Therapeutic Potential of Modulating Gene-MicroRNA Crosstalk in Burn Injury
Abstract
1. Introduction
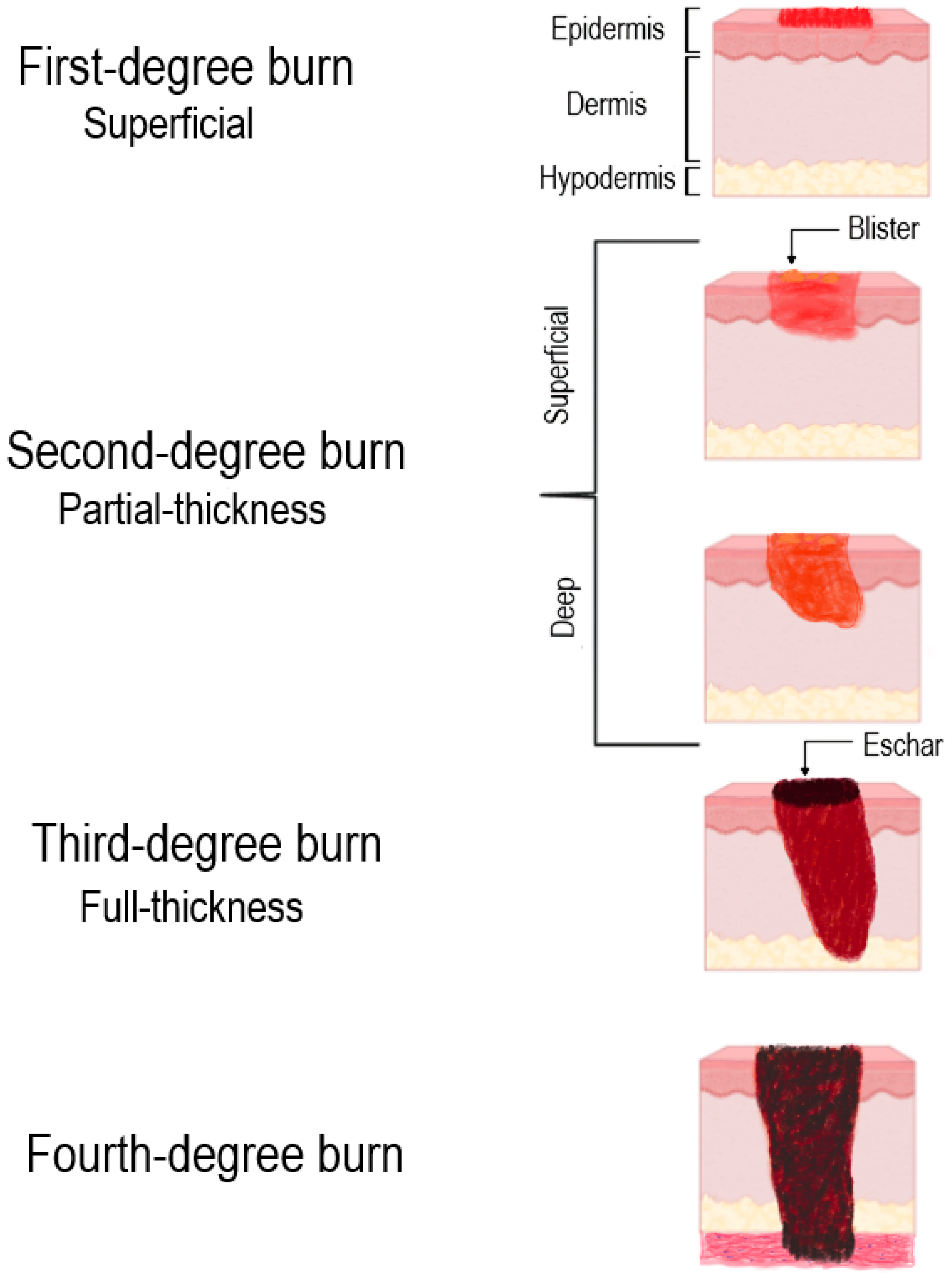
2. Classification of Burn Wounds
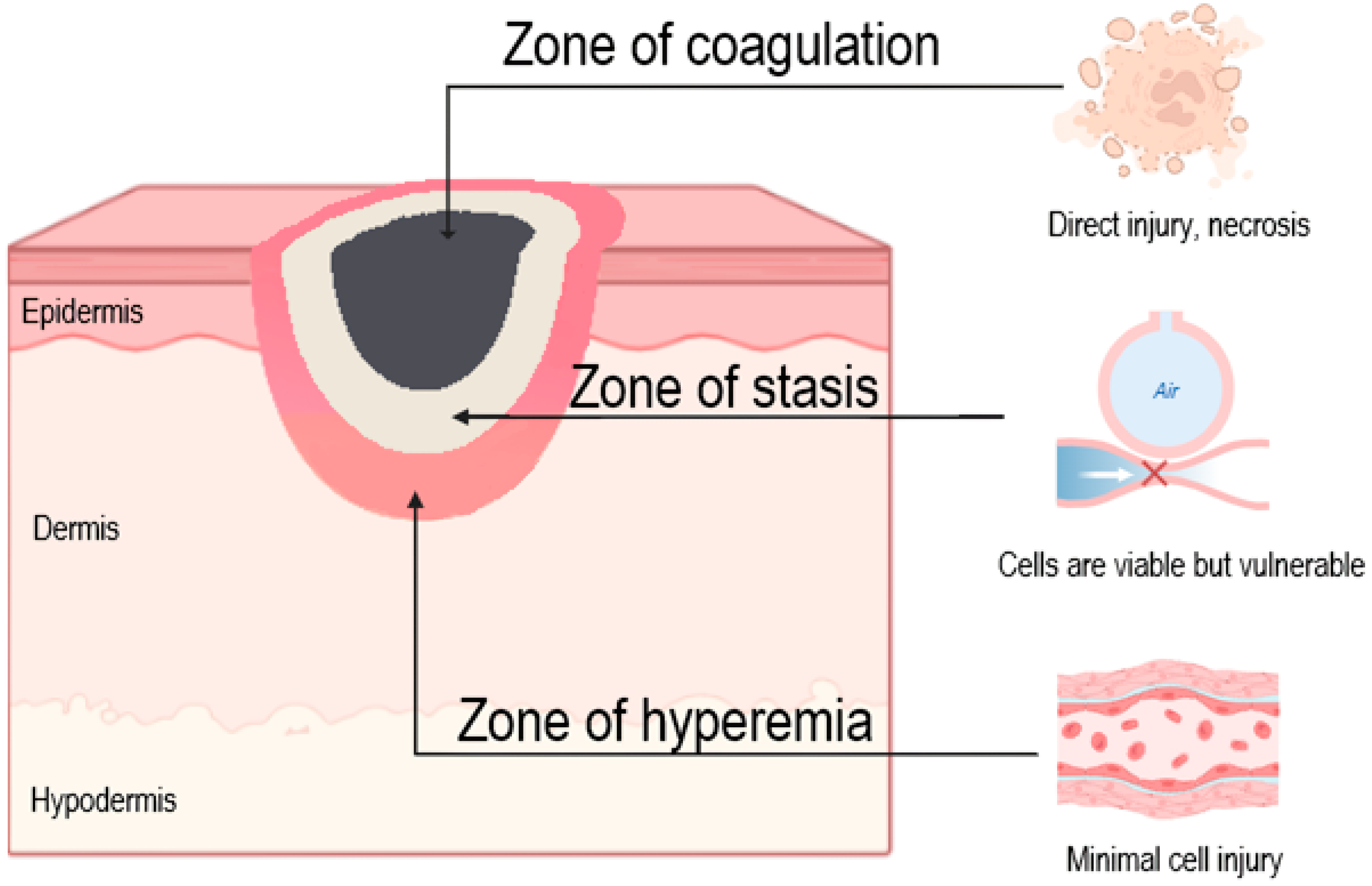
3. Pathogenetic Aspects of Burn Wound Damage
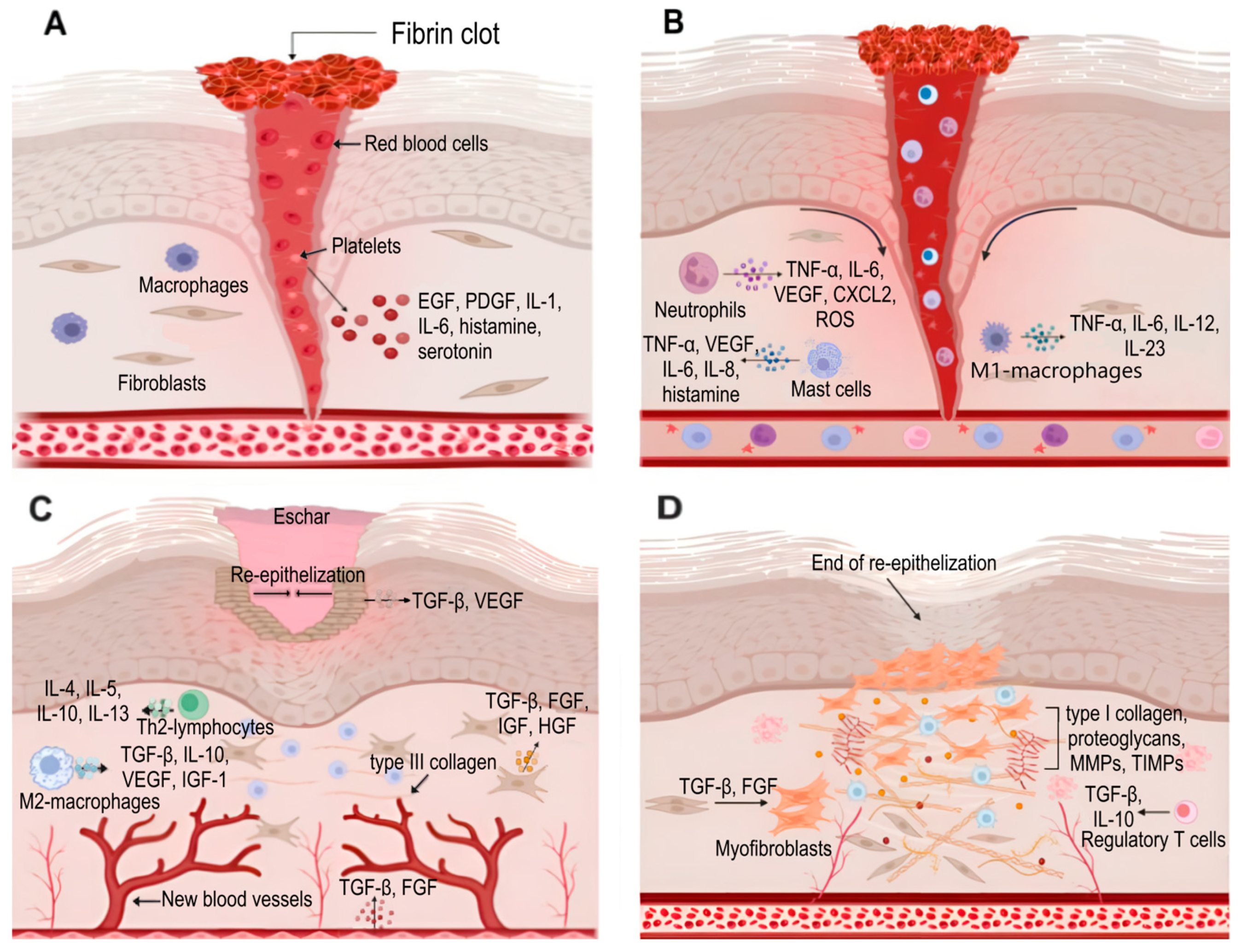
4. Skin Regeneration
5. Current Therapeutic Approaches in Burn Management: Challenges and Limitations
6. Role of MicroRNAs in Burn Wound Pathophysiology
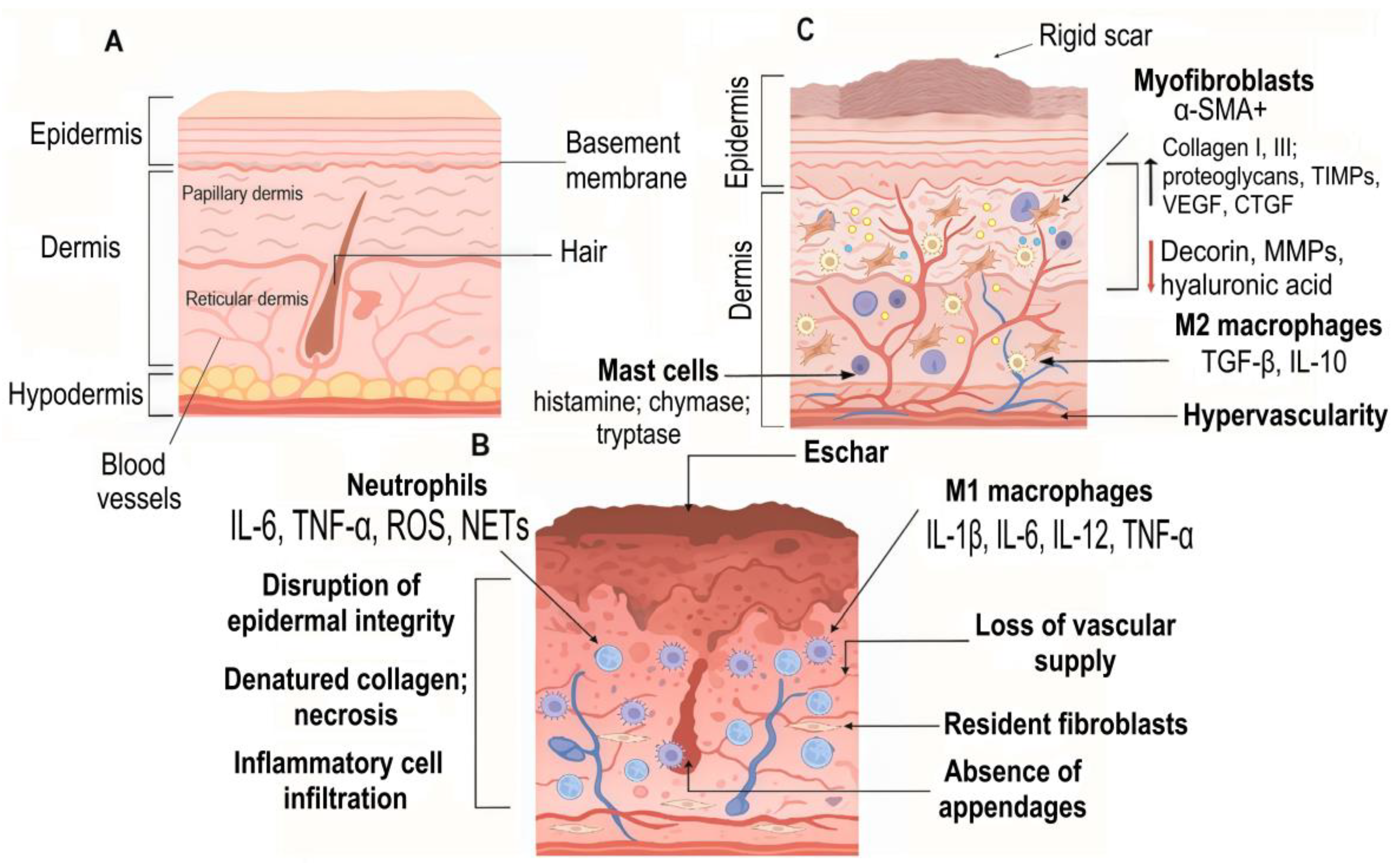
7. MicroRNAs and Scar Formation
8. MicroRNA-Based Therapeutic Strategies for Mitigating Burn Injury Pathogenesis
9. Current Limitations and Future Directions of microRNA Therapy After Burn
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKnight, G.; Shah, J.; Hargest, R. Physiology of the skin. Surgery 2022, 40, 8–12. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Roustit, M. Human Skin Microcirculation. Compr. Physiol. 2020, 10, 1105–1154. [Google Scholar] [CrossRef]
- Rognoni, E.; Watt, F. Skin Cell Heterogeneity in Development, Wound Healing, and Cancer. Trends Cell Biol. 2018, 28, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Potekaev, N.N.; Borzykh, O.B.; Medvedev, G.V.; Pushkin, D.V.; Petrova, M.M.; Petrov, A.V.; Dmitrenko, D.V.; Karpova, E.I.; Demina, O.M.; Shnayder, N.A. The Role of Extracellular Matrix in Skin Wound Healing. J. Clin. Med. 2021, 10, 5947. [Google Scholar] [CrossRef] [PubMed]
