Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds
Abstract
1. Introduction
2. Polysaccharide-Based Hydrogels: Formation and Delivery Mechanism
3. Marine-Derived Polysaccharides
3.1. Alginate
3.1.1. Alginate: Origin and Properties
3.1.2. Alginate-Based Hydrogel Applications
3.2. Carrageenan
3.2.1. Carrageenan: Origin and Properties
3.2.2. Carrageenan-Based Hydrogel Applications
3.3. Chitosan
3.3.1. Chitosan: Origin and Properties
3.3.2. Chitosan-Based Hydrogel Applications
3.4. Agarose
3.4.1. Agarose: Origin and Properties
3.4.2. Agarose-Based Hydrogel Applications
3.5. Marine Hyaluronan
3.5.1. Hyaluronan: Origin and Properties
3.5.2. Hyaluronan-Based Hydrogel Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Cai, Y.; Yu, L.; Zhou, J. Dual physically crosslinked hydrogels based on the synergistic effects of electrostatic and dipole-dipole interactions. J. Mater. Chem. B 2019, 7, 676–683. [Google Scholar] [CrossRef]
- Fredrick, R.; Podder, A.; Viswanathan, A.; Bhuniya, S. Synthesis and characterization of polysaccharide hydrogel based on hydrophobic interactions. J. Appl. Polym. Sci. 2019, 136, 47665. [Google Scholar] [CrossRef]
- Peng, G.; Wang, J.; Yang, F.; Zhang, S.; Hou, J.; Xing, W.; Lu, X.; Liu, C. In situ formation of biodegradable dextran-based hydrogel via Michael addition. J. Appl. Polym. Sci. 2013, 127, 577–584. [Google Scholar] [CrossRef]
- Pasqui, D.; De Cagna, M.; Barbucci, R. Polysaccharide-Based Hydrogels: The Key Role of Water in Affecting Mechanical Properties. Polymers 2012, 4, 1517–1534. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D. The functional response of alginate-gelatin-nanocrystalline cellulose injectable hydrogels toward delivery of cells and bioactive molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.X.; von Recum, H.A. Affinity-based drug delivery. Macromol. Biosci. 2011, 11, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; De Luise, A.; Valentino, A.; Di Cristo, F.; Petillo, O.; Riccitiello, F.; Di Salle, A.; Calarco, A.; Peluso, G. Chapter 10—Hydrogel Nanocomposite Systems: Characterization and Application in Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–349. [Google Scholar]
- Valentino, A.; Di Cristo, F.; Bosetti, M.; Amaghnouje, A.; Bousta, D.; Conte, R.; Calarco, A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Appl. Sci. 2021, 11, 5122. [Google Scholar] [CrossRef]
- Raffaele, C.; Anna Di, S.; Francesco, R.; Orsolina, P.; Gianfranco, P.; Anna, C. Biodegradable polymers in dental tissue engineering and regeneration. AIMS Mater. Sci. 2018, 5, 1073–1101. [Google Scholar] [CrossRef]
- Conte, R.; Petillo, O.; Rengo, C.; Riccitiello, F.; Di Salle, A.; Calarco, A.; Peluso, G. Biodegradable polymers for dental tissue engineering and regeneration. In Biodegradable Polymers: Recent Developments and New Perspectives; Rohman, G., Ed.; IAPC: Thane, Maharashtra, 2017. [Google Scholar]
- De Luca, I.; Di Cristo, F.; Conte, R.; Peluso, G.; Cerruti, P.; Calarco, A. In-Situ Thermoresponsive Hydrogel Containing Resveratrol-Loaded Nanoparticles as a Localized Drug Delivery Platform for Dry Eye Disease. Antioxidants 2023, 12, 993. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. J. Funct. Biomater. 2023, 14, 82. [Google Scholar] [CrossRef]
- Valentino, A.; Conte, R.; De Luca, I.; Di Cristo, F.; Peluso, G.; Bosetti, M.; Calarco, A. Thermo-Responsive Gel Containing Hydroxytyrosol-Chitosan Nanoparticles (Hyt@tgel) Counteracts the Increase of Osteoarthritis Biomarkers in Human Chondrocytes. Antioxidants 2022, 11, 1210. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Chambin, O.; Dupuis, G.; Champion, D.; Voilley, A.; Pourcelot, Y. Colon-specific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int. J. Pharm. 2006, 321, 86–93. [Google Scholar] [CrossRef]
