Enhancing Industrial Hemp (Cannabis sativa) Leaf By-Products: Bioactive Compounds, Anti-Inflammatory Properties, and Potential Health Applications
Abstract
1. Introduction
2. Results
2.1. Chemical Composition and Antioxidant Capacity of C. sativa Leaves Extract
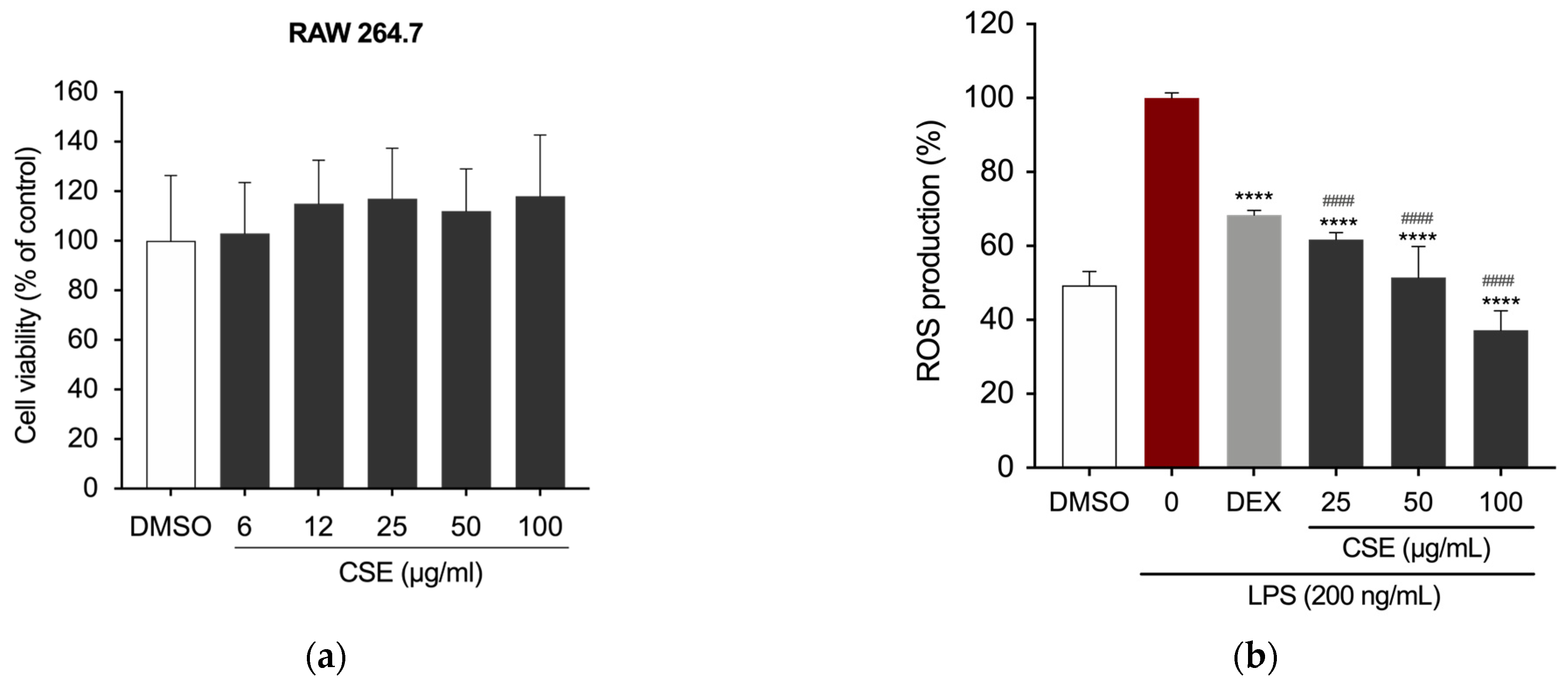
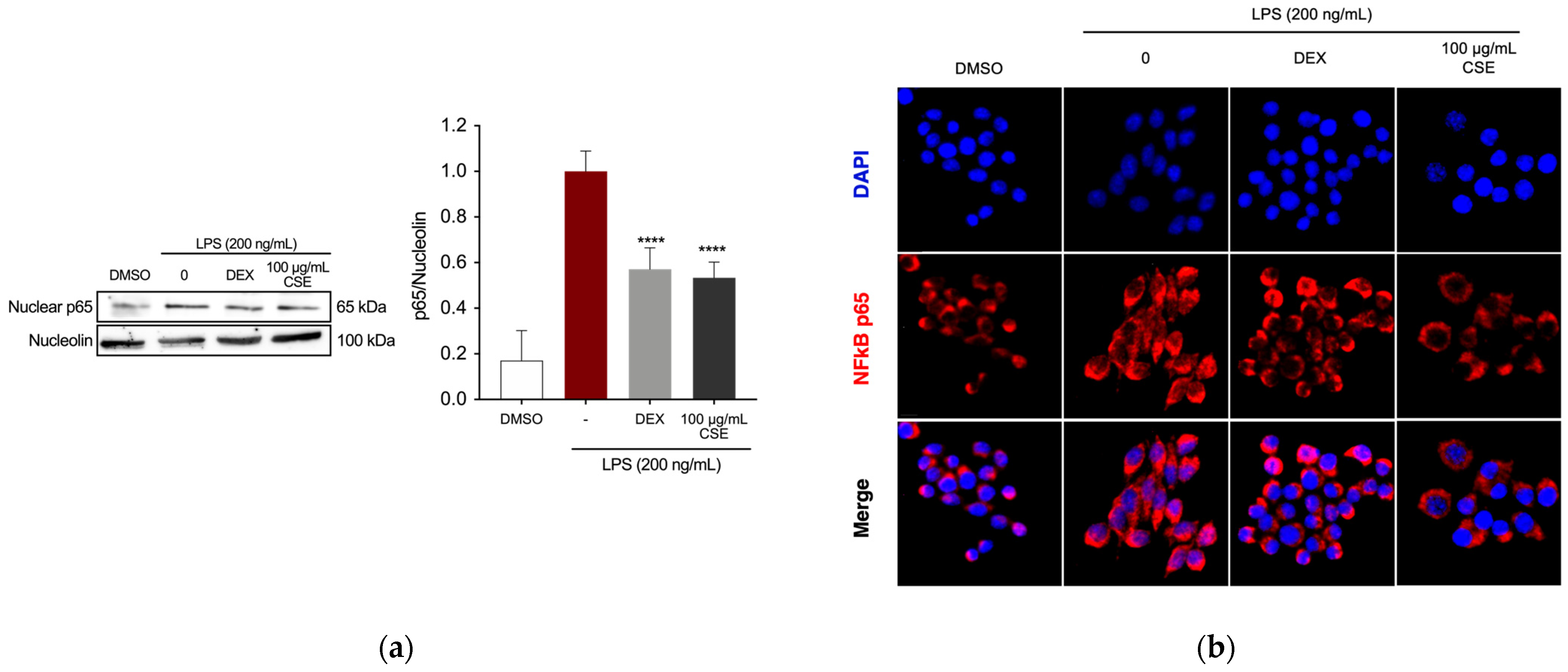
2.2. CSE Reduced LPS-Induced Inflammation in RAW 264.7 Cells
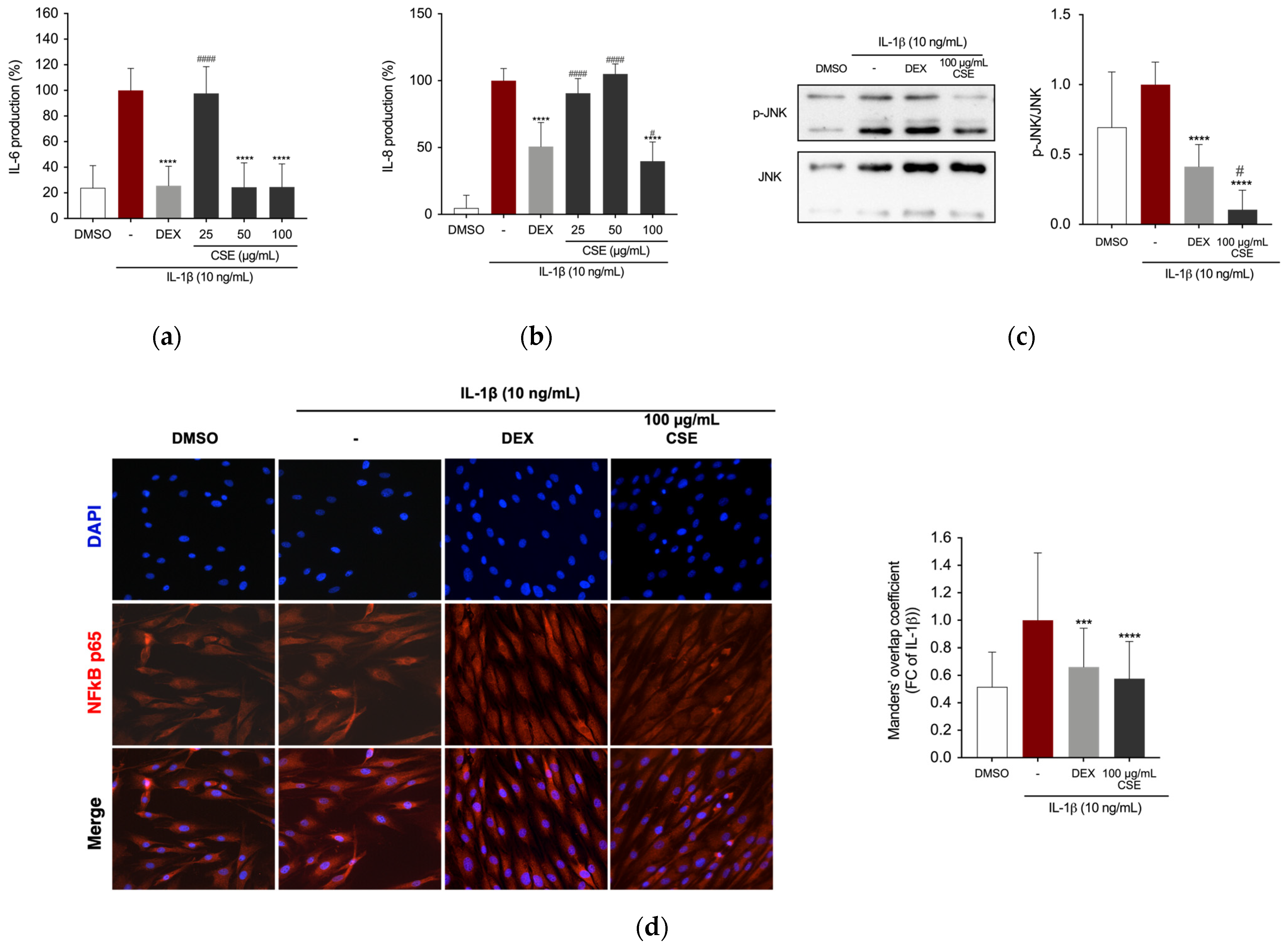
2.3. CSE Reduced IL-1β-Stimulated Production of Inflammatory Mediators in Human Chondrocytes
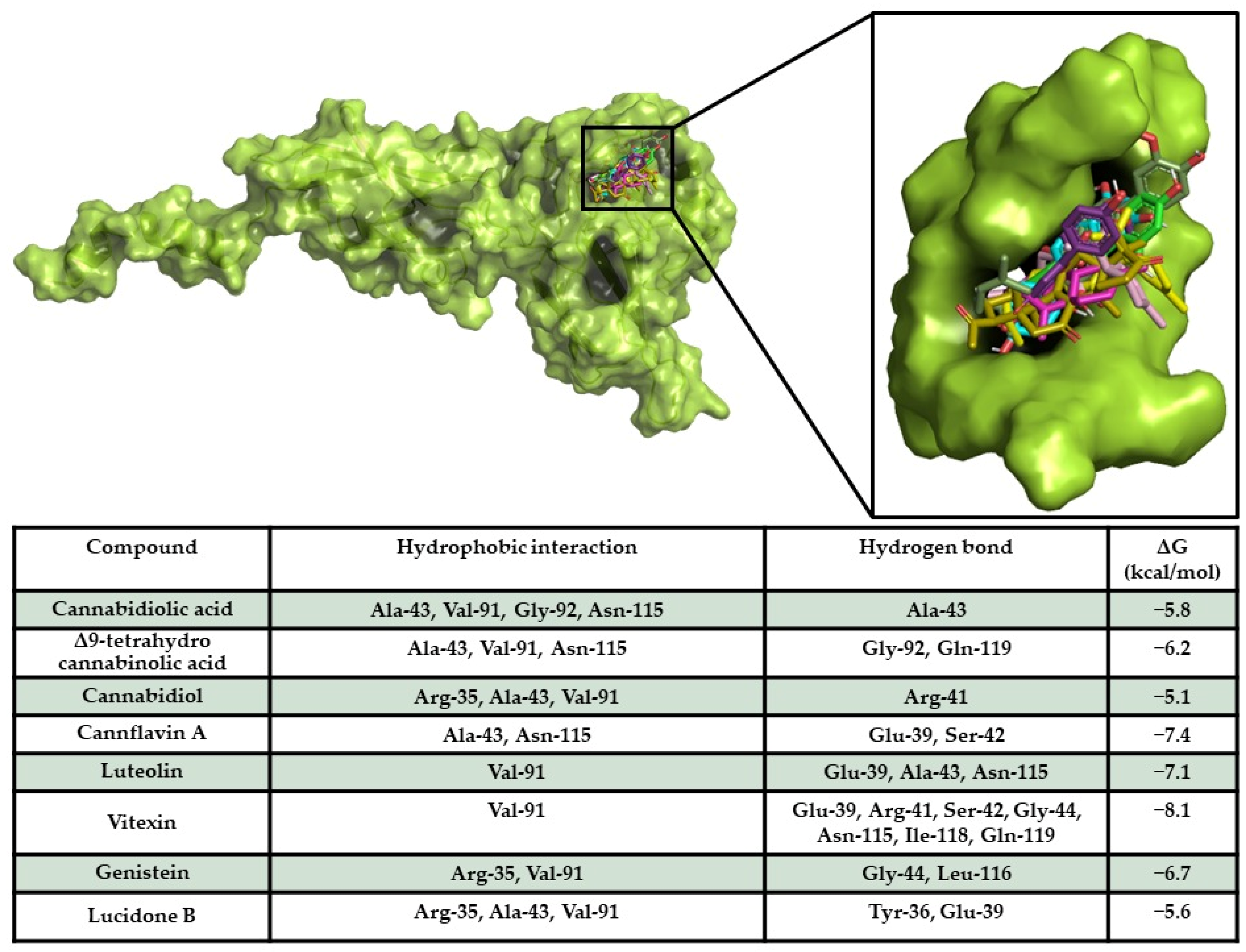
2.4. In Silico Results
Target/Compound Interaction
2.5. A HA-Based CSE Formulation Reduced LPS-Induced Inflammation in RAW 264.7 Cells
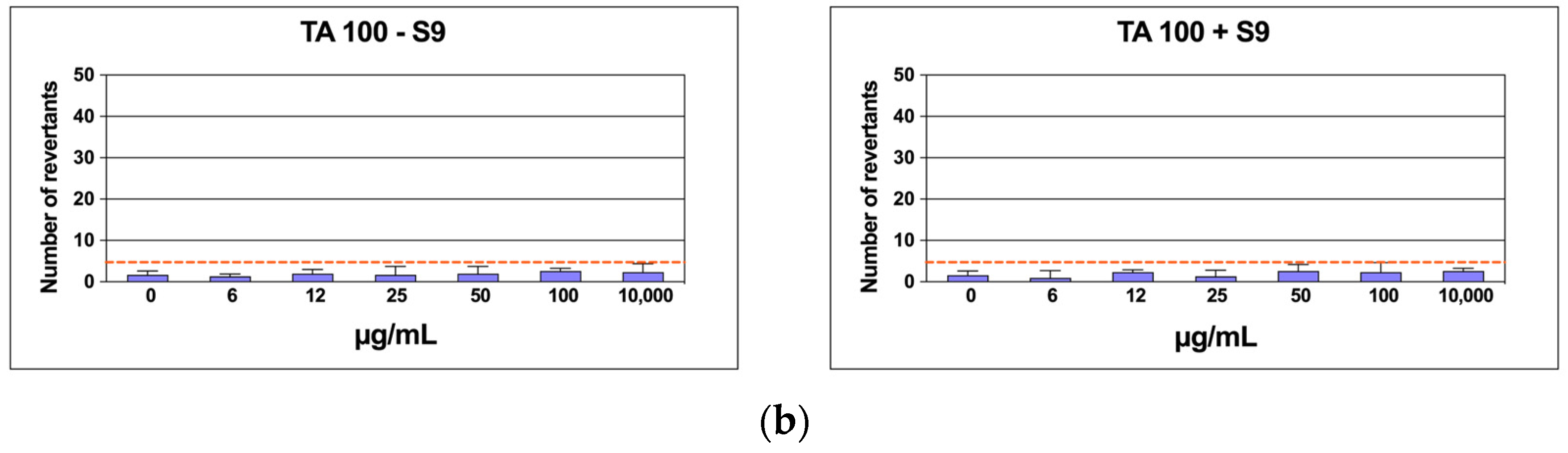
2.6. Mutagenicity Assay: Ames Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of C. sativa Hydroethanolic (CSE) Extract
4.3. Total Phenolic Content (TPC)
4.4. Total Flavonoid Content (TFC)
4.5. Determination of Reducing Power
4.6. ABTS•+ Free-Radical Scavenging Activity
4.7. DPPH Free-Radical Scavenging Activity
4.8. UPLC-MS/MS
4.9. Cell Cultures
4.9.1. Cell Viability
4.9.2. Cell Stimulation
4.9.3. Quantification of Intracellular ROS Formation
4.9.4. Determination of NO Production
4.9.5. Enzyme-Linked Immunosorbent (ELISA) Assay
4.9.6. Protein Extraction
4.9.7. Western Blotting
4.9.8. Immunofluorescence Study
4.10. Preparation of the HA-Based CSE Formulation and RAW 264.7 Cells Treatment
4.11. Mutagenicity Assay: Ames Test
4.12. Statistical Analysis
4.13. In Silico Studies
Structural Resources and Docking Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, B.; Chen, Z.S.; Hu, Y.; Yong, Q. Insight in the Recent Application of Polyphenols from Biomass. Front. Bioeng. Biotechnol. 2021, 9, 753898. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Nouri, K.; Momtaz, S.; Roufogalis, B.D.; Alidadi, M.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Plant-Derived Fermented Products: An Interesting Concept for Human Health. Curr. Dev. Nutr. 2024, 8, 102162. [Google Scholar] [CrossRef]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Chagas, M.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Palai, S.; Rudrapal, M. Nanodeliveries of Food Polyphenols as Nutraceuticals. In Polyphenols: Food, Nutraceutical, and Nanotherapeutic Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 155–175. [Google Scholar]
