How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy?
Abstract
1. Introduction
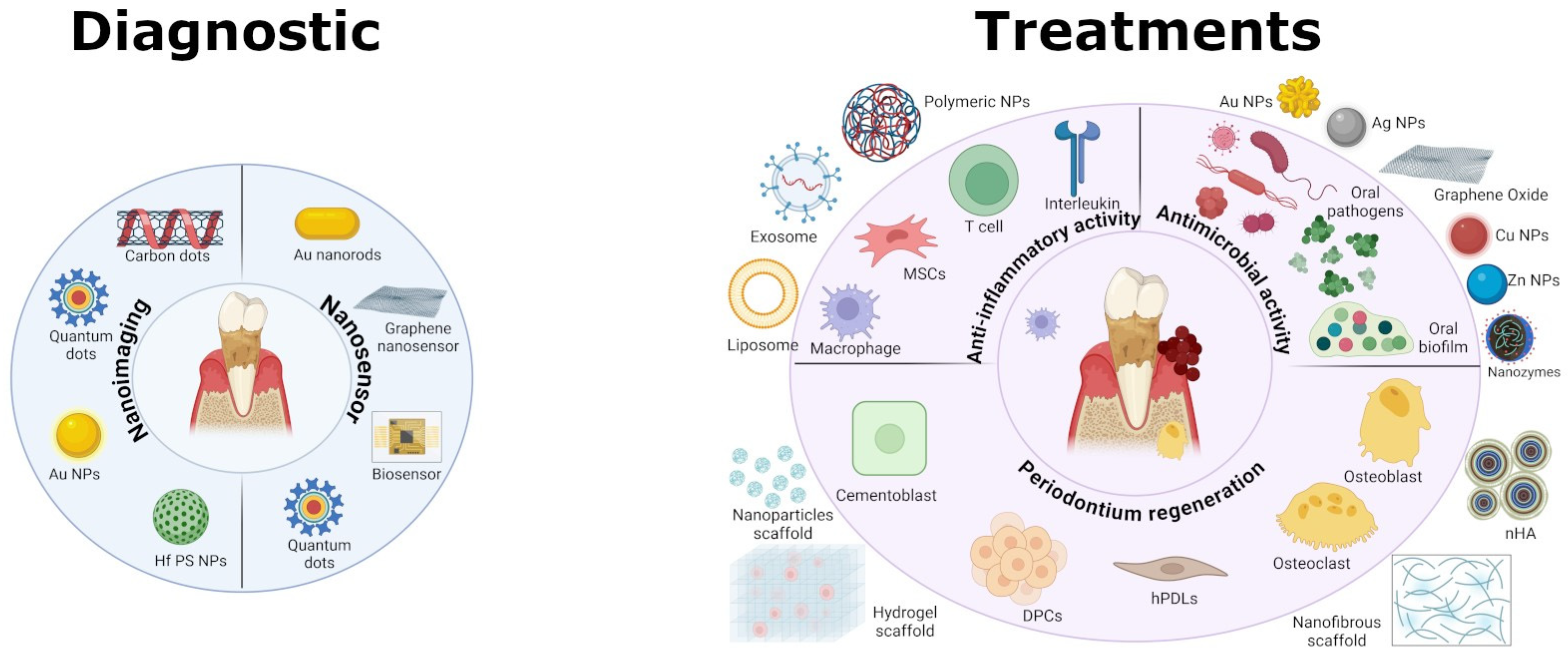
2. Nanotechnology for Diagnostic Tools
2.1. Nanotechnology-Enhanced Imaging
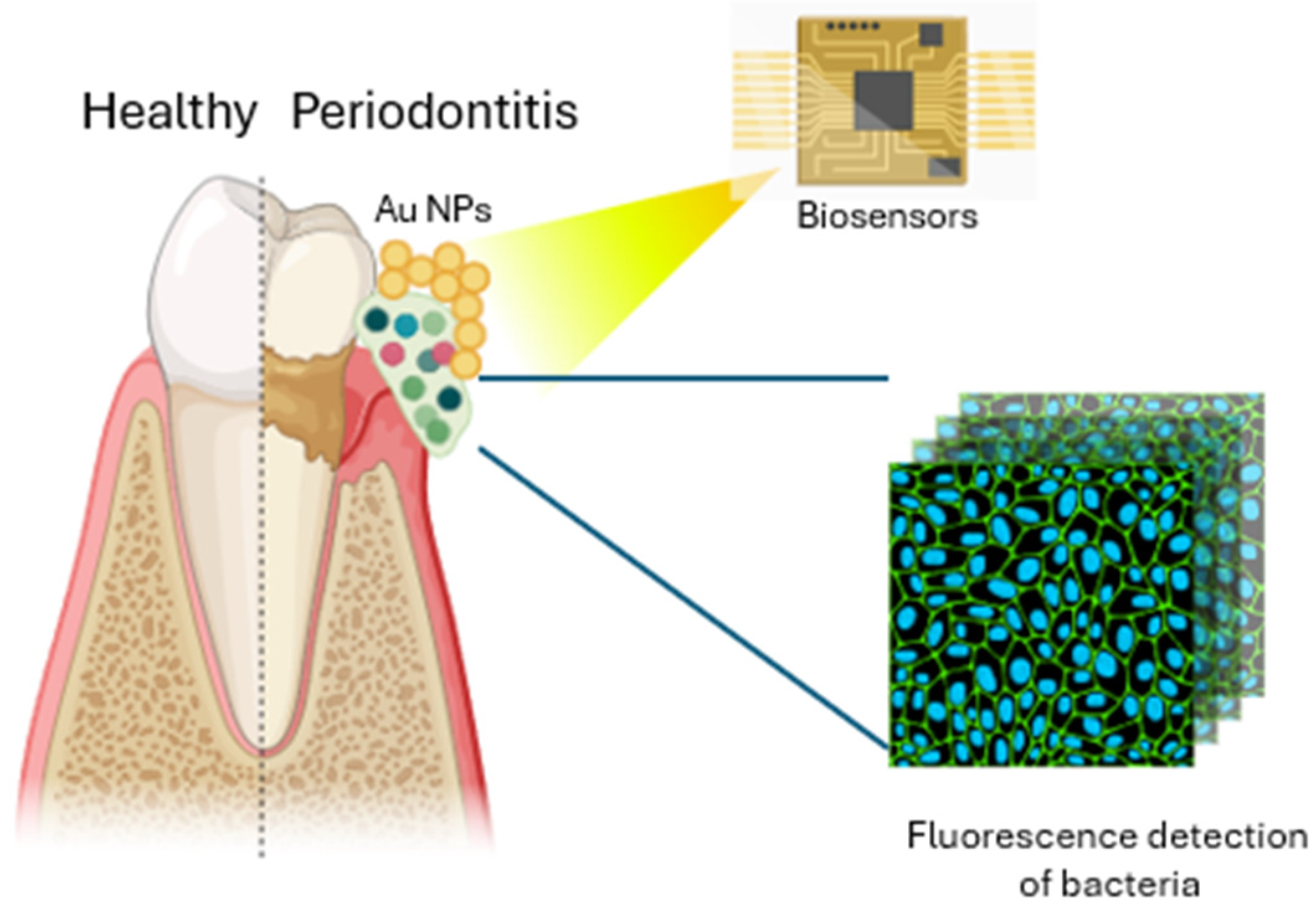
2.2. Nano-Biosensors
| Nanoparticles | Study Type | Methods | Effects | References |
|---|---|---|---|---|
| Hf PS NPs | In vitro | Polymeric-silane-conjugated hafnium oxide nanoparticles | Precise method of identifying and localizing the bacterial pathogen using molecularly targeted X-ray imaging | [23] |
| CDs | In vitro/in vivo (S. aureus-infected mouse model) | One-pot solvothermal method to prepare quaternized CDs | Inactivation of Gram+ bacteria via disrupting the bacterial walls/membranes | [24] |
| N,Cl-CDs | In vitro | Synthesized metal-free N,Cl-doped carbon dots using Impatiens balsamina L. stems as green precursors in a deep eutectic solvent (DES) | High selectivity for Gram+ bacteria through selective fluorescence imaging, and antibacterial effects | [25] |
| Au@Ag Nanorods–PDMS | In vitro/in vivo (human) | Nanomaterial consisting of gold–silver nanorods (Au@Ag NRs) and PDMS | Visualization of the presence of dental lesions through a color change at the affected sites | [29] |
| Functionalized graphene with AMPs | In vitro | Graphene nanosensors functionalized with dodecapeptide graphene, triglycine linker, and the AMP odorranin-HP (OHP) | Highly sensitive, selective, and wireless sensor that can be used for detecting bacteria in oral or tracking health conditions in real time | [30] |
3. Nanomaterials for Antibacterial Therapy
3.1. Nanoparticle–Membrane Interaction
3.2. Nanoparticles Target Efflux System
3.3. Nanoparticle-Induced Oxidative Stress
3.4. Combination of Therapies
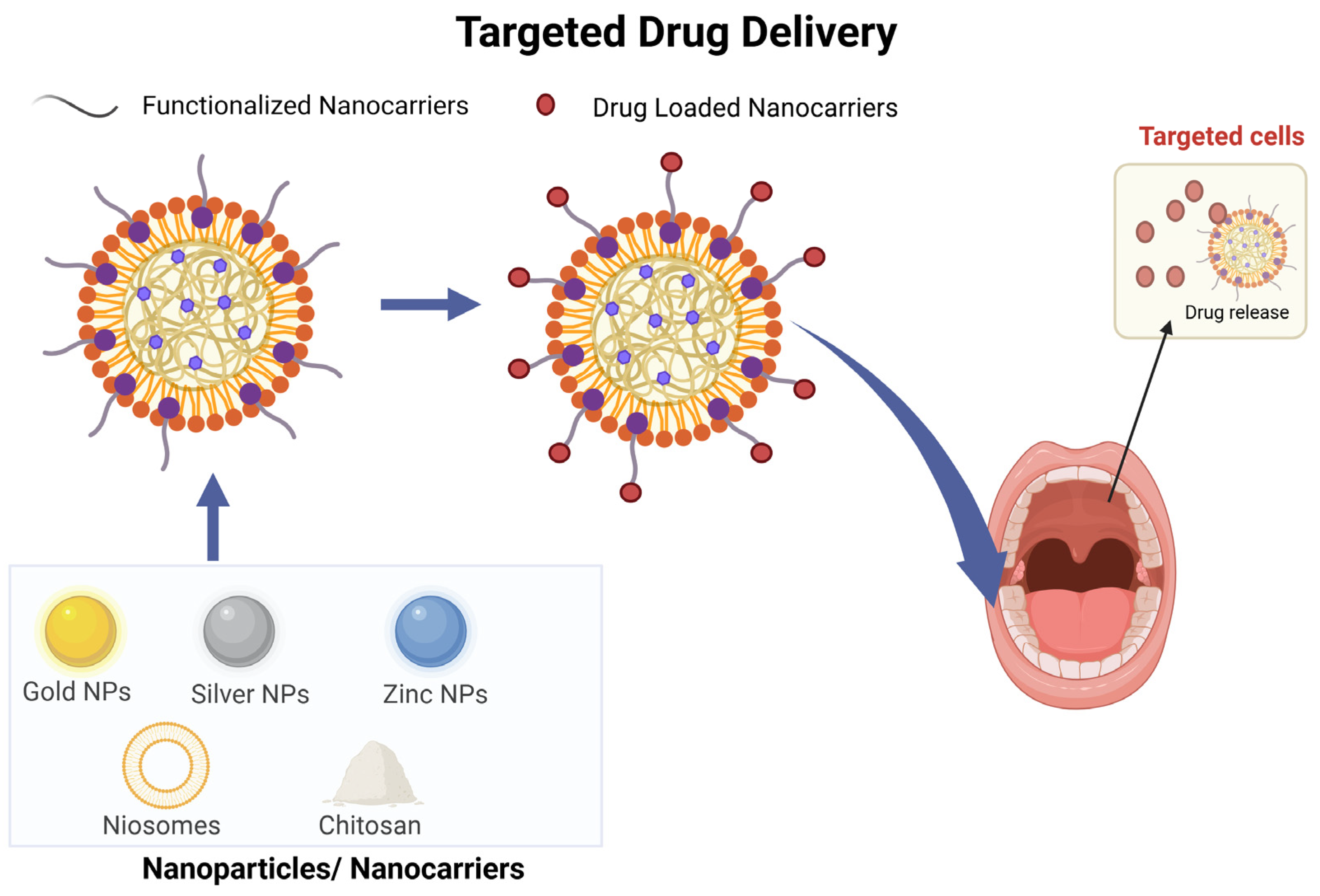
3.5. Targeted Drug Delivery
| Nanoparticles | Study Type | Methods | Effects | References |
|---|---|---|---|---|
| AuNPs | In vitro | LPS selectively synergize with 10 nm AuNPs | AuNPs and LPS augment cellular responses in neutrophils and induce a canonical process of NET formation | [43] |
| AgNPs | In vitro | Biological pathway using various plant extracts to produce AgNPs | Antibacterial efficacy against E. coli and P. aeruginosa, B. subtilis and S. epidermidis by membrane damage | [48] |
| AuNCs | In vitro/in vivo (male C57 BL/6 mice) | AuNCs composed of 25 Au atoms and 18 thiolate ligands with ultra-small structure | Lysis of the F. nucleatum membrane, ROS generation, and inhibition of biofilm formation | [49] |
| AgNP-sp, AgNR | In vitro | Spherical silver nanoparticles (AgNP-sp) | Structural damage to bacterial cell walls of K. pneumoniae | [50] |
| AgNPs | In vitro | AgNPs in the form of nanocubes, nanospheres, and nanowires prepared via microwave-assisted method | Nanocubes and nanospheres showed stronger antibacterial activity than the nanowires with low specific surface areas against E. coli | [52] |
| AuNPs | In vitro | AuNPs characterized by face-centered cubic lattice structures and truncated-octahedron morphology | Reduction of F. nucleatum growth and damage of cell wall integrity by membrane depolarization mechanism | [55] |
| pAgNCs | In vitro | Highly monodispersed, ultra-small (<3 nm) pAgNCs | Penetration of pAgNCs in the bacterial cell membrane of F. nucleatum and S. sanguinis | [57] |
