Targeting Inflammation: Cytosporone B Modulates Imatinib-Driven Biochemical Alterations in Rat Heart
Abstract
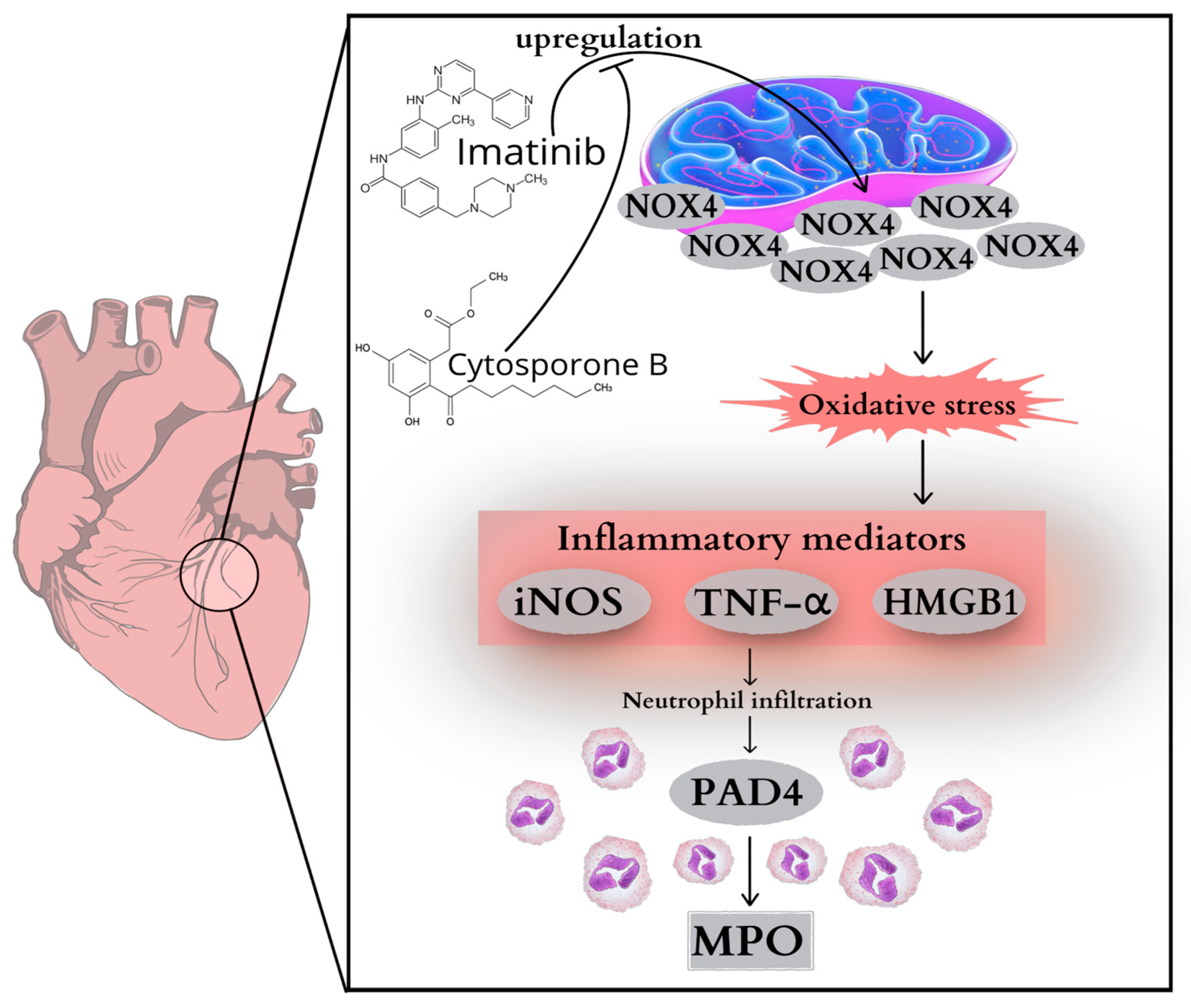
1. Introduction
2. Results
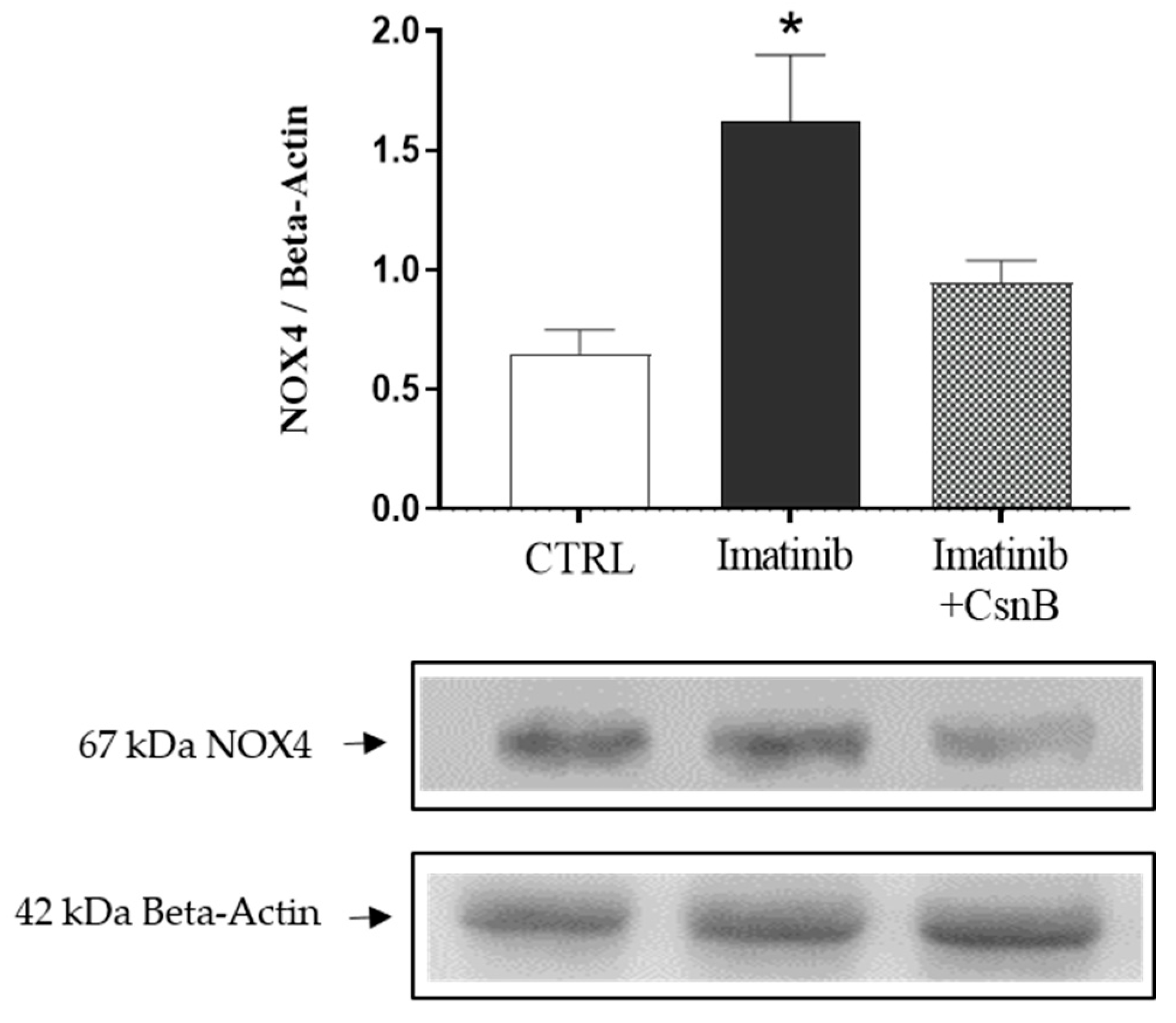
2.1. Cardiac NOX4 Expression
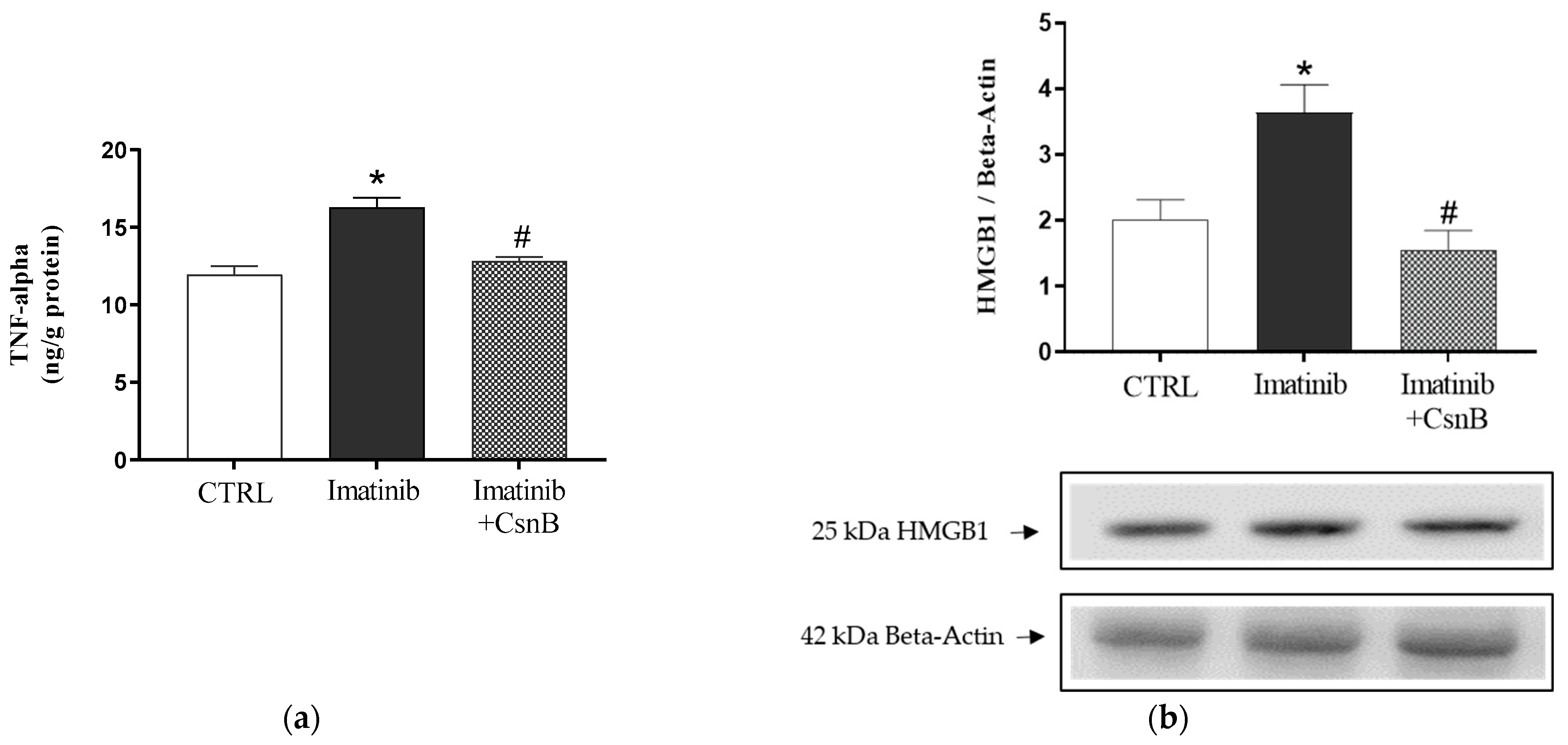
2.2. TNF-α Concentration and HMGB1 Expression of the Heart
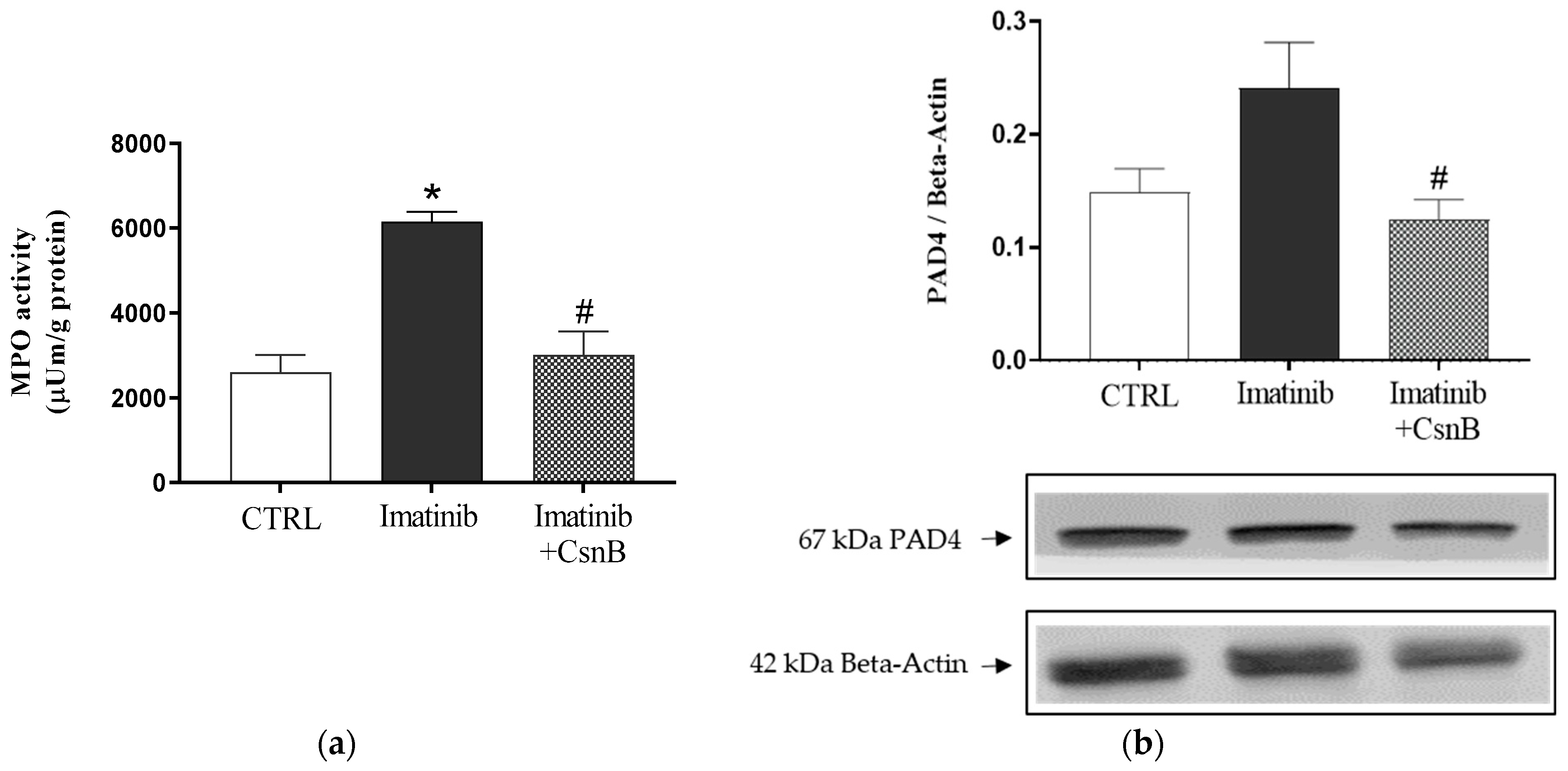
2.3. MPO Activity and PAD4 Expression of the Heart
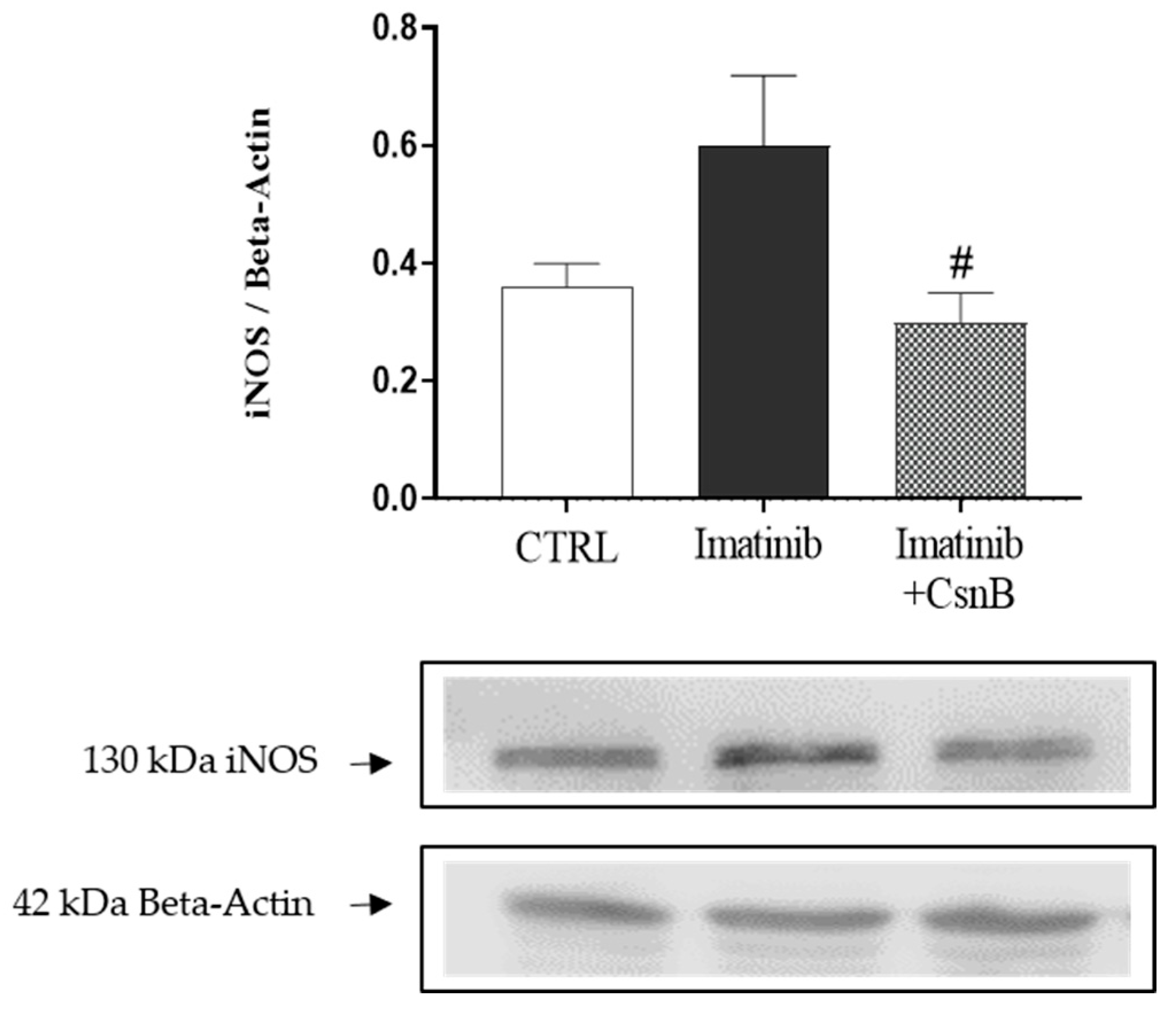
2.4. Cardiac iNOS Expression
3. Discussion
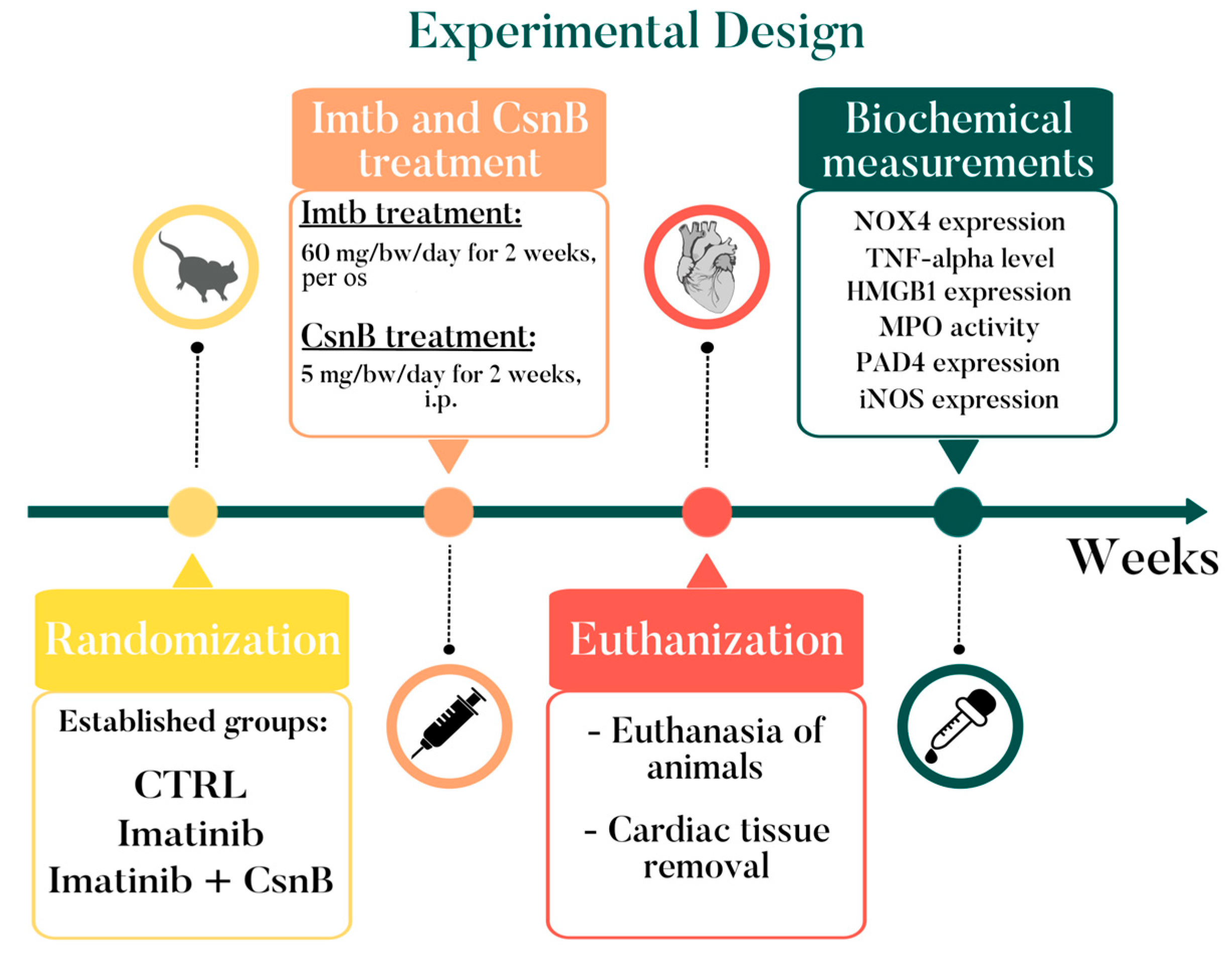
4. Materials and Methods
4.1. Animals
4.2. Determining Cardiac HMGB1, PAD4, NOX4, and iNOS Expressions
4.3. Measurement of Cardiac TNF-α Concentration
4.4. Measurement of Cardiac MPO Enzymatic Activity
4.5. Statistical Analysis
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CsnB | Cytosporone B |
| CTRL | control |
| DAMP | damage-associated molecular pattern |
| ELISA | enzyme-linked immunosorbent assay |
| Imtb | Imatinib |
| iNOS | inducible nitric oxide synthase |
| MPO | myeloperoxidase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NET | neutrophil extracellular trap |
| NO | nitric oxide |
| NOX4 | NADPH oxidase |
| NR4A | nuclear receptor subfamily 4 group A |
| H2O2 | hydrogen peroxide |
| HMGB1 | high mobility group box 1 |
| PAD4 | peptidylarginine deiminase 4 |
| PBS | phosphate buffer |
| TNF-α | tumor necrosis factor-alpha |
References
