NOS3 Gene Polymorphisms (rs2070744 and rs1799983) and Differentiated Thyroid Cancer: Investigating Associations with Clinical Outcomes
Abstract
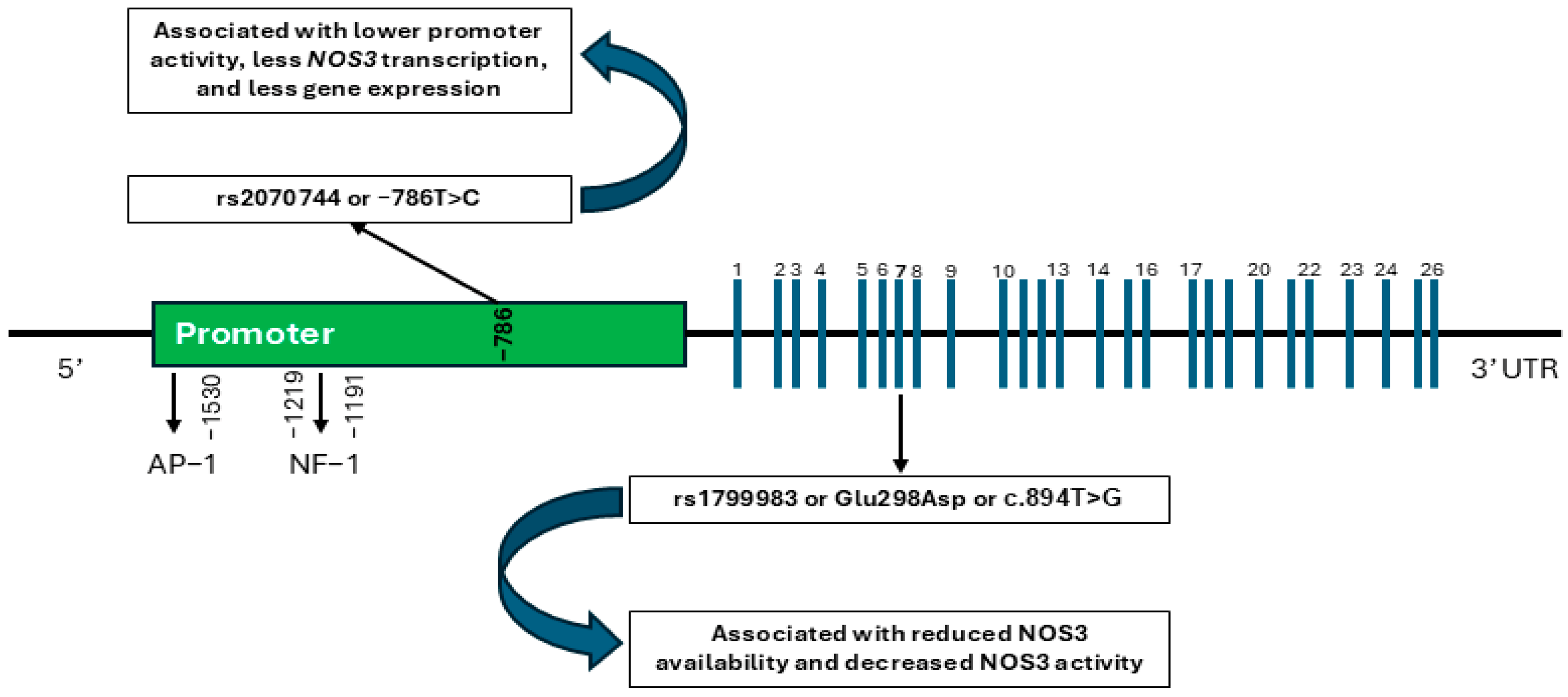
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
- (a)
- Case group with documented DTC diagnoses ranging from 1978 to 2022.
- I.
- Inclusion criteria: patients over 18 years old with a confirmed pathological diagnosis of DTC.
- II.
- Exclusion criteria: incomplete information on the pathological examination, as well as missing information about the biochemical and structural responses following therapy.
- (b)
- Control group
- I.
- Inclusion criteria: subjects over 18 years old.
- II.
- Exclusion criteria: personal history of any thyroid disease, personal history of any malignant disease.