| bPEI-AuNPs | In vitro | Interaction of cationic bPEI-AuNPs with wall teichoic acids of Gram-positive bacterial cell walls | Interaction and damage of bacterial wall of B. subtilis | [58] |
| ZnONPs | In vitro | Green synthesis from natural sweetener S. rebaudiana | Action on efflux activity of Enterococci | [58] |
| CuNPs | In vitro | 110 nm casein-stabilized CuNPs | Action as efflux pump inhibitor and anti-biofilm agent on S. aureus | [61] |
| Co–ZnO | In vitro | Synthesis of 40–60 nm Co–ZnO particles by modified co-precipitation method | Photo-inactivation and efflux pump inhibition of methicillin-resistant S. aureus | [63] |
| GONPs | In vitro | Natural shellac-derived GO coatings | Draw-up of electrons from the cell membrane and transfer to GO with consequent ROS production | [66] |
| GONPs, rGONPs | In vitro | GO dispersion obtained by sonication of graphite (Gt) powders (20 µm) | Loss of cell viability, induced oxidative stress, and DNA fragmentation on P. aeruginosa | [67] |
| Au25 NCs | In vitro | Ultra-small gold nanoclusters Au25 | Destruction of membrane integrity, disruption of antioxidant defense system, metabolic inactivation, DNA damage on E. coli | [68] |
| Dex-IONP, Dex-IONP-GOx | In vitro | Dextran-coated iron oxide nanozymes (Dex-IONP) that display strong catalytic peroxidase-like activity at acidic pH values | Nanohybrid system to increase intrinsic H2O2 production and trigger pH-dependent ROS generation to kill pathogenic bacteria | [70] |
| IONzymes, ISNzymes | In vitro | Dextran-coated iron oxide nanozymes (Dex-IONP) that display strong catalytic peroxidase-like activity at acidic pH values | Combination of iron-based nanozymes and H2O2 provide elimination of oral biofilm | [71] |
| Ce6, TAT–Ce6 NPs | In vitro/in vivo (female Sprague–Dawley rats) | TAT–Ce6 self-assembled nanoparticles for loading TDZ | Synergistic anti-periodontitis effects of PDT and antibiotic therapy: killing of P. gingivalis and the reduced adsorption of alveolar bone in rat | [73] |
| Ce6, C6, and Fe3O4 NPs | In vitro | Ce6 and C6 co-loaded into the Fe3O4–silane core–shell structure to form multifunctional nanoparticles | Strong anti-biofilm activity against S. sanguinis, P. gingivalis, and F. nucleatum, with magnetically targeting capacities | [74] |
| Cu2O@rGO | In vitro | Nanosystem designed via the in situ growth of Cu2O on rGO | Generation of charge carriers and improved electron–hole separation showed enhanced antibacterial rates against E. coli and S. aureus | [75] |
| PCL/ZnO | In vitro | Membrane using polycaprolactone (PCL), a biodegradable polymer, and zinc oxide (ZnO) nanoparticles | Inhibition of bacterial adhesion of P. gingivalis without affecting the viability of osteoblasts | [76] |
| Au NBPs | In vitro | Mixing of mesoporous silica-coated Au NBPs (Au NBPs@SiO2) with gelatin methacrylate (GelMA-Au NBPs@SiO2) to deliver minocycline | Higher antibacterial efficacy of the antibiotic and photothermal treatment against P. gingivalis | [97] |
| SPEEK + NH2–ZrO2 + Cur | In vitro | Amine-functionalized zirconia-nanoparticle-loaded curcumin-incorporated SPEEK nanofibrous scaffolds | Antibacterial activity against S. oralis | [79] |
| CNDs | In vitro | Combination of CNDs with near-infrared | Inactivation of S. aureus, E. faecalis, and methicillin-resistant S. aureus due to ROS generation | [81] |
