Long Non-Coding RNA LOC113219358 Regulates Immune Responses in Apis mellifera Through Protein Interactions
Abstract
1. Introduction
2. Results
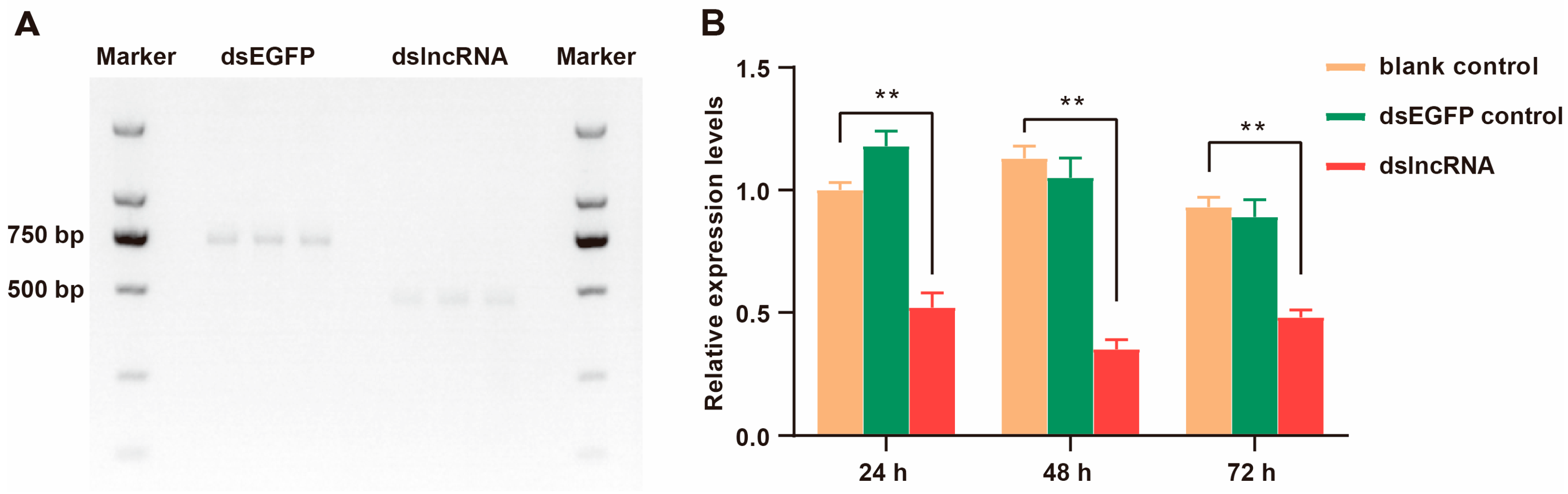
2.1. Results of dsRNA Synthesis and Interference Efficiency
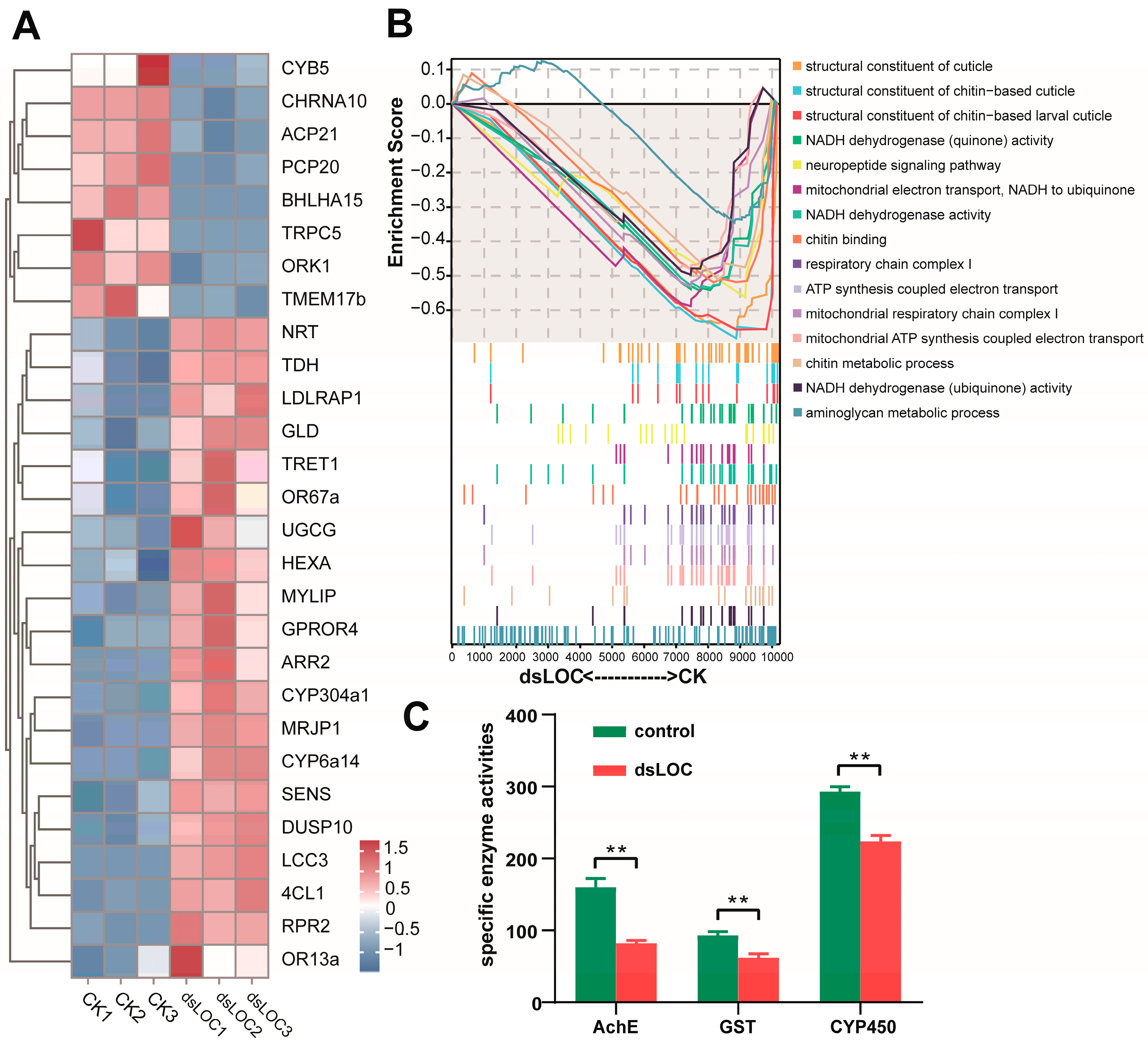
2.2. Effects of Silencing lncLOC113219358 on Gene Expression and Enzymatic Activities in the Honeybee
2.3. Analysis of lncLOC113219358 Coding Potential
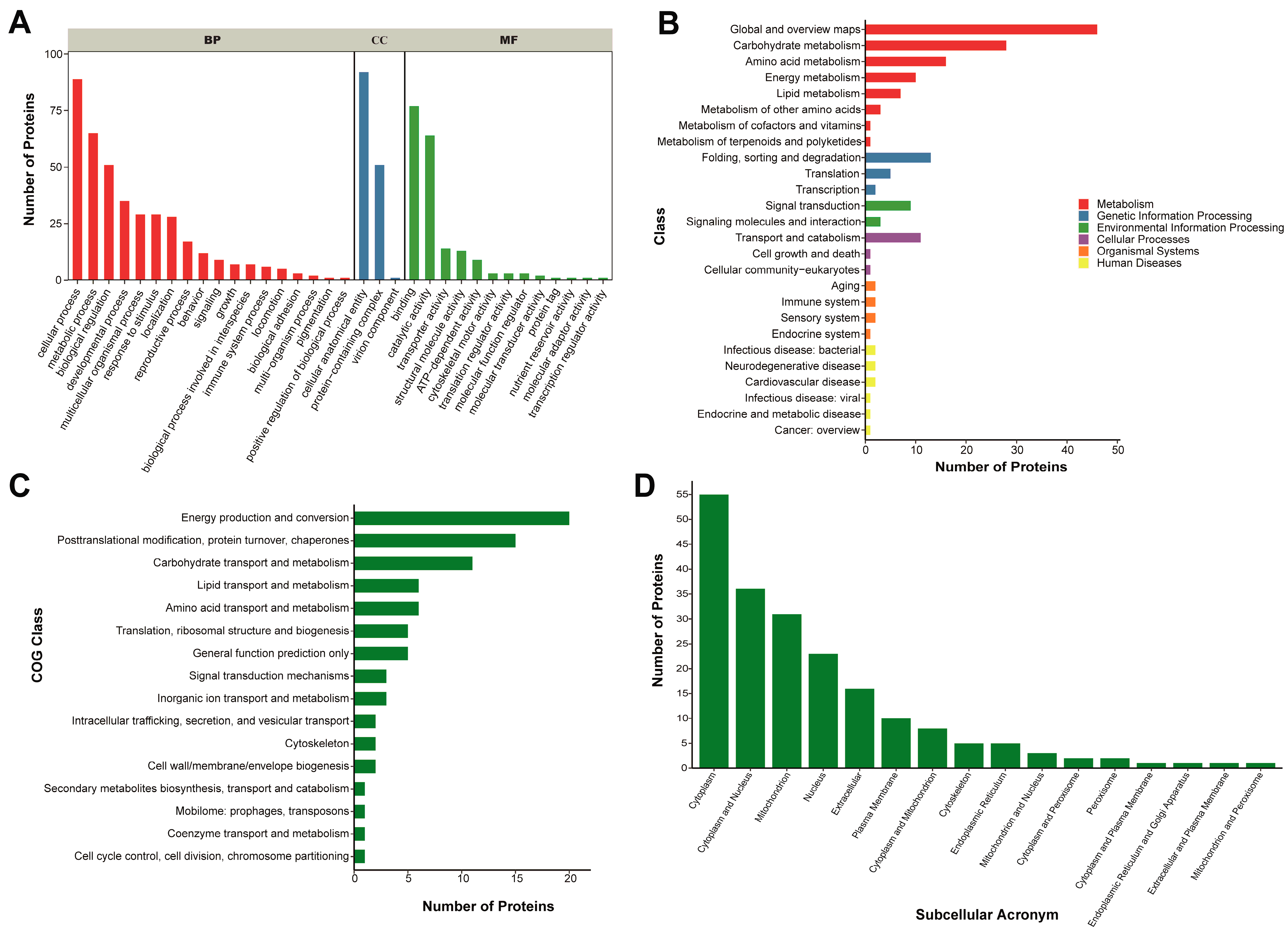
2.4. Proteomics Analysis Reveals Binding Proteins and Potential Roles
3. Discussion
4. Materials and Methods
4.1. Honeybee Rearing
4.2. dsRNA Synthesis, and RNAi Treatments
4.3. Sample Collection, RNA Extraction, Sequencing, and Enzyme-Linked Immunosorbent Assay
4.4. Potential Coding Analysis and RNA Pull-Down
4.5. qPCR and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Schmuck, R.; Lewis, G. Review of field and monitoring studies investigating the role of nitro-substituted neonicotinoid insecticides in the reported losses of honey bee colonies (Apis mellifera). Ecotoxicology 2016, 25, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Manea-Karga, E.; Machera, K. Occurrence and human health risk assessment of mineral elements and pesticides residues in bee pollen. Food Chem. Toxicol. 2022, 161, 112826. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.A.; Daisley, B.A.; Pitek, A.P.; Thompson, G.J.; Reid, G. Understanding the Effects of Sublethal Pesticide Exposure on Honey Bees: A Role for Probiotics as Mediators of Environmental Stress. Front. Ecol. Evol. 2020, 8, 22. [Google Scholar] [CrossRef]
- Koch, L. Screening for lncRNA function. Nat. Rev. Genet. 2017, 18, 70. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, W. Genome-wide characterization of coding and non-coding RNAs in the ovary of honeybee workers and queens. Apidologie 2020, 51, 777–792. [Google Scholar] [CrossRef]
