Sodium Hypochlorite-Assisted Photooxidation of Salicylic Acid: Degradation Kinetics, Formation, and Ecotoxicological Assessment of Intermediates
Abstract
1. Introduction
2. Results and Discussion
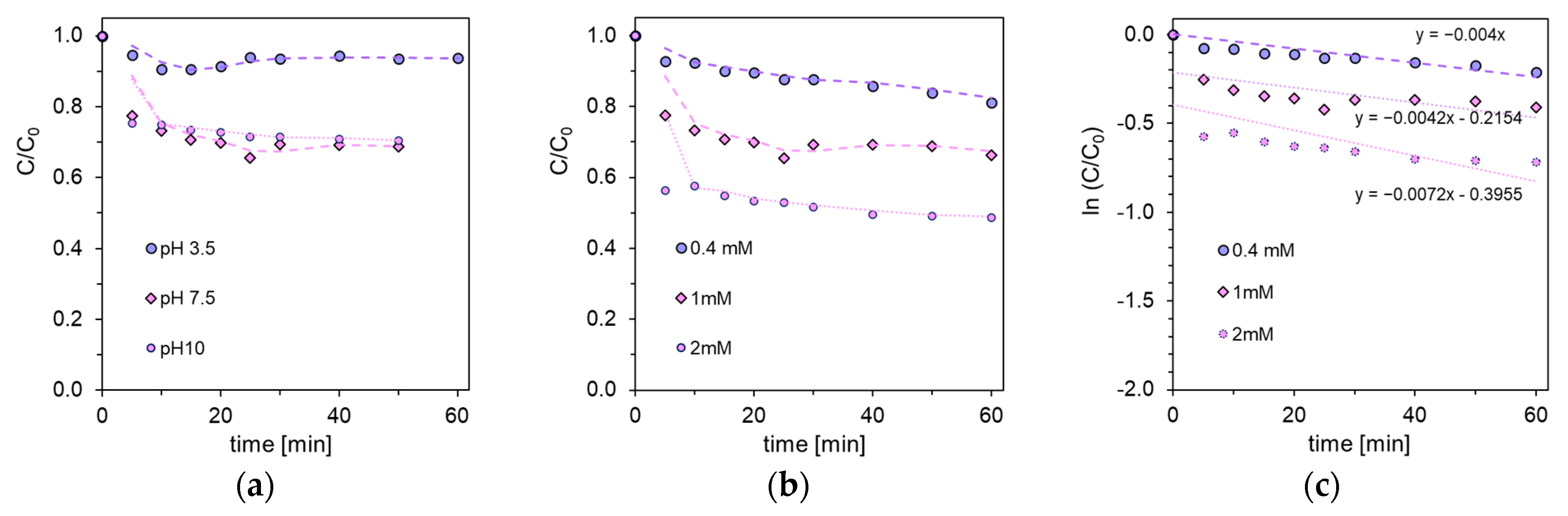
2.1. The Effect of NaOCl on Salicylic Acid Degradation
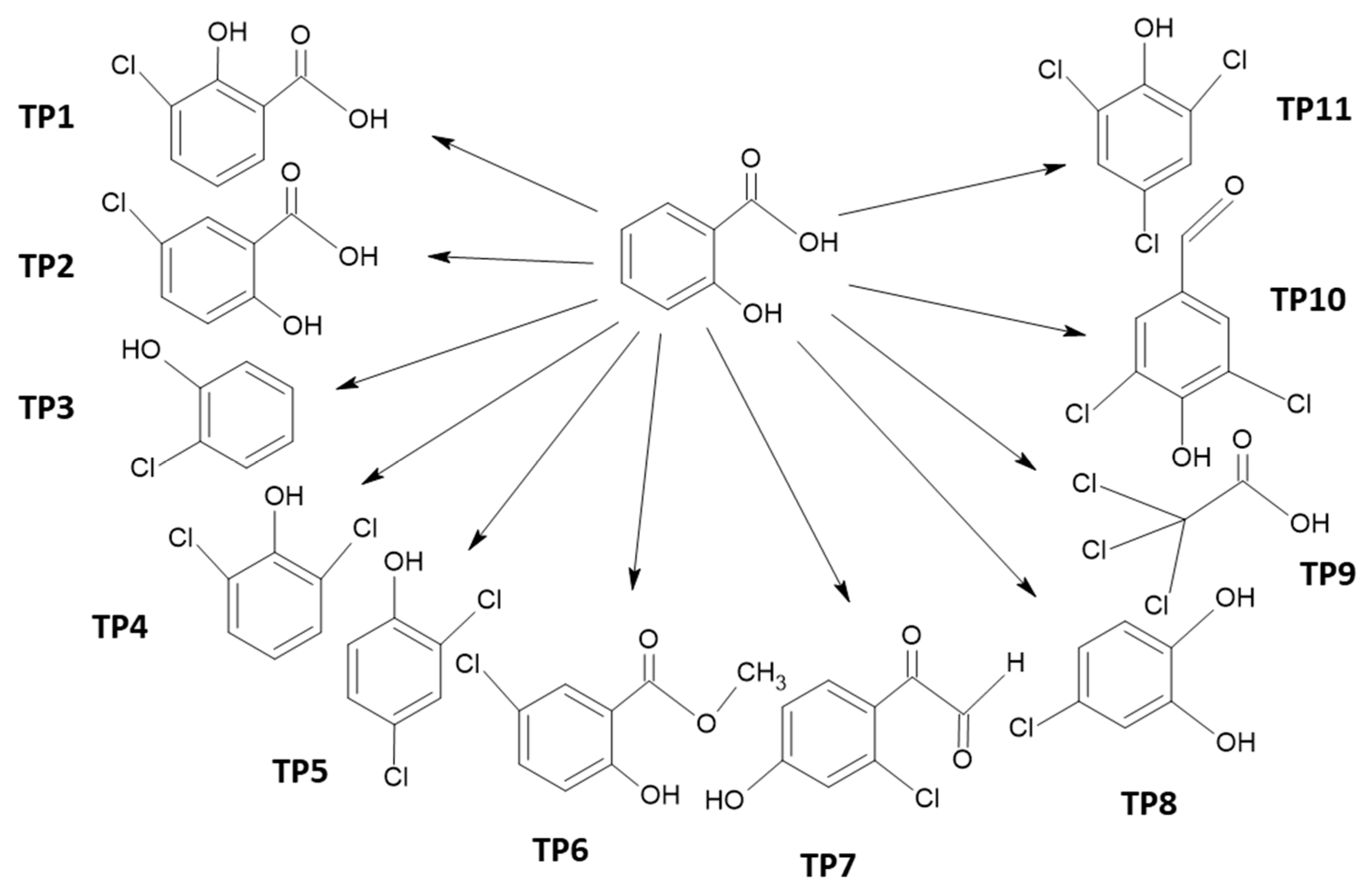
2.2. Chloro-Organic Products of SA Photodegradation in the Presence of NaOCl
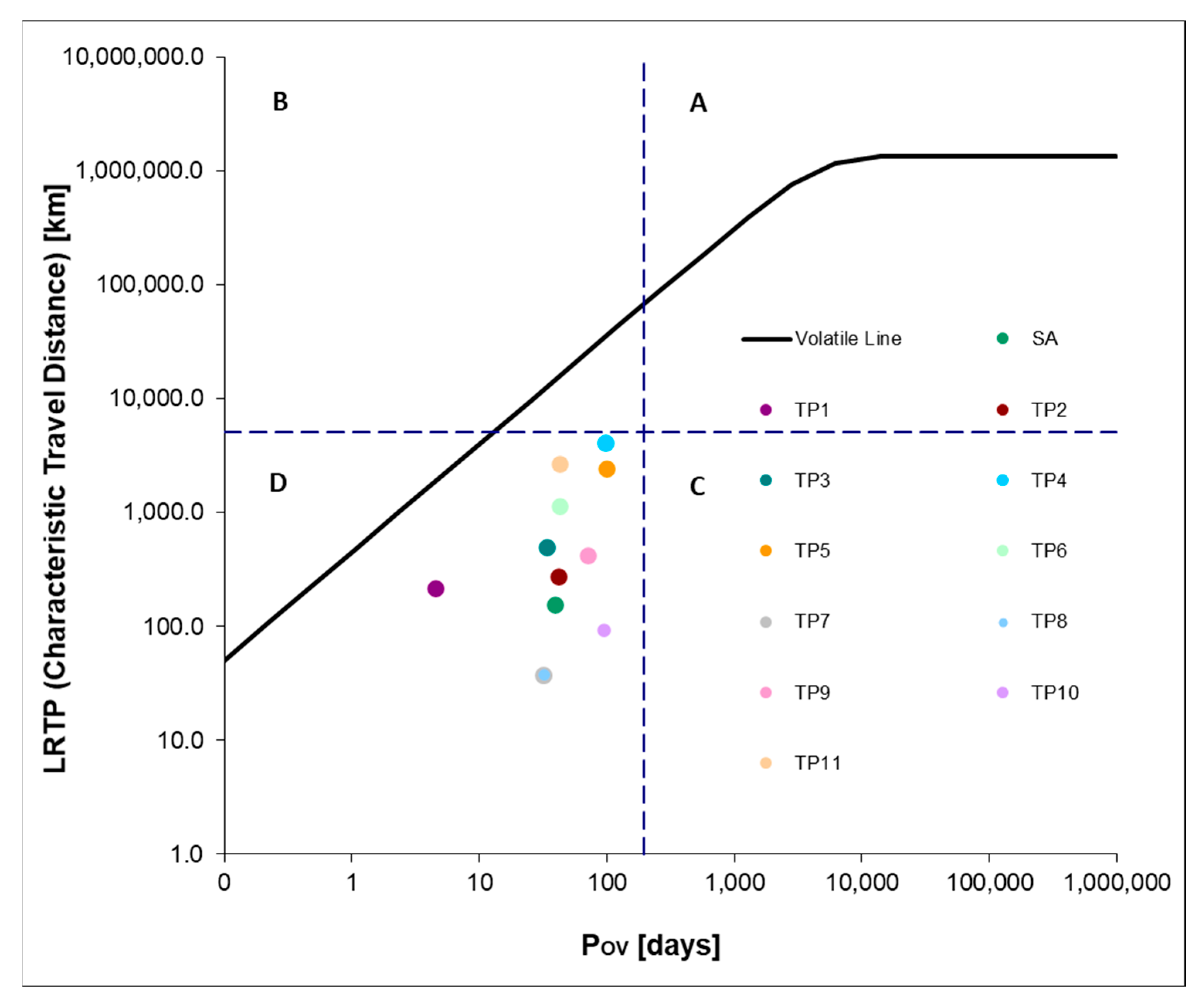
2.3. Environmental Assessment of Chloroproducts of Salicylic Acid Intermediates
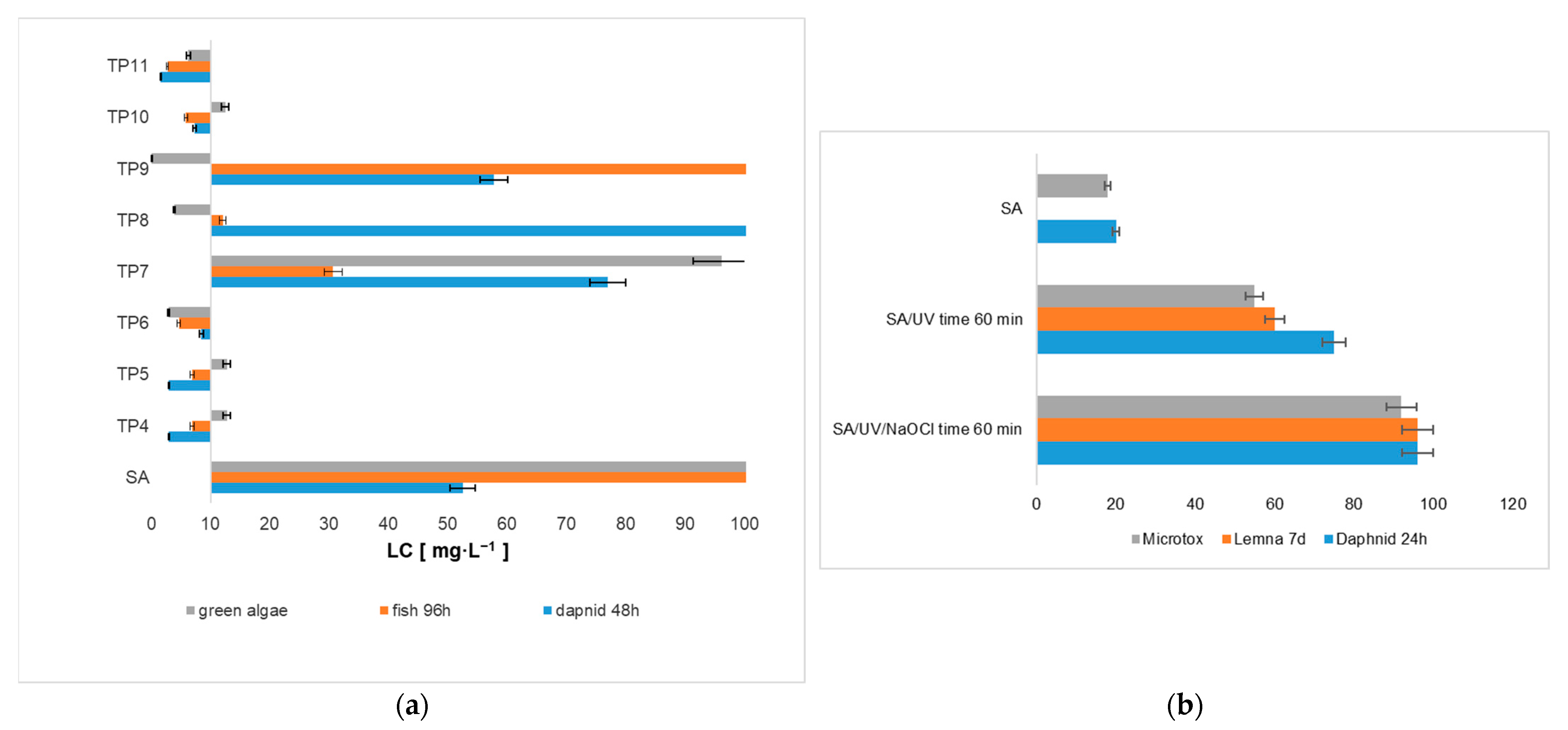
2.4. Toxicological Evaluation of Chloroproducts of Salicylic Acid Transformation
3. Materials and Methods
3.1. Reagents
3.2. Experiments on the Photodegradation of Salicylic Acid and Its Transformation Products
3.3. Detection of Salicylic Acid Transformation Products
3.4. Determination of Physicochemical and Ecotoxicological Parameters of Intermediate Compounds
3.5. Toxicity Assessment of Intermediate Compounds
3.6. Data Analysis and Replication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, L.H.M.L.M.; Gros, M.; Rodriguez-Mozaz, S.; Delerue-Matos, C.; Pena, A.; Barceló, D.; Montenegro, M.C.B.S.M. Contribution of Hospital Effluents to the Load of Pharmaceuticals in Urban Wastewaters: Identification of Ecologically Relevant Pharmaceuticals. Sci. Total Environ. 2013, 461–462, 302–316. [Google Scholar] [CrossRef]
- Heberer, T.; Feldmann, D. Contribution of Effluents from Hospitals and Private Households to the Total Loads of Diclofenac and Carbamazepine in Municipal Sewage Effluents—Modeling versus Measurements. J. Hazard. Mater. 2005, 122, 211–218, Erratum in J. Hazard. Mater. 2005, 127, 249. [Google Scholar] [CrossRef]
- Ferreira, B.L.; Ferreira, D.P.; Borges, S.F.; Ferreira, A.M.; Holanda, F.H.; Ucella-Filho, J.G.M.; Cruz, R.A.S.; Birolli, W.G.; Luque, R.; Ferreira, I.M. Diclofenac, Ibuprofen, and Paracetamol Biodegradation: Overconsumed Non-Steroidal Anti-Inflammatories Drugs at COVID-19 Pandemic. Front. Microbiol. 2023, 14, 1207664. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Wang, B.; Duan, L.; Zhang, Y.; Zhao, W.; Wang, F.; Sui, Q.; Chen, Z.; Xu, D.; et al. Occurrence, Source and Ecotoxicological Risk Assessment of Pesticides in Surface Water of Wujin District (Northwest of Taihu Lake), China. Environ. Pollut. 2020, 265, 114953. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in the Aquatic Environment: A Review of Recent Research Data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Acuña, V.; Ginebreda, A.; Mor, J.R.; Petrovic, M.; Sabater, S.; Sumpter, J.; Barceló, D. Balancing the Health Benefits and Environmental Risks of Pharmaceuticals: Diclofenac as an Example. Environ. Int. 2015, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Jankunaite, D.; Tichonovas, M.; Buivydiene, D.; Radziuniene, I.; Racys, V.; Krugly, E. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil. Pollut. 2017, 228, 353. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the Marine Environment: A Review of Its Occurrence and Effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef]
