Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate
Abstract
1. Introduction
2. Results
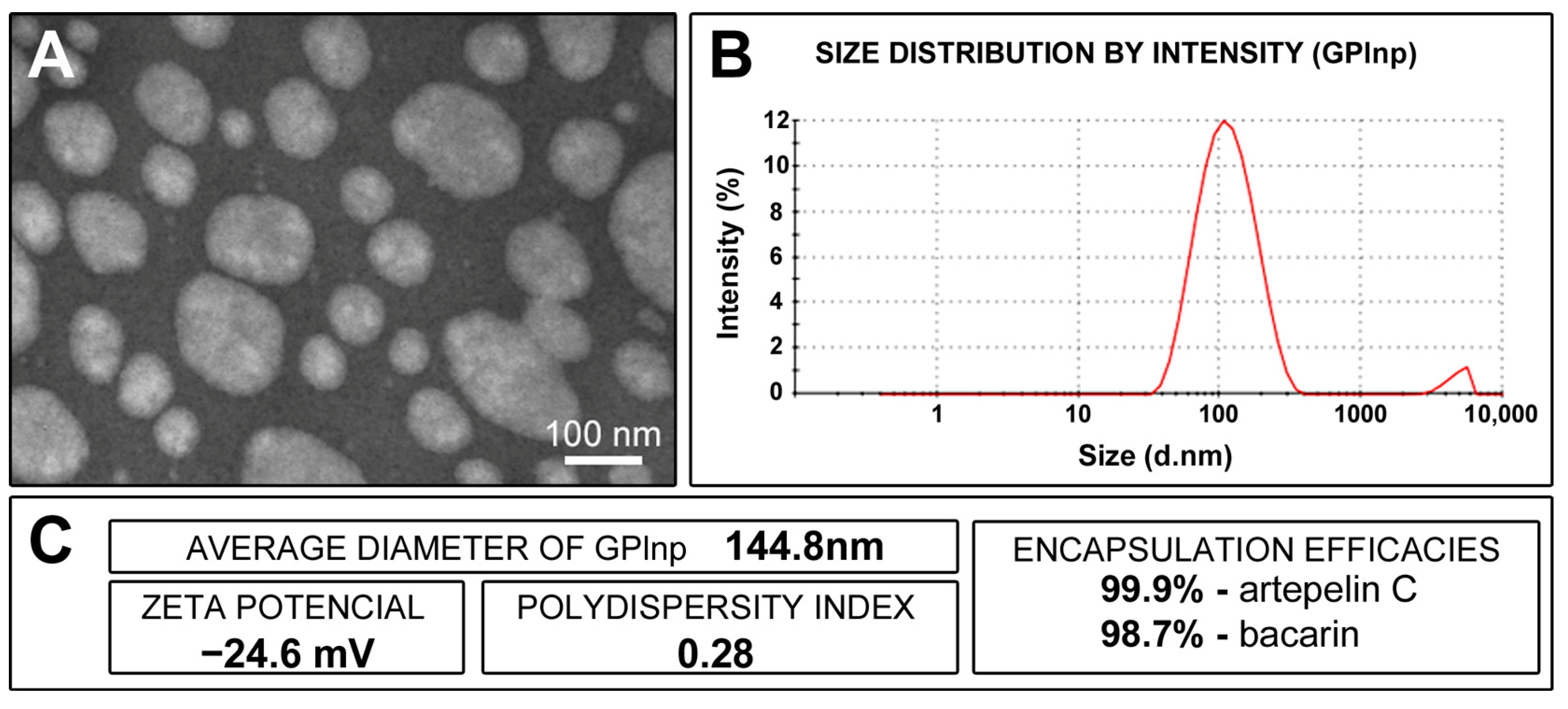
2.1. Physicochemical Characterization of GPlnp
2.2. Availability of General Health and Intraoral Condition of the Animals
2.3. Histopathological Analyses of Periodontal Tissues
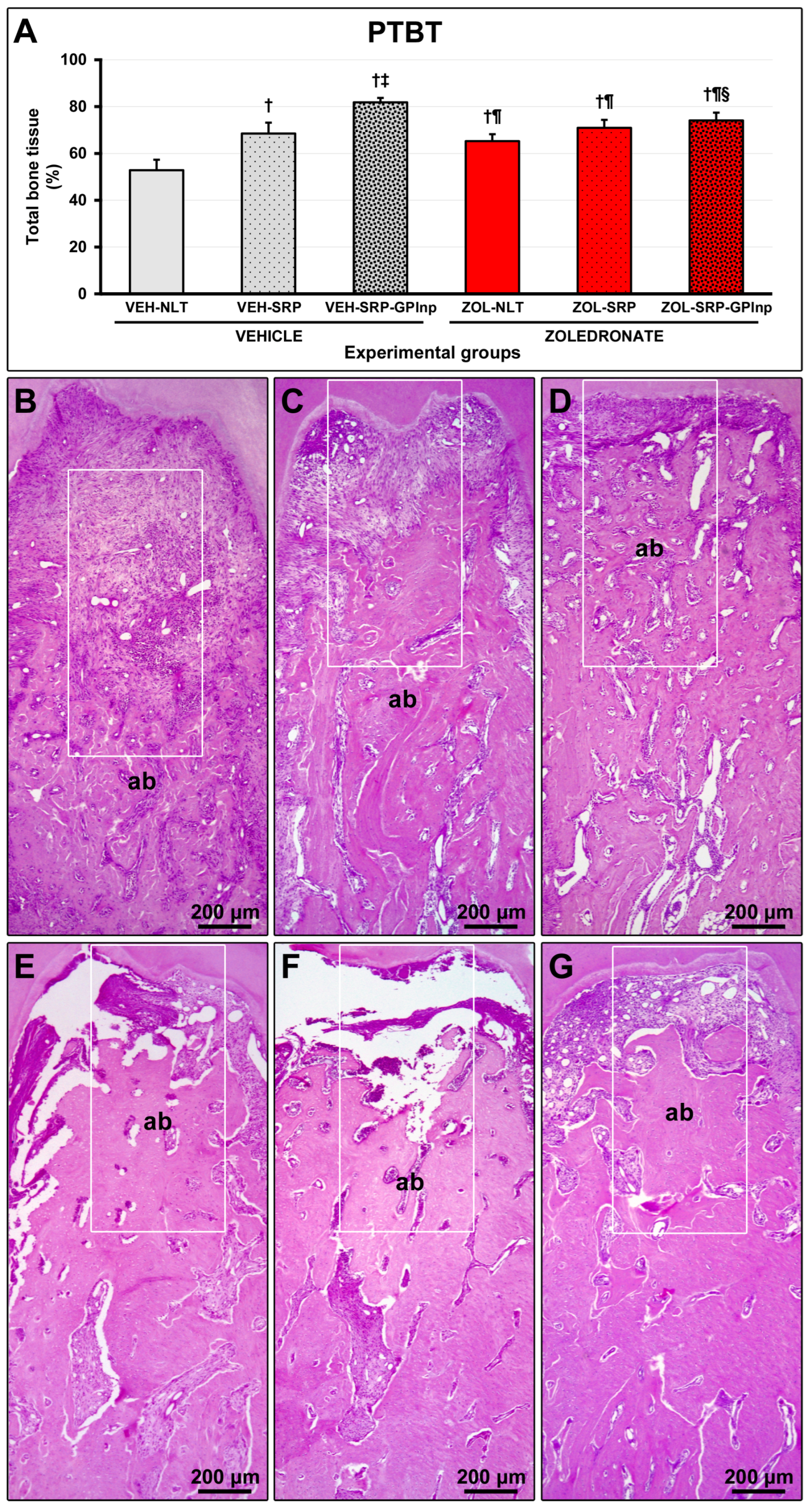
2.4. PTBT
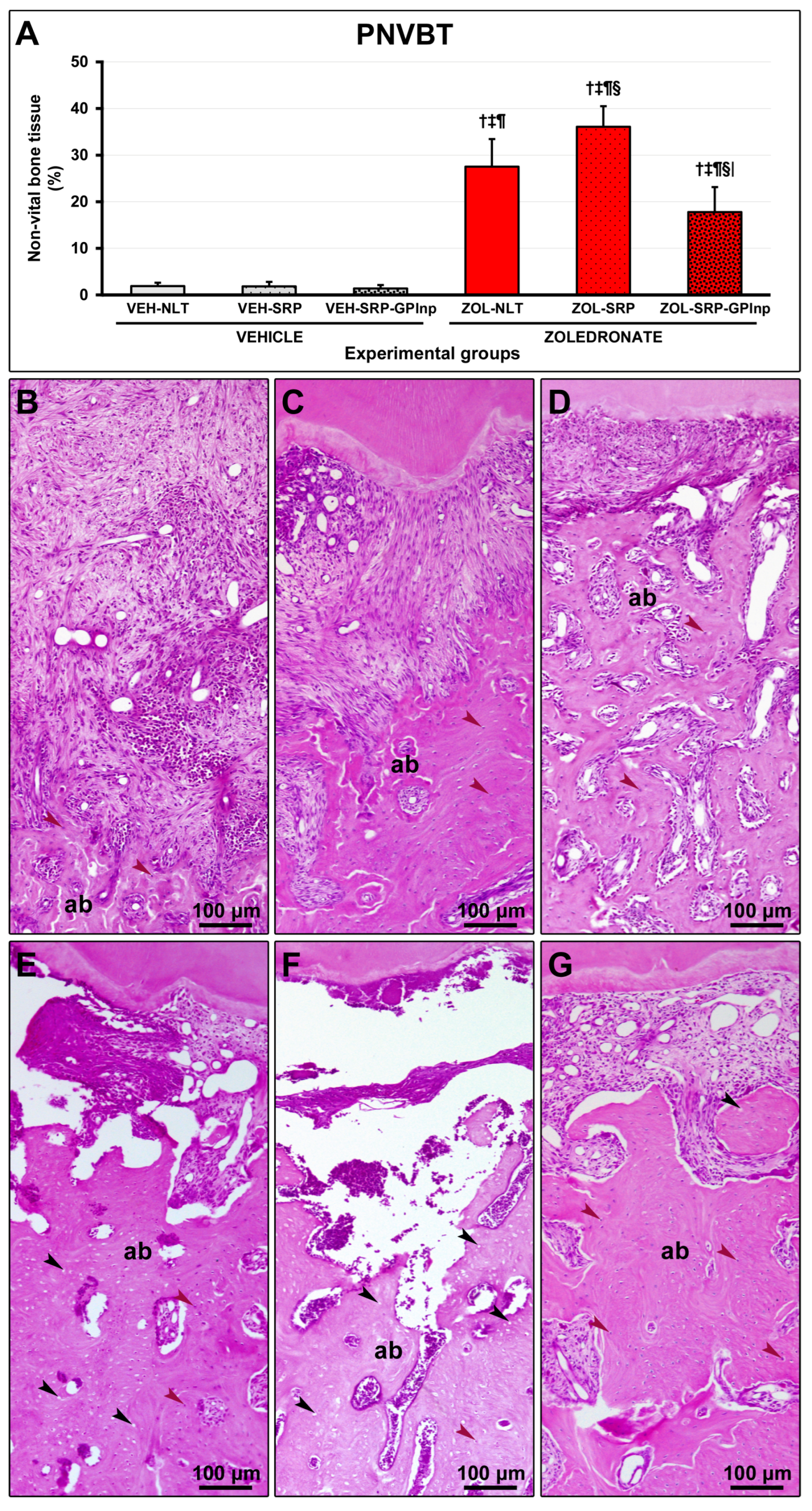
2.5. PNVBT
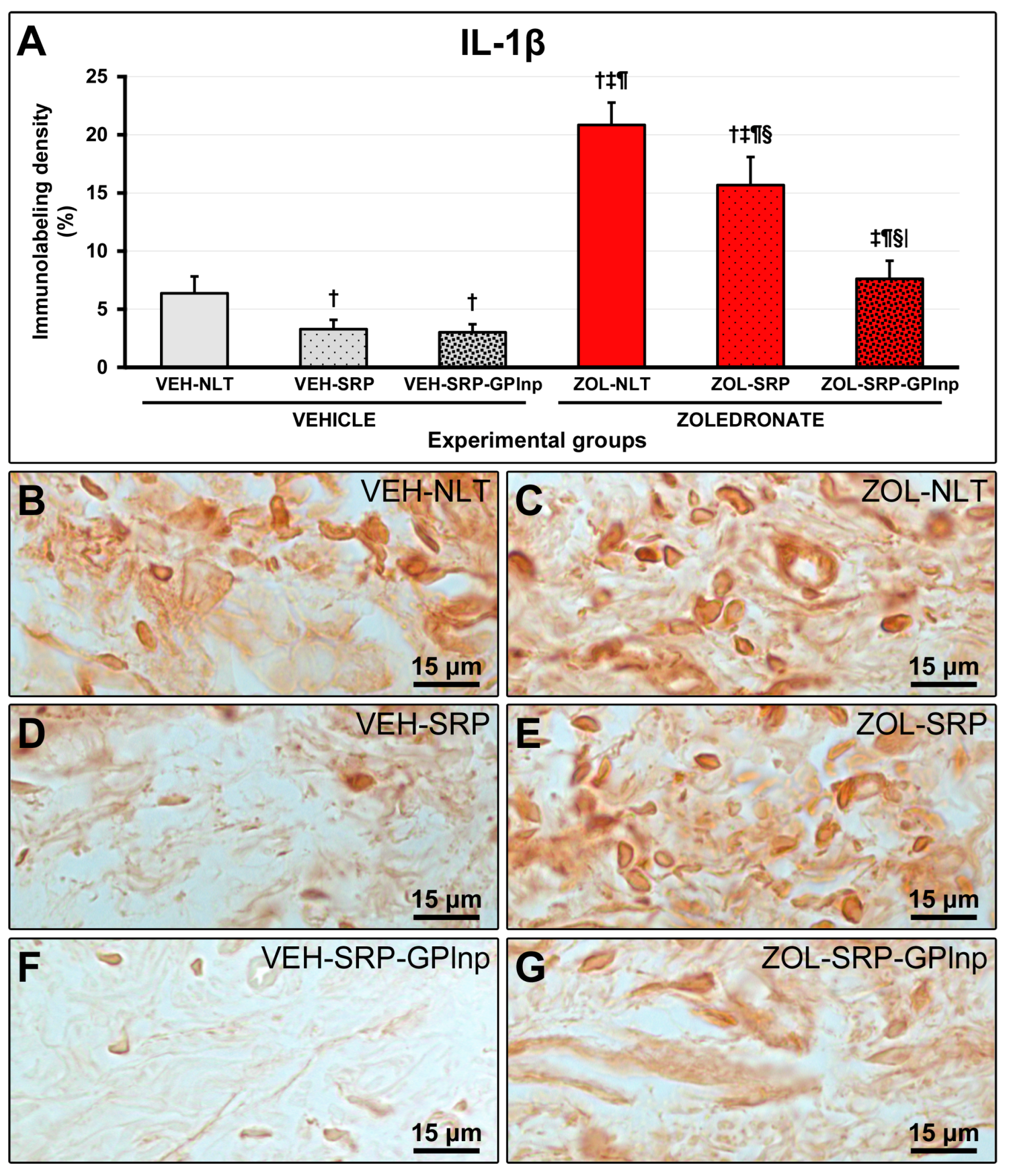
2.6. Immunolabeling Density for TNFα and IL-1β
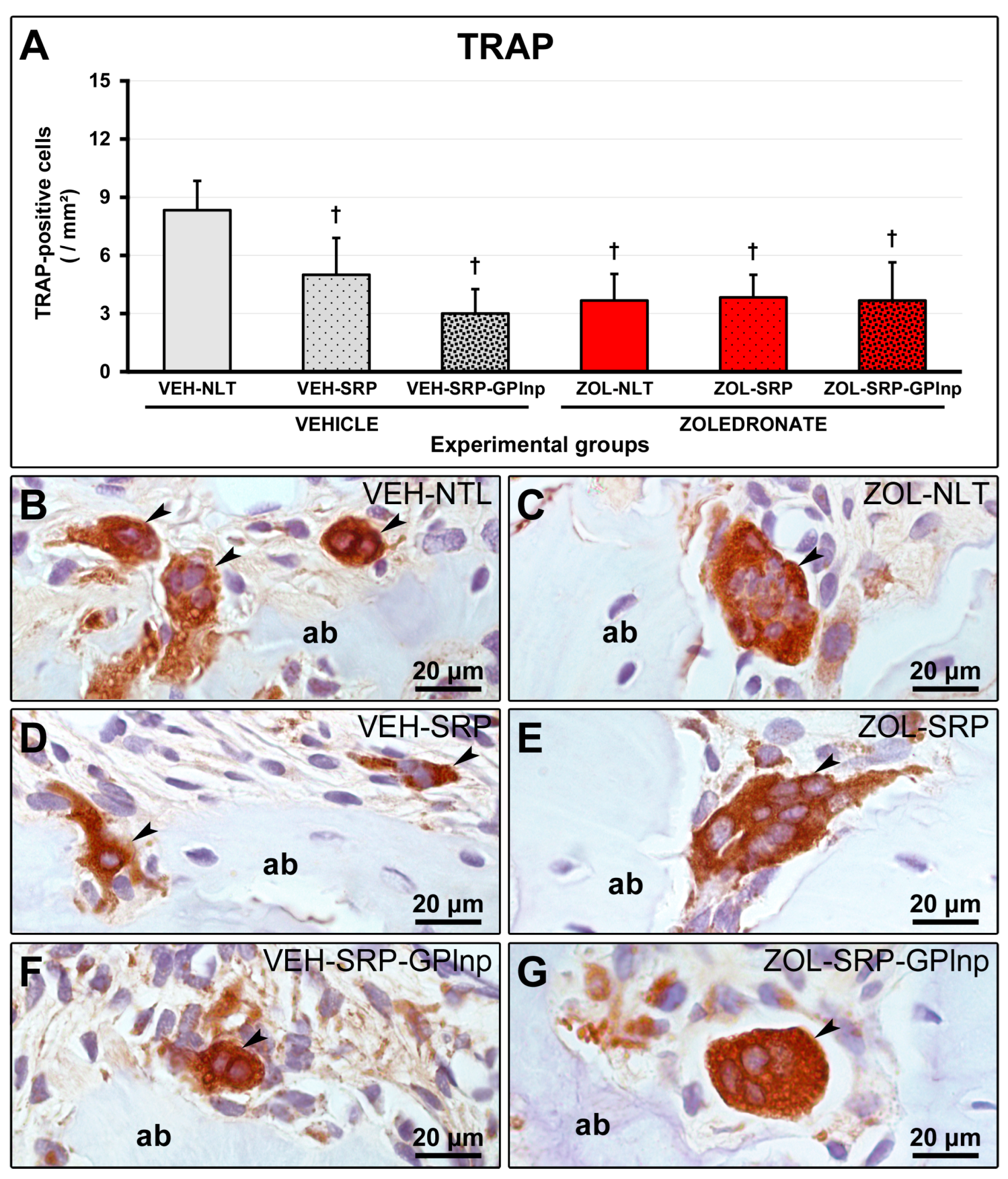
2.7. TRAP Immunolabeling
3. Discussion
4. Materials and Methods
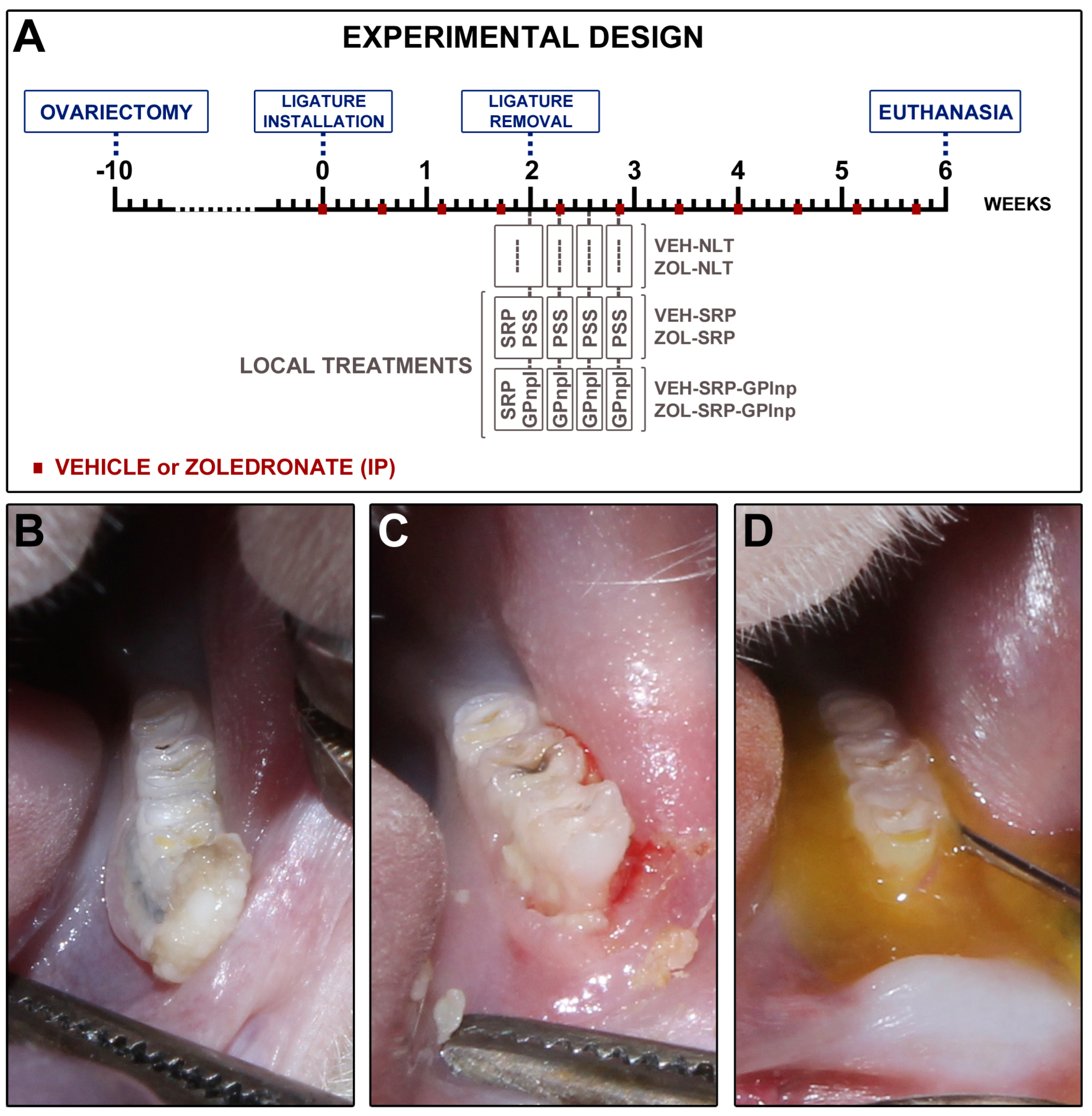
4.1. Animals, Sample Calculation, Randomization, and Ethics
4.2. Anesthesia
4.3. Ovariectomy
4.4. Ligature-Induced Periodontitis
4.5. Drug Regimen
4.6. Experimental Groups
4.7. Scaling and Root Planing
4.8. Green Propolis-Loaded Lipid Nanoparticles
4.9. Subgingival Irrigation with Physiological Saline Solution or GPlnp
4.10. Euthanasia and Sample Collection
4.11. Histological Processing
4.12. Immunohistochemistry Processing
5. Analysis of the Results
5.1. General Health and Intraoral Condition of the Animals
5.2. Histopathological Analyses
5.3. Histometric Analysis of the Percentage of Total Bone Tissue (PTBT)
5.4. Histometric Analysis of the Percentage of Non-Vital Bone Tissue (PNVBT)
5.5. Immunolabeling for TNFα, IL-1β, and TRAP in the Furcation Area
5.6. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral. Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Tetradis, S.; Allen, M.R.; Ruggiero, S.L. Pathophysiology of Medication-Related Osteonecrosis of the Jaw-A Minireview. JBMR Plus 2023, 7, e10785. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Mannion, C.J. Medication-related osteonecrosis of the jaws and quality of life: Review and structured analysis. Br. J. Oral. Maxillofac. Surg. 2020, 58, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Halpern, L.R.; Adams, D.R. Treatment of medication-related osteonecrosis of the jaw: Controversies in causality and therapy. Dent. Clin. N. Am. 2024, 68, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, CD012432. [Google Scholar] [CrossRef]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Kim, H.Y. Review and Update of the Risk Factors and Prevention of Antiresorptive-Related Osteonecrosis of the Jaw. Endocrinol. Metab. 2021, 36, 917–927. [Google Scholar] [CrossRef]