- LeValley, P.J.; Sutherland, B.P.; Jaje, J.; Gibbs, S.; Jones, M.; Gala, R.; Kloxin, C.J.; Kiick, K.L.; Kloxin, A.M. On-demand and tunable dual wavelength release of antibody using light-responsive hydrogels. ACS Appl. Bio Mater. 2020, 3, 6944–6958. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.C. In Situ Synthesis of Magnetic Field-Responsive Hemicellulose Hydrogels for Drug Delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Qi, X.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a multi-sensitive polysaccharide hydrogel for drug delivery. Carbohydr. Polym. 2017, 177, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, Z.; Zhang, D.; Deng, M.; Chen, X. pH and redox dual-sensitive polysaccharide nanoparticles for the efficient delivery of doxorubicin. Biomater. Sci. 2017, 5, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Knipe, J.M.; Chen, F.; Peppas, N.A. Enzymatic biodegradation of hydrogels for protein delivery targeted to the small intestine. Biomacromolecules 2015, 16, 962–972. [Google Scholar] [CrossRef]
- Lima, C.S.A.; Balogh, T.S.; Varca, J.; Varca, G.H.C.; Lugão, A.B.; A. Camacho-Cruz, L.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef]
- Vulic, K.; Shoichet, M.S. Affinity-based drug delivery systems for tissue repair and regeneration. Biomacromolecules 2014, 15, 3867–3880. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch. Pharmacal Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Coutinho, A.J.; Costa Lima, S.A.; Reis, S. Marine Polysaccharides in Pharmaceutical Applications: Fucoidan and Chitosan as Key Players in the Drug Delivery Match Field. Mar. Drugs 2019, 17, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation behavior and mechanism of alginate with calcium: Dependence on monovalent counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Bulut, E. Development and optimization of Fe3+-crosslinked sodium alginate-methylcellulose semi-interpenetrating polymer network beads for controlled release of ibuprofen. Int. J. Biol. Macromol. 2021, 168, 823–833. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.; Pal, N.; Chatterjee, J.; Mukherjee, R. Dual cross-linked honey coupled 3D antimicrobial alginate hydrogels for cutaneous wound healing. Mater. Sci. Eng. C 2020, 116, 111218. [Google Scholar] [CrossRef] [PubMed]
- Saberian, M.; Seyedjafari, E.; Zargar, S.; Mahdavi, F.; Sanaei-rad, P. Fabrication and characterization of alginate/chitosan hydrogel combined with honey and aloe vera for wound dressing applications. J. Appl. Polym. Sci. 2021, 138, 51398. [Google Scholar] [CrossRef]
- Rusu, A.G.; Niță, L.E.; Roșca, I.; Croitoriu, A.; Ghilan, A.; Mititelu-Tarțău, L.; Grigoraș, A.V.; Crețu, B.-E.-B.; Chiriac, A.P. Alginate-Based Hydrogels Enriched with Lavender Essential Oil: Evaluation of Physicochemical Properties, Antimicrobial Activity, and In Vivo Biocompatibility. Pharmaceutics 2023, 15, 2608. [Google Scholar] [CrossRef]
- Clemente, I.; Baglioni, M.; Bonechi, C.; Bisozzi, F.; Rossi, C.; Tamasi, G. Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers 2023, 15, 4455. [Google Scholar] [CrossRef]
- Cadena-Velandia, Z.G.; Montenegro-Alarcón, J.C.; Marquínez-Casas, X.; Mora-Huertas, C.E. Quercetin-loaded alginate microparticles: A contribution on the particle structure. J. Drug Deliv. Sci. Technol. 2020, 56, 101558. [Google Scholar] [CrossRef]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-loaded alginate hydrogels for cancer therapy and wound healing applications: A review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernández, M.A.; Häring, M.; Saldías, C.; Díaz, D.D. Niosomes encapsulated in biohydrogels for tunable delivery of phytoalexin resveratrol. RSC Adv. 2019, 9, 7601–7609. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W.; Wang, L.-F. Preparation and characterization of carrageenan-based nanocomposite films reinforced with clay mineral and silver nanoparticles. Appl. Clay Sci. 2014, 97–98, 174–181. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Rheology and microstructure of mixtures of iota and kappa-carrageenan. Food Hydrocoll. 2019, 89, 180–187. [Google Scholar] [CrossRef]
- Stenner, R.; Matubayasi, N.; Shimizu, S. Gelation of carrageenan: Effects of sugars and polyols. Food Hydrocoll. 2016, 54, 284–292. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int. J. Biol. Macromol. 2016, 93, 952–960. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, characterization and antibacterial activity of oxidized κ-carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in vitro antioxidant activity of kappa-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef]
