Gene-Exercise Interactions in Amyloid Metabolism and Clearance: Implications for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
3. Environmental Factors in AD
How Lifestyle Factors Influence Alzheimer Disease
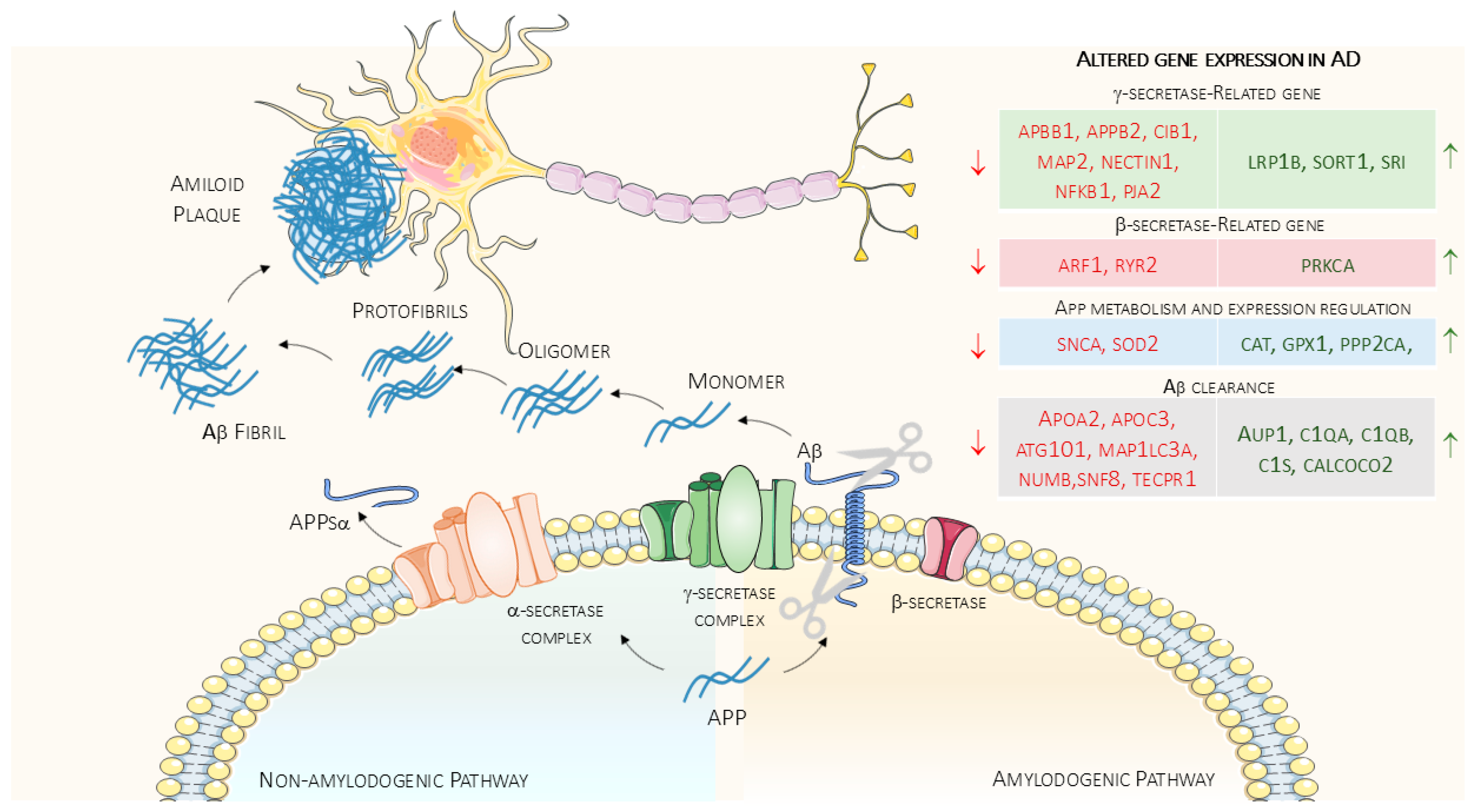
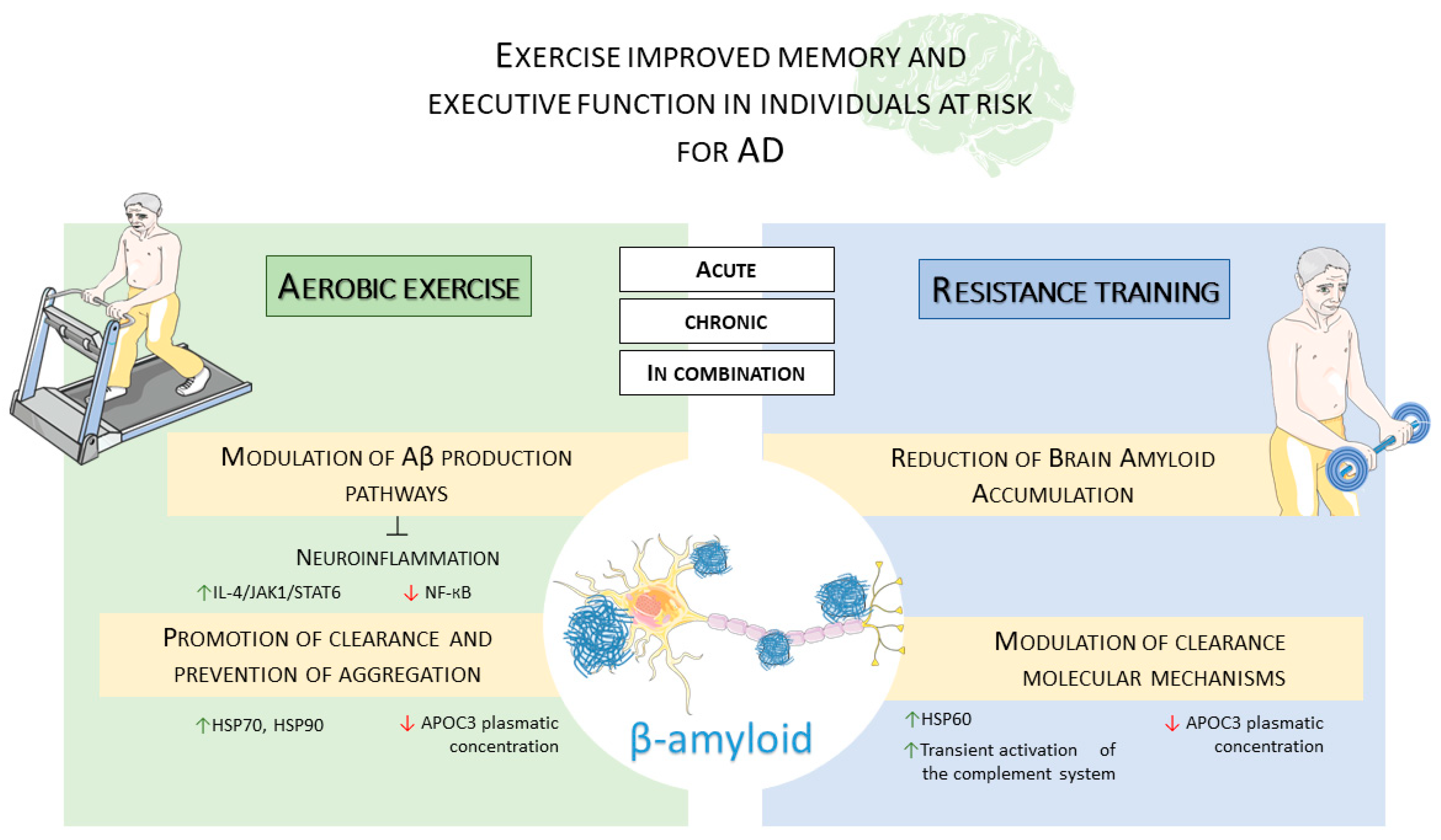
4. Amyloid Dis-Equilibrium in Alzheimer’s Disease and Physical Exercise Influences
4.1. Effects of Physical Exercise on Amyloid Production
4.2. Effects of Physical Exercise on the Regulation of Amyloid Precursor Protein Expression
4.3. Effects of Physical Exercise on Amyloid Clearance
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABCA7 | ATP binding cassette subfamily A member 7 |
| AD | Alzheimer’s Disease |
| ANAPC10 | Anaphase Promoting Complex Subunit 10 |
| ANKFY1 | Ankyrin Repeat And FYVE Domain Containing 1 |
| ANP32A | Acidic Nuclear Phosphoprotein 32 Family Member A |
| APBB1 | Amyloid Beta Precursor Protein Binding Family B Member 1 |
| APBB2 | Amyloid Beta Precursor Protein Binding Family B Member 2 |
| APOA2 | Apolipoprotein A-II |
| APOC3 | Apolipoprotein CIII |
| APOE | Apolipoprotein E |
| APP | Amyloid Precursor Protein |
| APPL2 | Adaptor Protein, Phosphotyrosine Interacting With PH Domain And Leucine Zipper 2 |
| ARF1 | ADP-Ribosylation Factor 1 |
| ARHGDIB | Rho GDP Dissociation Inhibitor Beta |
| ATG101 | Autophagy Related 101 |
| AUP1 | AUP1 Lipid Droplet Regulating VLDL Assembly Factor |
| Aβ | Amyloid-β |
| BACE1 | Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 |
| BBB | Blood–Brain Barrier |
| BIN1 | Bridging Integrator 1 |
| BRSK2 | BR Serine/Threonine Kinase 2 |
| BSN | Bassoon Presynaptic Cytomatrix Protein |
| C1QA | Complement C1q A Chain |
| C1QB | Complement C1q B Chain |
| C1S | Complement C1s |
| CALCOCO2 | Calcium Binding And Coiled-Coil Domain 2 |
| CAT | Catalase |
| CD99 | CD99 Molecule |
| CHMP4B | Charged Multivesicular Body Protein 4B |
| CIB1 | Calcium And Integrin Binding 1 |
| CIT | Citron Rho-Interacting Serine/Threonine Kinase |
| CLU | Clusterin |
| CNPY4 | Canopy FGF Signaling Regulator 4 |
| CNS | Central Nervous System |
| COCH | Cochlin |
| COG3 | Component Of Oligomeric Golgi Complex 3 |
| COMMD9 | COMM Domain Containing 9 |
| COX4I1 | Cytochrome C Oxidase Subunit 4I1 |
| CR1 | Complement Receptor 1 |
| CRELD1 | Cysteine Rich With EGF Like Domains 1 |
| CRHBP | Corticotropin Releasing Hormone Binding Protein |
| CTSF | Cathepsin F |
| DALYs | Disability-Adjusted Life Years |
| DAP3 | Death Associated Protein 3 |
| DIAPH1 | Diaphanous Related Formin 1 |
| DNER | Delta/Notch Like EGF Repeat Containing |
| ECHDC3 | Enoyl-CoA Hydratase Domain Containing 3 |