- Schmauss, D.; Rezaeian, F.; Finck, T.; Machens, H.-G.; Wettstein, R.; Harder, Y. Treatment of secondary burn wound progression in contact burns—A systematic review of experimental approaches. J. Burn. Care Res. 2015, 36, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Bae, Y.; Jang, S.; Lee, D.W.; Lee, S.W. Global, Regional, and National Burden of Burn Injury by Total Body Surface Area (TBSA) Involvement from 1990 to 2021, with Projections of Prevalence to 2050. Healthcare 2025, 13, 2077. [Google Scholar] [CrossRef]
- Lang, T.C.; Zhao, R.; Kim, A.; Wijewardena, A.; Vandervord, J.; Xue, M.; Jackson, C.J. A Critical Update of the Assessment and Acute Management of Patients with Severe Burns. Adv. Wound Care 2019, 8, 607–633. [Google Scholar] [CrossRef]
- Parry, I.; Bell, J. Associations between burn care services and impairment at discharge after burn injury: Analysis of the Global Burn Registry. Burns 2024, 50, 813–822. [Google Scholar] [CrossRef]
- Dobson, G.P.; Morris, J.L.; Letson, H.L. Pathophysiology of severe burn injuries: New therapeutic opportunities from a systems perspective. J. Burn Care Res. 2024, 45, 1041–1050. [Google Scholar] [CrossRef]
- Siu, M.C.; Voisey, J.; Zang, T.; Cuttle, L. MicroRNAs involved in human skin burns, wound healing and scarring. Wound Repair. Regen. 2023, 31, 439–453. [Google Scholar] [CrossRef]
- Rice, P.L.; Orgill, D. Assessment and classification of burn injury. UpToDate 2021, 8, 129–132. Available online: https://www.uptodate.com/contents/assessment-and-classification-of-burn-injury (accessed on 20 April 2025).