- Nagy, A.; Borzsei, D.; Hoffmann, A.; Torok, S.; Veszelka, M.; Almasi, N.; Varga, C.; Szabo, R. A Comprehensive Overview on Chemotherapy-Induced Cardiotoxicity: Insights into the Underlying Inflammatory and Oxidative Mechanisms. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef]
- Moslehi, J.J. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N. Engl. J. Med. 2016, 375, 1457–1467. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Mansour, H.H.; El Kiki, S.M.; Ibrahim, A.B.; Omran, M.M. Effect of l-carnitine on cardiotoxicity and apoptosis induced by imatinib through PDGF/ PPARgamma /MAPK pathways. Arch. Biochem. Biophys. 2021, 704, 108866. [Google Scholar] [CrossRef]
- Natorska, J.; Zabczyk, M.; Undas, A. Neutrophil extracellular traps (NETs) in cardiovascular diseases: From molecular mechanisms to therapeutic interventions. Kardiol. Pol. 2023, 81, 1205–1216. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Du, X.; Chen, H.; Liu, J.; Zhao, B.; Huang, D.; Li, G.; Xu, Q.; Zhang, M.; Weimer, B.C.; et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 2008, 4, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Volkers, M.; Din, S.; Avitabile, D.; Khan, M.; Gude, N.; Mohsin, S.; Bo, T.; Truffa, S.; Alvarez, R.; et al. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur. Heart J. 2011, 32, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.C.; Zhang, Z.; Pei, L.; Saito, T.; Tontonoz, P.; Pilch, P.F. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol. Endocrinol. 2007, 21, 2152–2163. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, M.; Yi, B.; You, X.; Yang, P.; Sun, J. Orphan nuclear receptor Nur77 is a novel negative regulator of endothelin-1 expression in vascular endothelial cells. J. Mol. Cell Cardiol. 2014, 77, 20–28. [Google Scholar] [CrossRef]
- Ismaiel, M.; Murphy, B.; Aldhafiri, S.; Giffney, H.E.; Thornton, K.; Mukhopadhya, A.; Keogh, C.E.; Fattah, S.; Mohan, H.M.; Cummins, E.P.; et al. The NR4A agonist, Cytosporone B, attenuates pro-inflammatory mediators in human colorectal cancer tissue ex vivo. Biochem. Biophys. Res. Commun. 2021, 554, 179–185. [Google Scholar] [CrossRef]
- Yan, J.; Huang, J.; Wu, J.; Fan, H.; Liu, A.; Qiao, L.; Shen, M.; Lai, X. Nur77 attenuates inflammatory responses and oxidative stress by inhibiting phosphorylated IkappaB-alpha in Parkinson’s disease cell model. Aging 2020, 12, 8107–8119. [Google Scholar] [CrossRef] [PubMed]
- Patino-Martinez, E.; Solis-Barbosa, M.A.; Santana, E.; Gonzalez-Dominguez, E.; Segovia-Gamboa, N.C.; Meraz-Rios, M.A.; Cordova, E.J.; Valdes, J.; Corbi, A.L.; Sanchez-Torres, C. The Nurr7 agonist Cytosporone B differentially regulates inflammatory responses in human polarized macrophages. Immunobiology 2022, 227, 152299. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Iqbal, N. Imatinib: A breakthrough of targeted therapy in cancer. Chemother. Res. Pract. 2014, 2014, 357027, Correction in Chemother. Res. Pract. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Samanci, N.S.; Guliyev, M.; Degerli, E.; Celik, E.; Turna, Z.H. A rare case of cardiac toxicity in a patient with imatinib treatment: Case report. J. Cancer Res. Ther. 2022, 18, 792–794. [Google Scholar] [CrossRef]
