Comprehensive Identification of AREB Gene Family in Populus euphratica Oliv. and Functional Analysis of PeAREB04 in Drought Tolerance
Abstract
1. Introduction
2. Results
2.1. Identification and Characterisation of PeAREB Genes
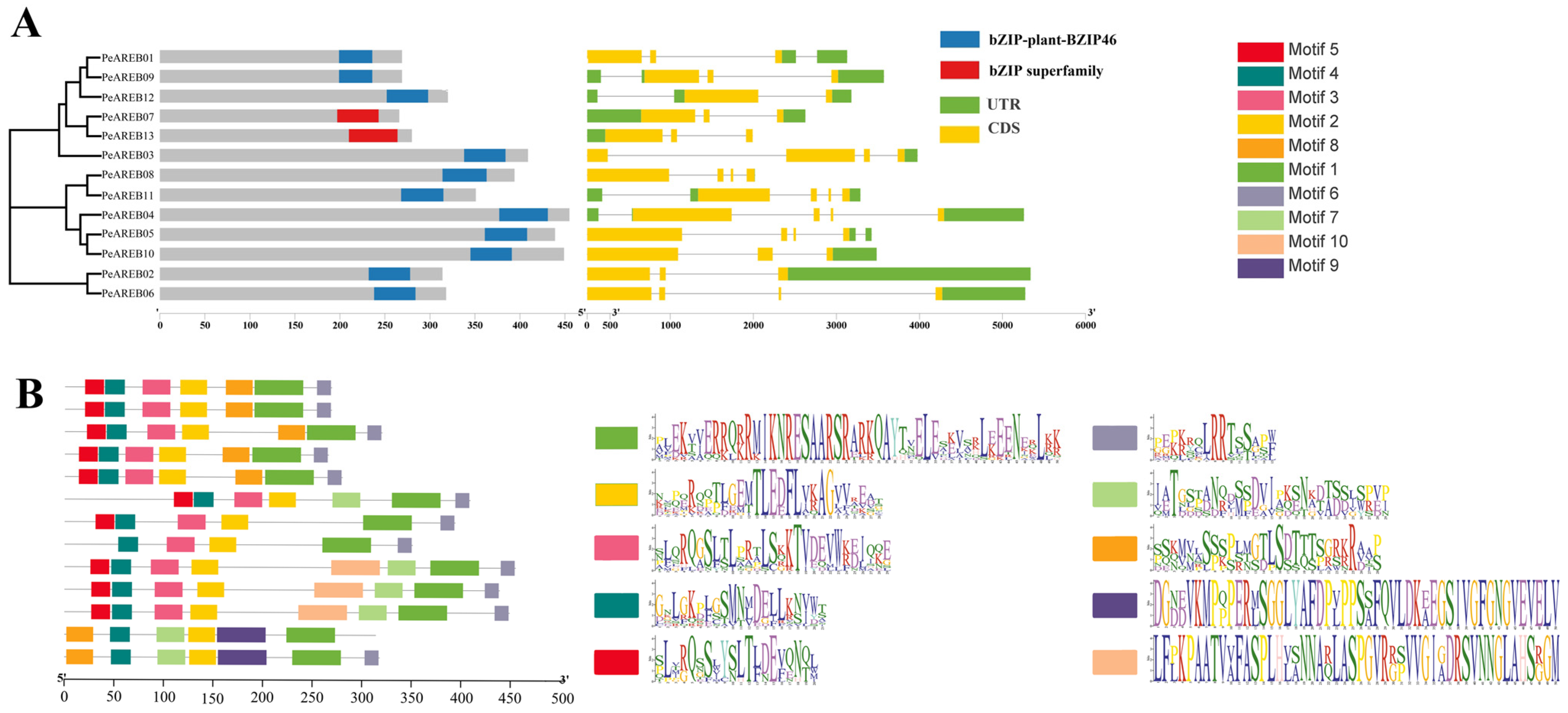
2.2. Phylogenetic Analysis and Structural Prediction of PeAREBs
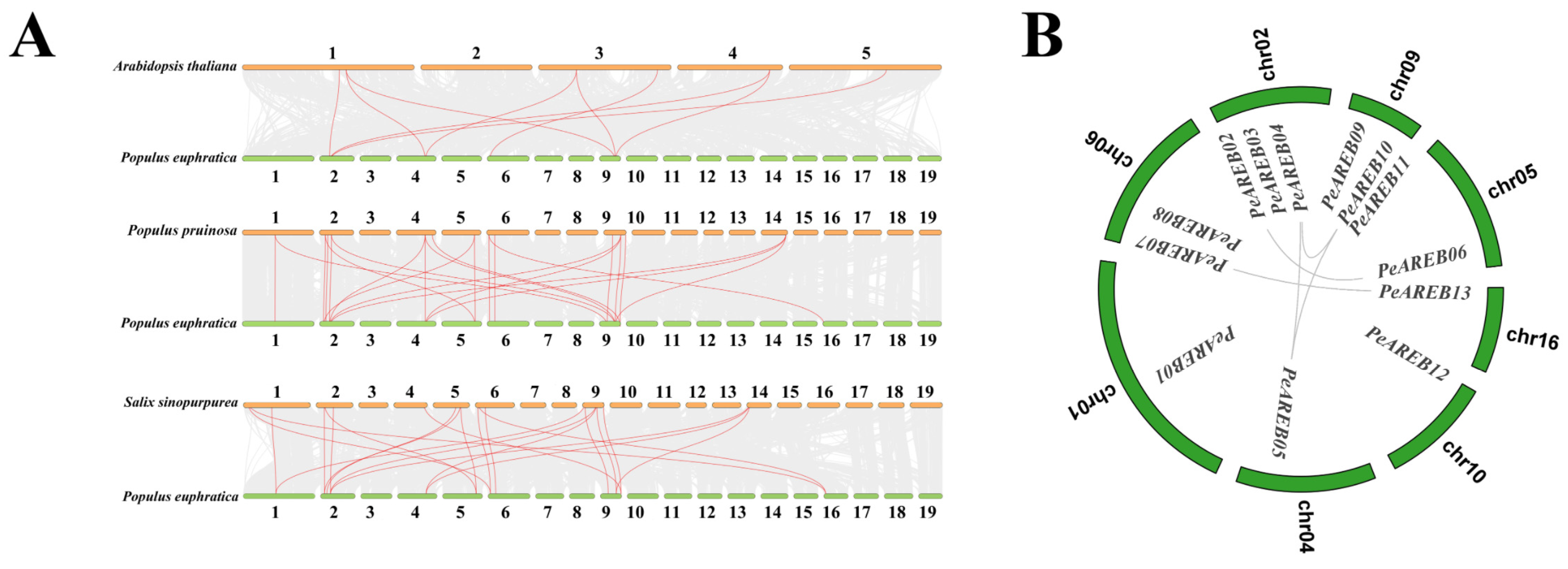
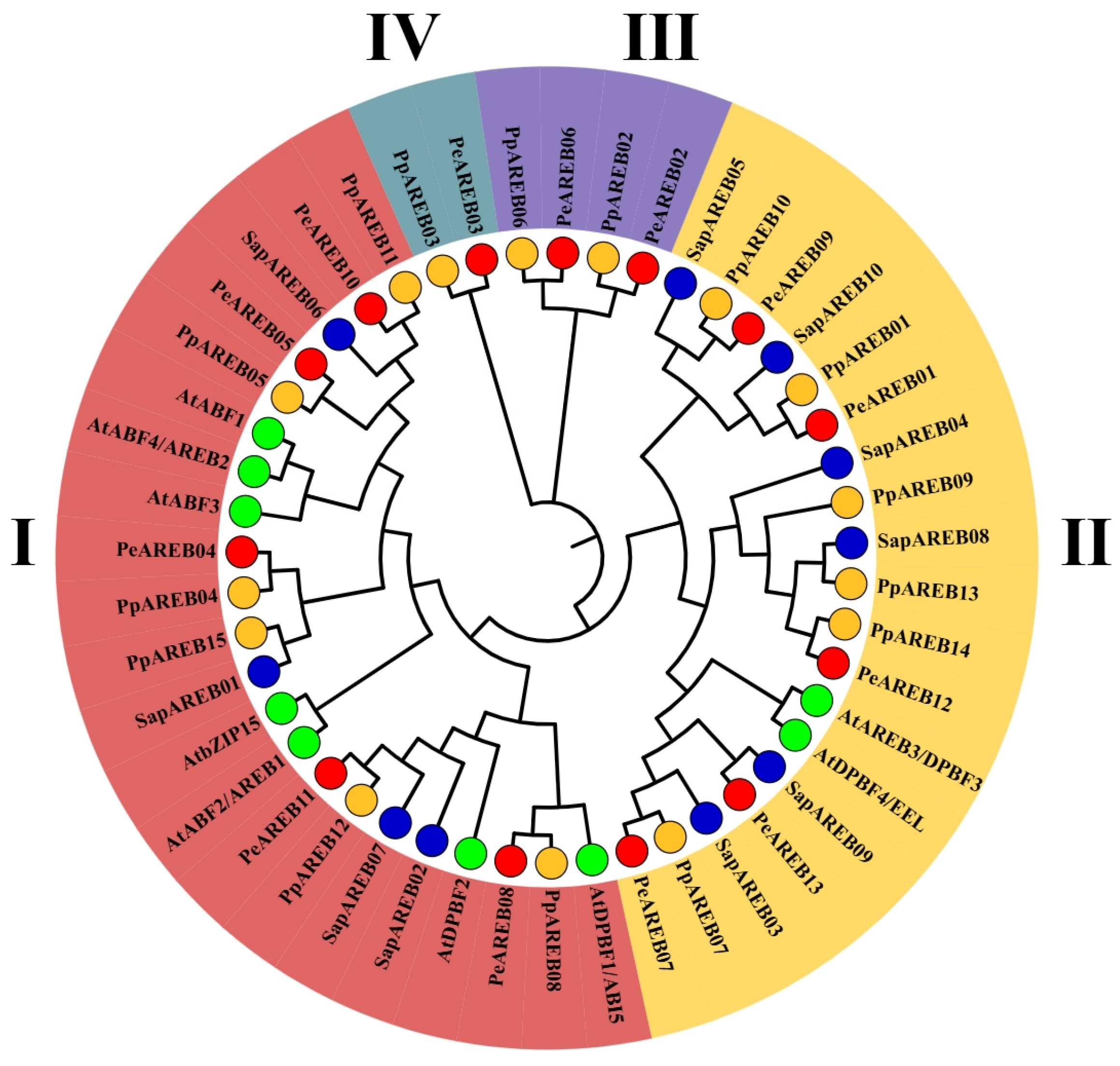
2.3. Colinear and Phylogenetic Analysis of AREB Genes Across Multiple Species
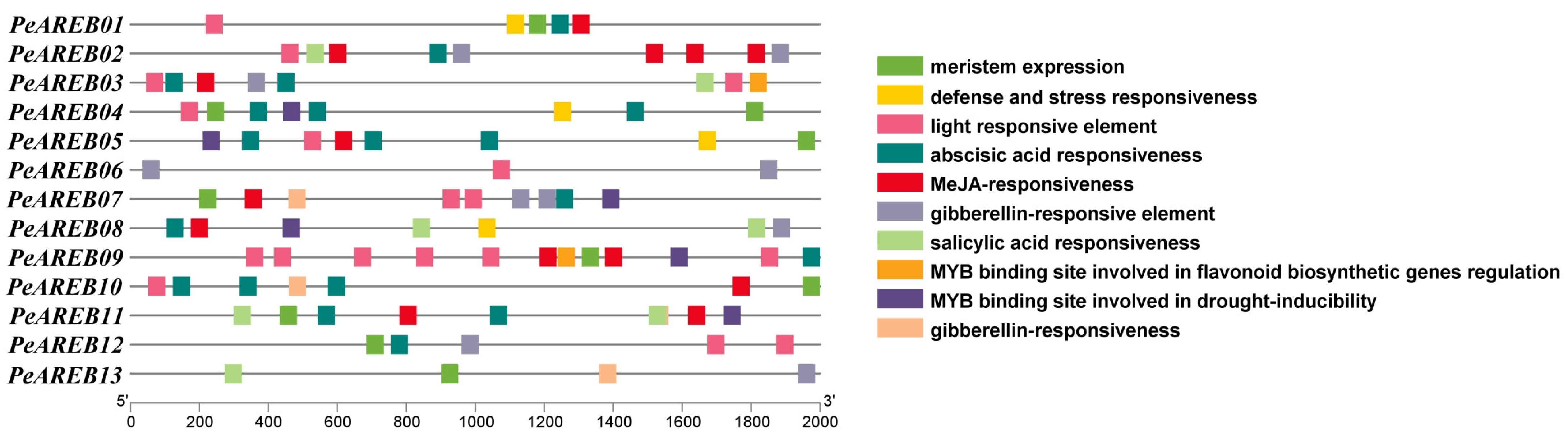
2.4. Prediction of Cis-Acting Elements in the Promoter Regions of PeAREBs
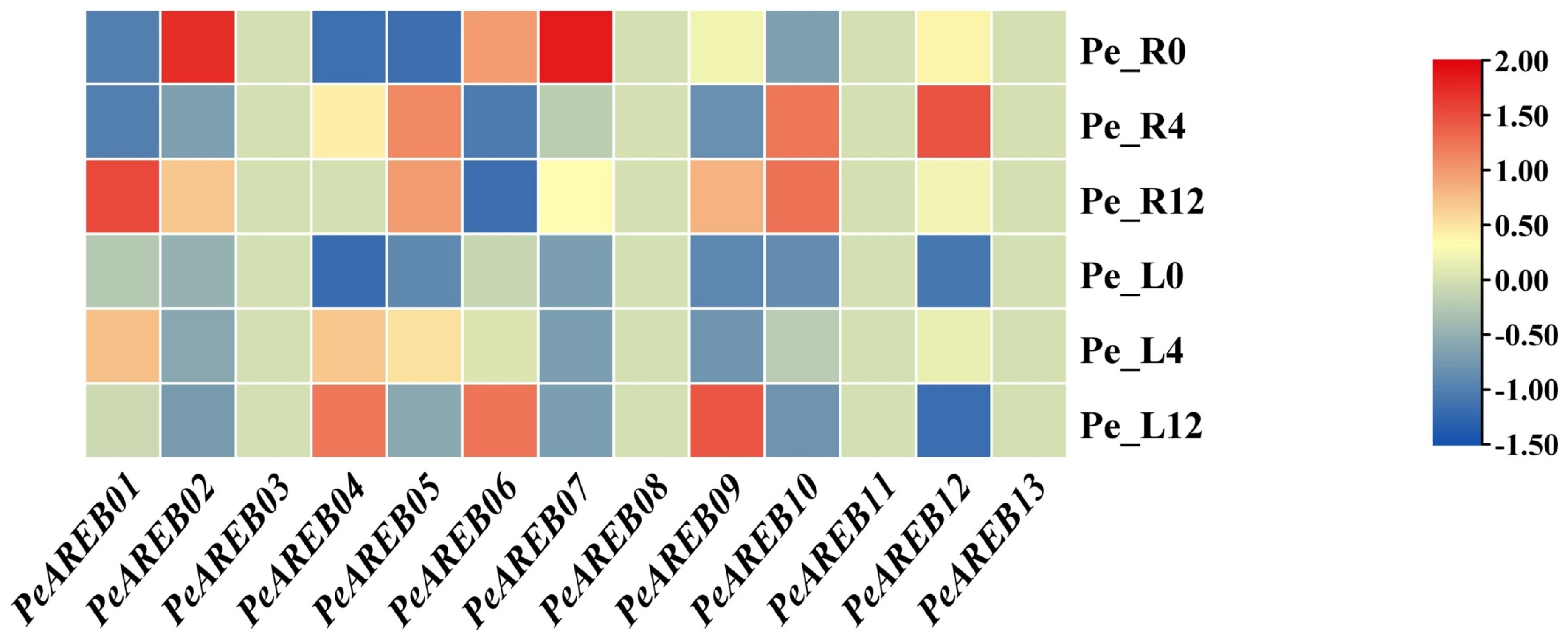
2.5. Transcriptional Expression Profile of PeAREB Genes Under Drought Stress
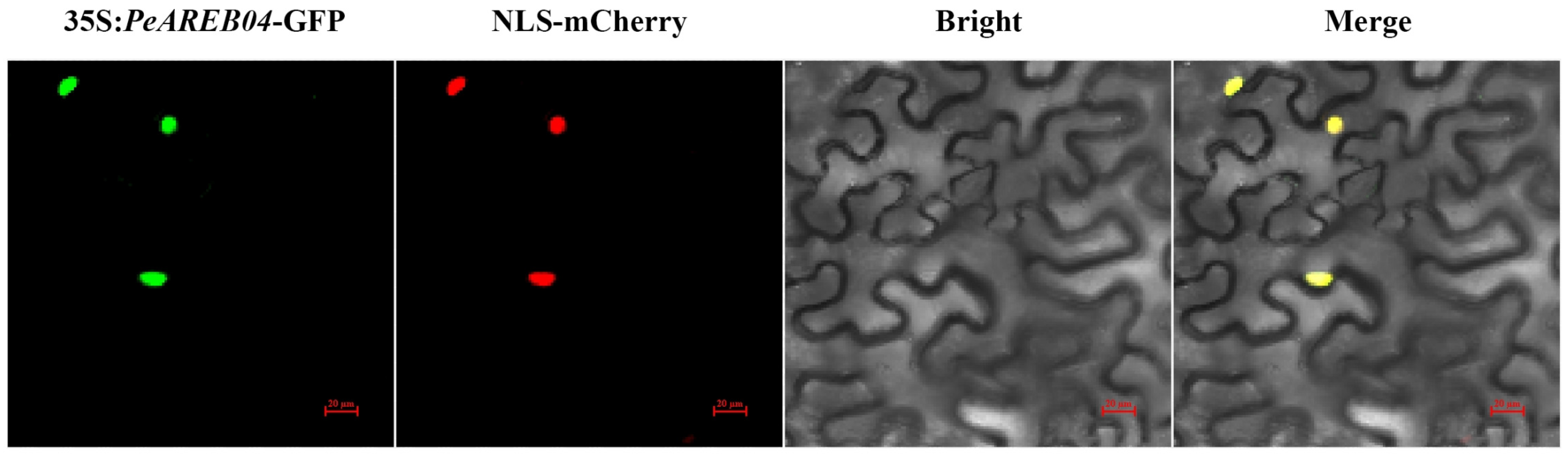
2.6. Subcellular Localisation of PeAREB04
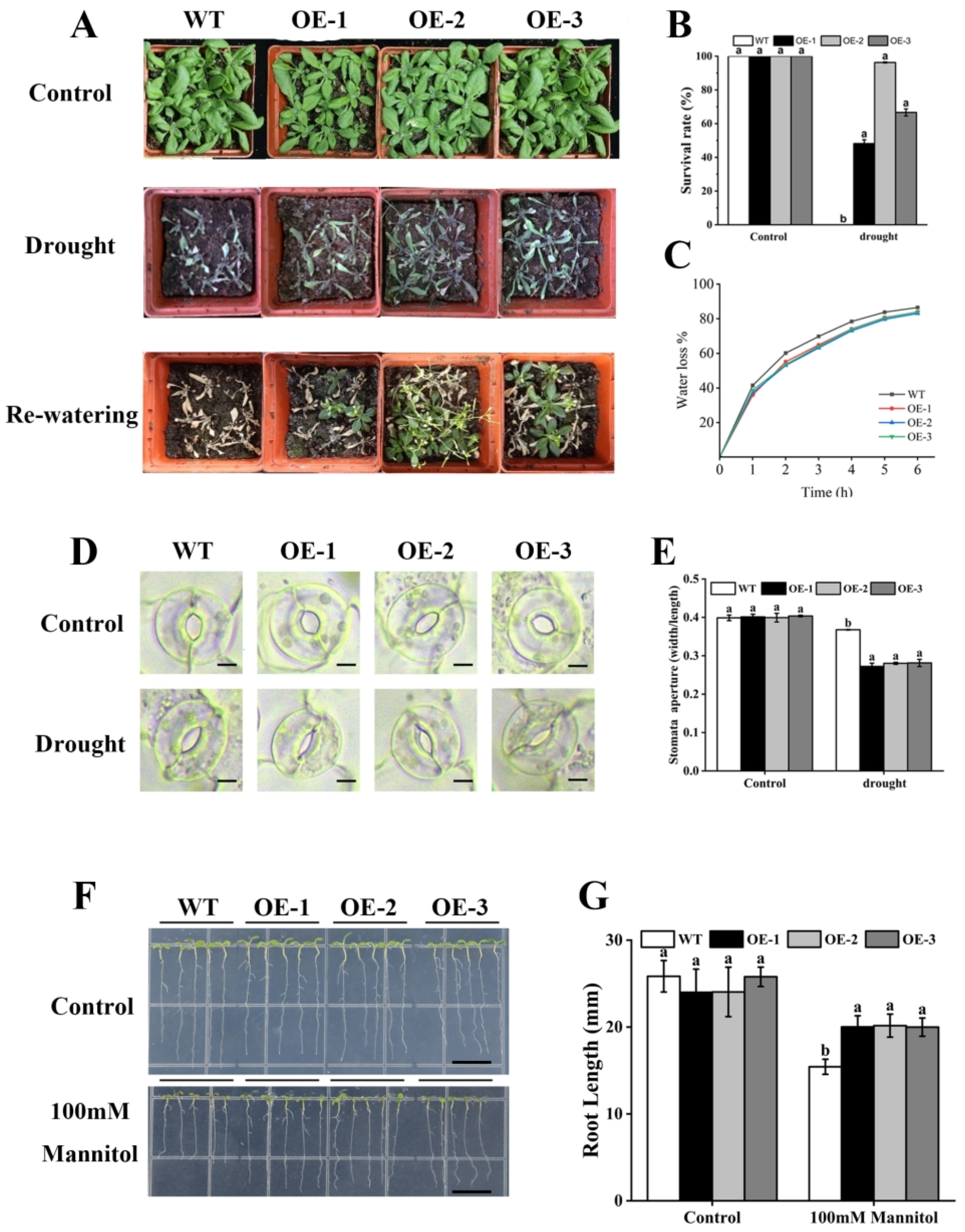
2.7. Overexpression of PeAREB04 Enhances Drought Tolerance in Plants
3. Discussion
3.1. Impact of Genome-Wide Duplication on the Expansion of PeAREBs in the Salicaceae Genus
3.2. PeAREBs Are Involved in the Regulation of Biological Processes
3.3. PeAREB04 Enhances Drought Tolerance in Plants
4. Materials and Methods
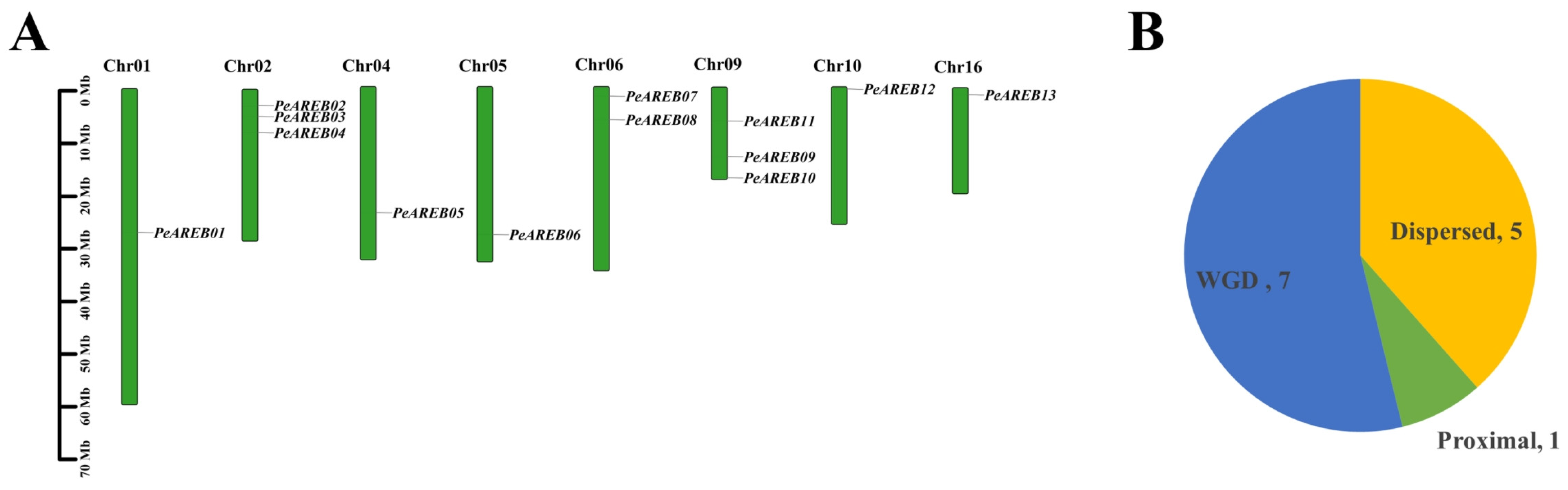
4.1. Identification of PeAREB Gene Family Members and Analysis of Chromosomal Localisation
4.2. Analysis of Replication Types and Physicochemical Properties of the PeAREB Gene Family
4.3. Prediction of Conserved Structural Domains, Gene Structure and Motifs of PeAREB Gene Family Members
4.4. Phylogenetic Analysis of the PeAREB Gene Family
4.5. Collinearity Analysis of Multi-Species AREB
4.6. Analysis of Cis-Acting Elements of PeAREBs
4.7. Cloning of the PeAREB04 Gene and Generation of Overexpression Plants
4.8. Subcellular Localisation of PeAREB04
4.9. Transcriptomic Expression Analysis of PeAREBs
4.10. Plant Growth Conditions and Drought Stress Treatments
4.11. Stomatal Phenotypic Analysis of Rosette Leaves
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hao, J.; Yue, N.; Zheng, C. Analysis of Changes in Anatomical Characteristics and Physiologic Features of Heteromorphic Leaves in a Desert Tree, Populus euphratica. Acta Physiol. Plant 2017, 39, 160. [Google Scholar] [CrossRef]