| SLNs | In vitro | Thermosensitive gel formulations containing clindamycin-loaded niosomes and solid lipid nanoparticles (SLNs) loaded with fluconazole (FLZ) | The gel formulation presented a slower release of both drugs compared to niosomes and SLN suspensions | [88] |
| CuNPs and ZnONPs | In vitro | CuNPs and ZnONPs combined with gentamicin | Stronger anti-biofilm activity of CuNPs and ZnONPs combined with gentamicin in their lowest concentrations than antibiotic itself | [89] |
| C-AuNp-Amp | In vitro | Chitosan-capped gold nanoparticles coupled with ampicillin | Better activity of C-AuNp-Amp compared to free ampicillin | [90] |
| Amp-AuNPs | In vitro | Synthesized ampicillin-capped gold nanoparticles (Amp-Au NPs) | Amp-AuNPs show successful accumulation onto the surface of the bacterial cell, as a result of which pores were formed into the bacterial membrane of E. coli | [91] |
| Kan-AuNPs | In vitro | Conjugation of kanamycin on the surface of AuNPs | Significant reduction in the MIC of Kan-AuNPs compared to free kanamycin against S. bovis, S. epidermidis, E. aerogenes, P. aeruginosa | [92] |
| DPP, DPPLM NPs | In vitro/in vivo (diabetic rat) | Self-assembled, dual-responsive, and dual-drug-loading nanocarrier system with minocycline loading | Co-delivery of antimicrobial/Mino and the antioxidant/ALA, disruption of dental-plaque biofilms, and suppression of periodontal bone loss | [93] |
| FPM NPs | In vitro/in vivo (male Wistar rats) | Fe3O4@PDA nanocomposites with minocycline loading | Robust antibacterial effect against S. sanguinis, F. nucleatum, and P. gingivalis, high biocompatibility, and low systemic toxicity of FPM NPs | [94] |
| CS-PA/CNP | In vitro/in vivo (mouse model of periodontitis complicated with hypertension) | CS with antibacterial properties cross-linked with AMP-modified PEG to form a dual antibacterial hydrogel (CS-PA) with curcumin loaded into CNP | Co-treatment of periodontitis and hypertension, and drug delivery platform to provide combinatorial therapeutic options | [96] |
4. Nanotechnology for Anti-Inflammatory Therapy
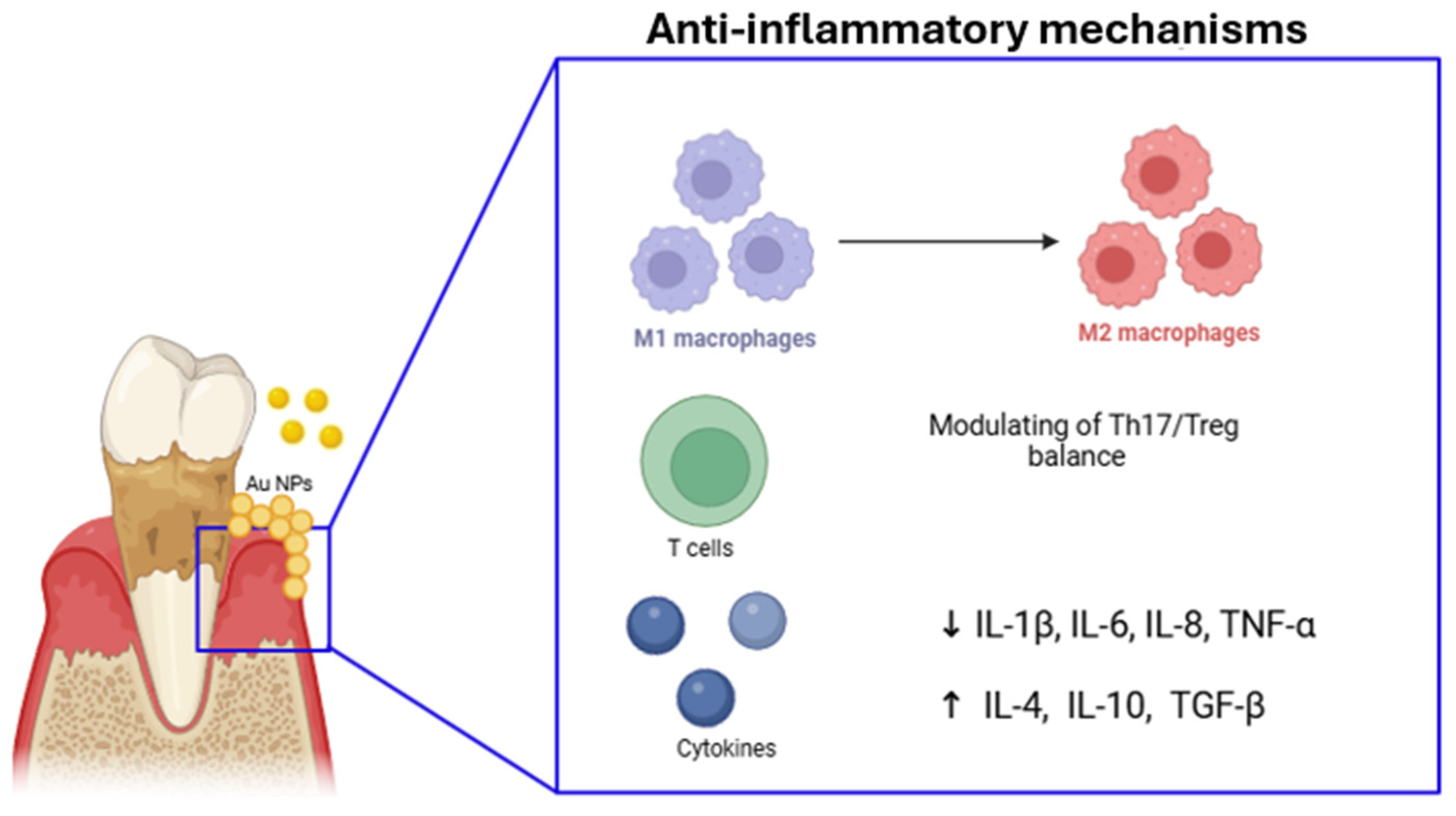
4.1. Immunomodulatory Action
4.2. Regulating Pro-/Anti-Inflammatory Environment
| Nanoparticles | Study Type | Methods | Effects | References |
|---|---|---|---|---|
| Lipo-RSV | In vitro | Resveratrol-loaded liposomal system | Re-education of macrophages from M1- to M2-like phenotype through activating p-STAT3 and downregulating p-STAT1. Reduction of ROS and inhibition of NF-κB signal and inflammasomes, reducing IL-1β, IL-6, and TNF-α. | [102] |
| CeO2@QU | In vitro/in vivo (P. gingivalis-infected rat model) | Quercetin onto nano-octahedral ceria | Significant downregulation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines | [103] |
| 3D-exos, 2D-exos | In vitro | Mesenchymal-stem-cell-derived exosomes produced using 2D and 3D culture systems | Improvement of the function of MSC-exos in the treatment of periodontitis | [106] |
| Exosomes | In vitro | Exosomes from PDLSCs stimulated by P. gingivalis and LPS | Influence of CD4+ T cells, by modulating the Th17/Treg balance through the miR-155-5p/SIRT1 pathway | [107] |
| Nano-BA, Nano-BE | In vitro | Baicalin (BA) and baicalein (BE) encapsulated in amine-modified mesoporous silica nanoparticles (MSNs) | Downregulation of IL-1β, IL-6, and IL-8 | [109] |
