Comparative Transcriptomic Analysis Reveals New Insights into Spawn Aging in Agaricus bisporus: Mitochondrial Dysfunction
Abstract
:1. Introduction
2. Results
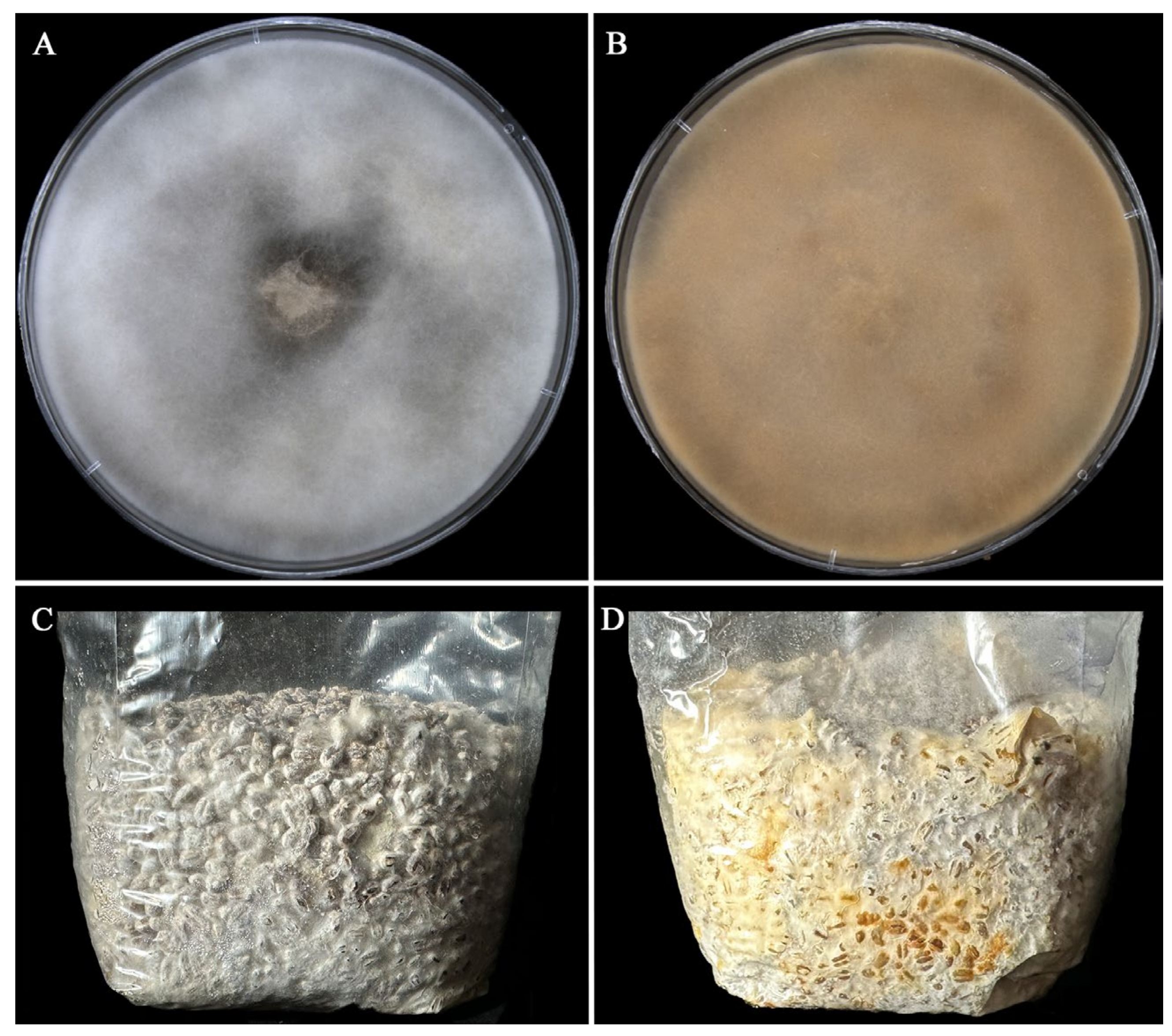
2.1. Aging Altered the Morphology of A. bisporus Mycelia
2.2. Overview of the Transcriptomic Sequencing and Assembly
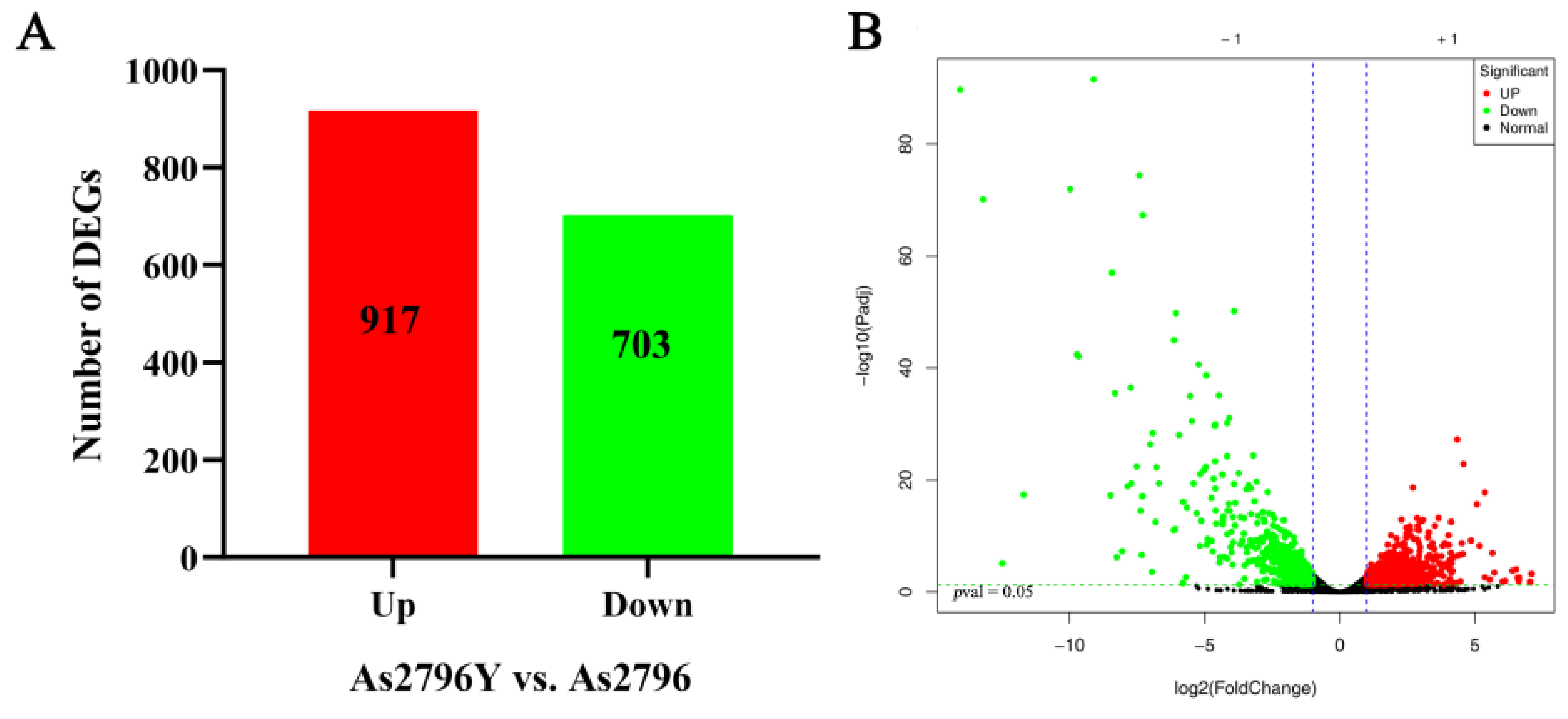
2.3. DEGs Identification
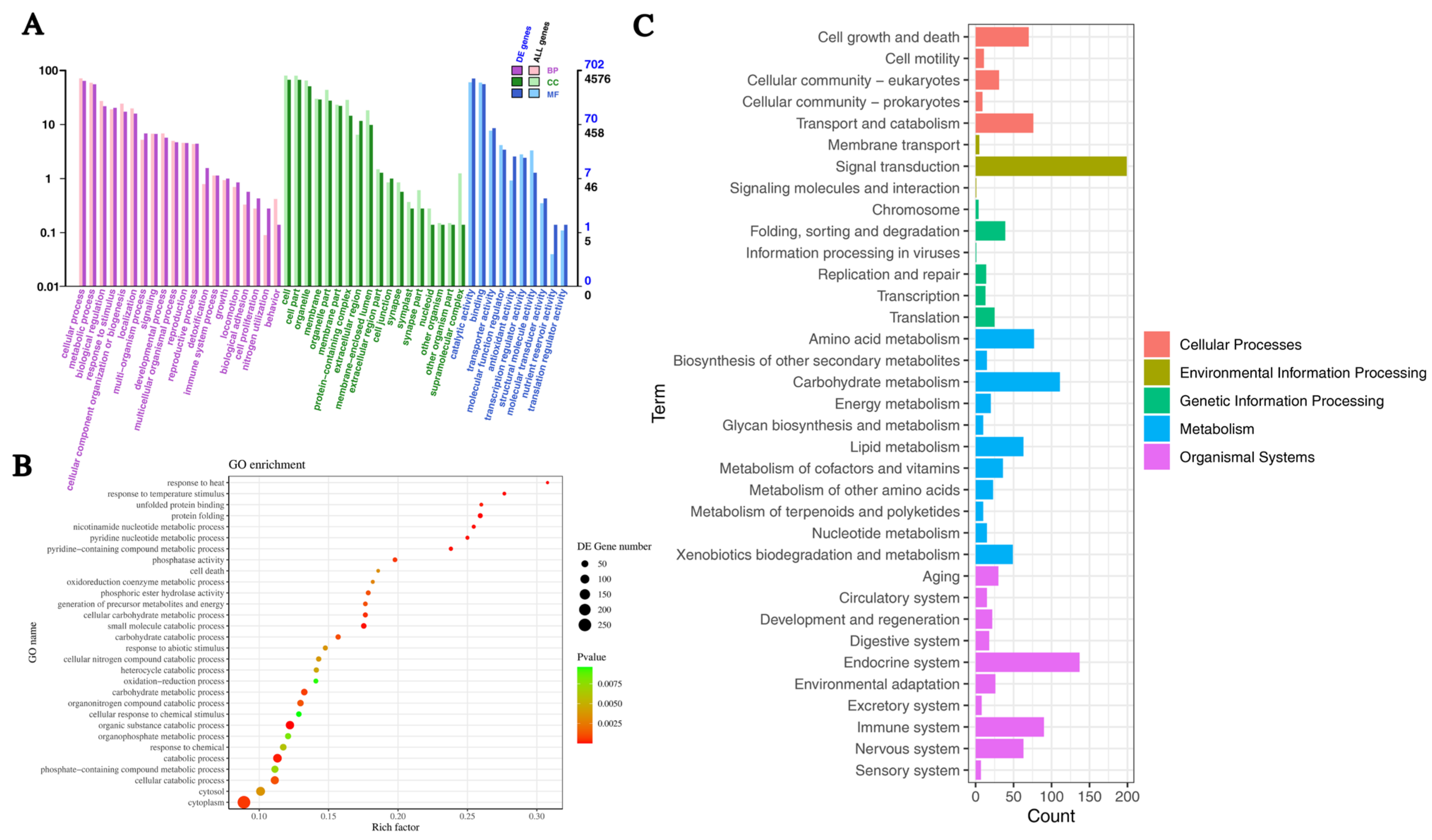
2.4. Functional Annotation of DEGs
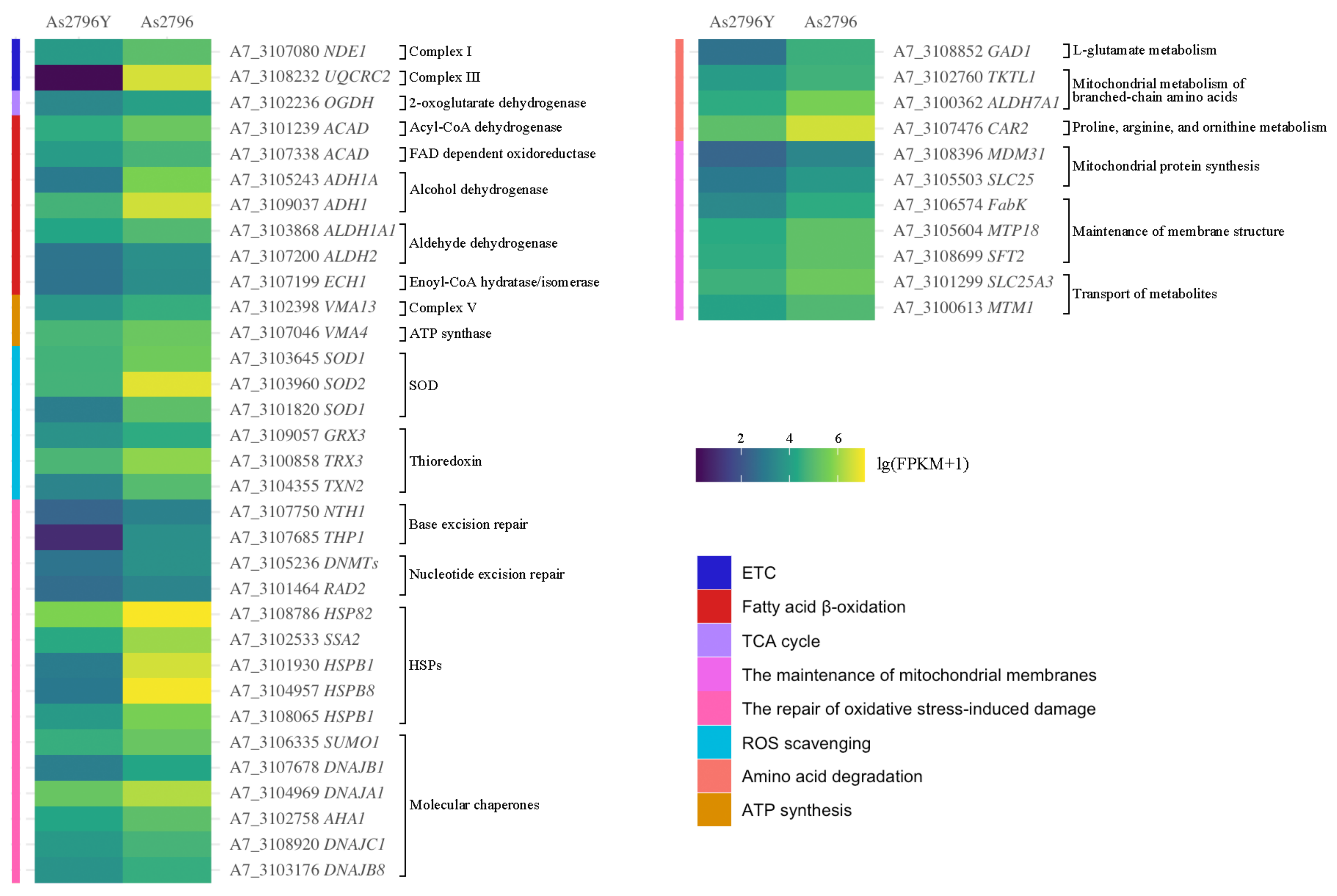
2.5. DEGs Related to Mitochondrial Function
2.5.1. DEGs Related to ETC
2.5.2. DEGs Related to TCA Cycle
2.5.3. DEGs Related to Fatty Acid β-Oxidation
2.5.4. DEGs Related to ATP Synthesis
2.5.5. DEGs Related to ROS Scavenging
2.5.6. DGEs Associated with the Repair of Oxidative Stress-Induced Damage
2.5.7. DEGs Related to Amino Acid Degradation
2.5.8. DEGs Related to the Maintenance of Mitochondrial Membranes
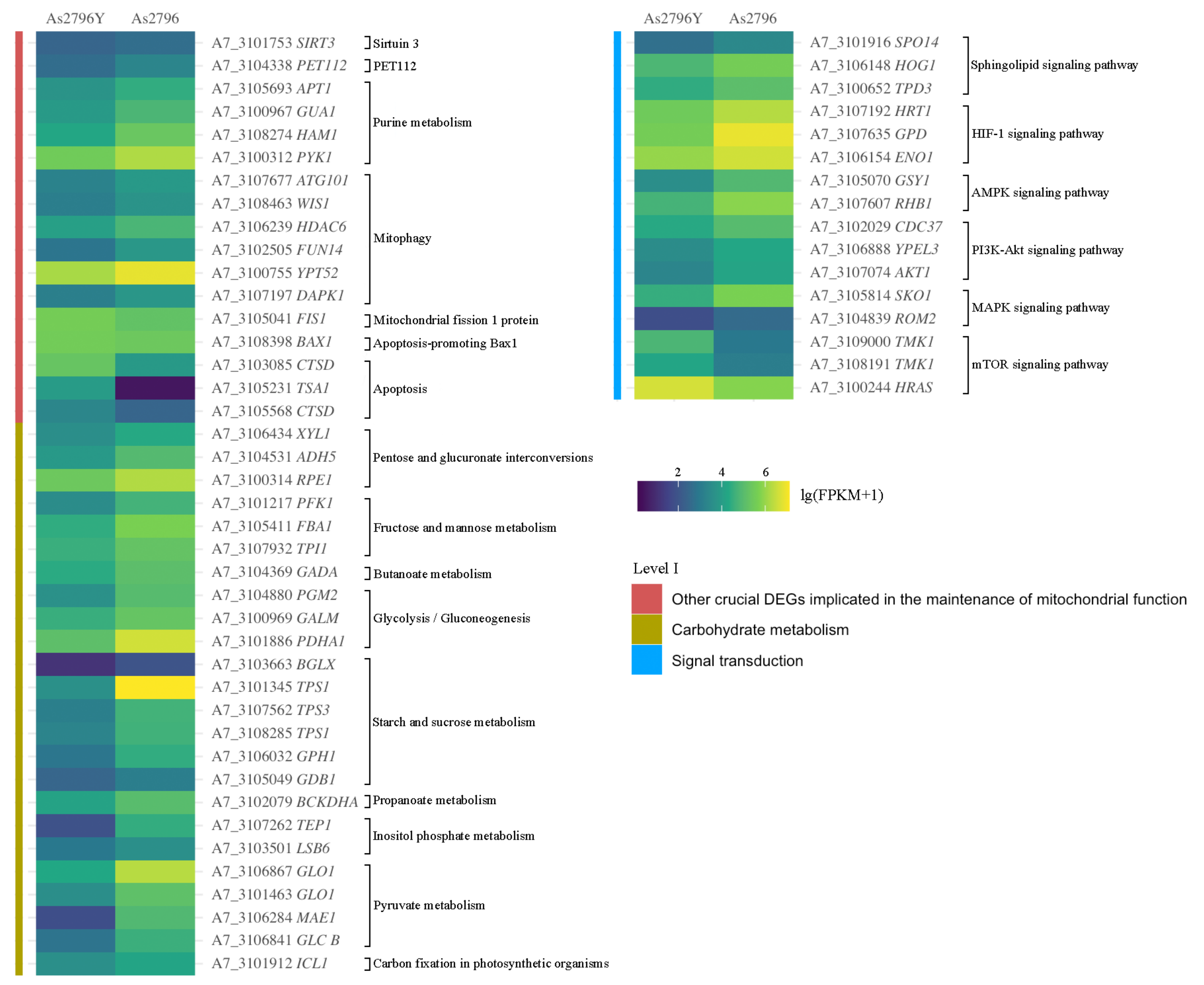
2.5.9. Other Crucial DEGs Implicated in the Maintenance of Mitochondrial Function
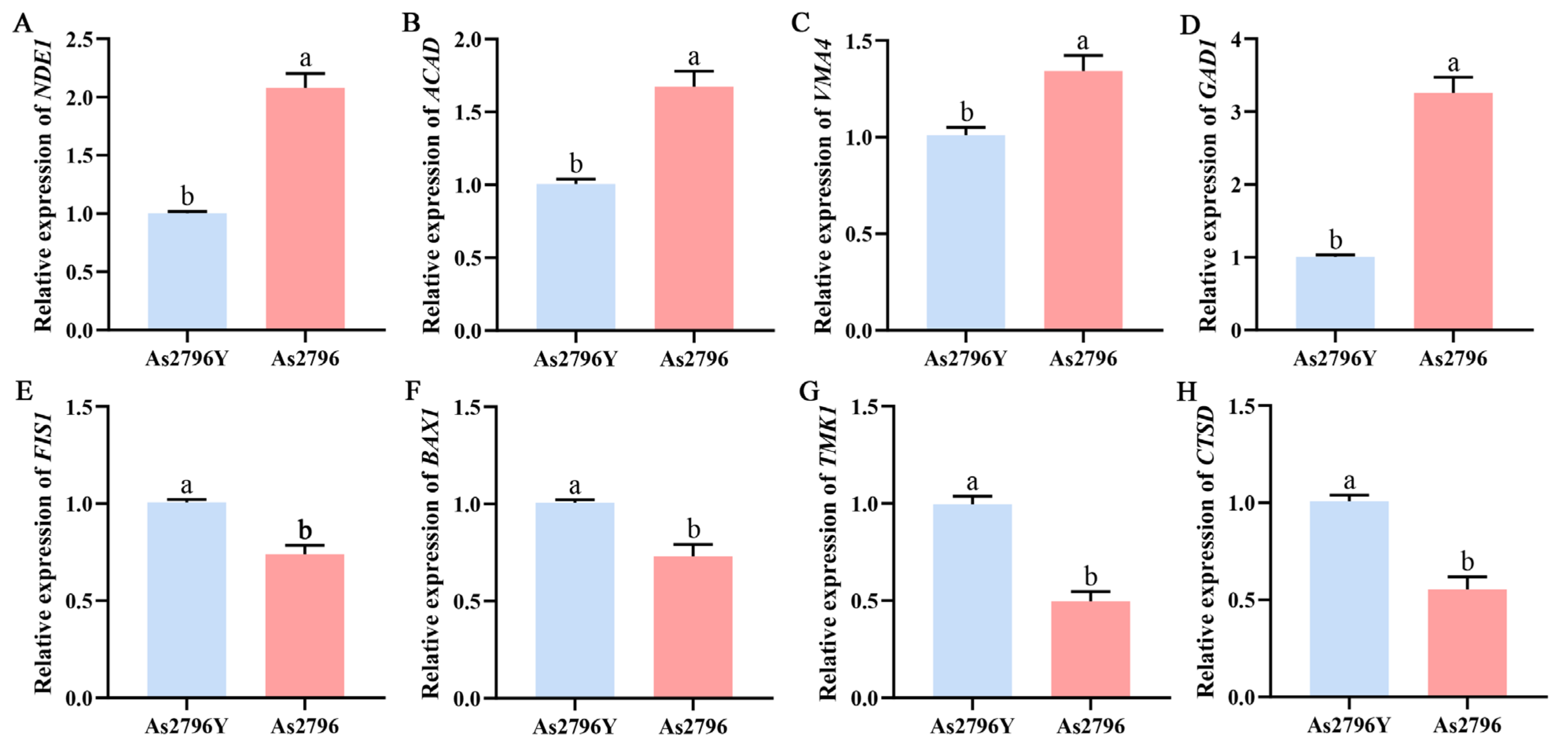
2.6. Validation of RNA-Seq Data by qRT-PCR
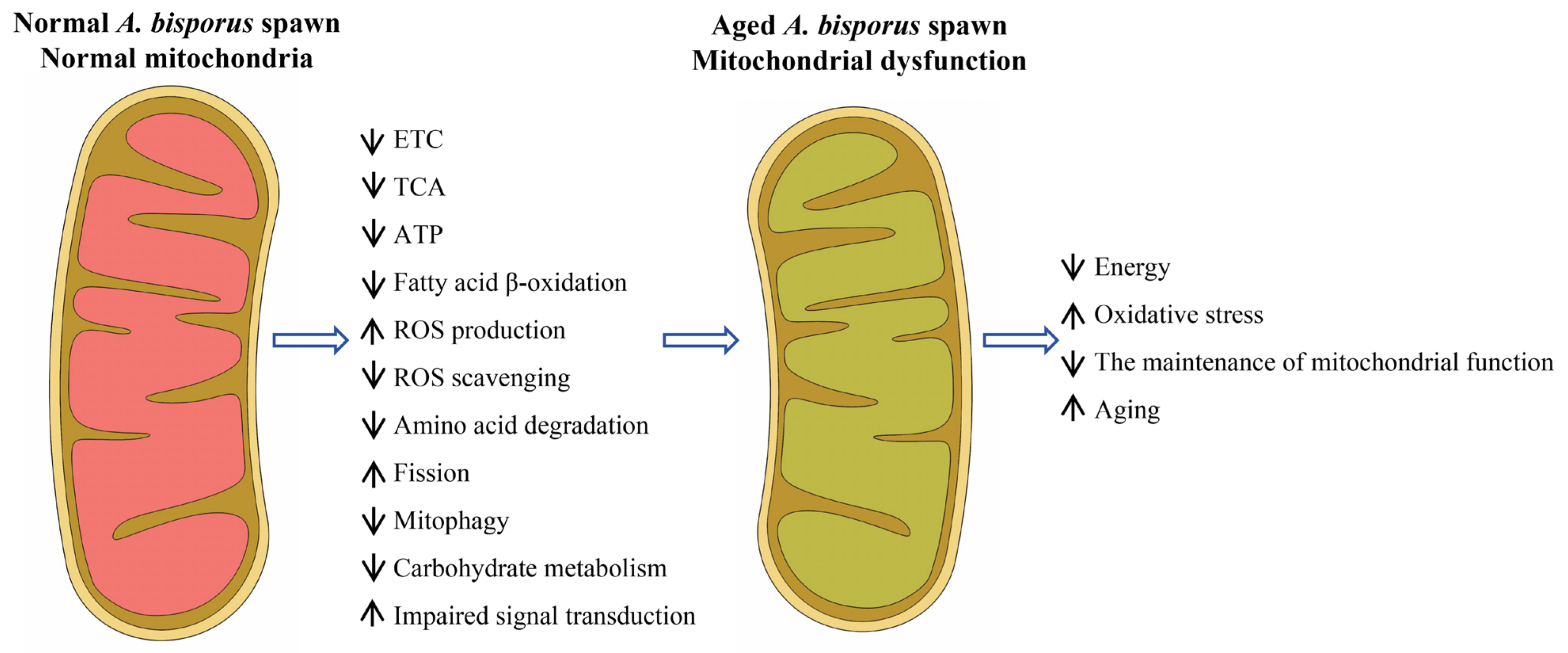