- Tang, S.; Liang, H.; Yan, D.; Zhao, Y.; Han, X.; Carlson, J.E.; Xia, X.; Yin, W. Populus euphratica: The Transcriptomic Response to Drought Stress. Plant Mol. Biol. 2013, 83, 539–557. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and Physiological Analysis of Drought Stress in Arabidopsis Reveals Early Responses Leading to Acclimation in Plant Growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; He, X. Effects of Salinity and Water Potential on Seed Germination and Radicle Growth of Riparian Populus euphratica. Arid Zone Res. 2005, 4, 104–109. [Google Scholar]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-Mediated Transcriptional Regulation in Response to Osmotic Stress in Plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Basic Leucine Zipper Transcription Factors Involved in an Abscisic Acid-Dependent Signal Transduction Pathway under Drought and High-Salinity Conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef]
- Melo, B.P.; Lourenço-Tessutti, I.T.; Paixão, J.F.R.; Noriega, D.D.; Silva, M.C.M.; Almeida-Engler, J.; Fontes, E.P.B.; Grossi-de-Sa, M.F. Transcriptional Modulation of AREB-1 by CRISPRa Improves Plant Physiological Performance under Severe Water Deficit. Sci. Rep. 2020, 10. [Google Scholar]
- Chang, H.-C.; Tsai, M.-C.; Wu, S.-S.; Chang, I.-F. Regulation of ABI5 Expression by ABF3 during Salt Stress Responses in Arabidopsis Thaliana. Bot. Stud. 2019, 60, 16231. [Google Scholar] [CrossRef]
- Lim, C.; Kang, K.; Shim, Y.; Yoo, S.-C.; Paek, N.-C. Inactivating Transcription Factor OsWRKY5 Enhances Drought Tolerance through Abscisic Acid Signaling Pathways. Plant Physiol. 2022, 188, 1900–1916. [Google Scholar] [CrossRef]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2, ABF3, and ABF4 Promote ABA-Mediated Chlorophyll Degradation and Leaf Senescence by Transcriptional Activation of Chlorophyll Catabolic Genes and Senescence-Associated Genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Veselova, S.; Hartung, W.; Farhutdinov, R.; Veselov, D.; Sharipova, G. Involvement of Root ABA and Hydraulic Conductivity in the Control of Water Relations in Wheat Plants Exposed to Increased Evaporative Demand. Planta 2010, 233, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bray, E.A. Plant Responses to Water Deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In Vitro Reconstitution of an Abscisic Acid Signalling Pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal Role of the Areb/Abf-Snrk2 Pathway in Abre-Mediated Transcription in Response to Osmotic Stress in Plants. Physiol. Plant. 