Knocking Down FRMD4A, a Factor Associated with the Brain Development Disorder and a Risk Factor for Alzheimer’s Disease, Using RNA-Targeting CRISPR/Cas13 Reveals Its Role in Cell Morphogenesis
Abstract
1. Introduction
2. Results
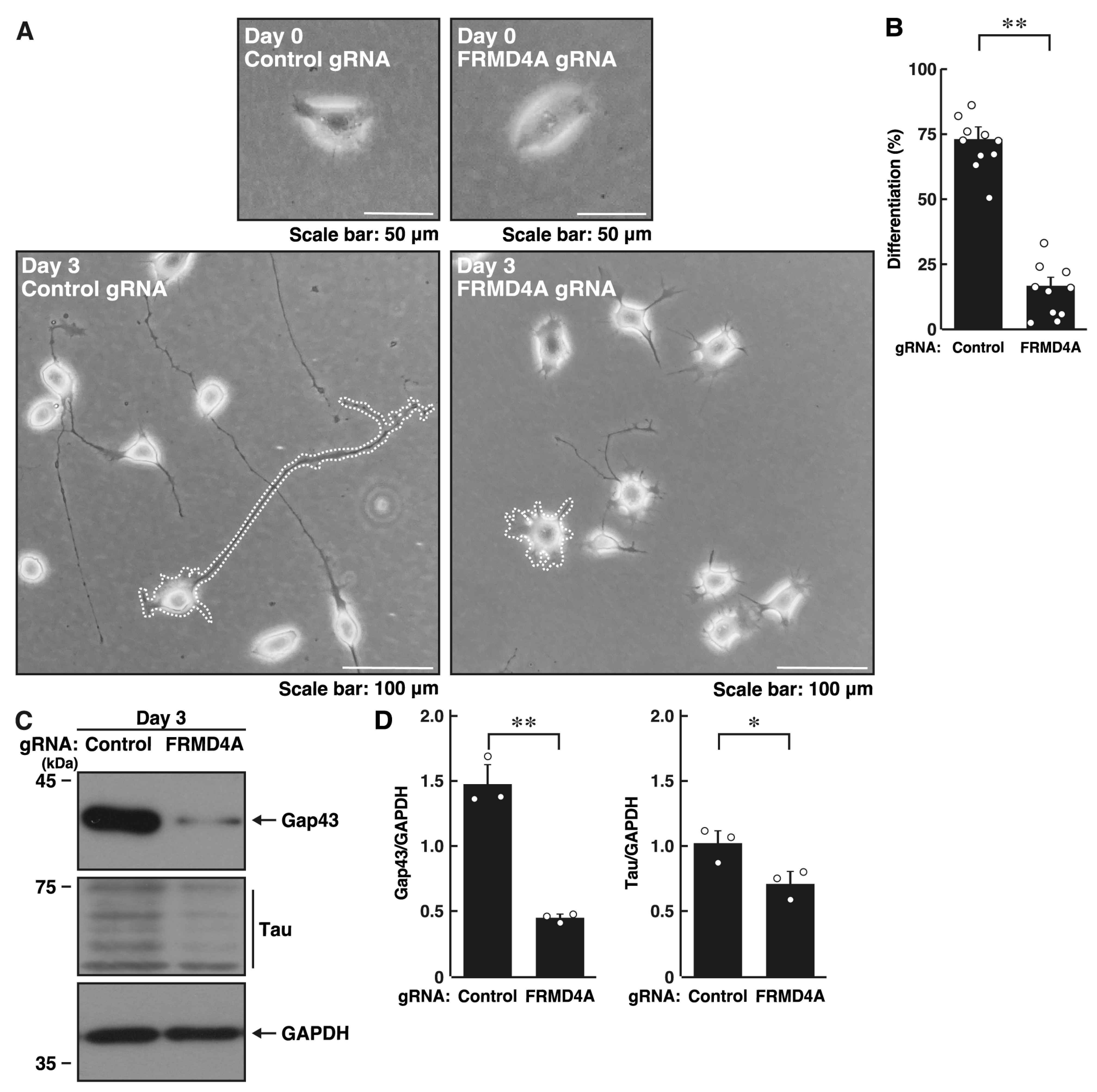
2.1. Knockdown of Frmd4a Leads to Decreased Process Elongation
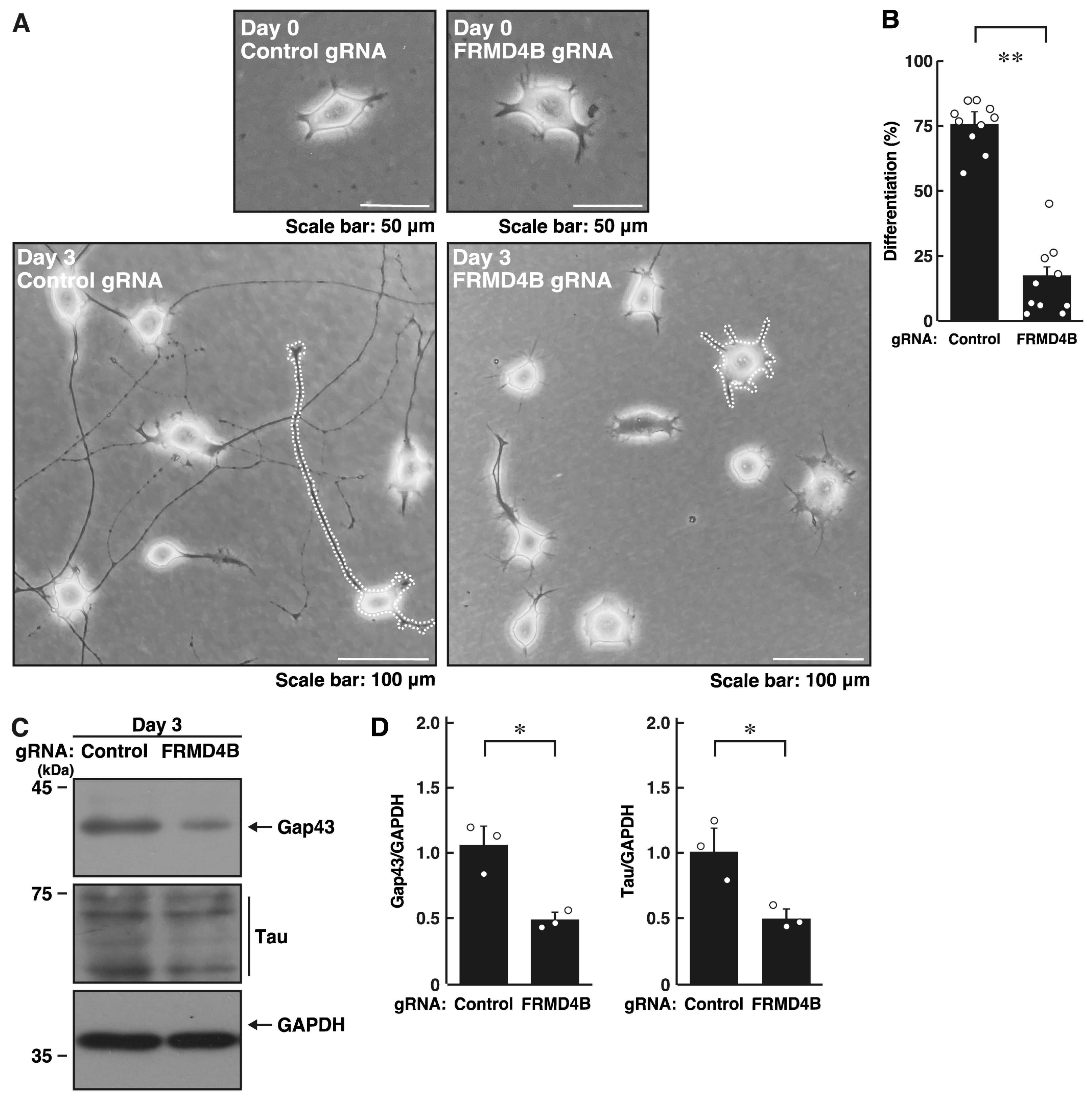
2.2. Knockdown of Frmd4b Leads to Decreased Process Elongation
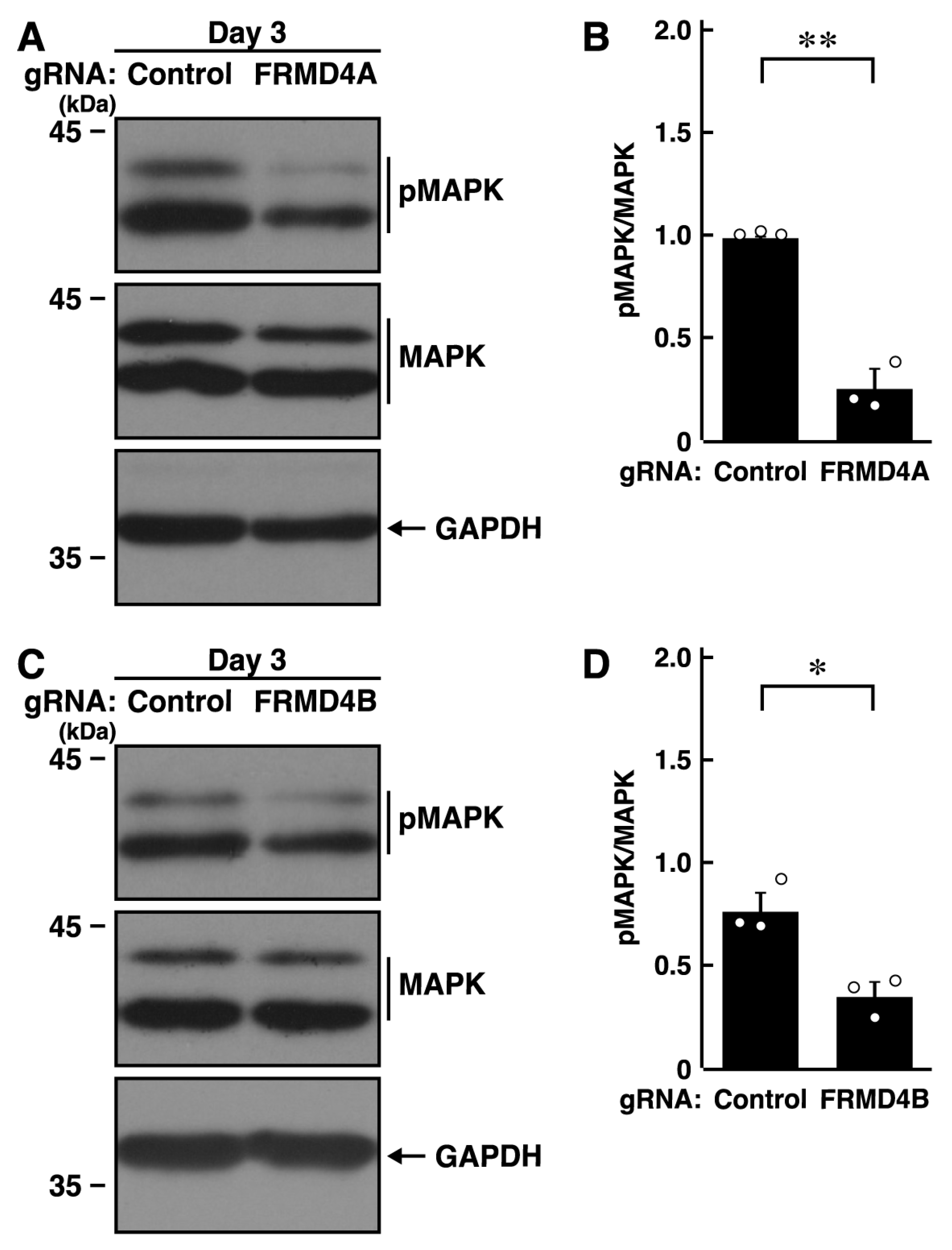
2.3. Knockdown of Frmd4a or Frmd4b Decreases the Phosphorylation Levels of MAPK/ERK
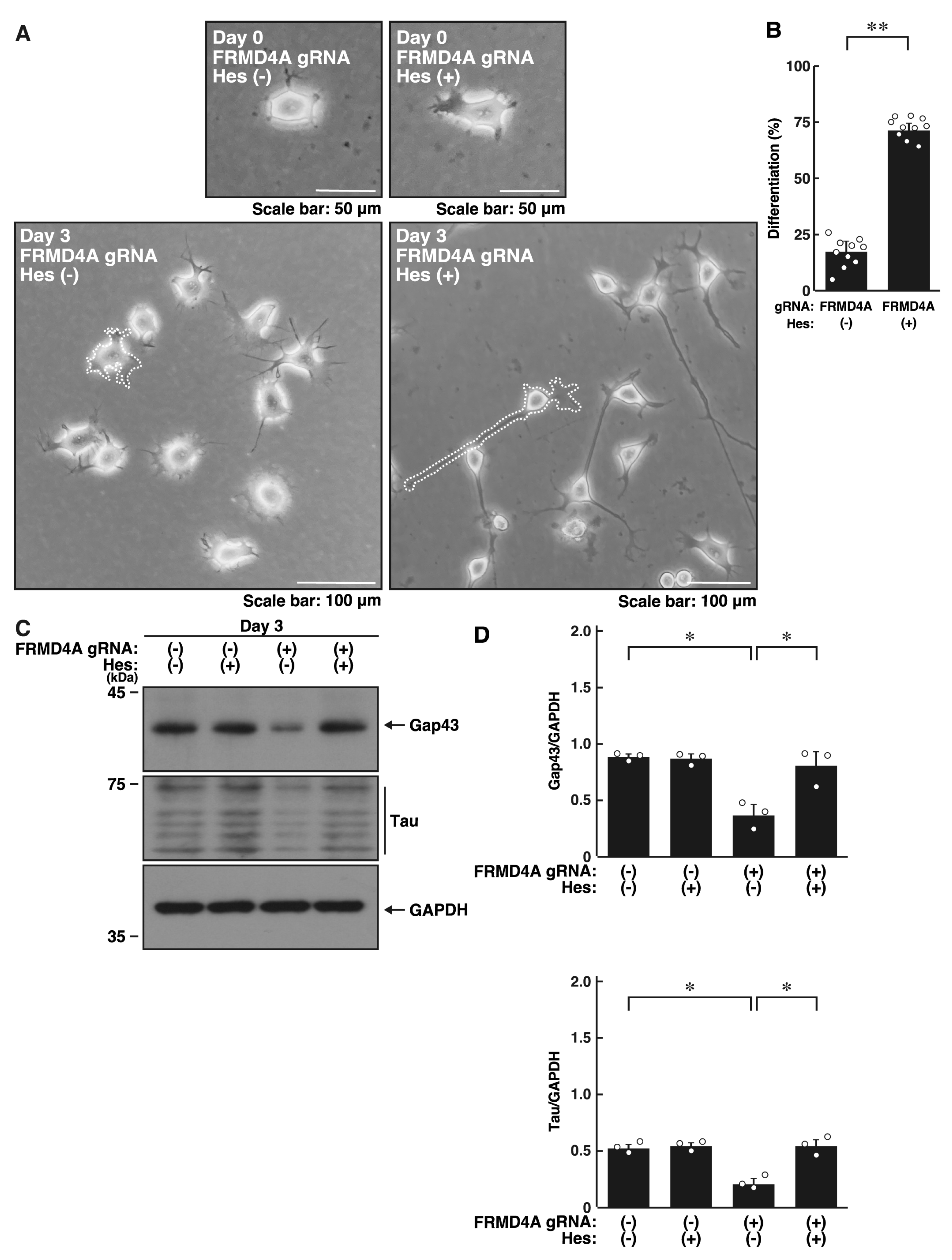
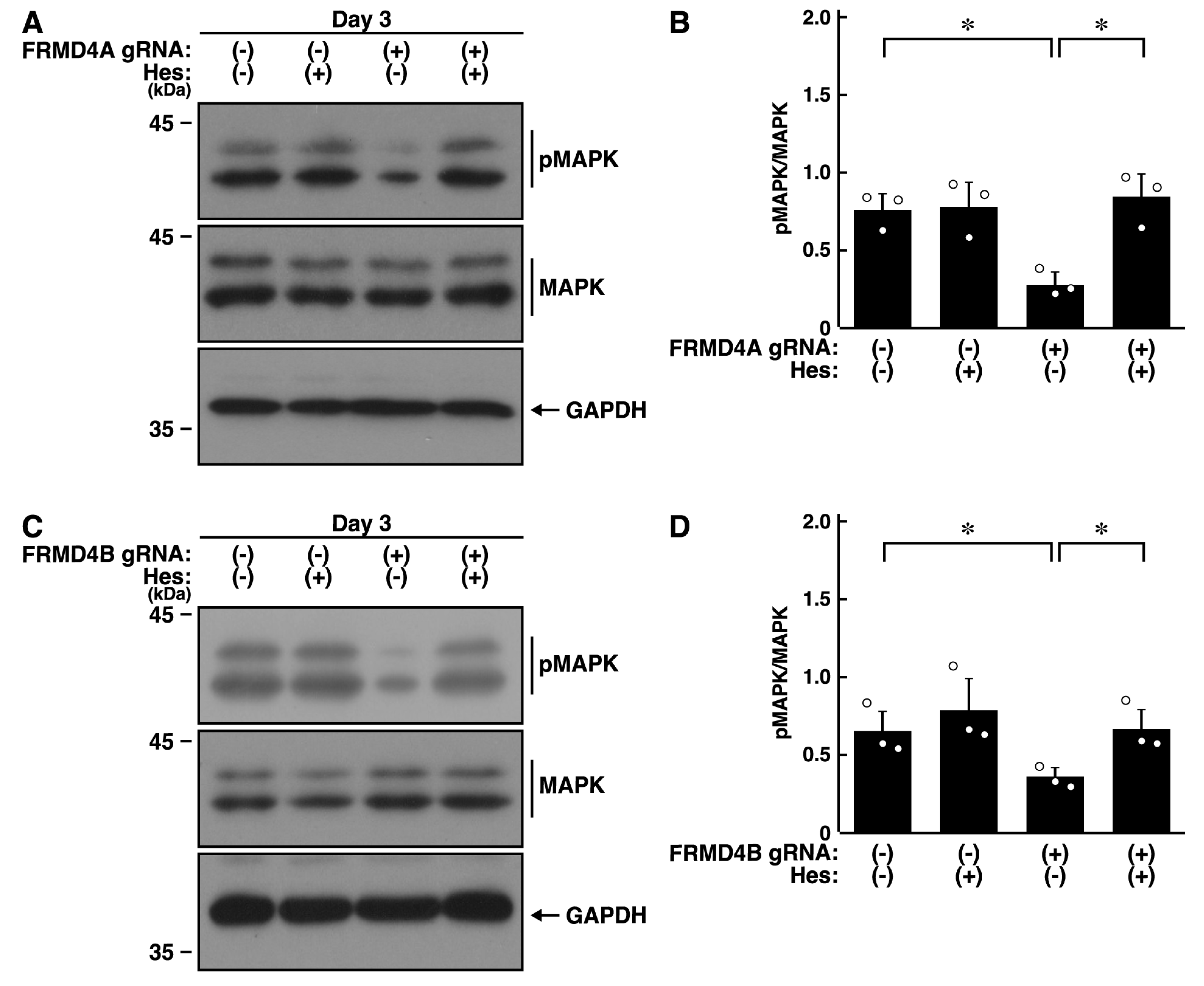
2.4. Hesperetin Recovers Cell Phenotypes Induced by Knockdown of Frmd4a or Frmd4b
2.5. Hesperetin Recovers Decreased MAPK/ERK Phosphorylation Induced by Knockdown of Frmd4a or Frmd4b
3. Discussion
4. Materials and Methods
4.1. Key Antibodies and Plasmids
4.2. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.3. Cell Line Culture and Differentiation
4.4. Primary Cell Culture and Process Elongation
4.5. Transfection of the Plasmid Encoding Designed gRNA and Cas13 into Cells
4.6. Polyacrylamide Gel Electrophoresis and Immunoblotting
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, D. Surface movements during the growth of single explanted neurons. Proc. Natl. Acad. Sci. USA 1970, 65, 905–910. [Google Scholar] [CrossRef]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial cell-axonal growth cone interactions in neurodevelopment and regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef]
- Craig, A.M.; Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 1994, 17, 267–310. [Google Scholar] [CrossRef]