- Hu, W.; Lu, S.; McAlpine, I.; Jamieson, J.D.; Lee, D.U.; Marroquin, L.D.; Heyen, J.R.; Jessen, B.A. Mechanistic investigation of imatinib-induced cardiac toxicity and the involvement of c-Abl kinase. Toxicol. Sci. 2012, 129, 188–199. [Google Scholar] [CrossRef][Green Version]
- Savi, M.; Frati, C.; Cavalli, S.; Graiani, G.; Galati, S.; Buschini, A.; Madeddu, D.; Falco, A.; Prezioso, L.; Mazzaschi, G.; et al. Imatinib mesylate-induced cardiomyopathy involves resident cardiac progenitors. Pharmacol. Res. 2018, 127, 15–25. [Google Scholar] [CrossRef]
- Kerkela, R.; Grazette, L.; Yacobi, R.; Iliescu, C.; Patten, R.; Beahm, C.; Walters, B.; Shevtsov, S.; Pesant, S.; Clubb, F.J.; et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat. Med. 2006, 12, 908–916. [Google Scholar] [CrossRef]
- Maharsy, W.; Aries, A.; Mansour, O.; Komati, H.; Nemer, M. Ageing is a risk factor in imatinib mesylate cardiotoxicity. Eur. J. Heart Fail. 2014, 16, 367–376. [Google Scholar] [CrossRef]
- Kobara, M.; Nessa, N.; Toba, H.; Nakata, T. Induction of autophagy has protective roles in imatinib-induced cardiotoxicity. Toxicol. Rep. 2021, 8, 1087–1097. [Google Scholar] [CrossRef]
- Herman, E.H.; Knapton, A.; Rosen, E.; Thompson, K.; Rosenzweig, B.; Estis, J.; Agee, S.; Lu, Q.A.; Todd, J.A.; Lipshultz, S.; et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicol. Pathol. 2011, 39, 1091–1106. [Google Scholar] [CrossRef]