- Vamanu, E. Polyphenolic Nutraceuticals to Combat Oxidative Stress Through Microbiota Modulation. Front Pharmacol. 2019, 10, 492. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Ayoubi, Y.; Sahiner, N. Therapeutic and Nutraceutical Effects of Polyphenolics from Natural Sources. Molecules 2022, 27, 6225. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Chávez-Delgado, E.L.; Jacobo-Velázquez, D.A. Essential Oils: Recent Advances on Their Dual Role as Food Preservatives and Nutraceuticals against the Metabolic Syndrome. Foods 2023, 12, 1079. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, M.; Ahmad, A. Flavonoids as Nutraceuticals. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–155. [Google Scholar]
- Bernardini, G.; Minetti, M.; Polizzotto, G.; Biazzo, M.; Santucci, A. Pro-Apoptotic Activity of French Polynesian Padina Pavonica Extract on Human Osteosarcoma Cells. Mar. Drugs 2018, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Minetti, M.; Bernardini, G.; Biazzo, M.; Gutierrez, G.; Geminiani, M.; Petrucci, T.; Santucci, A. Padina Pavonica Extract Promotes In Vitro Differentiation and Functionality of Human Primary Osteoblasts. Mar. Drugs 2019, 17, 473. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Moscariello, C.; Matassa, S.; Esposito, G.; Papirio, S. From Residue to Resource: The Multifaceted Environmental and Bioeconomy Potential of Industrial Hemp (Cannabis Sativa L.). Resour. Conserv. Recycl. 2021, 175, 105864. [Google Scholar] [CrossRef]
- Fordjour, E.; Manful, C.F.; Sey, A.A.; Javed, R.; Pham, T.H.; Thomas, R.; Cheema, M. Cannabis: A Multifaceted Plant with Endless Potentials. Front Pharmacol. 2023, 14, 1200269. [Google Scholar] [CrossRef]
- Groom, Q.; Clarke, R.C.; Merlin, M.D. Cannabis: Evolution and Ethnobotany. Plant Ecol. Evol. 2014, 147, 149. [Google Scholar] [CrossRef]