2013, 147, 15–27. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.; Cho, D.-I.; Park, J.H.; Kim, S.Y. ABF2, an ABRE-Binding BZIP Factor, Is an Essential Component of Glucose Signaling and Its Overexpression Affects Multiple Stress Tolerance. Plant J. 2004, 40, 75–87. [Google Scholar] [CrossRef]
- Kang, J.; Choi, H.; Im, M.; Kim, S.Y. Arabidopsis Basic Leucine Zipper Proteins That Mediate Stress-Responsive Abscisic Acid Signaling. Plant Cell 2002, 14, 343–357. [Google Scholar] [CrossRef]
- Kim, J.-B.; Kang, J.-Y.; Kim, S.Y. Over-Expression of a Transcription Factor Regulating Aba-Responsive Gene Expression Confers Multiple Stress Tolerance. Plant Biotechnol. J. 2004, 2, 459–466. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, R.; Chen, Y.; Wang, S.; Qin, F.; Wang, D.; Liu, Y.; Hu, L.; Meng, S. Identification and Characterization of the AREB/ABF/ABI5 Gene Family in Sandalwood (Santalum album L.) and Its Potential Role in Drought Stress and ABA Treatment. Forests 2023, 14, 1691. [Google Scholar] [CrossRef]
- Pan, X.; Wang, C.; Liu, Z.; Gao, R.; Feng, L.; Li, A.; Yao, K.; Liao, W. Identification of ABF/AREB Gene Family in Tomato (Solanum lycopersicum L.) and Functional Analysis of ABF/AREB in Response to ABA and Abiotic Stresses. Peer J 2023, 11, e15310. [Google Scholar] [CrossRef]
- Yong, X.; Zheng, T.; Zhuo, X.; Ahmad, S.; Li, L.; Li, P.; Yu, J.; Wang, J.; Cheng, T.; Zhang, Q. Genome-Wide Identification, Characterisation, and Evolution of ABF/AREB Subfamily in Nine Rosaceae Species and Expression Analysis in Mei. Peer J 2021, 9, e10785. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zhao, J.; Yang, Y.; Diao, K.; Zheng, G.; Li, T.; Dai, X.; Li, J. PeGSTU58, a Glutathione S-Transferase from Populus euphratica, Enhances Salt and Drought Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2023, 24, 9354. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Niu, M.-X.; Wang, T.; Li, J.-L.; Shi, Y.-J.; Zhao, J.-J.; Li, H.; Xiang, X.; Yang, P.; Wei, S.-Y.; et al. The Ubiquitin E3 Ligase RZFP1 Affects Drought Tolerance in Poplar by Mediating the Degradation of the Protein Phosphatase PP2C-9. Plant Physiol. 