Antiplasmodial Activity of a New Chemotype of Croton sylvaticus Hochst. Ex C. Krauss Essential Oil
Abstract
1. Introduction
2. Results
2.1. Extraction of Essential Oils from C. sylvaticus
2.2. Chemical Composition of Essential Oils from C. sylvaticus
2.2.1. Root Essential Oil
2.2.2. Trunk Bark Essential Oil
2.2.3. Leaf Essential Oil
2.3. Evaluation of the Antiplasmodial Activities of Essential Oils from C. sylvaticus
2.3.1. Inhibition of P. falciparum Asexual and Sexual-Blood Stages by Essential Oils from C. sylvaticus
2.3.2. Inhibition of the Asexual Stage of P. falciparum in the Blood by Compounds from Trunk Bark Essential Oil
2.3.3. Cytotoxicity Assay
3. Discussion
3.1. Extraction and Characterization of Essential Oils from C. sylvaticus
3.2. Antiplasmodial Activity and Cytotoxicity
4. Sustainability of C. sylvaticus Essential Oil
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Plant Material
5.3. Essential Oil Hydrodistillation
5.4. Essential Oil Analysis, GC-MS, and Data Analysis
5.5. Biological Assay
5.5.1. In Vitro Inhibition of P. falciparum Asexual-Blood Stages
5.5.2. In Vitro Assessment of the Gametocidal Activity
Induction of Gametocytogenesis and Maintenance of Parasite Cultures
Measurement of Gametocytocidal Activity
5.5.3. In Vitro Resazurin-Based Cytotoxicity Assay
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tali, M.B.T.; Kamdem, B.P.; Tchouankeu, J.C.; Boyom, F.F. Current Developments on the Antimalarial, Antileishmanial, and Antitrypanosomal Potential and Mechanisms of Action of Terminalia spp. S. Afr. J. Bot. 2023, 156, 309–333. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 11 April 2023).
- Mianda, S.M.; Invernizzi, L.; van der Watt, M.E.; Reader, J.; Moyo, P.; Birkholtz, L.-M.; Maharaj, V.J. In Vitro Dual Activity of Aloe marlothii Roots and Its Chemical Constituents against Plasmodium falciparum Asexual and Sexual Stage Parasites. J. Ethnopharmacol. 2022, 297, 115551. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 14 January 2025).
- Sinha, A.; Hughes, K.R.; Modrzynska, K.K.; Otto, T.D.; Pfander, C.; Dickens, N.J.; Religa, A.A.; Bushell, E.; Graham, A.L.; Cameron, R. A Cascade of DNA-Binding Proteins for Sexual Commitment and Development in Plasmodium. Nature 2014, 507, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E. History of the Discovery of the Malaria Parasites and Their Vectors. Parasit. Vectors 2010, 3, 5. [Google Scholar] [CrossRef]
- Dongmo, K.J.J.; Tali, M.B.T.; Fongang, Y.S.F.; Taguimjeu, P.L.K.T.; Kagho, D.U.K.; Bitchagno, G.T.; Lenta, B.N.; Boyom, F.F.; Sewald, N.; Ngouela, S.A. In Vitro Antiplasmodial Activity and Toxicological Profile of Extracts, Fractions and Chemical Constituents of Leaves and Stem Bark from Dacryodes edulis (Burseraceae). BMC Complement. Med. Ther. 2023, 23, 211. [Google Scholar] [CrossRef]
- Gouni, C.D.; Fongang, Y.S.F.; Dize, D.; Tabekoueng, G.B.; Kagho, D.U.K.; Bitchagno, G.T.M.; Bankeu, J.J.K.; Lenta, B.N.; Fekam, F.B.; Sewald, N. Bioguided Antiplasmodial Isolation of Six New Labdane-Type Diterpenoids from the Stem Bark of Croton sylvaticus Hochst. Ex. Krauss. Phytochem. Lett. 2024, 59, 92–100. [Google Scholar] [CrossRef]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A Review of Biological Activities and Phytochemistry of Six Ethnomedicinally Important South African Croton Species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, B.S.; Negri, G.; Cordeiro, I.; Motta, L.B.; Salatino, A. Taxonomic Significance of the Distribution of Constituents of Leaf Cuticular Waxes of Croton Species (Euphorbiaceae). Biochem. Syst. Ecol. 2020, 92, 104106. [Google Scholar] [CrossRef]
- Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef] [PubMed]
- Van Ee, B.W.; Riina, R.; Berry, P.E. A Revised Infrageneric Classification and Molecular Phylogeny of New World Croton (Euphorbiaceae). Taxon 2011, 60, 791–823. [Google Scholar] [CrossRef]