- McGowan, K.; McGowan, T.; Ivanovski, S. Risk Factors for Medication-Related Osteonecrosis of the Jaws: A Systematic Review. Oral. Dis. 2018, 24, 527–536. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Chamorro-Petronacci, C.; Gándara-Vila, P.; López-Jornet, P.; Carballo, J.; García-García, A. Association between periodontitis and medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Oral. Pathol. Med. 2020, 49, 190–200. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lin, K.C.; Liu, S.P.; Chang, C.T.; Muo, C.H.; Chang, P.J.; Tsai, C.H.; Wu, C.Z. The association between the severity of periodontitis and osteonecrosis of the jaw in patients with different cancer locations: A nationwide population-based study. Clin. Oral. Investig. 2022, 26, 3843–3852. [Google Scholar] [CrossRef]
- Thumbigere-Math, V.; Michalowicz, B.S.; Hodges, J.S.; Tsai, M.L.; Swenson, K.K.; Rockwell, L.; Gopalakrishnan, R. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J. Periodontol. 2014, 85, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.W.; Ho, K.; Lenon, A.; Kim, S.; Kim, T.; Gwack, Y.; Kim, R.H. Long-Term Ligature-Induced Periodontitis Exacerbates Development of Bisphosphonate-Related Osteonecrosis of the Jaw in Mice. J. Bone Miner. Res. 2022, 37, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.G.; Mendieta Calle, J.L.; Jiron, J.M.; Castillo, E.J.; Van Poznak, C.; Bhattacharyya, N.; Kimmel, D.B.; Aguirre, J.I. Zoledronic Acid Increases the Prevalence of Medication-Related Osteonecrosis of the Jaw in a Dose Dependent Manner in Rice Rats (Oryzomys palustris) with Localized Periodontitis. Bone 2018, 108, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Kang, B.; Sung, E.C.; Shoff, M.; Ronconi, M.; Gotcher, J.E.; Bezouglaia, O.; Dry, S.M.; Tetradis, S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J. Bone Miner. Res. 2011, 26, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef]
- Mello-Neto, J.M.; Ervolino, E.; Elangovan, G.; Toro, L.F.; Lee, J.; Gustafsson, A.; Figueredo, C.M.D.S. The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis. J. Clin. Med. 2023, 12, 4326. [Google Scholar] [CrossRef]
- Silveira, G.R.C.; de Lima, D.C.; Cintra, L.T.A.; Brigagão, M.R.P.L.; Ervolino, E.; Fernandes, L.A. Systemic and local effects of doxycycline and low-intensity laser treatment on periodontitis in rats. J. Periodontal Implant. Sci. 2022, 52, 39–53. [Google Scholar] [CrossRef]