- Żwierełło, W.; Piorun, K.; Skórka-Majewicz, M.; Maruszewska, A.; Antoniewski, J.; Gutowska, I. Burns: Classification, Pathophysiology, and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 3749. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, R.J.; Devi, A.; Hampson, P.; Wearn, C.M.; Bamford, A.L.; Hazeldine, J.; Bishop, J.; Ahmed, S.; Watson, C.; Lord, J.M.; et al. Changes in novel haematological parameters following thermal injury: A prospective observational cohort study. Sci. Rep. 2017, 7, 3211. [Google Scholar] [CrossRef] [PubMed]
- Rae, L.; Fidler, P.; Gibran, N. The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation. Crit. Care Clin. 2016, 32, 491–505. [Google Scholar] [CrossRef]
- Wong She, R.B.; Gibran, N.S. Burn Wound Bed Management. J. Burn Care Res. 2023, 44 (Suppl. S1), 13–18. [Google Scholar] [CrossRef]
- Palackic, A.; Jay, J.W.; Duggan, R.P.; Branski, L.K.; Wolf, S.E.; Ansari, N.; El Ayadi, A. Therapeutic Strategies to Reduce Burn Wound Conversion. Medicina 2022, 58, 922. [Google Scholar] [CrossRef]
- Tiwari, V.K. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhao, J.; Wang, X.; Zhang, J.; Shan, F.; Jiang, D. Research advances in prevention and treatment of burn wound deepening in early stage. Front. Surg. 2022, 9, 1015411. [Google Scholar] [CrossRef]
- Lanham, J.S.; Nelson, N.K.; Hendren, B.; Jordan, T.S. Outpatient burn care: Prevention and treatment. Am. Fam. Physician 2020, 101, 463–470. [Google Scholar] [PubMed]
- Mudgal, R.; Singh, S. Xanthine Oxidoreductase in the Pathogenesis of Endothelial Dysfunction: An Update. Curr. Hypertens. Rev. 2024, 20, 10–22. [Google Scholar] [CrossRef]
- Hellenthal, K.E.M.; Brabenec, L.; Wagner, N.M. Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation. Cells 2022, 11, 1935. [Google Scholar] [CrossRef]
- Foessl, I.; Haudum, C.W.; Vidakovic, I.; Prassl, R.; Franz, J.; Mautner, S.I.; Kainz, S.; Hofmann, E.; Obermayer-Pietsch, B.; Birngruber, T.; et al. miRNAs as Regulators of the Early Local Response to Burn Injuries. Int. J. Mol. Sci. 2021, 22, 9209. [Google Scholar] [CrossRef]
- Barrett, L.W.; Fear, V.S.; Waithman, J.C.; Wood, F.M.; Fear, M.W. Understanding acute burn injury as a chronic disease. Burns Trauma 2019, 7, 23. [Google Scholar] [CrossRef]
- Korkmaz, H.I.; Flokstra, G.; Waasdorp, M.; Pijpe, A.; Papendorp, S.G.; de Jong, E.; Rustemeyer, T.; Gibbs, S.; van Zuijlen, P.P.M. The Complexity of the Post-Burn Immune Response: An Overview of the Associated Local and Systemic Complications. Cells 2023, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.; Wall, B.; Edgar, D.W.; Fairchild, T.; Wood, F. Current understanding of the chronic stress response to burn injury from human studies. Burn. Trauma 2023, 11, tkad007. [Google Scholar] [CrossRef]
- Pi, L.; Fang, B.; Meng, X.; Qian, L. LncRNA XIST accelerates burn wound healing by promoting M2 macrophage polarization through targeting IL-33 via miR-19b. Cell Death Discov. 2022, 8, 220. [Google Scholar] [CrossRef]
- Bohr, S.; Patel, S.J.; Shen, K.; Vitalo, A.G.; Brines, M.; Cerami, A.; Berthiaume, F.; Yarmush, M.L. Alternative erythropoietin-mediated signaling prevents secondary microvascular thrombosis and inflammation within cutaneous burns. Proc. Natl. Acad. Sci. USA 2013, 110, 3513–3518. [Google Scholar] [CrossRef]
- Solomennikov, A.V.; Tyukavin, A.I.; Arseniev, N.A. The Effect of Platelet Degranulation on the Formation of Local Inflammatory Process. J. Med. Biol. Res. 2019, 7, 280289. [Google Scholar] [CrossRef]
- Strudwick, X.L.; Cowin, A.J. The Role of the Inflammatory Response in Burn Injury. In Hot Topics in Burn Injuries; IntechOpen: Rijeka, Croatia, 2018; Volume 3, pp. 38–61. [Google Scholar] [CrossRef]
- Mulder, P.P.G.; Vlig, M.; Boekema, B.K.H.L.; Stoop, M.M.; Pijpe, A.; van Zuijlen, P.P.M.; de Jong, E.; van Cranenbroek, B.; Joosten, I.; Koenen, H.J.P.M.; et al. Persistent Systemic Inflammation in Patients with Severe Burn Injury Is Accompanied by Influx of Immature Neutrophils and Shifts in T Cell Subsets and Cytokine Profiles. Front. Immunol. 2021, 11, 621222. [Google Scholar] [CrossRef]
- Das, P.; Pal, D.; Roy, S.; Chaudhuri, S.; Kesh, S.S.; Basak, P.; Nandi, S.K. Unveiling advanced strategies for therapeutic stem cell interventions in severe burn injuries: A comprehensive review. Int. J. Surg. 2024, 110, 6382–6401. [Google Scholar] [CrossRef] [PubMed]
- Faour, S.; Farahat, M.; Aijaz, A.; Jeschke, M.G. Fibrosis in burns: An overview of mechanisms and therapies. Am. J. Physiol. Cell Physiol. 2023, 325, 1545–1557. [Google Scholar] [CrossRef]
- Oliveira, A.; Simões, S.; Ascenso, A.; Reis, C.P. Therapeutic advances in wound healing. J. Dermatol. Treat. 2022, 33, 2–22. [Google Scholar] [CrossRef]
- Ji, S.; Xiao, S.; Xia, Z. Chinese Burn Association Tissue Repair of Burns and Trauma Committee, Cross-Straits Medicine Exchange Association of China. Consensus on the treatment of second-degree burn wounds (2024 edition). Burn. Trauma 2024, 12, tkad061. [Google Scholar] [CrossRef]
- Radzikowska-Büchner, E.; Łopuszyńska, I.; Flieger, W.; Tobiasz, M.; Maciejewski, R.; Flieger, J. An Overview of Recent Developments in the Management of Burn Injuries. Int. J. Mol. Sci. 2023, 24, 16357. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Chrisman, T.; Simman, R. Clinical indications of cultured epithelial autografts. Ann. Plast. Surg. 2023, 91, 433–440. [Google Scholar] [CrossRef]
- Esmaeili, A.; Soleimani, M.; Rouhani, M.; Noorkhajavi, G.; Aghaei-Zarch, S.M.; Hasannejad-Asl, B.; Bagheri-Mohammadi, S.; Ebrahimi, M.; Keshel, S.H. Xenograft-based skin substitutes: A critical review. J. Drug Deliv. Sci. Technol. 2024, 94, 105613. [Google Scholar] [CrossRef]
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Chang, K.; Nguyen, K.; Galvez, M.G.; Legrand, A.; Davis, C.; McGoldrick, R.; Long, C.; Pham, H.; Chang, J. An Inexpensive Bismuth-Petrolatum Dressing for Treatment of Burns. Plast. Reconstr. Surg. Glob. Open 2016, 4, e737. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef]
- Huynh, P.D. Hydrogel dressings: Revolutionizing burn care with innovative wound healing technology. Biomed. Res. Ther. 2025, 12, 7207–7223. [Google Scholar] [CrossRef]
- Shoham, Y.; Gasteratos, K.; Singer, A.J.; Krieger, Y.; Silberstein, E.; Goverman, J. Bromelain-based enzymatic burn debridement: A systematic review of clinical studies on patient safety, efficacy and long-term outcomes. Int. Wound J. 2023, 20, 4364–4383. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, D.L.; Zhao, Y.Q.; Li, Z.Y. Effectiveness of platelet rich plasma in burn wound healing: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 131–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; He, J.; Dong, J. Growth factor therapy in patients with partial-thickness burns: A systematic review and meta-analysis. Int. Wound J. 2016, 13, 354–366. [Google Scholar] [CrossRef]
- Gupta, S.; Moiemen, N.; Fischer, J.P.; Attinger, C.; Jeschke, M.G.; Taupin, P.; Orgill, D.P. Dermal Regeneration Template in the Management and Reconstruction of Burn Injuries and Complex Wounds: A Review. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5674. [Google Scholar] [CrossRef]