2024, 196, 2936–2955. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Park, I.H.; Jeong, S.; Choi, B.-H.; Han, S.-G.; Yoon, T.-M. Photosynthetic Traits and Plant Hydraulic Dynamics in Gamhong Apple Cultivar under Drought, Waterlogging, and Stress Recovery Periods. Sci. Hortic. 2023, 321, 112276. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a Family of ABA-Responsive Element Binding Factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef]
- Bastías, A.; Yañez, M.; Osorio, S.; Arbona, V.; Gómez-Cadenas, A.; Fernie, A.R.; Casaretto, J.A. The Transcription Factor AREB1 Regulates Primary Metabolic Pathways in Tomato Fruits. J. Exp. Bot. 2014, 65, 2351–2363. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 Is a Transcription Activator of Novel ABRE-Dependent ABA Signaling That Enhances Drought Stress Tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Cannon, S.; Mitra, A.; Baumgarten, A.; Young, N.; May, G. The Roles of Segmental and Tandem Gene Duplication in the Evolution of Large Gene Families in Arabidopsis thaliana. BMC Plant Biol. 2004, 20, 10. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, T.; Lian, M.; Liu, T.; Hou, J.; Ijaz, R.; Song, B. Genome-Wide Identification and Characterization of the AREB/ABF/ABI5 Subfamily Members from Solanum tuberosum. Int. J. Mol. Sci. 2019, 20, 311. [Google Scholar] [CrossRef]
- Bensmihen, S.; Rippa, S.; Lambert, G.; Jublot, D.; Pautot, V.; Granier, F.; Giraudat, J.; Parcy, F. The Homologous ABI5 and EEL Transcription Factors Function Antagonistically to Fine-Tune Gene Expression during Late Embryogenesis. Plant Cell 2002, 14, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Gao, C.; Zheng, X.; Han, B. Identification of OsbZIP72 as a Positive Regulator of ABA Response and Drought Tolerance in Rice. Planta 2008, 229, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wang, J.; Ye, M.; Li, Y.; Guo, B.; Chen, Z.; Li, H.; An, X. Identification and Characterization of the Populus AREB/ABF Subfamily. J. Integr. Plant Biol. 2012, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 Are Master Transcription Factors That Cooperatively Regulate ABRE-Dependent ABA Signaling Involved in Drought Stress Tolerance and Require ABA for Full Activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Li, X.