4.2. Data Collection
- (a)
- Indolent histological types: classic papillary carcinoma, papillary microcarcinoma, follicular variant of papillary microcarcinoma, follicular carcinoma, and Warthin-like variant.
- (b)
- Aggressive histological types: tall-cell variant, poorly differentiated component of follicular carcinoma, follicular carcinoma with insular carcinoma component, oncocytic carcinoma, and Hobnail variant.
4.3. Response to Therapy
4.4. Laboratory Methods
- rs1799983: T = wild-type allele; G = variant allele; TT = homozygous wild-type; GG = homozygous variant; GT = heterozygous variant.
- rs2070744: C = wild-type allele; T = variant allele; CC = homozygous wild-type; TT = homozygous variant; CT = heterozygous variant.
4.5. Statistical Analysis
4.6. Ethical Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The Epidemiological Landscape of Thyroid Cancer Worldwide: GLOBOCAN Estimates for Incidence and Mortality Rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Christofer Juhlin, C.; Mete, O.; Baloch, Z.W. The 2022 WHO Classification of Thyroid Tumors: Novel Concepts in Nomenclature and Grading. Endocr. Relat. Cancer 2022, 30, e220293. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid Cancer Incidence Trends by Histology in 25 Countries: A Population-Based Study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef]
- Lam, A.K. Papillary Thyroid Carcinoma: Current Position in Epidemiology, Genomics, and Classification. In Papillary Thyroid Carcinoma: Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2534, pp. 1–15. [Google Scholar]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared With a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Davies, L.; Sawka, A.M. Women and Thyroid Cancer Incidence: Overdiagnosis Versus Biological Risk. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 492–496. [Google Scholar] [CrossRef]
- Wynford-Thomas, D. Molecular Genetics of Thyroid Cancer. Trends Endocrinol. Metab. 1993, 4, 224–232. [Google Scholar] [CrossRef]
- DeLellis, R.A. Pathology and Genetics of Thyroid Carcinoma. J. Surg. Oncol. 2006, 94, 662–669. [Google Scholar] [CrossRef]
- Kalarani, I.B.; Sivamani, G.; Veerabathiran, R. Identification of Crucial Genes Involved in Thyroid Cancer Development. J. Egypt. Natl. Cancer Inst. 2023, 35, 15. [Google Scholar] [CrossRef]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int. J. Mol. Sci. 2021, 22, 1726. [Google Scholar] [CrossRef]
- Hlozek, J.; Pekova, B.; Rotnágl, J.; Holý, R.; Astl, J. Genetic Changes in Thyroid Cancers and the Importance of Their Preoperative Detection in Relation to the General Treatment and Determination of the Extent of Surgical Intervention—A Review. Biomedicines 2022, 10, 1515. [Google Scholar] [CrossRef]
- Sabi, E.M. The Role of Genetic and Epigenetic Modifications as Potential Biomarkers in the Diagnosis and Prognosis of Thyroid Cancer. Front. Oncol. 2024, 14, 1474267. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Gimm, O.; Hoang-Vu, C.; Dralle, H.; Pfeifer, G.P.; Dammann, R. Frequent Epigenetic Silencing of the CpG Island Promoter of RASSF1A in Thyroid Carcinoma. Cancer Res. 2002, 62, 3698–3701. [Google Scholar] [PubMed]
- Yu, X.; Zhang, H.; Zhang, H.; Hou, C.; Wang, X.; Gu, P.; Han, Y.; Yang, Z.; Zou, W. The Role of Epigenetic Methylations in Thyroid Cancer. World J. Surg. Oncol. 2024, 22, 281. [Google Scholar] [CrossRef]
