Influence of Coffee Roasting Degree from Four Mexican Regions on In Vitro Antioxidant Activity and Digestive Enzyme Inhibition and Its In Vivo Effects on Carbohydrate and Lipid Absorption
Abstract
1. Introduction
2. Results
3. Discussion
Limitations of This Study
4. Materials and Methods
4.1. Coffee Roasting Process and Physical Characterization
4.1.1. Yield
4.1.2. Moisture
4.1.3. Coffee Color
4.1.4. Spectrophotometric Analysis
4.1.5. Phenolic Profile by Ultra Performance Liquid Chromatography (UPLC)
4.2. Antioxidant Capacity
4.2.1. DPPH Assay
4.2.2. ABTS Assay
4.2.3. Lipid Peroxidation
4.3. Digestive Enzyme Inhibition Assessment
4.3.1. Pancreatic Lipase
4.3.2. α-Amylase
4.3.3. α-Glycosidase
4.4. Carbohydrates and Lipids Absorption Inhibition Assessment
4.4.1. Animal Subjects and Ethical Approval
4.4.2. Experimental Design
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| UN | Unroasted; |
| MR | Medium roasted; |
| HR | High roasted; |
| MRI | Maillard reaction index; |
| OSTT | Oral Starch Tolerance Test; |
| OLTT | Oral Glucose Tolerance Test. |
References
- International Coffee Organization (ICO). Coffee Development Report 2025. Available online: https://ico.org/ (accessed on 20 July 2025).
- Foreign Agriculture Service (FAS). U.S. Department of Agriculture. 2025. Available online: https://www.fas.usda.gov/ (accessed on 20 July 2025).
- Espitia-López, J.; Rogelio-Flores, F.; Angel-Cuapio, A.; Flores-Chávez, B.; Arce-Cervantes, O.; Hernández-León, S.; Garza-López, P.M. Characterization of sensory profile by the CATA method of Mexican coffee brew considering two preparation methods: Espresso and French press. Int. J. Food Prop. 2019, 22, 967–973. [Google Scholar] [CrossRef]
- Flores, V.F. La Producción de Café En México: Ventana de Oportunidad Para El Sector Agrícola de Chiapas. Rev. Espacio. I+D Innovación Más Desarro. 2015, 4, 174–194. [Google Scholar] [CrossRef]
- SADER-SIAP (Secretaria de Agricultura y Desarrollo Rural-Servicio de Información Agroalimentaria y Pesquera [Secretary of Agriculture and Rural Development-AgriFood and Fisheries Information Service]. Panorama Agroalimentario 2019; de I Agroalimentaria y Pesquera, S., Ed.; Secretaria de Agricultura y Desarrollo Social: Santiago de Querétaro, Mexico, 2024; Available online: https://www.gob.mx/agricultura/dgsiap (accessed on 20 July 2025).
- Pereira, L.L.; Marcate, J.P.P.; Caliman, A.D.C.; Guarçoni, R.C.; Moreli, A.P. Physical classification and sensory coffee analysis. In Quality Determinants in Coffee Production; Springer International Publishing: Cham, Switzerland, 2020; pp. 373–405. [Google Scholar]
- Poltronieri, P.; Rossi, F. Challenges in specialty coffee processing and quality assurance. Challenges 2016, 7, 19. [Google Scholar] [CrossRef]
- Worku, M.; de Meulenaer, B.; Duchateau, L.; Boeckx, P. Effect of altitude on biochemical composition and quality of green arabica coffee beans can be affected by shade and postharvest processing method. Food Res. Int. 2018, 105, 278–285. [Google Scholar] [CrossRef]
- Clarke, R.J. Roasting and grinding. In Coffee—Technology; Clarke, R.J., Macrae, R., Eds.; Elsevier Applied Science Publications: London, UK, 1987; pp. 73–108. [Google Scholar]
- Freitas, V.V.; Borges, L.L.R.; Castro, G.A.D.; Dos Santos, M.H.; Vidigal, M.C.T.R.; Fernandes, S.A.; Stringheta, P.C. Impact of different roasting conditions on the chemical composition, antioxidant activities, and color of Coffea canephora and Coffea arabica L. samples. Heliyon 2023, 9, e19580. [Google Scholar] [CrossRef] [PubMed]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, L.; Capcarová, M.; Curlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Capla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health B 2020, 55, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, K.S.; Nisha, P.; Zinia, S. An overview of conventional and emerging techniques of roasting: Effect on food bioactive signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef] [PubMed]
- Makiso, M.U.; Tola, Y.B.; Ogah, O.; Endale, F.L. Bioactive compounds in coffee and their role in lowering the risk of major public health consequences: A review. Food Sci. Nutr. 2024, 12, 734–764. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From plantation to cup: Changes in bioactive compounds during coffee processing. Foods 2021, 10, 2827. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Effect of coffee roasting on in vitro α-glucosidase activity: Inhibition and mechanism of action. Int. Food Res. J. 2018, 111, 480–487. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Muanprasat, C.; Pasachan, T.; Pongchaidecha, A.; Amornlerdpison, D.; Srimaroeng, C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine 2019, 52, 187–197. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular antioxidant and anti-inflammatory effects of coffee extracts with dif-ferent roasting levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef]
- Duangjai, A.; Trisat, K.; Saokaew, S. Effect of roasting degree, extraction time, and temperature of coffee beans on anti-hyperglycaemic and anti-hyperlipidaemic activities using ultrasound-assisted extraction. Prev. Nutr. Food Sci. 2021, 26, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Salamatullah, A.M. Relationship between the chemical composition and the biological functions of coffee. Molecules 2021, 26, 7634. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Alongi, M.; Cervantes-Flores, M.; Simental-Mendía, L.E.; Martínez-Aguilar, G.; Valenzuela-Ramírez, A.A.; Rojas-Contreras, J.J.; Guerrero-Romero, F.; Gamboa-Gómez, C.I. Influence of coffee roasting degree on inflammatory and oxidative stress markers in high-fructose and saturated fat-fed rats. Food Res. Int. 2023, 165, 112530. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gómez, C.I.; Barragán-Zúñiga, L.J.; Guerrero-Romero, F.; Martínez-Aguilar, G.; Luis Gónzalez, J.; Valenzuela-Ramírez, A.A.; Rojas-Contreras, J.A.; Anese, M.; Cervantes Flores, M.; Alongi, M. Effects of coffee with different roasting degrees on obesity and related metabolic disorders. J. Funct. Foods. 2023, 111, 105889. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Morales-Castro, J.; Barragan-Zuniga, J.; Herrera, M.D.; Zamilpa-Álvarez, A.; Gónzalez, J.L.; Marinez-Aguilar, G.; Morales-Castro, E.P.; Anese, M.; Alongi, M. Influence of coffee roasting degree on antioxidant and metabolic parameters: Comprehensive in vitro and in vivo analysis. Curr. Res. Food Sci. 2024, 9, 100861. [Google Scholar] [CrossRef]