- da Silva, J.S.; Dotti, C.G. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 2002, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Kaibuchi, K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Genovese, A.; Butler, M.G. Clinical assessment, genetics, and treatment approaches in autism spectrum disorder. Int. J. Mol. Sci. 2020, 21, 4726. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism spectrum disorder: Neurodevelopmental risk factors, biological mechanism, and precision therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Fine, D.; Flusser, H.; Markus, B.; Shorer, Z.; Gradstein, L.; Khateeb, S.; Langer, Y.; Narkis, G.; Birk, R.; Galil, A.; et al. A syndrome of congenital microcephaly, intellectual disability and dysmorphism with a homozygous mutation in FRMD4A. Eur. J. Hum. Genet. 2015, 23, 1729–1734. [Google Scholar] [CrossRef][Green Version]
- Nagase, T.; Kikuno, R.; Ishikawa, K.; Hirosawa, M.; Ohara, O. Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000, 7, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Umeda, M. FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc. Natl. Acad. Sci. USA 2010, 107, 748–753. [Google Scholar] [CrossRef]
- Yan, X.; Nykänen, N.P.; Brunello, C.A.; Haapasalo, A.; Hiltunen, M.; Uronen, R.L.; Huttunen, H.J. FRMD4A-cytohesin signaling modulates the cellular release of Tau. J. Cell Sci. 2016, 129, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Tago, K.; Fukatsu, S.; Okabe, M.; Shirai, R.; Oizumi, H.; Ohbuchi, K.; Yamamoto, M.; Mizoguchi, K.; Miyamoto, Y.; et al. CRISPR/CasRx-mediated RNA knockdown reveals that ACE2 Is involved in the regulation of oligodendroglial cell morphological differentiation. Noncoding RNA 2022, 8, 42. [Google Scholar] [CrossRef]