- Da Costa, L.S.; de Moraes, Â.A.B.; Cruz, J.N.; Mali, S.N.; Almeida, L.Q.; do Nascimento, L.D.; Ferreira, O.O.; Varela, E.L.P.; Percário, S.; de Oliveira, M.S. First Report on the Chemical Composition, Antioxidant Capacity, and Preliminary Toxicity to Artemia salina L. of Croton campinarensis Secco, A. Rosário & PE Berry (Euphorbiaceae) Essential Oil, and in Silico Study. Antioxidants 2022, 11, 2410. [Google Scholar] [CrossRef] [PubMed]
- Luu-Dam, N.A.; Le, C.V.C.; Satyal, P.; Le, T.M.H.; Bui, V.H.; Vo, V.H.; Ngo, G.H.; Bui, T.C.; Nguyen, H.H.; Setzer, W.N. Chemistry and Bioactivity of Croton Essential Oils: Literature Survey and Croton hirtus from Vietnam. Molecules 2023, 28, 2361. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.S.; do Nascimento, Y.M.; do Espirito-Santo, R.F.; Meira, C.S.; Santos, I.P.; Brandão, R.B.; Souto, A.L.; Guedes, M.L.S.; Soares, M.B.P.; Villarreal, C.F. Phenylpropanoids from Croton velutinus with Cytotoxic, Trypanocidal and Anti-Inflammatory Activities. Fitoterapia 2020, 145, 104632. [Google Scholar] [CrossRef]
- Araújo, A.C.; Freitas, P.R.; Rodrigues dos Santos Barbosa, C.; Muniz, D.F.; Esmeraldo Rocha, J.; Neto, J.B.d.A.; da Silva, M.M.C.; Moura, T.F.; Pereira, R.L.; Ribeiro-Filho, J. Essential Oil of Croton ceanothifolius Baill. Potentiates the Effect of Antibiotics against Multiresistant Bacteria. Antibiotics 2020, 9, 27. [Google Scholar] [CrossRef]
- de Almeida, T.S.; Rocha, J.B.T.; Rodrigues, F.F.G.; Campos, A.R.; da Costa, J.G.M. Chemical Composition, Antibacterial and Antibiotic Modulatory Effect of Croton campestris Essential Oils. Ind. Crops Prod. 2013, 44, 630–633. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Gracioso, J.S.; Nunes, D.S.; Brito, A.S. Effects of an Essential Oil from the Bark of Croton cajucara Benth. on Experimental Gastric Ulcer Models in Rats and Mice. J. Pharm. Pharmacol. 2010, 51, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.R.; Silva, M.A.P.D.; Santos, A.C.B.D.; Coutinho, H.D.M.; Duarte, A.E.; Costa, J.G.M.D. Antimicrobial, Modulatory and Chemical Analysis of the Oil of Croton limae. Pharm. Biol. 2017, 55, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; de Andrade Teles, R.B.; Silva, M.G.; Diniz, T.C.; Dos Santos, U.S.; de Souza, A.V.V.; Nunes, C.E.P.; Salvador, M.J. Neuropharmacological Effects of Essential Oil from the Leaves of Croton conduplicatus Kunth and Possible Mechanisms of Action Involved. J. Ethnopharmacol. 2018, 221, 65–76. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional Usage, Phytochemistry and Pharmacology of Croton sylvaticus Hochst. Ex, C. Krauss. Asian Paci. J. Trop. Med. 2017, 10, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, G.H.C. sylvaticus Hochst. Ex C. Krauss. In Plants Resources of Tropical Africa Medicinal Plants 1; Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA Foundation: Wageningen, The Netherlands, 2008; Volume 11, pp. 212–214. [Google Scholar]
- Lovett, J.C.; Ruffo, C.K.; Gereau, R.E.; Taplin, J.R. Field Guide to the Moist Forest Trees of Tanzania; Society for Environmental Exploration: London, UK, 2006. [Google Scholar]
- Kapingu, M.C.; Mbwambo, Z.H.; Moshi, M.J.; Magadula, J.J. Brine Shrimp Lethality of Alkaloids from Croton sylvaticus Hoechst. East Cent. Afr. J. Pharm. Sci. 2012, 15, 35–37. [Google Scholar]
- Beentje, H.J. Kenya: Trees, Shrubs and Lianas; Majestic Printing Works Ltd.: Nairobi, Kenya, 1994; p. 722. [Google Scholar]
- Schmidt, E.; Lotter, M.; McCleland, W. Trees and Shrubs of Mpumalanga and Kruger National Park; Jacana: Johannesburg, South Africa, 2002; p. 702. [Google Scholar]
- Bryant, A.T. Zulu Medicine and Medicine-Men. 1966. Available online: http://refhub.elsevier.com/S1995-7645(17)30422-4/sref21 (accessed on 24 May 2024).