| PDA NPs | In vitro/in vivo (LPS-induced periodontal disease in BALB/c nude mice) | Biodegradable polydopamine nanoparticles (PDA NPs) as smart ROS scavengers | Scavenging of multiple ROS and suppressing ROS-induced inflammation reactions | [110] |
| Dox-NPs | In vitro | Polymeric nanoparticles (NPs) produced by a polymerization/precipitation process and doped with doxycycline | Dox-NPs enhanced PDLSC differentiation into osteoblast/cementoblast lineages while providing an anti-inflammatory effect | [112] |
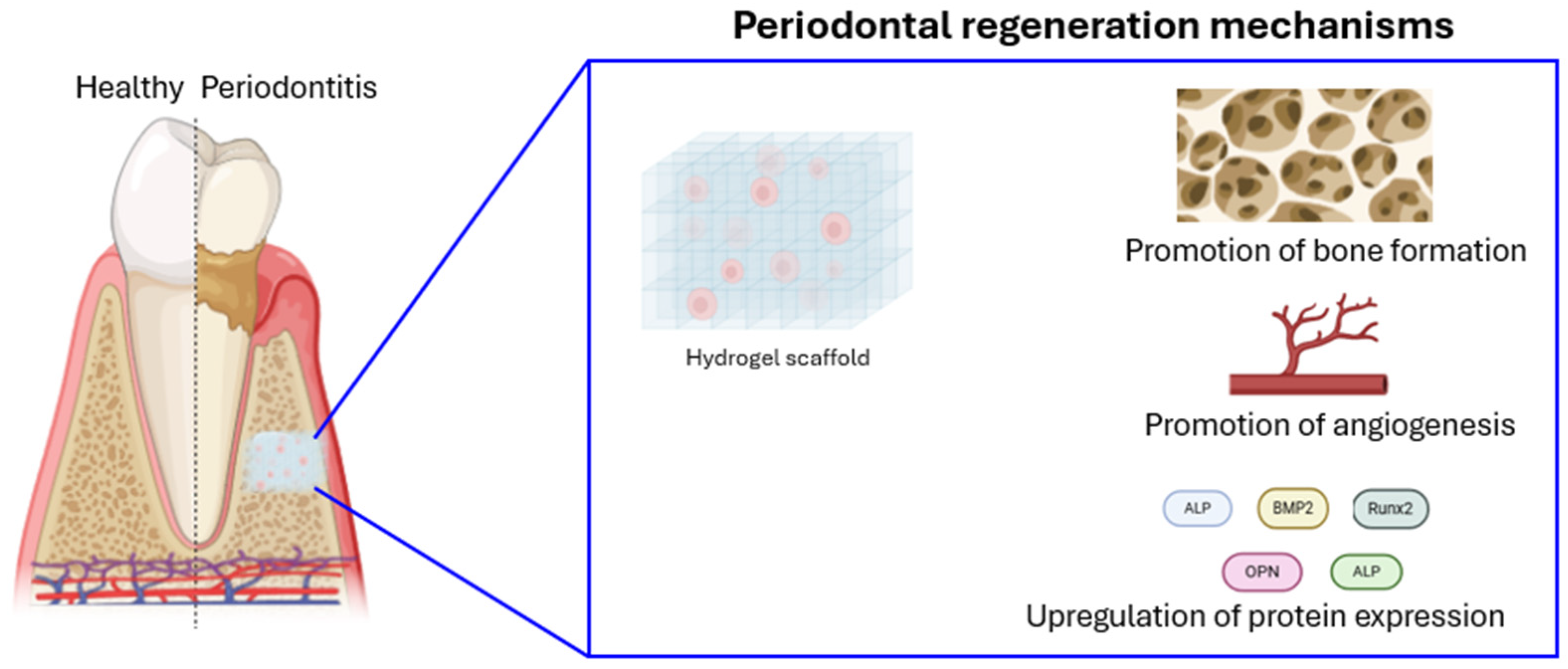
5. Nanomaterials for Regenerative Periodontal Therapy
5.1. Nanohydroxyapatite (nHA)
5.2. Nanostructured Scaffolds for Tissue Engineering
5.3. Nanofibrous Scaffolds
| Nanoparticles | Study Type | Methods | Effects | References |
|---|---|---|---|---|
| nHA | In vivo (diabetic rat model) | Nano-hydroxyapatite (nHA) coating implant surgically placed in tibias | nHA coatings stimulate cellular activity at genetic level of osteoblasts and osteoclast | [124] |
| nHA | In vivo (male Sprague–Dawley rats) | Nanopolymorphic crystalline hydroxyapatite (HA) coating on microroughened titanium implants | HA-coated implants showed significant improvements in bone–implant integration | [125] |
| HAp-PADH | In vitro/in vivo (female New Zealand white rabbits) | Hydrogel by a facile one-step PAAm and urethacrylate dextran (Dex-U), followed by the in situ mineralization of HAp nanocrystals | Promotion of osteogenic differentiation of M3CT3 cells, excellent osteoconductivity | [126] |
| NcHA | In vivo (human) | NcHA bone replacement graft (Sybograf®) in combination with bioresorbable collagen membrane (Periocol®) | Slight clinical improvement in CAL (clinical attachment level) gain | [127] |
| nano-HA paste | In vitro | Suspension of pure nanocrystalline HA in water | Stimulation of hPDL proliferation mediated by EGFR and followed by ERK1/2, Akt activation | [128] |
| MINO-PLGA | In vitro/in vivo (periodontitis rat model) | Minocycline-loaded poly(lactic-co-glycolic acid) electrospun membranes | Sustained diffusion of MINO, good support of osteoblast proliferation and adhesion, increased alveolar crest height | [134] |
| PPC, PP, PPβ | In vitro | Three-layer membranes structured with serial layers of electrospun chlorhexidine-doped PLGA/PCL (PPC), PLGA/PCL (PP), and β-tricalcium phosphate-doped PLGA/PCL (PPβ). | Better MC3T3 cell adhesion, and promoted osteoconductive properties | [135] |
| PCL/LAP | In vitro/in vivo (calvarial defect rat model) | Nanosilicate-incorporated PCL nanofibrous membranes | Mediation of osteogenesis and immunomodulation of PDLCs in vitro and accelerating periodontal regeneration in vivo | [136] |
| CsA-loaded PLGA | In vivo (ICR mouse) | Cyclosporine-A-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles | Innervation of 88.4% of the regenerated teeth using the CsA-loaded PLGA scaffold | [137] |
| Gel–nHA scaffold | In vitro | New gelatin (Gel)–nano-hydroxyapatite (nHA)-based scaffold | Increment of ALP in DPSCs | [121] |
| nHA/CG | In vitro/in vivo (minipig) | Nanohydroxyapatite/chitosan/gelatin (nHA/CG) three-dimensional porous scaffolds | Adhesion of hPDLSCs, increased new bone formation and generated large bones with normal architectures and vascularization | [138] |
6. Nanomaterials and Biocompatibility
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 6 December 2024).