| EGFR | Epidermal Growth Factor Receptor |
| EIF4A3 | Eukaryotic Translation Initiation Factor 4A3 |
| EOAD | Early-Onset Alzheimer’s Disease |
| FAF1 | FAS Associated Factor 1 |
| FBXW9 | F-Box And WD Repeat Domain Containing 9 |
| FHL5 | Four And A Half LIM Domains 5 |
| FLNC | Filamin C |
| FOXO1/3 | Forkhead Box Protein O1/3 |
| GABARAP | GABA Type A Receptor-Associated Protein |
| GPX1 | Glutathione Peroxidase 1 |
| GWAS | Genome-Wide Association Studies |
| HKDC1 | Hexokinase Domain Containing 1 |
| HSP | Heat Shock Protein (including HSP60, HSP70, HSP90) |
| HTRA1 | HtrA Serine Peptidase 1 |
| IL-4/IL-6 | Interleukin 4/6 |
| ITIH3 | Inter-Alpha-Trypsin Inhibitor Heavy Chain 3 |
| JAK1 | Janus Kinase 1 |
| LAMP2 | Lysosomal Associated Membrane Protein 2 |
| LOAD | Late-Onset Alzheimer’s Disease |
| LRP1 | Low-Density Lipoprotein Receptor-Related Protein-1 |
| LRP1B | LDL Receptor Related Protein 1B |
| LTA4H | Leukotriene A4 Hydrolase |
| LTP | Long-Term Potentiation |
| MADD | MAP-kinase activating death domain |
| MAP1LC3A | Microtubule Associated Protein 1 Light Chain 3 Alpha |
| MAP2 | Microtubule Associated Protein 2 |
| MAP2K6 | Mitogen-Activated Protein Kinase Kinase 6 |
| MAVS | Mitochondrial Antiviral Signaling Protein |
| MLLT11 | MLLT11, PHD Finger Containing |
| MRPL53 | Mitochondrial Ribosomal Protein L53 |
| MT-ATP8 | Mitochondrially Encoded ATP Synthase Membrane Subunit 8 |
| MVP | Major Vault Protein |
| NECTIN1 | Nectin Cell Adhesion Molecule 1 |
| NFKB1 | Nuclear Factor Kappa B Subunit 1 |
| NHEJ1 | Non-Homologous End Joining Factor 1 |
| NUMB | Numb Endocytic Adapter Protein |
| NUMBL | Numb Like Endocytic Adaptor Protein |
| NUP98 | Nucleoporin 98 |
| PRKN | Parkin RBR E3 Ubiquitin Protein Ligase |
| PD | Parkinson’s Disease |
| PDXP | Pyridoxal Phosphatase |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PINK1 | PTEN Induced Kinase 1 |
| PJA2 | Praja Ring Finger Ubiquitin Ligase 2 |
| PP2A | Protein Phosphatase 2A |
| PPP2CA | Protein Phosphatase 2 Catalytic Subunit Alpha |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRKCA | Protein Kinase C Alpha |
| PRKCB | Protein Kinase C Beta |
| PRPF3 | Pre-mRNA Processing Factor 3 |
| PRRT3 | Proline Rich Transmembrane Protein 3 |
| PSAP | Proactivator Polypeptide |
| PSEN1 | Presenilin 1 |
| PSEN2 | Presenilin 2 |
| RAC3 | Rac Family Small GTPase 3 |
| RALBP1 | RalA Binding Protein 1 |
| RFC2/4/5 | Replication Factor C Subunit 2, 4, 5 |
| RHOC | Ras Homolog Family Member C |
| RIPK2 | Receptor Interacting Serine/Threonine Kinase 2 |
| RPGR | Retinitis Pigmentosa GTPase Regulator |
| RYR2 | Ryanodine Receptor 2 |