3. Discussion
4. Materials and Methods
4.1. Strains, Media, Growth Conditions, and Sample Collection
4.2. RNA Extraction
4.3. cDNA Library Preparation, and Illumina Sequencing
4.4. Differential Expression Analysis and Functional Enrichment
4.5. Quantitative Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.; Jimenez Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef]
- Shu, L.; Zeng, Z.; Dai, J.; Cheng, Y.; Lu, Y.; Chen, M.; Zeng, H. Morphological and metabolic changes in an aged strain of Agaricus bisporus As2796. Appl. Microbiol. Biot. 2021, 105, 7997–8007. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A. Evaluation of spawn quality and doses on yield and biological efficiency of Blue Oyster Mushroom [Hypsizygus ulmarius (Bull.: Fr.) Redhead]. J. Eco-Friendly Agric. 2022, 17, 393–398. [Google Scholar] [CrossRef]
- Osiewacz, H. Genes, mitochondria and aging in filamentous fungi. Ageing Res. Rev. 2002, 1, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Navaraj, A. Senescence in fungi: The view from Neurospora. FEMS Microbiol. Lett. 2008, 280, 135–143. [Google Scholar] [CrossRef]
- Osiewacz, H.; Scheckhuber, C. Senescence in Podospora anserina; CRC Press: New York, NY, USA, 2002; pp. 87–108. [Google Scholar]
- Osiewacz, H. Mitochondrial quality control in aging and lifespan control of the fungal aging model Podospora anserina. Biochem. Soc. Trans. 2011, 39, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.; Greider, C.; Szostak, J. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Powers, E.; Morimoto, R.; Dillin, A.; Kelly, J.; Balch, W. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annu. Rev. Biochem. 2009, 78, 959–991. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.; Coppotelli, G.; Rolo, A.; Palmeira, C.; Ross, J.; Sinclair, D. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022, 18, 243–258. [Google Scholar] [CrossRef]
- Natarajan, V.; Chawla, R.; Mah, T.; Vivekanandan, R.; Tan, S.; Sato, P.; Mallilankaraman, K. Mitochondrial Dysfunction in Age-Related Metabolic Disorders. Proteomics 2020, 20, 1800404. [Google Scholar] [CrossRef] [PubMed]
- Skowronska-Krawczyk, D. Hallmarks of Aging: Causes and Consequences. Aging Biol. 2023, 1, 20230011. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.P.; Sharma, V.; Kumar, R.; Gupta, R.; Kumar, S.; Singh, R. Optimizations of parameters for quality spawn production. Mushroom Res. 2013, 22, 31–36. [Google Scholar]