- Gallardo-Ignacio, J.; Santibáñez, A.; Oropeza-Mariano, O.; Salazar, R.; Montiel-Ruiz, R.M.; Cabrera-Hilerio, S.; Gonzáles-Cortazar, M.; Cruz-Sosa, F.; Nicasio-Torres, P. Chemical and biological characterization of green and processed coffee beans from Coffea arabica varieties. Molecules 2023, 28, 4685. [Google Scholar] [CrossRef]
- Hong, S.J.; Boo, C.G.; Yoon, S.; Jeong, H.; Jo, S.M.; Youn, M.Y.; Kim, Y.J.; Shin, E.C. Impact of roasting conditions on physicochemical, taste, volatile, and odor-active compound profiles of Coffea arabica L. (cv. Yellow Bourbon) using electronic sensors and GC–MS-O using a multivariate approach. Food Chem. 2024, 21, 101119. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Williams, S.D.; Barkla, B.J.; Rose, T.J.; Liu, L. Does Coffee Have Terroir and How Should It Be Assessed? Foods 2022, 11, 1907. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.; Domingues, M.R.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef]
- Jung, S.; Gu, S.; Lee, S.H.; Jeong, Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from Coffea arabica cv. Javalab Appl. Sci. 2021, 11, 7025. [Google Scholar] [CrossRef]
- Awwad, S.; Issa, R.; Alnsour, L.; Albals, D.; Al-Momani, I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules 2021, 26, 7502. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Reyna-Gonzales, K.; Diaz, D.I.; Pajuelo-Muñoz, A.J.; Iliquin-Chavez, A.F.; Yoplac, I.; Medina-Mendoza, M.; Mori-Mestanza, D.; Cayo-Colca, I.S.; Castro-Alayo, E.M. Optimizing roasting time and temperature to enhance the physicochemical properties, and retention of bioactive compounds of three coffee arabica subvarieties. Appl. Food Res. 2025, 5, 100987. [Google Scholar] [CrossRef]
- Smrke, S.; Opitz, S.E.; Vovk, I.; Yeretzian, C. How does roasting affect the antioxidants of a coffee brew? Exploring the antioxidant capacity of coffee via on-line antioxidant assays coupled with size exclusion chromatography. Food Funct. 2013, 4, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-De Hond, A.; Casas, A.R.; del Castillo, M.D. Interest of coffee melanoidins as sustainable healthier food ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, S.; Silva, A.M.; Simões, C.; Almeida, D.; Félix, L.M.; Papetti, A.; Nunes, F.M. Chemical composition and potential biological activity of melanoidins from instant soluble coffee and instant soluble barley: A comparative study. Front. Nutr. 2022, 9, 825584. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Smrke, S.; Goodman, B.A.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2018, 106, 217–221. [Google Scholar] [CrossRef]
- Alongi, M.; Calligaris, S.; Anese, M. Fat concentration and high-pressure homogenization affect chlorogenic acid bioaccessibility and α-glucosidase inhibitory capacity of milk-based coffee beverages. J. Funct. Foods. 2019, 58, 130–137. [Google Scholar] [CrossRef]
- Alongi, M.; Celayeta, J.M.F.; Vriz, R.; Kinsella, G.K.; Rulikowska, A.; Anese, M. In vitro digestion nullified the differences triggered by roasting in phenolic composition and α-glucosidase inhibitory capacity of coffee. Food Chem. 2020, 342, 128289. [Google Scholar] [CrossRef]
- Petsiou, E.I.; Mitrou, P.I.; Raptis, S.A.; Dimitriadis, G.D. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr. Rev. 2014, 72, 651–661. [Google Scholar] [CrossRef]
- Cha, K.H.; Song, D.G.; Kim, S.M.; Pan, C.H. Inhibition of gastrointestinal lipolysis by green tea, coffee, and gomchui (Ligularia fischeri) tea polyphenols during simulated digestion. J. Agric. Food Chem. 2012, 60, 7152–7157. [Google Scholar] [CrossRef]
- Noh, H.L.; Okajima, K.; Molkentin, J.D.; Homma, S.; Goldberg, I.J. Acute lipoprotein lipase deletion in adult mice leads to dyslipidemia and cardiac dysfunction. Am. J. Physiol. Endocrinol. Metab. 2016, 291, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Iwai, K.; Fukunaga, T.; Nakagiri, O. Inhibitory activity of chlorogenic acids in decaffeinated green coffee beans against porcine pancreas lipase and effect of a decaffeinated green coffee bean extract on an emulsion of olive oil. Biosci. Biotechnol. Biochem. 2012, 76, 2329–2331. [Google Scholar] [CrossRef]
- Post, S.M.; de Wit, E.C.M.; Princen, H.M.G. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3064–3070. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, M.L.; Boekschoten, M.V.; Kreef, A.J.; Hooiveld, G.J.; Moen, C.J.; Muller, M.; Frants, R.R.; Kasanmoentalib, S.; Post, S.M.; Princen, H.M.G.; et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol. Endocrinol. 2007, 21, 1603–1616. [Google Scholar] [CrossRef]
- Association of Analytical Communities (AOAC®). Official Methods of Analysis of the AOAC, 15th ed.; Methods 932.06, 925.09, 985.29, 923.03; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Díaz-Rivas, J.O.; González-Laredo, R.F.; Chávez-Simental, J.A.; Montoya-Ayón, J.B.; Moreno-Jiménez, M.R.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E. Comprehensive characterization of extractable phenolic compounds by UPLC-PDA-ESI-QqQ of Buddleja scordioides plants elicited with salicylic acid. J. Chem. 2018, 2018, 4536970. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E.; Simental-Mendía, L.E.; Barragán-Zúñiga, L.J.; Ramírez-España, J.C.; Gallegos-Infante, J.A.; Lujan-Mendoza, C.I.; Gamboa-Gómez, C.I. Effect of Buddleja scordioides K. leaves infusion on lipid peroxidation in mice with ultraviolet light-induced oxidative stress. Med. Chem. Res. 2018, 27, 2379–2385. [Google Scholar] [CrossRef]
- McDougall, G.; Kulkarni, N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Coruh, N.; Celep, A.S.; Ӧzgӧkçe, F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2017, 100, 1237–1242. [Google Scholar] [CrossRef]
- Tamil, I.G.; Dineshkumar, B.; Nandhakumar, M.; Senthilkumar, M.; Mitra, A. In vitro study on α-amylase inhibitory activity of an Indian medicinal plant Phyllanthus amarus. Indian J. Pharmacol. 2010, 42, 280. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- National Institutes of Health. Office of Laboratory Animal Welfare, United States. Public Health Service. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Office of Laboratory Animal Welfare, National Institutes of Health, Department of Health, and Human Services. 2020. Available online: https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf (accessed on 20 July 2025).