- Cunningham, V.L.; Perino, C.; D’Aco, V.J.; Hartmann, A.; Bechter, R. Human Health Risk Assessment of Carbamazepine in Surface Waters of North America and Europe. Regul. Toxicol. Pharmacol. 2010, 56, 343–351. [Google Scholar] [CrossRef]
- Barceló, D.; Petrovic, M. Pharmaceuticals and Personal Care Products (PPCPs) in the Environment. Anal. Bioanal. Chem. 2007, 387, 1141–1142. [Google Scholar] [CrossRef]
- Wiese, B.; Massmann, G.; Jekel, M.; Heberer, T.; Dünnbier, U.; Orlikowski, D.; Grützmacher, G. Removal Kinetics of Organic Compounds and Sum Parameters under Field Conditions for Managed Aquifer Recharge. Water Res. 2011, 45, 4939–4950. [Google Scholar] [CrossRef]
- Fong, P.P.; Bury, T.B.; Dworkin-Brodsky, A.D.; Jasion, C.M.; Kell, R.C. The Antidepressants Venlafaxine (“Effexor”) and Fluoxetine (“Prozac”) Produce Different Effects on Locomotion in Two Species of Marine Snail, the Oyster Drill (Urosalpinx Cinerea) and the Starsnail (Lithopoma Americanum). Mar. Environ. Res. 2015, 103, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Archundia, D.; Martins, J.M.F.; Lehembre, F.; Morel, M.-C.; Duwig, C. Sulfamethoxazole Biodegradation and Impacts on Soil Microbial Communities in a Bolivian Arid High Altitude Catchment. Chemosphere 2021, 284, 131335. [Google Scholar] [CrossRef]
- Jebalbarezi, B.; Dehghanzadeh, R.; Sheikhi, S.; Shahmahdi, N.; Aslani, H.; Maryamabadi, A. Oxidative Degradation of Sulfamethoxazole from Secondary Treated Effluent by Ferrate(VI): Kinetics, by-Products, Degradation Pathway and Toxicity Assessment. J. Environ. Health Sci. Eng. 2022, 20, 205–218. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, G.; Liu, J.; Yan, Z.; Dong, H.; Zhou, R. Biodegradation of 2-Ethylhexyl-4-Methoxycinnamate in River Sediments and Its Impact on Microbial Communities. J. Environ. Sci. 2021, 104, 307–316. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and Personal Care Products (PPCPs): A Review on Environmental Contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Ghatak, H.R. Advanced Oxidation Processes for the Treatment of Biorecalcitrant Organics in Wastewater. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1167–1219. [Google Scholar] [CrossRef]
- Cheng, H.; Song, D.; Chang, Y.; Liu, H.; Qu, J. Chlorination of Tramadol: Reaction Kinetics, Mechanism and Genotoxicity Evaluation. Chemosphere 2015, 141, 282–289. [Google Scholar] [CrossRef]
- Broadwater, M.A.; Swanson, T.L.; Sivey, J.D. Emerging Investigators Series: Comparing the Inherent Reactivity of Often-Overlooked Aqueous Chlorinating and Brominating Agents toward Salicylic Acid. Environ. Sci. 2018, 4, 369–384, Erratum in Environ. Sci. 2018, 4, 750. [Google Scholar] [CrossRef]
