Vascular Disruption Therapy as a New Strategy for Cancer Treatment
Abstract
1. Introduction
2. Vascular Development
2.1. Main Mechanisms of Tumor Vascularization
2.2. Normal Blood Vessels vs. Tumor Blood Vessels Characteristics
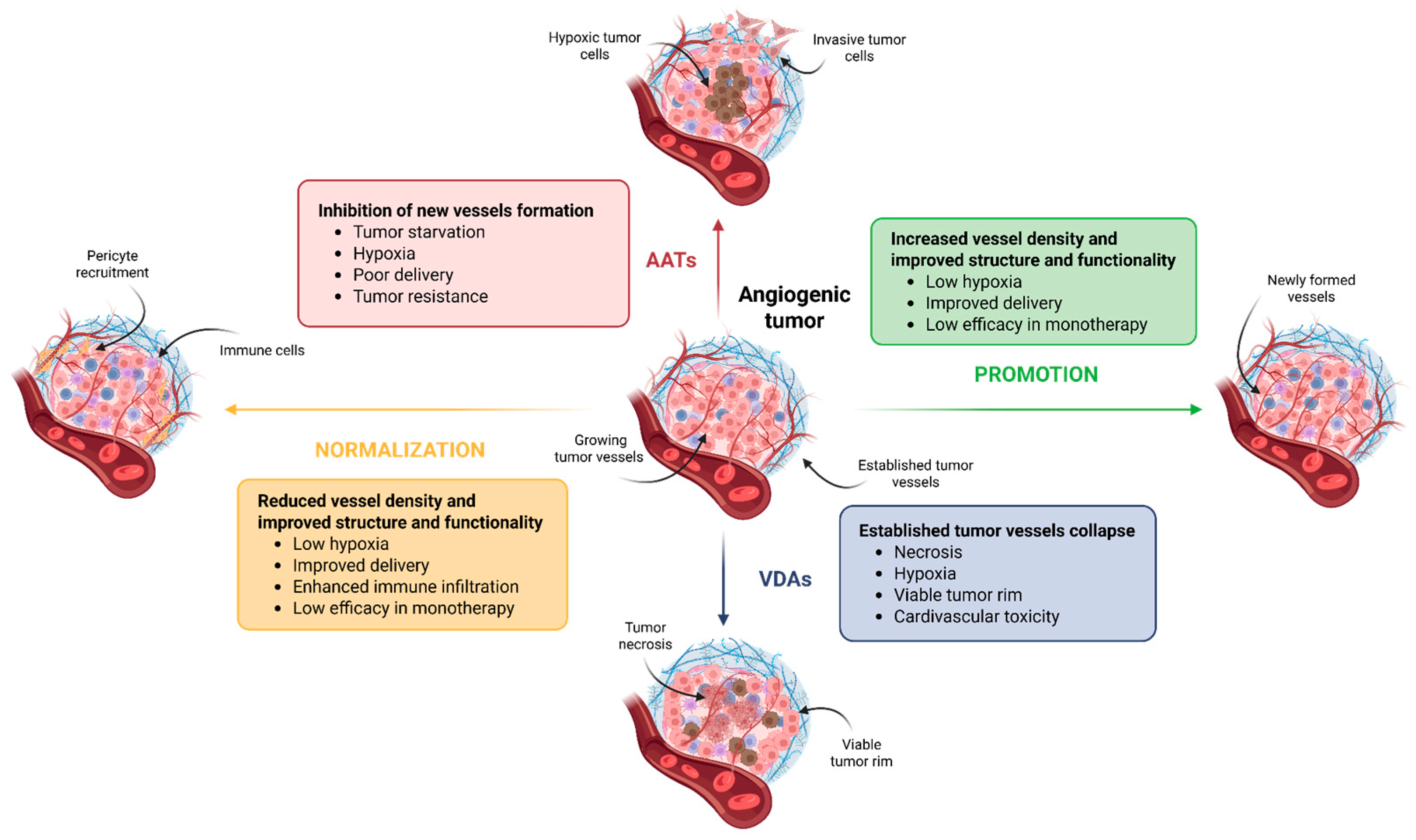
3. Strategies to Target Tumor Vasculature
3.1. Anti-Angiogenic Therapy
3.2. Vascular Normalization
3.3. Vascular Promotion
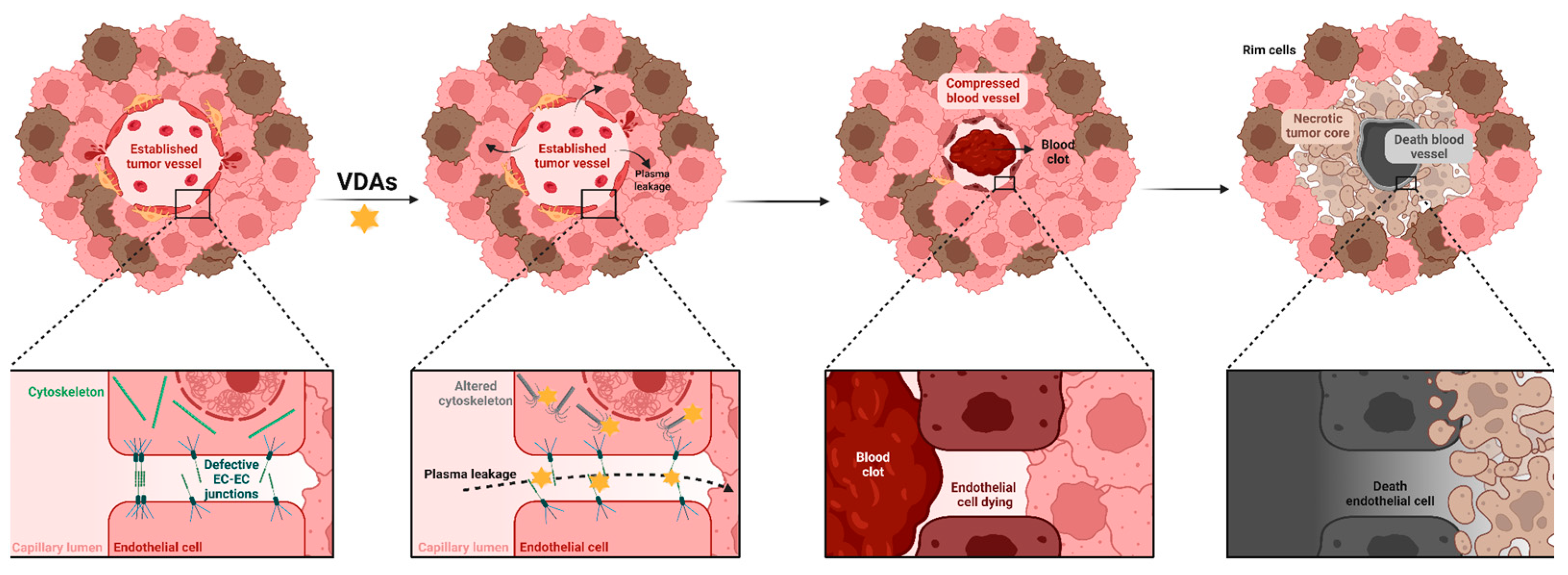
3.4. Vascular Disruption
4. Vascular Disrupting Agents
4.1. Microtubule-Destabilizing Agents
4.2. Flavonoids
4.3. Ligand-Directed Agents
5. Resistance to Vascular Disruption
5.1. Viable Tumor Rim
5.2. Hypoxia-Induced Adaptation
5.3. Cellular and Microenvironmental Factors
5.4. Strategies to Overcome Vascular Disruption Resistance
6. Current Status of Clinical Trial Studies of Vascular Disruption Agents
7. Conclusions and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAT | Anti-angiogenic therapy |
| ASA404 | Vadimezan |
| AVE8061 | Ombrabulin |
| CA4P | Fosbretab |
| DMXAA | Vadimezan |
| EC | Endothelial cell |
| EMT | Epithelial-to-mesenchymal transition |
| EPCs | Endothelial progenitor cells |
| HIF-1α | Hypoxia-inducible factor 1α |
| HREs | Hypoxia response elements |
| LLC | Lewis lung carcinoma |
| LNPs | Nucleic acid-loaded nanoparticles |
| MHC | Major histocompatibility complex |
| NPI-2358 | Plinabulin |
| NSCLC | Non-small cell lung cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| siRNA | Small interfering RNA |
| TAMs | Tumor-associated macrophages |
| TEMs | TIE2-expressing macrophages |
| TGF-β | Transforming growth factor β |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor α |
| Treg | Regulatory T cells |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| VDAs | Vascular disruption agents |
| VCO | Vessel co-option |
References
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor Angiogenesis: Molecular Pathways and Therapeutic Targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-P.; Bodrug, N.; Hodivala-Dilke, K.M. Explosring Novel Methods for Modulating Tumor Blood Vessels in Cancer Treatment. Curr. Biol. 2016, 26, R1161–R1166. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichoń, T. Vascular Disrupting Agents in Cancer Therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Shen, N.; Sun, J.; Tang, Z.; Chen, X. Destruction of Tumor Vasculature by Vascular Disrupting Agents in Overcoming the Limitation of EPR Effect. Adv. Drug Deliv. Rev. 2022, 183, 114138. [Google Scholar] [CrossRef]
- Pilat, M.J.; LoRusso, P.M. Vascular Disrupting Agents. J. Cell. Biochem. 2006, 99, 1021–1039. [Google Scholar] [CrossRef]
- Thorpe, P.E. Vascular Targeting Agents as Cancer Therapeutics. Clin. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Maione, P.; Rossi, E.; Castaldo, V.; Sacco, P.C.; Colantuoni, G. Vascular Disrupting Agents: A Novel Mechanism of Action in the Battle Against Non-Small Cell Lung Cancer. Oncologist 2009, 14, 612–620. [Google Scholar] [CrossRef]
- Liang, W.; Ni, Y.; Chen, F. Tumor Resistance to Vascular Disrupting Agents: Mechanisms, Imaging, and Solutions. Oncotarget 2016, 7, 15444–15459. [Google Scholar] [CrossRef]
- Guelfi, S.; Hodivala-Dilke, K.; Bergers, G. Targeting the Tumour Vasculature: From Vessel Destruction to Promotion. Nat. Rev. Cancer 2024, 24, 655–675. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.-E.; Andersen, S.; Rakaee, M.; Pedersen, M.I.; Lombardi, A.P.; Pøhl, M.; Kilvaer, T.; Busund, L.-T.; Pezzella, F.; Donnem, T. Impact of Microvessel Patterns and Immune Status in NSCLC: A Non-Angiogenic Vasculature Is an Independent Negative Prognostic Factor in Lung Adenocarcinoma. Front. Oncol. 2023, 13, 1157461. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Donnem, T.; Reynolds, A.R.; Kuczynski, E.A.; Gatter, K.; Vermeulen, P.B.; Kerbel, R.S.; Harris, A.L.; Pezzella, F. Non-Angiogenic Tumours and Their Influence on Cancer Biology. Nat. Rev. Cancer 2018, 18, 323–336. [Google Scholar] [CrossRef]
- Narang, A.S.; Varia, S. Role of Tumor Vascular Architecture in Drug Delivery. Adv. Drug Deliv. Rev. 2011, 63, 640–658. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Vasculature with Anti-Angiogenic Therapy: A New Paradigm for Combination Therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Burri, P.H.; Tarek, M.R. A Novel Mechanism of Capillary Growth in the Rat Pulmonary Microcirculation. Anat. Rec. 1990, 228, 35–45. [Google Scholar] [CrossRef]
- Ribatti, D.; Djonov, V. Intussusceptive Microvascular Growth in Tumors. Cancer Lett. 2012, 316, 126–131. [Google Scholar] [CrossRef]
- Ratajska, A.; Jankowska-Steifer, E.; Czarnowska, E.; Olkowski, R.; Gula, G.; Niderla-Bielińska, J.; Flaht-Zabost, A.; Jasińska, A. Vasculogenesis and Its Cellular Therapeutic Applications. Cells Tissues Organs 2017, 203, 141–152. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yi, T.; Li, H.; Tang, X.; Liu, D.; Wu, D.; Li, Y. Decoding Tumor Angiogenesis: Pathways, Mechanisms, and Future Directions in Anti-Cancer Strategies. Biomark. Res. 2025, 13, 62. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Reynolds, A.R. Vessel Co-Option and Resistance to Anti-Angiogenic Therapy. Angiogenesis 2020, 23, 55–74. [Google Scholar] [CrossRef]
