.J.; Baker, B.; Ryan, J.J. Mast cell production and response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Yang, H.; Zhang, Z.; Lin, Q.; Zheng, Y.; Chen, S.; Yang, P.; He, S. Modulation of mast cell proteinase-activated receptor expression and IL-4 release by IL-12. Immunol. Cell Biol. 2007, 85, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, H.K.; N’Deh, K.P.U.; Choi, Y.J.; Fan, M.; Kim, E.K.; Chung, K.H.; An, A.J.H. Inhibitory effect of Centella asiatica extract on DNCB-induced atopic dermatitis in HaCaT cells and BALB/c mice. Nutrients 2020, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, A.; Mrabet, Y.; Boulila, A. Caffeoylquinic acid derivatives: Chemical diversity and effect on the oxidative stress-related pathologies. Chem. Afr. 2024, 7, 4105–4126. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Mauricio, A.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation-A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Hossen, M.A.; Inoue, T.; Shinmei, Y.; Minami, K.; Fujii, Y.; Kamei, C. Caffeic acid inhibits compound 48/80-induced allergic symptoms in mice. Biol. Pharm. Bull. 2006, 29, 64–66. [Google Scholar] [CrossRef]
- Issac, J.; Elango, K. Evaluation of anti-asthmatic activity of caffeic acid in allergen induced murine allergic asthma model. Int. J. Sci. Res. 2017, 6, 1573–1580. [Google Scholar]
- Lee, Y.L.; Hsu, L.H.; Kuo, Y.H.; Lee, C.C. Caffeic amide derivatives inhibit allergen-induced bone marrow-derivaved dendritic cell maturation. Pharmacol. Rep. 2019, 71, 194–200. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Mao, Y.; Shao, Y.; Liu, J.; Tu, Z. A comparative study on the allergenic potential of β-lactoglobulin conjugated to glucose, caffeic acid and caffeoyl glucopyranose. Food Funct. 2023, 14, 4354–4367. [Google Scholar] [CrossRef]
- Wozniak, D.; Nawrot-Hadzik, I.; Kozlowska, W.; Slusarczyk, S.; Matkowski, A. Caffeoylquinic Acids. In Handbook of Dietary Phytochemicals; Xiao, J.-B., Sarker, S., Asakawa, Y., Eds.; Springer: Singapore, 2021; pp. 1065–1104. [Google Scholar]
- Zielinska, D.; Zielinski, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Gimenez-Bastida, J.A. Caffeic acid modulates processes associated with intestinal inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Fu, S.; Ni, S.; Wang, D.; Fu, M.; Hong, T. Berberine suppresses mast cell-mediated allergic responses via regulating FcɛRI-mediated and MAPK signaling. Int. Immunopharmacol. 2019, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.R.; Hsu, K.T.; Hsu, W.H.; Lee, B.H.; Li, T.L.; Chan, Y.L.; Wu, C.J. Immunomodulation and mechanisms of fucoidan from Cladosiphon okamuranus ameliorates atopic dermatitis symptoms. Int. J. Biol. Macromol. 2021, 189, 537–543. [Google Scholar] [CrossRef]
- Atakpa-Adaji, P.; Ivanova, A.; Kujawa, K.; Taylor, C.W. KRAP regulates mitochondrial Ca2+ uptake by licensing IP3 receptor activity and stabilizing ER-mitochondrial junctions. J. Cell Sci. 2024, 137, jcs261728. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.R.; Fan, G.; Arige, V.; Yule, D.I.; Serysheva, I.I. Understanding IP3R channels: From structural underpinnings to ligand-dependent conformational landscape. Cell Calcium 2023, 114, 102770. [Google Scholar] [CrossRef]
- Fujii, S.; Ushioda, R.; Nagata, K. Redox states in the endoplasmic reticulum directly regulate the activity of calcium channel, inositol 1,4,5-trisphosphate receptors. Proc. Natl. Acad. Sci. USA 2023, 120, e2216857120. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Atakpa-Adaji, P.; Rao, S.; Marti-Solano, M.; Taylor, C.W. Dual regulation of IP3 receptors by IP3 and PIP2 controls the transition from local to global Ca2+ signals. Mol. Cell 2024, 84, 3997–4015. [Google Scholar] [CrossRef]
