Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Results
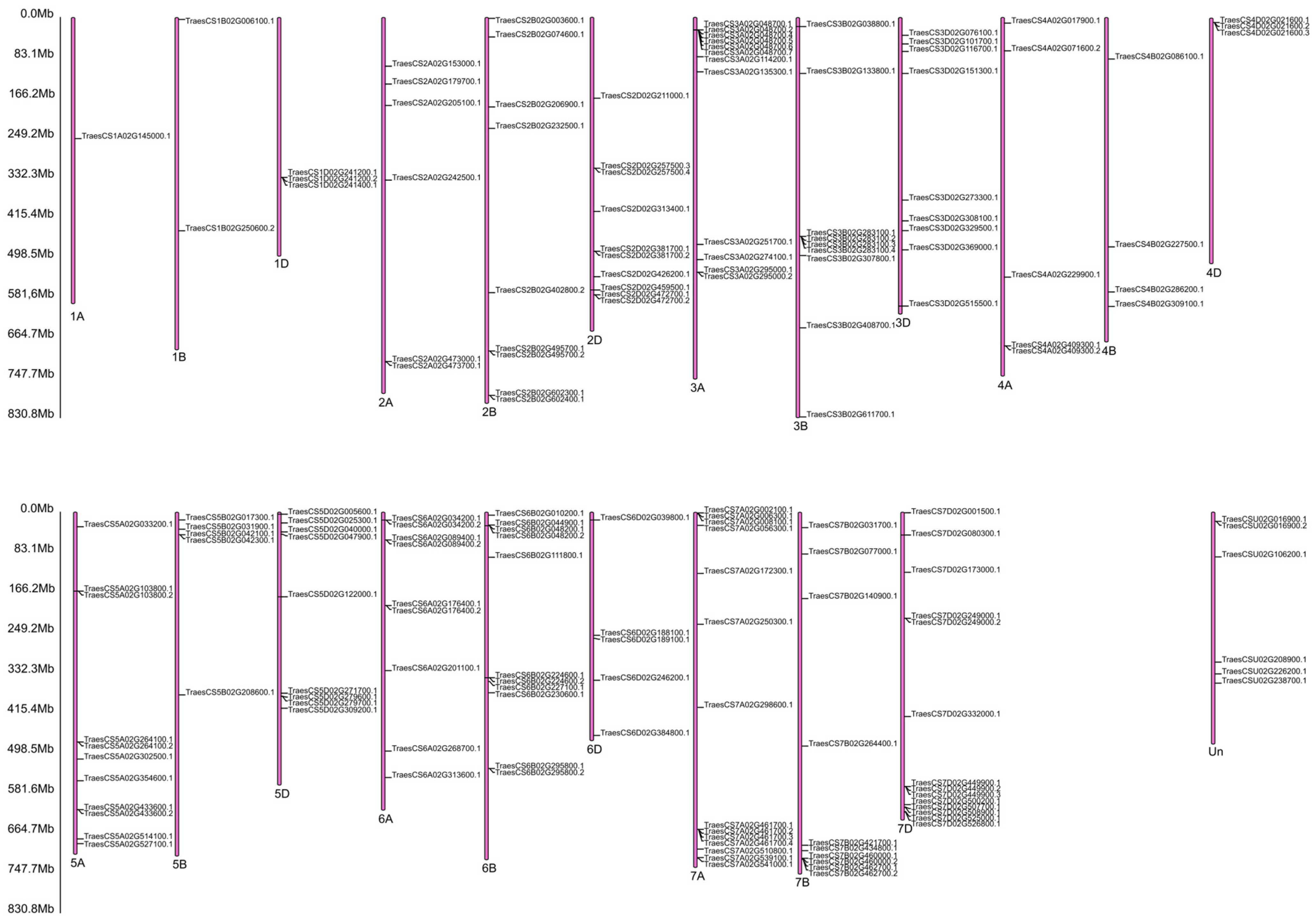
2.1. Identification of Molecular Markers Associated with APR Genes in Hybrid Forms
2.2. Observation and Evaluation of Infection Development by Pt
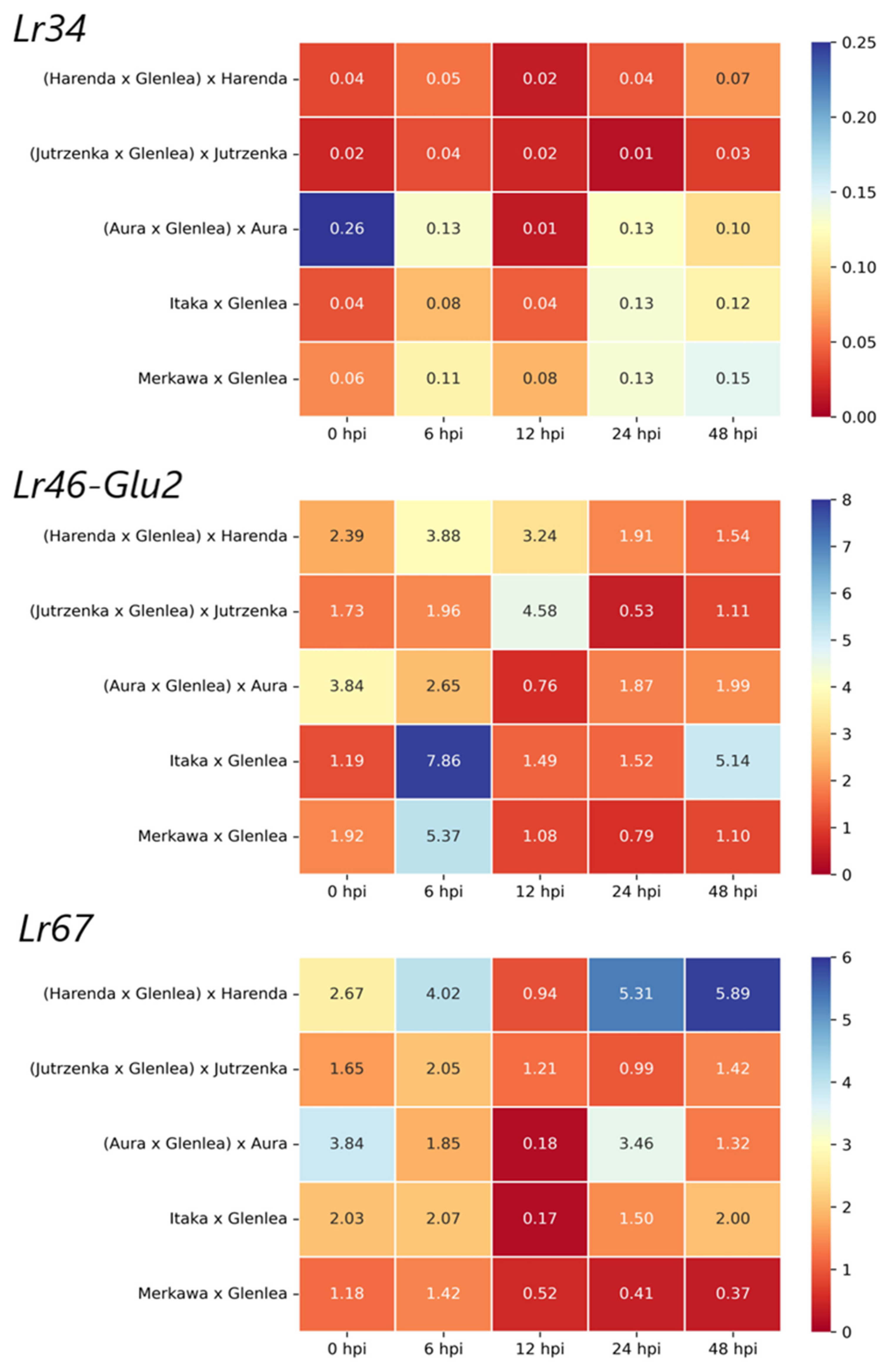
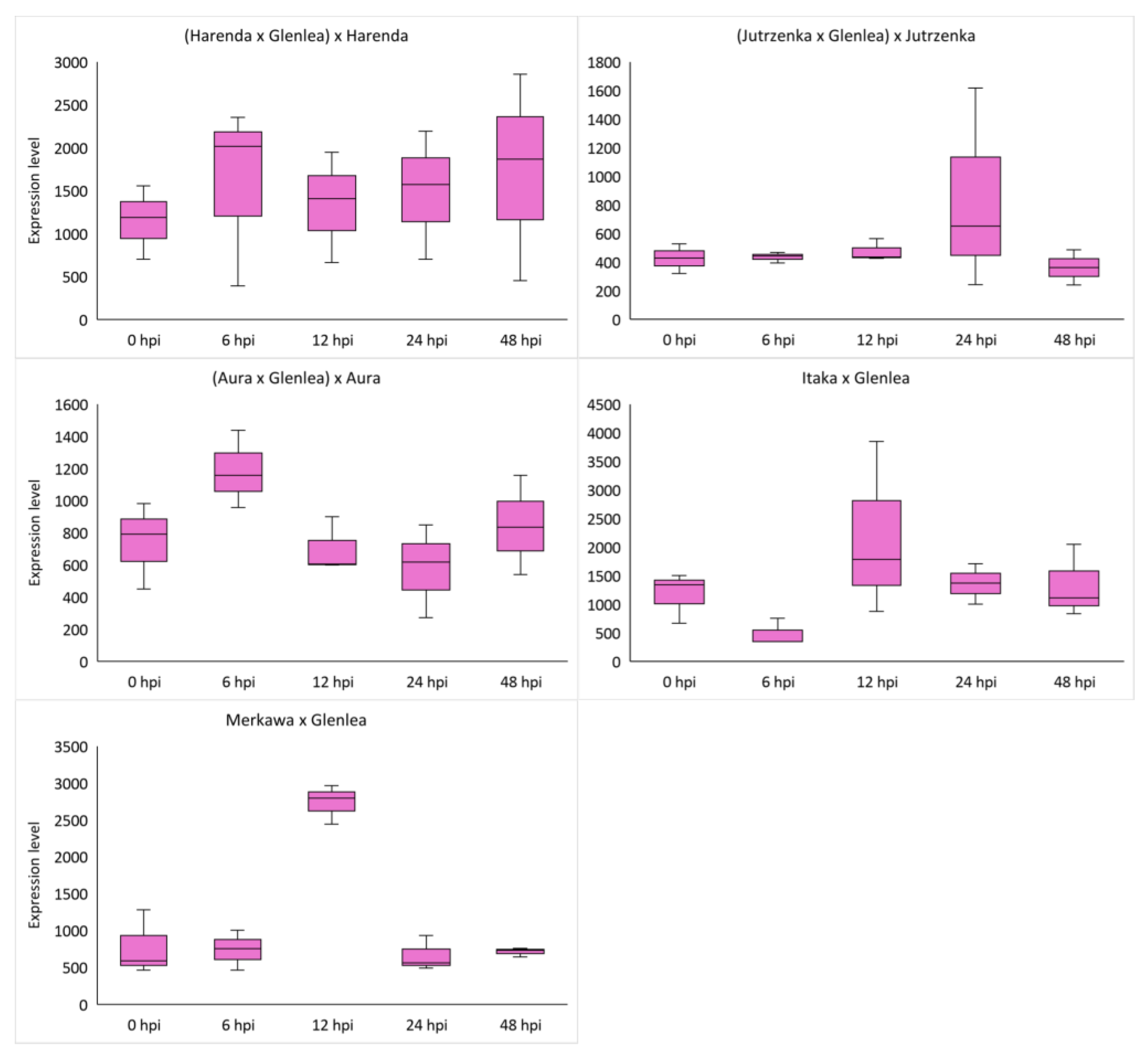
2.3. Analysis of APR Gene Expression in BC1F1 and F2 Generations
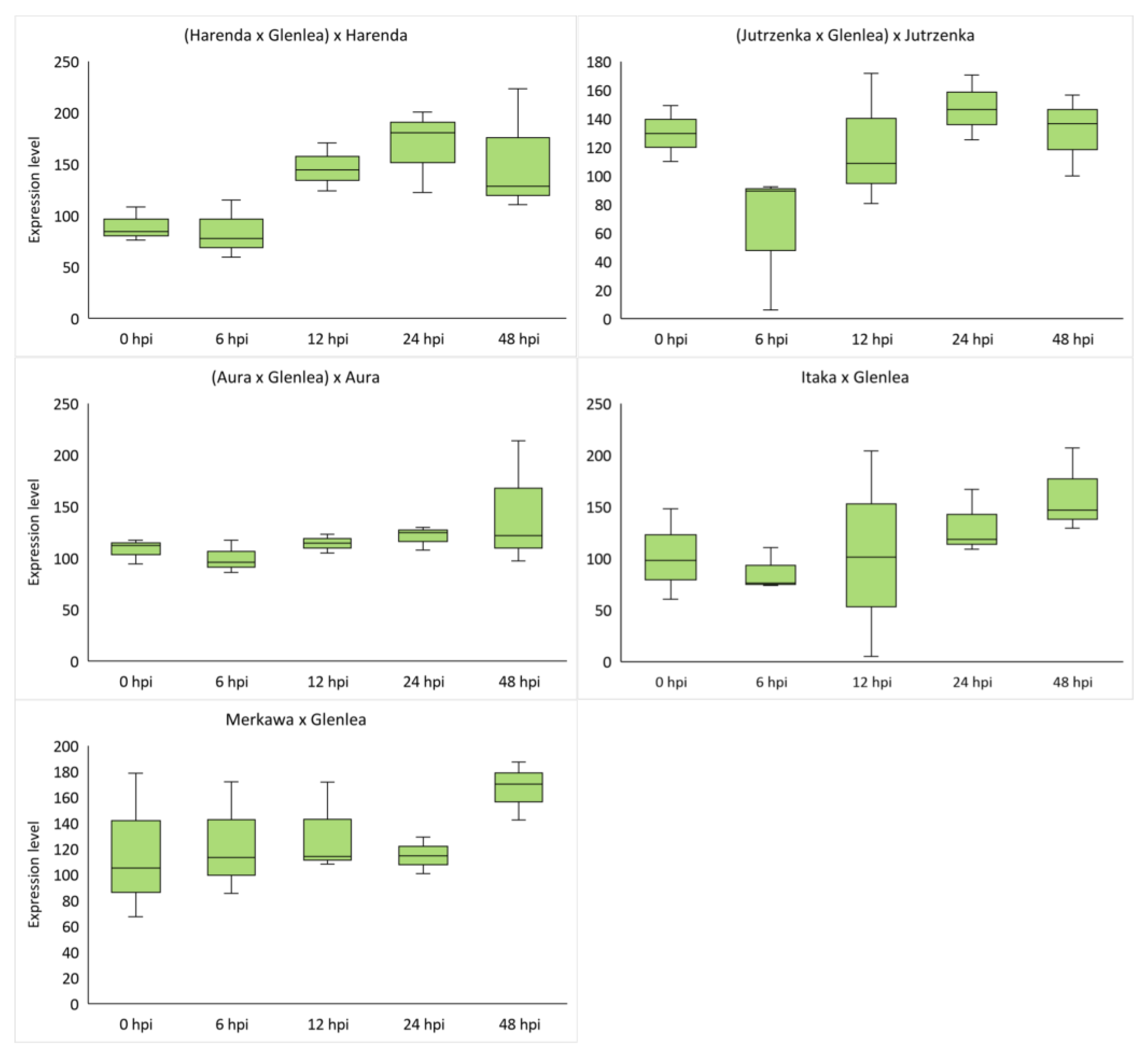
2.4. Analysis of miRNA Expression of Wheat Hybrid Forms of BC1F1 and F2 Generations
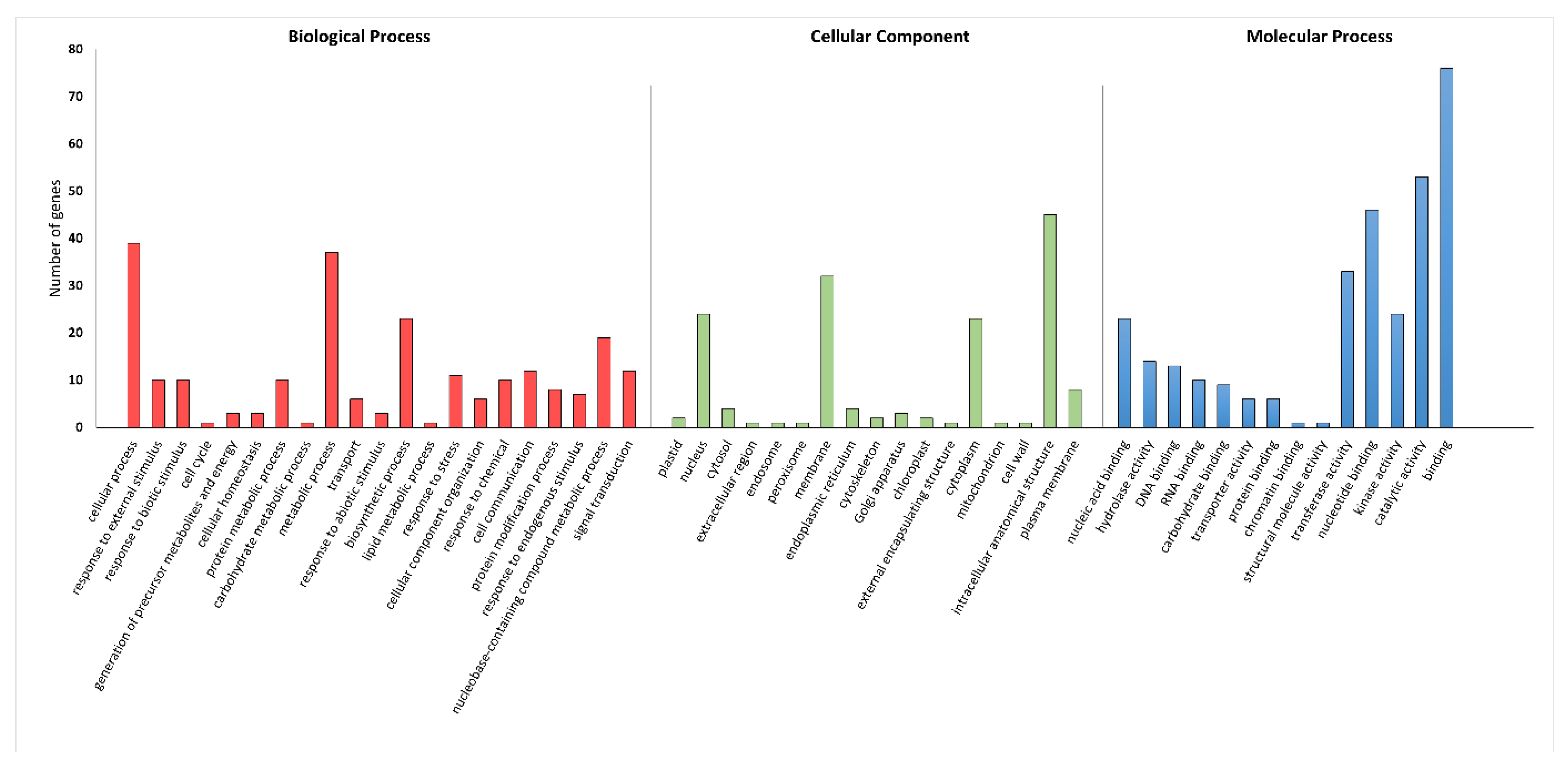
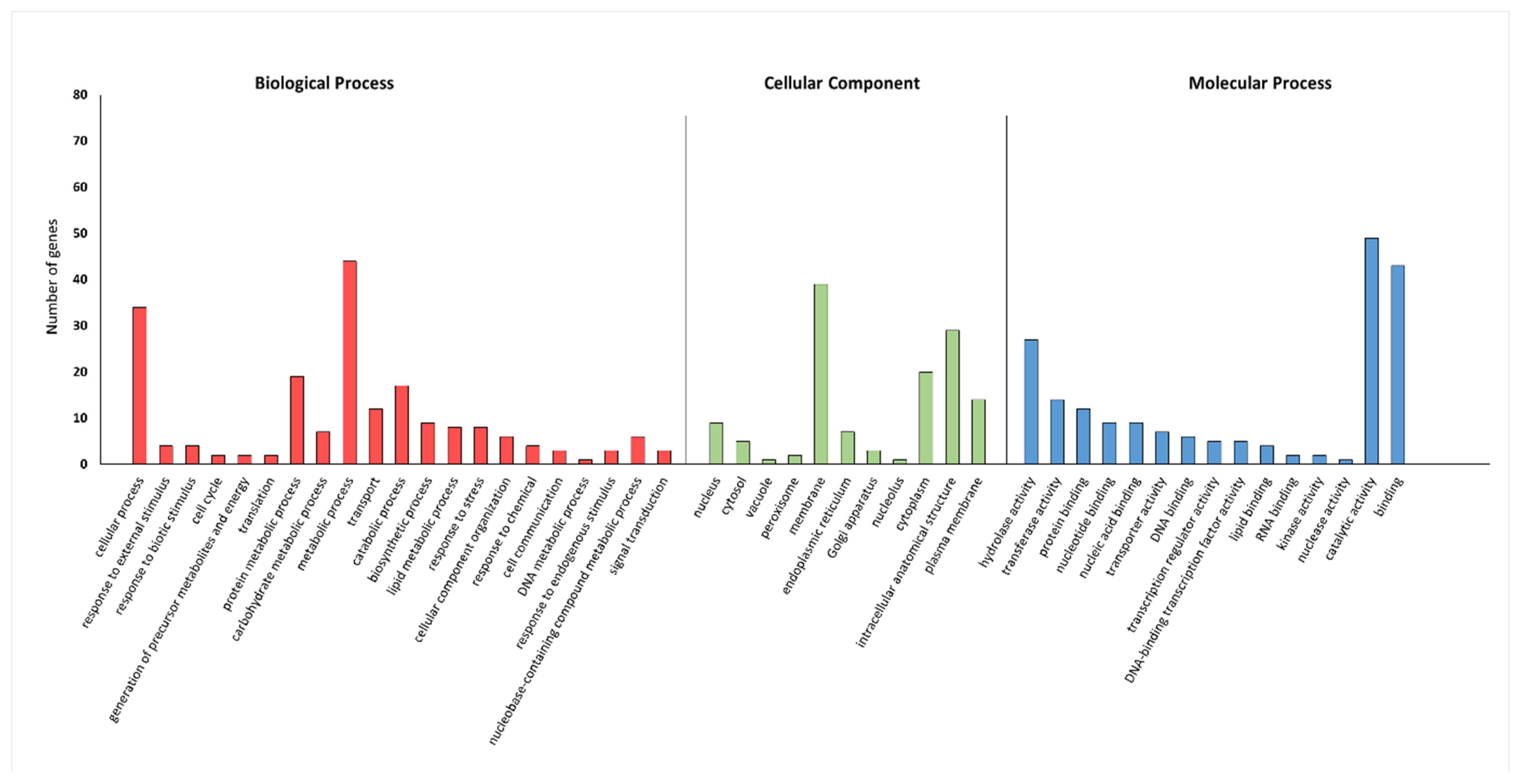
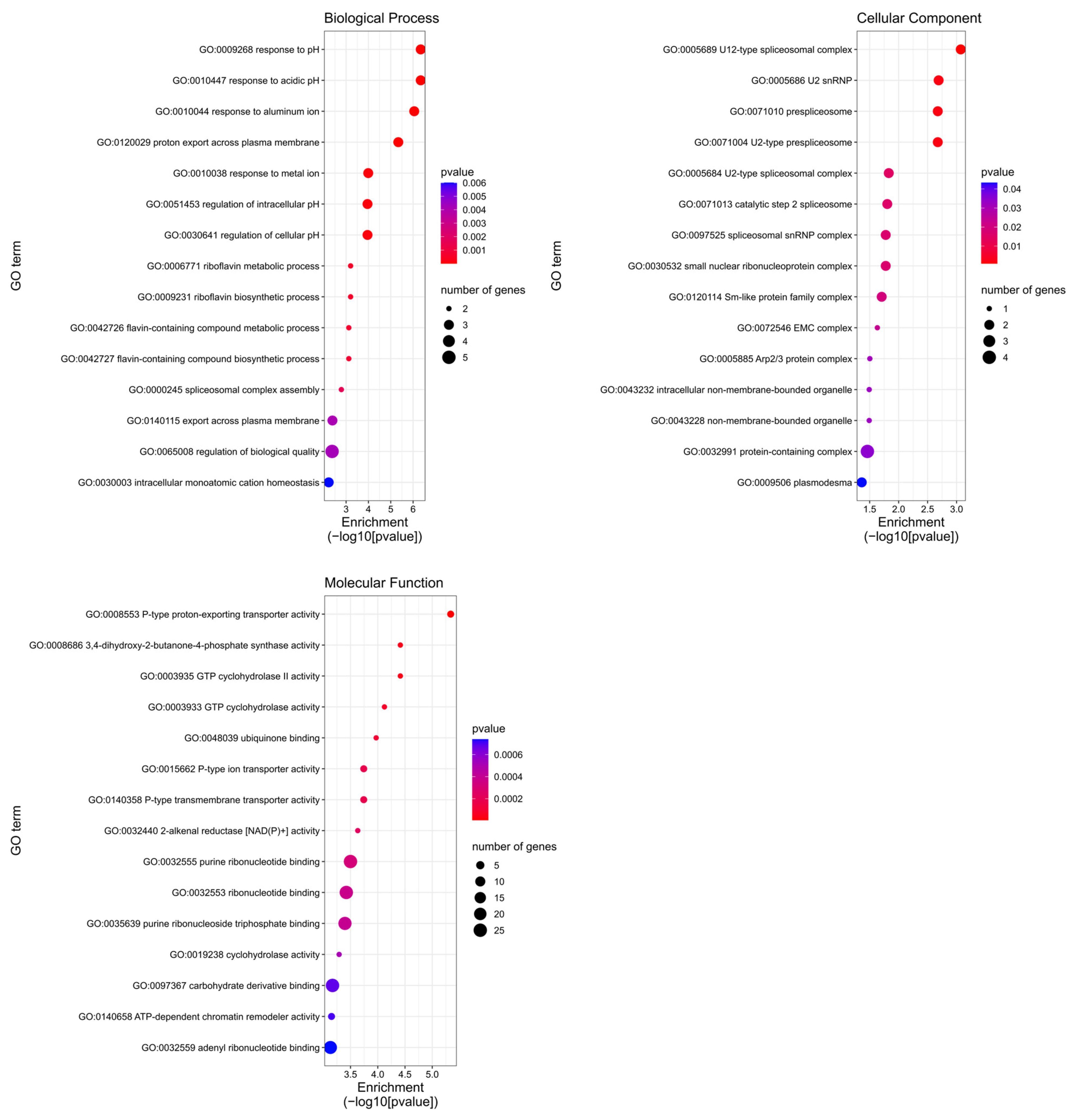
2.5. GO Results for Tested miRNAs
3. Discussion
4. Materials and Methods
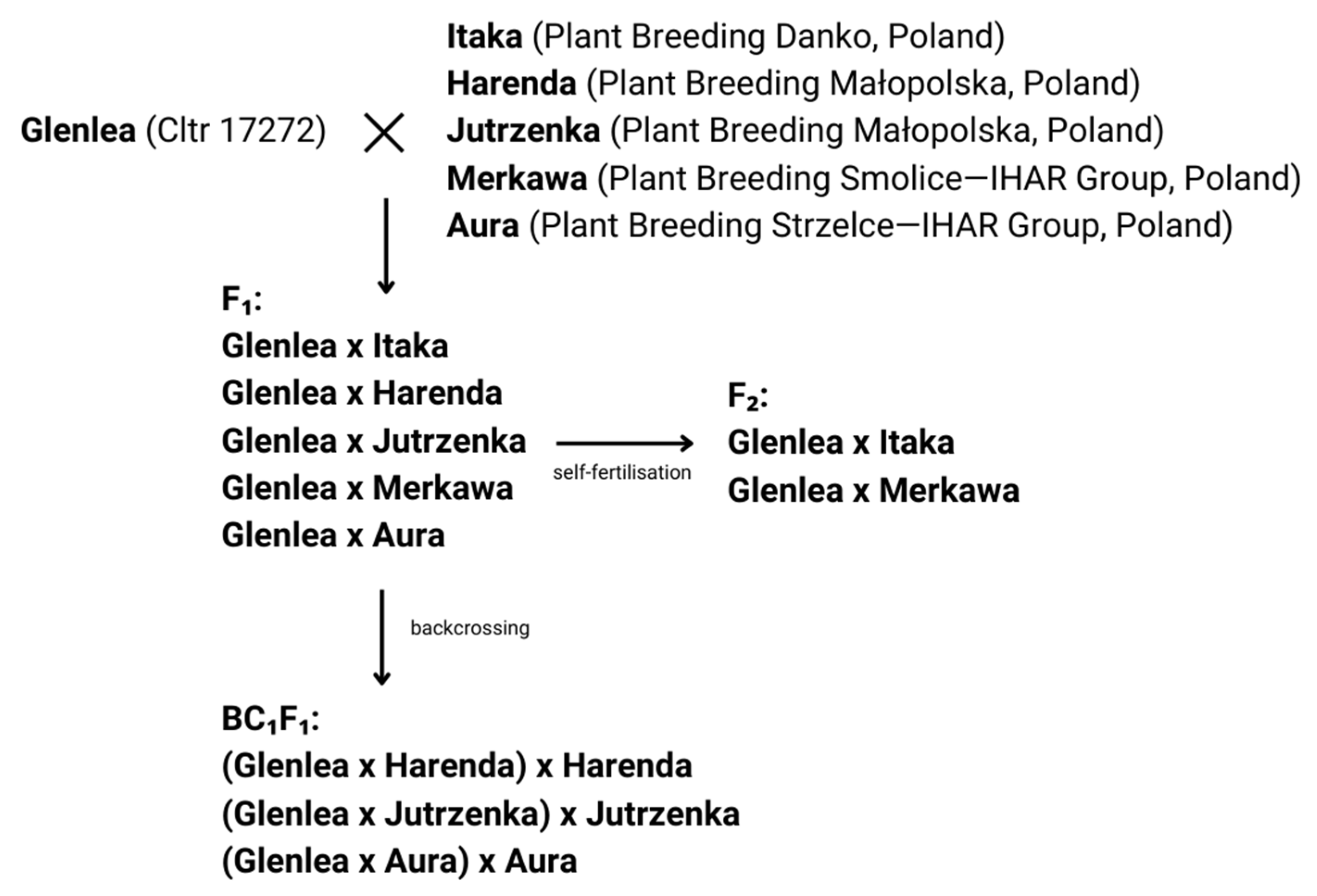
4.1. Crossbreeding Between Wheat Cultivars and Conducting Experiments in a Controlled Environment
4.2. Isolation of gDNA from Wheat Leaf Tissue
4.3. Identification of Molecular Markers Associated with APR Genes
4.4. Inoculation of Adult Plants with Pt Spores
4.5. Observation and Evaluation of Pt Infection
4.6. Total RNA Isolation and Reverse Transcription Reaction (cDNA Synthesis)
4.7. Quantitative Gene Expression Analysis Using RT-qPCR
4.8. miRNA Isolation and Reverse Transcription Reaction (Stem Loop RT-PCR)
4.9. ddPCR Expression Analysis
4.10. Statistical Analysis
4.11. Target Gene Prediction for Selected miRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The state of food security and nutrition in the world. In Building Climate Resilience for Food Security and Nutrition; FAO: Rome, Italy, 2018; ISBN 978-92-5-130571-3. [Google Scholar]
- Karaman, M.; Barutçular, C.; Mennan, H.; Caglayan, K.; Erman, M.; Yildirim, M.; Gazel, M.; Erdemci, İ.; Kendal, E.; Pala, F.; et al. Theoretical and Practical New Approaches in Cereal Science and Technology Theoretical and Practical New Approaches in Cereal Science and Technology Edited by Authors; Iksad Publishing House: Tremblay-en-France, France, 2021; ISBN 978-625-7562-37-9. [Google Scholar]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple Wheat Genomes Reveal Global Variation in Modern Breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Li, Y.; Xu, J.; Bi, A.; Kang, L.; Xu, D.; Chen, H.; Wang, Y.; Wang, Y.; et al. Triticum Population Sequencing Provides Insights into Wheat Adaptation. Nat. Genet. 2020, 52, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J. Food, Farming and Freedom: Sowing the Arab Spring. Aust. J. Int. Aff. 2012, 66, 403–404. [Google Scholar] [CrossRef]