| SERPINI1 | Serpin Family I Member 1 |
| SF3B5 | Splicing Factor 3b Subunit 5 |
| SH3BGRL3 | SH3 Domain Binding Glutamate-Rich Protein Like 3 |
| SIRT1 | Sirtuin 1 |
| SNCA | Synuclein Alpha |
| SNF8 | SNF8, ESCRT-II Complex Subunit |
| SNRPD2 | Small Nuclear Ribonucleoprotein D2 Polypeptide |
| SOD2 | Superoxide Dismutase 2 |
| SORT1 | Sortilin 1 |
| SOX10 | SRY-Box Transcription Factor 10 |
| SRI | Sorcin |
| STX2 | Syntaxin 2 |
| SUN2 | Sad1 And UNC84 Domain Containing 2 |
| SYAP1 | Synapse Associated Protein 1 |
| TAOK2/3 | TAO Kinase 2, 3 |
| TBC1D8 | TBC1 Domain Family Member 8 |
| TECPR1 | Tectonin Beta-Propeller Repeat Containing 1 |
| TECR | Trans-2,3-Enoyl-CoA Reductase |
| TNPO3 | Transportin 3 |
| TOR1A | Torsin Family 1 Member A |
| TREM2 | Triggering Receptor Expressed On Myeloid Cells 2 |
| U2AF2 | U2 Small Nuclear RNA Auxiliary Factor 2 |
| UBE2H | Ubiquitin Conjugating Enzyme E2 H |
| VPS36 | VPS36, ESCRT-II Complex Subunit |
| WBP11 | WW Domain Binding Protein 11 |
| WDR12 | WD Repeat Domain 12 |
| WHO | World Health Organization |
| YLDs | Years Lived with Disability |
| YLLs | Years of Life Lost. |
| ZFYVE20 | Zinc Finger FYVE-Type Containing 20 |
References
- Mertas, B.; Bosgelmez, I.I. The Role of Genetic, Environmental, and Dietary Factors in Alzheimer’s Disease: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 1222. [Google Scholar] [CrossRef] [PubMed]
- Better, M.A. Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Salahuddin, P.; Fatima, M.T.; Uversky, V.N.; Khan, R.H.; Islam, Z.; Furkan, M. The role of amyloids in Alzheimer’s and Parkinson’s diseases. Int. J. Biol. Macromol. 2021, 190, 44–55. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, S.; Li, Z.; Wang, R. Global, regional and national burden of gastroesophageal reflux disease, 1990–2019: Update from the GBD 2019 study. Ann. Med. 2022, 54, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Calabro, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef]
- Nicolas, G. Recent advances in Alzheimer disease genetics. Curr. Opin. Neurol. 2024, 37, 154–165. [Google Scholar] [CrossRef]
- Alonso Vilatela, M.E.; Lopez-Lopez, M.; Yescas-Gomez, P. Genetics of Alzheimer’s disease. Arch. Med. Res. 2012, 43, 622–631. [Google Scholar] [CrossRef]