- Gibs, J.; Stackelberg, P.E.; Furlong, E.T.; Meyer, M.; Zaugg, S.D.; Lippincott, R.L. Persistence of Pharmaceuticals and Other Organic Compounds in Chlorinated Drinking Water as a Function of Time. Sci. Total Environ. 2007, 373, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Bedner, M.; MacCrehan, W.A. Transformation of Acetaminophen by Chlorination Produces the Toxicants 1,4-Benzoquinone and N-Acetyl-p-Benzoquinone Imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef]
- Nam, S.-W.; Yoon, Y.; Choi, D.-J.; Zoh, K.-D. Degradation Characteristics of Metoprolol during UV/Chlorination Reaction and a Factorial Design Optimization. J. Hazard. Mater. 2015, 285, 453–463. [Google Scholar] [CrossRef]
- Gaffney, V.d.J.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Chlorination and Oxidation of Sulfonamides by Free Chlorine: Identification and Behaviour of Reaction Products by UPLC-MS/MS. J. Environ. Manag. 2016, 166, 466–477. [Google Scholar] [CrossRef]
- Krkošek, W.H.; Peldszus, S.; Huck, P.M.; Gagnon, G.A. Formation Kinetics of Gemfibrozil Chlorination Reaction Products: Analysis and Application. Water Environ. Res. 2014, 86, 654–662. [Google Scholar] [CrossRef]
- Sambyal, K.; Singh, R.V. Production of Salicylic Acid; a Potent Pharmaceutically Active Agent and Its Future Prospects. Crit. Rev. Biotechnol. 2021, 41, 394–405. [Google Scholar] [CrossRef]
- Styszko, K.; Durak, J.; Malicka, A.; Bochnia, T.; Żaba, T. The Occurrence of Chemicals of Emerging Concern in Samples of Surface Water and Wastewater Collected in Kraków, Poland. Desalination Water Treat. 2021, 232, 308–323. [Google Scholar] [CrossRef]
- Pulicharla, R.; Proulx, F.; Behmel, S.; Sérodes, J.-B.; Rodriguez, M.J. Occurrence and Seasonality of Raw and Drinking Water Contaminants of Emerging Interest in Five Water Facilities. Sci. Total Environ. 2021, 751, 141748. [Google Scholar] [CrossRef]
- Albarrán, G.; Mendoza, E. Ionizing Radiation Induced Degradation of Salicylic Acid in Aqueous Solution. Radiat. Phys. Chem. 2018, 147, 27–34. [Google Scholar] [CrossRef]
- Gackowska, A.; Studziński, W.; Shyichuk, A. Intermediates of Hydrogen Peroxide-Assisted Photooxidation of Salicylic Acid: Their Degradation Rates and Ecotoxicological Assessment. Int. J. Mol. Sci. 2025, 26, 697. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; von Gunten, U. Reactions of Chlorine with Inorganic and Organic Compounds during Water Treatment—Kinetics and Mechanisms: A Critical Review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Dao, Y.H.; Tran, H.N.; Tran-Lam, T.T.; Pham, T.Q.; Le, G.T. Degradation of Paracetamol by an UV/Chlorine Advanced Oxidation Process: Influencing Factors, Factorial Design, and Intermediates Identification. Int. J. Environ. Res. Public. Health 2018, 15, 2637. [Google Scholar] [CrossRef]
