HPV-Independent Cervical Cancer—A New Challenge of Modern Oncology
Abstract
1. Introduction
2. Pathogenesis and Molecular Mechanisms
2.1. Possible Reasons for HPV-Negative Status
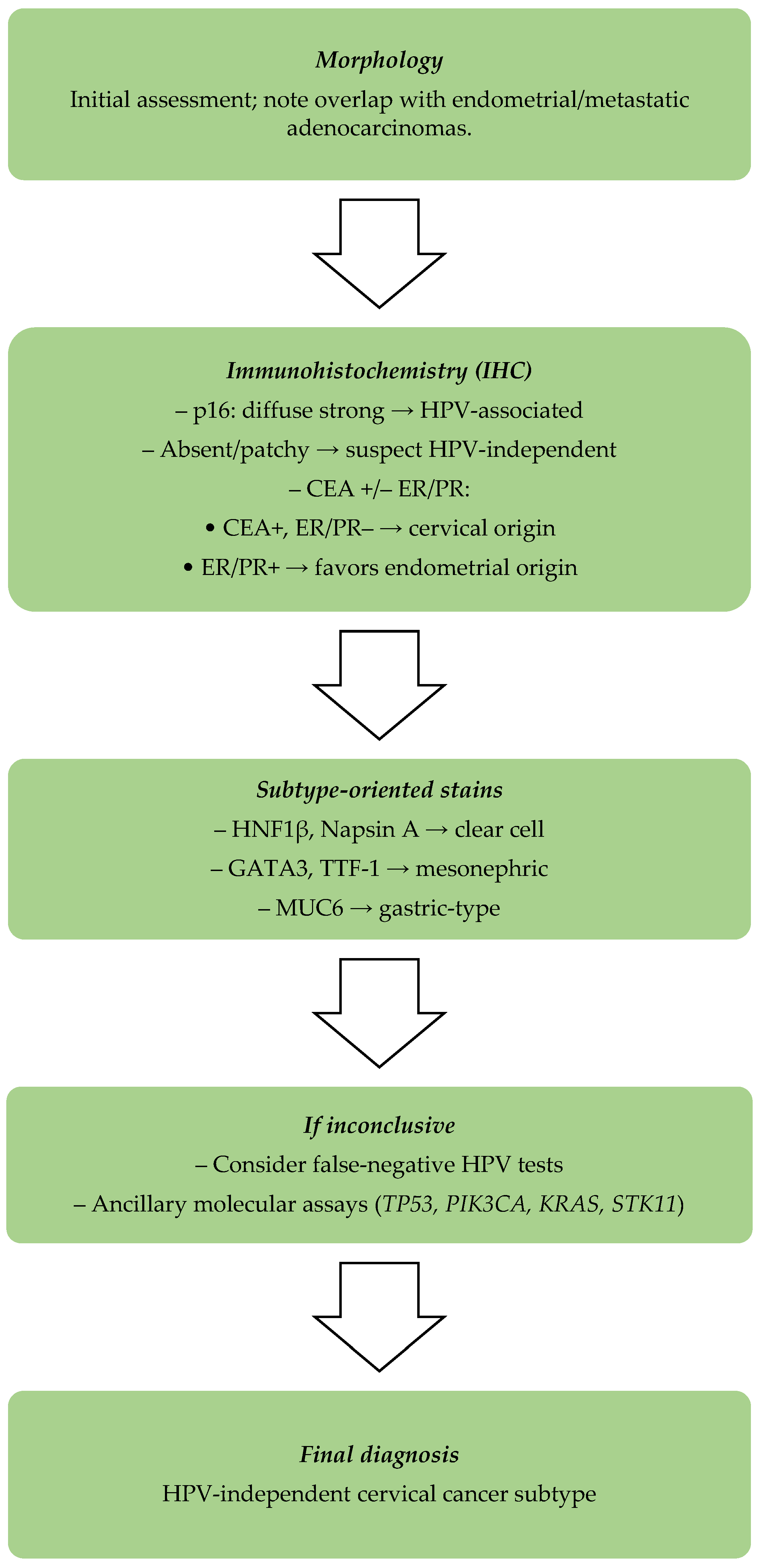
2.1.1. Inaccurate Tumor Classification
- Cervical cancers that are HPV-independent show positive immunoexpression for CEA (carcinoembryonic antigen) and p16, but lack immunopositive results for estrogen receptor (ER) or progesterone receptor (PR) [24].
- Endometrial adenocarcinomas usually show strong ER and PR immunoexpression, but do not present diffuse p16 immunostaining [25].
- In challenging cases, additional markers such as vimentin, CD10, and MUC6 can be used for a greater diagnostic accuracy [26].
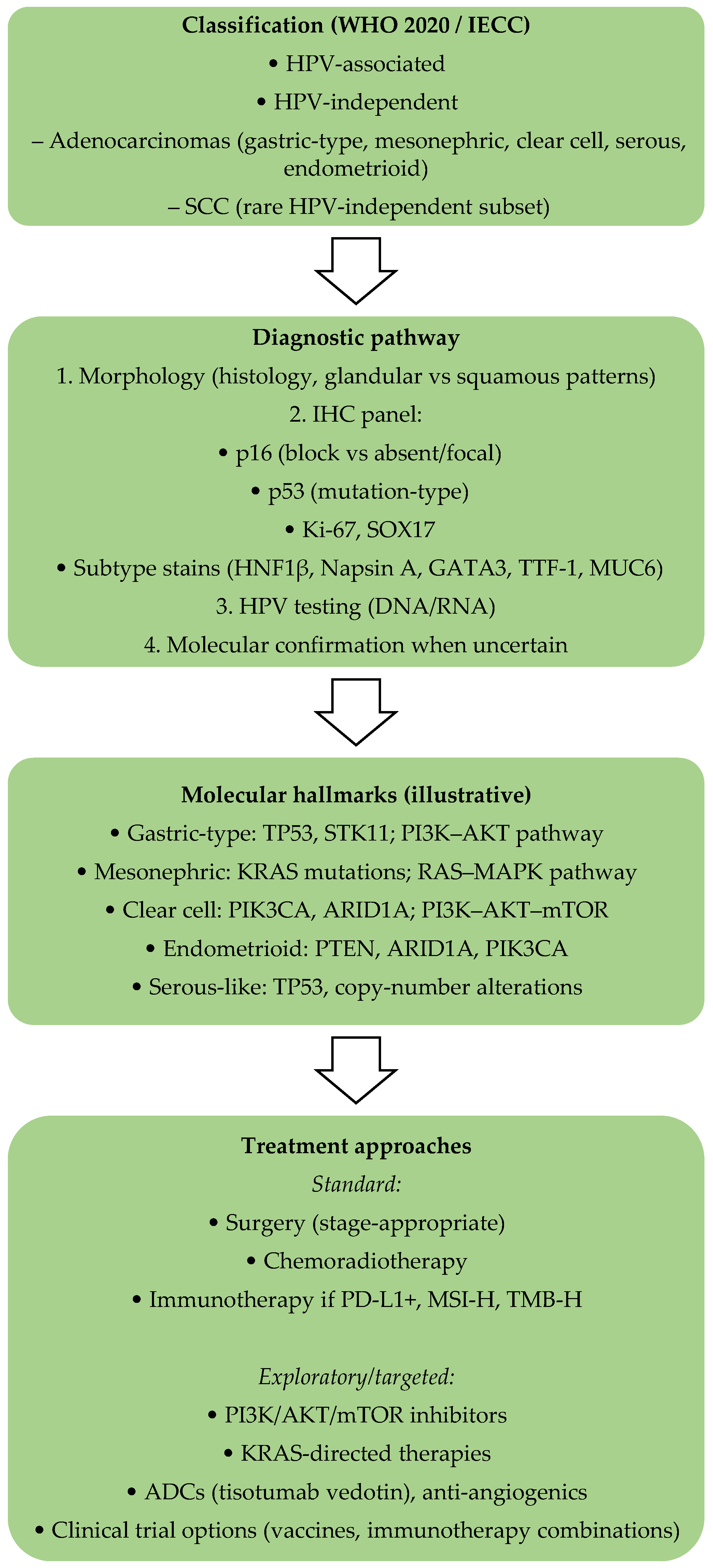
- The International Endocervical Adenocarcinoma Criteria and Classification (IECC) system has improved diagnostic precision by subclassifying adenocarcinomas into HPV-associated (HPVA) and non-HPV-associated types. The majority of HPV-independent types of cervical carcinoma are gastric-type adenocarcinomas, clear-cell carcinomas, mesonephric carcinomas, and endometrioid carcinomas, and they have distinct histological and molecular features, highlighting the importance of diagnosis based on subtypes [22,27,28].