- Wood, F.M. The Role of Cell-Based Therapies in Acute Burn Wound Skin Repair: A Review. J. Burn. Care Res. 2023, 44 (Suppl. S1), S42–S47. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Gu, Z.; Li, W.; Guo, L.; Ma, S.; Guo, L.; Zhang, W.; Han, B.; Chang, J. N-carboxymethyl chitosan/sodium alginate composite hydrogel loading plasmid DNA as a promising gene activated matrix for in-situ burn wound treatment. Bioact. Mater. 2021, 15, 330–342. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Therapeutic advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef]
- Sohel, M.M.H. Circulating microRNAs as biomarkers in cancer diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef]
- Komatsu, S.; Kitai, H.; Suzuki, H.I. Network Regulation of microRNA Biogenesis and Target Interaction. Cells 2023, 12, 306. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, A.K.; Bharti, R.; Kulshrestha, V.; Kalonia, A.; Shaw, P. Can miRNAs Serve as Potential Markers in Thermal Burn Injury: An in silico approach. J. Burn Care Res. 2019, 41, 57–64. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Norata, G.D.; Pinna, C.; Zappella, F.; Elia, L.; Sala, A.; Condorelli, G.; Catapano, A.L. MicroRNA 143-145 deficiency impairs vascular function. Int. J. Immunopathol. Pharmacol. 2012, 25, 467–474. [Google Scholar] [CrossRef]
- Kim, S.; Kang, H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep. 2013, 46, 550–554. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Nguyen, P.H.; Lagna, G.; Hata, A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J. Biol. Chem. 2009, 284, 3728–3738. [Google Scholar] [CrossRef]
- Fort, A.; Borel, C.; Migliavacca, E.; Antonarakis, S.E.; Fish, R.J.; Neerman-Arbez, M. Regulation of fibrinogen production by microRNAs. Blood 2010, 116, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yu, Y.; Wang, C.; Li, D.; Tai, Y.; Fang, L. microRNA-98 mediated microvascular hyperpermeability during burn shock phase via inhibiting FIH-1. Eur. J. Med. Res. 2015, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Ballarino, M.; Sorrentino, A.; Sthandier, O.; De Angelis, F.G.; Marchioni, M.; Masella, B.; Guarini, A.; Fatica, A.; Peschle, C.; et al. The interplay between the master transcription factor PU.1 and miR-424 regulates human monocyte/macrophage differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 19849–19854. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ganesh, K.; Khanna, S.; Sen, C.K.; Roy, S. Engulfment of apoptotic cells by macrophages: A role of microRNA-21 in the resolution of wound inflammation. J. Immunol. 2014, 192, 1120–1129. [Google Scholar] [CrossRef]
- Liu, G.; Friggeri, A.; Yang, Y.; Park, Y.J.; Tsuruta, Y.; Abraham, E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc. Natl. Acad. Sci. USA 2009, 106, 15819–15824. [Google Scholar] [CrossRef]
- Jablonski, K.A.; Gaudet, A.D.; Amici, S.A.; Popovich, P.G.; Guerau-de-Arellano, M. Control of the inflammatory macrophage transcriptional signature by miR-155. PLoS ONE 2016, 11, e0159724. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, M.; Xu, J.; Chen, L.; Li, J.; Li, Q.; Xie, X.; Wang, Q. MiR-711 and miR-183-3p as Potential Markers for Vital Reaction of Burned Skin. Forensic Sci. Res. 2022, 7, 503–509. [Google Scholar] [CrossRef]
- Li, D.; Wang, A.; Liu, X.; Meisgen, F.; Grünler, J.; Botusan, I.R.; Narayanan, S.; Erikci, E.; Li, X.; Blomqvist, L.; et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Investig. 2015, 125, 3008–3026. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Liu, J.; Wang, X.; Sun, F. Downregulation of miR-27b promotes skin wound healing in a rat model of scald burn by promoting fibroblast proliferation. Exp. Ther. Med. 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Tang, Y.; Wang, H.; Chen, C.; Yu, W.; Sun, H.; Duan, M.; Lin, X.; Liang, P. Down-regulation of long non-coding RNA HOTAIR promotes angiogenesis via regulating miR-126/SCEL pathways in burn wound healing. Cell Death Dis. 2020, 11, 61. [Google Scholar] [CrossRef]
- Wang, T.; Feng, Y.; Sun, H.; Zhang, L.; Hao, L.; Shi, C.; Wang, J.; Li, R.; Ran, X.; Su, Y.; et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am. J. Pathol. 2012, 181, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.I.; Wang, A.; Meisgen, F.; Pivarcsi, A.; Sonkoly, E.; Ståhle, M.; Landén, N.X. MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. J. Investig. Dermatol. 2015, 135, 1676–1685. [Google Scholar] [CrossRef]
- Sundaram, G.M.; Common, J.E.; Gopal, F.E.; Srikanta, S.; Lakshman, K.; Lunny, D.P.; Lim, T.C.; Tanavde, V.; Lane, E.B.; Sampath, P. ‘See-saw’ expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature 2013, 495, 103–106. [Google Scholar] [CrossRef]
- Jin, Y.; Tymen, S.D.; Chen, D.; Fang, Z.J.; Zhao, Y.; Dragas, D.; Dai, Y.; Marucha, P.T.; Zhou, X. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS ONE 2013, 8, e64434. [Google Scholar] [CrossRef]
- Ghatak, S.; Li, J.; Chan, Y.C.; Gnyawali, S.C.; Steen, E.; Yung, B.C.; Khanna, S.; Roy, S.; Lee, R.J.; Sen, C.K. AntihypoxamiR functionalized gramicidin lipid nanoparticles rescue against ischemic memory improving cutaneous wound healing. Nanomedicine 2016, 12, 1827–1831. [Google Scholar] [CrossRef]
- Chan, Y.C.; Roy, S.; Huang, Y.; Khanna, S.; Sen, C.K. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J. Biol. Chem. 2012, 287, 41032–41043. [Google Scholar] [CrossRef]
- Pastar, I.; Khan, A.A.; Stojadinovic, O.; Lebrun, E.A.; Medina, M.C.; Brem, H.; Kirsner, R.S.; Jimenez, J.J.; Leslie, C.; Tomic-Canic, M. Induction of specific microRNAs inhibits cutaneous wound healing. J. Biol. Chem. 2012, 287, 29324–29335. [Google Scholar] [CrossRef] [PubMed]
- Viticchiè, G.; Lena, A.M.; Cianfarani, F.; Odorisio, T.; Annicchiarico-Petruzzelli, M.; Melino, G.; Candi, E. MicroRNA-203 contributes to skin re-epithelialization. Cell Death Dis. 2012, 3, e435. [Google Scholar] [CrossRef]
- Chan, Y.C.; Khanna, S.; Roy, S.; Sen, C.K. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J. Biol. Chem. 2011, 286, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Truongcao, M.M.; Mallaredy, V.; Cimini, M.; Thej, C.; Joladarashi, D.; Gonzalez, C.; Benedict, C.; Verma, S.K.; Garikipati, V.N.S.; et al. Muscle-specific miR-499-5p delivered by small extracellular vesicles impairs endothelial function and ischemic hindlimb recovery in diabetic mice. Cardiovasc. Diabetol. 2025, 24, 273. [Google Scholar] [CrossRef]
- Wu, S.G.; Li, H.T.; Wang, L.L.; Yan, L. Lidocaine promotes fibroblast proliferation after thermal injury via up-regulating the expression of miR-663 and miR-486. Kaohsiung J. Med. Sci. 2020, 36, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Zhao, J.; Zhang, P.; Huang, X. MicroRNA-23b Inhibits the Proliferation and Migration of Heat-Denatured Fibroblasts by Targeting Smad3. PLoS ONE 2015, 10, e0131867. [Google Scholar] [CrossRef]
- Lü, M.H.; Hu, C.J.; Chen, L.; Peng, X.; Chen, J.; Hu, J.Y.; Teng, M.; Liang, G.P. miR-27b represses migration of mouse MSCs to burned margins and prolongs wound repair through silencing SDF-1a. PLoS ONE 2013, 8, e68972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, X.; Wu, W.; Zhang, F.; Wu, S. Let-7c miRNA Inhibits the Proliferation and Migration of Heat-Denatured Dermal Fibroblasts Through Down-Regulating HSP70. Mol. Cells 2016, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shi, K.; Zhang, X.; Zhao, J.; Yu, J. MiR-1908 promotes scar formation post-burn wound healing by suppressing Ski-mediated inflammation and fibroblast proliferation. Cell Tissue Res. 2016, 366, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Feng, Y. LncRNA XIST promotes extracellular matrix synthesis, proliferation and migration by targeting miR-29b-3p/COL1A1 in human skin fibroblasts after thermal injury. Biol. Res. 2019, 52, 52. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Wang, Y.; Li, L.; Wang, D.; Zhang, W.; Liu, L.; Jiang, H.; Yang, J.; Cheng, J. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl. Res. 2016, 178, 38–53. [Google Scholar] [CrossRef]