- Kim, J.H. Genetics of Alzheimer’s Disease. Dement. Neurocogn. Disord. 2018, 17, 131–136. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, F.; Nie, H.; Serohijos, A.W.; Sharma, S.; Wilcox, K.C.; Yin, S.; Dokholyan, N.V. Protein folding: Then and now. Arch. Biochem. Biophys. 2008, 469, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Haber, M.; Kula, P.; Juśkiewicz, A.; Grelewicz, O.; Kucy, N.; Servaas, E.; Czachor, A.; Kotula, A.; Siemiątkowski, R. Physical Exercise as a Strategy for Prevention and Management of Alzheimer’s Disease Progression. J. Educ. Health Sport 2024, 67, 55034. [Google Scholar] [CrossRef]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of physical exercise in Alzheimer’s disease. Biomed. Rep. 2016, 4, 403–407. [Google Scholar] [CrossRef]
- Khodadadi, D.; Gharakhanlou, R.; Naghdi, N.; Salimi, M.; Azimi, M.; Shahed, A.; Heysieattalab, S. Treadmill Exercise Ameliorates Spatial Learning and Memory Deficits Through Improving the Clearance of Peripheral and Central Amyloid-Beta Levels. Neurochem. Res. 2018, 43, 1561–1574. [Google Scholar] [CrossRef]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, K.; Majdi, A.; Baghaiee, B.; Hosseini, S.H.; Sadigh-Eteghad, S. Physical activity and beta-amyloid pathology in Alzheimer’s disease: A sound mind in a sound body. EXCLI J. 2017, 16, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Xia, J.; Xu, B. Physical exercise may exert its therapeutic influence on Alzheimer’s disease through the reversal of mitochondrial dysfunction via SIRT1-FOXO1/3-PINK1-Parkin-mediated mitophagy. J. Sport Health Sci. 2021, 10, 1–3. [Google Scholar] [CrossRef]
- Cass, S.P. Alzheimer’s Disease and Exercise: A Literature Review. Curr. Sports Med. Rep. 2017, 16, 19–22. [Google Scholar] [CrossRef]
- Cutuli, D.; Decandia, D.; Giacovazzo, G.; Coccurello, R. Physical Exercise as Disease-Modifying Alternative against Alzheimer’s Disease: A Gut-Muscle-Brain Partnership. Int. J. Mol. Sci. 2023, 24, 14686. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Wang, X.; Lin, H.; Zheng, L.; Zhang, Y.; Peng, L.; Huang, S.; Chen, L. Aerobic exercise improves clearance of amyloid-beta via the glymphatic system in a mouse model of Alzheimer’s Disease. Brain Res. Bull. 2025, 222, 111263. [Google Scholar] [CrossRef]
- Pahlavani, H.A. Exercise therapy to prevent and treat Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1243869. [Google Scholar] [CrossRef]
- Papatheodorou, I.; Moreno, P.; Manning, J.; Fuentes, A.M.; George, N.; Fexova, S.; Fonseca, N.A.; Fullgrabe, A.; Green, M.; Huang, N.; et al. Expression Atlas update: From tissues to single cells. Nucleic Acids Res. 2020, 48, D77–D83. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbaek, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Weis, B.K.; Balshaw, D.; Barr, J.R.; Brown, D.; Ellisman, M.; Lioy, P.; Omenn, G.; Potter, J.D.; Smith, M.T.; Sohn, L.; et al. Personalized exposure assessment: Promising approaches for human environmental health research. Environ. Health Perspect. 2005, 113, 840–848. [Google Scholar] [CrossRef]