2.1.2. Errors in HPV Detection Methods
- Low HPV DNA copy number: Sometimes, the amount of viral load in the tumor is too low for standard PCR or hybrid capture assays to detect. This is particularly relevant when HPV has been removed from the lesion, but oncogenic changes persist [32].
- Loss of HPV DNA fragments: While the HPV genome is integrated into the host genome, the L1 region of the HPV genome, which is usually subjected to diagnostic tests, is removed. Such deletions may result in false-negative outcomes when relying on L1-based detection assays. Rates of detection may be improved by testing for E6/E7 mRNA expression, which remains active post-integration [7].
- Errors in sampling and fixation: Negative HPV results can also arise from inadequate sample collection, tissue breakdown, or flawed fixation [6].
- Variability in HPV detection methods: The sensitivities and specificities of various HPV tests impact the possibility of identifying HPV within a sample. Therefore, it is advisable to carry out multiple approaches to the detection of HPV in both clinical and investigative contexts in order to reduce false-negative cases. E6/E7 mRNA-based tests, together with NGS and enhanced sampling methods, would reduce misdiagnosis of HPV-negative cases and improve overall case accuracy [6].
2.1.3. HPV Infection Latency and Non-High-Risk HPV Types
2.1.4. Screening Challenges and Adapted Protocols
2.2. Mechanisms of HPV-Independent Cervical Cancer
2.2.1. Immune Microenvironment Differences
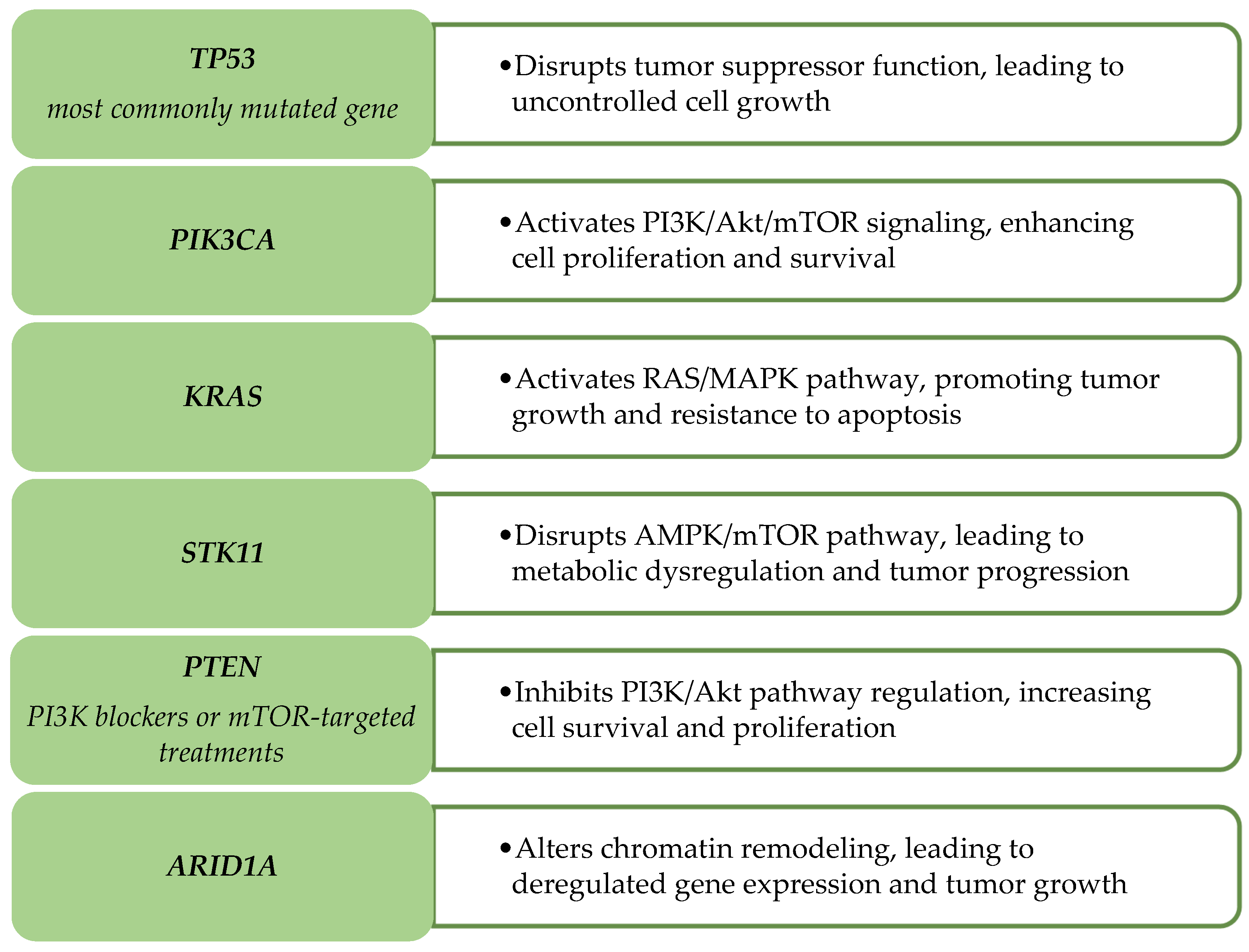
2.2.2. Key Genetic Alterations
2.2.3. Participation of Long Noncoding RNAs (lncRNAs)
3. Histopathological Features
| Subtype | Histological Features | Common Biomarkers | Clinical Implications |
|---|---|---|---|
| Gastric-Type Adenocarcinoma | Pale eosinophilic cytoplasm, clear or vacuolated cells, deep stromal invasion | MUC6, HNF1β, CDX2 (p16 negative or patchy) | Aggressive, late-stage diagnosis, high metastasis rate, poor response to therapy |
| Clear-Cell Carcinoma | Polygonal/hobnail cells, clear cytoplasm, central nuclei | HNF1 β, Napsin A, AMACR (p16 negative or focal) | Rare, may be linked to DES exposure, poor prognosis |
| Mesonephric Carcinoma | Tubular/ductal/papillary structures, eosinophilic luminal secretions | GATA3, TTF-1, CD10, AMACR (p16 negative) | Highly aggressive, high recurrence, poor prognosis |
| Endometrioid Carcinoma | Endometrial-like glands with villoglandular, secretory, or ciliated patterns, higher-grade tumors show solid growth, mucin commonly present | p16 negative or weak expression, ER and PR often positive, PAX8 positive | Early-stage diagnosis, better prognosis compared to other cervical adenocarcinomas, hormone receptor presence indicates possible benefit from hormonal treatment. |
- Key Points
- Diagnostic errors can arise from HPV testing limitations, viral latency, or confusion with endometrial cancer.