- Gras, C.; Ratuszny, D.; Hadamitzky, C.; Zhang, H.; Blasczyk, R.; Figueiredo, C. miR-145 Contributes to Hypertrophic Scarring of the Skin by Inducing Myofibroblast Activity. Mol. Med. 2015, 21, 296–304. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, X.; Feng, D.; Han, J.; Hu, D. MicroRNA let-7d attenuates hypertrophic scar fibrosis through modulation of iron metabolism by reducing DMT1 expression. J. Mol. Histol. 2023, 54, 77–87. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Pan, X.; Huang, X.; Yang, L.; Xue, Y. miR-506-3p regulates TGF- 1 and affects dermal fibroblast proliferation, migration and collagen formation after thermal injury. Tissue Cell 2021, 72, 101548. [Google Scholar] [CrossRef]
- Manasyan, A.; Stanton, E.W.; Malkoff, N.; Cannata, B.; Wallace, L.G.; Gillenwater, T.J. Use of platelet-rich plasma and platelet-rich fibrin in burn wound healing and skin grafting: A systematic review. Eur. J. Plast. Surg. 2024, 47, 59. [Google Scholar] [CrossRef]
- Sunderland, N.; Skroblin, P.; Barwari, T.; Huntley, R.P.; Lu, R.; Joshi, A.; Lovering, R.C.; Mayr, M. MicroRNA Biomarkers and Platelet Reactivity: The Clot Thickens. Circ. Res. 2017, 120, 418–435. [Google Scholar] [CrossRef]
- Zhang, L.J.; Hu, Y.X.; Huang, R.Z.; Xu, Y.Y.; Dong, S.H.; Guo, F.H.; Guo, J.J.; Qiu, J.J.; Cao, Z.Y.; Wei, L.J.; et al. Intraplatelet miRNA-126 regulates thrombosis and its reduction contributes to platelet inhibition. Cardiovasc. Res. 2024, 120, 1622–1635. [Google Scholar] [CrossRef]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217, 447–457. [Google Scholar] [CrossRef]
- Glémain, A.; Néel, M.; Néel, A.; André-Grégoire, G.; Gavard, J.; Martinet, B.; Le Bloas, R.; Riquin, K.; Hamidou, M.; Fakhouri, F.; et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J. Autoimmun. 2022, 129, 102826. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, X.P.; Luoreng, Z.M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.W. miR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int. J. Biol. Sci. 2021, 17, 2308–2322. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Gu, W.; Yao, L.; Li, L.; Zhang, J.; Place, A.T.; Minshall, R.D.; Liu, G. ICAM-1 regulates macrophage polarization by suppressing MCP-1 expression via miR-124 upregulation. Oncotarget 2017, 8, 111882–111901. [Google Scholar] [CrossRef]
- Dewberry, L.C.; Niemiec, S.M.; Hilton, S.A.; Louiselle, A.E.; Singh, S.; Sakthivel, T.S.; Hu, J.; Seal, S.; Liechty, K.W.; Zgheib, C. Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomedicine 2022, 40, 102483. [Google Scholar] [CrossRef] [PubMed]
- Luly, F.R.; Lévêque, M.; Licursi, V.; Cimino, G.; Martin-Chouly, C.; Théret, N.; Negri, R.; Cavinato, L.; Ascenzioni, F.; Del Porto, P. MiR-146a is over-expressed and controls IL-6 production in cystic fibrosis macrophages. Sci. Rep. 2019, 9, 16259. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Liu, Y.; Su, Y.; Xie, Y.; Du, J.; Wang, S.; Wang, H.; Liu, Y. MicroRNA-21 promotes wound healing via the Smad7-Smad2/3-Elastin pathway. Exp. Cell Res. 2018, 362, 245–251. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Li, W.D.; Lei, F.R.; Li, X.Q. The regulatory role of microRNAs in angiogenesis-related diseases. J. Cell. Mol. Med. 2018, 22, 4568–4587. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, O.; Mierzejewski, B.; Brzoska, E. MicroRNA-126: A key regulator of angiogenesis, inflammation, and tumorigenesis—Exploring its multifaceted functions in vascular health and cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167984. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Pal, A.K.; Roy, S.; Nandave, M.; Jain, K. Role of Angiogenesis and Its Biomarkers in Development of Targeted Tumor Therapies. Stem Cells Int. 2024, 2024, 9077926. [Google Scholar] [CrossRef]
- Chan, Y.C.; Roy, S.; Khanna, S.; Sen, C.K. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1372–1382. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Li, C.; Zhao, D.; Li, S.; Ma, L.; Cui, Y.; Wei, X.; Zhao, Y.; Gao, Y. MicroRNA-92a promotes vascular smooth muscle cell proliferation and migration through the ROCK/MLCK signalling pathway. J. Cell Mol. Med. 2019, 23, 3696–3710. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, Y.; Han, Y.; Wang, M.; Huang, Y. MiR-223-3p Expression in Deep Second-Degree Burn and its Role in the Wound Healing Process. J. Burn Care Res. 2025, 46, iraf083. [Google Scholar] [CrossRef]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Liang, P.; Ren, L.; Zeng, J.; Zhang, M.; Zhang, P.; Huang, X. Protective role of microRNA-29a in denatured dermis and skin fibroblast cells after thermal injury. Biol. Open 2016, 5, 211–219. [Google Scholar] [CrossRef]
- Pehrsson, M.; Mortensen, J.H.; Manon-Jensen, T.; Bay-Jensen, A.-C.; Karsdal, M.A.; Davies, M.J. Enzymatic cross-linking of collagens in organ fibrosis—Resolution and assessment. Expert Rev. Mol. Diagn. 2021, 21, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Basson, R.; Bayat, A. Skin scarring: Latest update on objective assessment and optimal management. Front. Med. 2022, 9, 942756. [Google Scholar] [CrossRef]
- Xie, F.; Teng, L.; Xu, J.; Lu, J.; Zhang, C.; Yang, L.; Ma, X.; Zhao, M. Adipose-derived mesenchymal stem cells inhibit cell proliferation and migration and suppress extracellular matrix synthesis in hypertrophic-scar and keloid fibroblasts. Exp. Ther. Med. 2021, 21, 139. [Google Scholar] [CrossRef]
- Rang, Z.; Wang, Z.Y.; Pang, Q.Y.; Wang, Y.W.; Yang, G.; Cui, F. MiR-181a Targets PHLPP2 to Augment AKT Signaling and Regulate Proliferation and Apoptosis in Human Keloid Fibroblasts. Cell Physiol. Biochem. 2016, 40, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guo, Z.; Gao, W. miR-181b-5p promotes proliferation and inhibits apoptosis of hypertrophic scar fibroblasts through regulating the MEK/ERK/p21 pathway. Exp. Ther. Med. 2019, 17, 1537–1544. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Zhang, J.; Zhao, D. MicroRNA-497-5p downregulation inhibits cell viability, reduces extracellular matrix deposition and induces apoptosis in human hyperplastic scar fibroblasts by regulating Smad7. Exp. Ther. Med. 2021, 21, 384. [Google Scholar] [CrossRef]
- Li, F.; Wan, D.W.; Hu, J.; Qin, R. Effect of artificial skin membrane on the expression of miR-155 and miR-506-3p in patients with second-degree burns. J. Clin. Lab. Anal. 2022, 36, e24564. [Google Scholar] [CrossRef]
- Yi, F.; Hao, Y.; Chong, X.; Zhong, W. Overexpression of microRNA-506-3p aggravates the injury of vascular endothelial cells in patients with hypertension by downregulating Beclin1 expression. Exp. Ther. Med. 2018, 15, 2844–2850. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Zhang, Y.; Fu, S.; Wang, C. Aberrant miR-21 and miR-200b expression and its pro-fibrotic potential in hypertrophic scars. Exp. Cell Res. 2015, 339, 360–366. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Li, C.; Bai, W.D.; Su, L.L.; Liu, J.Q.; Li, Y.; Shi, J.H.; Cai, W.X.; Bai, X.Z.; Jia, Y.H.; et al. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PLoS ONE 2014, 9, e97114. [Google Scholar] [CrossRef]