- Maishi, N.; Annan, D.A.; Kikuchi, H.; Hida, Y.; Hida, K. Tumor Endothelial Heterogeneity in Cancer Progression. Cancers 2019, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, X.; Li, J. Manipulation of Immune–Vascular Crosstalk: New Strategies towards Cancer Treatment. Acta Pharm. Sin. B 2020, 10, 2018–2036. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and Mechanisms of Vessel Normalization for Cancer and Other Angiogenic Diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Choyke, P.L.; Sato, N. Visualizing Vasculature and Its Response to Therapy in the Tumor Microenvironment. Theranostics 2023, 13, 5223–5246. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor Microenvironment Abnormalities: Causes, Consequences, and Strategies to Normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Tzeng, H.-T.; Huang, Y.-J. Tumor Vasculature as an Emerging Pharmacological Target to Promote Anti-Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 4422. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Huang, Y.; Kim, B.Y.S.; Shan, H.; Wu, D.; Jiang, W. Tumor Vasculatures: A New Target for Cancer Immunotherapy. Trends Pharmacol. Sci. 2019, 40, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the Role of the Tumor Vasculature in Antitumor Immunity and Immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Gerlowski, L.E.; Jain, R.K. Microvascular Permeability of Normal and Neoplastic Tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size1. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular Normalization by Vascular Endothelial Growth Factor Receptor 2 Blockade Induces a Pressure Gradient Across the Vasculature and Improves Drug Penetration in Tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Reynolds, A.R. Anti-Angiogenic Therapy for Cancer: Current Progress, Unresolved Questions and Future Directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Bilecz, A.; Daley, F.; Kostaras, E.; Nathan, M.R.; Wan, E.; Frentzas, S.; Schweiger, T.; et al. Vessel Co-Option Is Common in Human Lung Metastases and Mediates Resistance to Anti-Angiogenic Therapy in Preclinical Lung Metastasis Models: Vessel Co-Option in Lung Metastases. J. Pathol. 2017, 241, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Cleck, J.N. Adverse Effects of Anticancer Agents That Target the VEGF Pathway. Nat. Rev. Clin. Oncol. 2009, 6, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III Trial of Cisplatin Plus Gemcitabine With Either Placebo or Bevacizumab As First-Line Therapy for Nonsquamous Non–Small-Cell Lung Cancer: AVAiL. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Tebbutt, N.C.; Wilson, K.; Gebski, V.J.; Cummins, M.M.; Zannino, D.; Van Hazel, G.A.; Robinson, B.; Broad, A.; Ganju, V.; Ackland, S.P.; et al. Capecitabine, Bevacizumab, and Mitomycin in First-Line Treatment of Metastatic Colorectal Cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010, 28, 3191–3198. [Google Scholar] [CrossRef]
- Zheng, W.; Qian, C.; Tang, Y.; Yang, C.; Zhou, Y.; Shen, P.; Chen, W.; Yu, S.; Wei, Z.; Wang, A.; et al. Manipulation of the Crosstalk between Tumor Angiogenesis and Immunosuppression in the Tumor Microenvironment: Insight into the Combination Therapy of Anti-Angiogenesis and Immune Checkpoint Blockade. Front. Immunol. 2022, 13, 1035323. [Google Scholar] [CrossRef]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Swamy, K. Vascular Normalization and Immunotherapy: Spawning a Virtuous Cycle. Front. Oncol. 2022, 12, 1002957. [Google Scholar] [CrossRef]
- Nogami, N.; Barlesi, F.; Socinski, M.A.; Reck, M.; Thomas, C.A.; Cappuzzo, F.; Mok, T.S.K.; Finley, G.; Aerts, J.G.; Orlandi, F.; et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J. Thorac. Oncol. 2022, 17, 309–323. [Google Scholar] [CrossRef]
- Herbst, R.S.; Arkenau, H.-T.; Santana-Davila, R.; Calvo, E.; Paz-Ares, L.; Cassier, P.A.; Bendell, J.; Penel, N.; Krebs, M.G.; Martin-Liberal, J.; et al. Ramucirumab plus Pembrolizumab in Patients with Previously Treated Advanced Non-Small-Cell Lung Cancer, Gastro-Oesophageal Cancer, or Urothelial Carcinomas (JVDF): A Multicohort, Non-Randomised, Open-Label, Phase 1a/b Trial. Lancet Oncol. 2019, 20, 1109–1123. [Google Scholar] [CrossRef]
- Li, A.; Fang, J. Anti-angiogenic Therapy Enhances Cancer Immunotherapy: Mechanism and Clinical Application. Interdiscip. Med. 2024, 2, e20230025. [Google Scholar] [CrossRef]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Wyser Rmili, C.; Kiialainen, A.; Kienast, Y.; Mueller, H.-J.; Ooi, C.-H.; Laoui, D.; et al. Dual Angiopoietin-2 and VEGFA Inhibition Elicits Antitumor Immunity That Is Enhanced by PD-1 Checkpoint Blockade. Sci. Transl. Med. 2017, 9, eaak9670. [Google Scholar] [CrossRef]
- Schmittnaegel, M.; De Palma, M. Reprogramming Tumor Blood Vessels for Enhancing Immunotherapy. Trends Cancer 2017, 3, 809–812. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, I.-K.; Han, S.; Park, I.; Kim, C.; Bae, J.; Oh, S.J.; Lee, S.; Kim, J.H.; Woo, D.-C.; et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment. Cancer Cell 2016, 30, 953–967. [Google Scholar] [CrossRef]