- Consider HPV-independent disease when encountering gastric-type, clear-cell, mesonephric, or true endometrioid carcinomas.
- Use immunostains such as p16, ER/PR, and CEA, along with molecular profiling (TP53, PIK3CA, KRAS), to support accurate classification.
- These tumors are often detected at advanced stages, show limited response to standard chemoradiation, and have poorer outcomes than HPV-associated tumors.
4. Prognosis
5. Key Clinical Challenges
6. Management and Therapeutic Strategies
6.1. Surgical Approaches
6.2. Radiotherapy and Chemotherapy
6.3. Targeted Therapy and Immunotherapy
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferrara, P.; Dallagiacoma, G.; Alberti, F.; Gentile, L.; Bertuccio, P.; Odone, A. Prevention, diagnosis and treatment of cervical cancer: A systematic review of the impact of COVID-19 on patient care. Prev. Med. 2022, 164, 107264. [Google Scholar] [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Ahmed, H.G.; Bensumaidea, S.H.; Alshammari, F.D.; Alenazi, F.S.H.; ALmutlaq, B.A.; Alturkstani, M.Z.; Aladani, I.A. Prevalence of Human Papillomavirus subtypes 16 and 18 among Yemeni Patients with Cervical Cancer. Asian Pac. J. Cancer Prev. 2017, 18, 1543–1548. [Google Scholar]
- Brandt, H.M.; Footman, A.; Adsul, P.; Ramanadhan, S.; Kepka, D. Implementing interventions to start HPV vaccination at age 9: Using the evidence we have. Hum. Vaccin. Immunother. 2023, 19, 2180250. [Google Scholar] [CrossRef]
- Giannella, L.; Grelloni, C.; Natalini, L.; Sartini, G.; Bordini, M.; Delli Carpini, G.; Di Giuseppe, J.; Dugo, E.; Piva, F.; Ciavattini, A. Molecular Features of HPV-Independent Cervical Cancers. Pathogens 2025, 14, 668. [Google Scholar] [CrossRef]
- Macios, A.; Nowakowski, A. False Negative Results in Cervical Cancer Screening-Risks, Reasons and Implications for Clinical Practice and Public Health. Diagnostics 2022, 12, 1508. [Google Scholar] [CrossRef]
- Shao, N. Research progress on human papillomavirus-negative cervical cancer: A review. Medicine 2024, 103, e39957. [Google Scholar] [CrossRef]
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef]
- Nishio, H.; Matsuda, R.; Iwata, T.; Yamagami, W. Gastric-type adenocarcinoma of the uterine cervix: Clinical features and future directions. Jpn. J. Clin. Oncol. 2024, 54, 516–520. [Google Scholar] [CrossRef]
- Yim, E.K.; Park, J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Litwin, T.R.; Clarke, M.A.; Dean, M.; Wentzensen, N. Somatic Host Cell Alterations in HPV Carcinogenesis. Viruses 2017, 9, 206. [Google Scholar] [CrossRef]
- Schabath, M.B.; Welsh, E.A.; Fulp, W.J.; Chen, L.; Teer, J.K.; Thompson, Z.J.; Engel, B.E.; Xie, M.; Berglund, A.E.; Creelan, B.C.; et al. Differential association of STK11 and TP53 with KRAS mutation-associated gene expression, proliferation and immune surveillance in lung adenocarcinoma. Oncogene 2016, 35, 3209–3216. [Google Scholar] [CrossRef]
- Arezzo, F.; Cormio, G.; Loizzi, V.; Cazzato, G.; Cataldo, V.; Lombardi, C.; Ingravallo, G.; Resta, L.; Cicinelli, E. HPV-Negative Cervical Cancer: A Narrative Review. Diagnostics 2021, 11, 952. [Google Scholar] [CrossRef]
- Katki, H.A.; Kinney, W.K.; Fetterman, B.; Lorey, T.; Poitras, N.E.; Cheung, L.; Demuth, F.; Schiffman, M.; Wacholder, S.; Castle, P.E. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: A population-based study in routine clinical practice. Lancet Oncol. 2011, 12, 663–672, Correction in Lancet Oncol. 2011, 12, 722. [Google Scholar] [CrossRef]
- González-Bosquet, E.; Muñoz, A.; Suñol, M.; Lailla, J.M. Cervical cancer and low-risk HPV; a case report. Eur. J. Gynaecol. Oncol. 2006, 27, 193–194. [Google Scholar]
- Yanaranop, M.; Ayuwat, S.; Nakrangsee, S. Differential Diagnosis between Primary Endocervical and Endometrial Adenocarcinoma using Immunohistochemical Staining of Estrogen Receptor, Vimentin, Carcinoembryonic Antigen and p16. J. Med. Assoc. Thai. 2016, 99 (Suppl. 2), S106–S115. [Google Scholar]
- Lee, J.E.; Chung, Y.; Rhee, S.; Kim, T.H. Untold story of human cervical cancers: HPV-negative cervical cancer. BMB Rep. 2022, 55, 429–438. [Google Scholar] [CrossRef]
- Giannella, L.; Di Giuseppe, J.; Delli Carpini, G.; Grelloni, C.; Fichera, M.; Sartini, G.; Caimmi, S.; Natalini, L.; Ciavattini, A. HPV-Negative Adenocarcinomas of the Uterine Cervix: From Molecular Characterization to Clinical Implications. Int. J. Mol. Sci. 2022, 23, 15022. [Google Scholar] [CrossRef]
- Evans, A.M.; Salnikov, M.; Gameiro, S.F.; Maleki Vareki, S.; Mymryk, J.S. HPV-Positive and -Negative Cervical Cancers Are Immunologically Distinct. J. Clin. Med. 2022, 11, 4825. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2021, 10, 606335. [Google Scholar] [CrossRef]
- Hopenhayn, C.; Christian, A.; Christian, W.J.; Watson, M.; Unger, E.R.; Lynch, C.F.; Peters, E.S.; Wilkinson, E.J.; Huang, Y.; Copeland, G.; et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 US cancer registries before vaccine introduction. J. Low. Genit. Tract. Dis. 2014, 18, 182–189. [Google Scholar]
- Pirog, E.C. Cervical Adenocarcinoma: Diagnosis of Human Papillomavirus-Positive and Human Papillomavirus-Negative Tumors. Arch. Pathol. Lab. Med. 2017, 141, 1653–1667. [Google Scholar]
- Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. Obgyn 2018, 10, 107–113. [Google Scholar]
- Park, K.J.; Kiyokawa, T.; Soslow, R.A.; Lamb, C.A.; Oliva, E.; Zivanovic, O.; Juretzka, M.M.; Pirog, E.C. Unusual endocervical adenocarcinomas: An immunohistochemical analysis with molecular detection of human papillomavirus. Am. J. Surg. Pathol. 2011, 35, 633–646. [Google Scholar]
- Reid-Nicholson, M.; Iyengar, P.; Hummer, A.; Linkov, I.; Asher, M.; Soslow, R.A. Immunophenotypic diversity of endometrial adenocarcinomas: Implications for differential diagnosis. Mod. Pathol. 2006, 19, 1091–1100. [Google Scholar] [CrossRef]