- Fan, X.; Gao, X.; Zang, H.; Guo, S.; Jing, X.; Zhang, Y.; Liu, X.; Zou, P.; Chen, M.; Huang, Z.; et al. Diverse Regulatory Manners and Potential Roles of lncRNAs in the Developmental Process of Asian Honey Bee (Apis cerana) Larval Guts. Int. J. Mol. Sci. 2023, 24, 15399. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, T.; Qi, L.; Su, X.; Wang, D.; Dong, J.; Huang, Z.Y. lncRNA profile of Apis mellifera and its possible role in behavioural transition from nurses to foragers. BMC Genom. 2019, 20, 393. [Google Scholar] [CrossRef] [PubMed]
- Humann, F.C.; Tiberio, G.J.; Hartfelder, K. Sequence and Expression Characteristics of Long Noncoding RNAs in Honey Bee Caste Development—Potential Novel Regulators for Transgressive Ovary Size. PLoS ONE 2013, 8, e78915. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, D.; Xiong, C.; Hou, C.; Zheng, Y.; Fu, Z.; Diao, Q.; Zhang, L.; Wang, H.; Hou, Z.; et al. Identification of long non-coding RNAs in the chalkbrood disease pathogen Ascospheara apis. J. Invertebr. Pathol. 2018, 156, 1–5. [Google Scholar] [CrossRef]
- Guo, R.; Chen, H.; Du, Y.; Zhou, D.; Geng, S.; Wang, H.; Zhu, Z.; Shi, C.; Wan, J.; Xiong, C.; et al. Genome-wide identification of long non-coding RNAs and their regulatory networks involved in Apis mellifera ligustica response to Nosema ceranae infection. Insects 2019, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Tadano, H.; Kohno, H.; Takeuchi, H.; Kubo, T. Unique spatially and temporary-regulated/sex-specific expression of a long ncRNA, Nb-1, suggesting its pleiotropic functions associated with honey bee lifecycle. Sci. Rep. 2024, 14, 8701. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Li, Y.; Wei, G.; Yuan, J.; Sun, Y.; Wang, H.; Qin, Q.; Zeng, Z.; Zhang, S.; et al. Lateralization of gene expression in the honeybee brain during olfactory learning. Sci. Rep. 2016, 6, 34727. [Google Scholar] [CrossRef]