- Aparna, V.; Melge, A.R.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Gopi Mohan, C. Carboxymethylated ɩ-carrageenan conjugated amphotericin B loaded gelatin nanoparticles for treating intracellular Candida glabrata infections. Int. J. Biol. Macromol. 2018, 110, 140–149. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Gelatin-carrageenan hydrogels: Role of pore size distribution on drug delivery process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Keppeler, S.; Ellis, A.; Jacquier, J.C. Cross-linked carrageenan beads for controlled release delivery systems. Carbohydr. Polym. 2009, 78, 973–977. [Google Scholar] [CrossRef]
- Santo, V.E.; Frias, A.M.; Carida, M.; Cancedda, R.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules 2009, 10, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; McClements, D.J.; Li, J.; Su, Y.; Yang, Y.; Li, J. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Silva, R.C.; Trevisan, M.G.; Garcia, J.S. β-galactosidase Encapsulated in Carrageenan, Pectin and Carrageenan/Pectin: Comparative Study, Stability and Controlled Release. An. Acad. Bras. Cienc. 2020, 92, e20180609. [Google Scholar] [CrossRef]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Miletić Kovačević, M.; Gazdić Janković, M.; Stanić, Z.D. pH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef]
- Mahdavinia, G.; Rahmani, Z.; Karami, S.; Pourjavadi, A. Magnetic/pH-sensitive κ-carrageenan/sodium alginate hydrogel nanocomposite beads: Preparation, swelling behavior, and drug delivery. J. Biomater. Sci. Polym. Ed. 2014, 25, 1891–1906. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; Ying, R.; Wang, Y.; Wang, Z.; Huang, M. Binding a chondroitin sulfate-based nanocomplex with kappa-carrageenan to enhance the stability of anthocyanins. Food Hydrocoll. 2020, 100, 105448. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with k-carrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Condrò, G.; Perugini, P. Evaluation of the Mucoadhesive Properties of Chitosan-Based Microstructured Lipid Carrier (CH-MLC). Pharmaceutics 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Xu, J.; Li, Y.; Yang, J.; Zhou, S.; Situ, W. Plasma etching effect on the molecular structure of chitosan-based hydrogels and its biological properties. Int. J. Biol. Macromol. 2023, 230, 123257. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef]
- Polez, R.T.; Ajiboye, M.A.; Österberg, M.; Horn, M.M. Chitosan hydrogels enriched with bioactive phloroglucinol for controlled drug diffusion and potential wound healing. Int. J. Biol. Macromol. 2024, 265, 130808. [Google Scholar] [CrossRef]
- Xu, N.; Wang, L.; Guan, J.; Tang, C.; He, N.; Zhang, W.; Fu, S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018, 117, 102–107. [Google Scholar] [CrossRef]
- Bektas, N.; Şenel, B.; Yenilmez, E.; Özatik, O.; Arslan, R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rasool, B.K.; Shehab, N.G.; Khan, S.A.; Bayoumi, F.A. A new natural gel of Fagonia indica Burm f. extract for the treatment of burn on rats. Pak. J. Pharm. Sci. 2014, 27, 73–81. [Google Scholar] [PubMed]
- Rocasalbas, G.; Francesko, A.; Touriño, S.; Fernández-Francos, X.; Guebitz, G.M.; Tzanov, T. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 2013, 92, 989–996. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Shalaby, A.S.G.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Li, J.; Zhang, C.; Gao, F.; Ma, X.; Zhang, J.; Fu, C.; Geng, F. A feasible biocompatible hydrogel film embedding Periplaneta americana extract for acute wound healing. Int. J. Pharm. 2019, 571, 118707. [Google Scholar] [CrossRef]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Salehi, M.; Zamiri, S.; Samadian, H.; Ai, J.; Foroutani, L.; Ai, A.; Khanmohammadi, M. Chitosan hydrogel loaded with gel and tetrasodium ethylenediaminetetraacetic acid (EDTA) as the wound healing material: In vitro and in vivo study. J. Appl. Polym. Sci. 2021, 138, 50225. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Jaromin, A.; Czopek, A.; Zagórska, A.; Tyliszczak, B. Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract. Materials 2021, 14, 3379. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, F.; Wang, L.; Yang, Y.; Khan, B.M.; Cheong, K.L.; Liu, Y. Preparation and evaluation of Bletilla striata polysaccharide/carboxymethyl chitosan/Carbomer 940 hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 132, 729–737. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In vitro biosafety of pro-ecological chitosan-based hydrogels modified with natural substances. J. Biomed. Mater. Res. Part A 2019, 107, 2501–2511. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F.; Rizvi, M.A.; Zafaryab, M. Ethyl acetate Salix alba leaves extract-loaded chitosan-based hydrogel film for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2015, 26, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Khan, A.; Khan, M.K.; Braga, V.A. Preparation and properties of High sheared Poly(Vinyl Alcohol)/Chitosan blended Hydrogels films with Lawsonia inermis extract as wound dressing. J. Drug Deliv. Sci. Technol. 2021, 61, 102227. [Google Scholar] [CrossRef]
- Lucca, L.G.; de Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga, V.F., Jr.; de Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech 2018, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Noshad, M.; Falah, F. Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microb. Pathog. 2019, 136, 103716. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and evaluation of chitosan/gelatin/PVA hydrogel incorporating honey for wound healing applications: An in vitro, in vivo study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef]
- Lavanya, K.; Balagangadharan, K.; Chandran, S.V.; Selvamurugan, N. Chitosan-coated and thymol-loaded polymeric semi-interpenetrating hydrogels: An effective platform for bioactive molecule delivery and bone regeneration in vivo. Biomater. Adv. 2023, 146, 213305. [Google Scholar] [CrossRef]
- Arpornmaeklong, P.; Jaiman, N.; Apinyauppatham, K.; Fuongfuchat, A.; Boonyuen, S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15, 416. [Google Scholar] [CrossRef]
- Haloi, P.; Chawla, S.; Konkimalla, V.B. Thermosensitive smart hydrogel of PEITC ameliorates the therapeutic efficacy in rheumatoid arthritis. Eur. J. Pharm. Sci. 2023, 181, 106367. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, T.; Xiong, M.; Li, S.; Li, W.X.; Liu, J.; Zhou, X.; Qi, J.; Jiang, G.B. Cannabidiol-loaded injectable chitosan-based hydrogels promote spinal cord injury repair by enhancing mitochondrial biogenesis. Int. J. Biol. Macromol. 2022, 221, 1259–1270. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, G.; Wang, J.; Zhang, R.; Zhong, S.; Wang, J.; Cui, X. Injectable chitosan-based self-healing supramolecular hydrogels with temperature and pH dual-responsivenesses. Int. J. Biol. Macromol. 2023, 227, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, G.; Wang, Y.; Tang, M.; Ma, X.; Chang, X.; Guo, J.; Gui, S. Thermosensitive acetylated carboxymethyl chitosan gel depot systems sustained release caffeic acid phenethyl ester for periodontitis treatment. Polym. Adv. Technol. 2023, 34, 155–165. [Google Scholar] [CrossRef]
- Maghsoudi, A.; Yazdian, F.; Shahmoradi, S.; Ghaderi, L.; Hemati, M.; Amoabediny, G. Curcumin-loaded polysaccharide nanoparticles: Optimization and anticariogenic activity against Streptococcus mutans. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhou, H.Y.; Ren, L.; Zheng, H.; Tong, J.; Chen, Y.; Park, H. Preparation and antibacterial properties of curcumin-loaded cyclodextrin-grafted chitosan hydrogel. J. Sol-Gel Sci. Technol. 2023, 106, 877–894. [Google Scholar] [CrossRef]
- Wang, M.; Muhammad, T.; Gao, H.; Liu, J.; Liang, H. Targeted pH-responsive chitosan nanogels with Tanshinone IIA for enhancing the antibacterial/anti-biofilm efficacy. Int. J. Biol. Macromol. 2023, 237, 124177. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, J.; Zhang, Y.; Zheng, P.; Tang, T.; Luo, J.K.; Cui, H.J.; Song, R.R.; Wang, Y. Chitosan Hydrogel Delivery System Containing Herbal Compound Functions as a Potential Antineuroinflammatory Agent. ACS Omega 2019, 4, 10185–10191. [Google Scholar] [CrossRef]