- Okabe, M.; Sato, T.; Takahashi, M.; Honjo, A.; Okawa, M.; Ishida, M.; Kukimoto-Niino, M.; Shirouzu, M.; Miyamoto, Y.; Yamauchi, J. Autism spectrum disorder- and/or intellectual disability-associated semaphorin-5A exploits the mechanism by which Dock5 signalosome molecules control cell shape. Curr. Issues Mol. Biol. 2024, 46, 3092–3107. [Google Scholar] [CrossRef]
- Numakawa, T.; Yokomaku, D.; Kiyosue, K.; Adachi, N.; Matsumoto, T.; Numakawa, Y.; Taguchi, T.; Hatanaka, H.; Yamada, M. Basic fibroblast growth factor evokes a rapid glutamate release through activation of the MAPK pathway in cultured cortical neurons. J. Biol. Chem. 2002, 277, 28861–28869. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.E.; Ahn, S.G.; Lee, G.H. Psoralidin stimulates expression of immediate-early genes and synapse development in primary cortical neurons. Neurochem. Res. 2018, 43, 2460–2472, Erratum in Neurochem. Res. 2019, 44, 509. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.R.; Kudo, T.A.; Tominami, K.; Izumi, S.; Tanaka, T.; Hayashi, Y.; Noguchi, T.; Matsuzawa, A.; Nakai, J.; Hong, G.; et al. SP600125 enhances temperature-controlled repeated thermal stimulation-induced neurite outgrowth in PC12-P1F1 cells. Int. J. Mol. Sci. 2022, 23, 15602. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, J.; Kawaguchi, Y.; Kawakami, Y.; Takei, K. Brain-derived neurotrophic factor (BDNF) induces antagonistic action to Nogo signaling by the upregulation of lateral olfactory tract usher substance (LOTUS) expression. J. Neurochem. 2023, 164, 29–43. [Google Scholar] [CrossRef]