- Prasse, C.; von Gunten, U.; Sedlak, D.L. Chlorination of Phenols Revisited: Unexpected Formation of α,β-Unsaturated C 4 -Dicarbonyl Ring Cleavage Products. Environ. Sci. Technol. 2020, 54, 826–834. [Google Scholar] [CrossRef]
- Bulloch, D.N.; Nelson, E.D.; Carr, S.A.; Wissman, C.R.; Armstrong, J.L.; Schlenk, D.; Larive, C.K. Occurrence of Halogenated Transformation Products of Selected Pharmaceuticals and Personal Care Products in Secondary and Tertiary Treated Wastewaters from Southern California. Environ. Sci. Technol. 2015, 49, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; He, Q.; Liu, C.; Deng, Y.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Prednisolone Degradation by UV/Chlorine Process: Influence Factors, Transformation Products and Mechanism. Chemosphere 2018, 212, 56–66. [Google Scholar] [CrossRef]
- Gackowska, A.; Przybyłek, M.; Studziński, W.; Gaca, J. Formation of Chlorinated Breakdown Products during Degradation of Sunscreen Agent, 2-Ethylhexyl-4-Methoxycinnamate in the Presence of Sodium Hypochlorite. Environ. Sci. Pollut. Res. 2016, 23, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Ambauen, N.; Muff, J.; Mai, N.L.; Hallé, C.; Trinh, T.T.; Meyn, T. Insights into the Kinetics of Intermediate Formation during Electrochemical Oxidation of the Organic Model Pollutant Salicylic Acid in Chloride Electrolyte. Water 2019, 11, 1322. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodil, R.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Investigating the Chlorination of Acidic Pharmaceuticals and By-Product Formation Aided by an Experimental Design Methodology. Water Res. 2010, 44, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Chen, H.-F.; Kuo, Y.-S.; Wang, C.-T. Degradation of Salicylic Acid Using Electrochemically Assisted UV/Chlorine Process: Effect of Operating Conditions, Reaction Kinetics, and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108714. [Google Scholar] [CrossRef]
- Smith, K.; Hegazy, A.S.; El-Hiti, G.A. The Use of Polymeric Sulfides as Catalysts for the Para-Regioselective Chlorination of Phenol and 2-Chlorophenol. J. Sulfur. Chem. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Gallard, H.; von Gunten, U. Chlorination of Phenols: Kinetics and Formation of Chloroform. Environ. Sci. Technol. 2002, 36, 884–890. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, L.; Chen, H. Effects of PH on the Chlorination Process of Phenols in Drinking Water. J. Hazard. Mater. 2006, 133, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhu, L.; Wang, J. Distribution of Chlorination Products of Phenols under Various PHs in Water Disinfection. Desalination 2008, 225, 156–166. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Estimation Programs Interface SuiteTM for Microsoft® Windows; v 4.11; United States Environmental Protection Agency: Washington, DC, USA, 2012. Available online: https://Www.Epa.Gov/Tsca-Screening-Tools/Epi-Suitetm-Estimation-Program-Interface (accessed on 9 September 2025).