- Lim, M.K.; Lee, S.; Kim, J.Y.; Jeong, J.; Han, E.H.; Lee, S.H.; Ryu, J.H.; Lee, J. Neuroprotective and anti-neuroinflammatory effects of ethanolic extract from leaves and stems of Aster glehni. J. Funct. Foods 2021, 79, 104400. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr. Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Ghosh, B.; Bhattacharya, D.; Mukhopadhyay, M. A hydrogel sheet mask with tea tree essential oil entrapment and targeted dose delivery capability. Mater. Today Proc. 2022, 57, 77–83. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.; Guo, Y.; Zhao, P.; Wang, J.; Zhang, R.; Wang, X. Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023, 405, 134856. [Google Scholar] [CrossRef]
- Sabzini, M.; Pourmadadi, M.; Yazdian, F.; Khadiv-Parsi, P.; Rashedi, H. Development of chitosan/halloysite/graphitic carbon nitride nanovehicle for targeted delivery of quercetin to enhance its limitation in cancer therapy: An in vitro cytotoxicity against MCF-7 cells. Int. J. Biol. Macromol. 2023, 226, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Issarachot, O.; Bunlung, S.; Kaewkroek, K.; Wiwattanapatapee, R. Superporous hydrogels based on blends of chitosan and polyvinyl alcohol as a carrier for enhanced gastric delivery of resveratrol. Saudi Pharm. J. SPJ 2023, 31, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kurtulbaş, E.; Albarri, R.; Torun, M.; Şahin, S. Encapsulation of Moringa oleifera leaf extract in chitosan-coated alginate microbeads produced by ionic gelation. Food Biosci. 2022, 50, 102158. [Google Scholar] [CrossRef]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; Martín de Llano, J.J.; Gisbert Roca, F.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef]
- Ghebremedhin, M.; Seiffert, S.; Vilgis, T.A. Physics of agarose fluid gels: Rheological properties and microstructure. Curr. Res. Food Sci. 2021, 4, 436–448. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef]
- Zhao, J.; Marczynski, M.; Henkel, M.; Lieleg, O. Agarose-based hydrogels with tunable, charge-selective permeability properties. J. Appl. Polym. Sci. 2023, 140, e54303. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.-W.; Chen, F.-Q.; Weng, H.-F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.-F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar. Drugs 2023, 21, 299. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Ye, J.; Zhao, P.; Xiao, M. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Forget, A.; Pique, R.-A.; Ahmadi, V.; Lüdeke, S.; Shastri, V.P. Mechanically Tailored Agarose Hydrogels through Molecular Alloying with β-Sheet Polysaccharides. Macromol. Rapid Commun. 2015, 36, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Heinze, T. Homogeneous tosylation of agarose as an approach toward novel functional polysaccharide materials. Carbohydr. Polym. 2015, 127, 236–245. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Agarose, Alginate and Chitosan Nanostructured Aerogels for Pharmaceutical Applications: A Short Review. Front. Bioeng. Biotechnol. 2021, 9, 688477. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Kolanthai, E.; Abinaya Sindu, P.; Thanigai Arul, K.; Sarath Chandra, V.; Manikandan, E.; Narayana Kalkura, S. Agarose encapsulated mesoporous carbonated hydroxyapatite nanocomposites powder for drug delivery. J. Photochem. Photobiol. B Biol. 2017, 166, 220–231. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/agarose/graphitic carbon nitride nanocomposite as an efficient pH-sensitive drug delivery system for anticancer curcumin releasing. J. Drug Deliv. Sci. Technol. 2022, 74, 103443. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible pH-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Samadi, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Eufrasio-da-Silva, T. Ameliorating quercetin constraints in cancer therapy with pH-responsive agarose-polyvinylpyrrolidone -hydroxyapatite nanocomposite encapsulated in double nanoemulsion. Int. J. Biol. Macromol. 2021, 182, 11–25. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M. Marine Biomaterials: Hyaluronan. Mar. Drugs 2023, 21, 426. [Google Scholar] [CrossRef] [PubMed]