- Agnihotri, A.; Aruoma, O.I. Alzheimer’s Disease and Parkinson’s Disease: A Nutritional Toxicology Perspective of the Impact of Oxidative Stress, Mitochondrial Dysfunction, Nutrigenomics and Environmental Chemicals. J. Am. Coll. Nutr. 2020, 39, 16–27. [Google Scholar] [CrossRef]
- Grant, W.B.; Campbell, A.; Itzhaki, R.F.; Savory, J. The significance of environmental factors in the etiology of Alzheimer’s disease. J. Alzheimers Dis. 2002, 4, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Martin-Hernandez, J.; Seisdedos, M.M.; Garcia-Lopez, O.; Toschi, N.; Di Giuliano, F.; Garaci, F.; Mercuri, N.B.; et al. Physical Exercise and Alzheimer’s Disease: Effects on Pathophysiological Molecular Pathways of the Disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, T.; Liu, Z.; Liang, Y.; Li, F.; Li, Y.; Liu, W.; Li, F.; Shi, S.; Zhou, C.; et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 2023, 380, e072691. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Hayes, S.M.; Alosco, M.L.; Forman, D.E. The Effects of Aerobic Exercise on Cognitive and Neural Decline in Aging and Cardiovascular Disease. Curr. Geriatr. Rep. 2014, 3, 282–290. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; Garcia-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Maliszewska-Cyna, E.; Xhima, K.; Aubert, I. A Comparative Study Evaluating the Impact of Physical Exercise on Disease Progression in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 243–257. [Google Scholar] [CrossRef]
- Wolfe, K.J.; Cyr, D.M. Amyloid in neurodegenerative diseases: Friend or foe? Semin. Cell Dev. Biol. 2011, 22, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Koszla, O.; Solek, P. Misfolding and aggregation in neurodegenerative diseases: Protein quality control machinery as potential therapeutic clearance pathways. Cell Commun. Signal. 2024, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Estrada, L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008, 65, 184–189. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Protein folding and aggregation into amyloid: The interference by natural phenolic compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef]
- Hiroaki, H. Molecular mechanisms of amyloid-beta peptide fibril and oligomer formation: NMR-based challenges. Biophys. Physicobiol. 2023, 20, e200007. [Google Scholar] [CrossRef] [PubMed]
- Cristovao, J.S.; Santos, R.; Gomes, C.M. Metals and Neuronal Metal Binding Proteins Implicated in Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2016, 2016, 9812178. [Google Scholar] [CrossRef]