- Koppmann, R. (Ed.) Volatile Organic Compounds in the Atmosphere; Wiley: Hoboken, NJ, USA, 2007; ISBN 9781405131155. [Google Scholar]
- Studziński, W.; Gackowska, A.; Kudlek, E. Determination of Environmental Properties and Toxicity of Octyl-Dimethyl-Para-Aminobenzoic Acid and Its Degradation Products. J. Hazard. Mater. 2021, 403, 123856. [Google Scholar] [CrossRef]
- Gackowska, A.; Studziński, W.; Kudlek, E.; Przybyłek, M. Environmental Fate and Ecotoxicity of Diclofenac Degradation Products Generated by Photo-Assisted Advanced Oxidation Processes. J. Hazard. Mater. 2025, 489, 137708. [Google Scholar] [CrossRef]
- Park, H.; Kim, K. Concentrations of 2,4-Dichlorophenol and 2,5-Dichlorophenol in Urine of Korean Adults. Int. J. Environ. Res. Public. Health 2018, 15, 589. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, S.; Haritash, A.K. A Comprehensive Review of Chlorophenols: Fate, Toxicology and Its Treatment. J. Environ. Manag. 2023, 342, 118254. [Google Scholar] [CrossRef]
- Bogdanowicz, N.; Lusina, A.; Nazim, T.; Cegłowski, M. Rapid Quantification of 2,4-Dichlorophenol in River Water Samples Using Molecularly Imprinted Polymers Coupled to Ambient Plasma Mass Spectrometry. J. Hazard. Mater. 2023, 450, 131068. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierek, K.Ś.A.D.L. Removal of 2, 4, 6-Trichlorophenol from Aqueous Solutions Using Agricultural Waste as Low-Cost Adsorbents. Environ. Prot. Eng. 2017, 43, 149–164. [Google Scholar] [CrossRef]
- Hallaj, T.; Amjadi, M. Determination of 2,4-Dichlorophenol in Water Samples Using a Chemiluminescence System Consisting of Graphene Quantum Dots, Rhodamine B and Cerium(IV) Ion. Microchim. Acta 2016, 183, 1219–1225. [Google Scholar] [CrossRef]
- WHO. Chlorophenols in Drinking-Water, Background Document for Preparation of WHO Guidelines for Drinking-Water Quality (WHO/SDE/WSH/03.04/47); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Xu, Z.; Wei, J.; Abid, A.; Liu, Z.; Wu, Y.; Gu, J.; Ma, D.; Zheng, M. Formation and Toxicity Contribution of Chlorinated and Dechlorinated Halobenzoquinones from Dichlorophenols after Ozonation. Sci. Total Environ. 2024, 914, 169860. [Google Scholar] [CrossRef]
- Ju, Z.; Liu, S.-S.; Xu, Y.-Q.; Li, K. Combined Toxicity of 2,4-Dichlorophenoxyacetic Acid and Its Metabolites 2,4-Dichlorophenol (2,4-DCP) on Two Nontarget Organisms. ACS Omega 2019, 4, 1669–1677. [Google Scholar] [CrossRef]
- Otitoju, O.B.; Alfred, M.O.; Ogunlaja, O.O.; Olorunnisola, C.G.; Olukanni, O.D.; Ogunlaja, A.; Omorogie, M.O.; Unuabonah, E.I. Pollution and Risk Assessment of Phenolic Compounds in Drinking Water Sources from South-Western Nigeria. Environ. Sci. Pollut. Res. 2023, 30, 76798–76817. [Google Scholar] [CrossRef]
- Mhlongo, N.L.; Akharame, M.O.; Pereao, O.; Human, I.S.; Opeolu, B.O. Phenolic Compounds Occurrence and Human Health Risk Assessment in Potable and Treated Waters in Western Cape, South Africa. Front. Toxicol. 2024, 5, 1269601. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ferreira, N.G.C.; Saha, N.C. Effects of 2,4,6-Trichlorophenol on Clarias Batrachus: A Biomarkers Approach. Environ. Sci. Pollut. Res. 2022, 29, 47011–47024. [Google Scholar] [CrossRef]
- Available online: https://www.oecd.org/en/topics/assessment-of-chemicals.html (accessed on 9 September 2025).
