The Modulatory Effect of Selol (Se IV) on Pro-Inflammatory Pathways in RAW 264.7 Macrophages
Abstract
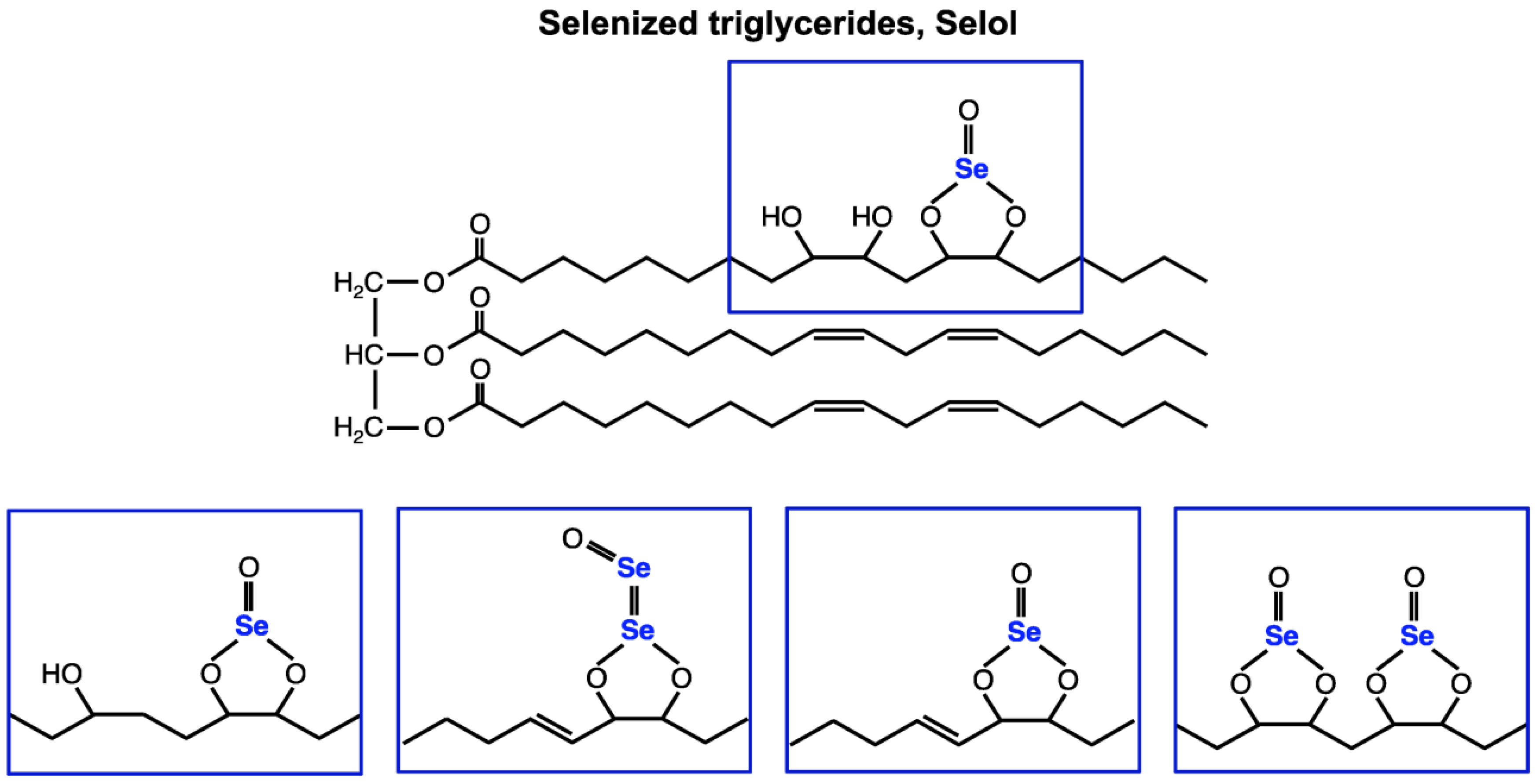
1. Introduction
2. Results and Discussion
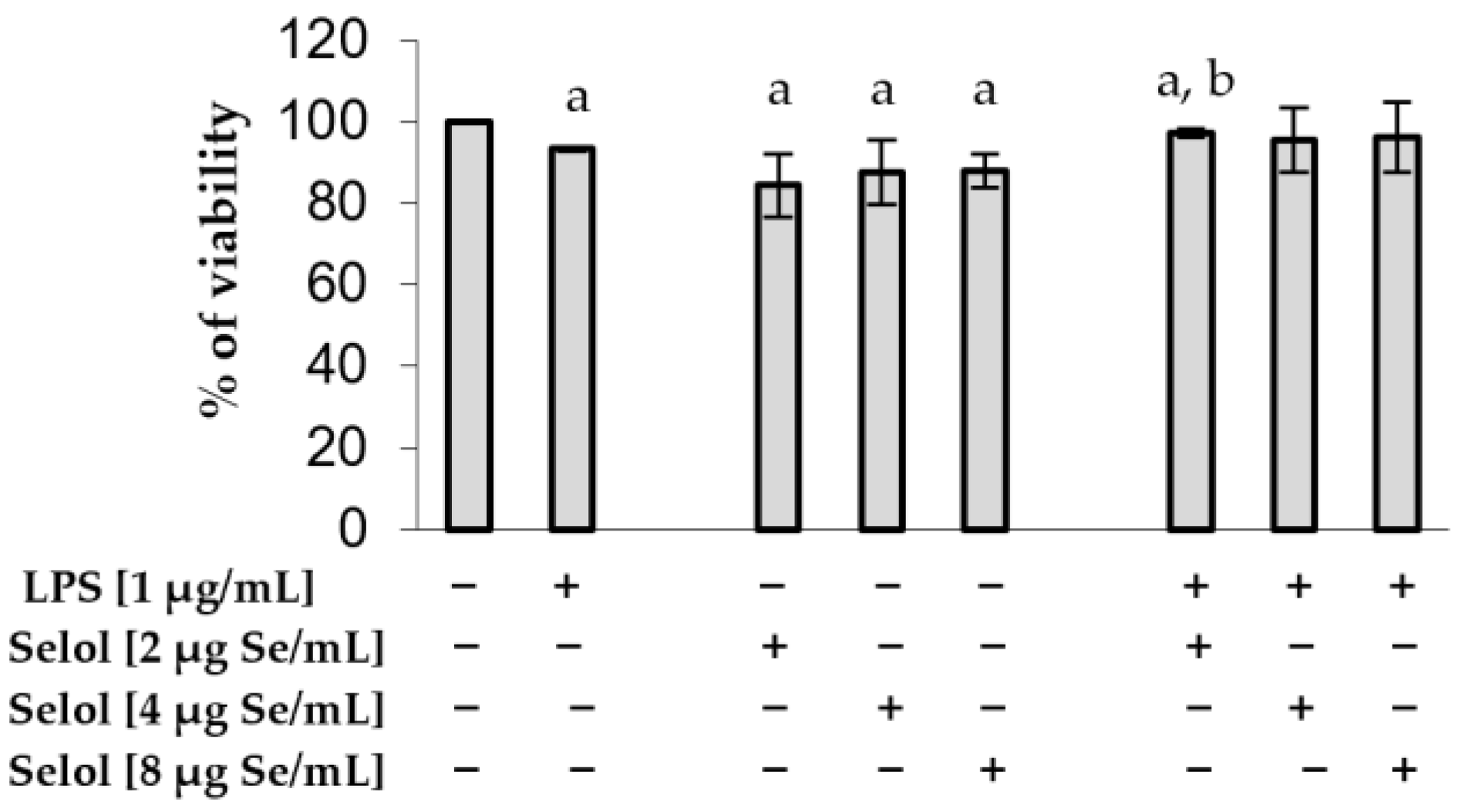
2.1. Effect of Selol on RAW 264.7 Viability
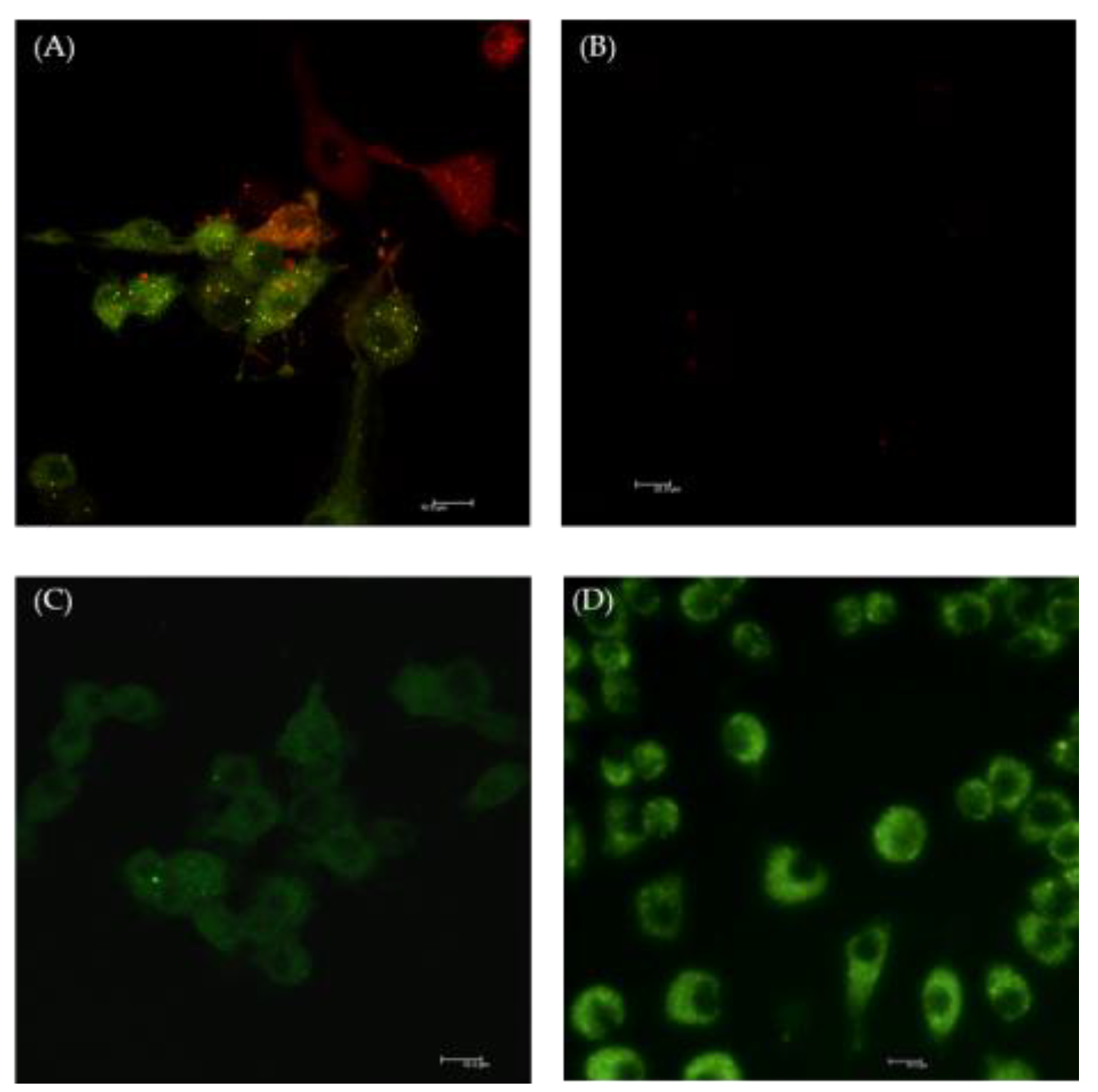
2.2. The Effect of Selol on NF-κB Activation
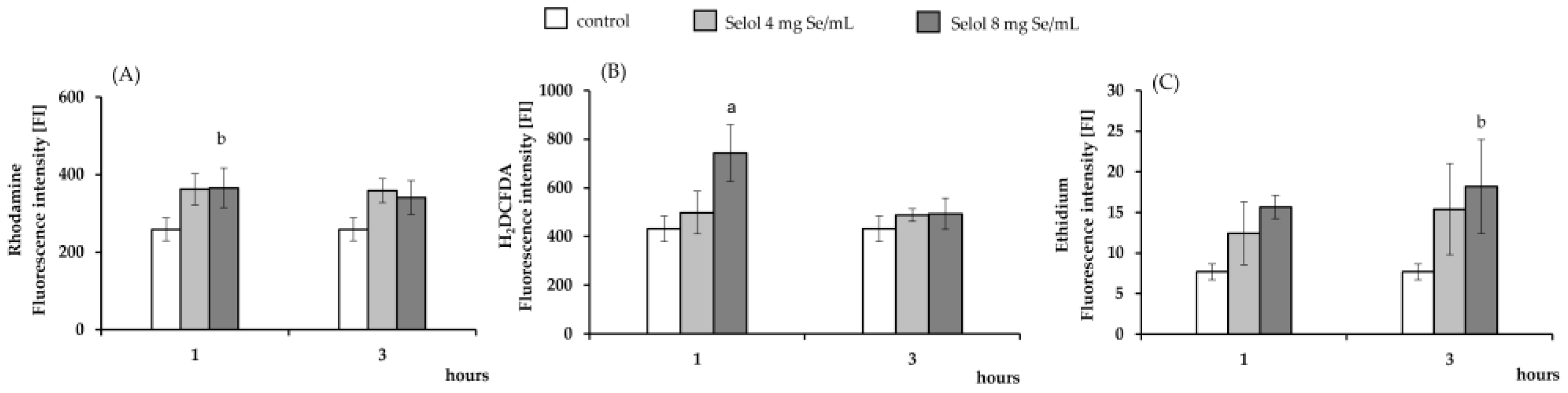
2.3. The Effect of Selol on ROS Production
2.4. The Effect of Selol on Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Concentration and Thioredoxin (Txn) Level
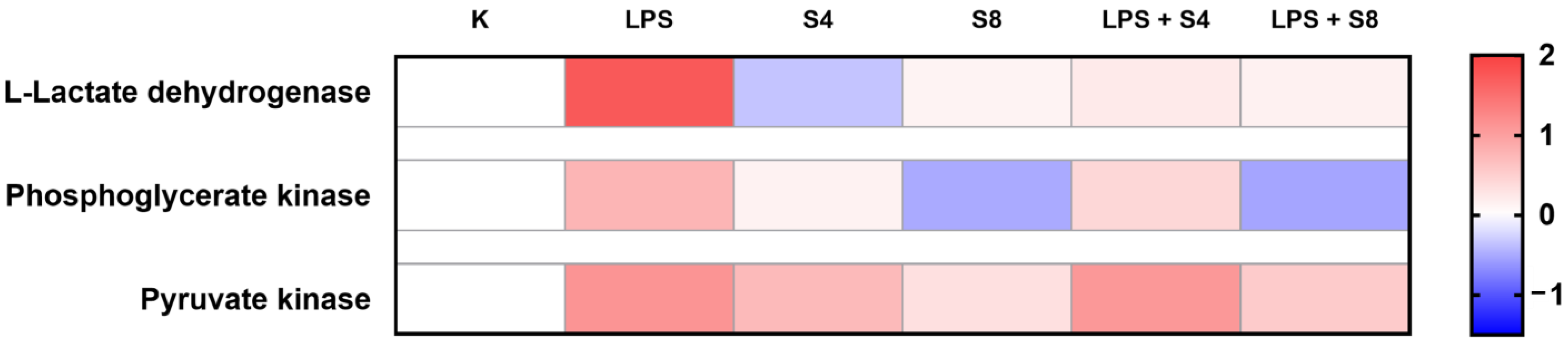
2.5. The Effect of Selol on Cellular Energy Metabolism
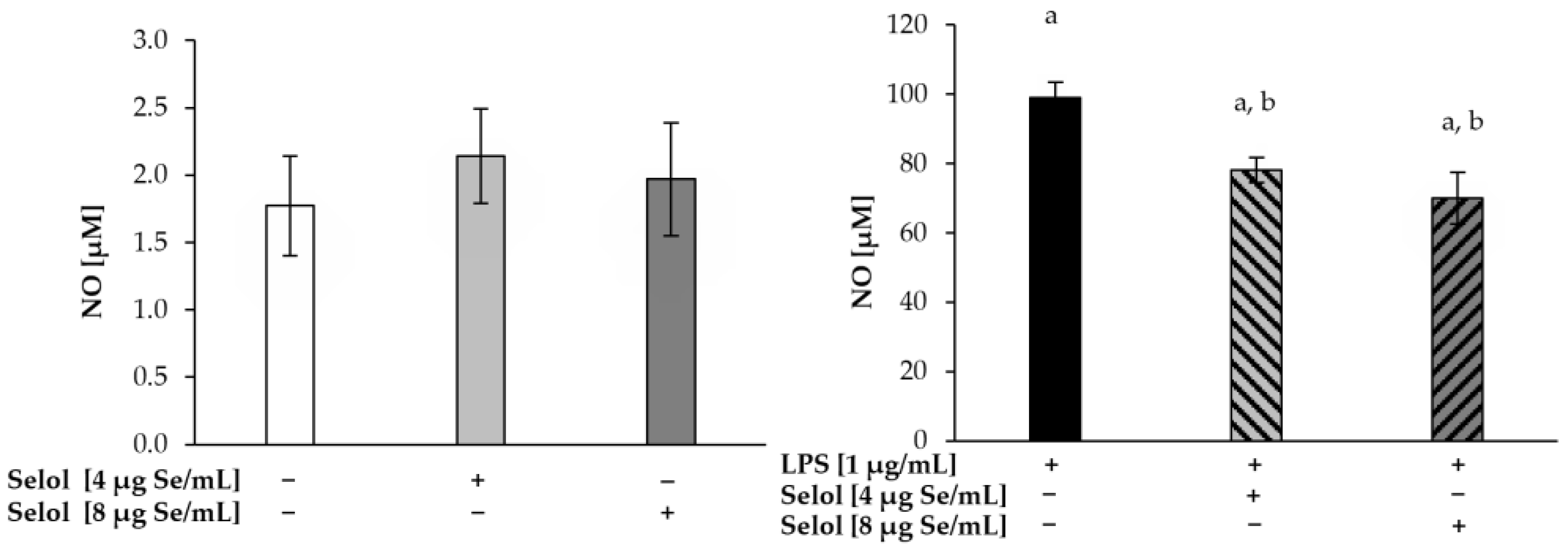
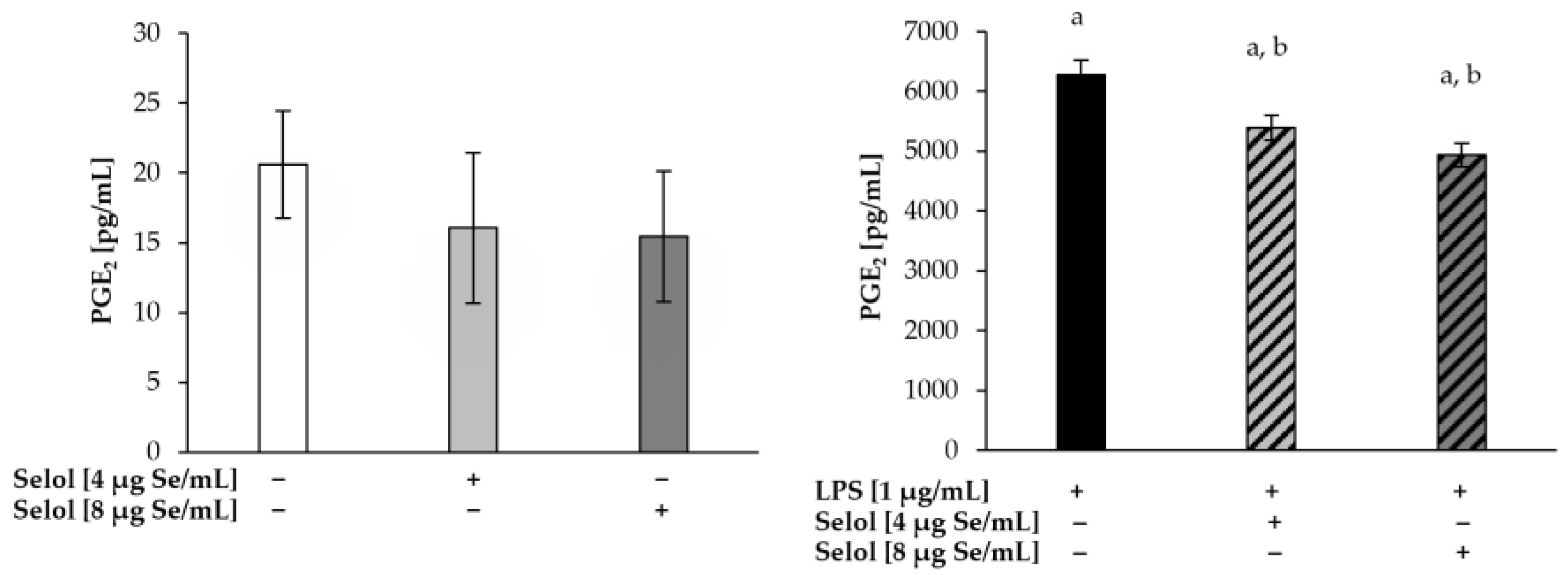
2.6. Effect of Selol on NO/PGE2 Produciton
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Cell Viability
3.4. ROS Detection by Confocal Microscopy
3.5. ROS Detection: DHR 123, DCFH-DA, and HE Assay
3.6. Colorimetric Determination of Total (GSHt) and Oxidized (GSSG) Glutathione and GSH/GSSG Ratio in RAW 264.7 Cells After Selol Treatment
3.7. Analysis of NF-κB Induction with Laser Scanning Confocal Microscope (LSCM)
3.8. LC-MS Proteome Analysis
3.9. NO and PGE2 Production
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontol. 2000 2013, 63, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Huang, W.; Glass, C.K. Nuclear receptors and inflammation control: Molecular mechanisms and pathophysiological relevance. Arter. Thromb. Vasc. Biol. 2010, 30, 1542–1549. [Google Scholar] [CrossRef]
- Ahmed, A. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Chojnacka, K.; Lewandowska, U. The influence of polyphenol-rich extracts on the production of pro-inflammatory mediators in macrophages. J. Physiol. Pharmacol. 2021, 72, 167–176. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007, 282, 17964–17973. [Google Scholar] [CrossRef]