- Bentley, A. Broken Bread—Avert Global Wheat Crisis Caused by Invasion of Ukraine. Nature 2022, 603, 551. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Bokore, F.E.; Knox, R.E.; Hiebert, C.W.; Cuthbert, R.D.; DePauw, R.M.; Meyer, B.; N’Diaye, A.; Pozniak, C.J.; McCallum, B.D. A Combination of Leaf Rust Resistance Genes, Including Lr34 and Lr46, Is the Key to the Durable Resistance of the Canadian Wheat Cultivar, Carberry. Front. Plant Sci. 2022, 12, 775383. [Google Scholar] [CrossRef] [PubMed]
- Figlan, S.; Ntushelo, K.; Mwadzingeni, L.; Terefe, T.; Tsilo, T.; Shimelis, H. Breeding Wheat for Durable Leaf Rust Resistance in Southern Africa: Variability, Distribution, Current Control Strategies, Challenges and Future Prospects. Front. Plant Sci. 2020, 11, 549. [Google Scholar] [CrossRef]
- Pequeno, D.N.L.; Ferreira, T.B.; Fernandes, J.M.C.; Singh, P.K.; Pavan, W.; Sonder, K.; Robertson, R.; Krupnik, T.J.; Erenstein, O.; Asseng, S. Production Vulnerability to Wheat Blast Disease under Climate Change. Nat. Clim. Change 2024, 14, 178–183. [Google Scholar] [CrossRef]
- Singh, J.; Chhabra, B.; Raza, A.; Yang, S.H.; Sandhu, K.S. Important Wheat Diseases in the US and Their Management in the 21st Century. Front. Plant Sci. 2023, 13, 1010191. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Gupta, V.; Prakash Gupta, O.; Sendhil, R.; Gopalareddy, K.; Jasrotia, P.; Singh, G.P. (Eds.) New Horizons in Wheat and Barley Research: Crop Protection and Resource Management; Springer Nature: Singapore, 2022; ISBN 978-981-16-4133-6. [Google Scholar]
- Lorrain, C.; Gonçalves dos Santos, K.C.; Germain, H.; Hecker, A.; Duplessis, S. Advances in Understanding Obligate Biotrophy in Rust Fungi. New Phytol. 2019, 222, 1190–1206. [Google Scholar] [CrossRef]
- Petersen, G.; Seberg, O.; Yde, M.; Berthelsen, K. Phylogenetic Relationships of Triticum and Aegilops and Evidence for the Origin of the A, B, and D Genomes of Common Wheat (Triticum aestivum). Mol. Phylogenet. Evol. 2006, 39, 70–82. [Google Scholar] [CrossRef]
- Katamadze, A.; Vergara-Díaz, O.; Uberegui, E.; Yoldi-Achalandabaso, A.; Araus, J.L.; Vicente, R. Evolution of Wheat Architecture, Physiology, and Metabolism during Domestication and Further Cultivation: Lessons for Crop Improvement. Crop J. 2023, 11, 1080–1096. [Google Scholar] [CrossRef]
- Alonge, M.; Shumate, A.; Puiu, D.; Zimin, A.V.; Salzberg, S.L. Chromosome-Scale Assembly of the Bread Wheat Genome Reveals Thousands of Additional Gene Copies. Genetics 2020, 216, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Juery, C.; Concia, L.; De Oliveira, R.; Papon, N.; Ramírez-González, R.; Benhamed, M.; Uauy, C.; Choulet, F.; Paux, E. New Insights into Homoeologous Copy Number Variations in the Hexaploid Wheat Genome. Plant Genome 2021, 14, e20069. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.A.; Feldman, M. Evolution and Origin of Bread Wheat. Plant Cell 2022, 34, 2549–2567. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F.; Zhang, Z.-B.; Wang, Z.-H.; Li, N.; Sha, Y.; Wang, X.-F.; Ding, N.; Li, Y.; Zhao, J.; Wu, Y.; et al. Genome Sequences of Five Sitopsis Species of Aegilops and the Origin of Polyploid Wheat B Subgenome. Mol. Plant 2022, 15, 488–503. [Google Scholar] [CrossRef]
- Cavalet-Giorsa, E.; González-Muñoz, A.; Athiyannan, N.; Holden, S.; Salhi, A.; Gardener, C.; Quiroz-Chávez, J.; Rustamova, S.M.; Elkot, A.F.; Patpour, M.; et al. Origin and Evolution of the Bread Wheat D Genome. Nature 2024, 633, 848–855. [Google Scholar] [CrossRef]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-Adaptive Traits Derived from Wheat Wild Relatives and Landraces. J. Exp. Bot. 2007, 58, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Schierscher-Viret, B.; Fossati, D.; Brabant, C.; Schori, A.; Keller, B.; Krattinger, S.G. Unlocking the Diversity of Genebanks: Whole-Genome Marker Analysis of Swiss Bread Wheat and Spelt. Theor. Appl. Genet. 2018, 131, 407–416. [Google Scholar] [CrossRef]
- Dinglasan, E.; Periyannan, S.; Hickey, L.T. Harnessing Adult-Plant Resistance Genes to Deploy Durable Disease Resistance in Crops. Essays Biochem. 2022, 66, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Jan, I.; Saripalli, G.; Sharma, P.K.; Mir, R.R.; Balyan, H.S.; Gupta, P.K. An Update on Resistance Genes and Their Use in the Development of Leaf Rust Resistant Cultivars in Wheat. Front. Genet. 2022, 13, 816057. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.C.; Gangwar, O.P.; Prasad, P.; Kumar, S.; Pal, D. Immunity to Rusts in Wheat: Theory, Fact and Practice. Indian Phytopathol. 2021, 74, 355–363. [Google Scholar] [CrossRef]
- Haq, I.U.; Ijaz, S. Plant Disease Management Strategies for Sustainable Agriculture Through Traditional and Modern Approaches; Springer Nature: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-35955-3. [Google Scholar]
- Bazzini, A.A.; Hopp, H.E.; Beachy, R.N.; Asurmendi, S. Infection and Coaccumulation of Tobacco Mosaic Virus Proteins Alter microRNA Levels, Correlating with Symptom and Plant Development. Proc. Natl. Acad. Sci. USA 2007, 104, 12157–12162. [Google Scholar] [CrossRef]
- Kumar, R.R.; Pathak, H.; Sharma, S.K.; Kala, Y.K.; Nirjal, M.K.; Singh, G.P.; Goswami, S.; Rai, R.D. Novel and Conserved Heat-Responsive microRNAs in Wheat (Triticum aestivum L.). Funct. Integr. Genom. 2015, 15, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Small RNAs in Plants: Recent Development and Application for Crop Improvement. Front. Plant Sci. 2015, 6, 208. [Google Scholar] [CrossRef]