- Klasmeier, J.; Matthies, M.; Macleod, M.; Fenner, K.; Scheringer, M.; Stroebe, M.; Le Gall, A.C.; Mckone, T.; Van De Meent, D.; Wania, F. Application of Multimedia Models for Screening Assessment of Long-Range Transport Potential and Overall Persistence. Environ. Sci. Technol. 2006, 40, 53–60. [Google Scholar] [CrossRef]
- Graumans, M.H.F.; Hoeben, W.F.L.M.; Ragas, A.M.J.; Russel, F.G.M.; Scheepers, P.T.J. In Silico Ecotoxicity Assessment of Pharmaceutical Residues in Wastewater Following Oxidative Treatment. Environ. Res. 2024, 243, 117833. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Kong, J.; Li, Y.; Xin, X.; Zhou, J.; Zhang, R.; Lee, K.S.; Jin, B.R.; Gui, Z. Comprehensive Analysis of Biotransformation Pathways and Products of Chloramphenicol by Raoultella Ornithinolytica CT3: Pathway Elucidation and Toxicity Assessment. J. Hazard. Mater. 2024, 480, 136199. [Google Scholar] [CrossRef]
- Studziński, W.; Narloch, I.; Dąbrowski, Ł. Determination of the Efficiency of Electrolyzed Water Devices for the Removal of Pesticides in Aqueous Solutions and the Characteristics of the Pesticide Residues and Their Transformation Products. J. Water Process Eng. 2024, 61, 105372. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (US). Toxicological Profile for Chlorophenols; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2022.
- Department of Health Australian Industrial Chemicals Introduction Scheme. Phenol, 2,4-Dichloro-Evaluation Statement; Department of Health: Canberra, Australia, 2022.
- Huff, J. Long-Term Toxicology and Carcinogenicity of 2,4,6-Trichlorophenol. Chemosphere 2012, 89, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Tang, Y.; Wang, H.; Zhu, L.; Zhang, G.; Zhou, Y.; Tang, H. A Catalytic Strategy for Rapid Cleavage of C-Cl Bond under Mild Conditions: Effects of Active Hydrogen Induced by Pd Nanoparticles on the Complete Dechlorination of Chlorobenzenes. Chem. Eng. J. 2021, 419, 129510. [Google Scholar] [CrossRef]
- Beck, U.; Löser, E. Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Available online: http://Chm.Pops.Int/Portals/0/Docs/From_old_website/Documents/Meetings/Bat_bep/1st_session/EGB1_INF10_overview.Pdf (accessed on 9 September 2025).
- Alabdalall, A.H.; Aldakheel, F.A.; Ababutain, I.M.; Chakroun, H.; Alghamdi, A.I.; Hammami, I.; Al Dosary, S.K.; Youssef, T.E.; Albarrag, A.M.; Aldakeel, S.A.; et al. Degradation of 2,6-dicholorophenol by Trichoderma longibraciatum Isolated from an industrial Soil Sample in Dammam, Saudi Arabia. Sci. Rep. 2022, 12, 2940. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, M.; Meng, Q.; Yan, L.; Feng, R.; Zhang, Y.; Fan, D.; Pang, X.; Du, B.; Wei, Q. Aerobic biodegradation of 2,6-dichlorophenol in a nitrifying granular sludge reactor: System performance and microbial community evolution. J. Water Process Eng. 2020, 37, 101524. [Google Scholar] [CrossRef]
- Miles, S.L. Methyl Salicylate. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Oxford, UK, 2011; pp. 1–6. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Ventura, P.; Checa-Fernández, A.; Santos, A. Comprehensive Study of Acute Toxicity Using Microtox® Bioassay in Soils Contaminated by Lindane Wastes. Sci. Total Environ. 2023, 856, 159146. [Google Scholar] [CrossRef]
- Juan-García, A.; Pakkanen, H.; Juan, C.; Vehniäinen, E.-R. Alterations in Daphnia Magna Exposed to Enniatin B and Beauvericin Provide Additional Value as Environmental Indicators. Ecotoxicol. Environ. Saf. 2023, 249, 114427. [Google Scholar] [CrossRef]
- Mkandawire, M.; Teixeira da Silva, J.A.; Dudel, E.G. The Lemna Bioassay: Contemporary Issues as the Most Standardized Plant Bioassay for Aquatic Ecotoxicology. Crit. Rev. Environ. Sci. Technol. 2014, 44, 154–197. [Google Scholar] [CrossRef]
- Available online: https://One.Oecd.Org/Document/ENV/JM/MONO(2004)5/En/Pdf (accessed on 9 September 2025).
- ISO 6341:2012; Water Quality—Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. ISO: Geneva, Switzerland, 2021.
- OECD 202; OECD Guidelines 202 for the Testing of chemicals. Daphnia sp. Acute Immobilisation Test. OECD: Paris, France, 2004.