- Wong, P.-P.; Demircioglu, F.; Ghazaly, E.; Alrawashdeh, W.; Stratford, M.R.L.; Scudamore, C.L.; Cereser, B.; Crnogorac-Jurcevic, T.; McDonald, S.; Elia, G.; et al. Dual-Action Combination Therapy Enhances Angiogenesis While Reducing Tumor Growth and Spread. Cancer Cell 2015, 27, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.R.; Hart, I.R.; Watson, A.R.; Welti, J.C.; Silva, R.G.; Robinson, S.D.; Da Violante, G.; Gourlaouen, M.; Salih, M.; Jones, M.C.; et al. Stimulation of Tumor Growth and Angiogenesis by Low Concentrations of RGD-Mimetic Integrin Inhibitors. Nat. Med. 2009, 15, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Takara, K.; Eino, D.; Ando, K.; Yasuda, D.; Naito, H.; Tsukada, Y.; Iba, T.; Wakabayashi, T.; Muramatsu, F.; Kidoya, H.; et al. Lysophosphatidic Acid Receptor 4 Activation Augments Drug Delivery in Tumors by Tightening Endothelial Cell-Cell Contact. Cell Rep. 2017, 20, 2072–2086. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, Y.; Okamoto, K.; Adachi, Y.; Semba, T.; Uesugi, M.; Ozawa, Y.; Tohyama, O.; Uehara, T.; Kimura, T.; Watanabe, H.; et al. Eribulin Mesylate Reduces Tumor Microenvironment Abnormality by Vascular Remodeling in Preclinical Human Breast Cancer Models. Cancer Sci. 2014, 105, 1334–1342. [Google Scholar] [CrossRef]
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for Its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- Tozer, G.M.; Kanthou, C.; Lewis, G.; Prise, V.E.; Vojnovic, B.; Hill, S.A. Tumour Vascular Disrupting Agents: Combating Treatment Resistance. Br. J. Radiol. 2008, 81, S12–S20. [Google Scholar] [CrossRef]
- Newport, E.L.; Pedrosa, A.R.; Njegic, A.; Hodivala-Dilke, K.M.; Muñoz-Félix, J.M. Improved Immunotherapy Efficacy by Vascular Modulation. Cancers 2021, 13, 5207. [Google Scholar] [CrossRef]
- Baluk, P.; Morikawa, S.; Haskell, A.; Mancuso, M.; McDonald, D.M. Abnormalities of Basement Membrane on Blood Vessels and Endothelial Sprouts in Tumors. Am. J. Pathol. 2003, 163, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, P.; Eskens, F.A.L.M. Vascular Disrupting Agents in Clinical Development. Br. J. Cancer 2007, 96, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Chaplin, D.J.; Horsman, M.R. Vascular-targeting Therapies for Treatment of Malignant Disease. Cancer 2004, 100, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pérez, T.; Ortiz-Ferrón, G.; López-Rivas, A. Mitotic Arrest and JNK-Induced Proteasomal Degradation of FLIP and Mcl-1 Are Key Events in the Sensitization of Breast Tumor Cells to TRAIL by Antimicrotubule Agents. Cell Death Differ. 2010, 17, 883–894. [Google Scholar] [CrossRef]
- Singh, A.V.; Bandi, M.; Raje, N.; Richardson, P.; Palladino, M.A.; Chauhan, D.; Anderson, K.C. A Novel Vascular Disrupting Agent Plinabulin Triggers JNK-Mediated Apoptosis and Inhibits Angiogenesis in Multiple Myeloma Cells. Blood 2011, 117, 5692–5700. [Google Scholar] [CrossRef]
- Patterson, D.M.; Rustin, G.J.S. Vascular Damaging Agents. Clin. Oncol. 2007, 19, 443–456. [Google Scholar] [CrossRef]
- Iversen, A.B.; Busk, M.; Horsman, M.R. Induction of Hypoxia by Vascular Disrupting Agents and the Significance for Their Combination with Radiation Therapy. Acta Oncol. 2013, 52, 1320–1326. [Google Scholar] [CrossRef]
- Gill, J.H.; Rockley, K.L.; De Santis, C.; Mohamed, A.K. Vascular Disrupting Agents in Cancer Treatment: Cardiovascular Toxicity and Implications for Co-Administration with Other Cancer Chemotherapeutics. Pharmacol. Ther. 2019, 202, 18–31. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S.; et al. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, 2105254. [Google Scholar] [CrossRef]
- Shenoi, M.M.; Iltis, I.; Choi, J.; Koonce, N.A.; Metzger, G.J.; Griffin, R.J.; Bischof, J.C. Nanoparticle Delivered Vascular Disrupting Agents (VDAs): Use of TNF-Alpha Conjugated Gold Nanoparticles for Multimodal Cancer Therapy. Mol. Pharm. 2013, 10, 1683–1694. [Google Scholar] [CrossRef]
- Liang, Y.; Hao, Y.; Wu, Y.; Zhou, Z.; Li, J.; Sun, X.; Liu, Y.-N. Integrated Hydrogel Platform for Programmed Antitumor Therapy Based on Near Infrared-Triggered Hyperthermia and Vascular Disruption. ACS Appl. Mater. Interfaces 2019, 11, 21381–21390. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Su, L.; Hu, Q.; Li, J.; Zhu, X. An Erythrocyte Membrane-Modified Biomimetic Synergistic Nanosystem for Cancer Anti-Vascular Therapy and Initial Efficacy Monitoring. J. Mater. Chem. B 2023, 11, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Luong, R.; Paulmurugan, R.; Lin, F.I.; Kempen, P.; Zavaleta, C.; Chu, P.; Massoud, T.F.; Sinclair, R.; Gambhir, S.S. The Fate and Toxicity of Raman-Active Silica-Gold Nanoparticles in Mice. Sci. Transl. Med. 2011, 3, 79ra33. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, A.B.; Rojas, J.D.; Dayton, P.A.; Huang, L. Enhancing Nanoparticle Accumulation and Retention in Desmoplastic Tumors via Vascular Disruption for Internal Radiation Therapy. Theranostics 2017, 7, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Dragulska, S.A.; Poursharifi, M.; Chen, Y.; Wlodarczyk, M.T.; Acosta Santiago, M.; Dottino, P.; Martignetti, J.A.; Mieszawska, A.J. Engineering and Validation of a Peptide-Stabilized Poly(Lactic- Co -Glycolic) Acid Nanoparticle for Targeted Delivery of a Vascular Disruptive Agent in Cancer Therapy. Bioconjug. Chem. 2022, 33, 2348–2360. [Google Scholar] [CrossRef]
- Sidorenko, V.; Scodeller, P.; Uustare, A.; Ogibalov, I.; Tasa, A.; Tshubrik, O.; Salumäe, L.; Sugahara, K.N.; Simón-Gracia, L.; Teesalu, T. Targeting Vascular Disrupting Agent-Treated Tumor Microenvironment with Tissue-Penetrating Nanotherapy. Sci. Rep. 2024, 14, 17513. [Google Scholar] [CrossRef]