- Ko, W.; Liu, Z.; Kim, K.W.; Dong, L.; Lee, H.; Kim, N.Y.; Lee, D.S.; Woo, E.R. Kuwanon T and Sanggenon a Isolated from Morus alba Exert Anti-Inflammatory Effects by Regulating NF-kappaB and HO-1/Nrf2 Signaling Pathways in BV2 and RAW264.7 Cells. Molecules 2021, 26, 7642. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Yu, Y.; Lu, X.; Yang, Y.; Liu, Z.; Wang, D. Preventive effects of matrine on LPS-induced inflammation in RAW 264.7 cells and intestinal damage in mice through the TLR4/NF-kappaB/MAPK pathway. Int. Immunopharmacol. 2024, 143, 113432. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.B.; Silva, R.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two Sides of the Same Coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Gurgul, I.; Oszajca, M.; Polaczek, J.; Kieca, K.; Bieszczad-Zak, E.; Martyka, T.; Stochel, G. Nitric Oxide Signaling and Sensing in Age-Related Diseases. Antioxidants 2024, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fu, S.; Yang, R.; Yang, K.; Lei, W.; Yang, Y.; Zhang, Q.; Zhao, Y.; Yu, J.; Yu, L.; et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J. Inflamm. 2023, 20, 33. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021, 9, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef]
- Korwar, A.M.; Hossain, A.; Lee, T.J.; Shay, A.E.; Basrur, V.; Conlon, K.; Smith, P.B.; Carlson, B.A.; Salis, H.M.; Patterson, A.D.; et al. Selenium-dependent metabolic reprogramming during inflammation and resolution. J. Biol. Chem. 2021, 296, 100410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Djalalinia, S.; Hasani, M.; Asayesh, H.; Ejtahed, H.S.; Malmir, H.; Kasaeian, A.; Zarei, M.; Baygi, F.; Rastad, H.; Mahdavi Gorabi, A.; et al. The effects of dietary selenium supplementation on inflammatory markers among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2021, 20, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Khoso, P.A.; Zhang, Y.; Yin, H.; Teng, X.; Li, S. Selenium Deficiency Affects Immune Function by Influencing Selenoprotein and Cytokine Expression in Chicken Spleen. Biol. Trace Elem. Res. 2019, 187, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Sonet, J.; Mosca, M.; Bierla, K.; Modzelewska, K.; Flis-Borsuk, A.; Suchocki, P.; Ksiazek, I.; Anuszewska, E.; Bulteau, A.L.; Szpunar, J.; et al. Selenized Plant Oil Is an Efficient Source of Selenium