- Chen, L.; Simões, A.; Chen, Z.; Zhao, Y.; Wu, X.; Dai, Y.; DiPietro, L.A.; Zhou, X. Overexpression of the oral mucosa-specific microRNA-31 promotes skin wound closure. Int. J. Mol. Sci. 2019, 20, 3679. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, K.A.; Chan, S.Y.; Loscalzo, J. Hypoxamirs and mitochondrial metabolism. Antioxid. Redox Signal 2014, 21, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Loscalzo, J. MicroRNA-210: A unique and pleiotropic hypoxamir. Cell Cycle 2010, 9, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Zhang, Y.Y.; Hemann, C.; Mahoney, C.E.; Zweier, J.L.; Loscalzo, J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009, 10, 273–284. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, X.C.; Wang, J.J.; Hong, X.D.; Wang, G.Y.; Xia, Z.F. Burn-related dysregulation of inflammation and immunity in experimental and clinical studies. J. Burn Care Res. 2017, 38, e892–e899. [Google Scholar] [CrossRef] [PubMed]
- Jibing, C.; Weiping, L.; Yuwei, Y.; Bingzheng, F.; Zhiran, X. Exosomal microRNA-Based therapies for skin diseases. Regen. Ther. 2023, 25, 101–112. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Wang, A.; Chu, T.; Lohcharoenkal, W.; Zheng, X.; Grünler, J.; Narayanan, S.; Eliasson, S.; Herter, E.K.; et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J. Investig. Dermatol. 2017, 137, 2630–2638. [Google Scholar] [CrossRef]
- Yang, L.L.; Liu, J.Q.; Bai, X.Z.; Fan, L.; Han, F.; Jia, W.B.; Su, L.L.; Shi, J.H.; Tang, C.W.; Hu, D.H. Acute downregulation of miR-155 at wound sites leads to a reduced fibrosis through attenuating inflammatory response. Biochem. Biophys. Res. Commun. 2014, 453, 153–159. [Google Scholar] [CrossRef]
- Liu, J.; Sun, F.; Wang, X.; Bi, Q. miR-27b promotes angiogenesis and skin repair in scalded rats through regulating VEGF-C expression. Lasers Med. Sci. 2020, 35, 1577–1588. [Google Scholar] [CrossRef]
- Radmanesh, F.; Sadeghi Abandansari, H.; Ghanian, M.H.; Pahlavan, S.; Varzideh, F.; Yakhkeshi, S.; Alikhani, M.; Moradi, S.; Braun, T.; Baharvand, H. Hydrogel-Mediated Delivery of MicroRNA-92a Inhibitor Polyplex Nanoparticles Induces Localized Angiogenesis. Angiogenesis 2021, 24, 657–676. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Yang, X.; Bai, X.; Shi, J.; Gao, J.; Li, Y.; Han, S.; Zhang, Y.; Han, F.; et al. miR-155 inhibits the formation of hypertrophic scar fibroblasts by targeting HIF-1α via PI3K/AKT pathway. J. Mol. Histol. 2018, 49, 377–387. [Google Scholar] [CrossRef]
- Fedorova, O.; Parfenyev, S.; Daks, A.; Shuvalov, O.; Barlev, N.A. The Role of PTEN in Epithelial-Mesenchymal Transition. Cancers 2022, 14, 3786. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef]
- Shi, H.; Wang, M.; Sun, Y.; Yang, D.; Xu, W.; Qian, H. Exosomes: Emerging Cell-Free Based Therapeutics in Dermatologic Diseases. Front. Cell Dev. Biol. 2021, 9, 736022. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Quiñones-Vico, M.I.; Sanabria-de la Torre, R.; Sánchez-Díaz, M.; Sierra-Sánchez, Á.; Montero-Vílchez, T.; Fernández-González, A.; Arias-Santiago, S. The Role of Exosomes Derived From Mesenchymal Stromal Cells in Dermatology. Front. Cell Dev. Biol. 2021, 9, 647012. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, J.; Zhang, M.; Chen, Y.; Wang, X.; Huang, M.; Zhang, L. Effect of iPSCs-derived keratinocytes on healing of full-thickness skin wounds in mice. Exp. Cell Res. 2019, 385, 111627. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, L.; Liu, B.; Tian, G.; Li, C.; Zhang, L.; Yan, Y. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J. Nanobiotechnol. 2022, 20, 291. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ishii, T.; Otani, T.; Inai, Y.; Matsuura, T.; Inai, T. JNK inhibition enhances cell–cell adhesion impaired by desmoglein 3 gene disruption in keratinocytes. Histochem. Cell Biol. 2024, 161, 345–357. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, R.; Bo, Y.; Zhang, M.; Chen, Y.; Wang, X.; Huang, M.; Liu, B.; Zhang, L. Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration. Theranostics 2020, 10, 9970–9983. [Google Scholar] [CrossRef]
- Badoiu, S.C.; Miricescu, D.; Stanescu-Spinu, I.I.; Ripszky Totan, A.; Badoiu, S.E.; Costagliola, M.; Greabu, M. Glucose Metabolism in Burns-What Happens? Int. J. Mol. Sci. 2021, 22, 5159. [Google Scholar] [CrossRef]
- Yu, Y.; Chai, J.; Zhang, H.; Chu, W.; Liu, L.; Ma, L.; Duan, H.; Li, B.; Li, D. miR-194 Promotes burn-induced hyperglycemia via attenuating IGF-IR expression. Shock 2014, 42, 578–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, B.; Shu, B.; Liu, Z.; Ding, H.; Jia, C. Differential expression of microRNA let-7b-5p regulates burn-induced hyperglycemia. Oncotarget 2017, 8, 72886–72892. [Google Scholar] [CrossRef]
- Ray, J.J.; Meizoso, J.P.; Allen, C.J.; Teisch, L.F.; Yang, E.Y.; Foong, H.Y.; Mundra, L.S.; Namias, N.; Pizano, L.R.; Schulman, C.I. Admission Hyperglycemia Predicts Infectious Complications After Burns. J. Burn Care Res. 2017, 38, 85–89. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.A.; Martins, P.N. RNA interference therapeutics in organ transplantation: The dawn of a new era. Am. J. Transplant. 2020, 20, 931–941. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Noferesti, L.; Hussen, B.M.; Moghadam, M.H.B.; Taheri, M.; Rashnoo, F. Nanoparticle-mediated delivery of microRNAs-based therapies for treatment of disorders. Pathol. Res. Pract. 2023, 248, 154667. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, J.; Zhao, Y.; Yan, X.; Zhang, L.; Choy, K.; Hu, J.; Sant, H.J.; Gale, B.K.; Tang, T. Transdermal Delivery of siRNA through Microneedle Array. Sci. Rep. 2016, 6, 21422. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Malik, G.; Gulati, D.; Kaushik, P.; Arora, S. Formulation and evaluation of fusidic acid based transferosome for burn wound infection. Mater. Today Proc. 2022, 68, 836–841. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, W.; Zhong, W.; Xi, C.; Ye, J.; Zhang, Q.; Wu, H.; Du, S. Study of the skin-penetration promoting effect and mechanism of combined system of curcumin liposomes prepared by microfluidic chip and skin penetrating peptides TD-1 for topical treatment of primary melanoma. Int. J. Pharm. 2023, 643, 123256. [Google Scholar] [CrossRef]
- Ullah, I.; Ullah, I.; Aman, S.; Siraj, S.; Rehmat, S.; Ullah, A.; Ullah, N. Nanoparticle-infused wound dressings: A novel alternative to injectable therapies for enhanced healing and drug delivery. Nanomedicine 2025, 69, 102852, Advance online publication. [Google Scholar] [CrossRef]
- Li, J.; Ghatak, S.; El Masry, M.S.; Das, A.; Liu, Y.; Roy, S.; Lee, R.J.; Sen, C.K. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound. Mol. Ther. 2018, 26, 2178–2188. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair. Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, S.; Fullard, N.; Roger, M.; Degnan, A.; Todryk, S.; Przyborski, S.; O’Reilly, S. MicroRNAs in the skin: Role in development, homoeostasis and regeneration. Clin. Sci. 2017, 131, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Żwierełło, W.; Styburski, D.; Maruszewska, A.; Piorun, K.; Skórka-Majewicz, M.; Czerwińska, M.; Maciejewska, D.; Baranowska-Bosiacka, I.; Krajewski, A.; Gutowska, I. Bioelements in the treatment of burn injuries—The complex review of metabolism and supplementation (copper, selenium, zinc, iron, manganese, chromium and magnesium). J. Trace Elem. Med. Biol. 2020, 62, 126616. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.M.; Mageed, S.S.A.; Awad, F.A.; Sadek, M.M.; Adel, S.A.; Ashraf, A.; Alam-Eldein, K.M.; Ahmed, N.E.; Abdelaziz, R.Y.; Tolba, E.F.; et al. miRNAs and their multifaceted role in cutaneous wound healing. Funct. Integr. Genom. 2025, 25, 33. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Deka Dey, A.; Yousefiasl, S.; Kumar, A.; Dabbagh Moghaddam, F.; Rahimmanesh, I.; Samandari, M.; Jamwal, S.; Maleki, A.; Mohammadi, A.; Rabiee, N.; et al. miRNA-encapsulated abiotic materials and biovectors for cutaneous and oral wound healing: Biogenesis, mechanisms, and delivery nanocarriers. Bioeng. Transl. Med. 2022, 8, e10343. [Google Scholar] [CrossRef]