- Ji, Y.-T.; Liu, Y.-N.; Liu, Z.-P. Tubulin Colchicine Binding Site Inhibitors as Vascular Disrupting Agents in Clinical Developments. Curr. Med. Chem. 2015, 22, 1348–1360. [Google Scholar] [CrossRef]
- Wei, X.-L.; Wu, H.-X.; Ruan, D.-Y.; Wang, F.; Xu, L.; Li, Y.-H.; Ma, Y.-X.; Wang, Z.-Q.; Yang, Y.-P.; Tang, L.-W.; et al. First-in-Human Phase 1 Study of an Orally Bioavailable Vascular-Disrupting Agent DX1002 in Patients with Advanced Solid Tumors. Cell Rep. Med. 2025, 6, 101969. [Google Scholar] [CrossRef]
- Prieto, L.; Gaviña, D.; Escolano, M.; Cánovas-Belchí, M.; Sánchez-Roselló, M.; Del Pozo, C.; Falomir, E.; Díaz-Oltra, S. Synthesis and Biological Evaluation of New Cis-Restricted Triazole Analogues of Combretastatin A-4. Molecules 2025, 30, 317. [Google Scholar] [CrossRef]
- Lippert, J.W. Vascular Disrupting Agents. Bioorg. Med. Chem. 2007, 15, 605–615. [Google Scholar] [CrossRef]
- Patterson, D.M.; Zweifel, M.; Middleton, M.R.; Price, P.M.; Folkes, L.K.; Stratford, M.R.L.; Ross, P.; Halford, S.; Peters, J.; Balkissoon, J.; et al. Phase I Clinical and Pharmacokinetic Evaluation of the Vascular-Disrupting Agent OXi4503 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2012, 18, 1415–1425. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, D.; Song, W.; Tang, Z.; Zhu, J.; Ma, Z.; Wang, X.; Chen, X.; Tong, T. A Poly(l-Glutamic Acid)-Combretastatin A4 Conjugate for Solid Tumor Therapy: Markedly Improved Therapeutic Efficiency through Its Low Tissue Penetration in Solid Tumor. Acta Biomater. 2017, 53, 179–189. [Google Scholar] [CrossRef]
- Su, X.-Z.; Qiao, E.-Q.; Teng, G.-J.; Xiong, F. Quaternary Ammonium Salt Microspheres Loaded with Vascular Disrupting Agents for Targeted Interventional Therapy of Hepatocellular Carcinoma. Acta Biomater. 2025, 203, 591–603. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, K. Targeting Angiogenesis in Gastrointestinal Tumors: Strategies from Vascular Disruption to Vascular Normalization and Promotion Strategies Angiogenesis Strategies in GI Tumor Therapy. Front. Immunol. 2025, 16, 1550752. [Google Scholar] [CrossRef]
- Chu, H.; Xu, Y.; Shan, Y.; Sun, M.; Zhao, W.; Fang, X.; Shen, N.; Tang, Z. Platelet Hitchhiking Vascular-Disrupting Agents for Self-Amplified Tumor-Targeting Therapy. J. Nanobiotechnology 2025, 23, 197. [Google Scholar] [CrossRef]
- Endo, R.; Ueda, T.; Nagaoki, T.; Sato, Y.; Maishi, N.; Hida, K.; Harashima, H.; Nakamura, T. Selective Vascular Disrupting Therapy by Lipid Nanoparticle-Mediated Fas Ligand Silencing and Stimulation of STING. Biomaterials 2025, 321, 123297. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Shi, Y.-K.; Feinstein, T.; Feng, D.; Mitchell, D.; Lelorier, Y.; Du, L.; Huang, L.; Mohanlal, R.; Sun, Y. LBA48 DUBLIN-3 (BPI-2358-103): A Global Phase (Ph) III Trial with the Plinabulin/Docetaxel (Plin/Doc) Combination vs. Doc in 2nd/3rd Line NSCLC Patients (Pts) with EGFR-Wild Type (Wt) Progressing on a Prior Platinum-Based Regimen. Ann. Oncol. 2021, 32, S1326. [Google Scholar] [CrossRef]
- Han, B.; Feinstein, T.; Shi, Y.; Chen, M.; Huang, L.; Mohanlal, R.W.; Sun, Y. Subgroup Analysis in Patients (Pts) with Non-Squamous (N-Sq), EGFR-Wild Type (Wt), Second/Third-Line NSCLC from the Global Phase (Ph) 3 Trial DUBLIN-3 (BPI-2358-103) with the Plinabulin/Docetaxel (Plin/Doc) Combination versus Doc Alone. J. Clin. Oncol. 2022, 40, 9090. [Google Scholar] [CrossRef]
- Blayney, D.W.; Mohanlal, R.; Huang, L. Protective-2 (BPI-2358-106): A Confirmatory Trial to Demonstrate Superiority of the Plinabulin+Pegfilgrastim (Plin/Peg) Combination Versus Standard of Care Pegfilgrastim for the Prevention of Chemotherapy-Induced Neutropenia (CIN) in Breast Cancer (BC) Patients (Pts). Blood 2020, 136, 16. [Google Scholar] [CrossRef]
- Rehman, F.; Rustin, G. ASA404: Update on Drug Development. Expert Opin. Investig. Drugs 2008, 17, 1547–1551. [Google Scholar] [CrossRef]
- Daei Farshchi Adli, A.; Jahanban-Esfahlan, R.; Seidi, K.; Samandari-Rad, S.; Zarghami, N. An Overview on Vadimezan (DMXAA): The Vascular Disrupting Agent. Chem. Biol. Drug Des. 2018, 91, 996–1006. [Google Scholar] [CrossRef]
- Burrows, F.J.; Thorpe, P.E. Eradication of Large Solid Tumors in Mice with an Immunotoxin Directed against Tumor Vasculature. Proc. Natl. Acad. Sci. USA 1993, 90, 8996–9000. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, F.; Haruta, Y.; Kondo, M.; Tsai, H.; Barcos, M.; Seon, B.K. Induction of Lasting Complete Regression of Preformed Distinct Solid Tumors by Targeting the Tumor Vasculature Using Two New Anti-Endoglin Monoclonal Antibodies1. Clin. Cancer Res. 1999, 5, 371–382. [Google Scholar] [PubMed]
- Seon, B.K.; Haba, A.; Matsuno, F.; Takahashi, N. Endoglin-Targeted Cancer Therapy. Curr. Drug Deliv. 2011, 8, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Gao, B.; Duffy, S.; Watkins, L.; Rote, N.; Thorpe, P.E. Infarction of Solid Hodgkin’s Tumors in Mice by Antibody-Directed Targeting of Tissue Factor to Tumor Vasculature1. Cancer Res. 1998, 58, 4646–4653. [Google Scholar]