- Stolnicu, S.; Park, K.J.; Kiyokawa, T.; Oliva, E.; McCluggage, W.G.; Soslow, R.A. Tumor Typing of Endocervical Adenocarcinoma: Contemporary Review and Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021, 40 (Suppl. 1), S75–S91. [Google Scholar] [CrossRef]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C.; et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef]
- Stolnicu, S.; Karpathiou, G.; Guerra, E.; Mateoiu, C.; Reques, A.; Garcia, A.; Bart, J.; Felix, A.; Fanni, D.; Gama, J.; et al. Clear Cell Carcinoma (CCC) of the Cervix Is a Human Papillomavirus (HPV)-independent Tumor Associated with Poor Outcome: A Comprehensive Analysis of 58 Cases. Am. J. Surg. Pathol. 2022, 46, 765–773. [Google Scholar] [CrossRef]
- Bekkers, R.L.; Bulten, J.; Wiersma-van Tilburg, A.; Mravunac, M.; Schijf, C.P.; Massuger, L.F.; Quint, W.G.; Melchers, W.J. Coexisting high-grade glandular and squamous cervical lesions and human papillomavirus infections. Br. J. Cancer 2003, 89, 886–890. [Google Scholar] [CrossRef]
- Yu, L.; Fei, L.; Liu, X.; Pi, X.; Wang, L.; Chen, S. Application of p16/Ki-67 dual-staining cytology in cervical cancers. J. Cancer 2019, 10, 2654–2660. [Google Scholar] [CrossRef]
- Mühr, L.S.A.; Guerendiain, D.; Cuschieri, K.; Sundström, K. Human Papillomavirus Detection by Whole-Genome Next-Generation Sequencing: Importance of Validation and Quality Assurance Procedures. Viruses 2021, 13, 1323. [Google Scholar]
- Abreu, A.L.; Souza, R.P.; Gimenes, F.; Consolaro, M.E. A review of methods for detect human Papillomavirus infection. Virol. J. 2012, 9, 262. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Peng, X.; Woodhouse, I.; Hancock, G.; Parker, R.; Marx, K.; Müller, J.; Salatino, S.; Partridge, T.; Nicastri, A.; Liao, H.; et al. Novel canonical and non-canonical viral antigens extend current targets for immunotherapy of HPV-driven cervical cancer. iScience 2023, 26, 106101. [Google Scholar]
- U.S. Preventive Services Task Force. Draft Recommendation Statement: Cervical Cancer Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/cervical-cancer-screening-adults-adolescents (accessed on 10 December 2024).
- American Society for Colposcopy and Cervical Pathology. New ASCCP Cervical Cancer Management Guidelines Now Include Dual-Stain Triage Testing. Available online: https://www.asccp.org/clinical-practice/guidelines/screening-guidelines (accessed on 11 March 2024).
- American Society for Colposcopy and Cervical Pathology. New Guidelines Released for Self-Collected HPV Screening. Available online: https://www.asccp.org/Assets/3b07f8a7-d292–4836-b5d3-72ee9996a96d/638757404555370000/hpv-self-collection-guidelines-press-release-final-pdf (accessed on 21 February 2024).
- World Health Organization. New Evidence on Cervical Cancer Screening and Treatment for Women with HIV. Available online: https://www.who.int/news/item/12-12-2023-new-evidence-on-cervical-cancer-screening-and-treatment-for-women-with-hiv (accessed on 12 December 2024).
- Schreiberhuber, L.; Barrett, J.E.; Wang, J.; Redl, E.; Herzog, C.; Vavourakis, C.D.; Sundström, K.; Dillner, J.; Widschwendter, M. Cervical cancer screening using DNA methylation triage in a real-world population. Nat. Med. 2024, 30, 2251–2257. [Google Scholar] [CrossRef]
- Brăila, A.D.; Poalelungi, C.-V.; Albu, C.-C.; Damian, C.M.; Dȋră, L.M.; Bănățeanu, A.-M.; Bogdan-Andreescu, C.F. The relationship between cervicovaginal infection, human papillomavirus infection and cervical intraepithelial neoplasia in Romanian women. Diseases 2025, 13, 18. [Google Scholar] [CrossRef]
- Lou, J.; Guo, F. The characteristics of high-risk HPV-negative cervical cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1452834. [Google Scholar]
- He, J.; Tu, L.; Weng, G.; Zeng, L. Analysis of the differences between HPV-independent and HPV-related cervical adenocarcinoma. Front. Oncol. 2025, 15, 1544207. [Google Scholar] [CrossRef]
- Tahboub, R.; Sanchez-Ortiz, J.; Lai, M.; Clark, J.L.; Zou, T. Something old, something new: Cervical cytopathology in the new era. Hum. Pathol. Rep. 2024, 37, 300756. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Wu, X. Gastric-type endocervical adenocarcinoma: A report and literature review. Oncol. Lett. 2024, 27, 247. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef]
- Kang, Y.J.; O’Haire, S.; Franchini, F.; Ijzerman, M.; Zalcberg, J.; Macrae, F.; Canfell, K.; Steinberg, J. A scoping review and meta-analysis on the prevalence of pan-tumour biomarkers (dMMR, MSI, high TMB) in different solid tumours. Sci. Rep. 2022, 12, 20495. [Google Scholar] [CrossRef]
- Yang, S.M.; Wu, M.; Han, F.Y.; Sun, Y.M.; Yang, J.Q.; Liu, H.X. Role of HPV status and PD-L1 expression in prognosis of laryngeal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2021, 14, 107–115. [Google Scholar]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor Immunity and Immunotherapy for HPV-Related Cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Saglam, O.; Conejo-Garcia, J. PD-1/PD-L1 immune checkpoint inhibitors in advanced cervical cancer. Integr. Cancer Sci. Ther. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liang, H.; Hu, J.; Liu, S.; Hao, X.; Wong, M.S.K.; Li, X.; Hu, L. PD-L1 Expression Correlates with Tumor Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Cervical Cancer. J. Cancer 2018, 9, 2938–2945. [Google Scholar] [CrossRef]
- Senba, M.; Mori, N. Mechanisms of virus immune evasion lead to development from chronic inflammation to cancer formation associated with human papillomavirus infection. Oncol. Rev. 2012, 6, e17. [Google Scholar] [CrossRef]
- Jamison, B.L.; Lawrance, M.; Wang, C.J.; DeBerg, H.A.; Ziegler, L.J.; Sansom, D.M.; Gavin, M.A.; Walker, L.S.K.; Campbell, D.J. An IL-2 mutein increases regulatory T cell suppression of dendritic cells via IL-10 and CTLA-4 to promote T cell anergy. Cell Rep. 2024, 43, 114938. [Google Scholar] [CrossRef]