for Selenoprotein Biosynthesis in Human Cell Lines. Nutrients 2019, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Rahden-Staron, I.; Suchocki, P.; Czeczot, H. Evaluation of mutagenic activity of the organo-selenium compound Selol by use of the Salmonella typhimurium mutagenicity assay. Mutat. Res. 2010, 699, 44–46. [Google Scholar] [CrossRef]
- Sochacka, M.; Giebultowicz, J.; Remiszewska, M.; Suchocki, P.; Wroczynski, P. Effects of Selol 5% supplementation on the activity or concentration of antioxidants and malondialdehyde level in the blood of healthy mice. Pharmacol. Rep. 2014, 66, 301–310. [Google Scholar] [CrossRef]
- Flis, A.; Suchocki, P.; Krolikowska, M.A.; Suchocka, Z.; Remiszewska, M.; Sliwka, L.; Ksiazek, I.; Sitarz, K.; Sochacka, M.; Hoser, G.; et al. Selenitetriglycerides—Redox-active agents. Pharmacol. Rep. 2015, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, I.; Sitarz, K.; Roslon, M.; Anuszewska, E.; Suchocki, P.; Wilczynska, J.D. The influence of Selol on the expression of oxidative stress genes in normal and malignant prostate cells. Cancer Genom. Proteom. 2013, 10, 225–232. [Google Scholar]
- de Souza, L.R.; Muehlmann, L.A.; Matos, L.C.; Simon-Vazquez, R.; Lacava, Z.G.; De-Paula, A.M.; Mosiniewicz-Szablewska, E.; Suchocki, P.; Morais, P.C.; Gonzalez-Fernandez, A.; et al. Antitumor activity and systemic effects of PVM/MA-shelled selol nanocapsules in lung adenocarcinoma-bearing mice. Nanotechnology 2015, 26, 505101. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.R.; Muehlmann, L.A.; Dos Santos, M.S.; Ganassin, R.; Simon-Vazquez, R.; Joanitti, G.A.; Mosiniewicz-Szablewska, E.; Suchocki, P.; Morais, P.C.; Gonzalez-Fernandez, A.; et al. PVM/MA-shelled selol nanocapsules promote cell cycle arrest in A549 lung adenocarcinoma cells. J. Nanobiotechnology 2014, 12, 32. [Google Scholar] [CrossRef]