-Y.; Liu, X.; Yao, Y.; Li, Y.-H.; Liu, S.; He, C.-Y.; Li, J.-M.; Lin, Y.-Y.; Li, L. Overexpression of Arachis hypogaea AREB1 Gene Enhances Drought Tolerance by Modulating ROS Scavenging and Maintaining Endogenous ABA Content. Int. J. Mol. Sci. 2013, 14, 12827–12842. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Zandkarimi, H.; Ebadi, A.; Salami, S.A.; Alizade, H.; Baisakh, N. Analyzing the Expression Profile of AREB/ABF and DREB/CBF Genes under Drought and Salinity Stresses in Grape (Vitis vinifera L.). PLoS ONE 2015, 10, e0134288. [Google Scholar] [CrossRef]
- Hattori, T.; Totsuka, M.; Hobo, T.; Kagaya, Y.; Yamamoto-Toyoda, A. Experimentally Determined Sequence Requirement of ACGT-Containing Abscisic Acid Response Element. Plant Cell Physiol. 2002, 43, 136–140. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Furihata, T.; Maruyama, K.; Fujita, Y.; Umezawa, T.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Abscisic Acid-Dependent Multisite Phosphorylation Regulates the Activity of a Transcription Activator AREB1. Proc. Natl. Acad. Sci. USA 2006, 103, 1988–1993. [Google Scholar] [CrossRef]
- Vysotskii, D.; de Vries-van Leeuwen, I.J.; Souer, E.; Babakov, A.V.; de Boer, A.H. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Plant Signal Behav. 2013, 8, e22672. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.G.G.; Leite, J.P.; Marin, S.R.R.; Marinho, J.P.; de Fátima Corrêa Carvalho, J.; Fuganti-Pagliarini, R.; Farias, J.R.B.; Neumaier, N.; Marcelino-Guimarães, F.C.; de Oliveira, M.C.N.; et al. Overexpression of the ABA-Dependent AREB1 Transcription Factor from Arabidopsis thaliana Improves Soybean Tolerance to Water Deficit. Plant Mol. Biol. Rep. 2012, 31, 719–730. [Google Scholar] [CrossRef]
- Jin, X.F.; Xiong, A.S.; Peng, R.H.; Liu, J.G.; Gao, F.; Chen, J.M.; Yao, Q.H. OsAREB1, an ABRE-Binding Protein Responding to ABA and Glucose, Has Multiple Functions in Arabidopsis. BMB Rep. 2010, 43, 34–39. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Q.; Xu, S.; Liu, W.; Zhu, X.; Song, C. Trehalose-6-Phosphate Phosphatase E Modulates ABA-Controlled Root Growth and Stomatal Movement in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 1518–1534. [Google Scholar] [CrossRef]