- Small, E. Morphological Variation of Achenes of Cannabis. Can. J. Bot. 1975, 53, 978–987. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A Practical and Natural Taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis Domestication, Breeding History, Present-Day Genetic Diversity, and Future Prospects. CRC Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Grassi, G.; McPartland, J.M. Chemical and Morphological Phenotypes in Breeding of Cannabis Sativa L. In Cannabis sativa L.-Botany and Biotechnology; Springer International Publishing: Cham, Germany, 2017; pp. 137–160. [Google Scholar]
- Durán-Zuazo, V.H.; Rodríguez, B.C.; García-Tejero, I.F.; Ruiz, B.G. Suitability and Opportunities for Cannabis Sativa L. as an Alternative Crop for Mediterranean Environments. In Current Applications, Approaches, and Potential Perspectives for Hemp; Academic Press: Cambridge, MA, USA, 2023; pp. 3–47. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis Sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed]
- Cicaloni, V.; Salvini, L.; Vitalini, S.; Garzoli, S. Chemical Profiling and Characterization of Different Cultivars of Cannabis Sativa L. Inflorescences by SPME-GC-MS and UPLC-MS. Separations 2022, 9, 90. [Google Scholar] [CrossRef]
- Lorensen, M.D.B.B.; Hayat, S.Y.; Wellner, N.; Bjarnholt, N.; Janfelt, C. Leaves of Cannabis Sativa and Their Trichomes Studied by DESI and MALDI Mass Spectrometry Imaging for Their Contents of Cannabinoids and Flavonoids. Phytochem. Anal. 2023, 34, 269–279. [Google Scholar] [CrossRef]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic Acid as a Selective Cyclooxygenase-2 Inhibitory Component in Cannabis. Drug Metab. Dispos. 2008, 36, 1917–1921. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)Δ9-Tetrahydrocannabinol Are Neuroprotective Antioxidants. Proc. Natl Acad. Sci. USA 1998, 95, 8268. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In Vivo Cannabidiol Treatment Improves Endothelium-Dependent Vasorelaxation in Mesenteric Arteries of Zucker Diabetic Fatty Rats. Front Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol Improves Vasorelaxation in Zucker Diabetic Fatty Rats through Cyclooxygenase Activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in Medical Marijuana: Research Vistas and Potential Opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Lee, Y.-H.; Chen, L.-G.; Lee, C.-J.; Wang, C.-C. In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum Multiflorum. Molecules 2018, 23, 571. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; McDougall, J.J. Cannabis and Joints: Scientific Evidence for the Alleviation of Osteoarthritis Pain by Cannabinoids. Curr. Opin. Pharmacol. 2018, 40, 104–109. [Google Scholar] [CrossRef]
- Deckey, D.G.; Lara, N.J.; Gulbrandsen, M.T.; Hassebrock, J.D.; Spangehl, M.J.; Bingham, J.S. Prevalence of Cannabinoid Use in Patients with Hip and Knee Osteoarthritis. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e20. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of Chronic Pain in Europe: Prevalence, Impact on Daily Life, and Treatment. Eur. J. Pain. 2006, 10, 287. [Google Scholar] [CrossRef]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a Treatment for Arthritis and Joint Pain: An Exploratory Cross-Sectional Study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Dunn, S.L.; Wilkinson, J.M.; Crawford, A.; Bunning, R.A.D.; Le Maitre, C.L. Expression of Cannabinoid Receptors in Human Osteoarthritic Cartilage: Implications for Future Therapies. Cannabis Cannabinoid. Res. 2016, 1, 3–15. [Google Scholar] [CrossRef]
- Soliman, N.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Liao, J.; Macleod, M.; Segelcke, D.; Sena, C.; Thomas, J.; Vollert, J.; et al. Systematic Review and Meta-Analysis of Cannabinoids, Cannabis-Based Medicines, and Endocannabinoid System Modulators Tested for Antinociceptive Effects in Animal Models of Injury-Related or Pathological Persistent Pain. Pain 2021, 162, S26. [Google Scholar] [CrossRef]
- Vela, J.; Dreyer, L.; Petersen, K.K.; Arendt-Nielsen, L.; Duch, K.S.; Kristensen, S. Cannabidiol Treatment in Hand Osteoarthritis and Psoriatic Arthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Pain 2022, 163, 1206–1214. [Google Scholar] [CrossRef]