- Suchocki, P.; Misiewicz, I.; Skupinska, K.; Waclawek, K.; Fijalek, Z.; Kasprzycka-Guttman, T. The activity of Selol in multidrug-resistant and sensitive human leukemia cells. Oncol. Rep. 2007, 18, 893–899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dudkiewicz-Wilczynska, J.; Grabowska, A.; Ksiazek, I.; Sitarz, K.; Suchocki, P.; Anuszewska, E. Comparison of selected gene expression profiles in sensitive and resistant cancer cells treated with doxorubicin and Selol. Contemp. Oncol. 2014, 18, 90–94. [Google Scholar] [CrossRef]
- Ganassin, R.; Souza, L.R.; Py-Daniel, K.R.; Longo, J.P.F.; Coelho, J.M.; Rodrigues, M.C.; Jiang, C.S.; Gu, J.; Morais, P.C.; Mosiniewicz-Szablewska, E.; et al. Decoration of a Poly(methyl vinyl ether-co-maleic anhydride)-Shelled Selol Nanocapsule with Folic Acid Increases Its Activity Against Different Cancer Cell Lines In Vitro. J. Nanosci. Nanotechnol. 2018, 18, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Ganassin, R.; Merker, C.; Rodrigues, M.C.; Guimaraes, N.F.; Sodre, C.S.C.; Ferreira, Q.D.S.; da Silva, S.W.; Ombredane, A.S.; Joanitti, G.A.; Py-Daniel, K.R.; et al. Nanocapsules for the co-delivery of selol and doxorubicin to breast adenocarcinoma 4T1 cells in vitro. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Ganassin, R.; Horst, F.H.; Camargo, N.S.; Chaves, S.B.; Morais, P.C.; Mosiniewicz-Szablewska, E.; Suchocki, P.; Figueiro Longo, J.P.; Azevedo, R.B.; Muehlmann, L.A. Selol nanocapsules with a poly(methyl vinyl ether-co-maleic anhydride) shell conjugated to doxorubicin for combinatorial chemotherapy against murine breast adenocarcinoma in vivo. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Sochacka, M.; Giebultowicz, J.; Remiszewska, M.; Suchocki, P.; Wroczynski, P. Effects of Selol 5% supplementation on tissue antioxidant enzyme levels and peroxidation marker in healthy mice. Pharmacol. Rep. 2018, 70, 1073–1078. [Google Scholar] [CrossRef]
- Sochacka, M.; Hoser, G.; Remiszewska, M.; Suchocki, P.; Sikora, K.; Giebułtowicz, J. Effect of Selol on Tumor Morphology and Biochemical Parameters Associated with Oxidative Stress in a Prostate Tumor-Bearing Mice Model. Nutrients 2024, 16, 2860. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, W.O.; Costa do Santos, M.S.; Farias, G.R.; Fascineli, M.L.; Ramos, K.L.V.; Duarte, E.C.B.; Damasceno, E.A.M.; da Silva, J.R.; Joanitti, G.A.; de Azevedo, R.B.; et al. Combination of selol nanocapsules and magnetic hyperthermia hinders breast tumor growth in aged mice after a short-time treatment. Nanotechnology 2022, 33, 205101. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, W.O.; Fascineli, M.L.; Ramos, K.L.V.; de Andrade, L.R.; Farias, G.R.; Neta, M.S.B.; Correa, L.H.; Duarte, E.C.B.; Damasceno, E.A.M.; Magalhaes, K.G.; et al. Synergistic Antitumor Efficacy of Magnetohyperthermia and Poly(lactic-co-glycolic acid)-Encapsulated Selol in Ehrlich Breast Adenocarcinoma Treatment in Elderly Swiss Mice. J. Biomed. Nanotechnol. 2020, 16, 179–192. [Google Scholar] [CrossRef]
- Grosicka-Maciąg, E.; Kurpios-Piec, D.; Woźniak, K.; Kowalewski, C.; Szumiło, M.; Drela, N.; Kiernozek, E.; Suchocki, P.; Rahden-Staroń, I. Selol (Se IV) modulates adhesive molecules in control and TNF-α-stimulated HMEC-1 cells. J. Trace Elem. Med. Biol. 2019, 51, 106–114. [Google Scholar] [CrossRef]
- Dominiak, A.; Wilkaniec, A.; Jesko, H.; Czapski, G.A.; Lenkiewicz, A.M.; Kurek, E.; Wroczynski, P.; Adamczyk, A. Selol, an organic selenium donor, prevents lipopolysaccharide-induced oxidative stress and inflammatory reaction in the rat brain. Neurochem. Int. 2017, 108, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Dudkiewicz-Wilczyńska, J.; Książek, I.; Nowak, K.; Suchocki, P.; Flis, S.; Kiljan, M.; Anuszewska, E. Badania wpływu Selolu i seleninu sodu na komórki HeLa in vitro. CHEMIK 2011, 65, 105–114. [Google Scholar]
- Meplan, C.; Johnson, I.T.; Polley, A.C.; Cockell, S.; Bradburn, D.M.; Commane, D.M.; Arasaradnam, R.P.; Mulholland, F.; Zupanic, A.; Mathers, J.C.; et al. Transcriptomics and proteomics show that selenium affects inflammation, cytoskeleton, and cancer pathways in human rectal biopsies. FASEB J. 2016, 30, 2812–2825. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Bjornstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Alkan, Z. Regulation of redox signaling by selenoproteins. Biol. Trace Elem. Res. 2010, 134, 235–251. [Google Scholar] [CrossRef]
