Analysis of Selected Serum Cytokines to Evaluate the Early Efficacy of Benralizumab, Dupilumab, and Mepolizumab in Severe Eosinophilic Asthma Treatment
Abstract
1. Introduction
2. Results
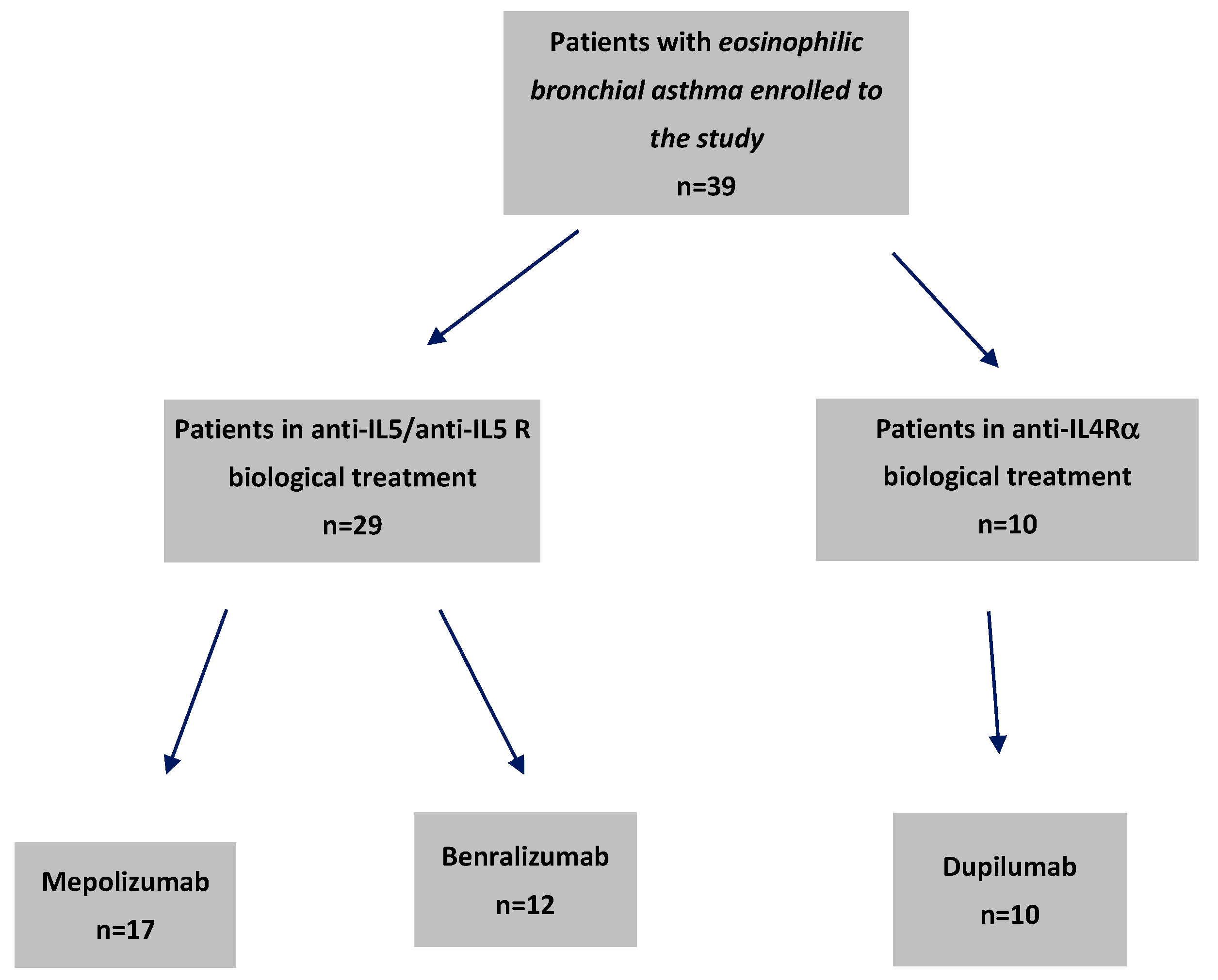
2.1. Baseline Patient Characteristics and Their Relevance to Biologic Treatment Efficacy in Severe Bronchial Asthma
2.2. Differences Across Treatment Groups
2.3. Cytokine Profile Analyzed
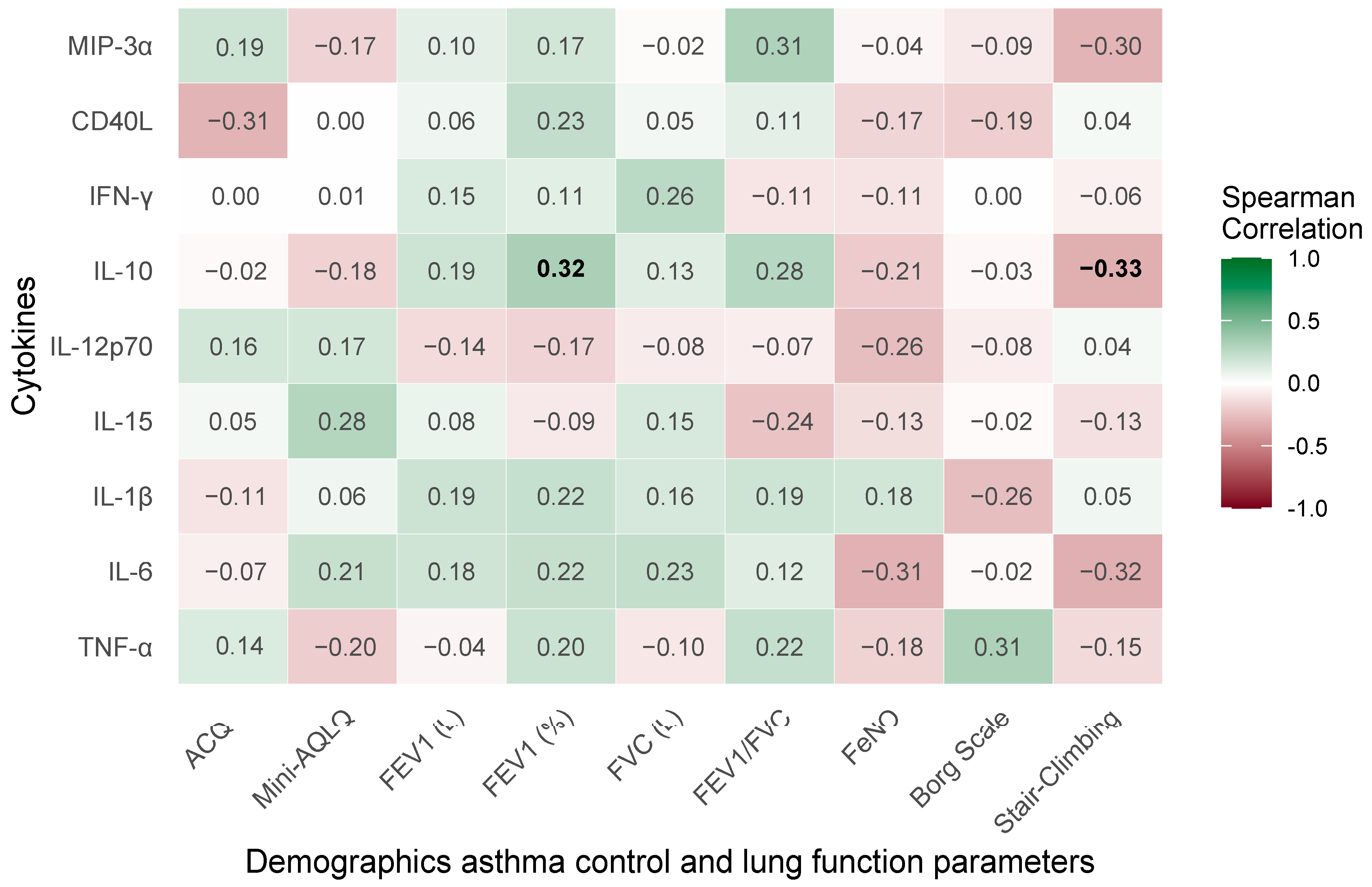
2.4. Baseline Correlations of Demographic, Asthma Control, and Pulmonary Function Parameters with Cytokine Profiles in Severe Bronchial Asthma
2.5. Baseline Concentrations of Serum Cytokines
2.6. Efficacy Outcomes of Biologic Treatments in Severe Bronchial Asthma: Changes from Baseline to Follow-Up
2.6.1. CD40L
2.6.2. IL-10
2.6.3. IL-6
2.6.4. TNF-α
2.6.5. IL-12p70
2.7. Clinical Parameter Changes
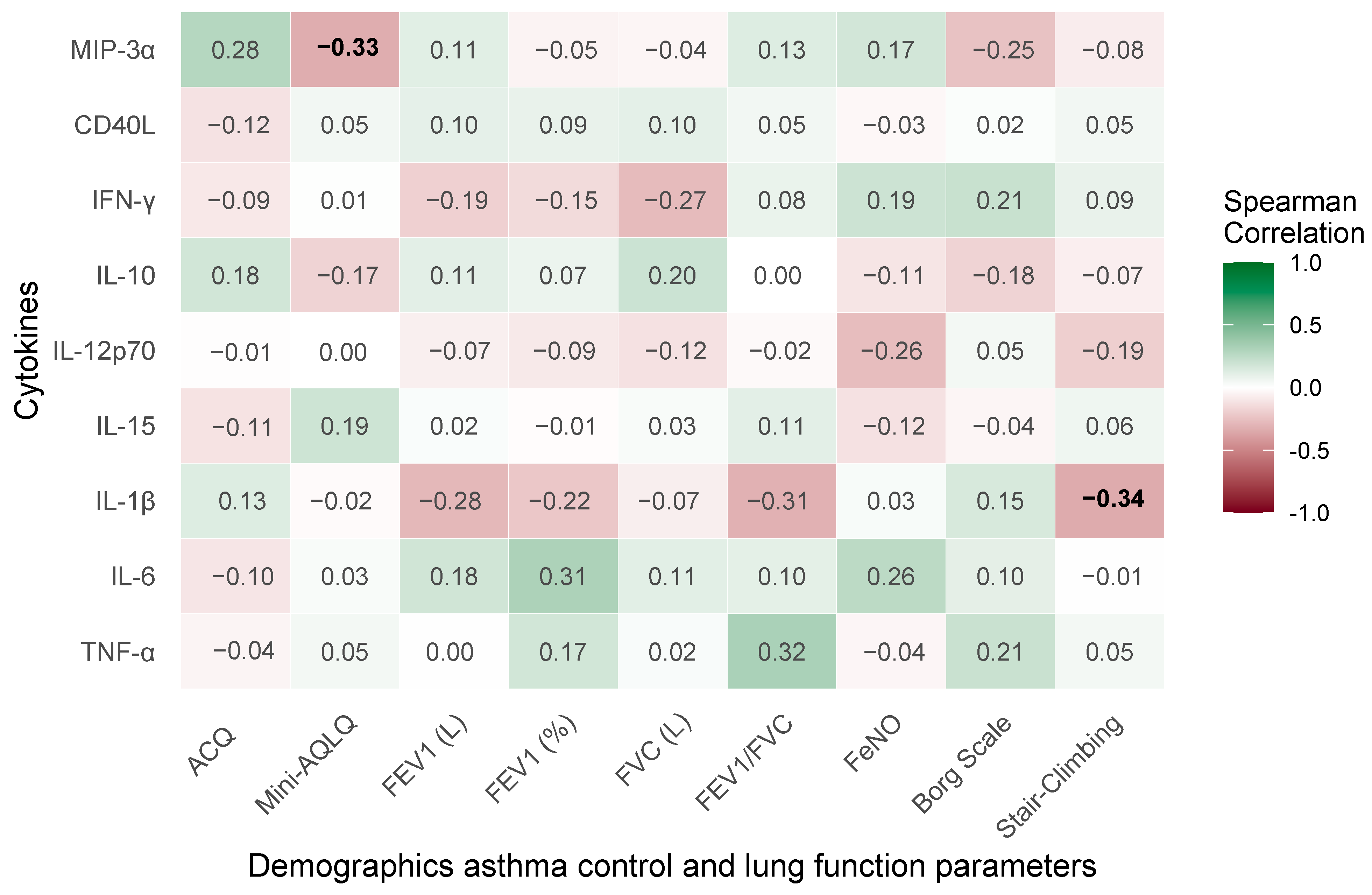