- Norma Oficial MexiNOM-062-ZOO-1999. Especificaciones Técnicas Para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 20 July 2025).
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
 Chiapas (CH),
Chiapas (CH),  Colima (CO),
Colima (CO),  Oaxaca (O), and
Oaxaca (O), and  Hidalgo (H) with respect to unroasted (UN), medium-roast (MR), and high-roast (HR) brews. (1–3) DPPH radical scavenging; (4–6) ABTS assay, and (7–9) lipid peroxidation inhibition. Values are expressed as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (p < 0.05; ANOVA with Tukey’s post hoc test).
Hidalgo (H) with respect to unroasted (UN), medium-roast (MR), and high-roast (HR) brews. (1–3) DPPH radical scavenging; (4–6) ABTS assay, and (7–9) lipid peroxidation inhibition. Values are expressed as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (p < 0.05; ANOVA with Tukey’s post hoc test).
 Chiapas (CH),
Chiapas (CH),  Colima (CO),
Colima (CO),  Oaxaca (O), and
Oaxaca (O), and  Hidalgo (H) with respect to unroasted (UN), medium-roast (MR), and high-roast (HR) brews. (1–3) DPPH radical scavenging; (4–6) ABTS assay, and (7–9) lipid peroxidation inhibition. Values are expressed as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (p < 0.05; ANOVA with Tukey’s post hoc test).
Hidalgo (H) with respect to unroasted (UN), medium-roast (MR), and high-roast (HR) brews. (1–3) DPPH radical scavenging; (4–6) ABTS assay, and (7–9) lipid peroxidation inhibition. Values are expressed as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (p < 0.05; ANOVA with Tukey’s post hoc test).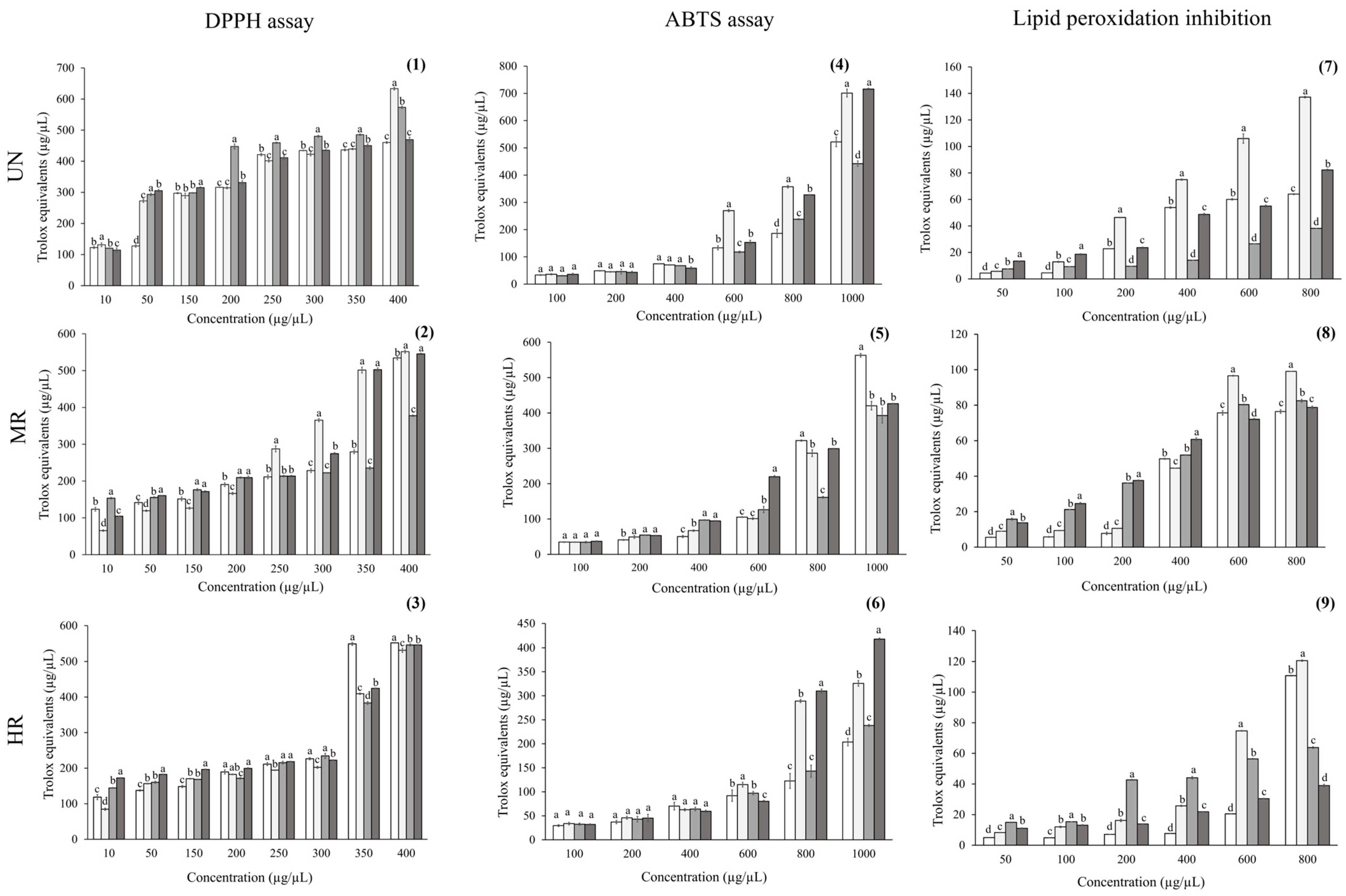
 Chiapas (CH),
Chiapas (CH),  Colima (CO),
Colima (CO),  Oaxaca (O), and
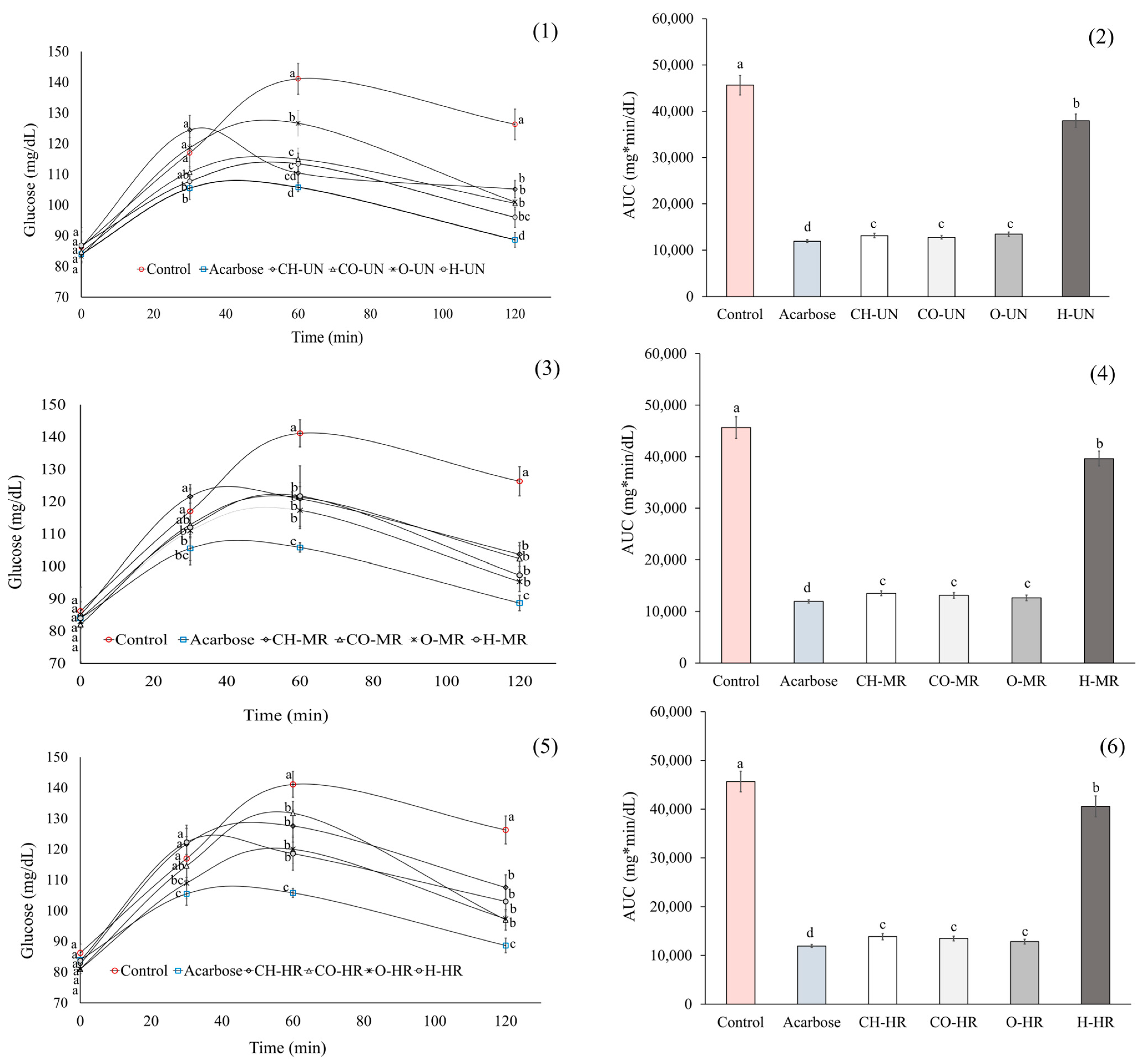
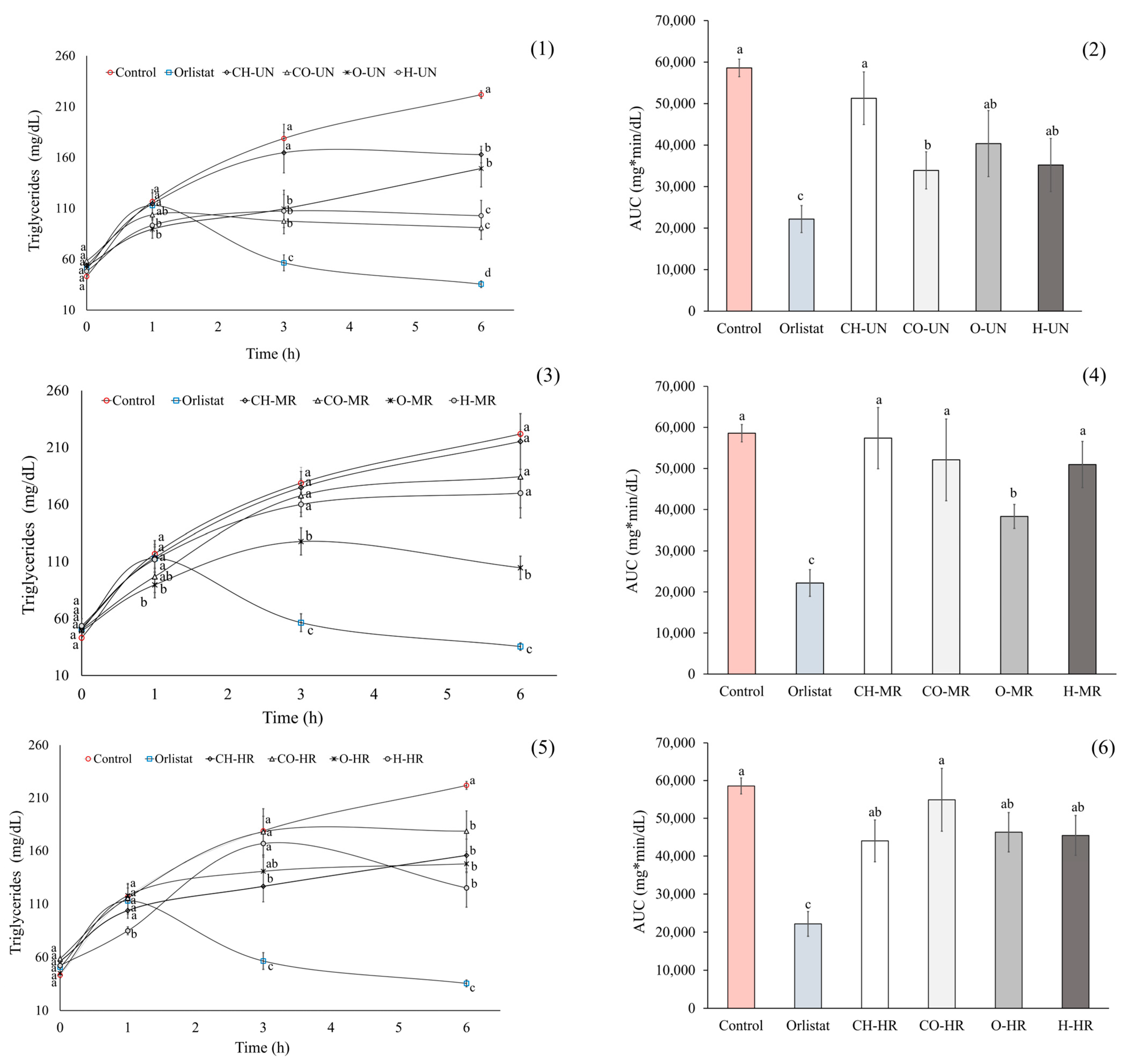
Oaxaca (O), and  Hidalgo (H) of unroasted (UN), medium-roast (MR), and high-roast (HR) brews.
Hidalgo (H) of unroasted (UN), medium-roast (MR), and high-roast (HR) brews.  Acarbose or
Acarbose or  orlistat was used as a positive control. (1–3) Lipase; (4–6) α-amylase, and (7–9) α-glucosidase inhibition assessment. Data are presented as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (ANOVA with Tukey’s test, p < 0.05).