- Summanwar, A.; Basu, U.; Rahman, H.; Kav, N.N.V. Non-Coding RNAs as Emerging Targets for Crop Improvement. Plant Sci. 2020, 297, 110521. [Google Scholar] [CrossRef] [PubMed]
- Lagudah, E.S.; Krattinger, S.G.; Herrera-Foessel, S.; Singh, R.P.; Huerta-Espino, J.; Spielmeyer, W.; Brown-Guedira, G.; Selter, L.L.; Keller, B. Gene-Specific Markers for the Wheat Gene Lr34/Yr18/Pm38 Which Confers Resistance to Multiple Fungal Pathogens. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2009, 119, 889–898. [Google Scholar] [CrossRef]
- Spychała, J.; Tomkowiak, A.; Noweiska, A.; Bobrowska, R.; Bocianowski, J.; Sobiech, A.; Kwiatek, M.T. Diversity of Expression Patterns of Lr34, Lr67, and Candidate Genes towards Lr46 with Analysis of Associated miRNAs in Common Wheat Hybrids in Response to Puccinia Triticina Fungus. Curr. Issues Mol. Biol. 2024, 46, 5511–5529. [Google Scholar] [CrossRef]
- Hiebert, C.W.; Thomas, J.B.; McCallum, B.D.; Humphreys, D.G.; DePauw, R.M.; Hayden, M.J.; Mago, R.; Schnippenkoetter, W.; Spielmeyer, W. An Introgression on Wheat Chromosome 4DL in RL6077 (Thatcher*6/PI 250413) Confers Adult Plant Resistance to Stripe Rust and Leaf Rust (Lr67). Theor. Appl. Genet. 2010, 121, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- McCallum, B.D.; Hiebert, C.W. Interactions Between Lr67 or Lr34 and Other Leaf Rust Resistance Genes in Wheat (Triticum aestivum). Front. Plant Sci. 2022, 13, 871970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, G.; Zang, Y.; Bhavani, S.; Bai, B.; Liu, W.; Zhao, M.; Cheng, Y.; Li, S.; Chen, W.; et al. Lr34/Yr18/Sr57/Pm38 Confers Broad-Spectrum Resistance to Fungal Diseases via Sinapyl Alcohol Transport for Cell Wall Lignification in Wheat. Plant Commun. 2024, 5, 101077. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.; Crespo-Herrera, L.A.; Villaseñor-Mir, H.E.; Rodriguez-Garcia, M.F.; Dreisigacker, S.; Barcenas-Santana, D.; Lagudah, E. Adult Plant Slow Rusting Genes Confer High Levels of Resistance to Rusts in Bread Wheat Cultivars From Mexico. Front. Plant Sci. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Spychała, J.; Tomkowiak, A.; Noweiska, A.; Bobrowska, R.; Rychel-Bielska, S.; Bocianowski, J.; Wolko, Ł.; Kowalczewski, P.Ł.; Nowicki, M.; Kwiatek, M.T. Expression Patterns of Candidate Genes for the Lr46/Yr29 “Slow Rust” Locus in Common Wheat (Triticum aestivum L.) and Associated miRNAs Inform of the Gene Conferring the Puccinia triticina Resistance Trait. PLoS ONE 2024, 19, e0309944. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory Network of miRNA on Its Target: Coordination between Transcriptional and Post-Transcriptional Regulation of Gene Expression. Cell Mol. Life Sci. 2019, 76, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tomkowiak, A.; Bobrowska, R.; Kwiatek, M.; Spychała, J.; Kuczyński, J.; Tyczewska, A.; Kowalczewski, P.; Weigt, D.; Kosiada, T. Analysis of miRNA Expression Associated with Gene Lr34 Responsible for Resistance Mechanisms to Wheat Leaf Rust. Pak. J. Bot. 2023, 55, 379–385. [Google Scholar] [CrossRef]