- Sprott, H.; Fleck, C. Hyaluronic Acid in Rheumatology. Pharmaceutics 2023, 15, 2247. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Adv. Mater. 2018, 30, e1705388. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose-hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Jin, X.; Wei, C.; Li, K.; Yin, P.; Wu, C.; Zhang, W. Polyphenol-mediated hyaluronic acid/tannic acid hydrogel with short gelation time and high adhesion strength for accelerating wound healing. Carbohydr. Polym. 2024, 342, 122372. [Google Scholar] [CrossRef]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; De Luca, I.; Soares Pontes, G.; Calarco, A.; Cerruti, P. Thermo-Responsive Hydrogel Containing Microfluidic Chitosan Nanoparticles Loaded with Opuntia ficus-indica Extract for Periodontitis Treatment. Int. J. Mol. Sci. 2024, 25, 9374. [Google Scholar] [CrossRef]

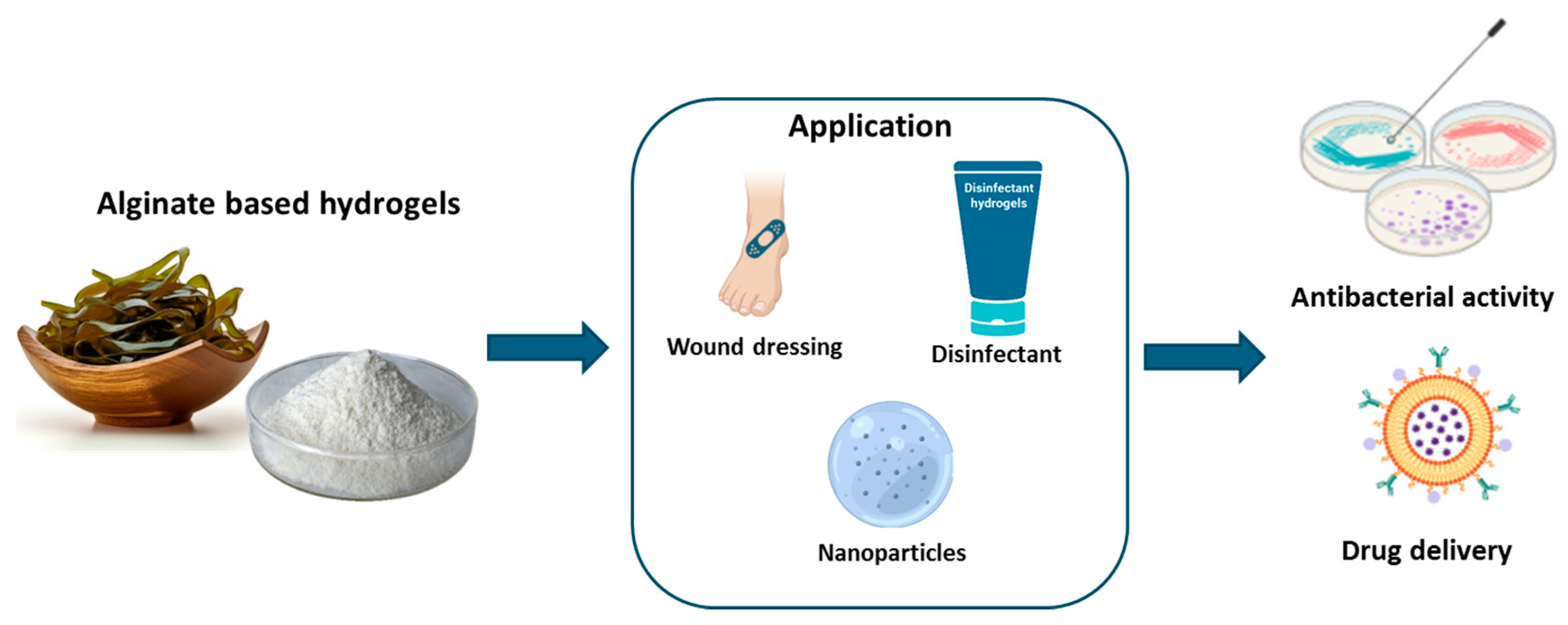

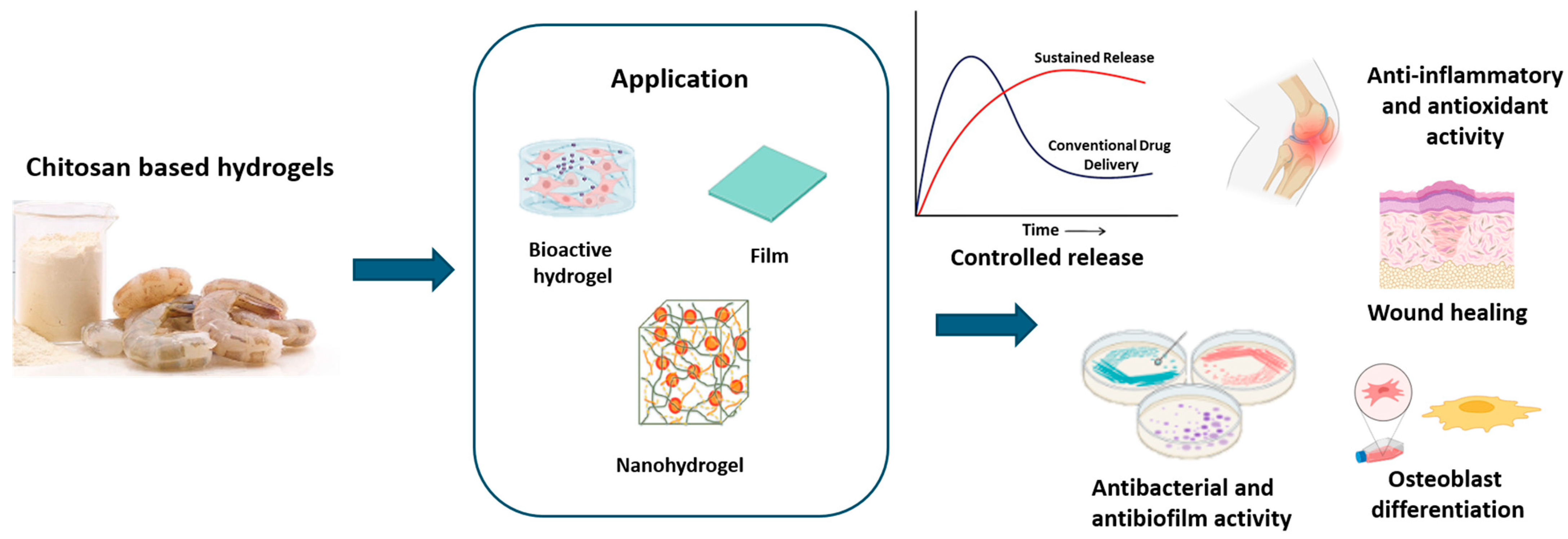
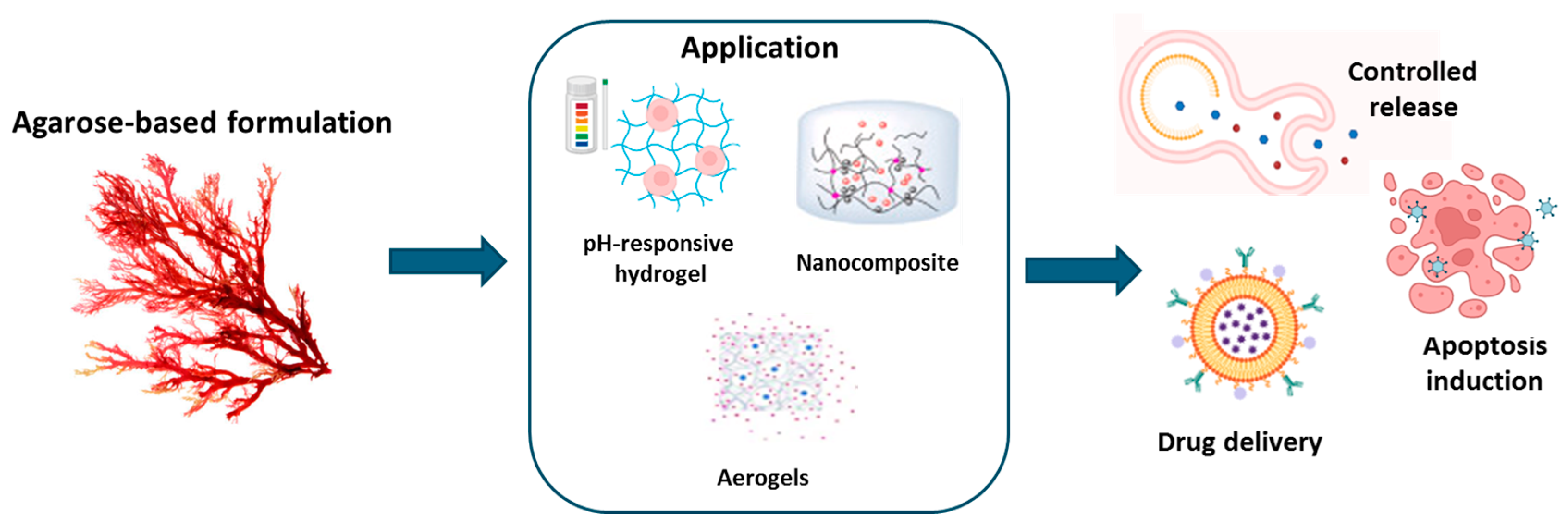
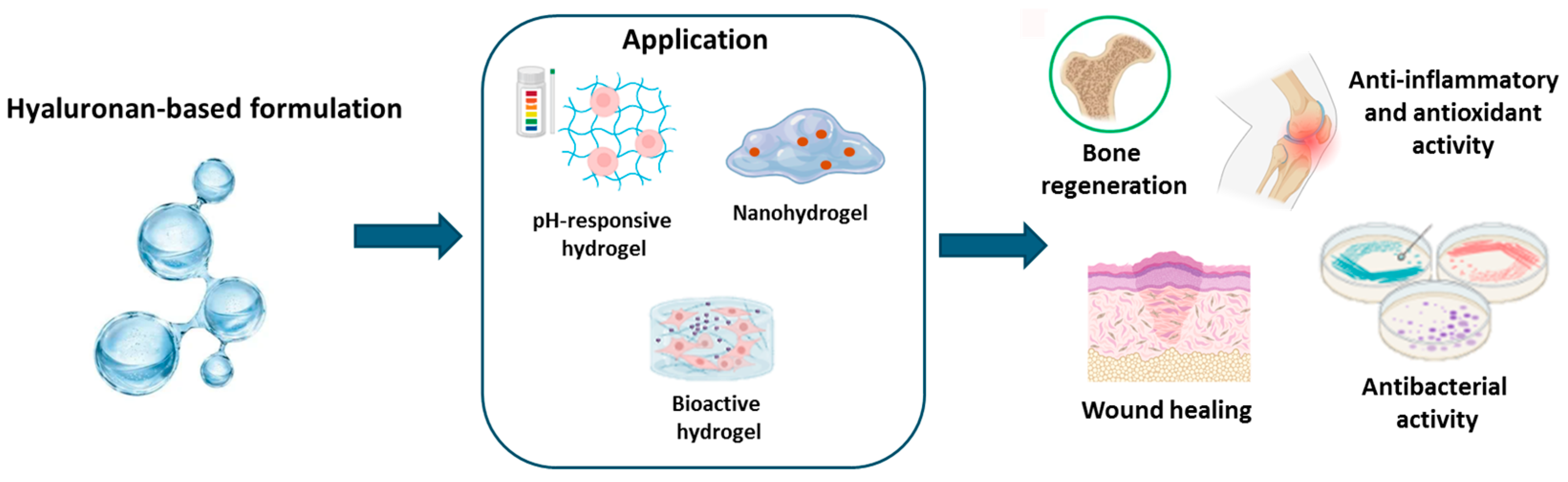
| Application | Alginate-Based Formulation | Benefits | References |
|---|---|---|---|
| Wound dressing | Alginate hydrogel with honey | Strong antibacterial activity against methicillin-resistant strains of S. aureus and E. coli | [45] |
| Wound dressing | Alginate and chitosan with aloe vera extract and honey | Prevention of bacterial infections from Staphylococcus aureus and Pseudomonas aeruginosa | [46] |
| Multifunction hydrogel | Lavender Essential Oil in alginate-modified hydrogel | Antibacterial effectiveness against S. aureus and C. albicans | [47] |
| Biocompatible disinfectants | Cross-linked hydrogel beads encapsulating glycoalkaloids derived from tomato and potato leaves | Antibacterial properties | [48] |
| Multifunction hydrogel | Alginate-based hydrogels for the sustained release of quercetin | Versatile delivery system for single and combination therapies | [49] |
| Multifunction hydrogel | Alginate-based hydrogels for the sustained release of curcumin | Versatile delivery system for single and combination therapies | [50] |
| Application | Carrageenan-Based Formulation | Benefits | References |
|---|---|---|---|
| Multifunction hydrogel | Alginate/carrageenan microgels delivering natural immunoglobulins | Improved pharmacokinetics and pharmacological action | [66] |
| Multifunction hydrogel | Carrageenan/pectin gels delivering natural enzymes (β-galactosidase) | Decreased enzyme sensitivity and increased compound stability during digestion | [67] |
| Multifunction hydrogel | pH-responsive hydrogel beads based on alginate, κ-carrageenan, and Poloxamer delivering curcumin | Enhanced encapsulation and controlled release efficiency | [68] |
| Multifunction hydrogel | Hydrogel composed of κ-carrageenan-containing nanocomplexes able to encapsulate blueberry anthocyanins | Safeguarded against anthocyanin degradation at low pH | [70] |
| Colon-targeted device | Whey protein/κ-carrageenan hydrogels delivering curcumin | Preserved curcumin from degradation during digestion | [71] |
| Hydrogel beads | Hydrogel beads made of cross-linked carrageenan and alginate delivering curcumin | Enhanced drug solubility, metabolic stability, non-toxicity, and capacity to target cancer cells more specifically | [72] |
| Application | Chitosan-Based Formulation | Benefits | References |
|---|---|---|---|
| Dressing for wound healing | Chitosan-based hydrogel containing phloroglucinol | Improved antioxidant and antimicrobial activities | [81] |
| Dressing for wound healing | Chitosan/silk hydrogel loaded with purified polysaccharide from Curcuma zedoaria and platelet-rich plasma–exosomes | Improved antioxidant and antimicrobial activities | [82] |
| Treatment of excisional wounds | Chitosan-based gel containing vitexin | Improved antioxidant, anti-inflammatory, antiviral, and antibacterial properties | [83] |
| Dressing for wound healing | Chitosan hydrogel incorporating Fagonia indica | Improved skin wound re-epithelialization and wound healing abilities | [84] |
| Functionalized platform for wound reparation | Cross-linked chitosan/gelatin hydrogel containing polyphenolic extract from Hamamelis virginiana | Inhibition of metalloproteases and reactive oxygen species Antibacterial activity against P. aeruginosa and S. aureus | [85] |