- Holmannova, D.; Borsky, P.; Parova, H.; Stverakova, T.; Vosmik, M.; Hruska, L.; Fiala, Z.; Borska, L. Non-Genomic Hallmarks of Aging—The Review. Int. J. Mol. Sci. 2023, 24, 15468. [Google Scholar] [CrossRef]
- Chen, M. Mitochondria in aging: A review of structure, function, and interorganelle relationships. Theoret. Nat. Sci. 2024, 29, 69–81. [Google Scholar] [CrossRef]
- Silaghi, C.; Farcaș, M.; Craciun, A. Sirtuin 3 (SIRT3) Pathways in Age-Related Cardiovascular and Neurodegenerative Diseases. Biomedicines 2021, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bai, M.; Xie, X.; Wang, J.; Weng, C.; Dai, H.; Chen, J.; Han, F.; Lin, W. Impaired Amino Acid Metabolism and Its Correlation with Diabetic Kidney Disease Progression in Type 2 Diabetes Mellitus. Nutrients 2022, 14, 3345. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, C.; Fernández, J.; Herrero-Sánchez, C.; Alvarez, Y.; Alonso, S.; Sandoval, T.; Cubillos-Ruiz, J.; Montero, O.; Fernández, N.; Crespo, M. Fungal Patterns Induce Cytokine Expression through Fluxes of Metabolic Intermediates That Support Glycolysis and Oxidative Phosphorylation. J. Immunol. 