- Verrico, C.D.; Wesson, S.; Konduri, V.; Hofferek, C.J.; Vazquez-Perez, J.; Blair, E.; Dunner, K.; Salimpour, P.; Decker, W.K.; Halpert, M.M. A Randomized, Double-Blind, Placebo-Controlled Study of Daily Cannabidiol for the Treatment of Canine Osteoarthritis Pain. Pain 2020, 161, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of Cannflavins A and B from Cannabis Sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Povedano, M.M.; Sánchez-Carnerero Callado, C.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted Characterization of Extracts from Cannabis Sativa L. Cultivars by Gas and Liquid Chromatography Coupled to Mass Spectrometry in High Resolution Mode. Talanta 2020, 208, 120384. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Ocmín, P.G.; Marti, G.; Bonhomme, M.; Mathis, F.; Fournier, S.; Bertani, S.; Maciuk, A. Cannabinoids vs. Whole Metabolome: Relevance of Cannabinomics in Analyzing Cannabis Varieties. Anal. Chim. Acta. 2021, 1184, 339020. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional Profiling of the LPS Induced NF-ΚB Response in Macrophages. BMC Immunol. 2007, 8, 1–17. [Google Scholar] [CrossRef]
- Scapin, G.; Patel, S.B.; Lisnock, J.; Becker, J.W.; LoGrasso, P.V. The Structure of JNK3 in Complex with Small Molecule Inhibitors. Chem. Biol. 2003, 10, 705–712. [Google Scholar] [CrossRef]
- Shiroma, Y.; Fujita, G.; Yamamoto, T.; Takahashi, R.; Kumar, A.; Zhang, K.Y.J.; Ito, A.; Osada, H.; Yoshida, M.; Tahara, H. Identification of a Selective RelA Inhibitor Based on DSE-FRET Screening Methods. Int. J. Mol. Sci. 2020, 21, 9150. [Google Scholar] [CrossRef]
- Hadley Kershaw, E.; Hartley, S.; McLeod, C.; Polson, P. The Sustainable Path to a Circular Bioeconomy. Trends Biotechnol. 2021, 39, 542–545. [Google Scholar] [CrossRef]
- Bioeconomy-Research and Innovation-European Union European Union. Available online: https://research-and-innovation.ec.europa.eu/research-area/environment/bioeconomy_en (accessed on 19 July 2024).