- Wang, Q.; Guan, Y.; Wu, Y.; Chen, H.; Chen, F.; Chu, C. Overexpression of a Rice OsDREB1F Gene Increases Salt, Drought, and Low Temperature Tolerance in Both Arabidopsis and Rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Noman, M.; Jameel, A.; Qiang, W.-D.; Ahmad, N.; Liu, W.; Wang, F.; Li, H. Overexpression of GmCAMTA12 Enhanced Drought Tolerance in Arabidopsis and Soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhang, J.; Ma, X.; Li, Y.; Li, M.; Wang, D.; Kang, M. Improved Genome Assembly Provides New Insights into Genome Evolution in a Desert Poplar (Populus euphratica). Mol. Ecol. Resour. 2020, 20, 781–794. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Qu, W.; Han, X.; Qiu, C.; Gai, Z.; Zhai, J.; Qin, R.; Liu, H.; Wu, Z.; et al. Genome-Wide Analysis of R2R3-MYB Transcription Factors Reveals Their Differential Responses to Drought Stress and ABA Treatment in Desert Poplar (Populus euphratica). Gene 2022, 855, 147124. [Google Scholar] [CrossRef]
- Dai, X.; Hu, Q.; Cai, Q.; Feng, K.; Ye, N.; Tuskan, G.A.; Milne, R.; Chen, Y. The Willow Genome and Divergent Evolution from Poplar after the Com-Mon Genome Duplication. Cell Res. 2014, 24, 1274–1277. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Jin, H.; Li, J.; Song, T.; Chen, Y.; Yuan, Y.; Hu, H.; Li, R.; Wu, Z.; et al. Comparative Genomics Analysis of the Populus Epidermal Pattern Factor (EPF) Family Revealed Their Regulatory Effects in Populus euphratica Stomatal Development. Int. J. Mol. Sci. 2024, 25, 10052. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Qiu, C.; Jiao, P.; Wang, H.; Wu, Z.; Li, Z. Comparative Genomic Analysis of the Growth-Regulating Factors-Interacting Factors (GIFs) in Six Salicaceae Species and Functional Analysis of PeGIF3 Reveals Their Regulatory Role in Populus Heteromorphic Leaves. BMC Genom. 2024, 25, 317. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Qiu, C.; Zhai, J.; Zhang, S.; Zhang, X.; Wu, Z.; Li, Z. The Chromosome-Scale Genome and Population Genomics Reveal the Adaptative Evolution of Populus pruinosa to Desertification Environment. Hortic. Res. 2024, 11, uhae034. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wan, H.h.; Huang, W.; Yousaf, Z.; Huang, H.; Ying, W. Characterization of B- and C-Class MADS-Box Genes in Medicinal Plant Epimedium sagittatum. Med. Plant Biol. 2023, 2, 1. [Google Scholar] [CrossRef]