- Sonet, J.; Bulteau, A.L.; Touat-Hamici, Z.; Mosca, M.; Bierla, K.; Mounicou, S.; Lobinski, R.; Chavatte, L. Selenoproteome Expression Studied by Non-Radioactive Isotopic Selenium-Labeling in Human Cell Lines. Int. J. Mol. Sci. 2021, 22, 7308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhang, Y.; Dong, P.Y.; Chen Yan, Y.M.; Liu, J.; Zhang, B.Q.; Chen, M.M.; Zhang, S.E.; Zhang, X.F. A comprehensive review on potential role of selenium, selenoproteins and selenium nanoparticles in male fertility. Heliyon 2024, 10, e34975. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Filippini, T.; Vinceti, M. Selenium status and immunity. Proc. Nutr. Soc. 2023, 82, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic. Free Radic. Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hamid, S.; Liu, Q.; Cai, J.; Xu, S.; Zhang, Z. Gene expression of selenoproteins can be regulated by thioredoxin(Txn) silence in chicken cardiomyocytes. J. Inorg. Biochem. 2017, 177, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Thut, H.; Feng, Q.; Kopf, M. Thioredoxin-1 distinctly promotes NF-kappaB target DNA binding and NLRP3 inflammasome activation independently of Txnip. Elife 2020, 9, e53627. [Google Scholar] [CrossRef] [PubMed]
- Isakov, E.; Weisman-Shomer, P.; Benhar, M. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim. Biophys. Acta 2014, 1840, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Holmgren, A. Thioredoxin and related molecules—From biology to health and disease. Antioxid. Redox Signal. 2007, 9, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kwon, O.K.; Kim, J.H.; Oh, S.R.; Kim, J.H.; Paik, J.H.; Marwoto, B.; Widjhati, R.; Juniarti, F.; Irawan, D.; et al. Rhododendron album Blume inhibits iNOS and COX-2 expression in LPS-stimulated RAW264.7 cells through the downregulation of NF-kappaB signaling. Int. J. Mol. Med. 2015, 35, 987–994. [Google Scholar] [CrossRef]
- Diwakar, B.T.; Korwar, A.M.; Paulson, R.F.; Prabhu, K.S. The Regulation of Pathways of Inflammation and Resolution in Immune Cells and Cancer Stem Cells by Selenium. Adv. Cancer Res. 2017, 136, 153–172. [Google Scholar]