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; Lucia, A.D.; Colucci, G.; La Cara, F.; Morana, A. Chestnut (Castanea sativa Mill.) Industrial Wastes as a Valued Bioresource for the Production of Active Ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Afraz, M.; Muhammad, F.; Nisar, J.; Shah, A.; Munir, S.; Ali, G.; Ahmad, A. Production of Value Added Products from Biomass Waste by Pyrolysis: An Updated Review. Waste Manag. Bull. 2024, 1, 30–40. [Google Scholar] [CrossRef]
- Pérez-Marroquín, X.A.; Estrada-Fernández, A.G.; García-Ceja, A.; Aguirre-Álvarez, G.; León-López, A. Agro-Food Waste as an Ingredient in Functional Beverage Processing: Sources, Functionality, Market and Regulation. Foods 2023, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Jaouhari, Y.; Travaglia, F.; Giovannelli, L.; Picco, A.; Oz, E.; Oz, F.; Bordiga, M. From Industrial Food Waste to Bioactive Ingredients: A Review on the Sustainable Management and Transformation of Plant-Derived Food Waste. Foods 2023, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Longo, S.; Cellura, M.; Luu, L.Q.; Nguyen, T.Q.; Rincione, R.; Guarino, F. Circular Economy and Life Cycle Thinking Applied to the Biomass Supply Chain: A Review. Renew. Energy 2024, 220, 119598. [Google Scholar] [CrossRef]
- Fava, F.; Gardossi, L.; Brigidi, P.; Morone, P.; Carosi, D.A.R.; Lenzi, A. The Bioeconomy in Italy and the New National Strategy for a More Competitive and Sustainable Country. N. Biotechnol. 2021, 61, 124–136. [Google Scholar] [CrossRef]
- Wee, Y.B.; Berkowitz, O.; Whelan, J.; Jost, R. Same, yet Different: Towards Understanding Nutrient Use in Hemp- and Drug-Type Cannabis. J. Exp. Bot. 2024, 76, 94–108. [Google Scholar] [CrossRef]
- Grassa, C.J.; Weiblen, G.D.; Wenger, J.P.; Dabney, C.; Poplawski, S.G.; Timothy Motley, S.; Michael, T.P.; Schwartz, C.J. A New Cannabis Genome Assembly Associates Elevated Cannabidiol (CBD) with Hemp Introgressed into Marijuana. New Phytol. 2021, 230, 1665–1679. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of Fibre Hemp (Cannabis sativa L.) in Europe. Ind. Crops. Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Blandinières, H.; Amaducci, S. Adapting the Cultivation of Industrial Hemp (Cannabis sativa L.) to Marginal Lands: A Review. GCB Bioenergy 2022, 14, 1004–1022. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Mandal, N.; Benhiba, F.; Bahadur, I.; Dagdag, O. Anticorrosive Properties of a Green and Sustainable Inhibitor from Leaves Extract of Cannabis sativa Plant: Experimental and Theoretical Approach. Colloids Surf. Physicochem. Eng. Asp. 2021, 614, 126211. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Substances Generally Recognized as Safe (Final Rule). Fed. Regist. 2016, 19164, 54960–55055. [Google Scholar]
- Amin, I.; Mukhrizah, O. Antioxidant Capacity of Methanolic and Water Extracts Prepared from Food-Processing by-Products. J. Sci. Food Agric. 2006, 86, 778–784. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.F.; Javeed, A. Phytochemical Screening, Total Phenolic and Flavonoids Contents and Antioxidant Activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Luangpraditkun, K.; Pimjuk, P.; Phimnuan, P.; Wisanwattana, W.; Wisespongpand, C.; Waranuch, N.; Viyoch, J. Anti-Aging Properties of Cannabis sativa Leaf Extract against UVA Irradiation. Cosmetics 2024, 11, 45. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Bioactive Compound Extraction of Hemp (Cannabis sativa L.) Leaves through Response Surface Methodology Optimization. AgriEngineering 2024, 6, 1300–1318. [Google Scholar] [CrossRef]
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of Extraction Conditions on Total Polyphenol Contents, Antioxidant and Antimicrobial Activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2012, 11, 300–307. [Google Scholar]
- Chen, L.; Li, H.-L.; Zhou, H.-J.; Zhang, G.-Z.; Zhang, Y.; Wang, Y.-M.; Wang, M.-Y.; Yang, H.; Gao, W. Feature-Based Molecular Network-Assisted Cannabinoid and Flavonoid Profiling of Cannabis sativa Leaves and Their Antioxidant Properties. Antioxidants 2024, 13, 749. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (–)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Schneider, M.; Pongratz, G. Joints for Joints: Cannabinoids in the Treatment of Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2019, 31, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.-G.; Hwang, S.; Yun, S.-H.; Uprety, L.P.; Oh, K.-I.; Singh, S.; Yoo, J.; Jeong, H.; Yong, Y.; et al. Anti-Osteoarthritic Effects of Prunella Vulgaris and Gentiana Lutea In Vitro and In Vivo. Antioxidants 2022, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Hu, S.; Cai, Y.; Yang, Z.; Peng, K. Scoparone Prevents IL-1β-Induced Inflammatory Response in Human Osteoarthritis Chondrocytes through the PI3K/Akt/NF-ΚB Pathway. Biomed. Pharmacother. 2018, 106, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Zhang, Y.; Dai, B.-L.; Ma, Y.-J.; Zhang, Q.; Wang, Y.; Yang, H. Chlorogenic Acid Prevents Inflammatory Responses in IL-1β-Stimulated Human SW-1353 Chondrocytes, a Model for Osteoarthritis. Mol. Med. Rep. 2017, 16, 1369–1375. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471. [Google Scholar] [CrossRef]
- Cadet, C.; Maheu, E. Non-Steroidal Anti-Inflammatory Drugs in the Pharmacological Management of Osteoarthritis in the Very Old: Prescribe or Proscribe? Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X2110221. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The Evaluation of Oxidative Stress in Osteoarthritis. Med. Pharm. Rep. 2020, 93, 12. [Google Scholar] [CrossRef]