- Stevenson, M.D.; Canugovi, C.; Vendrov, A.E.; Hayami, T.; Bowles, D.E.; Krause, K.H.; Madamanchi, N.R.; Runge, M.S. NADPH Oxidase 4 Regulates Inflammation in Ischemic Heart Failure: Role of Soluble Epoxide Hydrolase. Antioxid. Redox Signal 2019, 31, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Vendrov, K.C.; Smith, A.; Yuan, J.; Sumida, A.; Robidoux, J.; Runge, M.S.; Madamanchi, N.R. NOX4 NADPH Oxidase-Dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease. Antioxid. Redox Signal 2015, 23, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Matsushima, S.; Zhai, P.; Schneider, M.D.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. USA 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Higashi, M.; Shimokawa, H.; Hattori, T.; Hiroki, J.; Mukai, Y.; Morikawa, K.; Ichiki, T.; Takahashi, S.; Takeshita, A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: Effect on endothelial NAD(P)H oxidase system. Circ. Res. 2003, 93, 767–775. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Monestier, M.; Esmon, N.L.; Esmon, C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011, 187, 2626–2631. [Google Scholar] [CrossRef]
- Du, M.; Yang, L.; Gu, J.; Wu, J.; Ma, Y.; Wang, T. Inhibition of Peptidyl Arginine Deiminase-4 Prevents Renal Ischemia-Reperfusion-Induced Remote Lung Injury. Mediators Inflamm. 2020, 2020, 1724206. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Kostin, S.; Krizanic, F.; Kelesidis, T.; Pagonas, N. The role of NETosis in heart failure. Heart Fail. Rev. 2024, 29, 1097–1106. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Ja, K.; Chua, J.; Cong, S.; Shim, W.; Hausenloy, D.J. Myeloperoxidase As a Multifaceted Target for Cardiovascular Protection. Antioxid. Redox Signal 2020, 32, 1135–1149. [Google Scholar] [CrossRef]
- Nettersheim, F.S.; Schluter, J.D.; Kreuzberg, W.; Mehrkens, D.; Grimm, S.; Nemade, H.; Braumann, S.; Hof, A.; Guthoff, H.; Peters, V.; et al. Myeloperoxidase is a critical mediator of anthracycline-induced cardiomyopathy. Basic. Res. Cardiol. 2023, 118, 36. [Google Scholar] [CrossRef]
- Farahani, A.; Farahani, A.; Kashfi, K.; Ghasemi, A. Inducible nitric oxide synthase (iNOS): More than an inducible enzyme? Rethinking the classification of NOS isoforms. Pharmacol. Res. 2025, 216, 107781. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, V.; Scheiper, S.; Roehr, W.; Niess, C.; Kippenberger, S.; Steinhorst, K.; Verhoff, M.A.; Kauferstein, S. Increased inducible nitric oxide synthase (iNOS) expression in human myocardial infarction. Int. J. Legal Med. 2020, 134, 575–581. [Google Scholar] [CrossRef] [PubMed]
- de Leseleuc, L.; Denis, F. Inhibition of apoptosis by Nur77 through NF-kappaB activity modulation. Cell Death Differ. 2006, 13, 293–300. [Google Scholar] [CrossRef]