orlistat was used as a positive control. (1–3) Lipase; (4–6) α-amylase, and (7–9) α-glucosidase inhibition assessment. Data are presented as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (ANOVA with Tukey’s test, p < 0.05).
 Chiapas (CH),
Chiapas (CH),  Colima (CO),
Colima (CO),  Oaxaca (O), and
Oaxaca (O), and  Hidalgo (H) of unroasted (UN), medium-roast (MR), and high-roast (HR) brews.
Hidalgo (H) of unroasted (UN), medium-roast (MR), and high-roast (HR) brews.  Acarbose or
Acarbose or  orlistat was used as a positive control. (1–3) Lipase; (4–6) α-amylase, and (7–9) α-glucosidase inhibition assessment. Data are presented as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (ANOVA with Tukey’s test, p < 0.05).
orlistat was used as a positive control. (1–3) Lipase; (4–6) α-amylase, and (7–9) α-glucosidase inhibition assessment. Data are presented as mean ± standard error. Different letters indicate statistically significant differences among coffee samples within each region at the same concentration (ANOVA with Tukey’s test, p < 0.05).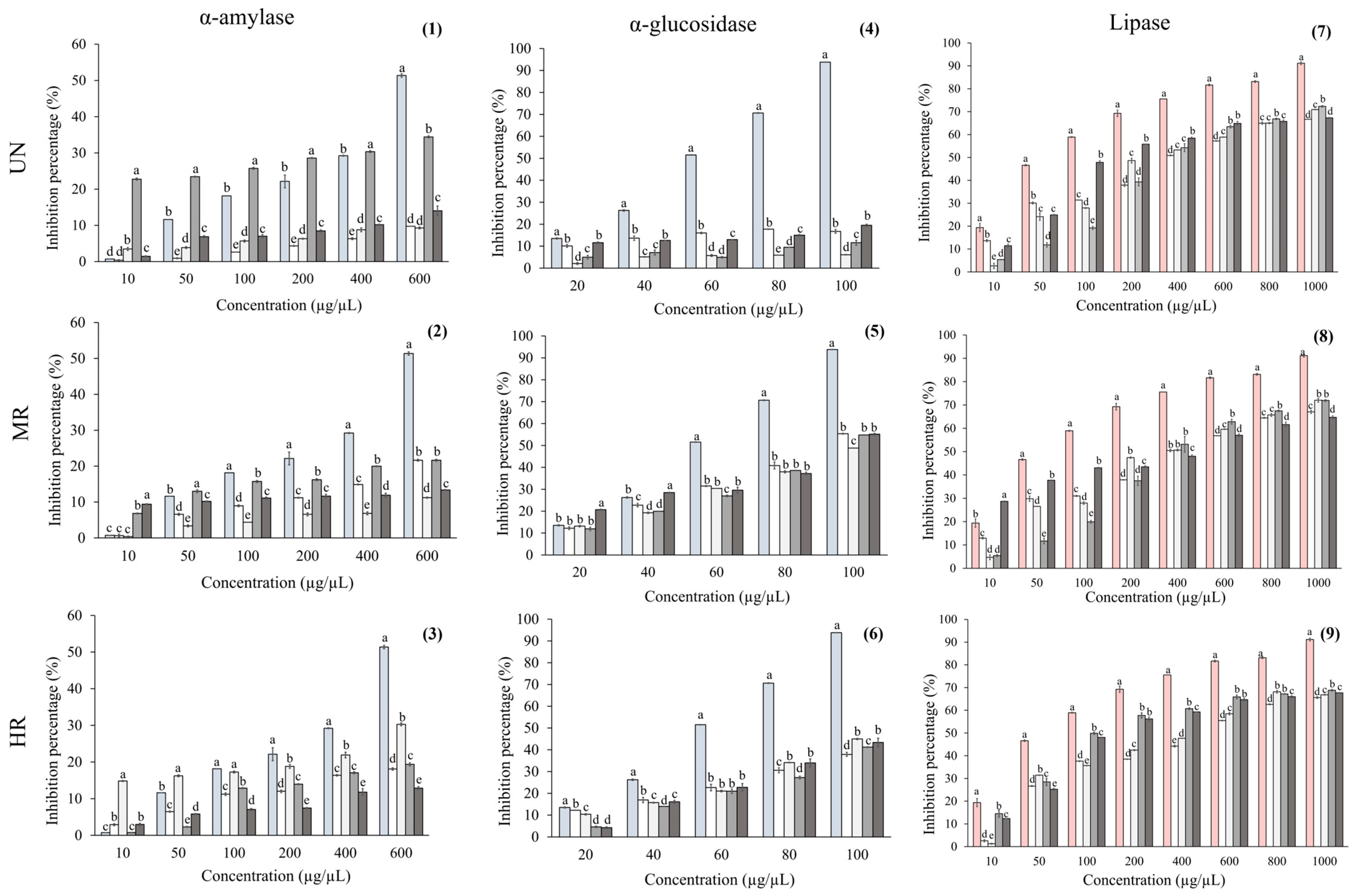
| UN | MR | HR | |
|---|---|---|---|
| Coffee from Chiapas | |||
| Moisture (%) | 10.0 ± 0.03 a | 4.5 ± 0.01 b | 3.9 ± 0.00 c |
| a* | 2.1 ± 0.02 c | 9.0 ± 0.07 a | 7.2 ± 0.11 b |
| b* | 16.9 ± 0.12 a | 2.9 ± 0.17 b | 1.1 ± 0.13 c |
| L* | 65.4 ± 0.48 a | 27.4 ± 0.12 b | 23.2 ± 0.24 b |
| Hue angle | 1.4 ± 0.01 a | 0.3 ± 0.01 b | 0.1 ± 0.02 b |
| MRI280 | 68.0 ± 1.2 c | 177.0 ± 7 b | 206.0 ± 7.5 a |
| MRI360 | 58.0 ± 1.2 a | 61.0 ± 1.8 a | 48.0 ± 1.7 b |
| MRI420 | 2.0 ± 0.1 c | 8.5 ± 0.1 b | 18.0 ± 0.7 a |
| Coffee from Colima | |||
| Moisture (%) | 8.8 ± 0.03 a | 4.9 ± 0.07 b | 3.6 ± 0.0 c |
| a* | 1.7 ± 0.03 b | 6.0 ± 0.06 a | 1.9 ± 0.11 b |
| b* | 12.6 ± 0.06 a | 3.4 ± 0.09 c | 5.3 ± 0.04 b |
| L* | 53.6 ± 0.09 a | 22.1 ± 0.05 b | 21.0 ± 0.20 b |
| Hue angle | 1.4 ± 0.01 a | 0.5 ± 0.01 b | 0.3 ± 0.01 c |
| MRI280 | 58.0 ± 2.2 c | 190.0 ± 6.0 a | 116.0 ± 3.1 b |
| MRI360 | 51.0 ± 1.3 b | 61.0 ± 0.9 a | 41.0 ± 1.1 c |
| MRI420 | 2.0 ± 0.0 c | 9.0 ± 0.1 a | 8.0 ± 0.0 b |