- Folkers, U.; Kirik, V.; Schöbinger, U.; Falk, S.; Krishnakumar, S.; Pollock, M.A.; Oppenheimer, D.G.; Day, I.; Reddy, A.S.; Jürgens, G.; et al. The Cell Morphogenesis Gene ANGUSTIFOLIA Encodes a CtBP/BARS-like Protein and Is Involved in the Control of the Microtubule Cytoskeleton. EMBO J. 2002, 21, 1280–1288. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-Mediated Transformation of Arabidopsis thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Jiao, P.; Wu, Z.; Wang, X.; Jiang, Z.; Wang, Y.; Liu, H.; Qin, R.; Li, Z. Short-Term Transcriptomic Responses of Populus euphratica Roots and Leaves to Drought Stress. J. For. Res. J. 2020, 32, 841–853. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, X.; Li, Y.; Zhang, L.; Guan, P.; Kou, X.; Wang, X.; Xin, M.; Hu, Z.; Yao, Y.; et al. Molecular and Functional Characterization of Wheat ARGOS Genes Influencing Plant Growth and Stress Tolerance. Front. Plant Sci. 2017, 8, 170. [Google Scholar] [CrossRef]
- Franks, P.J.; Doheny-Adams, T.W.; Britton-Harper, Z.J.; Gray, J.E. Increasing Water-Use Efficiency Directly through Genetic Manipulation of Stomatal Density. New Phytol. 2015, 207, 188–195. [Google Scholar] [CrossRef]
- Ling, Y.; Alshareef, S.; Butt, H.; Lozano-Juste, J.; Li, L.; Galal, A.A.; Moustafa, A.; Momin, A.A.; Tashkandi, M.; Richardson, D.N.; et al. Pre-MRNA Splicing Repression Triggers Abiotic Stress Signaling in Plants. Plant J. 2017, 89, 291–309. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, Z.; Cai, Z.; Li, L.; Cheng, Y.; Liu, J.; Nian, H. GmWRKY16 Enhances Drought and Salt Tolerance through an ABA-Mediated Pathway in Arabidopsis thaliana. Front. Plant Sci. 2019, 9, 1979. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, H.; Xia, X.; Yin, W. Expression Profiling and Functional Characterization of a CBL-Interacting Protein Kinase Gene from Populus euphratica. Plant Cell Rep. 2014, 33, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Aoki, S.; Ando, E.; Kitatsuji, A.; Watanabe, A.; Ohnishi, M.; Takahashi, K.; Inoue, S.; Nakamichi, N.; Tamada, Y.; et al. A Flowering Integrator, SOC1, Affects Stomatal Opening in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 640–649. [Google Scholar] [CrossRef] [PubMed]
| Sequence ID | Gene ID | Number of Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | GRAVY |