- Gerstner, J. A Preliminary Check List Of Zulu Names of Plants: With Short Notes. Bantu Stud. 1941, 15, 277–301. [Google Scholar] [CrossRef]
- Frum, Y.; Viljoen, A.M. In Vitro 5-Lipoxygenase and Anti-Oxidant Activities of South African Medicinal Plants Commonly Used Topically for Skin Diseases. Ski. Pharmacol. Physiol. 2006, 19, 329–335. [Google Scholar] [CrossRef]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; De Oliveira, M.S., Da Costa, W.A., Silva, G.S., Eds.; Museu Paraense Emílio Goeldi: Belém, Brazil, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plants; El-Shemy, H.: Cairo University, Egypt, 2021; pp. 1–39. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Madeley, L.G.; Van Wyk, B.-E. Volatiles from African Species of Croton (Euphorbiaceae), Including New Diterpenes in Essential Oil from Croton gratissimus. Heliyon 2019, 5, e02677. [Google Scholar] [CrossRef]
- Mwangi, J.W.; Thoithi, G.N.; Addae-Mensah, I.; Achenbach, H.; Lwande, W.; Hassanali, A. Aromatic Plants of Kenya III: Volatile and Some Non-Volatile Constituents of the Croton sylvaticus Hochst. East Cent. Afr. J. Pharm. Sci. 1998, 1, 41–43. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Séguin, J.-C.; Gagnon, D.; Bélanger, S.; Richard, D.; Fernandez, X.; Boudreau, S.; Voyer, N. Chemical Composition and Antiplasmodial Activity of the Essential Oil of Rhododendron subarcticum Leaves from Nunavik, Québec, Canada. ACS Omega 2023, 8, 16729–16737. [Google Scholar] [CrossRef]
- Muganza, D.M.; Fruth, B.; Nzunzu, J.L.; Tuenter, E.; Foubert, K.; Cos, P.; Maes, L.; Kanyanga, R.C.; Exarchou, V.; Apers, S. In Vitro Antiprotozoal Activity and Cytotoxicity of Extracts and Isolated Constituents from Greenwayodendron suaveolens. J. Ethnopharmacol. 2016, 193, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Do Vale, J.P.C.; Vasconcelos, M.A.; Arruda, F.V.S.; Firmino, N.C.S.; Pereira, A.L.; Andrade, A.L.; Saker-Sampaio, S.; Sampaio, A.H.; Marinho, E.S.; Teixeira, A.M.R.; et al. Evaluation of Antimicrobial and Antioxidant Potential of Essential Oil from Croton piauhiensis Müll. Arg. Curr. Microbiol. 2021, 78, 1926–1938. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Viljoen, A.M. In Vitro Evidence of Phyto-Synergy for Plant Part Combinations of Croton gratissimus (Euphorbiaceae) Used in African Traditional Healing. J. Ethnopharmacol. 2008, 119, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Huy Hung, N.; Ngoc Dai, D.; Satyal, P.; Thi Huong, L.; Thi Chinh, B.; Quang Hung, D.; Anh Tai, T.; Setzer, W.N. Lantana camara Essential Oils from Vietnam: Chemical Composition, Molluscicidal, and Mosquito Larvicidal Activity. Chem. Biodivers. 2021, 18, e2100145. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Demirci, B.; Demirci, F.; Baser, K.H.C. Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules 2019, 24, 4421. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-I.; Hong, J.-M.; Choi, J.-W.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. β-Caryophyllene Alleviates D-Galactosamine and Lipopolysaccharide-Induced Hepatic Injury through Suppression of the TLR4 and RAGE Signaling Pathways. Eur. J. Pharmacol. 2015, 764, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 Receptor Agonist Produces Multiple Behavioral Changes Relevant to Anxiety and Depression in Mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef]
- Chang, H.-J.; Kim, J.-M.; Lee, J.-C.; Kim, W.-K.; Chun, H.S. Protective Effect of β-Caryophyllene, a Natural Bicyclic Sesquiterpene, Against Cerebral Ischemic Injury. J. Med. Food 2013, 16, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb Rebey, I.; Bourgou, S.; Aidi Wannes, W.; Hamrouni Selami, I.; Saidani Tounsi, M.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative Assessment of Phytochemical Profiles and Antioxidant Properties of Tunisian and Egyptian Anise (Pimpinella anisum L.) Seeds. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 971–978. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Wognin, E.L.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, Z.F.; Fauconnier, M.-L. A New Chemotype of Lantana rhodesiensis Moldenke Essential Oil from Côte d’Ivoire: Chemical Composition and Biological Activities. Ind. Crops Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Dodoš, T.; Rajčević, N.; Janaćković, P.; Vujisić, L.; Marin, P.D. Essential Oil Profile in Relation to Geographic Origin and Plant Organ of Satureja Kitaibelii Wierzb. Ex Heuff. Ind. Crops Prod. 2019, 139, 111549. [Google Scholar] [CrossRef]
- Torras, J.; Grau, M.D.; López, J.F.; De Las Heras, F.X.C. Analysis of Essential Oils from Chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007, 87, 2327–2333. [Google Scholar] [CrossRef]
- Amorese, V.; Donadu, M.; Usai, D.; Sanna, A.; Milia, F.; Pisanu, F.; Molicotti, P.; Zanetti, S.; Doria, C. In Vitro Activity of Essential Oils against Pseudomonas aeruginosa Isolated from Infected Hip Implants. J. Infect. Dev. Ctries 2018, 12, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical Composition, Antimicrobial, Antioxidant and Antitumor Activity of Thymus serpyllum L.; Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. Essential Oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Kaloustian, J.; Abou, L.; Mikail, C.; Amiot, M.J.; Portugal, H. Southern French Thyme Oils: Chromatographic Study of Chemotypes. J. Sci. Food Agric. 2005, 85, 2437–2444. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial Activity and Chemical Composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis Essential Oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Thompson, J.D.; Chalchat, J.-C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and Quantitative Variation in Monoterpene Co-Occurrence and Composition in the Essential Oil of Thymus vulgaris Chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef]