- Zamamiri-Davis, F.; Lu, Y.; Thompson, J.T.; Prabhu, K.S.; Reddy, P.V.; Sordillo, L.M.; Reddy, C.C. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic. Biol. Med. 2002, 32, 890–897. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Kaushal, N.; Ravindra, K.C.; Hegde, S.; Nelson, S.M.; Narayan, V.; Vunta, H.; Paulson, R.F.; Prabhu, K.S. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J. Biol. Chem. 2011, 286, 27471–27482. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: Role of nuclear factor-kappaB in up-regulation. Biochem. J. 2002, 366, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, K.; Zhang, C.; Talukder, M.; Lv, M.W.; Li, J.Y.; Li, J.L. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci. Total Environ. 2021, 773, 145442. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Indiano-Romacho, P.; Mora-Gutiérrez, I.; Pérez-Rodríguez, L.; Ortega Moreno, L.; Marin, A.C.; Baldán-Martín, M.; Moreno-Monteagudo, J.A.; Santander, C.; Chaparro, M.; et al. Lunasin Peptide is a Modulator of the Immune Response in the Human Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, 2001034. [Google Scholar] [CrossRef]
- Fitak, B.; Grabowski, M.; Suchocki, P. Preparat Przeciwnowotworowy i Sposób Jego Wytwarzania. Polish Patent 1765301994, 6 January 1999. [Google Scholar]
- Bierla, K.; Flis-Borsuk, A.; Suchocki, P.; Szpunar, J.; Lobinski, R. Speciation of Selenium in Selenium-Enriched Sunflower Oil by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry/Electrospray-Orbitrap Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 4975–4981. [Google Scholar] [CrossRef] [PubMed]
- Suchocki, P.; Jakoniuk, D.; Fitak, B.A. Specific spectrophotometric method with trifluoroacetic acid for the determination of selenium(IV) in selenitetriglycerides. J. Pharm. Biomed. Anal. 2003, 32, 1029–1036. [Google Scholar] [CrossRef]
- Kurpios-Piec, D.; Woźniak, K.; Kowalewski, C.; Gajewska, B.; Rahden-Staroń, I. Thiram modulates pro-inflammatory mediators in RAW 264.7 murine macrophage cells. Immunopharmacol. Immunotoxicol. 2015, 37, 90–102. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Jensen, P.E.; Møller, I.M.; Schulz, A. Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol. Plant. 2009, 136, 369–383. [Google Scholar] [CrossRef]