- Popichak, K.A.; Hammond, S.L.; Moreno, J.A.; Afzali, M.F.; Backos, D.S.; Slayden, R.D.; Safe, S.; Tjalkens, R.B. Compensatory Expression of Nur77 and Nurr1 Regulates NF-kappaB-Dependent Inflammatory Signaling in Astrocytes. Mol. Pharmacol. 2018, 94, 1174–1186. [Google Scholar] [CrossRef]
- Tian, H.; Chen, F.; Wang, Y.; Liu, Y.; Ma, G.; Zhao, Y.; Ma, Y.; Tian, T.; Ma, R.; Yu, Y.; et al. Nur77 Prevents Osteoporosis by Inhibiting the NF-kappaB Signalling Pathway and Osteoclast Differentiation. J. Cell Mol. Med. 2022, 26, 2163–2176. [Google Scholar] [CrossRef]
- Kurakula, K.; Vos, M.; Logiantara, A.; Roelofs, J.J.; Nieuwenhuis, M.A.; Koppelman, G.H.; Postma, D.S.; van Rijt, L.S.; de Vries, C.J. Nuclear Receptor Nur77 Attenuates Airway Inflammation in Mice by Suppressing NF-kappaB Activity in Lung Epithelial Cells. J. Immunol. 2015, 195, 1388–1398. [Google Scholar] [CrossRef]
- Cortes, J.E.; Baccarani, M.; Guilhot, F.; Druker, B.J.; Branford, S.; Kim, D.W.; Pane, F.; Pasquini, R.; Goldberg, S.L.; Kalaycio, M.; et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: Tyrosine kinase inhibitor optimization and selectivity study. J. Clin. Oncol. 2010, 28, 424–430. [Google Scholar] [CrossRef]
- Yoo, C.; Ryu, M.H.; Ryoo, B.Y.; Beck, M.Y.; Kang, Y.K. Efficacy, safety, and pharmacokinetics of imatinib dose escalation to 800 mg/day in patients with advanced gastrointestinal stromal tumors. Investig. New Drugs 2013, 31, 1367–1374. [Google Scholar] [CrossRef]