- De Pooter, H.L.; Vermeesch, J.; Schamp, N.M. The Essential Oils of Tanacetum vulgare L. and Tanacetum parthenium (L.) Schultz-Bip. J. Essent. Oil Res. 1989, 1, 9–13. [Google Scholar] [CrossRef]
- Dragland, S.; Rohloff, J.; Mordal, R.; Iversen, T.-H. Harvest Regimen Optimization and Essential Oil Production in Five Tansy (Tanacetum vulgare L.) Genotypes under a Northern Climate. J. Agric. Food Chem. 2005, 53, 4946–4953. [Google Scholar] [CrossRef]
- Rohloff, J.; Mordal, R.; Dragland, S. Chemotypical Variation of Tansy (Tanacetum vulgare L.) from 40 Different Locations in Norway. J. Agric. Food Chem. 2004, 52, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.; Yang, B. Extraction of Essential Oils from Five Cinnamon Leaves and Identification of Their Volatile Compound Compositions. Innov. Food Sci. Emerg. Technol. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Moarefian, M.; Barzegar, M.; Sattari, M. Cinnamomum zeylanicum essential oil as a natural antioxidant and antibactrial in cooked sausage: Cinnamomum zeylanicum essential oil as a natural preservative. J. Food Biochem. 2013, 37, 62–69. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wimalasena, S.; Jayatilake, G.S.; Jayawardena, A.L.; Senanayake, U.M.; Mubarak, A.M. A Comparison of Essential Oil Constituents of Bark, Leaf, Root and Fruit of Cinnamon (Cinnamomum zeylanicum Blum) Grown in Sri Lanka. J. Natl. Sci. Found. Sri. 2001, 29, 147–153. [Google Scholar] [CrossRef]
- Boyom, F.F.; Ngouana, V.; Zollo, P.H.A.; Menut, C.; Bessiere, J.M.; Gut, J.; Rosenthal, P.J. Composition and Anti-Plasmodial Activities of Essential Oils from Some Cameroonian Medicinal Plants. Phytochemistry 2003, 64, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Boyom, F.F.; Ngouana, V.; Kemgne, E.A.M.; Zollo, P.H.A.; Menut, C.; Bessiere, J.M.; Gut, J.; Rosenthal, P.J. Antiplasmodial Volatile Extracts from Cleistopholis patens Engler & Diels and Uvariastrum pierreanum Engl. (Engl. & Diels) (Annonaceae) Growing in Cameroon. Parasitol. Res. 2011, 108, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, N.R.; Bingel, A.S. Problems and Prospects of Discovering New Drugs from Higher Plants by Pharmacological Screening. In New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity; Wagner, H., Wolff, P., Eds.; Proceedings in Life Sciences; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–22. ISBN 978-3-642-66684-1. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Terpenes and the Lipid–Protein–Partitioning Theory of Skin Penetration Enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.A.; Barry, B.W. Sesquiterpene Components of Volatile Oils as Skin Penetration Enhancers for the Hydrophilic Permeant 5-Fluorouracil. J. Pharm. Pharmacol. 1994, 46, 261–269. [Google Scholar] [CrossRef]
- Santoro, G.F.; Das Graças Cardoso, M.; Guimarães, L.G.L.; Salgado, A.P.S.P.; Menna-Barreto, R.F.S.; Soares, M.J. Effect of Oregano (Origanum vulgare L.) and Thyme (Thymus vulgaris L.) Essential Oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) Growth and Ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Martínez, F.; Lu, P.; Cortés-Selva, F.; Pérez-Victoria, J.M.; Jiménez, I.A.; Ravelo, A.G.; Sharom, F.J.; Gamarro, F.; Castanys, S. Celastraceae Sesquiterpenes as a New Class of Modulators That Bind Specifically to Human P-Glycoprotein and Reverse Cellular Multidrug Resistance. Cancer Res. 2004, 64, 7130–7138. [Google Scholar] [CrossRef]
- Lopes, N.P.; Kato, M.J.; Eloísa, H.d.A.; Maia, J.G.; Yoshida, M.; Planchart, A.R.; Katzin, A.M. Antimalarial Use of Volatile Oil from Leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J. Ethnopharmacol. 1999, 67, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Somdej, K.; Kwanjai, K.; Ratsami, L. Bioactive constituents of the roots of Polyathia cerasoides. J. Nat. Prod. 2007, 70, 1536–1538. [Google Scholar] [CrossRef]
- da Silva, N.C.; Gonçalves, S.F.; de Araújo, L.S.; Kasper, A.A.M.; da Fonseca, A.L.; Sartoratto, A.; Castro, K.C.F.; Moraes, T.M.P.; Baratto, L.C.; Varotti, F.d.P. In Vitro and in Vivo Antimalarial Activity of the Volatile Oil of Cyperus Articulatus (Cyperaceae). Acta Amaz. 2019, 49, 334–342. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and caryophyllene oxide: A variety of chemical transformations and biological activities. Chem. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia Annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Zacchino, S.; Georgiev, M.I.; Liu, L.; Wagner, H.; Panossian, A. Nobel Prize for Artemisinin Brings Phytotherapy into the Spotlight. Phytomedicine 2015, 22, A1–A3. [Google Scholar] [CrossRef]
- Ekiert, H.; Kubica, P.; Kwiecień, I.; Szopa, A. Nagroda Nobla 2015 z Medycyny-Zwycięstwo Badań z Zakresu Fitochemii, Bakteriologii i Farmakologii. 2016. Available online: https://polishpharmacy.ptfarm.pl/en/PF/editions/-/16308 (accessed on 6 June 2024).