- Feng, W.; Huang, J.; Zhang, Z.; Nie, H.; Lin, Y.; Li, Z.; Su, S. Understanding of Waggle Dance in the Honey Bee (Apis mellifera) from the Perspective of Long Non-Coding RNA. Insects 2022, 13, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Fan, X.; Zhang, K.; Zhang, J.; Zhao, H.; Gao, X.; Zhang, Y.; Guo, S.; Zhou, D.; et al. Systematic Characterization and Regulatory Role of lncRNAs in Asian Honey Bees Responding to Microsporidian Infestation. Int. J. Mol. Sci. 2023, 24, 5886. [Google Scholar] [CrossRef]
- Huang, M.; Dong, J.; Guo, H.; Xiao, M.; Wang, D. Identification of long noncoding RNAs reveals the effects of dinotefuran on the brain in Apis mellifera (Hymenopptera: Apidae). BMC Genom. 2021, 22, 502. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. WIREs RNA 2018, 9, e1471. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Tao, M.; Shen, X.; Ju, S. Translatable circRNAs and lncRNAs: Driving mechanisms and functions of their translation products. Cancer Lett. 2020, 483, 59–65. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef]
- Tellis, M.B.; Kotkar, H.M.; Joshi, R.S. Regulation of trehalose metabolism in insects: From genes to the metabolite window. Glycobiology 2023, 33, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2014, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Cavener, D.R. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 2004, 207, 675–681. [Google Scholar] [CrossRef]
- Scholefield, J.A.; Shikano, I.; Lowenberger, C.A.; Cory, J.S. The impact of baculovirus challenge on immunity: The effect of dose and time after infection. J. Invertebr. Pathol. 2019, 167, 107232. [Google Scholar] [CrossRef]
- Paula, G.M.; da Silva Menegasso, A.R.; dos-Santos-Pinto, J.R.A.; Malaspina, O.; Palma, M.S. Profiling the neuroproteomics of honeybee brain: A clue for understanding the role of neuropeptides in the modulation of aggressivity. J. Proteom. 2024, 295, 105089. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.M.; Wanner, K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006, 16, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Sinkins, W.; Keightley, A.; Kinter, M.; Schilling, W.P. Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal Na+/K+-ATPase. Pflug. Arch. Eur. J. Phy. 2005, 451, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.-P.; Edelstein, S.J. Allosteric Mechanisms of Signal Transduction. Science 2005, 308, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.R.; Cooper, R.L. The Effect of Calcium Ions on Resting Membrane Potential. Biology 2024, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Winther, Å.M.E. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 2010, 92, 42–104. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J. Mitochondrial Complex I. Annu. Rev. Biochem. 2013, 82, 551–575. [Google Scholar] [CrossRef]
- Hedges, C.P.; Wilkinson, R.T.; Devaux, J.B.L.; Hickey, A.J.R. Hymenoptera flight muscle mitochondrial function: Increasing metabolic power increases oxidative stress. Comp. Biochem. Phys. A 2019, 230, 115–121. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.; Lee, J.H.; Han, S.H.; Lee, S.H. Differential expression of acetylcholinesterase 1 in response to various stress factors in honey bee workers. Sci. Rep. 2019, 9, 10342. [Google Scholar] [CrossRef]
- Moural, T.W.; Koirala B K, S.; Bhattarai, G.; He, Z.; Guo, H.; Phan, N.T.; Rajotte, E.G.; Biddinger, D.J.; Hoover, K.; Zhu, F. Architecture and potential roles of a delta-class glutathione S-transferase in protecting honey bee from agrochemicals. Chemosphere 2024, 350, 141089. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seong, K.M.; Lee, S.H. Acetylcholine titre regulation by non-neuronal acetylcholinesterase 1 and its putative roles in honey bee physiology. Insect Mol. Biol. 2023, 32, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, M.; He, J.; Zhao, X.; Chaimanee, V.; Huang, W.-F.; Nie, H.; Zhao, Y.; Su, S. Differential physiological effects of neonicotinoid insecticides on honey bees: A comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Orera, J.; Messeguer, X.; Subirana, J.A.; Alba, M.M. Long non-coding RNAs as a source of new peptides. eLife 2014, 3, e03523. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Rigoulet, M.; Bouchez, C.L.; Paumard, P.; Ransac, S.; Cuvellier, S.; Duvezin-Caubet, S.; Mazat, J.P.; Devin, A. Cell energy metabolism: An update. BBA Bioenerg. 2020, 1861, 148276. [Google Scholar] [CrossRef] [PubMed]
- Darveau, C.-A. Insect Flight Energetics and the Evolution of Size, Form, and Function. Integr. Comp. Biol. 2024, 64, 586–597. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. The Role of Reactive Oxygen Species in the Life Cycle of the Mitochondrion. Int. J. Mol. Sci. 2020, 21, 2173. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martinez, M.-A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef]
- Fent, K.; Schmid, M.; Hettich, T.; Schmid, S. The neonicotinoid thiacloprid causes transcriptional alteration of genes associated with mitochondria at environmental concentrations in honey bees. Environ. Pollut. 2020, 266, 115297. [Google Scholar] [CrossRef]
- Sargent, C.; Ebanks, B.; Hardy, I.C.W.; Davies, T.G.E.; Chakrabarti, L.; Stöger, R. Acute Imidacloprid Exposure Alters Mitochondrial Function in Bumblebee Flight Muscle and Brain. Front. Insect Sci. 2021, 1, 765179. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [CrossRef]
- Maya-Aguirre, C.A.; Torres, A.; Gutiérrez-Castañeda, L.D.; Salazar, L.M.; Abreu-Villaça, Y.; Manhães, A.C.; Arenas, N.E. Changes in the proteome of Apis mellifera acutely exposed to sublethal dosage of glyphosate and imidacloprid. Environ. Sci. Pollut. Res. 2024, 31, 45954–45969. [Google Scholar] [CrossRef] [PubMed]
- McAfee, A.; Milone, J.; Chapman, A.; Foster, L.J.; Pettis, J.S.; Tarpy, D.R. Candidate stress biomarkers for queen failure diagnostics. BMC Genom. 2020, 21, 571. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.W.; Melathopoulos, A.P.; Pernal, S.F.; Foster, L.J. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genom. 2009, 10, 387. [Google Scholar] [CrossRef]