- Robinson, M.A.; Kennedy, A.; Orozco, C.T.; Chen, H.C.; Waters, E.; Giovacchini, D.; Yeung, K.; Filer, L.; Hinze, C.; Lloyd, C.; et al. Rigid, bivalent CTLA-4 binding to CD80 is required to disrupt the cis CD80/PD-L1 interaction. Cell Rep. 2024, 43, 114768. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Zhang, Z.; Wu, P.; Zhang, Y.; Chen, X. Advances in targeting tumor microenvironment for immunotherapy. Front. Immunol. 2024, 15, 1472772. [Google Scholar] [CrossRef]
- Knutson, K.L. Regulation of tumor dendritic cells by programmed cell death 1 pathways. J. Immunol. 2024, 212, 1397–1405. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Bønløkke, S.; Stougaard, M.; Blaakær, J.; Bertelsen, J.; Andersen, K.; Fuglsang, K.; Steiniche, T. HPV is an essential driver in recurrence of cervical cancer. Pathol. Res. Pr. 2024, 264, 155672. [Google Scholar]
- Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and -Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef]
- Kashofer, K.; Winter, E.; Halbwedl, I.; Thueringer, A.; Kreiner, M.; Sauer, S.; Regauer, S. HPV-negative penile squamous cell carcinoma: Disruptive mutations in the TP53 gene are common. Mod. Pathol. 2017, 30, 1013–1020. [Google Scholar] [CrossRef]
- Park, D.J.; Wilczynski, S.P.; Paquette, R.L.; Miller, C.W.; Koeffler, H.P. p53 mutations in HPV-negative cervical carcinoma. Oncogene 1994, 9, 205–210. [Google Scholar] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar]
- Verlaat, W.; Snijders, P.J.; van Moorsel, M.I.; Bleeker, M.; Rozendaal, L.; Sie, D.; Ylstra, B.; Meijer, C.J.; Steenbergen, R.D.; Heideman, D.A. Somatic mutation in PIK3CA is a late event in cervical carcinogenesis. J. Pathol. Clin. Res. 2015, 1, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef]
- Ferreira, A.; Pereira, F.; Reis, C.; Oliveira, M.J.; Sousa, M.J.; Preto, A. Crucial Role of Oncogenic KRAS Mutations in Apoptosis and Autophagy Regulation: Therapeutic Implications. Cells 2022, 11, 2183. [Google Scholar] [CrossRef]
- Wu, J.N.; Roberts, C.W. ARID1A mutations in cancer: Another epigenetic tumor suppressor? Cancer Discov. 2013, 3, 35–43. [Google Scholar] [CrossRef]
- Annunziata, C.; Buonaguro, L.; Losito, S.; Buonaguro, F.M.; Tornesello, M.L. Somatic mutations of STK11 gene in human papillomavirus positive and negative penile cancer. Infect. Agent. Cancer 2013, 8, 2. [Google Scholar] [PubMed]
- Resta, N.; Pierannunzio, D.; Lenato, G.M.; Stella, A.; Capocaccia, R.; Bagnulo, R.; Lastella, P.; Susca, F.C.; Bozzao, C.; Loconte, D.C.; et al. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: Results of an Italian multicenter study. Dig. Liver Dis. 2013, 45, 606–611. [Google Scholar] [PubMed]
- Pons-Tostivint, E.; Lugat, A.; Fontenau, J.F.; Denis, M.G.; Bennouna, J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. Cells 2021, 10, 3129. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.X.; Cao, L.Y.; Chen, X.; Xiao, J.; Zou, Y.; Chen, Q. PTEN Inhibits Cell Proliferation, Promotes Cell Apoptosis, and Induces Cell Cycle Arrest via Downregulating the PI3K/AKT/hTERT Pathway in Lung Adenocarcinoma A549 Cells. Biomed Res. Int. 2016, 2016, 2476842. [Google Scholar] [CrossRef]
- Janku, F.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Naing, A.; Falchook, G.S.; Tsimberidou, A.M.; Stepanek, V.M.; Moulder, S.L.; Lee, J.J.; et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep. 2014, 6, 377–387. [Google Scholar]
- Groenewald, W.; Lund, A.H.; Gay, D.M. The Role of WNT Pathway Mutations in Cancer Development and an Overview of Therapeutic Options. Cells 2023, 12, 990. [Google Scholar] [CrossRef]
- Casarotto, M.; Fanetti, G.; Guerrieri, R.; Palazzari, E.; Lupato, V.; Steffan, A.; Polesel, J.; Boscolo-Rizzo, P.; Fratta, E. Beyond MicroRNAs: Emerging Role of Other Non-Coding RNAs in HPV-Driven Cancers. Cancers 2020, 12, 1246. [Google Scholar]
- Liang, W.C.; Ren, J.L.; Wong, C.W.; Chan, S.O.; Waye, M.M.; Fu, W.M.; Zhang, J.F. LncRNA-NEF antagonized epithelial to mesenchymal transition and cancer metastasis via cis-regulating FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene 2018, 37, 1445–1456. [Google Scholar]
- Ju, W.; Luo, X.; Zhang, N. LncRNA NEF inhibits migration and invasion of HPV-negative cervical squamous cell carcinoma by inhibiting TGF-β pathway. Biosci. Rep. 2019, 39, BSR20180878. [Google Scholar]
- Jin, L.; Ji, J.; Shi, L.; Jin, S.; Pei, L. lncRNA HAND2-AS1 inhibits cancer cell proliferation, migration and invasion by downregulating ROCK1 in HPV-positive and negative cervical squamous cell carcinoma. Exp. Ther. Med. 2019, 18, 2512–2518. [Google Scholar]
- Yan, Z.; Ruoyu, L.; Xing, L.; Hua, L.; Jun, Z.; Yaqin, P.; Lu, W.; Aili, T.; Yuzi, Z.; Lin, M.; et al. Long non-coding RNA GAS5 regulates the growth and metastasis of human cervical cancer cells via induction of apoptosis and cell cycle arrest. Arch. Biochem. Biophys. 2020, 684, 108320. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Zhang, L.; Cong, J.; Hou, J.; Liu, C. LncRNA PVT1 promotes the growth of HPV positive and negative cervical squamous cell carcinoma by inhibiting TGF-β1. Cancer Cell Intern. 2018, 18, 70. [Google Scholar]
- Liu, Y.; Liu, H.; Sheng, B.; Pan, S.; Wang, Z.W.; Zhu, X. The functions of lncRNAs in the HPV-negative cervical cancer compared with HPV-positive cervical cancer. Apoptosis 2022, 27, 685–696. [Google Scholar] [PubMed]
- Herrington, C.S.; Kim, K.-R.; Kong, C.S.; Longacre, T.A.; McCluggage, W.G.; Mikami, Y.; Ordi, J.; Soslow, R.A. Tumours of the uterine cervix. In WHO Classification of Tumours: Female Genital Tumours, 5th ed.; IARC Press: Lyon, France, 2020; pp. 342–386. [Google Scholar]
- Rodríguez-Carunchio, L.; Soveral, I.; Steenbergen, R.D.M.; Torné, A.; Martinez, S.; Fusté, P.; Pahisa, J.; Marimon, L.; Ordi, J.; del Pino, M. HPV-negative carcinoma of the uterine cervix: A distinct type of cervical cancer with poor prognosis. BJOG 2015, 122, 119–127. [Google Scholar] [PubMed]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Pilch, H.; Günzel, S.; Schäffer, U.; Tanner, B.; Brockerhoff, P.; Maeurer, M.; Höckel, M.; Hommel, G.; Knapstein, P.G. The presence of HPV DNA in cervical cancer: Correlation with clinico-pathologic parameters and prognostic significance: 10 years experience at the Department of Obstetrics and Gynecology of the Mainz University. Int. J. Gynecol. Cancer 2001, 11, 39–48. [Google Scholar] [PubMed]