- Ortiz, I.M.D.P.; Barros-Filho, M.C.; dos Reis, M.B.; Beltrami, C.M.; Marchi, F.A.; Kuasne, H.; Canto, L.M.D.; de Mello, J.B.H.; Abildgaard, C.; Pinto, C.A.L.; et al. Loss of DNA Methylation Is Related to Increased Expression of miR-21 and miR-146b in Papillary Thyroid Carcinoma. Clin. Epigenet. 2018, 10, 144. [Google Scholar] [CrossRef]
- Wei, Z.L.; Bin, G.A.; Wang, Q.; XE, L.; Zhao, J.; Lu, Q.J. MicroRNA-221 Promotes Papillary Thyroid Carcinoma Cell Migration and Invasion via Targeting RECK and Regulating Epithelial–Mesenchymal Transition. Onco Targets Ther. 2019, 12, 2323–2333. [Google Scholar] [CrossRef]
- Smith, R.A.; Lam, A.K. Single Nucleotide Polymorphisms in Papillary Thyroid Carcinoma: Clinical Significance and Detection by High-Resolution Melting. Methods Mol. Biol. 2022, 2534, 149–159. [Google Scholar]
- Ran, R.; Tu, G.; Li, H.; Wang, H.; Mou, E.; Liu, C. Genetic Variants Associated with Thyroid Cancer Risk: Comprehensive Research Synopsis, Meta-Analysis, and Cumulative Epidemiological Evidence. J. Oncol. 2021, 2021, 9967599. [Google Scholar] [CrossRef]
- Kyrodimos, E.; Chrysovergis, A.; Mastronikolis, N.; Papanastasiou, G.; Tsiambas, E.; Spyropoulou, D.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Pantos, P.; et al. The Landscape of Single Nucleotide Polymorphisms in Papillary Thyroid Carcinoma. Cancer Diagn. Progn. 2023, 3, 26–30. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Endothelial Nitric Oxide Synthase: From Biochemistry and Gene Structure to Clinical Implications of NOS3 Polymorphisms. Gene 2016, 575, 584–599. [Google Scholar] [CrossRef]
- Li, R.; Zhao, A.; Diao, X.; Song, J.; Wang, C.; Li, Y.; Qi, X.; Guan, Z.; Zhang, T.; He, Y. Polymorphism of NOS3 Gene and Its Association with Essential Hypertension in Guizhou Populations of China. PLoS ONE 2023, 18, e0278680. [Google Scholar] [CrossRef]
- García-Martín, E.; Navarro-Muñoz, S.; Rodriguez, C.; Serrador, M.; Alonso-Navarro, H.; Calleja, M.; Turpín-Fenoll, L.; Recio-Bermejo, M.; García-Ruiz, R.; Millán-Pascual, J.; et al. Association Between Endothelial Nitric Oxide Synthase (NOS3) rs2070744 and the Risk for Migraine. Pharmacogenom. J. 2020, 20, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Arda, E.; Ay, A.; Akdere, H.; Akdeniz, E. The Association of Intron 4 VNTR and Glu298Asp Polymorphisms of the Nitric Oxide Synthetase 3 Gene and Vasculogenic Erectile Dysfunction in Turkish Men. Syst. Biol. Reprod. Med. 2019, 65, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, T.S.; Kontic-Vucinic, O.; Nikolic, N.; Carkic, J.; Stamenkovic, J.; Soldatovic, I.; Milasin, J. Association Between Endothelial Nitric Oxide Synthase (eNOS) -786 T/C and 27-bp VNTR 4b/a Polymorphisms and Preeclampsia Development. Reprod. Sci. 2021, 28, 3529–3539. [Google Scholar] [CrossRef]
- Dobrijević, Z.; Stevanović, J.; Robajac, D.; Penezić, A.; Četić, D.; Baralić, M.; Nedić, O. Association Between Nitric Oxide Synthase (NOS3) Gene Polymorphisms and Diabetic Nephropathy: An Updated Meta-Analysis. Mol. Cell Endocrinol. 2024, 586, 112197. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.F.; Xu, Y.; Ren, R.; Heng, B.L.; Su, Z.X. Association Between Three eNOS Polymorphisms and Cancer Risk: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2014, 15, 5317–5324. [Google Scholar] [CrossRef]