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Vach, K. Healthier Smile: The Role of Diet and Nutrition in the Prevention and Therapy of Caries, Gingivitis, and Periodontitis. Nutrients 2023, 15, 4319. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.J.; Garzón, H.; Arboleda, S.; Rodríguez, A. Oral Dysbiosis and Autoimmunity: From Local Periodontal Responses to an Imbalanced Systemic Immunity. A Review. Front. Immunol. 2020, 11, 591255. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Loos, B.G.; Nicu, E.A. Emerging Concepts in the Resolution of Periodontal Inflammation: A Role for Resolvin E1. Front. Immunol. 2017, 8, 1682. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef]
- Díaz, C.M.; Bullon, B.; Ruiz-Salmerón, R.J.; Fernández-Riejos, P.; Fernández-Palacín, A.; Battino, M.; Cordero, M.D.; Quiles, J.L.; Varela-López, A.; Bullón, P. Molecular Inflammation and Oxidative Stress Are Shared Mechanisms Involved in Both Myocardial Infarction and Periodontitis. J. Periodontal. Res. 2020, 55, 519–528. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A Systematic Review and Meta-analysis of Epidemiologic Observational Evidence on the Effect of Periodontitis on Diabetes an Update of the EFP-AAP Review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Güler, B.; Doğan, E.; Onbaşı, K. The Relationship Between Monocyte Count to High-Density Lipoprotein Ratio and Severity of Inflammation in Aggressive Periodontitis: A Retrospective Analysis. Meandros Med. Dent. J. 2020, 21, 122–127. [Google Scholar] [CrossRef]
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proença, L.; Mendes, J.J. The 2018 Periodontitis Case Definition Improves Accuracy Performance of Full-Mouth Partial Diagnostic Protocols. Sci. Rep. 2020, 10, 7093. [Google Scholar] [CrossRef]
- Huck, O.; Stutz, C.; Gegout, P.-Y.; Özçelik, H.; Benkirane-Jessel, N.; Petit, C.; Batool, F. Nanomedicine and Periodontal Regenerative Treatment. Dent. Clin. North Am. 2022, 66, 131–155. [Google Scholar] [CrossRef]
- Dakhale, R.; Paul, P.; Achanta, A.; Ahuja, K.P.; Meshram, M. Nanotechnology Innovations Transforming Oral Health Care and Dentistry: A Review. Cureus 2023, 15, e46423. [Google Scholar] [CrossRef] [PubMed]
- Hooshiar, M.H.; Moghaddam, M.A.; Kiarashi, M.; Al-Hijazi, A.Y.; Hussein, A.F.; Alrikabi, H.A.; Salari, S.; Esmaelian, S.; Mesgari, H.; Yasamineh, S. Recent Advances in Nanomaterial-Based Biosensor for Periodontitis Detection. J. Biol. Eng. 2024, 18, 28. [Google Scholar] [CrossRef]
- Shi, R.; Zhu, Y.; Lu, W.; Zhai, R.; Zhou, M.; Shi, S.; Chen, Y. Nanomaterials: Innovative Approaches for Addressing Key Objectives in Periodontitis Treatment. RSC Adv. 2024, 14, 27904–27927. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Tang, M.; Zhang, C.; Fei, Y.; Li, M.; Li, M.; Gui, S.; Guo, J. New Insights into Nanotherapeutics for Periodontitis: A Triple Concerto of Antimicrobial Activity, Immunomodulation and Periodontium Regeneration. J. Nanobiotechnol. 2024, 22, 19. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A. Conventional Diagnostic Criteria for Periodontal Diseases (Plaque-induced Gingivitis and Periodontitis). Periodontol. 2000 2024, 95, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z. Digital Dentistry: Transformation of Oral Health and Dental Education with Technology. Eur. J. Dent. 2023, 17, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Mutreja, I.; Maalej, N.; Kaushik, A.; Kumar, D.; Raja, A. High Atomic Number Nanoparticles to Enhance Spectral CT Imaging Aspects. Mater. Adv. 2023, 4, 3967–3988. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Ostadhossein, F.; Misra, S.K.; Tripathi, I.; Kravchuk, V.; Vulugundam, G.; LoBato, D.; Selmic, L.E.; Pan, D. Dual Purpose Hafnium Oxide Nanoparticles Offer Imaging Streptococcus Mutans Dental Biofilm and Fight It In Vivo via a Drug Free Approach. Biomaterials 2018, 181, 252–267. [Google Scholar] [CrossRef]
- Yang, J.; Gao, G.; Zhang, X.; Ma, Y.-H.; Chen, X.; Wu, F.-G. One-Step Synthesis of Carbon Dots with Bacterial Contact-Enhanced Fluorescence Emission: Fast Gram-Type Identification and Selective Gram-Positive Bacterial Inactivation. Carbon NY 2019, 146, 827–839. [Google Scholar] [CrossRef]
- Liu, S.; Quan, T.; Yang, L.; Deng, L.; Kang, X.; Gao, M.; Xia, Z.; Li, X.; Gao, D. N,Cl-Codoped Carbon Dots from Impatiens Balsamina L. Stems and a Deep Eutectic Solvent and Their Applications for Gram-Positive Bacteria Identification, Antibacterial Activity, Cell Imaging, and ClO− Sensing. ACS Omega 2021, 6, 29022–29036. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Badiye, A.; Kapoor, N.; Shukla, R.K. Nano-Biosensors for Biochemical Analysis. In Nanobioanalytical Approaches to Medical Diagnostics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–343. [Google Scholar]
- Bakirhan, N.K.; Topal, B.D.; Ozcelikay, G.; Karadurmus, L.; Ozkan, S.A. Current Advances in Electrochemical Biosensors and Nanobiosensors. Crit. Rev. Anal. Chem. 2022, 52, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef]
- Ma, D.; Chen, B.; Li, Y.; Pang, X.; Fu, Q.; Xiao, Z.; Shi, Z.; Li, X.; Luo, C.; Zhou, Z.; et al. Au@Ag Nanorods-PDMS Wearable Mouthguard as a Visualized Detection Platform for Screening Dental Caries and Periodontal Diseases. Adv. Healthc. Mater. 2022, 11, 2102682. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-Based Wireless Bacteria Detection on Tooth Enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Botelho, J.; Machado, V.; Alves, R.; Mendes, J.J. Managing Oral Health in the Context of Antimicrobial Resistance. Int. J. Environ. Res. Public Health 2022, 19, 16448. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Wright, G.D. Intrinsic Antibiotic Resistance: Mechanisms, Origins, Challenges and Solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.C.; Romano, M.; Kerry, L.E.; Kwong, H.-S.; Low, W.-W.; Brett, S.J.; Clements, A.; Beis, K.; Frankel, G. OmpK36-Mediated Carbapenem Resistance Attenuates ST258 Klebsiella Pneumoniae in Vivo. Nat. Commun. 2019, 10, 3957. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus Aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance in Enterococci. Expert Rev. Anti. Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Sutaria, D.S.; Moya, B.; Green, K.B.; Kim, T.H.; Tao, X.; Jiao, Y.; Louie, A.; Drusano, G.L.; Bulitta, J.B. First Penicillin-Binding Protein Occupancy Patterns of β-Lactams and β-Lactamase Inhibitors in Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 2018, 62, e00282. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.-F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; Alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent Advances in Metal Nanoparticles to Treat Periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.K.; Gaur, S.; Sengupta, M.; Singh, M.S. Mechanistic Insights into Nanoparticle Surface-Bacterial Membrane Interactions in Overcoming Antibiotic Resistance. Front. Microbiol. 2023, 14, 1135579. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, N.; Zhu, Y.; Lu, Y.; Chen, Q.; Fan, S.; Huang, Q.; Chen, X.; Xia, L.; Wei, Y.; et al. Gold Nanoparticles Synergize with Bacterial Lipopolysaccharide to Enhance Class A Scavenger Receptor Dependent Particle Uptake in Neutrophils and Augment Neutrophil Extracellular Traps Formation. Ecotoxicol. Environ. Saf. 2021, 211, 111900. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Perreault, F.; de Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The Effect of Silver Nanoparticles Size, Produced Using Plant Extract from Arbutus Unedo, on Their Antibacterial Efficacy. Nanomaterials 2017, 7, 178. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Wang, Y.; Wang, P.; Pu, J.; Xu, X.; Chen, F.; Jiang, L.; Jiang, Q.; Yan, F. Antibiofilm Activity of Ultra-Small Gold Nanoclusters against Fusobacterium Nucleatum in Dental Plaque Biofilms. J. Nanobiotechnol. 2022, 20, 470. [Google Scholar] [CrossRef]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape Dependent Physical Mutilation and Lethal Effects of Silver Nanoparticles on Bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Pandey, P.; Mohanta, B. A Comparative Study on the Antibacterial Activity of Different Shaped Silver Nanoparticles. Chem. Pap. 2021, 75, 4907–4915. [Google Scholar] [CrossRef]