- Spychała, J.; Tomkowiak, A.; Noweiska, A.; Bobrowska, R.; Bocianowski, J.; Książkiewicz, M.; Sobiech, A.; Kwiatek, M.T. Expression Profiling of the Slow Rusting Resistance Genes Lr34/Yr18 and Lr67/Yr46 in Common Wheat (Triticum aestivum L.) and Associated miRNAs Patterns. Genes 2023, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yue, L.; Shen, Y.; Sheng, Y.; Zhan, X.; Xu, G.; Xing, B. Phenanthrene-Responsive microRNAs and Their Targets in Wheat Roots. Chemosphere 2017, 186, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, B.; Fard, E.M.; Gharechahi, J.; Safarzadeh, M.; Nikpay, N.; Fotovat, R.; Azimi, M.R.; Salekdeh, G.H. The Contrasting microRNA Content of a Drought Tolerant and a Drought Susceptible Wheat Cultivar. J. Plant Physiol. 2017, 216, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Biselli, C.; Bagnaresi, P.; Faccioli, P.; Hu, X.; Balcerzak, M.; Mattera, M.G.; Yan, Z.; Ouellet, T.; Cattivelli, L.; Valè, G. Comparative Transcriptome Profiles of Near-Isogenic Hexaploid Wheat Lines Differing for Effective Alleles at the 2DL FHB Resistance QTL. Front. Plant Sci. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Hajiahmadi, Z.; Abedi, A.; Wei, H.; Sun, W.; Ruan, H.; Zhuge, Q.; Movahedi, A. Identification, Evolution, Expression, and Docking Studies of Fatty Acid Desaturase Genes in Wheat (Triticum aestivum L.). BMC Genom. 2020, 21, 778. [Google Scholar] [CrossRef]
- Gidhi, A.; Mohapatra, A.; Fatima, M.; Jha, S.K.; Kumar, M.; Mukhopadhyay, K. Insights of Auxin Signaling F-Box Genes in Wheat (Triticum aestivum L.) and Their Dynamic Expression during the Leaf Rust Infection. Protoplasma 2022, 260, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Xu, J.; Zou, X.; Cheng, Y.; Nasir, M.; Zhang, X. Omics Approaches for Engineering Wheat Production under Abiotic Stresses. Int. J. Mol. Sci. 2018, 19, 2390. [Google Scholar] [CrossRef]
- Lagudah, E.S.; McFadden, H.; Singh, R.P.; Huerta-Espino, J.; Bariana, H.S.; Spielmeyer, W. Molecular Genetic Characterization of the Lr34/Yr18 Slow Rusting Resistance Gene Region in Wheat. Theor. Appl. Genet. 2006, 114, 21–30. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A Highly Sensitive RT-PCR Method for Detection and Quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. psRNATarget: A Plant Small RNA Target Analysis Server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]