- Mattson, M.P. Addendum: Pathways towards and away from Alzheimer’s disease. Nature 2004, 431, 107. [Google Scholar] [CrossRef]
- Maury, C.P. The emerging concept of functional amyloid. J. Intern. Med. 2009, 265, 329–334. [Google Scholar] [CrossRef]
- Murakami, K.; Ono, K. Interactions of amyloid coaggregates with biomolecules and its relevance to neurodegeneration. FASEB J. 2022, 36, e22493. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhao, N.; Xu, B.; Chen, X.H.; Li, T.J. Treadmill exercise inhibits amyloid-beta generation in the hippocampus of APP/PS1 transgenic mice by reducing cholesterol-mediated lipid raft formation. Neuroreport 2019, 30, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Filho, F.S.L.; da Rocha Oliveira, L.C.; de Freitas, T.B.C.; de Pontes, P.; Rocha, E.S.R.C.D.; Godinho, W.D.N.; Chaves, E.M.C.; da Silva, C.G.L.; Soares, P.M.; Ceccatto, V.M. Effect of involuntary chronic physical exercise on beta-amyloid protein in experimental models of Alzheimer’s disease: Systematic review and meta-analysis. Exp. Gerontol. 2021, 153, 111502. [Google Scholar] [CrossRef]
- Sepulveda-Lara, A.; Sepulveda, P.; Marzuca-Nassr, G.N. Resistance Exercise Training as a New Trend in Alzheimer’s Disease Research: From Molecular Mechanisms to Prevention. Int. J. Mol. Sci. 2024, 25, 7084. [Google Scholar] [CrossRef]
- Vidoni, E.D.; Morris, J.K.; Watts, A.; Perry, M.; Clutton, J.; Van Sciver, A.; Kamat, A.S.; Mahnken, J.; Hunt, S.L.; Townley, R.; et al. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: A 1-year randomized controlled trial. PLoS ONE 2021, 16, e0244893. [Google Scholar] [CrossRef]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Vassar, R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 2008, 283, 29621–29625. [Google Scholar] [CrossRef]
- Wolfe, M.S. Inhibition and modulation of gamma-secretase for Alzheimer’s disease. Neurotherapeutics 2008, 5, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cool, B.H.; Martin, G.M.; Hu, Q. A dominant role for FE65 (APBB1) in nuclear signaling. J. Biol. Chem. 2006, 281, 4207–4214. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Q.; Hearn, M.G.; Shimizu, K.; Ware, C.B.; Liggitt, D.H.; Jin, L.W.; Cool, B.H.; Storm, D.R.; Martin, G.M. Isoform-specific knockout of FE65 leads to impaired learning and memory. J. Neurosci. Res. 2004, 75, 12–24. [Google Scholar] [CrossRef]
- Strecker, P.; Ludewig, S.; Rust, M.; Mundinger, T.A.; Gorlich, A.; Krachan, E.G.; Mehrfeld, C.; Herz, J.; Korte, M.; Guenette, S.Y.; et al. FE65 and FE65L1 share common synaptic functions and genetically interact with the APP family in neuromuscular junction formation. Sci. Rep. 2016, 6, 25652. [Google Scholar] [CrossRef]