- Shinyuy, L.M.; Loe, G.E.; Jansen, O.; Mamede, L.; Ledoux, A.; Noukimi, S.F.; Abenwie, S.N.; Ghogomu, S.M.; Souopgui, J.; Robert, A. Secondary Metabolites Isolated from Artemisia afra and Artemisia annua and Their Anti-Malarial, Anti-Inflammatory and Immunomodulating Properties—Pharmacokinetics and Pharmacodynamics: A Review. Metabolites 2023, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Orjala, J.; Mutiso, P.C.; Soejarto, D.D. An Antimalarial Abietane Diterpene from Fuerstia africana TCE Fries. Biochem. Syst. Ecol. 2006, 34, 270–272. [Google Scholar] [CrossRef]
- Islam, M.T.; Da Mata, A.M.O.F.; De Aguiar, R.P.S.; Paz, M.F.C.J.; De Alencar, M.V.O.B.; Ferreira, P.M.P.; De Carvalho Melo-Cavalcante, A.A. Therapeutic Potential of Essential Oils Focusing on Diterpenes: Diterpenes, the Family Members of Essential Oils. Phytother. Res. 2016, 30, 1420–1444. [Google Scholar] [CrossRef]
- Moyo, P.; Mugumbate, G.; Eloff, J.N.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.M. Natural products: A potential source of Malaria transmission blocking drugs? Pharmaceuticals 2020, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Prapaporn, C.; Arisara, P.; Tachpon, T.; Parnpen, V.; Arnon, C.; Chuchard, P. Antiplasmodial activity and cytotoxicity of plant extracts from the Asteraceae and Rubiaceae families. Heliyon 2022, 8, e08848. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Falco, V.; Fraqueza, M.J.; Patarata, L. Synergistic Activity of Essential Oils from Herbs and Spices Used on Meat Products against Food Borne Pathogens. Nat. Prod. Commun. 2017, 12, 281–286. [Google Scholar] [CrossRef]
- Karakaya, S.; Yilmaz, S.V.; Özdemir, Ö.; Koca, M.; Pinar, M.N.; Demirci, B.; Yildirim, K.; Sytar, O.; Turkez, H.; Baser, C.H.K. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. Amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). J. Essent. Oil Res. 2020, 6, 512–525. [Google Scholar] [CrossRef]
- Moe, S.R.; Rutina, L.P.; Hytteborn, H.; Du Toit, J.T. What Controls Woodland Regeneration after Elephants Have Killed the Big Trees? J. Appl. Ecol. 2009, 46, 223–230. [Google Scholar] [CrossRef]
- Urbano, F.; Viterbi, R.; Pedrotti, L.; Vettorazo, E.; Movalli, C.; Corlatti, L. Enhancing biodiversity conservation and monitoring in protected areas through efficient data management. Environ. Monit. Assess. 2023, 196, 12. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Yao, X.-H.; Duan, M.-H.; Wei, F.-Y.; Wu, G.