| Coffee from Oaxaca | |||
| Moisture (%) | 9.2 ± 0.02 a | 5.0 ± 0.05 b | 2.8 ± 0.03 c |
| a* | 1.4 ± 0.02 c | 5.3 ± 0.09 b | 5.3 ± 0.09 a |
| b* | 11.6 ± 0.20 a | 4.2 ± 0.11 b | 1.1 ± 0.04 c |
| L* | 53.0 ± 0.26 a | 25.2 ± 0.12 b | 25.2 ± 0.12 b |
| Hue angle | 1.4 ± 0.01 c | 0.66 ± 0.01 b | 0.27 ± 0.1 a |
| MRI280 | 65.0 ± 2.0 c | 145.0 ± 7.0 a | 110.0 ± 5.6 b |
| MRI360 | 55 ± 1.0 a | 50.0 ± 0.5 b | 40.0 ± 2.1 c |
| MRI420 | 2.0 ± 0.1 c | 5.0 ± 0.0 b | 9.0 ± 0.1 a |
| Coffee from Hidalgo | |||
| Moisture (%) | 8.9 ± 0.03 a | 6.8 ± 0.01 b | 4.3 ± 0.12 c |
| a* | 1.6 ± 0.03 c | 6.1 ± 0.04 a | 4.7 ± 0.06 b |
| b* | 12.3 ± 0.17 a | 3.5 ± 0.07 b | 1.0 ± 0.08 c |
| L* | 54.3 ±0.26 a | 23.4 ±0.03 b | 21.8 ± 0.08 b |
| Hue angle | 1.4 ± 0.01 a | 0.5 ± 0.01 b | 0.2 ± 0.01 c |
| MRI280 | 65.0 ± 1.8 c | 117.0 ± 0.6 b | 167.0 ± 0.1 a |
| MRI360 | 52.0 ± 3.2 a | 51.0 ± 0.4 a | 48.0 ± 0.1 b |
| MRI420 | 2.0 ± 0.1 c | 7.0 ± 0.1 b | 9.0 ± 0.0 a |
| No. | Compound | Retention Time (min) | Molecular Weight | Main Transition (m/z) | λ Max (nm) | UN (µg/g) | MR (µg/g) | HR (µg/g) |
|---|---|---|---|---|---|---|---|---|
| Coffee from Chiapas | ||||||||
| 1. | Gallic acid | 1.08 | 169 | 79 > 125 | 270 | 64.5 ± 13.1 a | 55.1 ± 10.2 a | Traces |
| 2. | Chlorogenic acid | 3.42 | 353 | 191 > 85 | 320 | 231,990 ± 5032 a | 13,545.2 ± 482 b | 10,977.2 ± 52.6 c |
| 3. | Caffeic acid | 3.83 | 179 | 135 > 89 | 320 | 1125.9 ± 19.5 a | 63.9 ± 3.1 b | 26.8 ± 0.4 c |
| 4. | 3,4-Dicaffecoylquinic acid | 4.21 | 516 | 135 > 89 | 320 | 22,953.6 ± 78.5 a | 114.5 ± 14.7 b | Traces |
| 5. | Quercetin | 4.23 | 480 | 479 > 303 | 360 | 48,715 ± 836 a | 2289.9 ± 487.4 b | 346.5 ± 93.7 c |
| 6. | 2,4,6-trihydroxybenzoic acid | 4.69 | 170 | 137 > 137 | 270 | 35.3 ± 0.2 a | 40.2 ± 3.1 a | 22.1 ± 1.7 b |
| 7. | Coumaric acid | 4.90 | 193 | 178 > 134 | 320 | 315.0 ± 10.2 a | Traces | Traces |
| 8. | Ferulic acid | 5.50 | 193 | 178 > 134 | 320 | 1230.7 ± 58.8 a | 462.71 ± 43.3 b | Traces |
| 9. | Quercetin glucuronide | 6.45 | 480 | 479 > 303 | 360 | 5128.0 ± 548.2 a | 2077.9 ± 198.6 b | 892.9 ± 80.4 c |
| 10. | t-cinnamic acid | 8.39 | 148 | 148 > 149 | 320 | 235.5 ± 2.2 a | 148.0 ± 0.4 b | 159.0 ± 1.7 b |