2.8. Correlations of Treatment-Induced Changes in Clinical and Pulmonary Function Parameters with Cytokine Profiles in Severe Bronchial Asthma
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design and Participants
4.2. Inclusion Criteria
- Blood eosinophil count ≥ 350/µL in the last 12 months or ≥150 cells/μL if systemic glucocorticosteroids at a dose ≥ 5 mg per day had to be taken systematically in the 6 months prior to the qualification and the cumulative annual dose of oral glucocorticosteroids was ≥1.0 g (calculated as prednisone) due to a lack of asthma control.
- The need for high doses of inhaled glucocorticosteroids (>1000 mcg of beclomethasone dipropionate per day or another inhaled glucocorticosteroid at an equivalent dose determined according to current guidelines from The Global Initiative for Asthma (GINA)) in combination with another asthma control medication.
- Two or more exacerbations in the past year that required systemic glucocorticosteroids or an increase in their dose for more than three days in people who use them chronically.
- The patients met at least two of the following criteria:
- (a)
- Symptoms of uncontrolled asthma (lack of asthma control in the ACQ (Asthma Control Questionnaire) > 1.5 points).
- (b)
- Hospitalization in the last 12 months due to asthma exacerbation.
- (c)
- A life-threatening asthma attack incident in the past.
- (d)
- Persistent airway obstruction (first-second expiratory volume FEV1 < 80% of normal value or diurnal variation in peak expiratory flow PEF > 30%).
- (e)
- Impaired quality of life due to asthma (mean score on the asthma quality of life test mini-AQLQ < 5.0 points).
- The exclusion of other hypereosinophilic syndromes.
- Non-smoking.
- The exclusion of other clinically relevant pulmonary diseases [26].