- Silveira, G.R.C.; de Lima, D.C.; Cintra, L.T.A.; Brigagão, M.R.P.L.; Ervolino, E.; Fernandes, L.A. Influence of Doxycycline and InGaAlP Diode Laser at 660 nm Wavelength in the Treatment of Periodontitis Induced in Rats: In Vivo Study. Photochem. Photobiol. 2021, 97, 1104–1115. [Google Scholar] [CrossRef]
- Diniz-Freitas, M.; Fernández-Feijoo, J.; Diz Dios, P.; Pousa, X.; Limeres, J. Denosumab-related osteonecrosis of the jaw following non-surgical periodontal therapy: A case report. J. Clin. Periodontol. 2018, 45, 570–577. [Google Scholar] [CrossRef]
- Braun, E.; Iacono, V.J. Bisphosphonates: Case report of nonsurgical periodontal therapy and osteochemonecrosis. Int. J. Periodontics Restor. Dent. 2006, 26, 315–319. [Google Scholar]
- Araujo, N.J. Experimental Periodontitis in Rats Treated with an Oncological Dose of Zoledronate: Analysis of Disease Progression and Evaluation of Periodontal Response to Conventional Mechanical Treatment. Master’s Thesis, School of Dentistry, São Paulo State University, São Paulo, Brazil, 2017. [Google Scholar]
- Eghbali Zarch, R.; Askari, M.; Boostani, H.; Mirzaii-Dizgah, I. Effect of propolis extract on clinical parameters and salivary level of matrix metalloproteinase 8 in periodontitis patients: A randomized controlled clinical trial. J. Adv. Periodontol. Implant. Dent. 2021, 13, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of Topical Administration of Propolis in Chronic Periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Ozan, F.; Ozer, H.; Ozdemir, H.; Eren, K.; Yeler, H. A morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2008, 79, 1089–1094. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- dos Santos, F.F.; Morais-Urano, R.P.; Cunha, W.R.; de Almeida, S.G.; Cavallari, P.S.D.S.R.; Manuquian, H.A.; Pereira, H.A.; Furtado, R.; Santos, M.F.C.; Amdrade e Silva, M.L. A review on the anti-inflammatory activities of Brazilian green, brown and red propolis. J. Food Biochem. 2022, 46, e14350. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Soni, A.K.; Jha, R.K. Nanotechnology’s Applications and Potential in Various Fields. Cureus 2024, 16, e59234. [Google Scholar] [CrossRef]