- Ulm, L.; Vrzina, J.; Schiesl, V.; Puntaric, D.; Smit, Z. Sensitivity Comparison of the Conventional Acute Daphnia Magna Immobilization Test with the Daphtoxkit FTM Microbiotest for Household Products. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Springer: Boston, MA, USA, 2000; pp. 247–252. [Google Scholar]
- Białk-Bielińska, A.; Grabarczyk, Ł.; Mulkiewicz, E.; Puckowski, A.; Stolte, S.; Stepnowski, P. Mixture Toxicity of Six Pharmaceuticals towards Aliivibrio fischeri, Daphnia magna, and Lemna minor. Environ. Sci. Pollut. Res. 2022, 29, 26977–26991. [Google Scholar] [CrossRef] [PubMed]
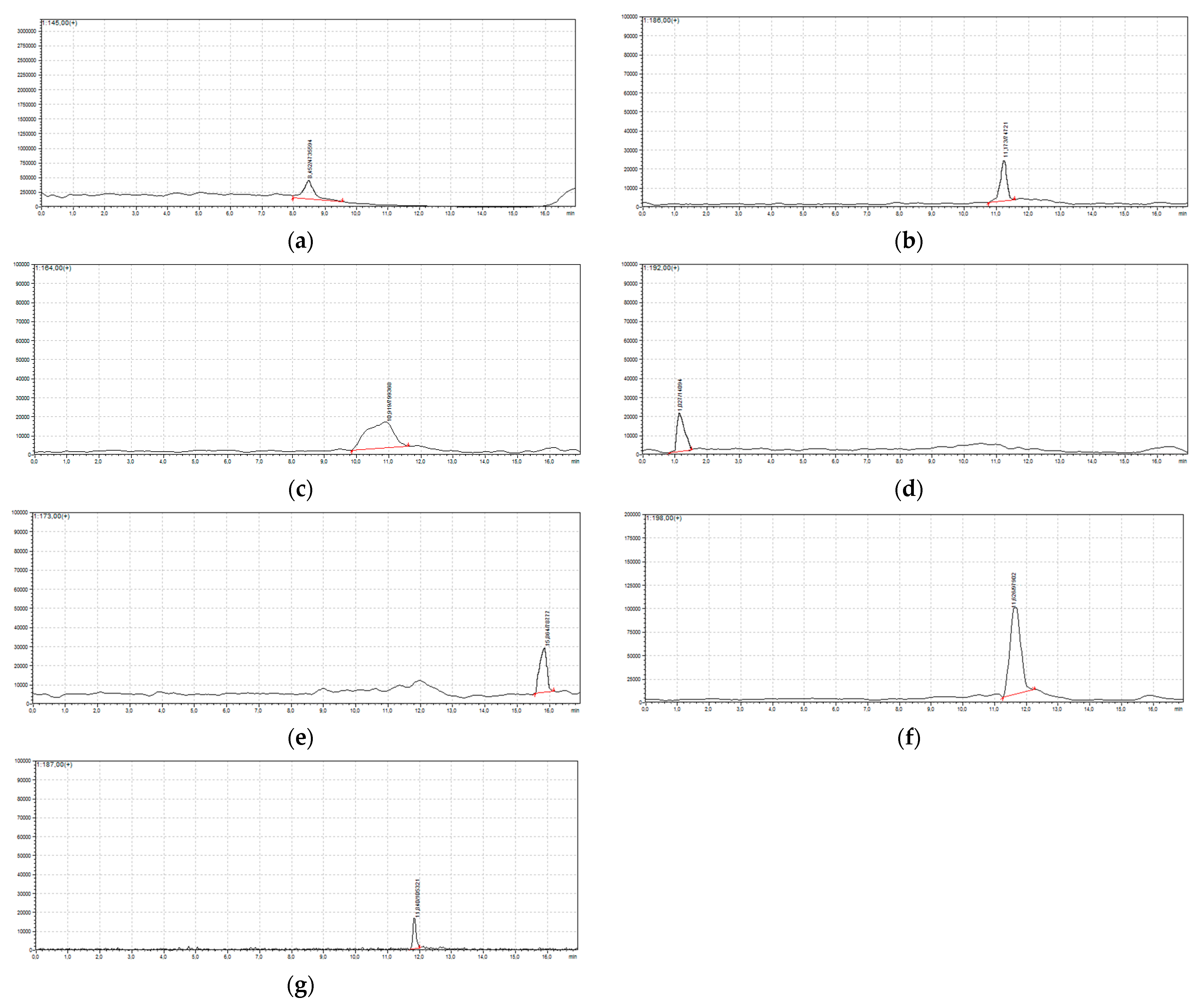

| Retention Time, TR | Compound | Molecular Weight | [M + H]+ |
|---|---|---|---|
| 8.45 | 4-chlorobenzene-1,2-diol | 144.50 | 145 |
| 11.17 | (2-chloro-4-hydroxyphenyl)(oxo)acetaldehyde | 185.63 | 186 |
| 10.91 | trichloroacetic acid | 163.00 | 164 |
| 1.02 | 3,5-dichloro-4-hydroxybenzaldehyde | 191.00 | 192 |
| 15.80 | 5-chloro-2-hydroxybenzoic acid | 172.56 | 173 |
| 15.80 | 3-chloro-2-hydroxybenzoic acid | 172.56 | 173 |
| 11.60 | 2,4,6-trichlorophenol | 197.50 | 198 |
| 10.85 | methyl 5-chlorosalicylate | 186.59 | 187 |
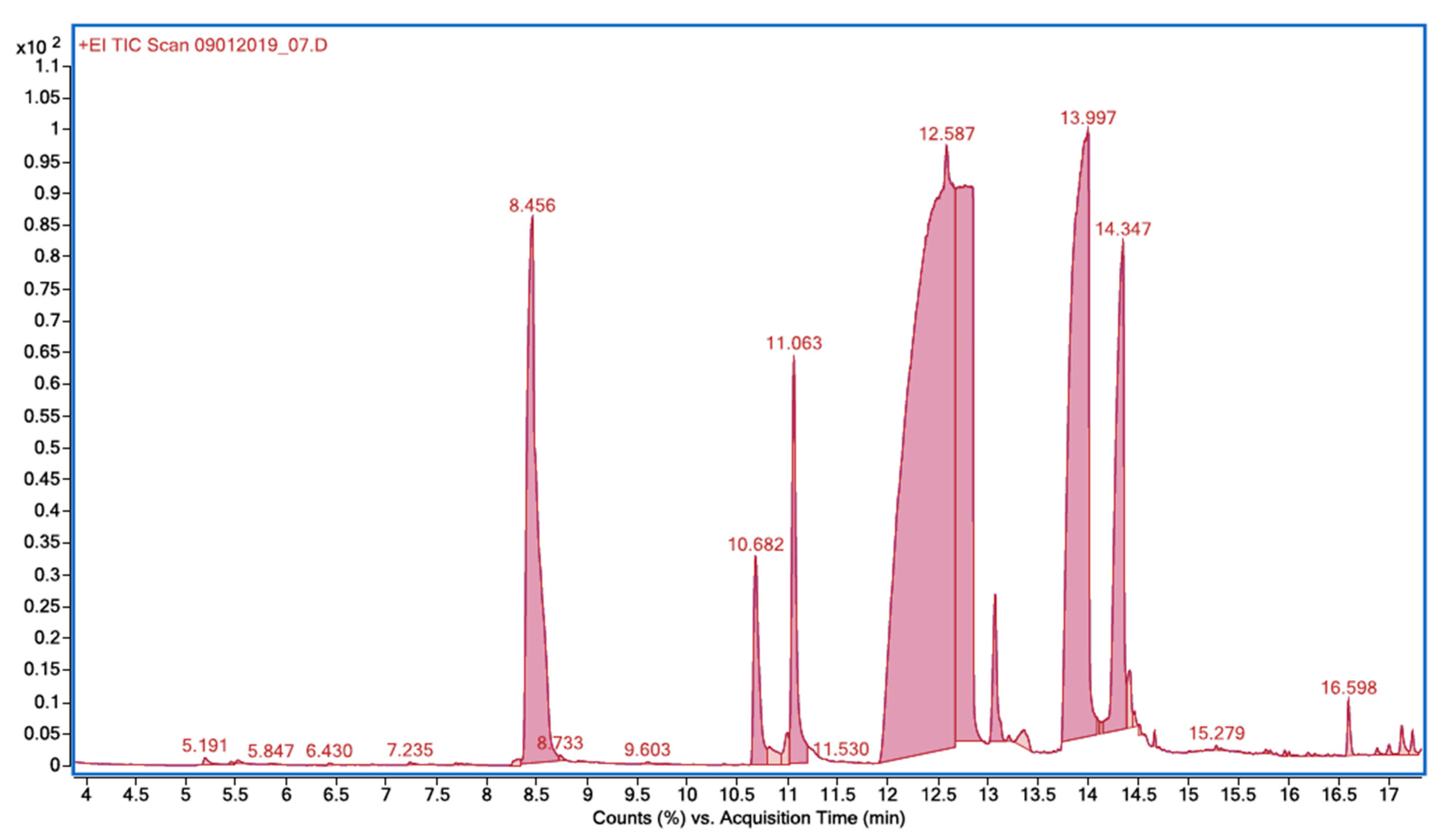
| Retention Time, TR | Compound | Molecular Weight | m/z |
|---|---|---|---|
| 8.456 | 2-chlorophenol | 128.55 | 64, 92, 128, 130 |
| 10.682 | 2,6-dichlorophenol | 163.00 | 63, 98, 126, 162, 164 |
| 11.063 | 2,4-dichlorophenol | 163.00 | 63, 98, 162, 164 |
| 13.997 | 5-chloro-2-hydroxybenzoic acid | 172.56 | 63, 126, 154, 172 |
| 14.347 | 3-chloro-2-hydroxybenzoic acid | 172.56 | 154, 156, 172 |
| 16.598 | methyl 5-chlorosalicylate | 186.59 | 63, 126, 154, 186 |
| Compound | No. TP | BP, °C | MP, °C | VP, mmHg | WS, mg/L | Henry’s LC | Log KOW | Log KAW | Log KOA | Log KOC | BCF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | 298 | 94 | 8.2 × 10−5 | 3808 | 1.5 × 10−9 | 2.24 | −6.5 | 8.8 | 1.6 | 11.9 | |
| 3-chloro-2-hydroxybenzoic acid | TP1 | 320.7 | 114.4 | 2.6 × 10−5 | 775 | 1.1 × 10−8 | 2.89 | −6.4 | 9.3 | 1.9 | 34.9 |
| 5-chloro-2-hydroxybenzoic acid | TP2 | 320.7 | 114.4 | 2.6 × 10−5 | 775 | 1.1 × 10−8 | 2.89 | −6.4 | 9.3 | 1.9 | 34.9 |