|---|---|---|---|---|---|---|---|
| PeuTF01G02200.1 | PeAREB01 | 269 | 30,279.01 | 8.36 | 55.89 | 63.49 | −0.801 |
| PeuTF02G00444.1 | PeAREB02 | 314 | 34,540.42 | 4.94 | 54.01 | 67.07 | −0.754 |
| PeuTF02G00696.1 | PeAREB03 | 409 | 46,677.38 | 9.01 | 59.66 | 65.06 | −0.78 |
| PeuTF02G01055.1 | PeAREB04 | 455 | 49,251.12 | 9.42 | 49.37 | 68.99 | −0.668 |
| PeuTF04G01309.1 | PeAREB05 | 439 | 47,535.9 | 9.61 | 48.09 | 70.05 | −0.669 |
| PeuTF05G01689.1 | PeAREB06 | 318 | 35,007.38 | 5.64 | 60.93 | 67.45 | −0.744 |
| PeuTF06G00233.1 | PeAREB07 | 266 | 29,801.56 | 5.52 | 51.63 | 70.34 | −0.822 |
| PeuTF06G00810.1 | PeAREB08 | 394 | 43,160.06 | 9.09 | 52.53 | 62.46 | −0.776 |
| PeuTF09G00191.1 | PeAREB09 | 269 | 30,408.27 | 9.61 | 49.15 | 67.81 | −0.817 |
| PeuTF09G01018.1 | PeAREB10 | 449 | 49,487.98 | 10 | 46.02 | 72.96 | −0.586 |
| PeuTF09G01642.1 | PeAREB11 | 351 | 39,513.62 | 7.65 | 56.89 | 66.92 | −0.848 |
| PeuTF10G00079.1 | PeAREB12 | 320 | 36,118.93 | 6.93 | 49.86 | 68.22 | −0.767 |
| PeuTF16G00194.1 | PeAREB13 | 280 | 31,370.03 | 5.83 | 61.35 | 66.14 | −0.879 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Sun, J.; Qiu, C.; Han, X.; Li, Z. Comprehensive Identification of AREB Gene Family in Populus euphratica Oliv. and Functional Analysis of PeAREB04 in Drought Tolerance. Int. J. Mol. Sci. 2025, 26, 518. https://doi.org/10.3390/ijms26020518
Liu B, Sun J, Qiu C, Han X, Li Z. Comprehensive Identification of AREB Gene Family in Populus euphratica Oliv. and Functional Analysis of PeAREB04 in Drought Tolerance. International Journal of Molecular Sciences. 2025; 26(2):518. https://doi.org/10.3390/ijms26020518
Chicago/Turabian StyleLiu, Binglei, Jianhao Sun, Chen Qiu, Xiaoli Han, and Zhijun Li. 2025. "Comprehensive Identification of AREB Gene Family in Populus euphratica Oliv. and Functional Analysis of PeAREB04 in Drought Tolerance" International Journal of Molecular Sciences 26, no. 2: 518. https://doi.org/10.3390/ijms26020518
APA StyleLiu, B., Sun, J., Qiu, C., Han, X., & Li, Z. (2025). Comprehensive Identification of AREB Gene Family in Populus euphratica Oliv. and Functional Analysis of PeAREB04 in Drought Tolerance. International Journal of Molecular Sciences, 26(2), 518. https://doi.org/10.3390/ijms26020518