- Mercan, D.A.; Niculescu, A.G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery—An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Tang, M.; Zhang, C.; Fei, Y.; Li, M.; Li, M.; Gui, S.; Guo, J. New insights into nanotherapeutics for periodontitis: A triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration. J. Nanobiotechnol. 2024, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, J.; Liu, W.; Peng, B.; Cong, S.; Yu, H.; Zhang, B.; Li, Y. Advancements in Characterization Techniques for Microemulsions: From Molecular Insights to Macroscopic Phenomena. Molecules 2024, 29, 2901. [Google Scholar] [CrossRef] [PubMed]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Khuda, F.; Baharin, B.; Anuar, N.N.M.; Satimin, B.S.F.; Nasruddin, N.S. Effective Modalities of Periodontitis Induction in Rat Model. J. Vet. Dent. 2024, 41, 49–57. [Google Scholar] [CrossRef]
- Staubli, N.; Schmidt, J.C.; Rinne, C.A.; Signer-Buset, S.L.; Rodriguez, F.R.; Walter, C. Animal Experiments in Periodontal and Peri-Implant Research: Are There Any Changes? Dent. J. 2019, 7, 46. [Google Scholar] [CrossRef]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C., Jr. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral. Biol. 2012, 15, 117–132. [Google Scholar] [CrossRef]
- Hadad, H.; Matheus, H.R.; Pai, S.I.; Souza, F.A.; Guastaldi, F.P.S. Rodents as an animal model for studying tooth extraction-related medication-related osteonecrosis of the jaw: Assessment of outcomes. Arch. Oral. Biol. 2024, 159, 105875. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Castillo, E.J.; Kimmel, D.B. Biologic and pathologic aspects of osteocytes in the setting of medication-related osteonecrosis of the jaw (MRONJ). Bone 2021, 153, 116168. [Google Scholar] [CrossRef]
- Kuroshima, S.; Go, V.A.; Yamashita, J. Increased numbers of nonattached osteoclasts after long-term zoledronic acid therapy in mice. Endocrinology 2012, 153, 17–28. [Google Scholar] [CrossRef]
- Ervolino, E.; Olivo, M.B.; Toro, L.F.; Freire, J.O.A.; Ganzaroli, V.F.; Guiati, I.Z.; Nuernberg, M.A.A.; Franciscon, J.P.S.; Cintra, L.T.A.; Garcia, V.G.; et al. Effectiveness of antimicrobial photodynamic therapy mediated by butyl toluidine blue in preventing medication-related osteonecrosis of the jaws in rats. Photodiagnosis Photodyn. Ther. 2022, 40, 103172. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.Q.M.; Toro, L.F.; Ganzaroli, V.F.; de Oliveira Alvarenga Freire, J.; Matsumoto, M.A.; Casatti, C.A.; Cintra, L.T.A.; Buchaim, R.L.; Issa, J.P.M.; Garcia, V.G.; et al. Peri-implantitis increases the risk of medication-related osteonecrosis of the jaws associated with osseointegrated implants in rats treated with zoledronate. Sci. Rep. 2024, 14, 627. [Google Scholar] [CrossRef]