2022, 208, 2779–2794. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, A.; Song, Q.; Fang, H.; Liu, X.-Y.; Su, J.; Yang, L.; Yu, M.-D.; Wang, X.-J. Functional metabolomics discover pentose and glucuronate interconversion pathways as promising targets for Yang Huang syndrome treatment with Yinchenhao Tang. RSC Adv. 2018, 8, 36831–36839. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Sheng, H. Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems. CNS Neurosci. Ther. 2024, 30, e14576. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Gantner, B.; Sakiyama, M.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M. ROS production by mitochondria: Function or dysfunction? Oncogene 2023, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, L.; Zhang, Z.; Wang, Y.; Kouis, P.; Rasmussen, L.; Dai, F. Effects of reactive oxygen species and mitochondrial dysfunction on reproductive aging. Front. Cell Dev. Biol. 2024, 12, 1347286. [Google Scholar] [CrossRef] [PubMed]
- Dimmer, K.; Jakobs, S.; Vogel, F.; Altmann, K.; Westermann, B. Mdm31 and Mdm32 are inner membrane proteins required for maintenance of mitochondrial shape and stability of mitochondrial DNA nucleoids in yeast. J. Cell Biol. 2005, 168, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Scarcia, P.; Monné, M. Diseases Caused by Mutations in Mitochondrial Carrier Genes SLC25: A Review. Biomolecules 2020, 10, 655. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Chen, Z.; Haapalainen, A.; Wierenga, R.; Kastaniotis, A. Mitochondrial fatty acid synthesis—An adopted set of enzymes making a pathway of major importance for the cellular metabolism. Prog. Lipid Res. 2009, 49, 27–45. [Google Scholar] [CrossRef]
- Hao, K.; Chen, F.; Zhao, L.; Xu, S.; Xiong, Y.; Xu, R.; Xie, X.-H.; Huang, H.; Shu, C.; Liu, Z.; et al. Nicotinamide ameliorates mitochondria-related neuronal apoptosis and cognitive impairment via the NAD+/SIRT3 pathway. Schizophrenia 2023, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Suzuki, T.; Katoh, T.; Sakaguchi, Y.; Suzuki, T. Biogenesis of glutaminyl-mt tRNA(Gln) in human mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16209–16214. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Guberman, M.; Kirshenbaum, L. Mitochondrial quality control: The role of mitophagy in aging. Trends Cardiovasc. Med. 2017, 28, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Zhao, Y.; Yu, Y.; Zhao, S.; Xiang, S.; Lian, F. Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions. Front. Endocrinol. 2024, 15, 1361289. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K. Apoptosis and aging: Increased resistance to apoptosis enhances the aging process. Cell Mol. Life. Sci. 2011, 68, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Férandon, C.; Xu, J.; Barroso, G. The 135 kbp mitochondrial genome of Agaricus bisporus is the largest known eukaryotic reservoir of group I introns and plasmid-related sequences. Fungal Genet. Biol. 2013, 55, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2009, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; McCarthy, D.; Smyth, G. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xie, C.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, L.; Zeng, Z.; Chen, M.; Zhao, J.; Zhang, X.; Dai, J.; Cai, Z.; Lu, Y.; Qiu, Z.; Zeng, H. Comparative Transcriptomic Analysis Reveals New Insights into Spawn Aging in Agaricus bisporus: Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 849. https://doi.org/10.3390/ijms26020849
Shu L, Zeng Z, Chen M, Zhao J, Zhang X, Dai J, Cai Z, Lu Y, Qiu Z, Zeng H. Comparative Transcriptomic Analysis Reveals New Insights into Spawn Aging in Agaricus bisporus: Mitochondrial Dysfunction. International Journal of Molecular Sciences. 2025; 26(2):849. https://doi.org/10.3390/ijms26020849
Chicago/Turabian StyleShu, Lili, Zhiheng Zeng, Meiyuan Chen, Jiazhi Zhao, Xiaoyan Zhang, Jianqing Dai, Zhixin Cai, Yuanping Lu, Zhiheng Qiu, and Hui Zeng. 2025. "Comparative Transcriptomic Analysis Reveals New Insights into Spawn Aging in Agaricus bisporus: Mitochondrial Dysfunction" International Journal of Molecular Sciences 26, no. 2: 849. https://doi.org/10.3390/ijms26020849
APA StyleShu, L., Zeng, Z., Chen, M., Zhao, J., Zhang, X., Dai, J., Cai, Z., Lu, Y., Qiu, Z., & Zeng, H. (2025). Comparative Transcriptomic Analysis Reveals New Insights into Spawn Aging in Agaricus bisporus: Mitochondrial Dysfunction. International Journal of Molecular Sciences, 26(2), 849. https://doi.org/10.3390/ijms26020849