- McDonald, D.M.; Alp, N.J.; Channon, K.M. Functional Comparison of the Endothelial Nitric Oxide Synthase Glu298Asp Polymorphic Variants in Human Endothelial Cells. Pharmacogenetics 2004, 14, 831–839. [Google Scholar] [CrossRef]
- Tesauro, M.; Thompson, W.C.; Rogliani, P.; Qi, L.; Chaudhary, P.P.; Moss, J. Intracellular Processing of Endothelial Nitric Oxide Synthase Isoforms Associated with Differences in Severity of Cardiopulmonary Diseases: Cleavage of Proteins with Aspartate vs. Glutamate at Position 298. Proc. Natl. Acad. Sci. USA 2000, 97, 2832–2835. [Google Scholar] [CrossRef]
- Fairchild, T.A.; Fulton, D.; Fontana, J.T.; Gratton, J.P.; McCabe, T.J.; Sessa, W.C. Acidic Hydrolysis as a Mechanism for the Cleavage of the Glu(298)-->Asp Variant of Human Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 2001, 276, 26674–26679. [Google Scholar] [CrossRef]
- Joshi, M.S.; Mineo, C.; Shaul, P.W.; Bauer, J.A. Biochemical Consequences of the NOS3 Glu298Asp Variation in Human Endothelium: Altered Caveolar Localization and Impaired Response to Shear. FASEB J. 2007, 21, 2655–2663. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Saito, Y.; Nakayama, M.; Shimasaki, Y.; Yoshimura, T.; Yoshimura, M.; Harada, M.; Kajiyama, N.; Kishimoto, I.; Kuwahara, K.; et al. Replication Protein A1 Reduces Transcription of the Endothelial Nitric Oxide Synthase Gene Containing a -786T-->C Mutation Associated with Coronary Spastic Angina. Hum. Mol. Genet. 2000, 9, 2629–2637. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; Xue, P.; Liu, Y.; Xiong, T.; Yang, J.; Song, C.; He, Q.; Du, L. The -786T > C Polymorphism in the NOS3 Gene Is Associated with Increased Cancer Risk. Tumour Biol. 2014, 35, 3535–3540. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Guo, Y.; Guo, X.Y. Nitric Oxide Synthase 3 Gene Variants and Colorectal Cancer: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2014, 15, 3811–3815. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Choi, J.-Y.; Lee, J.E.; Noh, D.-Y.; Ahn, S.-H.; Han, W.; Yoo, K.-Y.; Hayes, R.B.; Kang, D. Genetic Polymorphisms of NOS3 Are Associated with the Risk of Invasive Breast Cancer with Lymph Node Involvement. Breast Cancer Res. Treat. 2007, 106, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Ramadhan, I.; Rahman Sulaiman, L.; Salihi, A. NOS3 and CTH Gene Mutations as New Molecular Markers for Detection of Lung Adenocarcinoma. PeerJ 2023, 11, e16209. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.R.D.; Fratelli, C.F.; Duarte, L.C.d.A.C.; Pereira, A.S.R.; de Morais, R.M.; Sobrinho, A.B.; Silva, C.M.d.S.; da Silva, I.C.R.; de Oliveira, J.R. The Impact of NOS3 Gene Polymorphism on Papillary Thyroid Cancer Susceptibility in Patients Undergoing Radioiodine Therapy. Int. J. Biol. Markers 2020, 35, 87–91. [Google Scholar] [CrossRef]
- Huang, Y.; Suguro, R.; Hu, W.; Zheng, J.; Liu, Y.; Guan, M.; Zhou, N.; Zhang, X. Nitric Oxide and Thyroid Carcinoma: A Review. Front. Endocrinol. 2023, 13, 1050656. [Google Scholar] [CrossRef]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative Stress in Thyroid Carcinomas: Biological and Clinical Significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Kościuszko, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer? Cancers 2023, 15, 3182. [Google Scholar] [CrossRef]
- Rajabi, S.; Dehghan, M.H.; Dastmalchi, R.; Jalali Mashayekhi, F.; Salami, S.; Hedayati, M. The Roles and Role-Players in Thyroid Cancer Angiogenesis. Endocr. J. 2019, 66, 277–293. [Google Scholar] [CrossRef]