| Dressing for chronic wound healing | Soft hydrogel based on modified chitosan containing P. granatum peel extract | Improved antioxidant and antimicrobial activities | [86] |
| Dressing for acute wound healing | Biocompatible hydrogel film embedding Periplaneta americana extract | Improved antioxidant and antimicrobial activities | [87] |
| Dressing for wound healing | Chitosan-based hydrogels containing aloe vera juice | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [88] |
| Dressing for wound healing | Chitosan-based hydrogels containing aloe vera gel | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [89] |
| Dressing for wound healing | Bee chitosan-based hydrogels modified with yellow tea extract | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [90] |
| Dressing for wound healing | Bletilla striata polysaccharide/carboxymethyl chitosan/carbomer 940 hydrogel | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [91] |
| Dressing for wound healing | Chitosan-based hydrogel containing Salvia officinalis | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [92] |
| Dressing for wound healing | Ethyl acetate Salix alba leaf extract-loaded chitosan-based hydrogel film | Regenerative effect on skin wounds; anti-inflammatory and antibacterial effect | [93] |
| Dressing for wound healing | Lawsonia inermis ethanolic extract loaded in chitosan/PVA hydrogel | Improved antioxidant and antimicrobial activities | [94] |
| Dressing for wound healing | Essential oil-rich copaiba loaded in chitosan-based hydrogel | Acceleration in wound healing and optimal antibacterial and anti-inflammatory effect | [95] |
| Dressing for wound healing | Essential oil from Eucalyptus, Ginger, and Cumin loaded in chitosan-based hydrogel | Acceleration in wound healing and optimal antibacterial and anti-inflammatory effect | [96,97] |
| Dressing for wound healing | Bee honey incorporated into chitosan-based hydrogel | Maintenance of well-structured epidermis and acceleration of wound healing | [98] |
| Gel for bone strengthening | Chitosan-coated semi-interpenetrating polymer hydrogels, containing sodium alginate and poly(2-ethyl-2-oxazoline) loaded with Thymol | Stimulation of osteoblastic differentiation without cytotoxicity on mesenchymal stem cells | [99] |
| Gel for bone disease treatment | Calcium carbonate microcapsules containing nanohydroxyapatite/chitosan/collagen hydrogel particles containing quercetin | Prolonged-release profiles of flavonoid; promotion of bone regeneration of bones | [100] |
| Hydrogel to reduce joint edema and advancement of arthritis and bone erosion | Phenethylisothiocyanate loaded in an injectable chitosan/pluronic hydrogel | Improvement of bioactive’s water solubility and half-life | [101] |
| In situ gelling device for the treatment of spinal cord lesions | In situ gelling chitosan hydrogels delivering cannabidiol | Prolonged release | [102] |
| Gel for treatment of periodontitis | Chitosan hydrogel loading caffeic acid phenyl ester | Effective reduction in inflammation and simultaneous repair of bone tissue | [104] |
| Gel for treatment of dental decay | Chitosan hydrogel encapsulating curcumin | Inhibitory effects against Streptococcus mutants | [105] |
| Antibacterial device | Turmeric cyclodextrin-grafted chitosan hydrogel | Antimicrobial activity against Staphylococcus aureus and E. coli | [106] |
| Antimicrobial device | Chitosan nanohydrogels loaded with tanshinone | Antibacterial and anti-biofilm activity against Streptococcus | [107] |
| Gel used as antineuroinflammatory agent | Rhein–chitosan hydrogel | Improved mechanical strength, sustained release, and low toxicity characteristics | [108] |
| Gel used for neuritis treatment | Chitosan gel containing Aster glehni leaf extract | Controlled release | [109] |
| Multifunction nanohydrogel | Chitosan nanohydrogels containing Yerba mate | Prolonged release of antioxidant compounds | [110] |
| Gel for acne treatment | Chitosan hydrogel containing tea tree oil for acne treatment | Improved antimicrobial activity | [111] |