- Xu, J.; Hao, Z.; Gou, X.; Tian, W.; Jin, Y.; Cui, S.; Guo, J.; Sun, Y.; Wang, Y.; Xu, Z. Imaging of reactive oxygen species burst from mitochondria using laser scanning confocal microscopy. Microsc. Res. Technol. 2013, 76, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

| GSHt [nmoL/106 Cells] | GSSG [nmoL/106 Cells] | GSH/GSSG | Thioredoxin [Protein Intensity, %] | |
|---|---|---|---|---|
| Control | 10.48 ± 1.49 | 0.13 ± 0.0071 | 81.52 ± 2.44 | 100 |
| S4 | 10.49 ± 0.70 | 0.13 ± 0.0063 | 75.72 ± 2.83 | 177.36 |
| S8 | 10.75 ± 0.99 | 0.12 ± 0.0059 | 84.56 ± 4.99 | 214.55 |
| LPS | 2.06 ± 0.48 a | 0.18 ± 0.0084 b | 9.36 ± 0.25 a | 235.97 |
| LPS + S4 | 4.73 ± 0.64 b, c# | 0.14 ± 0.0076 c# | 30.78 ± 1.47 a, b# | 82.17 |
| LPS + S8 | 5.47 ± 0.52 b, c# | 0.14 ± 0.01 c# | 37.50 ± 1.21 a, a# | 154.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, G.Y.; Grosicka-Maciąg, E.; Szumiło, M.; Graska, D.; Rahden-Staroń, I.; Kurpios-Piec, D. The Modulatory Effect of Selol (Se IV) on Pro-Inflammatory Pathways in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2025, 26, 559. https://doi.org/10.3390/ijms26020559
Lim GY, Grosicka-Maciąg E, Szumiło M, Graska D, Rahden-Staroń I, Kurpios-Piec D. The Modulatory Effect of Selol (Se IV) on Pro-Inflammatory Pathways in RAW 264.7 Macrophages. International Journal of Molecular Sciences. 2025; 26(2):559. https://doi.org/10.3390/ijms26020559
Chicago/Turabian StyleLim, Gwan Yong, Emilia Grosicka-Maciąg, Maria Szumiło, Daniel Graska, Iwonna Rahden-Staroń, and Dagmara Kurpios-Piec. 2025. "The Modulatory Effect of Selol (Se IV) on Pro-Inflammatory Pathways in RAW 264.7 Macrophages" International Journal of Molecular Sciences 26, no. 2: 559. https://doi.org/10.3390/ijms26020559
APA StyleLim, G. Y., Grosicka-Maciąg, E., Szumiło, M., Graska, D., Rahden-Staroń, I., & Kurpios-Piec, D. (2025). The Modulatory Effect of Selol (Se IV) on Pro-Inflammatory Pathways in RAW 264.7 Macrophages. International Journal of Molecular Sciences, 26(2), 559. https://doi.org/10.3390/ijms26020559