- Hong, X.; Wen, J.; Xiong, X.; Hu, Y. Shape Effect on the Antibacterial Activity of Silver Nanoparticles Synthesized via a Microwave-Assisted Method. Environ. Sci. Pollut. Res. 2016, 23, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Railsback, J.G.; Singh, A.; Pearce, R.C.; McKnight, T.E.; Collazo, R.; Sitar, Z.; Yingling, Y.G.; Melechko, A.V. Weakly Charged Cationic Nanoparticles Induce DNA Bending and Strand Separation. Adv. Mater. 2012, 24, 4261–4265. [Google Scholar] [CrossRef]
- Haidari, H.; Bright, R.; Kopecki, Z.; Zilm, P.S.; Garg, S.; Cowin, A.J.; Vasilev, K.; Goswami, N. Polycationic Silver Nanoclusters Comprising Nanoreservoirs of Ag + Ions with High Antimicrobial and Antibiofilm Activity. ACS Appl. Mater. Interfaces 2022, 14, 390–403. [Google Scholar] [CrossRef]
- Caudill, E.R.; Hernandez, R.T.; Johnson, K.P.; O’Rourke, J.T.; Zhu, L.; Haynes, C.L.; Feng, Z.V.; Pedersen, J.A. Wall Teichoic Acids Govern Cationic Gold Nanoparticle Interaction with Gram-Positive Bacterial Cell Walls. Chem. Sci. 2020, 11, 4106–4118. [Google Scholar] [CrossRef]
- Musiol, R. Efflux Systems as a Target for Anti-Biofilm Nanoparticles: Perspectives on Emerging Applications. Expert Opin. Ther. Targets 2023, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Sobhanipoor, M.H.; Ahmadrajabi, R.; Nave, H.H.; Saffari, F. Determination of Efflux Activity in Enterococci by Hoechst Accumulation Assay and the Role of Zinc Oxide Nanoparticles in Inhibition of This Activity. BMC Microbiol. 2022, 22, 195. [Google Scholar] [CrossRef]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V.; Sai Subramanian, N. Copper Nanoparticles as an Efflux Pump Inhibitor to Tackle Drug Resistant Bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Bui, V.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, G.; Faisal, S.; Khan, S.; Shams, D.F.; Nadhman, A. Photo-Inactivation and Efflux Pump Inhibition of Methicillin Resistant Staphylococcus Aureus Using Thiolated Cobalt Doped ZnO Nanoparticles. J. Photochem. Photobiol. B 2019, 192, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.-G.; Cho, M.H.; Lee, J. ZnO Nanoparticles Inhibit Pseudomonas Aeruginosa Biofilm Formation and Virulence Factor Production. Microbiol. Res. 2014, 169, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Woong Han, J.; Abdal Daye, A.; Eppakayala, V.; Kim, J. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial Mechanism and Transcriptome Analysis of Ultra-Small Gold Nanoclusters as an Alternative of Harmful Antibiotics against Gram-Negative Bacteria. J. Hazard. Mater. 2021, 416, 126236. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Hooshiar, M.; Badkoobeh, A.; Kolahdouz, S.; Tadayonfard, A.; Mozaffari, A.; Nasiri, K.; Salari, S.; Safaralizadeh, R.; Yasamineh, S. The Potential Use of Nanozymes as an Antibacterial Agents in Oral Infection, Periodontitis, and Peri-Implantitis. J. Nanobiotechnol. 2024, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Y.; Shah, S.; Kim, D.; Simon-Soro, A.; Ito, T.; Hajfathalian, M.; Li, Y.; Hsu, J.C.; Nieves, L.M.; et al. Precision Targeting of Bacterial Pathogen via Bi-Functional Nanozyme Activated by Biofilm Microenvironment. Biomaterials 2021, 268, 120581. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Ma, S.; Guo, Q.; Zhang, W.; Cheng, L.; Ding, L.; Xu, Z.; Jiang, J.; Gao, L. Oral Biofilm Elimination by Combining Iron-Based Nanozymes and Hydrogen Peroxide-Producing Bacteria. Biomater. Sci. 2020, 8, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Zhang, P.; Pathak, J.L.; Wang, X.; Wu, Y.; Yang, J.; Shen, Y. Photodynamic Therapy in Periodontitis: A Narrative Review. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12946. [Google Scholar] [CrossRef]
- Li, Z.; Pan, W.; Shi, E.; Bai, L.; Liu, H.; Li, C.; Wang, Y.; Deng, J.; Wang, Y. A Multifunctional Nanosystem Based on Bacterial Cell-Penetrating Photosensitizer for Fighting Periodontitis Via Combining Photodynamic and Antibiotic Therapies. ACS Biomater. Sci. Eng. 2021, 7, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, L.; Lynch, C.D.; Sun, X.; Li, X.; Qi, M.; Ma, C.; Li, C.; Dong, B.; Zhou, Y.; et al. Nanoparticles Having Amphiphilic Silane Containing Chlorin E6 with Strong Anti-Biofilm Activity against Periodontitis-Related Pathogens. J. Dent. 2019, 81, 70–84. [Google Scholar] [CrossRef]
- He, Y.; Zan, J.; He, Z.; Bai, X.; Shuai, C.; Pan, H. A Photochemically Active Cu2O Nanoparticle Endows Scaffolds with Good Antibacterial Performance by Efficiently Generating Reactive Oxygen Species. Nanomaterials 2024, 14, 452. [Google Scholar] [CrossRef]
- Seo, N.; Park, C.; Stahl, A.M.; Cho, H.; Park, S.-W.; Yim, S.-H.; Yun, K.-D.; Ji, M.-K.; Kim, H.; Yang, Y.P.; et al. Effect of Zinc Oxide Nanoparticle Addition to Polycaprolactone Periodontal Membranes on Antibacterial Activity and Cell Viability. J. Nanosci. Nanotechnol. 2021, 21, 3683–3688. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, X.; Xu, H.; Hua, F.; Hu, X.; Xie, Q.; Wang, W.; Jia, J. Development of a Doxycycline Hydrochloride-Loaded Electrospun Nanofibrous Membrane for GTR/GBR Applications. J. Nanomater. 2016, 2016, 6507459. [Google Scholar] [CrossRef]
- Nasajpour, A.; Ansari, S.; Rinoldi, C.; Rad, A.S.; Aghaloo, T.; Shin, S.R.; Mishra, Y.K.; Adelung, R.; Swieszkowski, W.; Annabi, N.; et al. A Multifunctional Polymeric Periodontal Membrane with Osteogenic and Antibacterial Characteristics. Adv. Funct. Mater. 2018, 28, 1703437. [Google Scholar] [CrossRef]
- Ekambaram, R.; Paraman, V.; Raja, L.; Suresh, M.K.; Dharmalingam, S. Design and Development of Electrospun SPEEK Incorporated with Aminated Zirconia and Curcumin Nanofibers for Periodontal Regeneration. J. Mech. Behav. Biomed. Mater. 2021, 123, 104796. [Google Scholar] [CrossRef]