| 2-chlorophenol | TP3 | 203.1 | 28.6 | 95.5 | 5165 | 1.2 | 2.16 | −3.3 | 5.5 | 2.3 | 14.1 |
| 2,6-dichlorophenol | TP4 | 233.7 | 46.8 | 3.02 | 1130 | 3.1 × 10−7 | 2.75 | −3.9 | 6.7 | 2.6 | 41.7 |
| 2,4-dichlorophenol | TP5 | 233.7 | 46.8 | 0.0657 | 614.2 | 3.1 × 10−7 | 3.06 | −3.8 | 6.8 | 2.8 | 33.9 |
| Methyl 5-chlorosalicylate | TP6 | 279 | 69.4 | 0.00128 | 327 | 3.4 × 10−6 | 3.25 | −3.9 | 7.1 | 2.8 | 20.9 |
| (2-chloro-4-hydroxyphenyl)(oxo)acetaldehyde | TP7 | 312 | 96.3 | 6.5 × 10−5 | 2.2 × 104 | 4.1 × 10−12 | 1.12 | −9.8 | 10.9 | 1 | 1.9 |
| 4-chlorobenzene-1,2-diol | TP8 | 258.4 | 69.9 | 7.3 × 10−4 | 1.1 × 104 | 4.3 × 10−11 | 1.68 | −8.8 | 10.4 | 2.2 | 2.9 |
| trichloroacetic acid | TP9 | 203.2 | 26.7 | 0.75 | 1.2 × 104 | 1.4 × 10−8 | 1.3 | −6.3 | 7.6 | 0.5 | 3.0 |
| 3,5-dichloro-4-hydroxybenzaldehyde | TP10 | 292.8 | 83.1 | 2.8 × 10−4 | 1.3 × 103 | 7.7 × 10−10 | 2.5 | −7.5 | 10.0 | 1.6 | 25.3 |
| 2,4,6-trichlorophenol | TP11 | 262.1 | 63.8 | 5.4 × 10−3 | 1.2 × 102 | 2.6 × 10−6 | 3.6 | −3.9 | 7.6 | 3.3 | 93.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studziński, W.; Gackowska, A. Sodium Hypochlorite-Assisted Photooxidation of Salicylic Acid: Degradation Kinetics, Formation, and Ecotoxicological Assessment of Intermediates. Int. J. Mol. Sci. 2025, 26, 10063. https://doi.org/10.3390/ijms262010063
Studziński W, Gackowska A. Sodium Hypochlorite-Assisted Photooxidation of Salicylic Acid: Degradation Kinetics, Formation, and Ecotoxicological Assessment of Intermediates. International Journal of Molecular Sciences. 2025; 26(20):10063. https://doi.org/10.3390/ijms262010063
Chicago/Turabian StyleStudziński, Waldemar, and Alicja Gackowska. 2025. "Sodium Hypochlorite-Assisted Photooxidation of Salicylic Acid: Degradation Kinetics, Formation, and Ecotoxicological Assessment of Intermediates" International Journal of Molecular Sciences 26, no. 20: 10063. https://doi.org/10.3390/ijms262010063
APA StyleStudziński, W., & Gackowska, A. (2025). Sodium Hypochlorite-Assisted Photooxidation of Salicylic Acid: Degradation Kinetics, Formation, and Ecotoxicological Assessment of Intermediates. International Journal of Molecular Sciences, 26(20), 10063. https://doi.org/10.3390/ijms262010063