-H.; Li, L. Variation of Essential Oil Content and Antioxidant Activity of Lonicera Species in Different Sites of China. Ind. Crops Prod. 2015, 77, 772–779. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Kenmogne, M.; Prost, E.; Harakat, D.; Jacquier, M.-J.; Frédérich, M.; Sondengam, L.B.; Zeches, M.; Waffo-Téguo, P. Five Labdane Diterpenoids from the Seeds of Aframomum zambesiacum. Phytochemistry 2006, 67, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.; Botha, M.; Theron, A.; Lauterbach, S.B.; Rossouw, C.; Engelbrecht, D.; Wepener, M.; Smit, A.; Leroy, D.; Mancama, D.; et al. Nowhere to Hide: Interrogating Different Metabolic Parameters of Plasmodium falciparum Gametocytes in a Transmission Blocking Drug Discovery Pipeline towards Malaria Elimination. Malar. J. 2015, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a Resazurin-Based High-Throughput Screening Assay for the Identification and Progression of New Treatments for Human African Trypanosomiasis. Int. J. Parasitol.-Drugs Drug 2012, 2, 262–270. [Google Scholar] [CrossRef] [PubMed]
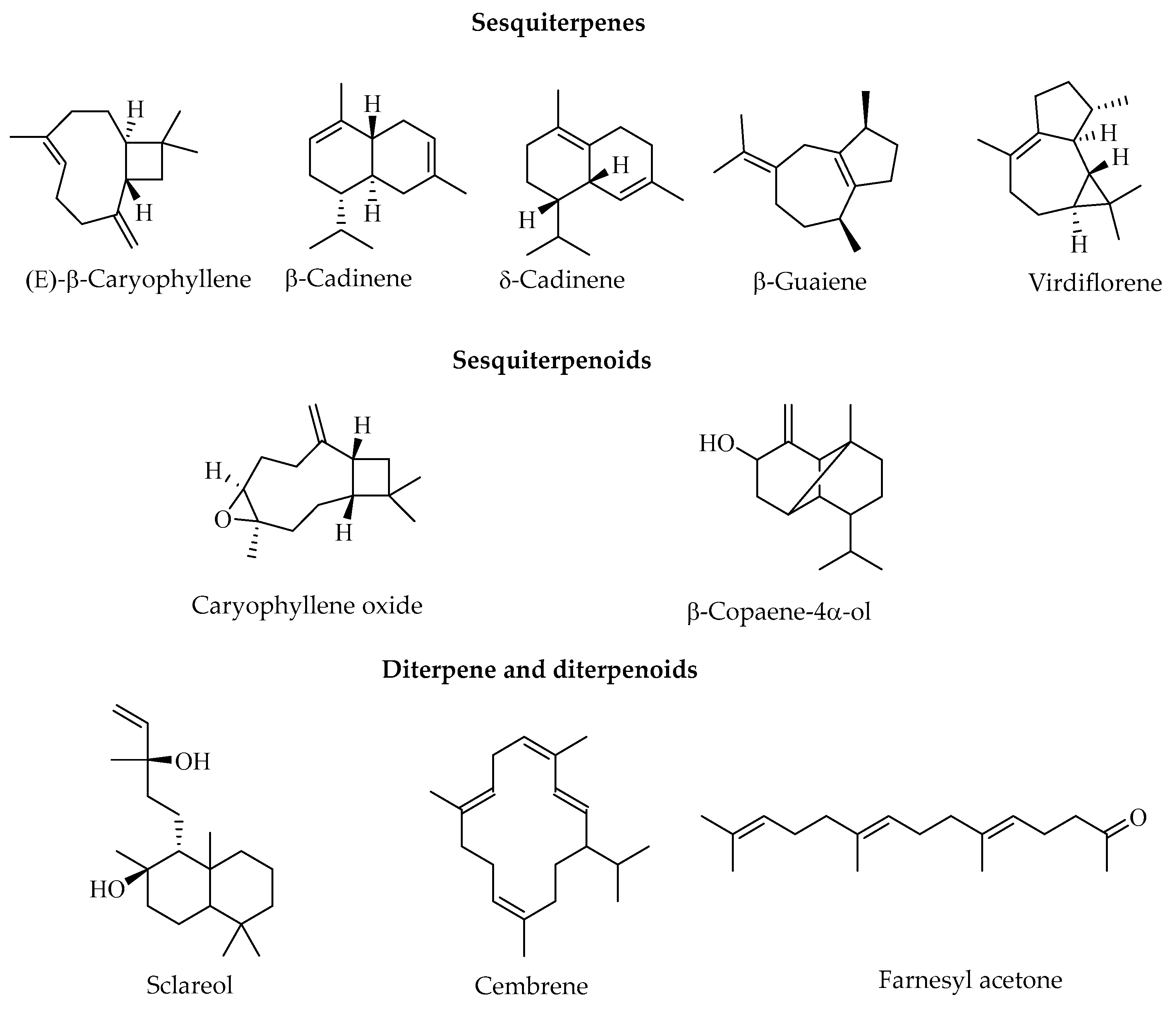
| N° | Compounds | N° CAS | RIs | Rel. Prop. d (% ± SD) | Identification c | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Polar Column a | Polar Column b | Plant Parts | ||||||||
| Exp. | Lit. e,f | Exp. | Lit. e,f | Leaves | Trunk Bark | Roots | ||||
| 1 | p-Cymene | 99-87-6 | 1055 | 1048 | 1343 | 1322 | - | - | 1.70 ± 0.12 | MS, RI i,j |
| 2 | α-Copaene | 3856-25-5 | 1379 | 1379 | 1529 | 1522 | 3.48 ± 0.07 | - | - | MS, RI i,j |
| 3 | α-Pinene | 80-56-8 | 937 | 936 | 1187 | 1050 | _ | 2.27 ± 1.70 | - | MS, RI i,j |
| 4 | β-Elemene | 515-13-9 | 1396 | 1396 | 1610 | 1606 | 5.99 ± 0.17 | - | - | MS, RI i,j |
| 5 | Isoborneol | 124-76-5 | 1164 | 1164 | 1692 | 1672 | - | - | 1.73 ± 0.03 | MS, RI i,j |
| 6 | Cyperene | 2387-78-2 | 1402 | 1402 | 1552 | 1562 | - | - | 3.03 ± 0.03 | MS, RI i,j |
| 7 | Episesquithujene | 15,9407-35-9 | 1395 | 1391 | 1569 | - | 3.0 ± 0.90 | - | MS, RI i | |
| 8 | (E)-β-Caryophyllene | 87-44-5 | 1422 | 1422 | 1609 | 1608 | 9.64 ± 0.15 | 18.40 ± 0.60 | 6.86 ± 0.06 | MS, RI i,j |
| 9 | (E)-α-Bergamotene | 13,474-59-4 | 1442 | 1442 | 1607 | 1585 | - | 3.89 ± 0.34 | - | MS, RI i,j |
| 10 | α-Humulene | 6753-98-6 | 1457 | 1457 | 1665 | 1665 | 8.40 ± 0.09 | 8.54 ± 0.23 | 2.49 ± 0.07 | MS, RI i,j |
| 11 | Germacrene D | 23,986-74-5 | 1489 | 1489 | - | 6.37 ± 0.24 | - | MS, RI i | ||
| 12 | cis-5-Decen-1-yl acetate | 67,446-07-5 | 1504 | 1591 | 1797 | - | 1.35 ± 0.06 | - | MS, RI i | |