- Turco, L.C.; Pedone Anchora, L.; Fedele, C.; Inzani, F.; Piermattei, A.; Martini, M.; Volpe, M.; Marchetti, S.; Santangelo, R.; Bizzarri, N.; et al. Human papillomavirus independent status on pathologic response and outcomes in locally advanced cervical cancer managed with chemoradiotherapy followed by surgery. Int. J. Gynecol. Cancer 2023, 33, 489–497. [Google Scholar] [CrossRef]
- McCluggage, W.G. New developments in endocervical glandular lesions. Histopathology 2013, 62, 138–160. [Google Scholar] [CrossRef]
- Fulmer, C.G.; Hoda, R.S.; Pirog, E.C.; Park, K.J.; Holcomb, K. Cytomorphology of Gastric-Type Cervical Adenocarcinoma on a ThinPrep Pap Test: Report of a p16-Positive Tumor Case. Diagn. Cytopathol. 2016, 44, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; Lindeman, N.; MacConaill, L.; Hirsch, M.; Dal Cin, P.; Gorman, M.; Barletta, J.A.; Nucci, M.R.; et al. Targeted Genomic Profiling Reveals Recurrent KRAS Mutations and Gain of Chromosome 1q in Mesonephric Carcinomas of the Female Genital Tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiraishi, K.; Kato, T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers 2021, 13, 6351. [Google Scholar] [CrossRef]
- Hanselaar, A.; van Loosbroek, M.; Schuurbiers, O.; Helmerhorst, T.; Bulten, J.; Bernheim, J. Clear Cell Adenocarcinoma of the Vagina and Cervix. Cancer 1997, 79, 2229–2236. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, Y.; Li, Y.; Huang, H.-F.; Wu, M.; Shen, K.; Pan, L.-Y. Clear Cell Carcinoma of the Uterine Cervix: Clinical Characteristics and Feasibility of Fertility-Preserving Treatment. Onco Targets Ther. 2014, 7, 111. [Google Scholar] [CrossRef]
- Tantitamit, T.; Hamontri, S.; Rangsiratanakul, L. Clear Cell Adenocarcinoma of the Cervix in Second Generation Young Women Who Are without Maternal Exposure to Diethylstilbestrol: A Case Report. Gynecol. Oncol. Rep. 2017, 20, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Howitt, B.E.; Park, K.J.; Lindeman, N.; Nucci, M.R.; Parra-Herran, C. Genomic Characterization of HPV-Related and Gastric-Type Endocervical Adenocarcinoma: Correlation with Subtype and Clinical Behavior. Int. J. Gynecol. Pathol. 2020, 39, 578–586. [Google Scholar] [CrossRef]
- Ueno, S.; Sudo, T.; Oka, N.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; Mikami, Y.; Nishimura, R. Absence of Human Papillomavirus Infection and Activation of PI3K-AKT Pathway in Cervical Clear Cell Carcinoma. Int. J. Gynecol. Cancer 2013, 23, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Selenica, P.; Alemar, B.; Matrai, C.; Talia, K.L.; Veras, E.; Hussein, Y.; Oliva, E.; Beets-Tan, R.G.H.; Mikami, Y.; McCluggage, W.G.; et al. Massively Parallel Sequencing Analysis of 68 Gastric-Type Cervical Adenocarcinomas Reveals Mutations in Cell Cycle-Related Genes and Potentially Targetable Mutations. Mod. Pathol. 2021, 34, 1213–1225. [Google Scholar] [CrossRef]
- Lee, E.K.; Lindeman, N.I.; Matulonis, U.A.; Konstantinopoulos, P.A. POLE-Mutated Clear Cell Cervical Cancer As-sociated with in-Utero Diethylstilbestrol Exposure. Gynecol. Oncol. Rep. 2019, 28, 15–17. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Park, K.J. Cervical Adenocarcinoma: Integration of HPV Status, Pattern of Invasion, Morphology and Molecular Markers into Classification. Histopathology 2020, 76, 112–127. [Google Scholar] [CrossRef]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric Adenocarcinomas of the Uterine Cervix and Corpus. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, S.; Kim, H.S. Comprehensive Immunohistochemical Analysis of Mesonephric Marker Expression in Low-grade Endometrial Endometrioid Carcinoma. Int. J. Gynecol. Pathol. 2024, 43, 221–232. [Google Scholar]
- Devarashetty, S.; Chennapragada, S.S.; Mansour, R. Not Your Typical Adenocarcinoma: A Case of Mesonephric Adenocarcinoma of the Cervix with Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation. Cureus 2022, 14, e25098. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shi, J.; Zhang, X.; Kong, F.; Liu, L.; Dong, X.; Wang, K.; Shen, D. Comprehensive Genomic Profiling and Prognostic Analysis of Cervical Gastric-Type Mucinous Adenocarcinoma. Virchows Arch. 2021, 479, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Molijn, A.; Kazem, S.; Pirog, E.C.; Alemany, L.; de Sanjosé, S.; Dinjens, W.; Quint, W. Molecular and Pathological Basis of HPV-negative Cervical Adenocarcinoma Seen in a Global Study. Int. J. Cancer 2020, 147, 2526–2536. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Song, Y. Clinical pathological and molecular features of 100 patients with gastric-type cervical adenocarcinoma. Diagn. Pathol. 2025, 20, 73. [Google Scholar] [CrossRef]
- Baek, J.Y.; Kim, H.S.; Cho, W.K.; Kim, B.G.; Lee, J.W.; Choi, C.H.; Kim, T.J.; Lee, Y.Y.; Park, W. Signifi-cance of HPV status on tumor response and treatment outcomes in endocervical adenocarcinoma treated with de-finitive chemoradiotherapy: A retrospective study. J. Gynecol. Oncol. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Fan, Y.; He, Y.; Sun, L.; Liu, T.; Shen, Y. Mesonephric adenocarcinoma of the uterine cervix with a prom-inent spindle cell component. Oncol. Lett. 2024, 28, 508. [Google Scholar] [CrossRef]
- Lambrou, N.C.; Amadeo, A. Surgical Gynecologic Oncology. In Breast Cancer and Gynecologic Cancer Rehabilitation; Cristian, A., Ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 171–188. [Google Scholar]
- Zeng, J.; Jiang, W.; Li, K.; Zhang, M.; Chen, J.; Duan, Y.; Li, Q.; Yin, R. Clinical and pathological character-istics of cervical clear cell carcinoma in patients not exposed to diethylstilbestrol: A comprehensive analysis of 49 cases. Front. Oncol. 2024, 14, 1430742. [Google Scholar] [CrossRef]