4.3. Exclusion Criteria
4.4. Assessment of Clinical Efficacy
4.5. Laboratory Tests
4.6. Evaluation of the Cytokine Screening Panel
4.7. Allergy Detection
4.8. Spirometry
4.9. Fractional Exhaled Nitric Oxide (FeNO)
4.10. Assessment of Functional Status and Exercise Tolerance
4.11. Evaluation
4.12. Ethics
4.13. Statistical Analysis
Characteristics of the Statistical Tool and List of Applied External Libraries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 535886. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a Role of Tumor Necrosis Factor α in Refractory Asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef]
- Carr, T.F.; Kraft, M. Use of Biomarkers to Identify Phenotypes and Endotypes of Severe Asthma. Ann. Allergy Asthma Immunol. 2018, 121, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.W.; et al. Type 2 Inflammation in Asthma and Other Airway Diseases. ERJ Open Res. 2022, 8, 00576-2021. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. Plasma Interleukin-6 Concentrations, Metabolic Dysfunction, and Asthma Severity: A Cross-Sectional Analysis of Two Cohorts. Lancet Respir. Med. 2016, 4, 574–584, Correction in Lancet Respir. Med. 2018, 6, e10. https://doi.org/10.1016/S2213-2600(18)30073-0. [Google Scholar] [CrossRef]
- Berry, M.; Brightling, C.; Pavord, I.; Wardlaw, A. TNF-α in Asthma. Curr. Opin. Pharmacol. 2007, 7, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.; Berry, M.; Amrani, Y. Targeting TNF-α: A Novel Therapeutic Approach for Asthma. J. Allergy Clin. Immunol. 2008, 121, 5–10. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Strieter, R.M.; Chensue, S.W.; Widmer, M.; Kunkel, S.L. TNF-Alpha Mediates Recruitment of Neutrophils and Eosinophils during Airway Inflammation. J. Immunol. 1995, 154, 5411–5417. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Platelet Activation and the CD40/CD40 Ligand Pathway: Mechanisms and Implications for Human Disease. Crit. Rev. Immunol. 2005, 25, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Matsushima, M.; Hashimoto, N.; Imaizumi, K.; Hasegawa, Y. CD40/CD40 ligand interactions in immune responses and pulmonary immunity. Nagoya J. Med. Sci. 2011, 73, 69. [Google Scholar]
- Saluk-Juszczak, J.; Królewska, K. The Role of CD40/CD40L Pathway in the Biological Activity of Blood Platelets: Part I. Menopause Rev. Przegląd Menopauzalny 2010, 9, 305–308. [Google Scholar]
- Hong, G.U.; Park, B.S.; Park, J.W.; Kim, S.Y.; Ro, J.Y. IgE Production in CD40/CD40L Cross-Talk of B and Mast Cells and Mediator Release via TGase 2 in Mouse Allergic Asthma. Cell Signal 2013, 25, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Geng, W.L.; Li, C.C.; Wu, J.H.; Gao, F.; Wang, Y. Progress of CCL20-CCR6 in the Airways: A Promising New Therapeutic Target. J. Inflamm. 2024, 21, 54, Correction in J. Inflamm. 2025, 22, 2. https://doi.org/10.1186/s12950-025-00428-y. [Google Scholar] [CrossRef]
- Chan, D.I.; Hunter, H.N.; Tack, B.F.; Vogel, H.J. Human Macrophage Inflammatory Protein 3α: Protein and Peptide Nuclear Magnetic Resonance Solution Structures, Dimerization, Dynamics, and Anti-Infective Properties. Antimicrob. Agents Chemother. 2008, 52, 883–894. [Google Scholar] [CrossRef]
- Shi, Z.R.; Mabuchi, T.; Riutta, S.J.; Wu, X.; Peterson, F.C.; Volkman, B.F.; Hwang, S.T. The Chemokine, CCL20, and Its Receptor, CCR6, in the Pathogenesis and Treatment of Psoriasis and Psoriatic Arthritis. J. Psoriasis Psoriatic Arthritis 2023, 8, 107. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Massacrier, C.; Homey, B.; Vanbervliet, B.; Pin, J.J.; Vicari, A.; Lebecque, S.; Dezutter-Dambuyant, C.; Schmitt, D.; Zlotnik, A.; et al. Macrophage Inflammatory Protein 3α Is Expressed at Inflamed Epithelial Surfaces and Is the Most Potent Chemokine Known in Attracting Langerhans Cell Precursors. J. Exp. Med. 2000, 192, 705. [Google Scholar] [CrossRef]