- Smith, H.A.; Thillaiappan, N.B.; Rossi, A.M. IP3 receptors: An “elementary” journey from structure to signals. Cell Calcium 2023, 113, 102761. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J. Type 3 IP3 receptor: Its structure, functions, and related disease implications. Channels 2023, 17, 2267416. [Google Scholar] [CrossRef]
- Lianto, P.; Ogutu, F.O.; Zhang, Y.; He, F.; Che, H. Inhibitory effects of quail egg on mast cells degranulation by suppressing PAR2-mediated MAPK and NF-kB activation. Food Nutr. Res. 2018, 62, 1084. [Google Scholar] [CrossRef]
- Wang, N.; Liu, R.; Liu, Y.; Zhang, R.; He, L. Sinomenine potentiates P815 cell degranulation via upregulation of Ca2+ mobilization through the Lyn/PLCγ/IP3R pathway. Int. J. Immunopathol. Pharmacol. 2016, 29, 676–683. [Google Scholar] [CrossRef]
- Adhikari, N.; Shim, W.S. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharmacal Res. 2022, 45, 644–657. [Google Scholar] [CrossRef]
- Pavlikova, N. Caffeic acid and diseases-Mechanisms of action. Int. J. Mol. Sci. 2023, 24, 588. [Google Scholar] [CrossRef]
- Socala, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaz, P. Neuroprotective effects of coffee bioactive compounds: A review. Int. J. Mol. Sci. 2021, 22, 107. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Kim, S.R.; Jung, U.J. Coffee and its major polyphenols in the prevention and management of type 2 diabetes: A comprehensive review. Int. J. Mol. Sci. 2025, 26, 5544. [Google Scholar] [CrossRef]
- Cortez, N.; Villegas, C.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; Gonzalez-Chavarria, I.; Nchiozem-Ngnitedem, V.A.; Paz, C. Adjuvant properties of caffeic acid in cancer treatment. Int. J. Mol. Sci. 2024, 25, 7631. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Lin, J.Y. Menthone inhalation alleviates local and systemic allergic inflammation in ovalbumin-sensitized and challenged asthmatic mice. Int. J. Mol. Sci. 2022, 23, 4011. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Lin, J.Y. Menthone supplementation ameliorates systemic allergic inflammation in asthmatic mice via regulating Th2-skewed immune balance. Phytomed. Plus 2022, 2, 100322. [Google Scholar] [CrossRef]
- Su, Y.H.; Lin, J.Y. Menthone supplementation protects from allergic inflammation in the lungs of asthmatic mice. Eur. J. Pharmacol. 2022, 931, 175222. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.J.; Han, E.T.; Han, J.H.; Park, W.S.; Kwon, Y.S.; Chun, W. Caffeic acid methyl ester inhibits mast cell activation through the suppression of MAPKs and NF-κB signaling in RBL-2H3 cells. Heliyon 2023, 9, e16529. [Google Scholar] [CrossRef]
- Duraisamy, K.; Kumar, M.; Nawabjan, A.; Lo, E.K.K.; Lin, M.H.; Lefranc, B.; Bonnafe, E.; Treilhou, M.; El-Nezami, H.; Leprince, J.; et al. MRGPRB2/X2 and the analogous effects of its agonist and antagonist in DSS-induced colitis in mice. Biomed. Pharmacother. 2024, 174, 116471. [Google Scholar] [CrossRef] [PubMed]
- Ronkko, J.; Rodriguez, Y.; Rasila, T.; Torregrosa-Munumer, R.; Pennonen, J.; Kvist, J.; Kuuluvainen, E.; Van Den Bosch, L.; Hietakangas, V.; Bultynck, G.; et al. Human IP3 receptor triple knockout stem cells remain pluripotent despite altered mitochondrial metabolism. Cell Calcium 2023, 114, 102782. [Google Scholar] [CrossRef] [PubMed]
- Staats, K.A.; Humblet-Baron, S.; Bento-Abreu, A.; Scheveneels, W.; Nikolaou, A.; Deckers, K.; Lemmens, R.; Goris, A.; Van Ginderachter, J.A.; Van Damme, P.; et al. Genetic ablation of IP3 receptor 2 increases cytokines and decreases survival of SOD1G93A mice. Hum. Mol. Genet. 2016, 25, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Chen, C.C.; Lin, J.Y. Anti-inflammatory in vitro activities of eleven selected caffeic acid derivatives based on a combination of pro-/anti-inflammatory cytokine secretions and Principal Component Analysis—A comprehensive evaluation. Food Chem. 2024, 458, 140201. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, C.C.; Lin, J.Y. Caffeic acid and 5-caffeoylquinic acid inhibit HT-29 colorectal cancer cell growth through immunotherapy by inducing the Bax/Bcl-2 apoptotic pathway in vitro. Bioorganic Chem. 2025, 163, 108723. [Google Scholar] [CrossRef]