- Almeida-Junior, S.; de Oliveira, K.R.P.; Marques, L.P.; Martins, J.G.; Ubeda, H.; Santos, M.F.C.; Rodrigues, M.A.; Andrade e Silva, M.L.; Ambrósio, S.R.; Bastos, J.K.; et al. In vivo anti-inflammatory activity of BACCHARIN from BRAZILIAN green PROPOLIS. Fitoterapia 2024, 175, 105975. [Google Scholar] [CrossRef] [PubMed]
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phytother. Res. 2021, 35, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Reis, M.B.; Coelho, G.D.P.; Gastaldello, G.H.; Peti, A.P.F.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Sorgi, C.A.; Faccioli, L.H.; et al. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021, 278, 114255. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.O.; Messias da Silva, L.C.F.; dos Santos, R.D.; Prado, B.A.; da Silva Montes, P.; Silva Rodrigues, L.F.; de Araújo, M.O.; Bilac, C.A.; Freire, D.O.; Gris, E.F.; et al. Chemical characterization and antibacterial activities of Brazilian propolis extracts from Apis mellifera bees and stingless bees (Meliponini). PLoS ONE 2024, 19, e0307289. [Google Scholar] [CrossRef]
- Jenny, J.C.; Kuś, P.M.; Szweda, P. Investigation of antifungal and antibacterial potential of green extracts of propolis. Sci. Rep. 2024, 14, 13613. [Google Scholar] [CrossRef]
- Veiga, R.S.; de Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Lira, I.S.; López, B.G.; Negrão, V.; Marcucci, M.C. Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef]
- de Sá Assis, M.A.; de Paula Ramos, L.; Abu Hasna, A.; de Queiroz, T.S.; Pereira, T.C.; Nagai de Lima, P.M.; Berretta, A.A.; Marcucci, M.C.; Talge Carvalho, C.A.; de Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef]
- Figueiredo, L.C.; Freitas Figueiredo, N.; da Cruz, D.F.; Baccelli, G.T.; Sarachini, G.E.; Bueno, M.R.; Feres, M.; Bueno-Silva, B. Propolis, Aloe Vera, Green Tea, Cranberry, Calendula, Myrrha and Salvia Properties against Periodontal Microorganisms. Microorganisms 2022, 10, 2172. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Cavagnola, M.A.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. A physico-chemical study of the interaction of ethanolic extracts of propolis with bacterial cells. Colloids Surf. B Biointerfaces 2021, 200, 111571. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Redden, P.; Traba, C. The mechanism of action of Russian propolis ethanol extracts against two antibiotic-resistant biofilm-forming bacteria. Lett. Appl. Microbiol. 2016, 62, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Hussain, E.A.; Shujaat, S.; Khan, U.M.; Ali, Q.; Malook, S.U.; Ali, D. Antibacterial potential of Propolis: Molecular docking, simulation and toxicity analysis. AMB Express. 2024, 14, 81. [Google Scholar] [CrossRef]