- Reibman, J.; Hsu, Y.; Chen, L.C.; Bleck, B.; Gordon, T. Airway Epithelial Cells Release MIP-3α/CCL20 in Response to Cytokines and Ambient Particulate Matter. Am. J. Respir. Cell Mol. Biol. 2003, 28, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of Immune Suppression by Interleukin-10 and Transforming Growth Factor-β: The Role of T Regulatory Cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Tian, T.; Xie, M.; Sun, G. Association of Systemic Immune-Inflammation Index with Asthma and Asthma-Related Events: A Cross-Sectional NHANES-Based Study. Front. Med. 2024, 11, 1400484. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Takahashi, K.; Kurihara, Y.; Sadamatsu, H.; Kimura, S.; Sueoka-Aragane, N. Efficacy of Dupilumab and Biomarkers for Systemic Corticosteroid Naïve Allergic Bronchopulmonary Mycosis. Allergol. Int. 2021, 70, 145–147. [Google Scholar] [CrossRef]
- Chen, C.C.; Buchheit, K.M.; Lee, P.Y.; Brodeur, K.E.; Sohail, A.; Cho, L.; Baloh, C.H.; Balestrieri, B.; Derakhshan, T.; Feng, C.; et al. IL-4Rα Signaling Promotes Barrier-Altering Oncostatin M and IL-6 Production in Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. 2024, 154, 458–467.e3. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Bartlett, N.W.; Upham, J.W.; Nichol, K.S.; Harrington, J.; Wark, P.A.B. Severe Asthma ILC2s Demonstrate Enhanced Proliferation That Is Modified by Biologics. Respirology 2023, 28, 758–766. [Google Scholar] [CrossRef]
- Rogliani, P.; Facciolo, F.; Melis, E.; Ritondo, B.L.; Gabriele, M.C.; Perduno, A.; Ora, J.; Calzetta, L.; Ora, J. Pharmacological Characterization of the Anti-Inflammatory Effect of Mepolizumab and Benralizumab in a Human Ex Vivo Model of Asthma. Eur. Respir. J. 2022, 60, 385. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23. [Google Scholar] [CrossRef]
- Ogawa, Y.; Duru, E.A.; Ameredes, B.T. Role of IL-10 in the Resolution of Airway Inflammation. Curr. Mol. Med. 2008, 8, 437. [Google Scholar] [CrossRef]
- Howell, I.; Yang, F.; Brown, V.; Cane, J.; Marchi, E.; Azim, A.; Busby, J.; McDowell, P.J.; Diver, S.E.; Borg, C.; et al. Airway Proteomics Reveals Broad Residual Anti-Inflammatory Effects of Prednisolone in Mepolizumab-Treated Asthma. J. Allergy Clin. Immunol. 2024, 154, 1146–1158. [Google Scholar]
- Čelakovská, J.; Čermáková, E.; Boudková, P.; Krejsek, J. Evaluation of the Levels of Interleukins IL-4, IL-13, IL-5, IL-10 and IL-33 in Atopic Dermatitis Patients with and without Dupilumab Therapy. Front. Immunol. 2025, 16, 1604883. [Google Scholar] [CrossRef] [PubMed]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2019, 50, 5. [Google Scholar] [CrossRef]
- Bergantini, L.; d’Alessandro, M.; Pianigiani, T.; Cekorja, B.; Bargagli, E.; Cameli, P. Benralizumab Affects NK Cell Maturation and Proliferation in Severe Asthmatic Patients. Clin. Immunol. 2023, 253, 109680. [Google Scholar] [CrossRef]
- Lyakh, L.; Trinchieri, G.; Provezza, L.; Carra, G.; Gerosa, F. Regulation of Interleukin-12/Interleukin-23 Production and the T-Helper 17 Response in Humans. Immunol. Rev. 2008, 226, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Gavett, S.H.; O’Hearn, D.J.; Li, X.; Huang, S.K.; Finkelman, F.D.; Wills-Karp, M. Interleukin 12 Inhibits Antigen-Induced Airway Hyperresponsiveness, Inflammation, and Th2 Cytokine Expression in Mice. J. Exp. Med. 1995, 182, 1527. [Google Scholar] [CrossRef]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Kusama, K.; Pawankar, R.; Yagi, T. Synergistic Induction of Macrophage Inflammatory Protein-3α/CCL20 Production by Interleukin-17A and Tumor Necrosis Factor-α in Nasal Polyp Fibroblasts. World Allergy Organ. J. 2009, 2, 218. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Peebles, R.S. Th17-Mediated Inflammation in Asthma. Curr. Opin. Immunol. 