| 13 | Caryophyllene oxide | 1139-30-6 | 1594 | 1594 | 1947 | 1955 | - | 12.61 ± 0.31 | - | MS, RI i,j |
| 14 | Epizonarene | 41,702-63-0 | 1529 | 1530 | 2.35 ± 0.06 | - | - | MS, RI i | ||
| 15 | β-Copaene-4α-ol | 124,753-76-0 | 1592 | 1591 | 2020 | 2135 | 14.23 ± 0.28 | - | - | MS, RI i,j |
| 16 | β-Selinene | 17,066-67-0 | 1513 | 1509 | 1705 | 1702 | 3.45 ± 0.09 | - | - | MS, RI i,j |
| 17 | γ-Selinene | 515-17-3 | 1478 | 1477 | 1708 | 1676 | 2.02 ± 0.22 | - | 5.34 ± 0.41 | MS, RI i,j |
| 18 | β-Guaiene | 88-84-6 | 1498 | 1499 | 1566 | 2.47 ± 0.15 | - | 15.38 ± 0.79 | MS, RI i | |
| 19 | β-Vetivenene | 27,840-40-0 | 1522 | 1527 | 1777 | 1885 | - | - | 3.19 ± 0.41 | MS, RI i,j |
| 20 | β-Cadinene | 523-47-7 | 1524 | 1523 | 1701 | 1692 | 10.80 ± 0.09 | - | MS, RI i,j | |
| 21 | δ-Cadinene | 483-76-1 | 1527 | 1527 | 1696 | 1708 | 12.57 ± 0.03 | 1.64 ± 0.05 | 4.45 ± 2.50 | MS, RI i,j |
| 22 | Virdiflorene | 21,747-46-6 | 1533 | 1534 | 2113 | 1719 | - | - | 18.13 ± 0.46 | MS, RI i,j |
| 23 | Isolongifolan-8-ol | 1139-08-8 | 1536 | 1531 | 1756 | - | - | 2.65 ± 0.40 | MS, RI i | |
| 24 | Germacrene B | 15,423-57-1 | 1553 | 1553 | 1981 | 1806 | - | - | 3.15 ± 0.09 | MS, RI i,j |
| 25 | Spathulenol | 6750-60-3 | 1589 | 1589 | 1809 | - | - | 9.58 ± 0.15 | MS, RI i | |
| 26 | τ-Muurolol | 19,912-62-0 | 1664 | 1662 | 2089 | 2160 | - | 1.27 ± 0.49 | - | MS, RI i,j |
| 27 | Neointermedeol | 5945-72-2 | 1670 | 1669 | - | 1.17 ± 0.40 | - | MS, RI i | ||
| 28 | Humulene epoxide II | 19,888-34-7 | 1620 | 1620 | - | 4.29 ± 0.16 | - | MS, RI i | ||
| 29 | Caryophylladienol II | 19,431-79-9 | 1681 | 1678 | 2136 | - | 2.28 ± 0.15 | - | MS, RI i | |
| 30 | Trans-longipinocarveol | 1,000,159-36-5 | 1615 | 1618 | 2241 | 7.62 ± 0.17 | - | 2.65 ± 0.18 | MS, RI i | |
| 31 | Selina-3,7(11) -diene | 6813-21-4 | 1666 | 1550 | 2090 | - | - | 6.32 ± 0.43 | MS, RI i | |
| 32 | Khusimyl methyl ether | 300,349-20-6 | 1690 | 1698 | 2123 | - | - | 2.83 ± 0.40 | MS, RI i | |
| 33 | 15,16-Dinorlab-12-ene, 8,13-epoxy- | 5153-92-4 | 1896 | 1894 | 2096 | - | 1.62 ± 0.04 | - | MS, RI i | |
| 34 | Cembrene | 1898-13-1 | 1946 | 1948 | 2055 | 2180 | 12.86 ± 0.29 | MS, RI i, j | ||
| 35 | m-Camphorene | 20,016-73-3 | 1958 | 1960 | 2074 | - | 1.30 ± 0.01 | - | MS, RI i | |
| 36 | Kolavelool | 19,941-81-2 | 1977 | 2079 | 2646 | - | 1.38 ± 0.06 | - | MS, RI i | |
| 37 | Sclareol | 515-03-7 | 2187 | 2198 | 2537 | - | 15.75 ± 0.49 | 9.23 ± 0.27 | MS, RI i | |
| 38 | Ylangenal | 41,610-68-8 | 1687 | 1674 | 2038 | 1.72 ± 0.43 | - | - | MS, RI i | |