- Wood, P.L. Metabolic and Lipid Biomarkers for Pathogenic Algae, Fungi, Cyanobacteria, Mycobacteria, Gram-Positive Bacteria, and Gram-Negative Bacteria. Metabolites 2024, 14, 378. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef]
- Sun, H.; Li, P.; Kong, Q.; Deng, F.; Yu, X. Zoledronic acid affects the process of Porphyromonas gingivalis infecting oral mucosal epithelial barrier: An in-vivo and in-vitro study. Front. Cell Infect. Microbiol. 2023, 28, 1104826. [Google Scholar] [CrossRef]
- Shang, J.; Liu, H.; Zheng, Y.; Zhang, Z. Role of oxidative stress in the relationship between periodontitis and systemic diseases. Front. Physiol. 2023, 14, 1210449. [Google Scholar] [CrossRef]
- Bagan, J.; Sáez, G.T.; Tormos, M.C.; Gavalda-Esteve, C.; Bagan, L.; Leopoldo-Rodado, M.; Calvo, J.; Camps, C. Oxidative stress in bisphosphonate-related osteonecrosis of the jaws. J. Oral. Pathol. Med. 2014, 43, 371–377. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evid. Based Complement. Alternat. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef]
- Salrian, A.A.; Behzadi, A.; Oloumi, M.M.; Farajli Abbasi, M.; Delshad, S.; Moghadaszadeh, M. Amplification of Wound Healing by Propolis and Honey Ointment in Healthy and Diabetic Rat Models; Histopathological and Morphometric Findings. Arch. Razi Inst. 2022, 77, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Wang, J.; Kurosawa, M.; Ogiso, N.; Shikama, Y.; Kanekura, T.; Matsushita, K. Effect of green propolis extracts on experimental aged gingival irritation in vivo and in vitro. J. Oral. Biosci. 2021, 63, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Kim, K.H.; Lee, Y.M.; Ku, Y.; Seol, Y.J. Periodontal wound healing and tissue regeneration: A narrative review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- Toro, L.F.; de Mello-Neto, J.M.; Santos, F.F.V.D.; Ferreira, L.C.; Statkievicz, C.; Cintra, L.T.A.; Issa, J.P.M.; Dornelles, R.C.M.; de Almeida, J.M.; Nagata, M.J.H.; et al. Application of Autologous Platelet-Rich Plasma on Tooth Extraction Site Prevents Occurrence of Medication-Related Osteonecrosis of the Jaws in Rats. Sci. Rep. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Ervolino, E.; Statkievicz, C.; Toro, L.F.; de Mello-Neto, J.M.; Cavazana, T.P.; Issa, J.P.M.; Dornelles, R.C.M.; de Almeida, J.M.; Nagata, M.J.H.; Okamoto, R.; et al. Antimicrobial photodynamic therapy improves the alveolar repair process and prevents the occurrence of osteonecrosis of the jaws after tooth extraction in senile rats treated with zoledronate. Bone 2019, 120, 101–113. [Google Scholar] [CrossRef]
- Novaes, V.C.N.; Ervolino, E.; Fernandes, G.L.; Cunha, C.P.; Theodoro, L.H.; Garcia, V.G.; de Almeida, J.M. Influence of the Treatment with the Antineoplastic Agents 5-Fluorouracil and Cisplatin on the Severity of Experimental Periodontitis in Rats. Support. Care Cancer 2022, 30, 1967–1980. [Google Scholar] [CrossRef]
- Garcia, V.G.; Novaes, V.C.; de Almeida, J.M.; Longo, M.; Ervolino, E.; Bomfim, S.R.; Theodoro, L.H. Evaluation of the progression and treatment of experimental periodontitis in rats subjected to chemotherapy with 5-fluorouracil. Support. Care Cancer 2015, 23, 2007–2017. [Google Scholar] [CrossRef]

| HISTOPATHOLOGICAL ANALYSES | ||||||
|---|---|---|---|---|---|---|
| PARAMETERS AND SCORES | QUANTITY OF SPECIMENS | |||||
| EXPERIMENTAL GROUPS | ||||||
| VEH-NLT | VEH-SRP | VEH-SRP-GPlnp | ZOL-NLT | ZOL-SRP | ZOL-SRP-GPlnp | |
| INTENSITY OF LOCAL INFLAMMATORY RESPONSE | ||||||
| (1) Absence of inflammation | - | - | 7 | - | - | 3 |
| (2) Small quantity of inflammatory cells (less than 1/3 of cells were inflammatory cells) | - | 7 | 1 | - | - | 5 |
| (3) Moderate quantity of inflammatory cells (1/3 to 2/3 were inflammatory cells) | 5 | 1 | - | 3 | 5 | - |
| (4) Large quantity of inflammatory cells (more than 2/3 were inflammatory cells) | 3 | - | - | 5 | 3 | - |
| MEDIAN | 3 | 2 † | 1 † | 4 ‡¶ | 3 ‡¶ | 2 †§| |
| EXTENSION OF INFLAMMATORY INFILTRATE | ||||||
| (1) Absence of inflammation | - | - | 7 | - | - | 3 |
| (2) Partial extension of connective tissue | - | 8 | 1 | - | - | 5 |
| (3) Entire extension of connective tissue, without reaching bone tissue in the furcation region | 5 | - | - | 3 | 3 | - |
| (4) Entire extension of connective tissue and bone tissue | 3 | - | - | 5 | 5 | - |
| MEDIAN | 3 | 2 † | 1 † | 4 ‡¶ | 4 ‡¶ | 2 †§| |
| PATTERN OF STRUCTURATION OF THE CONNECTIVE TISSUE IN THE FURCATION REGION | ||||||
| (1) Moderate number of fibroblasts and large amount of collagen fibers (dense connective tissue) | - | - | 7 | - | - | 1 |