2013, 25, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kang, M.J.; Jin, N.; Lee, S.Y.; Lee, Y.Y.; Jo, S.; Eom, J.Y.; Han, H.; Chung, S.I.; Jang, K.; et al. House Dust Mite-Induced Akt-ERK1/2-C/EBP Beta Pathway Triggers CCL20-Mediated Inflammation and Epithelial–Mesenchymal Transition for Airway Remodeling. FASEB J. 2022, 36, e22452. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Rasmussen, L.; Nolte, H.; Backer, V. Association of Airway Hyperresponsiveness with Reduced Quality of Life in Patients with Moderate to Severe Asthma. Ann. Allergy Asthma Immunol. 2007, 98, 44–50. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-Mediated, IL-1β-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Donnelly, R.P.; Young, H.A.; Rosenberg, A.S. An Overview of Cytokines and Cytokine Antagonists as Therapeutic Agents. Ann. N. Y. Acad. Sci. 2009, 1182, 1. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Sepiashvili, L. Cytokine Testing and Challenges for Diagnostic and Clinical Monitoring Use. J. Allergy Clin. Immunol. 2025, 155, 410–413. [Google Scholar] [CrossRef]
- Lou, Y.; Ke, Q.; Cui, H.; Shang, Y.; Yang, C. Correlation Study of Cytokine Levels in Alveolar Lavage Fluid with Exhaled Nitric Oxide and Lung Function in Children with Bronchial Asthma. Transl. Pediatr. 2021, 10, 2069. [Google Scholar] [CrossRef]
- Ora, J.; De Marco, P.; Motta, E.; Laitano, R.; Calzetta, L.; Rogliani, P. Real-World Efficacy of Biological Therapies in Severe Asthma: A Focus on Small Airways. J. Clin. Med. 2024, 13, 5883. [Google Scholar] [CrossRef]
- Niemiec-Górska, A.; Branicka, O.; Olszewska, P.; Mielcarska, S.; Glück, J.; Rymarczyk, B.; Gawlik, R. The Comparative Effectiveness of Mepolizumab and Benralizumab in the Treatment of Eosinophilic Asthma. Adv. Respir. Med. 2025, 93, 21. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Giacalone, A.; Ippolito, G.; Pastore, D.; Maglio, A.; Piazzetta, G.L.; Lobello, N.; Lombardo, N.; Vatrella, A.; Pelaia, G. Difficult-To-Treat and Severe Asthma: Can Real-World Studies on Effectiveness of Biological Treatments Change the Lives of Patients? Pragmat. Obs. Res. 2024, 15, 45–51. [Google Scholar] [CrossRef]
- Franceschi, E.; Drick, N.; Welte, J.F.T.; Suhling, H.; Santus, P.; Fischer, B.; Kayser, M. The Impact of Anti-Eosinophilic Therapy on Exercise Capacity and Inspiratory Muscle Strength in Patients with Severe Asthma. ERJ Open Res. 2023, 9, 00341-2022. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, M.; Koulouris, N.; Koutsoukou, A.; Rovina, N. Daily Physical Activity in Asthma and the Effect of Mepolizumab Therapy. J. Pers. Med. 2022, 12, 1692. [Google Scholar] [CrossRef]
- Kai, Y.; Hishida, Y. Dupilumab Treatment and 3-Dimensional Bronchial Tree Changes in Asthma-COPD Overlap. J. Allergy Clin. Immunol. Glob. 2025, 4, 100530. [Google Scholar] [CrossRef]
- Murugesan, N.; Saxena, D.; Dileep, A.; Adrish, M.; Hanania, N.A. Update on the Role of FeNO in Asthma Management. Diagnostics 2023, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Shirai, T.; Hirai, K.; Akamatsu, T.; Nakayasu, H.; Tamura, K.; Masuda, T.; Takahashi, S.; Tanaka, Y.; Kishimoto, Y.; et al. Blood Eosinophil Count and FeNO to Predict Benralizumab Effectiveness in Real-Life Severe Asthma Patients. J. Asthma 2022, 59, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (n = 39) | Benralizumab (n = 12) | Dupilumab (n = 10) | Mepolizumab (n = 17) | p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 56.0 (51.0, 64.0) | 57.0 (51.0, 64.5) | 55.0 (50.0, 63.0) | 54.0 (51.0, 64.0) | 0.931 |
| Sex | 0.159 | ||||
| Female | 30 (76.9%) | 7 (58.3%) | 8 (80.0%) | 15 (88.2%) | |
| Male | 9 (23.1%) | 5 (41.7%) | 2 (20.0%) | 2 (11.8%) | |
| Clinical Characteristics | |||||
| Oral Corticosteroid Use (days/year) | 21.0 (12.0, 80.0) | 14.0 (11.0, 55.0) | 23.0 (15.0, 40.0) | 25.0 (19.0, 120.0) | 0.725 |
| Disease Duration | 0.760 | ||||
| Over 10 years | 25 (64.1%) | 7 (58.3%) | 6 (60.0%) | 12 (70.6%) | |
| Up to 10 years | 14 (35.9%) | 5 (41.7%) | 4 (40.0%) | 5 (29.4%) | |