- Melaccio, A.; Sgaramella, L.I.; Pasculli, A.; Di Meo, G.; Gurrado, A.; Prete, F.P.; Vacca, A.; Ria, R.; Testini, M. Prognostic and Therapeutic Role of Angiogenic Microenvironment in Thyroid Cancer. Cancers 2021, 13, 2775. [Google Scholar] [CrossRef]
- Nan, J.; Liu, Y.; Xu, C.; Ge, D. Effects of eNOS Gene Polymorphisms on Individual Susceptibility to Cancer: A Meta-Analysis. Nitric Oxide 2019, 84, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gajowiec, A.; Chromik, A.; Furga, K.; Skuza, A.; Gąsior-Perczak, D.; Walczyk, A.; Pałyga, I.; Trybek, T.; Mikina, E.; Szymonek, M.; et al. Is Male Sex a Prognostic Factor in Papillary Thyroid Cancer? J. Clin. Med. 2021, 10, 2438. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, S.K.; Siraj, A.K.; Ahmed, S.O.; Annaiyappanaidu, P.; Al-Rasheed, M.; Al-Haqawi, W.; Qadri, Z.; Al-Sobhi, S.S.; Al-Dayel, F.; Al-Kuraya, K.S. Predicting Factors and Clinical Outcome of Biochemical Incomplete Response in Middle Eastern Differentiated Thyroid Carcinoma. Endocrine 2024, 86, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ryk, C.; Wiklund, N.P.; Nyberg, T.; de Verdier, P.J. Polymorphisms in Nitric-Oxide Synthase 3 May Influence the Risk of Urinary-Bladder Cancer. Nitric Oxide 2011, 25, 338–343. [Google Scholar] [CrossRef]
- Gao, X.; Wang, J.; Wang, W.; Wang, M.; Zhang, J. eNOS Genetic Polymorphisms and Cancer Risk: A Meta-Analysis and a Case-Control Study of Breast Cancer. Medicine 2015, 94, e972. [Google Scholar] [CrossRef]
- Branković, A.; Brajušković, G.; Nikolic, Z.; Vukotić, V.; Cerovic, S.; Savić-Pavićević, D.; Romac, S. Endothelial Nitric Oxide Synthase Gene Polymorphisms and Prostate Cancer Risk in Serbian Population. Int. J. Exp. Pathol. 2013, 94, 355–361. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, H.; Chen, Z.; Lu, B.; Li, J.; Peng, Y.; Shen, X. The Genetic Association Between iNOS and eNOS Polymorphisms and Gastric Cancer Risk: A Meta-Analysis. Onco Targets Ther. 2018, 11, 2497–2507. [Google Scholar] [CrossRef]
- Haque, S.; Mandal, R.K.; Akhter, N.; Panda, A.K.; Hussain, A.; Khan, S.; Lohani, M. G894T and 4a/b Polymorphisms of NOS3 Gene Are Not Associated with Cancer Risk: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 2929–2937. [Google Scholar] [CrossRef]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425–435. [Google Scholar] [CrossRef]
- Huang, G.; Chen, J.; Zhou, J.; Xiao, S.; Zeng, W.; Xia, J.; Zeng, X. Epigenetic Modification and BRAF Gene Mutation in Thyroid Carcinoma. Cancer Cell Int. 2021, 21, 687. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Tuttle, R.M. Differentiated Thyroid Cancer: Overview of Management. UpToDate 2022. Available online: https://www.uptodate.com/contents/differentiated-thyroid-cancer-overview-of-management (accessed on 18 August 2024).
- rs1799983 (SNP)—Population Genetics—Homo_Sapiens—Ensembl Genome Browser 113. Available online: https://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=7:150998523-150999523;v=rs1799983;vdb=variation;vf=480750083 (accessed on 18 November 2024).
- rs2070744 (SNP)—Population Genetics—Homo_Sapiens—Ensembl Genome Browser 113. Available online: https://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=7:150992491-150993491;v=rs2070744;vdb=variation;vf=480762918 (accessed on 18 November 2024).