- Uversky, V.N.; Albar, A.H.; Khan, R.H.; Redwan, E.M. Multifunctionality and intrinsic disorder of royal jelly proteome. Proteomics 2021, 21, 2000237. [Google Scholar] [CrossRef]
- Ćmielová, J.; Řezáčová, M. Protein and its function based on a subcellular localization. J. Cell. Biochem. 2011, 112, 3502–3506. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Grünewald, B. Neurotransmitter Systems in the Honey Bee Brain: Functions in Learning and Memory. In Honeybee Neurobiology and Behavior: A Tribute to Randolf Menzel; Galizia, C.G., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–169. [Google Scholar] [CrossRef]
- Nunes, F.M.F.; Aleixo, A.C.; Barchuk, A.R.; Bomtorin, A.D.; Grozinger, C.M.; Simões, Z.L.P. Non-Target Effects of Green Fluorescent Protein (GFP)-Derived Double-Stranded RNA (dsRNA-GFP) Used in Honey Bee RNA Interference (RNAi) Assays. Insects 2013, 4, 90–103. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Tan, X.; Yang, S.; Zhou, Z.; Wang, D.; Dong, J. Long Non-Coding RNA LOC113219358 Regulates Immune Responses in Apis mellifera Through Protein Interactions. Int. J. Mol. Sci. 2025, 26, 676. https://doi.org/10.3390/ijms26020676
Huang M, Tan X, Yang S, Zhou Z, Wang D, Dong J. Long Non-Coding RNA LOC113219358 Regulates Immune Responses in Apis mellifera Through Protein Interactions. International Journal of Molecular Sciences. 2025; 26(2):676. https://doi.org/10.3390/ijms26020676
Chicago/Turabian StyleHuang, Minjie, Xiaodong Tan, Shuyuan Yang, Zhenzhen Zhou, Deqian Wang, and Jie Dong. 2025. "Long Non-Coding RNA LOC113219358 Regulates Immune Responses in Apis mellifera Through Protein Interactions" International Journal of Molecular Sciences 26, no. 2: 676. https://doi.org/10.3390/ijms26020676
APA StyleHuang, M., Tan, X., Yang, S., Zhou, Z., Wang, D., & Dong, J. (2025). Long Non-Coding RNA LOC113219358 Regulates Immune Responses in Apis mellifera Through Protein Interactions. International Journal of Molecular Sciences, 26(2), 676. https://doi.org/10.3390/ijms26020676