| Coffee from Colima | ||||||||
| 1. | Gallic acid | 1.08 | 169 | 79 > 125 | 270 | 38.1 ± 1.4 a | 22.8 ± 6.3 a | Traces |
| 2. | Chlorogenic acid | 3.42 | 353 | 191 > 85 | 320 | 158,675.8 ± 790 a | 30,721 ± 633.8 b | 19,054 ± 321.8 c |
| 3. | Caffeic acid | 3.83 | 179 | 135 > 89 | 320 | 111.4 ± 10.4 a | 57.1 ± 3.1 b | 54.9 ± 0.4 b |
| 4. | 3,4-Dicaffecoylquinic acid | 4.21 | 516 | 135 > 89 | 320 | 12,549.9 ± 157.9 a | 53.9 ± 0.2 b | 21.6 ± 5.4 c |
| 5. | Quercetin | 4.23 | 480 | 479 > 303 | 360 | 422.1 ± 9.9 a | 397.1 ± 18.63 a | 215.8 ± 2.0 b |
| 6. | 2,4,6-trihydroxybenzoic acid | 4.69 | 170 | 137 > 137 | 270 | 72.1 ± 1.5 a | 44.7 ± 0.2 b | Traces |
| 7. | Coumaric acid | 4.90 | 193 | 178 > 134 | 320 | 70.3 ± 1.0 a | 43.8 ± 1.9 b | Traces |
| 8. | Ferulic acid | 5.50 | 193 | 178 > 134 | 320 | 1973.8 ± 213.7 a | 178.1 ± 0.6 b | 13.2 ± 0.1 c |
| 9. | Quercetin glucuronide | 6.45 | 480 | 479 > 303 | 360 | 2272.1 ± 49.4 a | 1861.3 ± 47.4 b | 1817.9 ± 55.8 b |
| 10. | t-cinnamic acid | 8.39 | 148 | 148 > 149 | 320 | 309.5 ± 0.2 a | 237.02 ± 5.7 b | Traces |
| Coffee from Oaxaca | ||||||||
| 1. | Gallic acid | 1.08 | 169 | 79 > 125 | 270 | 49.4 ± 4.9 a | 20.9 ± 0.9 b | 25.3 ± 1.4 b |
| 2. | Chlorogenic acid | 3.42 | 353 | 191 > 85 | 320 | 110,882 ± 2814 a | 70,133 ± 4441 b | 11,242 ± 556.6 c |
| 3. | Caffeic acid | 3.83 | 179 | 135 > 89 | 320 | 78.8 ± 9.1 a | 19.0 ± 1.0 b | 22.3 ± 0.3 b |
| 4. | 3,4-Dicaffecoylquinic acid | 4.21 | 516 | 135 > 89 | 320 | 5966.8 ± 241.4 a | 215.4 ± 16.8 b | Traces |
| 5. | Quercetin | 4.23 | 480 | 479 > 303 | 360 | 56,290.6 ± 2987 a | 509.1 ± 16.8 b | Traces |
| 6. | 2,4,6-trihydroxybenzoic acid | 4.69 | 170 | 137 > 137 | 270 | 42.1 ± 3.9 a | 11.3 ± 0.5 b | Traces |
| 7. | Coumaric acid | 4.90 | 193 | 178 > 134 | 320 | 27.7 ± 3.5 a | 4.9 ± 0.1 b | Traces |
| 8. | Ferulic acid | 5.50 | 193 | 178 > 134 | 320 | 1371.6 ± 168.6 a | 719.5 ± 51.2 b | 46.1 ± 4.6 c |
| 9. | Quercetin glucuronide | 6.45 | 480 | 479 > 303 | 360 | 5128.0 ± 548.2 a | 1670.6 ± 58.7 b | 1551.7 ± 9.3 b |
| 10. | t-cinnamic acid | 8.39 | 148 | 148 > 149 | 320 | 164.3 ± 19.4 a | 92.3 ± 2.8 b | Traces |