- Huijbers, E.J.M.; Van Beijnum, J.R.; Van Loon, K.; Griffioen, C.J.; Volckmann, R.; Bassez, A.; Lambrechts, D.; Nunes Monteiro, M.; Jimenez, C.R.; Hogendoorn, P.C.W.; et al. Embryonic Reprogramming of the Tumor Vasculature Reveals Targets for Cancer Therapy. Proc. Natl. Acad. Sci. USA 2025, 122, e2424730122. [Google Scholar] [CrossRef]
- Rakocevic, J.; Orlic, D.; Mitrovic-Ajtic, O.; Tomasevic, M.; Dobric, M.; Zlatic, N.; Milasinovic, D.; Stankovic, G.; Ostojić, M.; Labudovic-Borovic, M. Endothelial Cell Markers from Clinician’s Perspective. Exp. Mol. Pathol. 2017, 102, 303–313. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Popova, P.I.; Avdonin, P.P.; Kudryavtsev, I.V.; Serebryakova, M.K.; Korf, E.A.; Avdonin, P.V. Markers of Endothelial Cells in Normal and Pathological Conditions. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2020, 14, 167–183. [Google Scholar] [CrossRef]
- Takahashi, K.; Kobayashi, M.; Katsumata, H.; Tokizaki, S.; Anzai, T.; Ikeda, Y.; Alcaide, D.M.; Maeda, K.; Ishihara, M.; Tahara, K.; et al. CD40 Is Expressed in the Subsets of Endothelial Cells Undergoing Partial Endothelial–Mesenchymal Transition in Tumor Microenvironment. Cancer Sci. 2024, 115, 490–506. [Google Scholar] [CrossRef]
- Shaker, N.; Chen, W.; Sinclair, W.; Parwani, A.V.; Li, Z. Identifying SOX17 as a Sensitive and Specific Marker for Ovarian and Endometrial Carcinomas. Mod. Pathol. 2023, 36, 100038. [Google Scholar] [CrossRef]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Rio, G.D.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-Cancer Activity of Targeted pro-Apoptotic Peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Cichoń, T.; Graja, K.; Hucz, J.; Sochanik, A.; Szala, S. Antitumor Effect of RGD-4C-GG-D(KLAKLAK)2 Peptide in Mouse B16(F10) Melanoma Model. Acta Biochim. Pol. 2006, 53, 801–805. [Google Scholar] [CrossRef]
- Shibuya, M. Differential Roles of Vascular Endothelial Growth Factor Receptor-1 and Receptor-2 in Angiogenesis. BMB Rep. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Qiu, G.Q.; Xie, X.; Zhao, B.; Xu, L.Z.; Chen, Y.Q. Fusion Protein tTF-EG3287 Induces Occlusion of Tumor Vessels and Impairs Tumor Growth in Human Colon Cancer. Neoplasma 2019, 66, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kanthou, C.; Tozer, G.M. Microtubule Depolymerizing Vascular Disrupting Agents: Novel Therapeutic Agents for Oncology and Other Pathologies. Int. J. Exp. Pathol. 2009, 90, 284–294. [Google Scholar] [CrossRef] [PubMed]
- El-Emir, E.; Boxer, G.M.; Petrie, I.A.; Boden, R.W.; Dearling, J.L.J.; Begent, R.H.J.; Pedley, R.B. Tumour Parameters Affected by Combretastatin A-4 Phosphate Therapy in a Human Colorectal Xenograft Model in Nude Mice. Eur. J. Cancer 2005, 41, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Seidi, K.; Jahanban-Esfahlan, R.; Zarghami, N. Tumor Rim Cells: From Resistance to Vascular Targeting Agents to Complete Tumor Ablation. Tumor Biol. 2017, 39, 101042831769100. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Ma, W.; Gurung, K.; Guo, C.-H. Mechanisms of Tumor Resistance to Small-Molecule Vascular Disrupting Agents: Treatment and Rationale of Combination Therapy. J. Formos. Med. Assoc. 2013, 112, 115–124. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Z.; Xu, W.; Sun, T.; Zhuang, X.; Ding, J.; Chen, X. Spatiotemporally Targeted Nanomedicine Overcomes Hypoxia-Induced Drug Resistance of Tumor Cells after Disrupting Neovasculature. Nano Lett. 2020, 20, 6191–6198. [Google Scholar] [CrossRef]
- Chen, M.; Lei, X.; Shi, C.; Huang, M.; Li, X.; Wu, B.; Li, Z.; Han, W.; Du, B.; Hu, J.; et al. Pericyte-Targeting Prodrug Overcomes Tumor Resistance to Vascular Disrupting Agents. J. Clin. Investig. 2017, 127, 3689–3701. [Google Scholar] [CrossRef]
- Gee, M.S.; Procopio, W.N.; Makonnen, S.; Feldman, M.D.; Yeilding, N.M.; Lee, W.M.F. Tumor Vessel Development and Maturation Impose Limits on the Effectiveness of Anti-Vascular Therapy. Am. J. Pathol. 2003, 162, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Roswall, P.; Cortez, E.; Hanahan, D.; Pietras, K. Pericytes Promote Endothelial Cell Survival through Induction of Autocrine VEGF-A Signaling and Bcl-w Expression. Blood 2011, 118, 2906–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tan, Y.; He, C.; Liu, Y.; Duan, Y.; Zhu, W.; Zheng, T.; Li, D.; Xu, J.; Yang, D.-H.; et al. Discovery of a Novel Vascular Disrupting Agent Inhibiting Tubulin Polymerization and HDACs with Potent Antitumor Effects. J. Med. Chem. 2022, 65, 11187–11213. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, M.; Li, X.; Huang, M.; Nie, Q.; Ma, N.; Chen, H.; Xu, N.; Ye, W.; Zhang, D. A Vascular Disrupting Agent Overcomes Tumor Multidrug Resistance by Skewing Macrophage Polarity toward the M1 Phenotype. Cancer Lett. 2018, 418, 239–249. [Google Scholar] [CrossRef]
- Welford, A.F.; Biziato, D.; Coffelt, S.B.; Nucera, S.; Fisher, M.; Pucci, F.; Di Serio, C.; Naldini, L.; De Palma, M.; Tozer, G.M.; et al. TIE2-Expressing Macrophages Limit the Therapeutic Efficacy of the Vascular-Disrupting Agent Combretastatin A4 Phosphate in Mice. J. Clin. Investig. 2011, 121, 1969–1973. [Google Scholar] [CrossRef]
- Squadrito, M.L.; De Palma, M. Macrophage Regulation of Tumor Angiogenesis: Implications for Cancer Therapy. Mol. Aspects Med. 2011, 32, 123–145. [Google Scholar] [CrossRef]