| CASE GROUP | |
| Number of subjects | 88 |
| Sex distribution | F: 75 (85.2%) M: 13 (14.8%) |
| Mean age at the last evaluation (years) | 55.46 ± 13.65 |
| Median follow-up time (years) | 5 (4.0–7.6) |
| Smoking status | NS: 63 (71.6%) S: 25 (28.4%) |
| Type of surgical intervention | TT: 87 (98.9%) IL: 1 (1.1%) |
| Histological type of DTC | Indolent: 76 (86.4%) Aggressive: 12 (13.6%) |
| History of RAI administration | Yes: 68 (77.3%) No: 20 (22.7%) |
| Loco-regional or distant metastases at diagnosis | Yes: 26 (29.5%) No: 62 (70.5%) |
| Good biochemical control at last evaluation | Yes: 62 (70.5%) No: 26 (29.5%) |
| Good structural control at last evaluation | Yes: 65 (73.9%) No: 23 (26.1%) |
| Loco-regional or distant metastases after therapy | Yes: 6 (6.8%) No: 82 (93.2%) |
| CONTROL GROUP | |
| Number of subjects | 84 |
| Sex distribution | F: 68 (81%) M: 16 (19%) |
| Mean age | 54.41 ± 13.59 |
| Polymorphism | Genotype | DTC Cases n (%) | Population Controls n (%) | p Value |
|---|---|---|---|---|
| rs2070744 | CC (homozygous wild type) | 16 (18.2%) | 13 (15.5%) | 0.387 |
| CT (heterozygous variant) | 39 (44.3%) | 46 (54.7%) | ||
| TT (homozygous variant) | 33 (37.6%) | 25 (29.8%) | ||
| C (allele) | 71 (40.3%) | 72 (42.9%) | 0.716 | |
| T (allele) | 105 (59.7%) | 96 (57.1%) | ||
| rs1799983 | TT (homozygous wild type) | 8 (9.0%) | 8 (9.5%) | 0.329 |
| GT (heterozygous variant) | 40 (45.5%) | 29 (34.5%) | ||
| GG (homozygous variant) | 40 (45.5%) | 47 (56.0%) | ||
| G (allele) | 120 (68.2%) | 123 (62.4%) | 0.292 | |
| T (allele) | 56 (31.8%) | 74 (37.6%) |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| rs1799983 wild-type TT genotype | 0.191 | ||
| rs1799983 heterozygous variant GT genotype | 1.870 | 0.953–3.668 | 0.069 |
| rs1799983 homozygous variant GG genotype | 1.377 | 0.461–4.113 | 0.567 |
| rs2070744 wild-type CC genotype | 0.224 | ||
| rs2070744 heterozygous variant CT genotype | 0.701 | 0.298–1.650 | 0.416 |
| rs2070744 homozygous variant TT genotype | 1.299 | 0.511–3.301 | 0.582 |
| Polymorphism | Genotype | DTC Cases n (%) | p Value | Population Controls n (%) | p Value |
|---|---|---|---|---|---|
| rs2070744 | CC (homozygous wild type) | F: 13 (17.3%) M: 3 (23.1%) | 0.566 | F: 11 (16.2%) M: 2 (12.5%) | 0.787 |
| CT (heterozygous variant) | F: 35 (46.7%) M: 4 (30.8%) | F: 36 (52.9%) M: 10 (62.5%) | |||
| TT (homozygous variant) | F: 27 (36.0%) M: 6 (46.1%) | F: 21 (30.9%) M: 4 (25.0%) | |||
| rs1799983 | TT (homozygous wild type) | F: 7 (9.3%) M: 1 (7.7%) | 0.982 | F: 6 (8.8%) M: 2 (12.5%) | 0.080 |
| GT (heterozygous variant) | F: 34 (45.3%) M: 6 (46.1%) | F: 20 (29.4%) M: 9 (56.2%) | |||
| GG (homozygous variant) | F: 34 (45.3%) M: 6 (46.1%) | F: 42 (61.8%) M: 5 (31.3%) |
| NOS3 Gene Polymorphism | Clinicopathological Characteristic | p Value |
|---|---|---|
| rs1799983 | Male sex | 0.982 |
| Smoking | 0.354 | |
| Aggressive histological type | 0.944 | |
| Incomplete biochemical control | 0.592 | |
| Incomplete structural control | 0.204 | |
| History of RAI therapy | 0.856 | |
| Loco-regional/distance metastases | 0.725 | |
| rs2070744 | Male sex | 0.566 |
| Smoking | 0.958 | |
| Aggressive histological type | 0.349 | |
| Incomplete biochemical control | 0.907 | |
| Incomplete structural control | 0.382 | |
| History of RAI therapy | 0.436 | |
| Loco-regional/distance metastases | 0.246 |
| Genotype | Clinicopathological Characteristic | p Value |
|---|---|---|
| rs1799983 TT versus non-TT | Male sex | 0.849 |