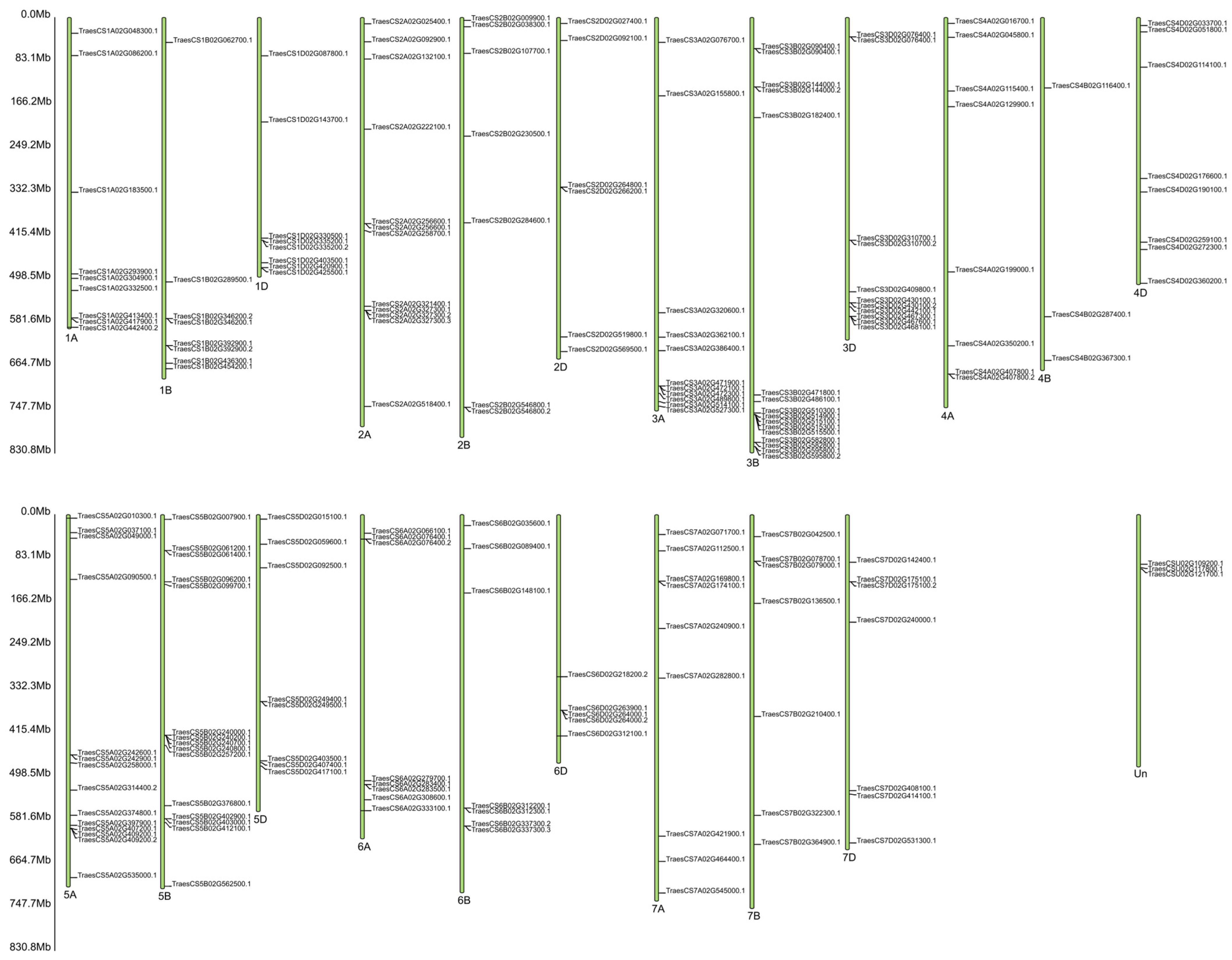

| No. | Plant Name | Breeding Company | Hybrid form BC1F1/F2 | Lr34 (csLV34) | Lr46 (csLV46G22) | Lr67 (cfd23) | Lr67 (cfd71) |
|---|---|---|---|---|---|---|---|
| 1. | H × G 1 I | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | + | - |
| 2. | H × G 1 II | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | - | - |
| 3. | H × G 1 III | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | - | + |
| 4. | H × G 1 IV | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | - | - |
| 5. | H × G 2 I | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | - | - |
| 6. | H × G 2 II | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | - | + |
| 7. | H × G 2 III | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | - | + |
| 8. | H × G 2 IV | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | - | - |
| 9. | H × G 3 I | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | - | - |
| 10. | H × G 3 II | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | - | - |
| 11. | H × G 3 III | Małopolska HR | (Harenda × Glenlea) × Harenda | - | + | + | - |
| 12. | H × G 3 IV | Małopolska HR | (Harenda × Glenlea) × Harenda | H | + | - | + |
| 13. | J × G 1 I | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | H | + | + | - |
| 14. | J × G 1 II | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | H | + | + | - |
| 15. | J × G 1 III | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | H | + | + | - |
| 16. | J × G 2 I | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | - | + | - | - |
| 17. | J × G 2 II | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | - | + | - | - |
| 18. | J × G 2 III | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | - | + | - | - |
| 19. | J × G 3 I | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | - | + | - | - |
| 20. | J × G 3 II | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | H | + | + | + |
| 21. | J × G 3 III | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | - | + | - | - |
| 22. | J × G 3 IV | Małopolska HR | (Jutrzenka × Glenlea) × Jutrzenka | H | + | + | - |
| 23. | 17 I | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 24. | 17 II | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 25. | 17 III | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 26. | 17 IV | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 27. | 17 V | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 28. | 18 I | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 29. | 18 II | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 30. | 18 III | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 31. | 18 IV | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 32. | 21 I | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 33. | 21 II | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 34. | 21 III | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 35. | 21 IV | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 36. | 21 V | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 37. | 22 I | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 38. | 22 II | Strzelce HR | (Aura × Glenlea) × Aura | - | + | + | + |
| 39. | 22 III | Strzelce HR | (Aura × Glenlea) × Aura | H | + | - | + |
| 40. | 22 IV | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 41. | 22 V | Strzelce HR | (Aura × Glenlea) × Aura | H | + | - | + |
| 42. | 24 I | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 43. | 24 II | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 44. | 24 III | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 45. | 24 IV | Strzelce HR | (Aura × Glenlea) × Aura | H | + | - | + |
| 46. | 24 V | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 47. | 25 I | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 48. | 25 II | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 49. | 25 III | Strzelce HR | (Aura × Glenlea) × Aura | H | + | - | + |
| 50. | 25 IV | Strzelce HR | (Aura × Glenlea) × Aura | - | + | - | + |
| 51. | 25 V | Strzelce HR | (Aura × Glenlea) × Aura | H | + | + | + |
| 52. | D1 I | Danko HR | Itaka × Glenlea | + | + | + | - |
| 53. | D1 II | Danko HR | Itaka × Glenlea | + | + | + | - |
| 54. | D1 III | Danko HR | Itaka × Glenlea | + | + | - | + |
| 55. | D1 IV | Danko HR | Itaka × Glenlea | H | + | + | - |
| 56. | D1 V | Danko HR | Itaka × Glenlea | - | + | + | + |
| 57. | D2 I | Danko HR | Itaka × Glenlea | H | + | + | + |
| 58. | D2 II | Danko HR | Itaka × Glenlea | + | + | - | - |
| 59. | D2 III | Danko HR | Itaka × Glenlea | - | + | + | - |
| 60. | D2 IV | Danko HR | Itaka × Glenlea | H | + | + | - |
| 61. | D2 V | Danko HR | Itaka × Glenlea | + | + | + | - |
| 62. | D3 I | Danko HR | Itaka × Glenlea | + | + | + | + |
| 63. | D3 II | Danko HR | Itaka × Glenlea | - | + | + | - |
| 64. | D3 III | Danko HR | Itaka × Glenlea | - | + | + | - |
| 65. | D3 IV | Danko HR | Itaka × Glenlea | - | + | + | - |
| 66. | D3 V | Danko HR | Itaka × Glenlea | H | + | + | + |
| 67. | M × G I | Smolice HR | Merkawa × Glenlea | H | + | - | + |
| 68. | M × G II | Smolice HR | Merkawa × Glenlea | H | + | + | + |
| 69. | M × G III | Smolice HR | Merkawa × Glenlea | - | + | + | + |
| 70. | M × G IV | Smolice HR | Merkawa × Glenlea | H | + | + | + |