- Liu, T.Y.; Yang, X.Y.; Zheng, L.T.; Wang, G.H.; Zhen, X.C. Activation of Nur77 in microglia attenuates proinflammatory mediators production and protects dopaminergic neurons from inflammation-induced cell death. J. Neurochem. 2017, 140, 589–604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börzsei, D.; Nagy, A.; Kiss, V.; Virág, Z.; Kis, G.; Almási, N.; Török, S.; Veszelka, M.; Varga, C.; Szabó, R. Targeting Inflammation: Cytosporone B Modulates Imatinib-Driven Biochemical Alterations in Rat Heart. Int. J. Mol. Sci. 2025, 26, 10018. https://doi.org/10.3390/ijms262010018
Börzsei D, Nagy A, Kiss V, Virág Z, Kis G, Almási N, Török S, Veszelka M, Varga C, Szabó R. Targeting Inflammation: Cytosporone B Modulates Imatinib-Driven Biochemical Alterations in Rat Heart. International Journal of Molecular Sciences. 2025; 26(20):10018. https://doi.org/10.3390/ijms262010018
Chicago/Turabian StyleBörzsei, Denise, András Nagy, Viktória Kiss, Zoltán Virág, Gyöngyi Kis, Nikoletta Almási, Szilvia Török, Médea Veszelka, Csaba Varga, and Renáta Szabó. 2025. "Targeting Inflammation: Cytosporone B Modulates Imatinib-Driven Biochemical Alterations in Rat Heart" International Journal of Molecular Sciences 26, no. 20: 10018. https://doi.org/10.3390/ijms262010018
APA StyleBörzsei, D., Nagy, A., Kiss, V., Virág, Z., Kis, G., Almási, N., Török, S., Veszelka, M., Varga, C., & Szabó, R. (2025). Targeting Inflammation: Cytosporone B Modulates Imatinib-Driven Biochemical Alterations in Rat Heart. International Journal of Molecular Sciences, 26(20), 10018. https://doi.org/10.3390/ijms262010018