| Smoking | 0.295 | |
| Aggressive histological type | 0.922 | |
| History of RAI | 0.872 | |
| Incomplete biochemical control | 0.768 | |
| Incomplete structural control | 0.939 | |
| Loco-regional/distant metastases | 0.422 | |
| rs1799983 GG versus non-GG | Male sex | 0.956 |
| Smoking | 0.517 | |
| Aggressive histological type | 0.734 | |
| History of RAI | 0.642 | |
| Incomplete biochemical control | 0.394 | |
| Incomplete structural control | 0.092 | |
| Loco-regional/distant metastases | 0.817 | |
| rs2070744 CC versus non-CC | Male sex | 0.620 |
| Smoking | 0.781 | |
| Aggressive histological type | 0.510 | |
| History of RAI | 0.280 | |
| Incomplete biochemical control | 0.660 | |
| Incomplete structural control | 0.170 | |
| Loco-regional/distant metastases | 0.232 | |
| rs2070744 TT versus non-TT | Male sex | 0.485 |
| Smoking | 0.855 | |
| Aggressive histological type | 0.336 | |
| History of RAI | 0.793 | |
| Incomplete biochemical control | 0.904 | |
| Incomplete structural control | 0.491 | |
| Loco-regional/distant metastases | 0.126 |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| rs1799983 wild-type TT genotype | 0.907 | ||
| rs1799983 heterozygous variant GT genotype | 1.342 | 0.359–5.018 | 0.662 |
| rs1799983 homozygous variant GG genotype | 1.237 | 0.172–8.883 | 0.832 |
| rs2070744 wild-type CC genotype | 0.989 | ||
| rs2070744 heterozygous variant CT genotype | 1.050 | 0.213–5.182 | 0.952 |
| rs2070744 homozygous variant TT genotype | 0.946 | 0.171–5.236 | 0.949 |
| Incomplete structural control | 8.707 | 2.154–35.193 | 0.002 |
| Male sex | 10.240 | 1.908–54.959 | 0.007 |
| Smoking | 3.151 | 0.899–11.049 | 0.073 |
| Aggressive histological type | 3.487 | 0.634–19.178 | 0.151 |
| History of RAI | 0.130 | 0.028–0.595 | 0.009 |
| Biological Data | Post-Therapeutic Evolution |
|---|---|
| Sex | Tumoral persistence |
| Age | Tumoral recurrence |
| Smoking status | Loco-regional or distant metastases |
| Histological type of the tumor | |
| Type of underwent treatment | |
| Presence of loco-regional or distant metastases at diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiucă, R.A.; Pop, R.M.; Tiucă, O.M.; Bănescu, C.; Cârstea, A.C.; Preda, C.; Pașcanu, I.M. NOS3 Gene Polymorphisms (rs2070744 and rs1799983) and Differentiated Thyroid Cancer: Investigating Associations with Clinical Outcomes. Int. J. Mol. Sci. 2025, 26, 759. https://doi.org/10.3390/ijms26020759
Tiucă RA, Pop RM, Tiucă OM, Bănescu C, Cârstea AC, Preda C, Pașcanu IM. NOS3 Gene Polymorphisms (rs2070744 and rs1799983) and Differentiated Thyroid Cancer: Investigating Associations with Clinical Outcomes. International Journal of Molecular Sciences. 2025; 26(2):759. https://doi.org/10.3390/ijms26020759
Chicago/Turabian StyleTiucă, Robert Aurelian, Raluca Monica Pop, Oana Mirela Tiucă, Claudia Bănescu, Ana Claudia Cârstea, Cristina Preda, and Ionela Maria Pașcanu. 2025. "NOS3 Gene Polymorphisms (rs2070744 and rs1799983) and Differentiated Thyroid Cancer: Investigating Associations with Clinical Outcomes" International Journal of Molecular Sciences 26, no. 2: 759. https://doi.org/10.3390/ijms26020759
APA StyleTiucă, R. A., Pop, R. M., Tiucă, O. M., Bănescu, C., Cârstea, A. C., Preda, C., & Pașcanu, I. M. (2025). NOS3 Gene Polymorphisms (rs2070744 and rs1799983) and Differentiated Thyroid Cancer: Investigating Associations with Clinical Outcomes. International Journal of Molecular Sciences, 26(2), 759. https://doi.org/10.3390/ijms26020759