- Higuchi, J.; Klimek, K.; Wojnarowicz, J.; Opalińska, A.; Chodara, A.; Szałaj, U.; Dąbrowska, S.; Fudala, D.; Ginalska, G. Electrospun Membrane Surface Modification by Sonocoating with HA and ZnO: Ag Nanoparticles—Characterization and Evaluation of Osteoblasts and Bacterial Cell Behavior In Vitro. Cells 2022, 11, 1582. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, L.; Wu, L.; Hao, X.; Liu, Q.; Lin, L.; Huang, Z.; Ruan, Z.; Weng, S.; et al. Quaternized Carbon Quantum Dots with Broad-Spectrum Antibacterial Activity for the Treatment of Wounds Infected with Mixed Bacteria. Acta Biomater. 2022, 138, 528–544. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Q.; Xue, J.; Ding, Y. Selectively Enhanced Antibacterial Effects and Ultraviolet Activation of Antibiotics with ZnO Nanorods Against Escherichia coli. J. Biomed. Nanotechnol. 2013, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Prasad, A.; Shivannavar, C.; Gaddad, S.M.; Thati, V.; Roy, A.S.; Prasad, M.V.N.A.; Shivannavar, C.T.; Gaddad, S.M. Nanostructured Zinc Oxide Enhances the Activity of Antibiotics Against Staphylococcus Aureus. J. Biosci. Technol. 2010, 1, 64–69. [Google Scholar]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjug. Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green Biosynthesis of Gold Nanoparticles Using Galaxaura elongata and Characterization of Their Antibacterial Activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Saeidi, Z.; Giti, R.; Emami, A.; Rostami, M.; Mohammadi, F. Thermosensitive and Mucoadhesive Gels Containing Solid Lipid Nanoparticles Loaded with Fluconazole and Niosomes Loaded with Clindamycin for the Treatment of Periodontal Diseases: A Laboratory Experiment. BMC Oral Health 2024, 24, 551. [Google Scholar] [CrossRef]
- Ashajyothi, C.; Harish, K.H.; Dubey, N.; Chandrakanth, R.K. Antibiofilm Activity of Biogenic Copper and Zinc Oxide Nanoparticles-Antimicrobials Collegiate against Multiple Drug Resistant Bacteria: A Nanoscale Approach. J. Nanostruct. Chem. 2016, 6, 329–341. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Sobhana, S.S.L.; Jacob, J.P.; Kumar, M.G.; Devi, M.P.; Sastry, T.P.; Mandal, A.B. Preparation, Characterization and Evaluation of a Biopolymeric Gold Nanocomposite with Antimicrobial Activity. Biotechnol. Appl. Biochem. 2010, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Chavan, C.; Kamble, S.; Murthy, A.V.R.; Kale, S.N. Ampicillin-Mediated Functionalized Gold Nanoparticles against Ampicillin-Resistant Bacteria: Strategy, Preparation and Interaction Studies. Nanotechnology 2020, 31, 215604. [Google Scholar] [CrossRef]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Ren, M.; Wang, X.; Li, L.; Liu, F.; Lan, Y.; Yang, S.; Song, J. PH and Lipase-Responsive Nanocarrier-Mediated Dual Drug Delivery System to Treat Periodontitis in Diabetic Rats. Bioact. Mater. 2022, 18, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Wang, P.; Chen, Z.; Liu, Y.; Wang, L.; Guo, J.; Li, Z.; Cai, H.; Wei, J. Combined Ferromagnetic Nanoparticles for Effective Periodontal Biofilm Eradication in Rat Model. Int. J. Nanomed. 2023, 18, 2371–2388. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of Periodontal Disease and Comorbidities: Evidence, Mechanisms, and Implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Xu, S.; Hu, B.; Dong, T.; Chen, B.; Xiong, X.; Du, L.; Li, Y.; Chen, Y.; Tian, G.; Bai, X.; et al. Alleviate Periodontitis and Its Comorbidity Hypertension Using a Nanoparticle-Embedded Functional Hydrogel System. Adv. Healthc. Mater. 2023, 12, e2203337. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and Autophagy: First Update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, B.-M.; Li, X.; Yu, Y.-C.; Deng, D.-K.; Sun, L.-J.; Qu, H.-L.; Wu, R.-X.; Xu, X.-Y.; Sun, H.-H.; et al. Gold Nanoparticles Targeting the Autophagy–Lysosome System to Combat the Inflammation-Compromised Osteogenic Potential of Periodontal Ligament Stem Cells: From Mechanism to Therapy. Biomaterials 2022, 288, 121743. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s Frontier in Combatting Infectious and Inflammatory Diseases: Prevention and Treatment. Signal. Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, J.; Cai, X.; Liou, Y.; Shen, H.; Hao, J.; Huang, C.; Luo, G.; He, W. Macrophage Plasticity: Signaling Pathways, Tissue Repair, and Regeneration. MedComm 2024, 5, e658. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhang, X.; Chen, R.; Wei, J.; Hou, J.; Wang, B.; Lai, H.; Huang, Y. Remodeling Immune Microenvironment in Periodontitis Using Resveratrol Liposomes as an Antibiotic-Free Therapeutic Strategy. J. Nanobiotechnol. 2021, 19, 429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Wan, Y.; Qi, M.; Chen, Q.; Sun, Y.; Sun, X.; Fang, J.; Fu, L.; Xu, L.; et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small 2021, 17, 2101505. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Nanoparticle-Mediated Drug Delivery Systems For The Treatment Of IBD: Current Perspectives. Int. J. Nanomed. 2019, 14, 8875–8889. [Google Scholar] [CrossRef]
- Huang, N.; Dong, H.; Luo, Y.; Shao, B. Th17 Cells in Periodontitis and Its Regulation by A20. Front. Immunol. 2021, 12, 742925. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Fu, H.; Kuang, S.; He, F.; Zhang, M.; Shen, Z.; Qin, W.; Lin, Z.; Huang, S. Exosomes Derived from 3D-Cultured MSCs Improve Therapeutic Effects in Periodontitis and Experimental Colitis and Restore the Th17 Cell/Treg Balance in Inflamed Periodontium. Int. J. Oral Sci. 2021, 13, 43. [Google Scholar] [CrossRef]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal MicroRNA-155-5p from PDLSCs Regulated Th17/Treg Balance by Targeting Sirtuin-1 in Chronic Periodontitis. J. Cell. Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Li, X.; Luo, W.; Ng, T.W.; Leung, P.C.; Zhang, C.; Leung, K.C.-F.; Jin, L. Nanoparticle-Encapsulated Baicalein Markedly Modulates pro-Inflammatory Response in Gingival Epithelial Cells. Nanoscale 2017, 9, 12897–12907. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Sun, Q.; Zhou, C.; Hu, S.; Lenahan, C.; Xu, W.; Deng, Y.; Li, G.; Tao, S. Update on Nanoparticle-Based Drug Delivery System for Anti-Inflammatory Treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.T.; Toledano, R.; Huang, H.; Toledano-Osorio, M.; Osorio, R.; Huang, C.-Y.C.; García-Godoy, F. Effect of Doxycycline Doped Nanoparticles on Osteogenic/Cementogenic and Anti-Inflammatory Responses of Human Cells Derived from the Periodontal Ligament. J. Dent. 2023, 137, 104668. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas de Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of Nanomaterial Applications in Oral and Maxillofacial Tissue Regeneration and Disease Treatment. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1669. [Google Scholar] [CrossRef]
- Vyas, S.P.; Sihorkar, V.; Mishra, V. Controlled and Targeted Drug Delivery Strategies towards Intraperiodontal Pocket Diseases. J. Clin. Pharm. Ther. 2000, 25, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Kozlova, D.; Ganesan, K.; Biewald, C.; Seipold, N.; Gaengler, P.; Arnold, W.H.; Epple, M. Chlorhexidine-Loaded Calcium Phosphatenanoparticles for Dental Maintenance Treatment: Combination of Mineralising and Antibacterial Effects. RSC Adv. 2012, 2, 870–875. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Lee, B.-S.; Lee, C.-C.; Wang, Y.-P.; Chen, H.-J.; Lai, C.-H.; Hsieh, W.-L. Controlled-Release of Tetracycline and Lovastatin by Poly(D,L-Lactide-Co-Glycolide Acid)-Chitosan Nanoparticles Enhances Periodontal Regeneration in Dogs. Int. J. Nanomed. 2016, 11, 285–297. [Google Scholar] [CrossRef]