- Taylor, M.; Billiot, F.; Marty, V.; Rouffiac, V.; Cohen, P.; Tournay, E.; Opolon, P.; Louache, F.; Vassal, G.; Laplace-Builhé, C.; et al. Reversing Resistance to Vascular-Disrupting Agents by Blocking Late Mobilization of Circulating Endothelial Progenitor Cells. Cancer Discov. 2012, 2, 434–449. [Google Scholar] [CrossRef]
- Horsman, M.R.; Siemann, D.W. Pathophysiologic Effects of Vascular-Targeting Agents and the Implications for Combination with Conventional Therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef]
- Drzyzga, A.; Cichoń, T.; Czapla, J.; Jarosz-Biej, M.; Pilny, E.; Matuszczak, S.; Wojcieszek, P.; Urbaś, Z.; Smolarczyk, R. The Proper Administration Sequence of Radiotherapy and Anti-Vascular Agent—DMXAA Is Essential to Inhibit the Growth of Melanoma Tumors. Cancers 2021, 13, 3924. [Google Scholar] [CrossRef]
- Clémenson, C.; Chargari, C.; Deutsch, E. Combination of Vascular Disrupting Agents and Ionizing Radiation. Crit. Rev. Oncol. Hematol. 2013, 86, 143–160. [Google Scholar] [CrossRef]
- Pedley, R.B.; Hill, S.A.; Boxer, G.M.; Flynn, A.A.; Boden, R.; Watson, R.; Dearling, J.; Chaplin, D.J.; Begent, R.H. Eradication of Colorectal Xenografts by Combined Radioimmunotherapy and Combretastatin A-4 3-O-Phosphate. Cancer Res. 2001, 61, 4716–4722. [Google Scholar]
- Meyer, T.; Gaya, A.M.; Dancey, G.; Stratford, M.R.L.; Othman, S.; Sharma, S.K.; Wellsted, D.; Taylor, N.J.; Stirling, J.J.; Poupard, L.; et al. A Phase I Trial of Radioimmunotherapy with 131I-A5B7 Anti-CEA Antibody in Combination with Combretastatin-A4-Phosphate in Advanced Gastrointestinal Carcinomas. Clin. Cancer Res. 2009, 15, 4484–4492. [Google Scholar] [CrossRef]
- Song, S.; Xiong, C.; Zhou, M.; Lu, W.; Huang, Q.; Ku, G.; Zhao, J.; Flores, L.G.; Ni, Y.; Li, C. Small-Animal PET of Tumor Damage Induced by Photothermal Ablation with64 Cu-Bis-DOTA-Hypericin. J. Nucl. Med. 2011, 52, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhang, J.; Sun, Z.; Chen, F.; Dai, X.; Li, Y.; Ni, Y.; Xu, K. Necrosis Targeted Radiotherapy with Iodine-131-Labeled Hypericin to Improve Anticancer Efficacy of Vascular Disrupting Treatment in Rabbit VX2 Tumor Models. Oncotarget 2015, 6, 14247–14259. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chu, T.; Wei, J.; Zhang, Y.; Qi, F.; Lu, Z.; Gao, C.; Zhang, T.; Jiang, E.; Xu, J.; et al. Platelet-Membrane-Coated Nanoparticles Enable Vascular Disrupting Agent Combining Anti-Angiogenic Drug for Improved Tumor Vessel Impairment. Nano Lett. 2021, 21, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Mita, M.M.; Sargsyan, L.; Mita, A.C.; Spear, M. Vascular-Disrupting Agents in Oncology. Expert Opin. Investig. Drugs 2013, 22, 317–328. [Google Scholar] [CrossRef]
- Nathan, P.; Zweifel, M.; Padhani, A.R.; Koh, D.-M.; Ng, M.; Collins, D.J.; Harris, A.; Carden, C.; Smythe, J.; Fisher, N.; et al. Phase I Trial of Combretastatin A4 Phosphate (CA4P) in Combination with Bevacizumab in Patients with Advanced Cancer. Clin. Cancer Res. 2012, 18, 3428–3439. [Google Scholar] [CrossRef]
- Morgan, R.D.; Banerjee, S.; Hall, M.; Clamp, A.R.; Zhou, C.; Hasan, J.; Orbegoso, C.; Taylor, S.; Tugwood, J.; Lyon, A.R.; et al. Pazopanib and Fosbretabulin in Recurrent Ovarian Cancer (PAZOFOS): A Multi-Centre, Phase 1b and Open-Label, Randomised Phase 2 Trial. Gynecol. Oncol. 2020, 156, 545–551. [Google Scholar] [CrossRef]
- Malhotra, J.; Chiappori, A.; Fujioka, N.; Hanna, N.H.; Feldman, L.E.; Patel, M.; Moore, D.; Chen, C.; Jabbour, S.K. Phase I/II Trial of Plinabulin in Combination with Nivolumab and Ipilimumab in Patients with Recurrent Small Cell Lung Cancer (SCLC): Big Ten Cancer Research Consortium (BTCRC-LUN17-127) Study. Lung Cancer 2024, 195, 107932. [Google Scholar] [CrossRef]
- Han, B.; Feinstein, T.; Shi, Y.; Chen, G.; Yao, Y.; Hu, C.; Shi, J.; Feng, J.; Wu, H.; Cheng, Y.; et al. Plinabulin plus Docetaxel versus Docetaxel in Patients with Non-Small-Cell Lung Cancer after Disease Progression on Platinum-Based Regimen (DUBLIN-3): A Phase 3, International, Multicentre, Single-Blind, Parallel Group, Randomised Controlled Trial. Lancet Respir. Med. 2024, 12, 775–786. [Google Scholar] [CrossRef]
- Jameson, M.B.; Thompson, P.I.; Baguley, B.C.; Evans, B.D.; Harvey, V.J.; Porter, D.J.; McCrystal, M.R.; Small, M.; Bellenger, K.; Gumbrell, L.; et al. Clinical Aspects of a Phase I Trial of 5,6-Dimethylxanthenone-4-Acetic Acid (DMXAA), a Novel Antivascular Agent. Br. J. Cancer 2003, 88, 1844–1850. [Google Scholar] [CrossRef]
- Jameson, M.B.; Sharp, D.M.; Sissingh, J.I.; Hogg, C.R.; Thompson, P.I.; McKeage, M.J.; Jeffery, M.; Waller, S.; Acton, G.; Green, C.; et al. Transient Retinal Effects of 5,6-Dimethylxanthenone-4-Acetic Acid (DMXAA, ASA404), an Antitumor Vascular-Disrupting Agent in Phase I Clinical Trials. Investig. Opthalmology Vis. Sci. 2009, 50, 2553. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Douillard, J.-Y.; Nakagawa, K.; Von Pawel, J.; McKeage, M.J.; Albert, I.; Losonczy, G.; Reck, M.; Heo, D.-S.; Fan, X.; et al. Randomized Phase III Placebo-Controlled Trial of Carboplatin and Paclitaxel With or Without the Vascular Disrupting Agent Vadimezan (ASA404) in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Von Pawel, J.; Gorbounova, V.; Reck, M.; Kowalski, D.M.; Allard, A.; Chadjaa, M.; Rey, A.; Bennouna, J.; Grossi, F. DISRUPT: A Randomised Phase 2 Trial of Ombrabulin (AVE8062) plus a Taxane–Platinum Regimen as First-Line Therapy for Metastatic Non-Small Cell Lung Cancer. Lung Cancer 2014, 85, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sunaga, Y.; Ecstein-Fraisse, E.; Fujiwara, K. Phase I Study of Ombrabulin in Combination with Paclitaxel and Carboplatin in Japanese Patients with Advanced Solid Tumors. Int. J. Gynecol. Cancer 2024, 34, 586–593. [Google Scholar] [CrossRef]