- Rychlik, A.; Querleu, D.; Bidzinski, M. Fertility Sparing Treatment in Gastric-Type Endocervical Carcinoma. Cancers 2021, 13, 5177. [Google Scholar] [CrossRef]
- Patel, D.; Tayade, S.; Tidke, V.P.; Toshniwal, S.; Tilva, H. Radiotherapy Versus Chemotherapy in Locally Advanced Cervical Cancer. Cureus 2023, 15, e44726. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, D.; Guo, M.; He, M.; He, H.; Li, X.; Zheng, Q.; Li, Q.; Mao, Z. HPV and radiosensitivity of cervical cancer: A narrative review. Ann. Transl. Med. 2022, 10, 1405. [Google Scholar] [CrossRef]
- Barlesi, F.; Scherpereel, A.; Gorbunova, V.; Gervais, R.; Vikström, A.; Chouaid, C.; Chella, A.; Kim, J.H.; Ahn, M.J.; Reck, M.; et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: Updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann. Oncol. 2014, 25, 1044–1052. [Google Scholar]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Huang, W.; Liu, J.; Xu, K.; Chen, H.; Bian, C. PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: From bench to bed. Front. Oncol. 2022, 12, 849352. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, S.; Zhi, W.; Chu, T.; Liu, B.; Peng, T.; Xu, M.; Ding, W.; Cao, C.; Wu, P. Genomic analysis of cervical carcinoma identifies Alpelisib as a therapeutic option for PIK3CA-mutant cervical carcinoma via the PI3K/AKT pathway. J. Med. Virol. 2023, 95, e28656. [Google Scholar] [CrossRef] [PubMed]
- Capivasertib Active against AKT1-Mutated Cancers. Cancer Discov. 2019, 9, OF7. [CrossRef] [PubMed]
- Tinker, A.V.; Ellard, S.; Welch, S.; Moens, F.; Allo, G.; Tsao, M.S.; Squire, J.; Tu, D.; Eisenhauer, E.A.; MacKay, H. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199). Gynecol. Oncol. 2013, 130, 269–274. [Google Scholar] [CrossRef]
- Li, G.; Huang, A.; Zhao, Y.; Li, B.; Ding, Y.; Lai, S.; Zhang, J.; Zhu, H.; Li, D.; Li, Q.; et al. 724P Preliminary outcomes from a phase Ib/II study of the highly potent PI3K-mTOR dual inhibitor WX390 combined with toripalimab in patients with advanced cervical cancer. Ann. Oncol. 2024, 35 (Suppl. 3), S724. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of WX390 Combined with Toripalimab in Patients with Advanced Cervical Cancer (NCT06117566). 2024. Available online: https://www.clinicaltrials.gov/study/NCT06117566 (accessed on 12 October 2025).
- PAN Foundation TrialFinder. A Study of WX390 Combined with Toripalimab in Advanced Gastric-Type En-Docervical Adenocarcinoma. 2024. Available online: https://clinicaltrials.gov/study/NCT06124963 (accessed on 12 October 2025).
- Passarelli, A.; Carbone, V.; Pignata, S.; Mazzeo, R.; Lorusso, D.; Scambia, G.; Canova, S.; Di Palma, T.; Tasca, G.; Mantiero, M.; et al. Alpelisib for PIK3CA-mutated advanced gynecological cancers: First clues of clinical activity. Gynecol. Oncol. 2024, 183, 61–67. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, J.; Liu, W.; Ashby, C.R.; Chen, Z.S., Jr.; Lin, L. Sotorasib: A treatment for non-small cell lung cancer with the KRAS G12C mutation. Drugs Today 2022, 58, 175–185. [Google Scholar] [CrossRef]
- de Guillebon, E.; Jimenez, M.; Mazzarella, L.; Betsou, F.; Stadler, P.; Peták, I.; Jeannot, E.; Chanas, L.; Servant, N.; Marret, G.; et al. Combining immunotherapy with an epidrug in squamous cell carcinomas of different locations: Rationale and design of the PEVO basket trial. ESMO Open 2021, 6, 100106. [Google Scholar] [CrossRef] [PubMed]
- Zahan, U.F.; Sohel, H.I.; Nakayama, K.; Ishikawa, M.; Nagase, M.; Razia, S.; Kanno, K.; Yamashita, H.; Sonia, S.B.; Kyo, S. A Comparative Analysis of Usual- and Gastric-Type Cervical Adenocarcinoma in a Japanese Population Reveals Distinct Clinicopathological and Molecular Features with Prognostic and Therapeutic Insights. Int. J. Mol. Sci. 2025, 26, 7469. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hao, Y.; Han, X.; Wang, B.; Li, L.; Chen, T.; Chen, S.; Zou, L.; Huang, J.; Wang, W.; et al. Therapeutic potential of T-cell receptor targeting the HLA-A*11:01-restricted KRASG12V neoantigen without cross-recognition of the self-antigen RAB7B in solid tumors. J. Immunother. Cancer 2025, 13, e011863. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Weekes, C.D.; Furqan, M.; Kasi, P.M.; Devoe, C.E.; Leal, A.D.; Chung, V.; Perry, J.R.; Kheoh, T.; McNeil, L.K.; et al. Lymph node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: Phase 1 AMPLIFY-201 trial final results. Nat. Med. 2025; online ahead of print. [Google Scholar]
- Elicio Therapeutics. Phase 2 AMPLIFY-7P Interim Immunogenicity Update. Available online: https://elicio.com/press_releases/elicio-therapeutics-reports-eli-002-7p-achieved-robust-mkras-specific-t-cell-responses-in-99-of-evaluable-patients-in-ongoing-phase-2-amplify-7p-trial/ (accessed on 20 September 2025).
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B.; et al. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef]
- Goloudina, A.; Burova, E.; Demidov, O. Shared neoantigens for cancer immunotherapy. Mol. Ther. Oncolytics 2025, 27, 100813. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Schrikkema, D.S.; Gadiot, J.; Gomez-Eerland, R.; Bies, L.; Walker, J.; Spaapen, R.M.; Kok, H.; Houg, D.; Viyacheva, M.; et al. Enabling next-generation engineered TCR-T therapies based on high-throughput TCR discovery from diagnostic tumor biopsies. Nat. Commun. 2025, 16, 649. [Google Scholar] [CrossRef]
- Sueangoen, N.; Thuwajit, P.; Yenchitsomanus, P.T.; Thuwajit, C. Public neoantigens in breast cancer immunotherapy (Review). Int. J. Mol. Med. 2024, 54, 65. [Google Scholar] [CrossRef]
- Vergote, I.; Fujiwara, K.; Kalbacher, E.; Bagaméri, A.; Ghamande, S.; Lee, J.-Y.; Banerjee, S.; Maluf, F.C.; Lorusso, D.; Yonemori, K.; et al. Tisotumab vedotin as second- or third-line therapy for recurrent cervical cancer. N. Engl. J. Med. 2024, 391, 44–55. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Tisotumab Vedotin-Tftv for Recurrent or Meta-Static Cervical Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer (accessed on 29 April 2024).