- Montero-Martin, N.; Girón, M.D.; Vílchez, J.D.; Salto, R. Sodium tungstate promotes neurite outgrowth and confers neuroprotection in Neuro2a and SH-SY5Y cells. Int. J. Mol. Sci. 2024, 25, 9150. [Google Scholar] [CrossRef]
- Nones, J.E.; Spohr, T.C.; Gomes, F.C. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem. Res. 2011, 36, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Zhen, A.X.; Ok, S.; Fernando, P.D.S.M.; Herath, H.M.U.L.; Piao, M.J.; Kang, K.A.; Hyun, J.W. Hesperidin protects SH-SY5Y neuronal cells against high glucose-induced apoptosis via regulation of MAPK signaling. Antioxidants 2022, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- William Raja, T.R.; Duraipandiyan, V.; Ignacimuthu, S.; Janakiraman, U.; Packiam, S.M. Role of polyphenols in alleviating Alzheimer’s disease: A review. Curr. Med. Chem. 2023, 30, 4032–4047. [Google Scholar] [CrossRef]
- Singh, A.; Singh, L.; Dalal, D. Neuroprotective potential of hispidulin and diosmin: A review of molecular mechanisms. Metab. Brain Dis. 2025, 40, 188. [Google Scholar] [CrossRef]
- Lambert, J.C.; Grenier-Boley, B.; Harold, D.; Zelenika, D.; Chouraki, V.; Kamatani, Y.; Sleegers, K.; Ikram, M.A.; Hiltunen, M.; Reitz, C.; et al. Genome-wide haplotype association study identifies the FRMD4A gene as a risk locus for Alzheimer’s disease. Mol. Psychiatry 2013, 18, 461–470. [Google Scholar] [CrossRef]
- Andrews, S.J.; Das, D.; Cherbuin, N.; Anstey, K.J.; Easteal, S. Association of genetic risk factors with cognitive decline: The PATH through life project. Neurobiol. Aging 2016, 41, 150–158. [Google Scholar] [CrossRef]