- Eskens, F.A.L.M.; Tresca, P.; Tosi, D.; Van Doorn, L.; Fontaine, H.; Van Der Gaast, A.; Veyrat-Follet, C.; Oprea, C.; Hospitel, M.; Dieras, V. A Phase I Pharmacokinetic Study of the Vascular Disrupting Agent Ombrabulin (AVE8062) and Docetaxel in Advanced Solid Tumours. Br. J. Cancer 2014, 110, 2170–2177. [Google Scholar] [CrossRef]
- Rischin, D.; Bibby, D.C.; Chong, G.; Kremmidiotis, G.; Leske, A.F.; Matthews, C.A.; Wong, S.S.; Rosen, M.A.; Desai, J. Clinical, Pharmacodynamic, and Pharmacokinetic Evaluation of BNC105P: A Phase I Trial of a Novel Vascular Disrupting Agent and Inhibitor of Cancer Cell Proliferation. Clin. Cancer Res. 2011, 17, 5152–5160. [Google Scholar] [CrossRef]
- Gregorc, V.; Cavina, R.; Novello, S.; Grossi, F.; Lazzari, C.; Capelletto, E.; Genova, C.; Salini, G.; Lambiase, A.; Santoro, A. NGR-hTNF and Doxorubicin as Second-Line Treatment of Patients with Small Cell Lung Cancer. Oncologist 2018, 23, 1133-e112. [Google Scholar] [CrossRef]
- Valentinis, B.; Porcellini, S.; Asperti, C.; Cota, M.; Zhou, D.; Di Matteo, P.; Garau, G.; Zucchelli, C.; Avanzi, N.R.; Rizzardi, G.P.; et al. Mechanism of Action of the Tumor Vessel Targeting Agent NGR-hTNF: Role of Both NGR Peptide and hTNF in Cell Binding and Signaling. Int. J. Mol. Sci. 2019, 20, 4511. [Google Scholar] [CrossRef]


| Strategies | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Anti-angiogenic therapy (AAT) | Inhibition of new blood vessel formation |
|
|
| Vascular normalization | Reduced vessel density and improved structure and functionality |
|
|
| Vascular promotion | Increased vessel density and improved structure and functionality |
|
|
| Vascular disruption | Collapse of established angiogenic vessels |
|
|
| VDAs | Compounds | Mechanism | Features | Enhancing Strategies | References |
|---|---|---|---|---|---|
| Microtubule-binding | CA4P, OXi4503 | Endothelial cytoskeleton collapse |
| Improved-selectivity analogs | Liu et al., 2017 [82] Wei et al., 2025 [78] Prieto et al., 2025 [79] |
| Improved delivery systems | Su et al., 2025 [83] Li et al., 2025 [84] | ||||
| Flavonoids | DMXAA | Cytokine induction, apoptosis |
| Cell-mediated delivery systems | Chu et al., 2025 [85] |
| Ligand-directed | Antibodies, peptides | Targeted toxin/pro-coagulant delivery |
| Lipid nanoparticle-mediated ligand silencing | Endo et al., 2025 [86] |
| Strategy | Mechanism of Action | Compound | Reported Benefits |
|---|---|---|---|
| Pericyte targeting | Selective ablation of pericyte-covered vessels | Z-GP-DAVLBH | Elimination of VDA-resistant tumor rim |
| EPC inhibition | Blockage of post-treatment vascular repair | TKR inhibitors + CA4P/VEGFR2 inhibitors + CA4P | Reduced neovascularization |
| Chemotherapy combination | Cytotoxic effect on surviving peripheral tumor cells | Conventional therapies (Carboplatin, Cisplatin, etc.) + VDAs | Enhanced tumor regression, including viable rim |
| Immunotherapy combination | Increased immune infiltration and activation | Immune check-point inhibitors + VDAs | Improved immune-mediated tumor clearance |
| Radiotherapy combination | Selective radiation of necrotic and hypoxic tumor regions | 131I-A5B7 + CA4P/131I-hypericin | Tumor necrosis targeting, imaging capability |
| Anti-angiogenic therapy | Inhibition of neovascularization | VDAs + AAT | Dual targeting of existing and forming vasculature |
| Nanoparticle co-delivery | Coordinated delivery of VDAs and cytotoxins | VDAs + drug-loaded nanoparticles | Improved penetration, reduced hypoxia-driven resistance |
| Clinical Trial Code | Treatment (Drug) | VDA Target | Pathology Conditions | Start Year, Phase and Status |
|---|---|---|---|---|
| NCT00003768 | Fosbretabulin (CA4P) | Tubulin | Unspecified Adult Solid Resistant Tumor | 1998 Phase I Completed |
| NCT00113438 | Fosbretabulin (CA4P) Paclitaxel Carboplatin | Tubulin | Advanced Imageable Malignancies | 2005 Phase II Completed |
| NCT00395434 | Fosbretabulin (CA4P) Bevacizumab (Avastin) | Tubulin VEGF | Advanced solid tumors | 2006 Phase I Completed |
| NCT00507429 | Fosbretabulin (CA4P) Carboplatin Paclitaxel | Tubulin | Anaplastic thyroid carcinoma | 2007 Phase II, III Terminated |
| NCT00653939 | Fosbretabulin (CA4P) Carboplatin Paclitaxel Bevacizumab | Tubulin VEGF | NSCLC Lung Cancer | 2008 Phase II Completed |
| NCT02279602 | Fosbretabulin (CA4P) | Tubulin | Neuroendocrine Tumors | 2014 Phase II Completed |
| NCT02055690 | Fosbretabulin (CA4P) Pazopanib | Tubulin | Advanced Recurrent Ovarian Cancer | 2014 Phase Ib/II Terminated |
| NCT03014297 | Everolimus Fosbretabulin (CA4P) | Tubulin | Neuroendocrine Tumors | 2017 Phase I Terminated |
| NCT00322608 | Plinabulin (NPI-2358) | Tubulin | Advanced Solid Tumor Malignancies Lymphoma | 2006 Phase I Completed |
| NCT00630110 | Plinabulin (NPI-2358) Docetaxel | Tubulin | Advanced NSCLC | 2008 Phase I, II Completed |