| pH-sensitive delivery platform for anticancer purposes | Chitosan gel containing carvacrol | Controlled release | [112] |
| pH-sensitive delivery platform for anticancer purposes | Chitosan gel containing quercetin | Controlled release | [113] |
| pH-sensitive delivery platform for anticancer purposes | Chitosan-based hydrogel containing resveratrol | Rapid absorption into gastric fluid | [114] |
| pH-sensitive delivery platform for anticancer purposes | Chitosan-coated alginate microbeads containing leaf extract of Moringa oleifera | Improved drug stability | [115] |
| Application | Agarose-Based Formulation | Benefits | References |
|---|---|---|---|
| Multifunction hydrogel | Agarose nanostructured aerogels delivering natural substances | Improved drug delivery characteristics | [127] |
| Anticancer hydrogel | Nanocomposite containing chitosan, agarose, and graphitic carbon nitride to load and release curcumin | Enhanced encapsulation and controlled release efficiency. | [130] |
| Multifunction hydrogel | Flexible pH-responsive agarose/succinoglycan hydrogels | Controlled drug release | [131] |
| Multifunction nanocomposite hydrogel | pH-responsive hydrogel nanocomposite made from agarose, polyvinylpyrrolidone, and hydroxyapatite loading quercetin | Improved loading capacity, sustained release, and apoptosis-inducing actions | [132] |
| Application | Hyaluronan-Based Formulation | Benefits | References |
|---|---|---|---|
| pH-sensitive hydrogel for transdermal drug delivery | pH-sensitive hydrogel containing isoliquiritigenin using hydroxyethyl cellulose and hyaluronic acid | pH-dependent drug release, with higher drug release at pH levels above 7 | [141] |
| Composite hydrogel for wound healing | Composite hydrogel incorporating tannic acid and dopamine-coated carbon particles rich in phenols | Rapid gelation time, exceptional adhesive strength, low hemolytic activity, minimal cytotoxicity, and ability to promote fibroblast proliferation and migration | [142] |
| Hydrogel for the treatment of lower limb skin wound in patients with diabetes mellitus | Hyaluronic acid-based nanohydrogel embedded with quercetin and oleic acid | Reduction in wound healing time without developing adverse drug reactions | [143] |
| Hydrogel for localized and sustained release in periodontal pockets | Opuntia ficus-indica extract encapsulated in chitosan nanoparticles embedded in pluronic–hyaluronic thermo-responsive hydrogel | System able to eradicate biofilms of S. mutans, P. aeruginosa, and P. gingivalis and disrupt extracellular polymeric substance formation. | [144] |
| Hydrogel for treatment of atopic dermatitis | Hyaluronic acid hydrogel containing resveratrol-loaded chitosan (CS) nanoparticles | Delayed hydrolytic degradation and slowed resveratrol release. Decreased oxidative damage in TNF-α/INF-γ-treated human keratinocytes (HaCaTs) and reduced secretion and gene expression of proinflammatory cytokines | [19] |
| Localized drug delivery platform | Hydroxytyrosol-loaded chitosan nanoparticles in an in situ hydrogel composed of pluronic F-127 and hyaluronic acid | Reduced oxidative and inflammatory effects in chondrocyte cellular model and influence on chondrocyte gene expression under pathological state | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. https://doi.org/10.3390/ijms26020764
Sepe F, Valentino A, Marcolongo L, Petillo O, Conte R, Margarucci S, Peluso G, Calarco A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. International Journal of Molecular Sciences. 2025; 26(2):764. https://doi.org/10.3390/ijms26020764
Chicago/Turabian StyleSepe, Fabrizia, Anna Valentino, Loredana Marcolongo, Orsolina Petillo, Raffaele Conte, Sabrina Margarucci, Gianfranco Peluso, and Anna Calarco. 2025. "Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds" International Journal of Molecular Sciences 26, no. 2: 764. https://doi.org/10.3390/ijms26020764
APA StyleSepe, F., Valentino, A., Marcolongo, L., Petillo, O., Conte, R., Margarucci, S., Peluso, G., & Calarco, A. (2025). Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. International Journal of Molecular Sciences, 26(2), 764. https://doi.org/10.3390/ijms26020764