- Duangnin, N.; Klangjorhor, J.; Tipparat, P.; Pinmanee, S.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Anti-Inflammatory Effect of Methanol Extracts of Hemp Leaf in IL-1β-Induced Synovitis. Trop. J. Pharm. Res. 2017, 16, 1553–1563. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β Signaling in Osteoarthritis–Chondrocytes in Focus. Cell Signal 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M.; Amin, A.R.; Clancy, R. Nitric Oxide and Inflammatory Mediators in the Perpetuation of Osteoarthritis. Curr. Rheumatol. Rep. 2001, 3, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zou, F.; Li, Y.; Liu, A.; Tu, M. JNK Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Recept. Signal Transduct. 2017, 37, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.; Rediske, J.; Koehne, C.; Stoyanovsky, D.; Amin, A.; Attur, M.; Iyama, K.; Abramson, S.B. Activation of Stress-Activated Protein Kinase in Osteoarthritic Cartilage: Evidence for Nitric Oxide Dependence. Osteoarthr. Cartil. 2001, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-Κb Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Vernarelli, J.A.; Lambert, J.D. Flavonoid Intake Is Inversely Associated with Obesity and C-Reactive Protein, a Marker for Inflammation, in US Adults. Nutr. Diabetes 2017, 7, e276. [Google Scholar] [CrossRef]
- Lefèvre-Arbogast, S.; Gaudout, D.; Bensalem, J.; Letenneur, L.; Dartigues, J.F.; Hejblum, B.P.; Féart, C.; Delcourt, C.; Samieri, C. Pattern of Polyphenol Intake and the Long-Term Risk of Dementia in Older Persons. Neurology 2018, 90, e1979–e1988. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from Hemp Sprouts, a Novel Cannabinoid-Free Hemp Food Product, Target Microsomal Prostaglandin E2 Synthase-1 and 5-Lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Barrett, M.L.; Gordon, D.; Evans, F.J. Isolation from Cannabis Sativa L. of Cannflavin—A Novel Inhibitor of Prostaglandin Production. Biochem. Pharmacol. 1985, 34, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.L.; Scutt, A.M.; Evans, F.J. Cannflavin A and B, Prenylated Flavones from Cannabis sativa L. Experientia 1986, 42, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Laufer, S.A.; Werz, O. Design and Development of Microsomal Prostaglandin E 2 Synthase-1 Inhibitors: Challenges and Future Directions. J. Med. Chem. 2016, 59, 5970–5986. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Pei, F.; Li, Z. Orientin and Vitexin Attenuate Lipopolysaccharide-Induced Inflammatory Responses in RAW264.7 Cells: A Molecular Docking Study, Biochemical Characterization, and Mechanism Analysis. Food Sci. Hum. Wellness 2022, 11, 1273–1281. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin Alleviates Interleukin-1β-induced Inflammatory Responses in Chondrocytes from Osteoarthritis Patients: Involvement of HIF-1α Pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Zhang, D.; Ning, T.; Wang, H. Vitexin Alleviates Inflammation and Enhances Apoptosis through the Regulation of the JAK/STAT/SOCS Signaling Pathway in the Arthritis Rat Model. J. Biochem. Mol. Toxicol. 2022, 36, e23201. [Google Scholar] [CrossRef]
- Kaux, J.-F.; Samson, A.; Crielaard, J.-M. Hyaluronic Acid and Tendon Lesions. Muscles Ligaments Tendons J. 2015, 5, 264. [Google Scholar] [CrossRef]
- Makvandi, P.; Della Sala, F.; di Gennaro, M.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. A Hyaluronic Acid-Based Formulation with Simultaneous Local Drug Delivery and Antioxidant Ability for Active Viscosupplementation. ACS Omega 2022, 7, 10039–10048. [Google Scholar] [CrossRef]
- Tijani, A.O.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.I.; Puri, A. Delivering Therapeutic Cannabinoids via Skin: Current State and Future Perspectives. J. Control. Release 2021, 334, 427–451. [Google Scholar] [CrossRef]
- Pluda, S.; Beninatto, R.; Soato, M.; Barbera, C.; di Lucia, A.; Fassina, L.; Gatti, F.; Guarise, C.; Galesso, D.; Pavan, M. Hyaluronic Acid-Alendronate Conjugate: A Macromolecular Drug Delivery System for Intra-Articular Treatment of Osteoarthritis. Osteoarthr Cart. Open 2021, 3, 100159. [Google Scholar] [CrossRef]
- d’Angelo, I.; Provenzano, R.; Florio, E.; Lombardi, A.; Trama, U.; Ungaro, F.; Quaglia, F.; Miro, A. Transmucosal Delivery of the Medical Cannabis Oil via a Nanoemulsion Formulation. J. Drug Deliv. Sci. Technol. 2023, 79, 104004. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H. Bin Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2020, 10, 3. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant Activity of Grape Seed (Vitis vinifera) Extracts on Peroxidation Models in Vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Frusciante, L.; Geminiani, M.; Shabab, B.; Olmastroni, T.; Scavello, G.; Rossi, M.; Mastroeni, P.; Nyong’a, C.N.; Salvini, L.; Lamponi, S.; et al. Exploring the Antioxidant and Anti-Inflammatory Potential of Saffron (Crocus sativus) Tepals Extract within the Circular Bioeconomy. Antioxidants 2024, 13, 1082. [Google Scholar] [CrossRef]
- Frusciante, L.; Geminiani, M.; Trezza, A.; Olmastroni, T.; Mastroeni, P.; Salvini, L.; Lamponi, S.; Bernini, A.; Grasso, D.; Dreassi, E.; et al. Phytochemical Composition, Anti-Inflammatory Property, and Anti-Atopic Effect of Chaetomorpha linum Extract. Mar Drugs 2024, 22, 226. [Google Scholar] [CrossRef]
- Ng, N.; Ooi, L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio. Protoc. 2021, 11, e3877. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087379. [Google Scholar] [CrossRef]