- Huovinen, J.; Helisalmi, S.; Paananen, J.; Laiterä, T.; Kojoukhova, M.; Sutela, A.; Vanninen, R.; Laitinen, M.; Rauramaa, T.; Koivisto, A.M.; et al. Alzheimer’s disease-related polymorphisms in shunt-responsive idiopathic normal pressure hydrocephalus. J. Alzheimer’s Dis. 2017, 60, 1077–1085. [Google Scholar] [CrossRef]
- Deczkowska, A.; Weiner, A.; Amit, I. The physiology, pathology, and potential therapeutic applications of the TREM2 signaling pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef]
- Bagyinszky, E.; An, S.S.A. Haploinsufficiency and Alzheimer’s disease: The possible pathogenic and protective genetic factors. Int. J. Mol. Sci. 2024, 25, 11959. [Google Scholar] [CrossRef] [PubMed]
- Martiskainen, H.; Viswanathan, J.; Nykänen, N.P.; Kurki, M.; Helisalmi, S.; Natunen, T.; Sarajärvi, T.; Kurkinen, K.M.; Pursiheimo, J.P.; Rauramaa, T.; et al. Transcriptomics and mechanistic elucidation of Alzheimer’s disease risk genes in the brain and in vitro models. Neurobiol. Aging 2015, 36, 1221.e15–1221.e28. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, X.; Zhou, X.; Liu, Y.; Lian, J.; Yang, T.; Huang, X.; He, F.; Zhang, J.; Wu, B.; et al. Case Report: A novel compound heterozygous mutation of the FRMD4A gene identified in a Chinese family with global developmental delay, intellectual disability, and ataxia. Front. Pediatr. 2021, 9, 775488. [Google Scholar] [CrossRef]
- Yoon, D.; Kim, Y.J.; Cui, W.Y.; Van der Vaart, A.; Cho, Y.S.; Lee, J.Y.; Ma, J.Z.; Payne, T.J.; Li, M.D.; Park, T. Large-scale genome-wide association study of Asian population reveals genetic factors in FRMD4A and other loci influencing smoking initiation and nicotine dependence. Hum. Genet. 2012, 131, 1009–1021. [Google Scholar] [CrossRef]