| Coffee from Hidalgo | ||||||||
| 1. | Gallic acid | 1.08 | 169 | 79 > 125 | 270 | 57.2 ± 3.1 a | 36.7 ± 6.1 b | 29.9 ± 0.1 b |
| 2. | Chlorogenic acid | 3.42 | 353 | 191 > 85 | 320 | 233,296 ± 1031 a | 39,374.0 ± 21.6 b | 3295.6 ± 20.3 c |
| 3. | Caffeic acid | 3.83 | 179 | 135 > 89 | 320 | 333.5 ± 43.9 a | 59.1 ± 1.9 b | 8.2 ± 0.2 c |
| 4. | 3,4-Dicaffecoylquinic acid | 4.21 | 516 | 135 > 89 | 320 | 30,509.4 ± 312.4 a | Traces | Traces |
| 5. | Quercetin | 4.23 | 480 | 479 > 303 | 360 | 8385.6 ± 1480 a | 996.6 ± 60.7 b | 649.0 ± 5.3 c |
| 6. | 2,4,6-trihydroxybenzoic acid | 4.69 | 170 | 137 > 137 | 270 | Traces | Traces | Traces |
| 7. | Coumaric acid | 4.90 | 193 | 178 > 134 | 320 | 191.6 ± 1.4 a | 5.5 ± 0.1 b | 2.9 ± 0.1 b |
| 8. | Ferulic acid | 5.50 | 193 | 178 > 134 | 320 | 1658.2 ± 168.0 a | 1426.3 ± 3.7 a | Traces |
| 9. | Quercetin glucuronide | 6.45 | 480 | 479 > 303 | 360 | 3383.7 ± 208.7 a | 3483.7 ± 178.6 a | 1987.7 ± 38.1 b |
| 10. | t-cinnamic acid | 8.39 | 148 | 148 > 149 | 320 | 173.1 ± 12.5 a | 192.4 ± 10.9 a | 44.0 ± 5.6 b |
| Acarbose | Orlistat | UN | MR | HR | |
|---|---|---|---|---|---|
| Coffee from Chiapas | |||||
| Lipase | -- | 65.0 ± 2.4 c | 338.0 ± 3.1 b | 344.5 ± 3.0 b | 385.4 ± 4.9 a |
| α-amylase | 752.0 ± 4.9 d | -- | 9017.0 ± 0.4 c | 19,853.0 ± 1.3 a | 18,041.0 ± 1.0 b |
| α-glucosidase | 56.2 ± 6.8 d | -- | 5843.0 ± 1.1 a | 95.6 ± 3.1 c | 173.6 ± 2.3 b |
| Coffee from Colima | |||||
| Lipase | -- | 65.0 ± 2.4 b | 309.6 ± 3.8 a | 307.2 ± 3.7 a | 315.0 ± 5.3 a |
| α-amylase | 752.0 ± 4.9 d | -- | 80,634.0 ± 2.8 a | 29,751.0 ±1.0 c | 60,778.0 ± 2.9 b |
| α-glucosidase | 56.2 ± 6.8 c | -- | 19,108.0 ± 0.7 a | 111.5 ± 2.3 b | 121.9 ± 3.3 b |
| Coffee from Oaxaca | |||||
| Lipase | -- | 65.0 ± 2.4 c | 354.9 ± 2.4 a | 363.3 ± 2.2 a | 170.8 ± 4 b |
| α-amylase | 752.0 ± 4.9 d | -- | 93,417 ± 1.5 a | 53,844.0 ± 0.9 b | 6930.0 ± 2.8 c |
| α-glucosidase | 56.2 ± 6.8 d | -- | 3155.0 ± 1.8 a | 99.5 ± 4.1 c | 131.6 ± 2.3 b |
| Coffee from Hidalgo | |||||
| Lipase | -- | 65.0 ± 2.4 c | 203.8 ± 5.0 b | 258.8 ± 3.4 a | 197.7 ± 4.8 b |