| 39 | Farnesyl acetone | 1117-52-8 | 1926 | 1927 | 2202 | 15.26 ± 0.25 | - | - | MS, RI i | |
| Hydrocarbons monoterpenes (%) | // | 2.27 | 1.70 | |||||||
| Oxygenated monoterpenes (%) | // | // | 1.73 | |||||||
| Hydrocarbons sesquiterpenes (%) | 61.17 | 41.84 | 53.04 | |||||||
| Oxygenated sesquiterpenes (%) | 23.57 | 21.62 | 33.01 | |||||||
| Diterpenoids (%) | 15.26 | 18.75 | 9.23 | |||||||
| Hydrocarbons diterpenes (%) | // | 14.16 | // | |||||||
| Carboxylic ester (%) | // | 1.35 | // | |||||||
| Identified compounds (%) | ˃99.99 | ˃99.99 | 98.71 | |||||||
| Organs | Antiplasmodial Activity Pf3D7/IC50 ± SD (µg/mL) | Gametocytes in Strain PfNF-54/IC50 (µg/mL) | Cytotoxicity CC50 ± SD (µg/mL) | Selectivity Index (SI) |
|---|---|---|---|---|
| Roots | 71.46 ± 30.78 | 1.85 | - | - |
| Trunk bark | 9.06 ± 2.15 | 0.56 | 15.98 ± 0.86 | 1.76 |
| Leaves | 74.04 ± 28.87 | 1.03 ± 0.73 | - | - |
| Compounds (n = 1) | ||||
| (E)-β-Caryophyllene | 1.15 | - | - | - |
| Caryophyllene oxide | 0.48 | - | - | - |
| Sclareol | 1.30 | - | - | - |
| Artemisinin (µM) | 0.03 ± 0.00 | - | - | - |
| Methylene Blue | - | 85.11 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguimjeu, P.L.K.T.; Fongang, Y.S.F.; Genva, M.; Shinyuy, L.M.; Held, J.; Frederich, M.; Ngouela, S.A.; Fauconnier, M.-L. Antiplasmodial Activity of a New Chemotype of Croton sylvaticus Hochst. Ex C. Krauss Essential Oil. Int. J. Mol. Sci. 2025, 26, 858. https://doi.org/10.3390/ijms26020858
Taguimjeu PLKT, Fongang YSF, Genva M, Shinyuy LM, Held J, Frederich M, Ngouela SA, Fauconnier M-L. Antiplasmodial Activity of a New Chemotype of Croton sylvaticus Hochst. Ex C. Krauss Essential Oil. International Journal of Molecular Sciences. 2025; 26(2):858. https://doi.org/10.3390/ijms26020858
Chicago/Turabian StyleTaguimjeu, Pierre Leonel K. Tafokeu, Yannick Stéphane Fotsing Fongang, Manon Genva, Lahngong Methodius Shinyuy, Jana Held, Michel Frederich, Silvère Augustin Ngouela, and Marie-Laure Fauconnier. 2025. "Antiplasmodial Activity of a New Chemotype of Croton sylvaticus Hochst. Ex C. Krauss Essential Oil" International Journal of Molecular Sciences 26, no. 2: 858. https://doi.org/10.3390/ijms26020858
APA StyleTaguimjeu, P. L. K. T., Fongang, Y. S. F., Genva, M., Shinyuy, L. M., Held, J., Frederich, M., Ngouela, S. A., & Fauconnier, M.-L. (2025). Antiplasmodial Activity of a New Chemotype of Croton sylvaticus Hochst. Ex C. Krauss Essential Oil. International Journal of Molecular Sciences, 26(2), 858. https://doi.org/10.3390/ijms26020858