| Comorbidities | |||||
| Hypertension | 21 (63.6%) n = 33 | 8 (72.7%) n = 11 | 6 (60.0%) | 7 (58.3%) n = 12 | 0.815 |
| Diabetes Mellitus | 7 (21.2%) n = 33 | 4 (36.4%) n = 11 | 2 (20.0%) | 1 (8.3%) n = 12 | 0.275 |
| Dyslipidemia | 12 (36.4%) n = 33 | 4 (36.4%) n = 11 | 4 (40.0%) | 4 (33.3%) n = 12 | 1.000 |
| Osteoporosis | 5 (13.5%) n = 37 | 2 (16.7%) | 2 (20.0%) | 1 (6.7%) n = 15 | 0.586 |
| Coronary Artery Disease | 3 (9.1%) n = 33 | 1 (9.1%) n = 11 | 0 (0.0%) | 2 (16.7%) n = 12 | 0.758 |
| Obstructive Sleep Apnea | 12 (33.3%) n = 36 | 5 (41.7%) | 4 (44.4%) n = 9 | 3 (20.0%) n = 15 | 0.439 |
| Allergy | 21 (53.8%) | 4 (33.3%) A | 9 (90.0%) B | 8 (47.1%) AB | 0.018 |
| Nasal Polyps | 18 (46.2%) | 4 (33.3%) | 3 (30.0%) | 11 (64.7%) | 0.128 |
| Complete Blood Count, (×103 cells/µL) | |||||
| White Blood Cell Count | 8.3 (6.6, 10.1) n = 37 | 9.2 (6.7, 11.4) | 7.4 (5.6, 9.5) | 8.4 (6.7, 10.1) n = 15 | 0.439 |
| Eosinophil Count | 0.4 (0.2, 0.7) n = 38 | 0.4 (0.3, 0.8) | 0.2 (0.2, 0.4) | 0.5 (0.2, 0.8) n = 16 | 0.219 |
| Neutrophil Count | 4.7 (3.7, 6.0) n = 37 | 5.5 (3.9, 7.7) | 4.2 (2.9, 5.4) | 4.7 (3.7, 5.8) n = 15 | 0.478 |
| Lymphocyte Count | 2.0 (1.7, 2.4) n = 37 | 1.9 (1.5, 2.4) | 2.0 (1.9, 2.4) | 2.2 (1.7, 2.4) n = 15 | 0.596 |
| Additional blood parameters | |||||
| Total IgE (IU/mL) | 182.0 (34.9, 558.0) n = 35 | 156.0 (125.0, 226.0) n = 9 | 558.0 (182.0, 714.0) n = 9 | 125.0 (32.0, 250.0) | 0.194 |
| C-Reactive Protein (mg/L) | 2.2 (1.1, 6.2) n = 38 | 1.2 (0.7, 5.5) | 3.4 (2.0, 8.7) | 2.1 (0.9, 4.5) n = 16 | 0.124 |
| Baseline | Changes After Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokines (pg/mL) | Overall (n = 39) | Benralizumab (n = 12) | Dupilumab (n = 10) | Mepolizuamb (n = 17) | p-Value | Overall (n = 39) | Benralizumab (n = 12) | Dupilumab (n = 10) | Mepolizumab (n = 17) | p-Value | ||
| Ben vs. Dup | Ben vs. Mep | Dup vs. Mep | ||||||||||
| MIP-3α | 3.3 (2.0, 5.8) | 2.5 (1.2, 7.0) | 4.4 (3.1, 6.3) | 3.3 (2.0, 5.7) | 0.333 | −0.7 (−1.6, 0.3) p = 0.192 | 0.6 (−2.1, 3.7) p = 0.677 | −1.6 (−3.7, 0.2) p = 0.105 | −0.7 (−2.4, 0.9) p = 0.224 | 0.089 | 0.234 | 0.178 |
| CD40L | 1359.9 (810.0, 2526.6) | 932.2 (349.8, 1650.6) A | 2573.5 (1421.0, 3741.5) B | 1146.0 (641.7, 2003.0) A | 0.012 | −149.0 (−560.0, 380.0) p = 0.494 | −155.0 (−430.0, 1240.0) p = 0.424 | −957.0 (−1868.0, −187.0) p = 0.027 | 277.0 (−639.0, 1246.0) p = 0.306 | 0.015 | 0.089 | 0.027 |
| IFN-γ | 0.5 (0.3, 1.2) | 0.5 (0.2, 1.2) | 0.5 (0.3, 1.0) | 0.4 (0.4, 1.4) | 0.603 | −0.1 (−0.3, 0.0) p = 0.105 | −0.1 (−0.3, 0.3) p = 0.756 | −0.0 (−3.5, 0.4) p = 0.721 | −0.2 (−1.8, 0.0) p = 0.064 | 0.456 | 0.567 | 0.612 |
| IL-10 | 8.5 (6.5, 12.5) | 7.5 (2.5, 10.5) | 10.5 (8.5, 12.5) | 8.5 (6.5, 12.5) | 0.215 | −3.0 (−5.1, −1.1) p = 0.002 | −2.0 (−6.1, 3.9) p = 0.564 | −5.1 (−6.6, −1.0) p = 0.024 | −3.0 (−11.2, −0.5) p = 0.015 | 0.178 | 0.345 | 0.267 |
| IL-12p70 | 0.3 (0.2, 0.7) | 0.3 (0.2, 1.0) | 0.3 (0.3, 1.3) | 0.3 (0.2, 0.7) | 0.725 | 0.5 (0.0, 0.8) p = 0.032 | 0.3 (−1.0, 1.1) p = 0.529 | 0.6 (0.3, 1.2) p = 0.181 | 0.5 (−0.5, 1.0) p = 0.124 | 0.623 | 0.701 | 0.789 |
| IL-15 | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.3) | 0.9 (0.5, 1.2) | 1.0 (0.7, 1.3) | 0.684 | −0.1 (−0.2, 0.1) p = 0.252 | −0.0 (−0.4, 0.3) p = 1.000 | −0.2 (−0.2, 0.0) p = 0.065 | −0.1 (−0.3, 0.2) p = 0.569 | 0.512 | 0.623 | 0.734 |
| IL-1β | 0.0 (0.0, 0.3) | 0.2 (0.0, 0.4) | 0.3 (0.0, 0.5) | 0.0 (0.0, 0.2) | 0.317 | 0.0 (−0.1, 0.1) p = 0.891 | 0.0 (−0.2, 0.2) p = 1.000 | −0.1 (−0.3, 0.2) p = 0.343 | 0.0 (−0.1, 0.2) p = 0.437 | 0.456 | 0.567 | 0.612 |
| IL-6 | 1.6 (0.8, 3.0) | 1.0 (0.5, 1.4) A | 2.5 (1.6, 3.0) B | 2.3 (0.8, 3.4) AB | 0.024 | 0.0 (−0.5, 0.4) p = 0.807 | 0.2 (−0.4, 0.9) p = 0.247 | −0.7 (−1.5, 0.0) p = 0.059 | 0.0 (−1.5, 0.9) p = 0.798 | 0.045 | 0.234 | 0.089 |