| α-amylase | 752.0 ± 4.9 d | -- | 59,707.0 ± 1.3 b | 22,321.0 ± 2.0 c | 84,878.0 ± 0.8 a |
| α-glucosidase | 56.2 ± 6.8 c | -- | 6761.0 ± 1.6 a | 112.5 ± 5.9 b | 119.1 ± 2.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa-Gómez, C.I.; Barragán-Zúñiga, J.; Herrera, M.D.; Alongi, M.; Rocha-Guzmán, N.E.; Hererra-Rocha, K.M.; Dominguez, D.; Valles-Araiza, K.F.; Anese, M.; Rodríguez-Morán, M.; et al. Influence of Coffee Roasting Degree from Four Mexican Regions on In Vitro Antioxidant Activity and Digestive Enzyme Inhibition and Its In Vivo Effects on Carbohydrate and Lipid Absorption. Int. J. Mol. Sci. 2025, 26, 10067. https://doi.org/10.3390/ijms262010067
Gamboa-Gómez CI, Barragán-Zúñiga J, Herrera MD, Alongi M, Rocha-Guzmán NE, Hererra-Rocha KM, Dominguez D, Valles-Araiza KF, Anese M, Rodríguez-Morán M, et al. Influence of Coffee Roasting Degree from Four Mexican Regions on In Vitro Antioxidant Activity and Digestive Enzyme Inhibition and Its In Vivo Effects on Carbohydrate and Lipid Absorption. International Journal of Molecular Sciences. 2025; 26(20):10067. https://doi.org/10.3390/ijms262010067
Chicago/Turabian StyleGamboa-Gómez, Claudia I., Jazel Barragán-Zúñiga, Mayra Denise Herrera, Marilisa Alongi, Nuria E. Rocha-Guzmán, Karen M. Hererra-Rocha, Deisy Dominguez, Karla F. Valles-Araiza, Monica Anese, Martha Rodríguez-Morán, and et al. 2025. "Influence of Coffee Roasting Degree from Four Mexican Regions on In Vitro Antioxidant Activity and Digestive Enzyme Inhibition and Its In Vivo Effects on Carbohydrate and Lipid Absorption" International Journal of Molecular Sciences 26, no. 20: 10067. https://doi.org/10.3390/ijms262010067
APA StyleGamboa-Gómez, C. I., Barragán-Zúñiga, J., Herrera, M. D., Alongi, M., Rocha-Guzmán, N. E., Hererra-Rocha, K. M., Dominguez, D., Valles-Araiza, K. F., Anese, M., Rodríguez-Morán, M., & Guerrero-Romero, F. (2025). Influence of Coffee Roasting Degree from Four Mexican Regions on In Vitro Antioxidant Activity and Digestive Enzyme Inhibition and Its In Vivo Effects on Carbohydrate and Lipid Absorption. International Journal of Molecular Sciences, 26(20), 10067. https://doi.org/10.3390/ijms262010067