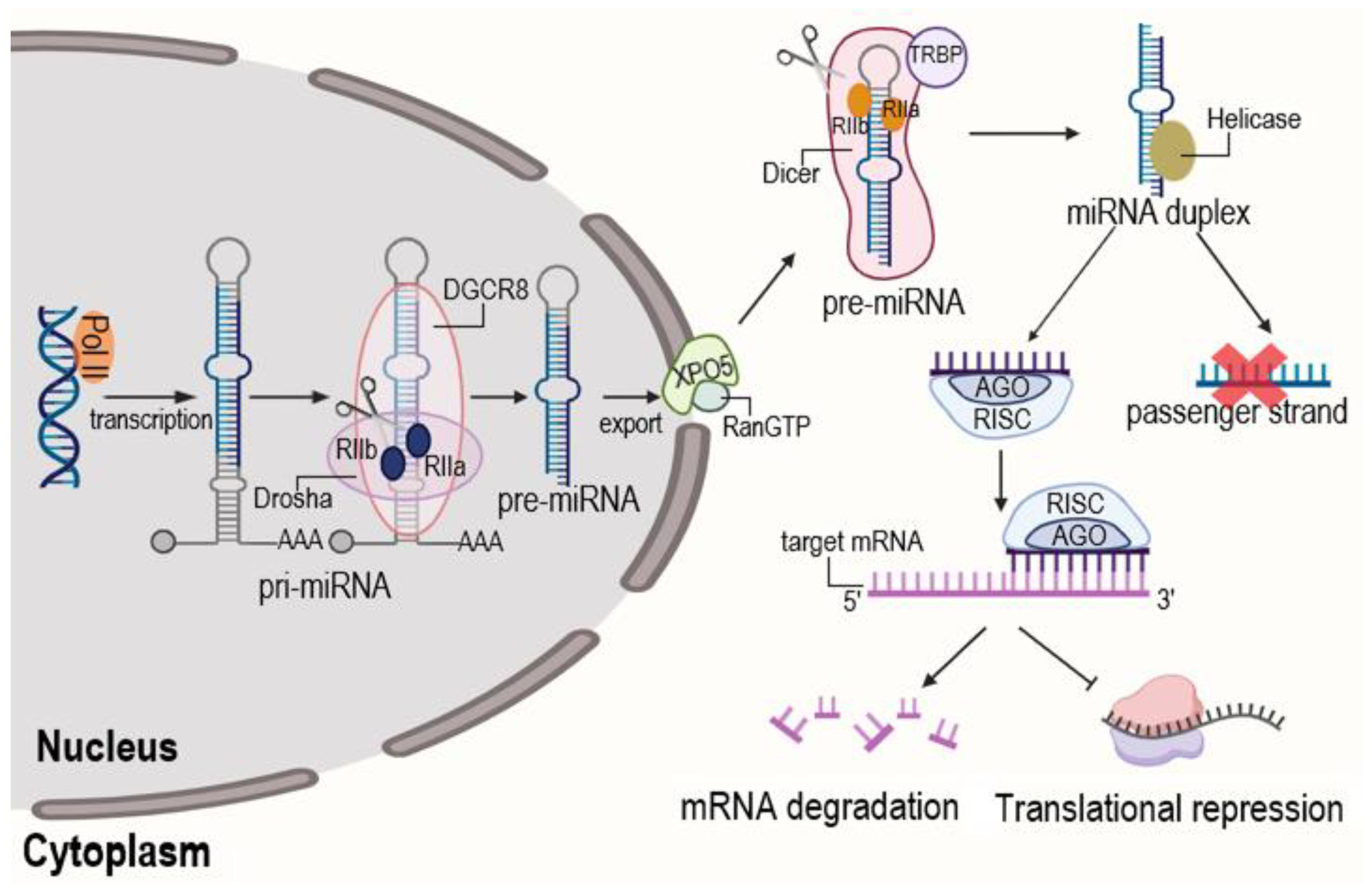
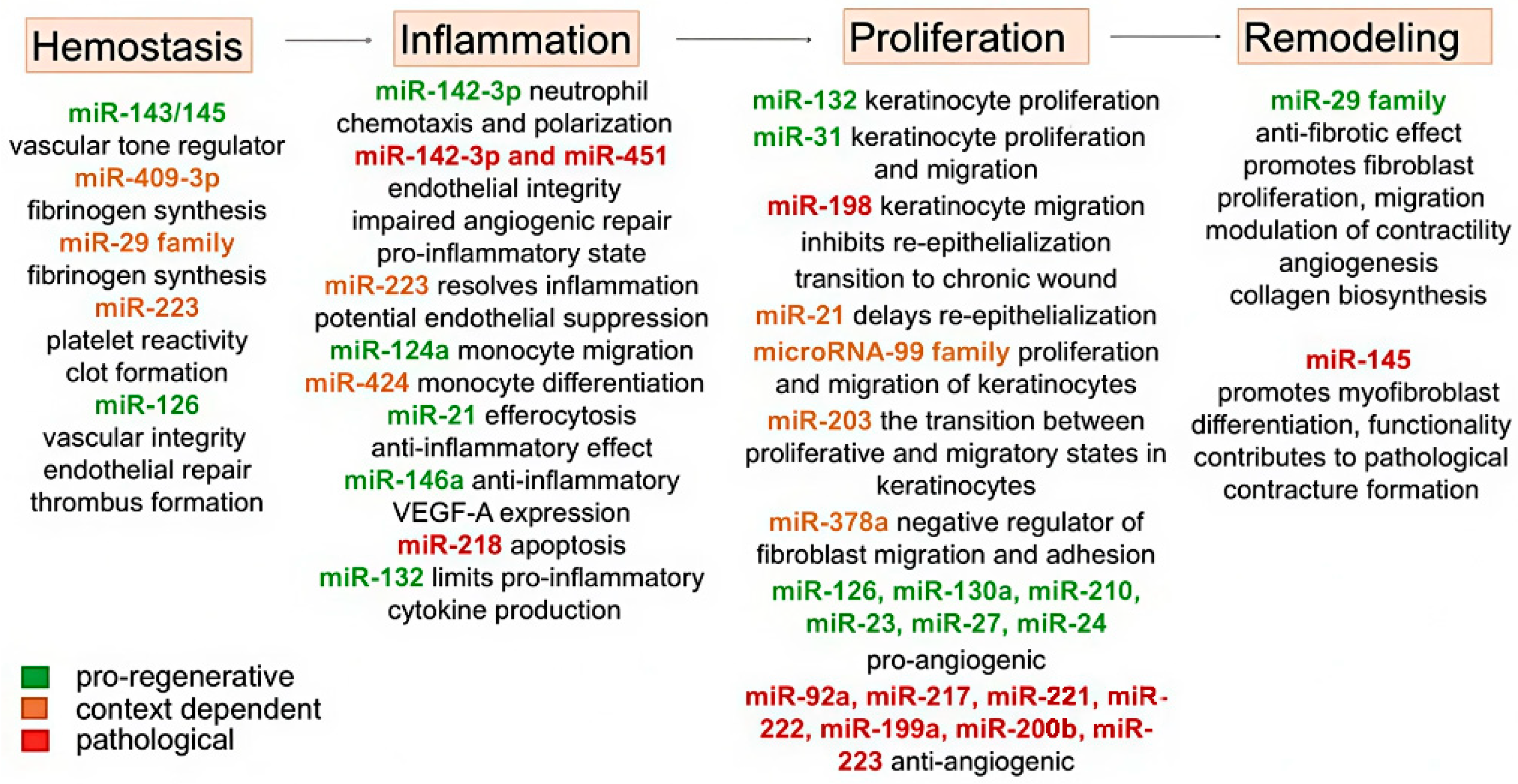
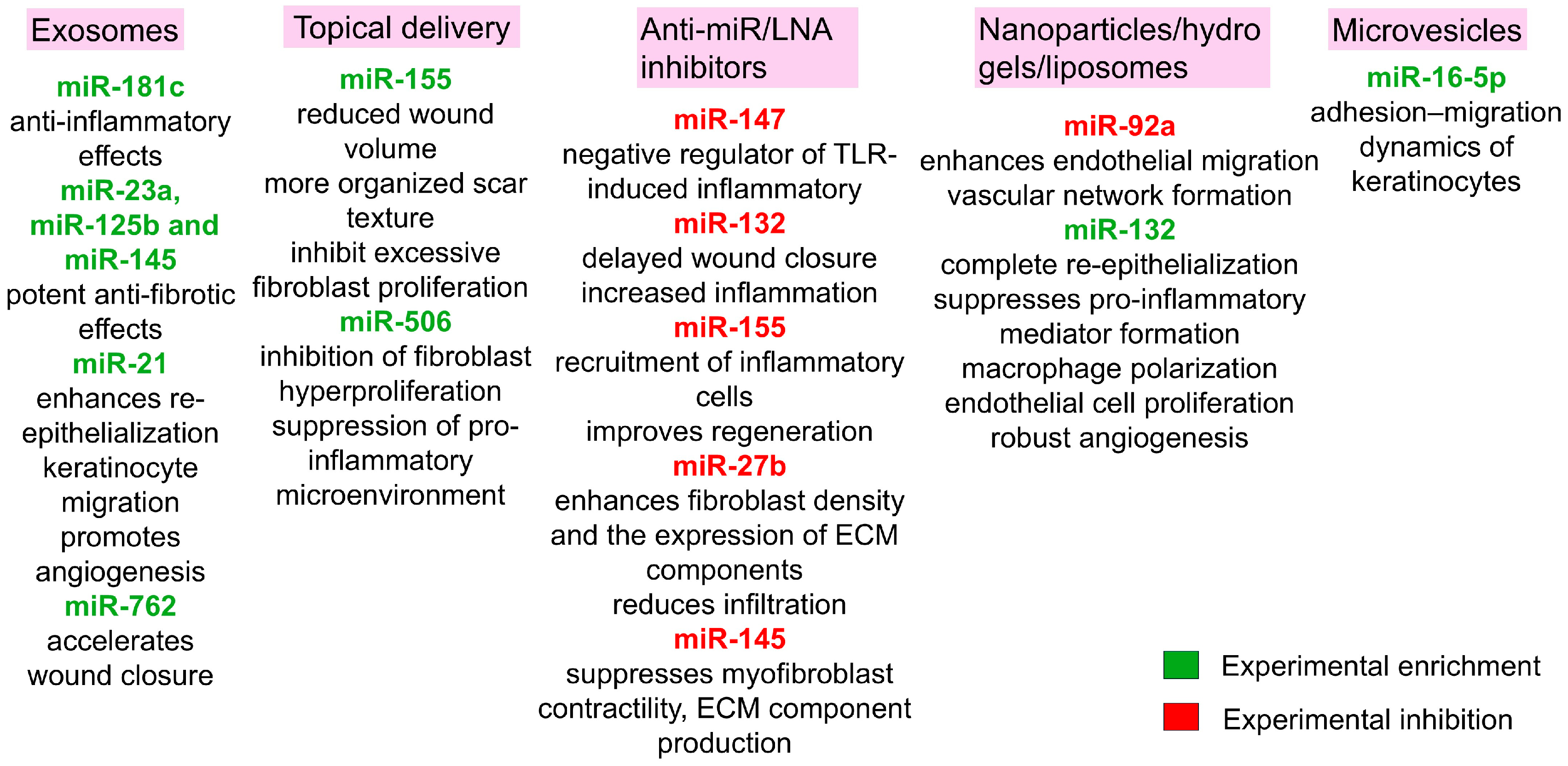
| Therapy | Typical Application | Advantages | Disadvantages | Limitations (Practical Notes) | References |
|---|---|---|---|---|---|
| Cooling with running water | Acute care of superficial and partial-thickness burns within first minutes | Reduces heat penetration, pain and depth progression; widely available and low cost | Limited to very early window (first 10–20 min) for maximal effect; insufficient for deep burns | Universal recommendation for initial management; avoid ice or prolonged refrigeration, which can worsen injury | [21] |
| Simple dressings and emollients | Superficial burns; temporary coverage | Inexpensive, easy to apply; maintain basic moist environment | Require frequent changes; limited antimicrobial activity; can adhere if improper | Suitable for small superficial burns; not optimal for exudative or infected wounds | [41] |
| Semipermeable film dressings (polyurethane: Tegaderm) | Superficial and some partial-thickness burns | Maintain moist microenvironment; permit wound inspection without removal; reduce pain | Not appropriate for heavily exuding wounds; potential for maceration if misused | Useful for small superficial burns and donor sites; contraindicated with high exudate or established infection | [42] |
| Hydrogels (amorphous gels and sheet hydrogel: IntraSite) | Partial-thickness burns and necrotic or dry wounds | Rehydrate necrotic tissue; facilitate autolytic debridement | Require secondary absorbent dressing for exudative wounds; may need frequent reapplication | Good for maintaining moist environment and pain control | |
| Hydrocolloid dressings (DuoDERM) | Low-to-moderate exudate partial-thickness wounds | Promote autolytic debridement; long wear time | Can be difficult to remove; not ideal for infected wounds | Effective where exudate is controlled and infection risk low | |
| Foam dressings (Mepilex) | Moderate-to-high exudate partial-thickness wounds | High absorbency; cushioning; reduced dressing change pain | Less transparent (harder to inspect wound) | Often used under secondary dressings or NPWT pads | |
| Antimicrobial dressings: silver (AQUACEL) | Partial-thickness burns at risk of infection | Broad-spectrum antimicrobial effect; prolonged activity; can reduce infection rates | Potential cytotoxicity at high concentrations | Indicated for contaminated wounds or high infection risk | [43] |
| Enzymatic debridement | Early selective debridement of deep partial-thickness and some full-thickness eschar | Selective removal of necrotic tissue; potentially reduces need for surgery; may preserve viable tissue | Pain during application in some patients; strict application protocols | Regional regulatory status varies; appropriate for controlled settings with experienced staff | [44] |
| Early tangential/surgical excision and autografting | Deep partial-thickness to full-thickness burns | Rapid removal of necrotic tissue, decreases infection risk, shortens hospital stay and definitive closure with autologous skin | Requires operating room; donor-site morbidity; risk of graft failure/infection | Gold standard for full-thickness burns and large, deep wounds; timing and technique tailored to patient stability | [12] |
| Negative-pressure wound therapy | Adjunct for graft fixation, large open wounds and exudative wounds | Promotes granulation tissue, reduces edema and bacterial load and secures grafts improving take | Device cost; requires vacuum system and trained personnel | Widely used as adjunct to surgical and dressing management; portable systems facilitate mobility | [37] |
| Platelet-rich plasma | Adjunct therapy for deep and partial-thickness burns | Delivers endogenous growth factors (PDGF, TGF-β, VEGF, FGF and EGF); accelerates epithelialization | Variability in preparation; limited standardization | Widely used autologous biotherapy; approved in multiple jurisdictions | [45] |
| Recombinant growth factor therapy | Chronic or delayed-healing burn wounds | Stimulates fibroblast proliferation, angiogenesis and epithelialization | Limited depth penetration; potential mitogenic risk; too-rapid degradation and clearance of GFs | FDA and EMA approved for chronic wounds; used off-label for burns | [46] |
| Bioengineered skin substitutes (Integra and Apligraf) | Deep partial- and full-thickness burns requiring dermal replacement | Provide dermal matrix scaffold, reduce graft requirement and promote neovascularization | Expensive and require careful storage | Clinically approved acellular and composite grafts | [47] |
| Cultured epithelial autografts (CEAs) | Extensive burns with limited donor sites | Permanent coverage using patient’s cells; reduces donor-site trauma | High cost; time for culture preparation; poor graft takes and stability | FDA approved (Epicel); used for extensive burns >30% TBSA | [38] |
| Allogeneic cell-based dressings | Temporary coverage in extensive burns | Provide bioactive molecules promoting healing | Immunological risk | Bridging therapy prior to autografting; facilitate tissue salvage by itself | [48] |
| Gene-activated matrix dressings | Advanced molecular therapy for severe burns | Sustained local release of angiogenic and reparative signals | High cost; specialized use; early regulatory adoption | Approved in selected markets (e.g., China) as advanced wound care devices | [49] |
| Phase | microRNA | Effect on Burn Wound Healing | Reported Manipulation | References | Other Pathological Conditions |
|---|---|---|---|---|---|
| Hemostasis | miR-143/145 | Necessary for differentiation of vascular smooth muscle cells, as well as for functional vasoconstriction and vasodilation | Knockout mice | [56] | Atherosclerosis; epithelial cancers; B-cell malignancies; coronary artery disease (all ↓) |
| miR-15b | Promotes proliferation of vascular smooth muscle cells | 5 nM miRNA mimic (chemically modified double-stranded RNA) 40 nM anti-miR (2′-O-methyl-modified RNA oligonucleotides) | [57] | Cardiac ischemia injury (↑); pulmonary fibrosis (↑); tongue squamous cell carcinoma | |
| miR-221 | Promotes proliferation and differentiation of vascular smooth muscle cells by inhibiting c-kit expression | 0.3 or 3 nm (chemically modified double-stranded RNA) 106 nm anti-miR (2′-O-methyl-modified RNA oligonucleotides) | [58] | Highly expressed in cancer-derived cells; inhibits normal erythropoiesis | |
| miR-409-3p/29 | Suppresses fibrinogen synthesis | 30 nM miRNA precursor molecules | [59] | miR-409-3p: cardiac fibrosis and acute coronary syndrome (↑); type 1 diabetes (↓) miR-29: pulmonary fibrosis (↓); osteosarcoma (↑) | |