- Klarlund, J.K.; Holik, J.; Chawla, A.; Park, J.G.; Buxton, J.; Czech, M.P. Signaling complexes of the FERM domain-containing protein GRSP1 bound to ARF exchange factor GRP1. J. Biol. Chem. 2001, 276, 40065–40070. [Google Scholar] [CrossRef]
- Thomason, E.J.; Escalante, M.; Osterhout, D.J.; Fuss, B. The oligodendrocyte growth cone and its actin cytoskeleton: A fundamental element for progenitor cell migration and CNS myelination. Glia 2020, 68, 1329–1346. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375, Erratum in Nat. Rev. Mol. Cell Biol. 2011, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Fukaya, M.; Okamoto, H.; Sakagami, H. Physiological and pathological roles of the cytohesin family in neurons. Int. J. Mol. Sci. 2022, 23, 5087. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Guo, Y.; Tang, P.; Li, H.; Chen, L. Arf6 as a therapeutic target: Structure, mechanism, and inhibitors. Acta. Pharm. Sin. B 2023, 13, 4089–4104. [Google Scholar] [CrossRef]
- Torii, T.; Miyamoto, Y.; Yamauchi, J. Myelination by signaling through Arf guanine nucleotide exchange factor. J. Neurochem. 2024, 168, 2201–2213. [Google Scholar] [CrossRef]
- Humphries, C.E.; Kohli, M.A.; Nathanson, L.; Whitehead, P.; Beecham, G.; Martin, E.; Mash, D.C.; Pericak-Vance, M.A.; Gilbert, J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Sofer, T.; Tarraf, W.; Bressler, J.; Faul, J.D.; Zhao, W.; Ratliff, S.M.; Lamar, M.; Launer, L.J.; Laurie, C.C.; et