- Wahlkvist, H.; Masjedi, K.; Gruvberger, B.; Zuber, B.; Karlberg, A.T.; Bruze, M.; Ahlborg, N. The lipophilic hapten parthenolide induces interferon-gamma and interleukin-13 production by peripheral blood-derived CD8+ T cells from contact allergic subjects in vitro. Br. J. Dermatol. 2008, 158, 70–77. [Google Scholar]
- Gajewski, T.F.; Markiewicz, M.A.; Uyttenhove, C. The P815 mastocytoma tumor model. Curr. Protoc. Immunol. 2001, 43, 20.4.1–20.4.18. [Google Scholar] [CrossRef]
- Demehri, S.; Corbin, A.; Loriaux, M.; Druker, B.J.; Deininger, M.W. Establishment of a murine model of aggressive systemic mastocytosis/mast cell leukemia. Exp. Hematol. 2006, 34, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Lunderius, C.; Xiang, Z.; Nilsson, G.; Hellman, L. Murine mast cell lines as indicators of early events in mast cell and basophil development. Eur. J. Immunol. 2000, 30, 3396–3402. [Google Scholar] [CrossRef]
- Lin, H.C.; Lin, J.Y. GSF3, a polysaccharide from guava (Psidium guajava L.) seeds, inhibits MCF-7 breast cancer cell growth via increasing Bax/Bcl-2 ratio or Fas mRNA expression levels. Int. J. Biol. Macromol. 2020, 161, 1261–1271. [Google Scholar] [CrossRef]
- Yeh, T.H.; Lin, J.Y. Acorus gramineusand and Euodia ruticarpa steam distilled essential oils exert anti-inflammatory effects through decreasing Th1/Th2 and pro-/anti-inflammatory cytokine secretion ratios in vitro. Biomolecules 2020, 10, 338. [Google Scholar] [CrossRef]
- Peng, B.; He, R.; Xu, Q.H.; Gao, J.; Lu, Y.L.; Li, J.R. Comparison of RBL-2H3 and P815 cell lines for establishing in vitro model of mast cell degranulation. Chin. J. Nat. Med. 2011, 9, 227–231. [Google Scholar]
- Sridharan, G.; Shankar, A.A. Toluidine blue: A review of its chemistry and clinical utility. J. Oral Maxillofac. Pathol. 2012, 16, 251–255. [Google Scholar] [CrossRef]
- Van der Leek, A.P.; Yanishevsky, Y.; Kozyrskyj, A.L. The Kynurenine pathway as a novel link between allergy and the gut microbiome. Front. Immunol. 2017, 8, 1374. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Conc. (μM) | IL-4 (pg/mL) | IL-6 (pg/mL) | |
|---|---|---|---|---|
| Vehicle control | 4.3 c | 6.3 ab | ||
| Control (C48/80 alone) | 7.6 ab | 8.1 a | ||
| C48/80 + CA | 1 | 8.0 a | 9.1 a | |
| 5 | 5.7 ab | 6.5 bc | ||
| 10 | 4.2 b | 4.2 c | ||
| 25 | 3.6 c | 1.0 d | ||
| Vehicle control | 3.8 a | 5.8 a | ||
| Control (5-HMF alone) | 8.1 a | 1.7 b | ||
| 5-HMF + CA | 1 | 5.0 a | 1.9 bc | |
| 5 | 0.9 a | 1.1 bc | ||
| 10 | 4.9 a | 1.0 bc | ||
| 25 | 5.1 a | 1.1 c | ||
| Treatments | Conc. (μM) | Relative Expressions (Fold) | |
|---|---|---|---|
| MRGP Receptor B2 | IP3 Receptor 2 | ||
| Vehicle control | 0.250.10 b | 0.660.12 b | |
| Control (C48/80 alone) | 1.000.04 a | 1.000.03 a | |
| C48/80 + CA | 10 | 1.110.04 a | 1.010.24 a |
| 25 | 1.400.58 a | 0.610.03 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-C.; Lin, J.-Y. Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells. Int. J. Mol. Sci. 2025, 26, 10086. https://doi.org/10.3390/ijms262010086
Chang K-C, Lin J-Y. Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells. International Journal of Molecular Sciences. 2025; 26(20):10086. https://doi.org/10.3390/ijms262010086
Chicago/Turabian StyleChang, Kai-Chi, and Jin-Yuarn Lin. 2025. "Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells" International Journal of Molecular Sciences 26, no. 20: 10086. https://doi.org/10.3390/ijms262010086
APA StyleChang, K.-C., & Lin, J.-Y. (2025). Caffeic Acid Inhibits Degranulation, Cytokine Secretions, and IP3 Receptor 2 Gene Expression in Compound 48/80-Stimulated Mouse P815 Mast Cells. International Journal of Molecular Sciences, 26(20), 10086. https://doi.org/10.3390/ijms262010086