- Chang, P.-C.; Tai, W.-C.; Luo, H.-T.; Lai, C.-H.; Lin, H.-H.; Lin, Z.-J.; Chang, Y.-C.; Lee, B.-S. Core-Shell Poly-(D,l-Lactide-Co-Glycolide)-Chitosan Nanospheres with Simvastatin-Doxycycline for Periodontal and Osseous Repair. Int. J. Biol. Macromol. 2020, 158, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhuo, J.; Xiao, L.; Xu, Y.; Yang, X.; Li, Y.; Du, Z.; Luo, K. Nanosilicate-Functionalized Polycaprolactone Orchestrates Osteogenesis and Osteoblast-Induced Multicellular Interactions for Potential Endogenous Vascularized Bone Regeneration. Macromol. Biosci. 2022, 22, 2100265. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in Dentistry: A Comprehensive Review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Dehghani, F.; Abdolahinia, E.D.; Sharifi, S.; Ahmadian, E.; Gajdács, M.; Kárpáti, K.; Dizaj, S.M.; Eftekhari, A.; Kavetskyy, T. Effect of Gelatinous Spongy Scaffold Containing Nano-Hydroxyapatite on the Induction of Odontogenic Activity of Dental Pulp Stem Cells. J. King Saud Univ.-Sci. 2022, 34, 102340. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and Characterization of Nano-Hydroxyapatite Powder Using Wet Chemical Precipitation Reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, X.; Zhang, Z.; Han, Y.; Luo, J.; Huang, M.; Zhang, B.; Hou, Y. Large-Scale and Fast Synthesis of Nano-Hydroxyapatite Powder by a Microwave-Hydrothermal Method. RSC Adv. 2019, 9, 13623–13630. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.F.P.; de Melo Soares, M.S.; Silveira e Souza, A.M.M.; Taba Jr, M.; Palioto, D.B.; Messora, M.R.; Ghiraldini, B.; Nunes, F.A.d.S.; de Souza, S.L.S. Influence of Nano-hydroxyapatite Coating Implants on Gene Expression of Osteogenic Markers and micro-CT Parameters. An in Vivo Study in Diabetic Rats. J. Biomed. Mater. Res. A 2021, 109, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Yamada, M.; Ueno, T.; Tsukimura, N.; Ikeda, T.; Nakagawa, K.; Hori, N.; Suzuki, T. Bone Integration Capability of Nanopolymorphic Crystalline Hydroxyapatite Coated on Titanium Implants. Int. J. Nanomedi. 2012, 7, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A Strong, Tough, and Osteoconductive Hydroxyapatite Mineralized Polyacrylamide/Dextran Hydrogel for Bone Tissue Regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef]
- Singh, V.; Nayak, D.; Uppoor, A.; Shah, D. Clinical and Radiographic Evaluation of Nano-Crystalline Hydroxyapatite Bone Graft (Sybograf ®) in Combination with Bioresorbable Collagen Membrane (Periocol ®) in Periodontal Intrabony Defects. Dent. Res. J. 2012, 9, 60. [Google Scholar] [CrossRef]
- Kasaj, A.; Willershausen, B.; Reichert, C.; Röhrig, B.; Smeets, R.; Schmidt, M. Ability of Nanocrystalline Hydroxyapatite Paste to Promote Human Periodontal Ligament Cell Proliferation. J. Oral Sci. 2008, 50, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured Polymer Scaffolds for Tissue Engineering and Regenerative Medicine. WIREs Nanomed. Nanobiotechnol. 2009, 1, 226–236. [Google Scholar] [CrossRef]
- Gupte, M.J.; Ma, P.X. Nanofibrous Scaffolds for Dental and Craniofacial Applications. J. Dent. Res. 2012, 91, 227–234. [Google Scholar] [CrossRef]
- Omidian, H.; Gill, E.J. Nanofibrous Scaffolds in Biomedicine. J. Compos. Sci. 2024, 8, 269. [Google Scholar] [CrossRef]
- Manea, L.R.; Popa, A.; Bertea, A. Technological Progress in Manufacturing Electrospun Nanofibers for Medical Applications. Key Eng. Mater. 2017, 752, 126–131. [Google Scholar] [CrossRef]
- Ilyas, R.; Zuhri, M.; Norrrahim, M.; Misenan, M.; Jenol, M.; Samsudin, S.; Nurazzi, N.; Asyraf, M.; Supian, A.; Bangar, S.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Ma, Y.; Song, J.; Almassri, H.N.S.; Zhang, D.; Zhang, T.; Cheng, Y.; Wu, X. Minocycline-Loaded PLGA Electrospun Membrane Prevents Alveolar Bone Loss in Experimental Peridontitis. Drug Deliv. 2020, 27, 151–160. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Sun, H.; Yang, J.; Chen, Y.; Li, C.; Wang, H.; Xing, T.; Zhang, F.; Gu, N. Biomimetic Domain-Active Electrospun Scaffolds Facilitating Bone Regeneration Synergistically with Antibacterial Efficacy for Bone Defects. ACS Appl. Mater. Interfaces 2018, 10, 3248–3259. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Z.; Xiao, L.; Xu, Y.; Xiao, N.; Jin, W.; Chen, Y.; Li, Y.; Luo, K. Nanosilicate-Functionalized Nanofibrous Membrane Facilitated Periodontal Regeneration Potential by Harnessing Periodontal Ligament Cell-Mediated Osteogenesis and Immunomodulation. J. Nanobiotechnol. 2023, 21, 223. [Google Scholar] [CrossRef] [PubMed]
- Kuchler-Bopp, S.; Larrea, A.; Petry, L.; Idoux-Gillet, Y.; Sebastian, V.; Ferrandon, A.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Promoting Bioengineered Tooth Innervation Using Nanostructured and Hybrid Scaffolds. Acta Biomater. 2017, 50, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, G.; Wang, T.; Jin, Y.; Lu, W.; Ji, J. Human Periodontal Ligament Stem Cells Transplanted with Nanohydroxyapatite/Chitosan/Gelatin 3D Porous Scaffolds Promote Jaw Bone Regeneration in Swine. Stem. Cells Dev. 2021, 30, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.M.T.; de Oliveira, E.M.N.; Pereira, T.C.B.; Papaléo, R.M.; Bogo, M.R. Implications of Exposure to Dextran-Coated and Uncoated Iron Oxide Nanoparticles to Developmental Toxicity in Zebrafish. J. Nanopart. Res. 2017, 19, 389. [Google Scholar] [CrossRef]
- Summer, M.; Ashraf, R.; Ali, S.; Bach, H.; Noor, S.; Noor, Q.; Riaz, S.; Khan, R.R.M. Inflammatory Response of Nanoparticles: Mechanisms, Consequences, and Strategies for Mitigation. Chemosphere 2024, 363, 142826. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Xiao, C.; Wang, H.; Dong, S. Nanoparticles in Periodontitis Therapy: A Review of the Current Situation. Int. J. Nanomed. 2024, 19, 6857–6893. [Google Scholar] [CrossRef]
- Sreenivasalu, P.K.P.; Dora, C.P.; Swami, R.; Jasthi, V.C.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K. Nanomaterials in Dentistry: Current Applications and Future Scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qi, Z.; Zou, Y.; Zhang, J.; Xia, W.; Zhang, R.; He, Z.; Cai, X.; Lin, Y.; Duan, S.-Z.; et al. Engineering DNA–Nanozyme Interfaces for Rapid Detection of Dental Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 30640–30647. [Google Scholar] [CrossRef]
- Patel, B.; Kamineni, Y.; Gorityala, S.; Bindu, M.H. Advances in Nanocarriers for Drug Delivery in Dental Therapies. Recent Prog. Mater. 2021, 3, 39. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, E.; Aceto, G.M.; Petrini, M.; Cinquini, C.; D’Ercole, S.; Iezzi, G.; Pierfelice, T.V. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? Int. J. Mol. Sci. 2025, 26, 592. https://doi.org/10.3390/ijms26020592
D’Amico E, Aceto GM, Petrini M, Cinquini C, D’Ercole S, Iezzi G, Pierfelice TV. How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? International Journal of Molecular Sciences. 2025; 26(2):592. https://doi.org/10.3390/ijms26020592
Chicago/Turabian StyleD’Amico, Emira, Gitana Maria Aceto, Morena Petrini, Chiara Cinquini, Simonetta D’Ercole, Giovanna Iezzi, and Tania Vanessa Pierfelice. 2025. "How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy?" International Journal of Molecular Sciences 26, no. 2: 592. https://doi.org/10.3390/ijms26020592
APA StyleD’Amico, E., Aceto, G. M., Petrini, M., Cinquini, C., D’Ercole, S., Iezzi, G., & Pierfelice, T. V. (2025). How Will Nanomedicine Revolutionize Future Dentistry and Periodontal Therapy? International Journal of Molecular Sciences, 26(2), 592. https://doi.org/10.3390/ijms26020592