| 71. | M × G V | Smolice HR | Merkawa × Glenlea | H | + | + | + |
| Plant Name | Hybrid Form from BC1F1/F2 Generations | Level of Infection |
|---|---|---|
| H × G 1 I | (Harenda × Glenlea) × Harenda | 8 |
| H × G 2 III | (Harenda × Glenlea) × Harenda | 6 |
| H × G 3 IV | (Harenda × Glenlea) × Harenda | 6 |
| J × G 1 I | (Jutrzenka × Glenlea) × Jutrzenka | 7 |
| J × G 1 III | (Jutrzenka × Glenlea) × Jutrzenka | 8 |
| J × G 3 II | (Jutrzenka × Glenlea) × Jutrzenka | 8 |
| 17 II | (Aura × Glenlea) × Aura | 6 |
| 18 II | (Aura × Glenlea) × Aura | 5 |
| 21 II | (Aura × Glenlea) × Aura | 5 |
| D1 III | Itaka × Glenlea | 7 |
| D3 I | Itaka × Glenlea | 6 |
| D2 V | Itaka × Glenlea | 5 |
| M × G II | Merkawa × Glenlea | 6 |
| M × G IV | Merkawa × Glenlea | 5 |
| M × G V | Merkawa × Glenlea | 7 |
| Hybrid Form | tae-miR9653b | tae-miR5384-3p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 hpi | 6 hpi | 12 hpi | 24 hpi | 48 hpi | 0 hpi | 6 hpi | 12 hpi | 24 hpi | 48 hpi | |
| (Harenda × Glenlea) × Harenda | 1149.51 | 1588.13 | 1340.06 | 1489.97 | 1726.36 | 89.77 | 84.14 | 146.39 | 167.93 | 154.10 |
| (Jutrzenka × Glenlea) × Jutrzenka | 425.41 | 434.56 | 475.90 | 836.50 | 362.28 | 129.60 | 62.57 | 120.24 | 147.38 | 130.92 |
| (Aura × Glenlea) × Aura | 741.59 | 1183.93 | 701.81 | 579.65 | 844.10 | 108.02 | 99.75 | 114.14 | 120.68 | 144.30 |
| Itaka × Glenlea | 1170.77 | 481.50 | 2169.09 | 1362.00 | 1332.23 | 102.19 | 86.76 | 103.48 | 131.42 | 160.96 |
| Merkawa × Glenlea | 774.80 | 738.21 | 2736.03 | 661.28 | 709.34 | 116.98 | 123.64 | 131.40 | 114.86 | 166.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spychała, J.; Noweiska, A.; Tomkowiak, A.; Bobrowska, R.; Szewczyk, K.; Kwiatek, M.T. Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2025, 26, 665. https://doi.org/10.3390/ijms26020665
Spychała J, Noweiska A, Tomkowiak A, Bobrowska R, Szewczyk K, Kwiatek MT. Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.). International Journal of Molecular Sciences. 2025; 26(2):665. https://doi.org/10.3390/ijms26020665
Chicago/Turabian StyleSpychała, Julia, Aleksandra Noweiska, Agnieszka Tomkowiak, Roksana Bobrowska, Katarzyna Szewczyk, and Michał Tomasz Kwiatek. 2025. "Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.)" International Journal of Molecular Sciences 26, no. 2: 665. https://doi.org/10.3390/ijms26020665
APA StyleSpychała, J., Noweiska, A., Tomkowiak, A., Bobrowska, R., Szewczyk, K., & Kwiatek, M. T. (2025). Unraveling Effects of miRNAs Associated with APR Leaf Rust Resistance Genes in Hybrid Forms of Common Wheat (Triticum aestivum L.). International Journal of Molecular Sciences, 26(2), 665. https://doi.org/10.3390/ijms26020665