- Pfizer. FDA Grants Full Approval for TIVDAK® to Treat Recurrent or Metastatic Cervical Cancer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/fda-grants-full-approval-tivdakr-treat-recurrent-or Pfizer (accessed on 29 April 2024).
- The ASCO Post. Recurrent Cervical Cancer: Tisotumab Vedotin in Second- or Third-Line Therapy. Available online: https://ascopost.com/news/july-2024/recurrent-cervical-cancer-tisotumab-vedotin-in-second-or-third-line-therapy/ (accessed on 16 July 2024).
- Andrikopoulou, A.; Zagouri, F.; Goula, K.; Haidopoulos, D.; Thomakos, N.; Svarna, A.; Dimopoulos, M.A.; Liontos, M. Real-world evidence of Trastuzumab Deruxtecan (T-DXd) efficacy in HER2-expressing gyneco-logical malignancies. BMC Cancer 2024, 24, 1503. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 Phase II trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Tostrud, L.; Howitt, B.E.; Mills, M.; Karam, A.; Bixel, K.L. Exceptional response to trastuzumab deruxtec-an in recurrent neuroendocrine cervical cancer: A case report. Gynecol. Oncol. Rep. 2025, 60, 101798. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, Y.; Ding, Y.; Liu, Y.; Wang, Y.; Liu, Y.; Liu, C. The clinicopathological and molecular characteristics of endocervical gastric-type adenocarcinoma and the use of Claudin18.2 as a potential therapeutic target. Mod. Pathol. 2024, 37, 100569. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2025 update. Int. J. Gynaecol. Obstet. 2025, 171 (Suppl. 1), 87–108. [Google Scholar] [CrossRef] [PubMed]

| Gene | Mutation Type | Relevance to Cancer Progression |
|---|---|---|
| TP53 | Missense mutations | Common in HPV-independent tumors; associated with aggressive phenotypes and poor prognosis |
| PIK3CA | Activating mutations | Highly prevalent in HPV-independent tumors; a potential target for PI3K inhibitors |
| KRAS | Point mutations (G12C, G12D) | Frequent in gastric-type and mesonephric carcinomas; linked to treatment resistance |
| STK11 | Loss-of-function mutations | Associated with immune evasion and poor response to therapy |
| PTEN | Loss-of-function mutations | Leads to increased tumor growth and resistance to therapy |
| ARID1A | Frameshift/Truncating mutations | Loss of function results in epigenetic deregulation, contributing to tumor aggressiveness |
| Subtype | Key Mutation Frequencies (Selected Genes) | Survival Outcomes | Treatment Response Rates | Sample Size/# Studies | Evidence Type/Trial Phase |
|---|---|---|---|---|---|
| Gastric-type adenocarcinoma | TP53 ~52%; TP53 73%, KRAS 46%, PIK3CA 27%, STK11 18% (small NGS subset, 2025) [103]. | 3-yr PFS 16.7% (HPVI) vs. 49.8% (HPVA); 3-yr OS 42.3% vs. 90.7% (definitive CCRT cohort) [104]. | Definitive CCRT CR 27.8% (HPVI) vs. 81.8% (HPVA); limited targeted/IO data; HER2/PI3K-selected early reports [103,104]. | NGS n ≈ 11; CCRT cohort n ≈ 40 (HPVI n ≈ 18); multiple small series [103,104]. | Retrospective cohorts; molecular series; no randomized GAS-specific trials. |
| Mesonephric adenocarcinoma | KRAS 75–100% (codons 12/13); occasional PIK3CA; ARID1A/B, SMARCA4 reported [105]. | 5-yr OS ~74%; 5-yr PFS ~60%; high recurrence (~30%) [5,105]. | Surgery ± adjuvant therapy; systemic response rates not well defined [105]. | Aggregated literature ~30–60 cases; single-institution series often <20 [105]. | Retrospective case series; no randomized trials. |
| Clear-cell carcinoma | HNF1β /Napsin A expression; ARID1A/PIK3CA/PTEN variably reported [106]. | 5-yr PFS ~87%; 5-yr OS ~88% (single-center cohort, n ≈ 49) [107]. | Early-stage surgery predominant; systemic therapy/IO response not robustly quantified. | Single-center cohort n ≈ 49; smaller series [107]. | Retrospective cohort; no randomized trials. |
| True endometrioid adenocarcinoma | PTEN, PIK3CA, KRAS, CTNNB1 common; TP53 variable [5]. | Cervix-specific PFS/OS limited; intermediate; many NR in 2024–2025 [5]. | Subtype-specific chemo/targeted/IO rates not established [5]. | Sparse subtype-dedicated cohorts; embedded in mixed series [5]. | Narrative/retrospective; no prospective subtype-focused trials. |
| Feature | HPV-Associated | HPV-Independent |
|---|---|---|
| Stage at diagnosis | Often detected at earlier stages through HPV-based screening programs, since precursor lesions can be identified | More frequently diagnosed at advanced FIGO stages due to absence of precursor lesions and lower detectability |
| Nodal/peritoneal spread | Lymphovascular and nodal spread may occur but are less commonly highlighted | Higher rates of lymphovascular invasion, parametrial extension, and peritoneal or distant spread |
| Response to chemotherapy/radiation | Generally more responsive; viral oncogenes (E6/E7) enhance radiosensitivity and increase treatment efficacy | Reduced responsiveness; mutant/wild-type p53 aids DNA repair, while PI3K/Akt/mTOR and RAS/MAPK pathways drive resistance |
| Survival (stage-adjusted) | Better overall outcomes and higher survival at comparable stages | Worse prognosis with higher recurrence rates, particularly in gastric-type and mesonephric subtypes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurjui, R.M.; Hurjui, I.A.; Buțureanu, T.A.; Popovici, D.; Avădănei, E.-R.; Balan, R.A. HPV-Independent Cervical Cancer—A New Challenge of Modern Oncology. Int. J. Mol. Sci. 2025, 26, 10051. https://doi.org/10.3390/ijms262010051
Hurjui RM, Hurjui IA, Buțureanu TA, Popovici D, Avădănei E-R, Balan RA. HPV-Independent Cervical Cancer—A New Challenge of Modern Oncology. International Journal of Molecular Sciences. 2025; 26(20):10051. https://doi.org/10.3390/ijms262010051
Chicago/Turabian StyleHurjui, Ruxandra Maria, Ion Andrei Hurjui, Tudor Andrei Buțureanu, Diana Popovici, Elena-Roxana Avădănei, and Raluca Anca Balan. 2025. "HPV-Independent Cervical Cancer—A New Challenge of Modern Oncology" International Journal of Molecular Sciences 26, no. 20: 10051. https://doi.org/10.3390/ijms262010051
APA StyleHurjui, R. M., Hurjui, I. A., Buțureanu, T. A., Popovici, D., Avădănei, E.-R., & Balan, R. A. (2025). HPV-Independent Cervical Cancer—A New Challenge of Modern Oncology. International Journal of Molecular Sciences, 26(20), 10051. https://doi.org/10.3390/ijms262010051