- Mastroeni, P.; Geminiani, M.; Olmastroni, T.; Frusciante, L.; Trezza, A.; Visibelli, A.; Santucci, A. An in Vitro Cell Model for Exploring Inflammatory and Amyloidogenic Events in Alkaptonuria. J. Cell. Physiol. 2024, 239, e31449. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, P.; Trezza, A.; Geminiani, M.; Frusciante, L.; Visibelli, A.; Santucci, A. HGA Triggers SAA Aggregation and Accelerates Fibril Formation in the C20/A4 Alkaptonuria Cell Model. Cells 2024, 13, 1501. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/Microsome Mutagenicity Assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, L.; Geminiani, M.; Olmastroni, T.; Mastroeni, P.; Trezza, A.; Salvini, L.; Lamponi, S.; Spiga, O.; Santucci, A. Repurposing Castanea sativa Spiny Burr By-Products Extract as a Potentially Effective Anti-Inflammatory Agent for Novel Future Biotechnological Applications. Life 2024, 14, 763. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic. Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Janson, G.; Paiardini, A. PyMod 3: A Complete Suite for Structural Bioinformatics in PyMOL. Bioinformatics 2021, 37, 1471–1472. [Google Scholar] [CrossRef]
- Trezza, A.; Barletta, R.; Geminiani, M.; Frusciante, L.; Olmastroni, T.; Sannio, F.; Docquier, J.-D.; Santucci, A. Chestnut Burrs as Natural Source of Antimicrobial Bioactive Compounds: A Valorization of Agri-Food Waste. Appl. Sci. 2024, 14, 6552. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Trezza, A.; Geminiani, M.; Cutrera, G.; Dreassi, E.; Frusciante, L.; Lamponi, S.; Spiga, O.; Santucci, A. A Drug Discovery Approach to a Reveal Novel Antioxidant Natural Source: The Case of Chestnut Burr Biomass. Int. J. Mol. Sci. 2024, 25, 2517. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Koebel, M.R.; Schmadeke, G.; Posner, R.G.; Sirimulla, S. AutoDock VinaXB: Implementation of XBSF, New Empirical Halogen Bond Scoring Function, into AutoDock Vina. J. Cheminform. 2016, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Pessina, F.; Gamberucci, A.; Chen, J.; Liu, B.; Vangheluwe, P.; Gorelli, B.; Lorenzini, S.; Spiga, O.; Trezza, A.; Sgaragli, G.; et al. Negative Chronotropism, Positive Inotropism and Lusitropism of 3,5-Di-t-Butyl-4-Hydroxyanisole (DTBHA) on Rat Heart Preparations Occur through Reduction of RyR2 Ca2+ Leak. Biochem. Pharmacol. 2018, 155, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Spiga, O.; Trezza, A.; Frosini, M.; Floriddia, E.; Teodori, E.; Dei, S.; Saponara, S. In Vitro and in Silico Analysis of the Vascular Effects of Asymmetrical N, N-Bis(Alkanol)Amine Aryl Esters, Novel Multidrug Resistance-Reverting Agents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Cuong, N.M.; Son, N.T.; Nhan, N.T.; Khanh, P.N.; Huong, T.T.; Tram, N.T.T.; Sgaragli, G.; Ahmed, A.; Trezza, A.; Spiga, O.; et al. Vasorelaxing Activity of R-(−)-3′-Hydroxy-2,4,5-Trimethoxydalbergiquinol from Dalbergia Tonkinensis: Involvement of Smooth Muscle CaV1.2 Channels. Planta Med. 2020, 86, 284–293. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Trezza, A.; Spiga, O.; Mugnai, P.; Saponara, S.; Sgaragli, G.; Fusi, F. Functional, Electrophysiology, and Molecular Dynamics Analysis of Quercetin-Induced Contraction of Rat Vascular Musculature. Eur. J. Pharmacol. 2022, 918, 174778. [Google Scholar] [CrossRef]






| Antioxidant Capacity | |||||
|---|---|---|---|---|---|
| TPC (mg GAE/g) | TFC (mg QE/g) | RP (mg AAE/g) | ABTS (IC50 µg/mL) | DPPH (IC50 µg/mL) | |
| CSE | 65.31 ± 2.83 | 55.18 ± 10.6 | 58.50 ± 2.4 | 128.35 ± 3.1 | 67.93 ± 3.6 |
| Name | Retention Time (min) | Formula | Calculated MW | m/z | Reference Ion | Mass Error (ppm) |
|---|---|---|---|---|---|---|
| Cannabidiolic acid | 29.787 | C22H30O4 | 358.21483 | 357.2076 | [M−H]− | 1.17 |
| Δ9-tetrahydrocannabinolic acid | 40.725 | C22H30O4 | 358.21456 | 359.2218 | [M+H]+ | 0.41 |
| Cannabidiol | 45.903 | C21H30O2 | 314.22572 | 315.233 | [M+H]+ | 3.64 |
| Cannflavin A | 40.63 | C26H28O6 | 436.18919 | 437.1965 | [M+H]+ | 1.38 |
| Luteolin | 16.598 | C15H10O6 | 286.04839 | 287.0557 | [M+H]+ | 2.27 |
| Vitexin | 15.777 | C21H20O10 | 432.10671 | 433.114 | [M+H]+ | 2.45 |
| Genistein | 17.997 | C15H10O5 | 270.05291 | 269.0456 | [M−H]− | 0.33 |
| Lucidone B | 40.665 | C24H32O5 | 400.22536 | 401.2328 | [M+H]+ | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frusciante, L.; Geminiani, M.; Shabab, B.; Olmastroni, T.; Roncucci, N.; Mastroeni, P.; Salvini, L.; Lamponi, S.; Trezza, A.; Santucci, A. Enhancing Industrial Hemp (Cannabis sativa) Leaf By-Products: Bioactive Compounds, Anti-Inflammatory Properties, and Potential Health Applications. Int. J. Mol. Sci. 2025, 26, 548. https://doi.org/10.3390/ijms26020548
Frusciante L, Geminiani M, Shabab B, Olmastroni T, Roncucci N, Mastroeni P, Salvini L, Lamponi S, Trezza A, Santucci A. Enhancing Industrial Hemp (Cannabis sativa) Leaf By-Products: Bioactive Compounds, Anti-Inflammatory Properties, and Potential Health Applications. International Journal of Molecular Sciences. 2025; 26(2):548. https://doi.org/10.3390/ijms26020548
Chicago/Turabian StyleFrusciante, Luisa, Michela Geminiani, Behnaz Shabab, Tommaso Olmastroni, Neri Roncucci, Pierfrancesco Mastroeni, Laura Salvini, Stefania Lamponi, Alfonso Trezza, and Annalisa Santucci. 2025. "Enhancing Industrial Hemp (Cannabis sativa) Leaf By-Products: Bioactive Compounds, Anti-Inflammatory Properties, and Potential Health Applications" International Journal of Molecular Sciences 26, no. 2: 548. https://doi.org/10.3390/ijms26020548
APA StyleFrusciante, L., Geminiani, M., Shabab, B., Olmastroni, T., Roncucci, N., Mastroeni, P., Salvini, L., Lamponi, S., Trezza, A., & Santucci, A. (2025). Enhancing Industrial Hemp (Cannabis sativa) Leaf By-Products: Bioactive Compounds, Anti-Inflammatory Properties, and Potential Health Applications. International Journal of Molecular Sciences, 26(2), 548. https://doi.org/10.3390/ijms26020548