| miR-98 | Enhances endotheliocyte permeability by suppressing the synthesis of hypoxia-inducible factor 1-alpha (HIF-1α) inhibitor protein | 10 to 50 nM anti-miR | [60] | Systemic lupus erythematosus; osteoarthritis; stroke (all ↓) | |
| Inflammation | miR-424 | Differentiation of monocytes into macrophages; activation of the macrophage colony-stimulating factor receptor (M-CSFR) gene due to suppression of nuclear factor I type A (NFI-A) translation | - | [61] | Hepatocellular carcinoma (↓); deep vein thrombosis (↑); obesity (↑) |
| miR-21 | Enhances efferocytosis; suppresses the activation of NF-kB and synthesis of TNF-α; enhances the production of IL-10 | - | [62] | Contributes to cardiac fibrosis; acute myocardial infarction; stroke; atherosclerosis (all ↑) | |
| miR-147 | Anti-inflammatory effect; regulates toll-like receptor (TLR)-induced inflammatory reactions in macrophages | 40 nM miRNA mimic and 40 nM locked nucleic acid (LNA) miR-147 inhibitor | [63] | Rheumatoid arthritis (↑); pulmonary tuberculosis (↑); coronary artery disease (↓) | |
| miR-155 | Pro-inflammatory effect; enhances the production of TNF-α/IL-6 and the differentiation of macrophages into the M1 | Knockout mice | [64] | Multiple sclerosis, Alzheimer’s disease; atherosclerosis; heart failure (all ↑) | |
| miR-183-3p | Regulation of skin capillary permeability and inflammatory mediators release | In vivo burned skin model; qRT-PCR analysis | [65] | Non-small-cell lung carcinoma (↑); psoriasis (↓) | |
| miR-132 | Reduces the production of chemokines by keratinocytes; limits the excessive production of pro-inflammatory cytokines by macrophages; polarization of macrophages into M2 | 20 nM pre-miR-132 and 20 nM LNA inhibitor | [66,67] | Alzheimer’s disease; heart failure; post-traumatic stress disorder (all ↑) | |
| miR-27b | Inhibits fibroblast proliferation | 4 µg/kg mimic; 4 µg/kg inhibitor | [68] | Kawasaki disease (↑); breast and prostate cancers (↓) | |
| Proliferation | miR-126 | Induces neoangiogenesis | - | [69] | Atherosclerosis (↓); acute myocardial infarction (↓); aerobic exercises (↑); knee osteoarthritis (↑) |
| miR-21 | Promotes migration of keratinocytes and fibroblasts | miR-21 antagomir (16 μg dissolved in 100 μL of PBS); 20 μg of miR-21 plasmid DNA | [70] | Contributes to cardiac fibrosis; acute myocardial infarction; stroke; atherosclerosis (all ↑) | |
| miR-31 | Promotes keratinocytes proliferation and migration | 20 nM miR-31 precursor | [71] | Diabetic wounds (↓); oral cancer (↑) | |
| miR-198 | Suppresses keratinocytes proliferation and migration | - | [72] | Pancreatic cancer (↓); osteosarcoma (↓); non-healing diabetic ulcers (↑) | |
| miR-99 family | 20 μM mimic; 20 μM LNA-inhibitor | [73] | Cardiac hypertrophy (↑); enhances HBV replication; highly expressed in hematopoietic and acute myeloid leukemia stem cells | ||
| miR-210 | Suppresses keratinocyte proliferation; promotes angiogenesis | 500 nM LNA-based anti-miR | [74] | Cardiac stress (↑); solid tumors (↑); retinal degeneration (↓) | |
| miR-199a-5p | Suppresses angiogenesis | Mimic (50 nm); inhibitor (100 nm) | [75] | Fibrosis (↑); myocardial infarction (↓) | |
| miR-130a | Inhibits re-epithelialization and granulation tissue formation; promotes angiogenesis | 5 μM mimic | [76] | Endothelial cell senescence; diabetic vascular disease; ischemic stroke (all ↓) | |
| miR-203 | Inhibits keratinocytes proliferation and migration | 80 mg/kg | [77] | Melanoma (↓); psoriasis (↑); ER-positive breast cancer (↑) | |
| miR-200b | Activation of angiogenic transcription factor ETS-1 and intensification of angiogenesis | 50 nM mimic; 100 nM inhibitor | [78] | Lung cancer; nonalcoholic fatty liver disease (all ↓) | |
| miR-499-5p | Impairs the angiogenic properties of endothelial cells and reduces blood perfusion | 3 μL/mL mimic 3 μL/mL inhibitor | [79] | Myocardial infarction (in ischemic tissue ↓; in the blood ↑) | |
| miR-663 | Inhibit apoptosis and promote proliferation of fibroblasts and keratinocytes | - | [80] | Hematologic malignancies (↓); non-small-cell lung carcinoma (↑); vascular smooth muscle cells differentiation (↑) | |
| miR-486 | Non-small-cell lung carcinoma; Duchenne muscular dystrophy (all ↓) | ||||
| miR-23b | Promotes fibroblast proliferation and migration through Smad3 | - | [81] | Rheumatoid arthritis; ovarian cancer; Parkinson’s disease (all ↓) | |
| miR-27b | Suppresses the directed migration of mesenchymal stem cells from bone marrow to damaged tissues by regulating the synthesis of stromal cell-derived factor-1 (SDF-1a) | 100 nM miRNA mimic | [82] | Kawasaki disease (↑); breast and prostate cancers (↓) | |
| miR-let-7c | Inhibits the proliferation and migration of dermal fibroblasts by binding to heat shock protein 70 (HSP70), increasing Bcl-2 and decreasing Bax level | 50 nM mimic and inhibitor | [83] | Prostate cancer (↓); coronary artery disease (↓); predictor of acute chest syndrome (↑) | |
| Remodeling | miR-1908 | Increases production of TGF-β1, IL-1a, TNF-α and collagen I by fibroblasts | 2 μg/kg mimic and inhibitor | [84] | Glioblastoma (↑); myocardial infarction (↓); HBV infection (↑) |
| miR-29b-3p | Affects the expression of collagen genes to improve extracellular matrix remodeling | - | [85] | Pre-eclampsia (↑); congenital heart disease (↑); prostate cancer (↓) | |
| miR-29a | Reduces the levels of collagen type I | 100 nM mimic and 2′-OMe chemically modified inhibitors | [86] | Metastatic prostate cancer; oral squamous carcinoma; cardiac fibrosis (all ↓) | |
| miR-145 | Decreases Krüppel-like factor 4 (KLF4) level, thereby suppressing α-SMA synthesis and fibroblast differentiation into myofibroblasts | 5 nM inhibitor | [87] | Prostate cancer; coronary artery disease; type-2 diabetes mellitus (all ↓) | |
| miR-let-7d | Reduction of iron absorption through divalent metal transporter 1 (DMT1) and stabilization of new collagen production | 100 nM mimic and 100 nM anti-miR | [88] | Fibromyalgia syndrome (↑); idiopathic pulmonary fibrosis (↓) | |
| miR-506-3p | Regulates the autophagy of fibroblasts, their migration and proliferation and the synthesis of ECM components | - | [89] | Osteosarcoma (↓); hepatic steatosis (↑) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badanina, D.M.; Bubnova, A.M.; Kozlov, D.S.; Krylov, D.P.; Mozherov, A.M.; Vosough, M.; Timashev, P.S.; Kuznetsova, D.S. Therapeutic Potential of Modulating Gene-MicroRNA Crosstalk in Burn Injury. Int. J. Mol. Sci. 2025, 26, 10060. https://doi.org/10.3390/ijms262010060
Badanina DM, Bubnova AM, Kozlov DS, Krylov DP, Mozherov AM, Vosough M, Timashev PS, Kuznetsova DS. Therapeutic Potential of Modulating Gene-MicroRNA Crosstalk in Burn Injury. International Journal of Molecular Sciences. 2025; 26(20):10060. https://doi.org/10.3390/ijms262010060
Chicago/Turabian StyleBadanina, Dariya M., Anastasia M. Bubnova, Dmitry S. Kozlov, Dmitry P. Krylov, Artem M. Mozherov, Massoud Vosough, Peter S. Timashev, and Daria S. Kuznetsova. 2025. "Therapeutic Potential of Modulating Gene-MicroRNA Crosstalk in Burn Injury" International Journal of Molecular Sciences 26, no. 20: 10060. https://doi.org/10.3390/ijms262010060
APA StyleBadanina, D. M., Bubnova, A. M., Kozlov, D. S., Krylov, D. P., Mozherov, A. M., Vosough, M., Timashev, P. S., & Kuznetsova, D. S. (2025). Therapeutic Potential of Modulating Gene-MicroRNA Crosstalk in Burn Injury. International Journal of Molecular Sciences, 26(20), 10060. https://doi.org/10.3390/ijms262010060