al. Genome-wide association study of cognitive function in diverse Hispanics/Latinos: Results from the Hispanic community health study/study of Latinos. Transl. Psychiatry 2020, 10, 245. [Google Scholar] [CrossRef]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Choi, J.-S. Structural bases for hesperetin derivatives: Inhibition of protein tyrosine phosphatase 1B, kinetics mechanism and molecular molecules. Molecules 2021, 26, 7433. [Google Scholar] [CrossRef] [PubMed]
- Figlia, G.; Gerber, D.; Suter, U. Myelination and mTOR. Glia 2018, 66, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Iijima, S.; Hoshikawa, S.; Miura, T.; Yamamoto, S.; Oda, H.; Nakamura, K.; Tanaka, S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004, 24, 6724–6732. [Google Scholar] [CrossRef]
- Dahl, K.D.; Almeida, A.R.; Hathaway, H.A.; Bourne, J.; Brown, T.L.; Finseth, L.T.; Wood, T.L.; Macklin, W.B. mTORC2 loss in oligodendrocyte progenitor cells results in regional hypomyelination in the central nervous system. J. Neurosci. 2023, 43, 540–558. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, Y.; Zhang, H.; Ren, K.; Liu, W.; An, Y.; Yao, J.; Fan, H. Hesperetin inhibits TGFbeta1-induced migration and invasion of triple negative breast cancer MDA-MB-231 cells via suppressing Fyn/Paxillin/RhoA pathway. Integr. Cancer Ther. 2022, 21, 15347354221086900. [Google Scholar] [CrossRef]
- Lu, Q.; Kishi, H.; Zhang, Y.; Morita, T.; Kobayashi, S. Hesperetin inhibits sphingosylphosphorylcholine-induced vascular smooth muscle contraction by regulating the Fyn/Rho-Kinase pathway. J. Cardiovasc. Pharmacol. 2022, 79, 456–466. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honjo, A.; Yako, H.; Miyamoto, Y.; Yagi, M.; Yamamoto, M.; Nishi, A.; Sakagami, H.; Yamauchi, J. Knocking Down FRMD4A, a Factor Associated with the Brain Development Disorder and a Risk Factor for Alzheimer’s Disease, Using RNA-Targeting CRISPR/Cas13 Reveals Its Role in Cell Morphogenesis. Int. J. Mol. Sci. 2025, 26, 10083. https://doi.org/10.3390/ijms262010083
Honjo A, Yako H, Miyamoto Y, Yagi M, Yamamoto M, Nishi A, Sakagami H, Yamauchi J. Knocking Down FRMD4A, a Factor Associated with the Brain Development Disorder and a Risk Factor for Alzheimer’s Disease, Using RNA-Targeting CRISPR/Cas13 Reveals Its Role in Cell Morphogenesis. International Journal of Molecular Sciences. 2025; 26(20):10083. https://doi.org/10.3390/ijms262010083
Chicago/Turabian StyleHonjo, Asahi, Hideji Yako, Yuki Miyamoto, Moeri Yagi, Masahiro Yamamoto, Akinori Nishi, Hiroyuki Sakagami, and Junji Yamauchi. 2025. "Knocking Down FRMD4A, a Factor Associated with the Brain Development Disorder and a Risk Factor for Alzheimer’s Disease, Using RNA-Targeting CRISPR/Cas13 Reveals Its Role in Cell Morphogenesis" International Journal of Molecular Sciences 26, no. 20: 10083. https://doi.org/10.3390/ijms262010083
APA StyleHonjo, A., Yako, H., Miyamoto, Y., Yagi, M., Yamamoto, M., Nishi, A., Sakagami, H., & Yamauchi, J. (2025). Knocking Down FRMD4A, a Factor Associated with the Brain Development Disorder and a Risk Factor for Alzheimer’s Disease, Using RNA-Targeting CRISPR/Cas13 Reveals Its Role in Cell Morphogenesis. International Journal of Molecular Sciences, 26(20), 10083. https://doi.org/10.3390/ijms262010083