| NCT02812667 | Plinabulin (NPI-2358) Nivolumab | Tubulin | NSCLC Metastatic | 2016 Phase I Active, not recruiting |
| NCT02846792 | Plinabulin (NPI-2358) Nivolumab | Tubulin | Stage IIIB-IV NSCLC | 2017 Phase I, II Terminated |
| NCT03575793 | Plinabulin (NPI-2358) Nivolumab Ipilimumab | Tubulin | Recurrent Small Cell Lung Cancer | 2018 Phase I, II Completed |
| NCT05130827 | Plinabulin (NPI-2358) Pegfilgrastim | Tubulin | Multiple Myeloma | 2021 Phase II Active, not recruiting |
| NCT00863733 | Vadimezan (DMXAA/ASA404) | Pro-inflammatory cytokine activation | Solid Tumors | 1996 Phase I Completed |
| NCT00856336 | Vadimezan (DMXAA/ASA404) | Pro-inflammatory cytokine activation | Refractory Tumors | 2003 Phase I Completed |
| NCT00832494 | Vadimezan (DMXAA/ASA404) Paclitaxel Carboplatin | Pro-inflammatory cytokine activation | NSCLC | 2004 Phase I, II Completed |
| NCT00111618 | Vadimezan (DMXAA/ASA404) Docetaxel | Pro-inflammatory cytokine activation | Hormone Refractory Metastatic Prostate Cancer | 2005 Phase II Completed |
| NCT00630110 | Vadimezan (DMXAA/ASA404) Docetaxel | Pro-inflammatory cytokine activation | Advanced or Recurrent Solid Tumors | 2009 Phase I Completed |
| NCT01299415 | Vadimezan (DMXAA/ASA404) Fluvoxamine | Pro-inflammatory cytokine activation | Solid Tumors | 2009 Phase I Terminated |
| NCT01240642 | Vadimezan (DMXAA/ASA404) Paclitaxel + Carboplatin Docetaxel | Pro-inflammatory cytokine activation | Metastatic Cancer With Impaired or Normal Renal Function | 2010 Phase I Terminated |
| NCT01057342 | Vadimezan (DMXAA/ASA404) Carboplatin Paclitaxel | Pro-inflammatory cytokine activation | Extensive-Stage Small-Cell Lung Cancer | 2010 Phase II Completed |
| NCT00699517 | Ombrabulin (AVE8062) | Tubulin | Advanced Soft Tissue Sarcoma | 2008 Phase III Completed |
| NCT00968916 | Ombrabulin (AVE8062) | Tubulin | Advanced Solid Tumors | 2009 Phase I Completed |
| NCT01293630 | Ombrabulin (AVE8062) Placlitaxel Carboplatin | Tubulin | Advanced Solid Tumors | 2011 Phase I Completed |
| NCT01263886 | Ombrabulin (AVE8062) | Tubulin | NSCLC Metastatic | 2011 Phase II Completed |
| NCT01332656 | Ombrabulin (AVE8062) Placlitaxel Carboplatin | Tubulin | Platinum-sensitive recurrent ovarian cancer | 2011 Phase II Completed |
| NCT01034631 | BNC105P Everolimus | Tubulin | Renal Cell Carcinoma | 2010 Phase I, II Completed |
| NCT03454165 | BNC105P Ibrutinib | Tubulin | Chronic Lymphocytic Leukemia | 2018 Phase I Completed |
| NCT03647839 | BNC 105P Nivolumab BBI608 | Tubulin | Refractory Colorectal Cancer | 2018 Phase II Completed |
| NCT00419328 | NGR-hTNF | CD13 (aminopeptidase N) | Advanced Solid Tumors | 2005 Phase I Completed |
| NCT00483080 | NGR-hTNF | CD13 (aminopeptidase N) | Colorectal Cancer | 2006 Phase II Completed |
| NCT00484211 | NGR-hTNF | CD13 (aminopeptidase N) | Advanced or Metastatic Hepatocellular Carcinoma | 2006 Phase II Completed |
| NCT00483093 | NGR-hTNF Cisplatin | CD13 (aminopeptidase N) | Advanced or Metastatic Solid Tumor | 2007 Phase I Completed |
| NCT00484276 | NGR-hTNF | CD13 (aminopeptidase N) | Advanced or Metastatic Malignant Pleural Mesothelioma | 2007 Phase II Completed |
| NCT00675012 | NGR-hTNF Oxaliplatin Capecitabine | CD13 (aminopeptidase N) | Metastatic Colorectal Cancer | 2007 Phase II Completed |
| NCT00484432 | NGR-hTNF Doxorubicin | CD13 (aminopeptidase N) | Advanced or Metastatic Ovarian Cancer | 2008 Phase II Completed |
| NCT00994097 | NGR-hTNF Cisplatin Gemcitabine Pemetrexed | CD13 (aminopeptidase N) | Advanced NSCLC | 2009 Phase II Completed |
| NCT00484341 | Low-dose NGR-hTNF High-dose NGR-hTNF Doxorubicin | CD13 (aminopeptidase N) | Advanced Soft Tissue Sarcoma | 2010 Phase II Completed |
| NCT03804866 | NGR-hTNF Pegylated liposomal doxorubicin Doxorubicin | CD13 (aminopeptidase N) | Platinum-resistant Ovarian Cancer | 2013 Phase II Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Escudero, J.; Berlana-Galán, P.; Guerra-Paes, E.; Torre-Cea, I.; Marcos-Zazo, L.; Carrera-Aguado, I.; Cáceres-Calle, D.; Sánchez-Juanes, F.; Muñoz-Félix, J.M. Vascular Disruption Therapy as a New Strategy for Cancer Treatment. Int. J. Mol. Sci. 2025, 26, 10085. https://doi.org/10.3390/ijms262010085
Gómez-Escudero J, Berlana-Galán P, Guerra-Paes E, Torre-Cea I, Marcos-Zazo L, Carrera-Aguado I, Cáceres-Calle D, Sánchez-Juanes F, Muñoz-Félix JM. Vascular Disruption Therapy as a New Strategy for Cancer Treatment. International Journal of Molecular Sciences. 2025; 26(20):10085. https://doi.org/10.3390/ijms262010085
Chicago/Turabian StyleGómez-Escudero, Jesús, Patricia Berlana-Galán, Elena Guerra-Paes, Irene Torre-Cea, Laura Marcos-Zazo, Iván Carrera-Aguado, Daniel Cáceres-Calle, Fernando Sánchez-Juanes, and José M. Muñoz-Félix. 2025. "Vascular Disruption Therapy as a New Strategy for Cancer Treatment" International Journal of Molecular Sciences 26, no. 20: 10085. https://doi.org/10.3390/ijms262010085
APA StyleGómez-Escudero, J., Berlana-Galán, P., Guerra-Paes, E., Torre-Cea, I., Marcos-Zazo, L., Carrera-Aguado, I., Cáceres-Calle, D., Sánchez-Juanes, F., & Muñoz-Félix, J. M. (2025). Vascular Disruption Therapy as a New Strategy for Cancer Treatment. International Journal of Molecular Sciences, 26(20), 10085. https://doi.org/10.3390/ijms262010085