| TNF-α | 3.1 (1.1, 4.3) | 2.3 (1.0, 3.1) | 2.4 (0.8, 4.3) | 3.4 (2.2, 4.3) | 0.224 | 0.0 (−0.6, 0.5) p = 0.916 | 0.8 (−0.2, 2.2) p = 0.110 | 0.2 (−1.6, 1.2) p = 0.695 | −0.9 (−1.9, 0.0) p = 0.050 | 0.178 | 0.008 | 0.045 |
| Baseline | Changes After Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall (n = 39) | Benralizumab (n = 12) | Dupilumab (n = 10) | Mepolizumab (n = 17) | p Value | Overall (n = 39) | Benralizumab (n = 12) | Dupilumab (n = 10) | Mepolizumab (n = 17) | p-Value | ||
| Ben vs. Dup | Ben vs. Mep | Dup vs. Mep | ||||||||||
| Asthma Control and Quality of Life | ||||||||||||
| Asthma Control Questionnaire Score | 3.4 (2.9, 4.0) | 3.4 (3.2, 4.1) | 3.4 (2.6, 3.6) | 3.4 (2.7, 4.0) | 0.686 | −1.1 (−1.4, −0.8) n = 38, p < 0.001 | −1.0 (−1.7, −0.6) n = 11, p = 0.004 | −1.2 (−1.9, −0.8) p = 0.002 | −1.0 (−1.6, −0.4) p = 0.003 | 0.612 | 0.789 | 0.705 |
| Mini-Asthma Quality of Life Questionnaire Score | 2.9 (2.4, 3.4) | 2.7 (2.4, 3.4) | 3.1 (2.7, 3.4) | 2.7 (2.1, 3.5) | 0.452 | 1.1 (0.7, 1.5) n = 38, p < 0.001 | 1.3 (0.7, 2.1) n = 11, p = 0.008 | 1.1 (0.6, 1.8) p = 0.002 | 0.9 (0.1, 1.7) p = 0.015 | 0.523 | 0.456 | 0.612 |
| Lung Function | ||||||||||||
| FEV1 (% Predicted) | 64.0 (52.0, 75.0) | 67.0 (50.0, 74.5) | 55.5 (51.0, 66.0) | 69.0 (53.0, 79.0) | 0.515 | 10.5 (4.5, 16.5) n = 37, p = 0.002 | 8.0 (−7.0, 16.5) p = 0.208 | 10.5 (−0.5, 19.5) p = 0.059 | 15.5 (−2.5, 28.0) n = 15, p = 0.079 | 0.567 | 0.432 | 0.523 |
| FeNO (ppb) | 23.0 (7.0, 63.0) n = 27 | 13.5 (10.0, 63.0) n = 10 | 25.0 (7.0, 35.0) n = 7 | 34.5 (7.0, 80.0) n = 10 | 0.694 | −2.0 (−12.5, 6.0) n = 25, p = 0.648 | 8.5 (−14.5, 79.0) n = 10, p = 0.275 | −13.0 (−27.0, 1.0) n = 6, p = 0.178 | −8.0 (−35.0, 5.0) n = 9, p = 0.236 | 0.045 | 0.112 | 0.267 |
| Functional Status | ||||||||||||
| Borg’s Dyspnea Scale Score | 7.0 (6.0, 8.0) | 6.0 (5.5, 7.0) | 6.5 (5.0, 8.0) | 7.0 (6.0, 8.0) | 0.478 | −2.5 (−2.5, −2.0) n = 37, p < 0.001 | −2.0 (−3.0, −1.5) n = 11, p = 0.004 | −2.0 (−3.0, −1.5) n = 9, p = 0.013 | −2.5 (−3.0, −2.0) n = 16, p = 0.001 | 0.678 | 0.589 | 0.456 |
| Stair-Climbing Capacity | 1.0 (0.5, 1.0) n = 37 | 1.0 (0.8, 1.5) n = 12 | 1.0 (1.0, 2.0) n = 10 | 1.0 (0.5, 1.0) n = 15 | 0.242 | 1.3 (1.0, 2.0) n = 35, p < 0.001 | 2.0 (1.0, 3.0) n = 11, p = 0.009 | 1.3 (1.0, 1.5) n = 9, p = 0.020 | 1.0 (0.8, 2.0) n = 14, p = 0.002 | 0.367 | 0.289 | 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemiec-Górska, A.; Labus, Ł.; Mielcarska, S.; Glück, J.; Czuba, Z.; Cyrnek, M.; Branicka, O.; Rymarczyk, B.; Gawlik, R. Analysis of Selected Serum Cytokines to Evaluate the Early Efficacy of Benralizumab, Dupilumab, and Mepolizumab in Severe Eosinophilic Asthma Treatment. Int. J. Mol. Sci. 2025, 26, 10075. https://doi.org/10.3390/ijms262010075
Niemiec-Górska A, Labus Ł, Mielcarska S, Glück J, Czuba Z, Cyrnek M, Branicka O, Rymarczyk B, Gawlik R. Analysis of Selected Serum Cytokines to Evaluate the Early Efficacy of Benralizumab, Dupilumab, and Mepolizumab in Severe Eosinophilic Asthma Treatment. International Journal of Molecular Sciences. 2025; 26(20):10075. https://doi.org/10.3390/ijms262010075
Chicago/Turabian StyleNiemiec-Górska, Aleksandra, Łukasz Labus, Sylwia Mielcarska, Joanna Glück, Zenon Czuba, Marcin Cyrnek, Olga Branicka, Barbara Rymarczyk, and Radosław Gawlik. 2025. "Analysis of Selected Serum Cytokines to Evaluate the Early Efficacy of Benralizumab, Dupilumab, and Mepolizumab in Severe Eosinophilic Asthma Treatment" International Journal of Molecular Sciences 26, no. 20: 10075. https://doi.org/10.3390/ijms262010075
APA StyleNiemiec-Górska, A., Labus, Ł., Mielcarska, S., Glück, J., Czuba, Z., Cyrnek, M., Branicka, O., Rymarczyk, B., & Gawlik, R. (2025). Analysis of Selected Serum Cytokines to Evaluate the Early Efficacy of Benralizumab, Dupilumab, and Mepolizumab in Severe Eosinophilic Asthma Treatment. International Journal of Molecular Sciences, 26(20), 10075. https://doi.org/10.3390/ijms262010075