| (2) Moderate amount of both fibroblasts and collagen fibers | - | 7 | 1 | - | - | 6 |
| (3) Small amount of both fibroblasts and collagen fibers | 7 | 1 | - | 2 | 2 | 1 |
| (4) Severe tissue disorganization with necrosis areas | 1 | - | - | 6 | 6 | - |
| MEDIAN | 3 | 2 | 1 † | 4 ‡¶ | 4 ‡¶ | 2 †§| |
| PATTERN OF STRUCTURATION OF THE ALVEOLAR BONE IN THE FURCATION REGION | ||||||
| (1) Bone trabeculae with regular contours coated with active osteoblasts, including areas of new bone formation | - | 1 | 8 | - | - | - |
| (2) Bone trabeculae with predominantly vital bone tissue, with few areas comprising non-vital bone tissue | 8 | 7 | - | - | - | 8 |
| (3) Bone trabeculae composed of equivalent amounts of vital bone tissue and non-vital bone tissue | - | - | - | 3 | 1 | - |
| (4) Bone trabeculae composed predominantly of non-vital bone tissue, with few areas consisting of vital bone tissue | - | - | - | 5 | 7 | - |
| MEDIAN | 2 | 2 | 1 †‡ | 4 †‡¶ | 4 †‡¶ | 2 ¶§| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, G.R.C.; Ganzaroli, V.F.; Toro, L.F.; Lopes-Pereira, E.; Costa, L.L.d.; Mello-Neto, J.M.d.; Buchaim, R.L.; Garcia, V.G.; Theodoro, L.H.; Sforcin, J.M.; et al. Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate. Int. J. Mol. Sci. 2024, 25, 12443. https://doi.org/10.3390/ijms252212443
Silveira GRC, Ganzaroli VF, Toro LF, Lopes-Pereira E, Costa LLd, Mello-Neto JMd, Buchaim RL, Garcia VG, Theodoro LH, Sforcin JM, et al. Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate. International Journal of Molecular Sciences. 2024; 25(22):12443. https://doi.org/10.3390/ijms252212443
Chicago/Turabian StyleSilveira, Glauco Rodrigues Carmo, Vinícius Franzão Ganzaroli, Luan Felipe Toro, Estevão Lopes-Pereira, Leandro Lemes da Costa, João Martins de Mello-Neto, Rogério Leone Buchaim, Valdir Gouveia Garcia, Leticia Helena Theodoro, José Maurício Sforcin, and et al. 2024. "Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate" International Journal of Molecular Sciences 25, no. 22: 12443. https://doi.org/10.3390/ijms252212443
APA StyleSilveira, G. R. C., Ganzaroli, V. F., Toro, L. F., Lopes-Pereira, E., Costa, L. L. d., Mello-Neto, J. M. d., Buchaim, R. L., Garcia, V. G., Theodoro, L. H., Sforcin, J. M., Marcato, P. D., & Ervolino, E. (2024). Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate. International Journal of Molecular Sciences, 25(22), 12443. https://doi.org/10.3390/ijms252212443