- Santiago, J.A.; Quinn, J.P.; Potashkin, J.A. Physical Activity Rewires the Human Brain against Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 6223. [Google Scholar] [CrossRef]
- Tan, J.Z.A.; Fourriere, L.; Wang, J.; Perez, F.; Boncompain, G.; Gleeson, P.A. Distinct anterograde trafficking pathways of BACE1 and amyloid precursor protein from the TGN and the regulation of amyloid-beta production. Mol. Biol. Cell 2020, 31, 27–44. [Google Scholar] [CrossRef]
- Covington, J.D.; Galgani, J.E.; Moro, C.; LaGrange, J.M.; Zhang, Z.; Rustan, A.C.; Ravussin, E.; Bajpeyi, S. Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS ONE 2014, 9, e91675. [Google Scholar] [CrossRef]
- Tomizawa, I.; Chiu, Y.W.; Hori, Y.; Tomita, T. Identification of novel regulators involved in AD pathogenesis using the CRISPR-Cas9 system. Nihon Yakurigaku Zasshi 2023, 158, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Ranganathan, S.; Robinson, S.; Strickland, D.K. gamma-Secretase-mediated release of the low density lipoprotein receptor-related protein 1B intracellular domain suppresses anchorage-independent growth of neuroglioma cells. J. Biol. Chem. 2007, 282, 7504–7511. [Google Scholar] [CrossRef]
- Taylor-Walker, G.; Lynn, S.A.; Keeling, E.; Munday, R.; Johnston, D.A.; Page, A.; Scott, J.A.; Goverdhan, S.; Lotery, A.J.; Ratnayaka, J.A. The Alzheimer’s-related amyloid beta peptide is internalised by R28 neuroretinal cells and disrupts the microtubule associated protein 2 (MAP-2). Exp. Eye Res. 2016, 153, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Capetillo-Zarate, E.; Lin, M.T.; Milner, T.A.; Gouras, G.K. Accumulation of intraneuronal beta-amyloid 42 peptides is associated with early changes in microtubule-associated protein 2 in neurites and synapses. PLoS ONE 2013, 8, e51965. [Google Scholar] [CrossRef]
- Salame, S.; Garcia, P.C.; Real, C.C.; Borborema, J.; Mota-Ortiz, S.R.; Britto, L.R.; Pires, R.S. Distinct neuroplasticity processes are induced by different periods of acrobatic exercise training. Behav. Brain Res. 2016, 308, 64–74. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ingano, L.A.; Kovacs, D.M. Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J. Biol. Chem. 2002, 277, 49976–49981. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Hu, G.; Lin, C.S.; ShiDu Yan, S. PINK1-dependent NFKB signaling contributes to amyloid pathology in Alzheimer disease. Autophagy 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Munoz, V.R.; Vieira, R.F.L.; Katashima, C.K.; Gaspar, R.C.; Lino, M.; Trombeta, J.; Duft, R.G.; Azevedo Macedo, A.P.; da Silva, A.S.R.; Ropelle, E.R.; et al. Rho-Kinase Is Differentially Expressed in the Adipose Tissue of Rodent and Human in Response to Aging, Sex, and Acute Exercise. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2024, 79, glae001. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Ye, S.; Li, W.X.; Zhang, J.; Liu, Y.; Zhu, J.; Lv, W.; Zhang, H.; Wang, J.; Lu, A.; et al. Regulatory function of praja ring finger ubiquitin ligase 2 mediated by the P2rx3/P2rx7 axis in mouse hippocampal neuronal cells. Am. J. Physiol. Cell Physiol. 2020, 318, C1123–C1135. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.I.; Callender, J.A.; Hooli, B.; Antal, C.E.; Mullin, K.; Sherman, M.A.; Lesne, S.E.; Leitges, M.; Newton, A.C.; Tanzi, R.E.; et al. Gain-of-function mutations in protein kinase Calpha (PKCalpha) may promote synaptic defects in Alzheimer’s disease. Sci. Signal. 2016, 9, ra47. [Google Scholar] [CrossRef]
- Hayley, M.; Perspicace, S.; Schulthess, T.; Seelig, J. Calcium enhances the proteolytic activity of BACE1: An in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim. Biophys. Acta 2009, 1788, 1933–1938. [Google Scholar] [CrossRef]
- Bussiere, R.; Lacampagne, A.; Reiken, S.; Liu, X.; Scheuerman, V.; Zalk, R.; Martin, C.; Checler, F.; Marks, A.R.; Chami, M. Amyloid beta production is regulated by beta2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor. J. Biol. Chem. 2017, 292, 10153–10168. [Google Scholar] [CrossRef]
- Babaee Bigi, M.A.U.; Faramarzi, H.U.; Gaeini, A.A.U.; Asghar Ravasi, A.U.; Izadi, M.R.U.; Delfan, M.U.; Izadi, E.U. Upregulation of Ryanodine Receptor Calcium Channels (RyR2) in Rats with Induced Diabetes after 4 Weeks of High Intensity Interval Training. Int. Cardiovasc. Res. J. 2016, 10, e10311. [Google Scholar] [CrossRef]
- Overby, M.; Serrano-Rodriguez, A.; Dadras, S.; Christiansen, A.K.; Ozcelik, G.; Lichtenthaler, S.F.; Weick, J.P.; Muller, H.K. Neuron-specific gene NSG1 binds to and positively regulates sortilin ectodomain shedding via a metalloproteinase-dependent mechanism. J. Biol. Chem. 2023, 299, 105446. [Google Scholar] [CrossRef]
- Berrocal, M.; Saez, L.; Mata, A.M. Sorcin Activates the Brain PMCA and Blocks the Inhibitory Effects of Molecular Markers of Alzheimer’s Disease on the Pump Activity. Int. J. Mol. Sci. 2021, 22, 6055. [Google Scholar] [CrossRef]
- Chang, Y.; Tesco, G.; Jeong, W.J.; Lindsley, L.; Eckman, E.A.; Eckman, C.B.; Tanzi, R.E.; Guenette, S.Y. Generation of the beta-amyloid peptide and the amyloid precursor protein C-terminal fragment gamma are potentiated by FE65L1. J. Biol. Chem. 2003, 278, 51100–51107. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Lepsch, L.B.; Boaventura, M.F.; Munhoz, C.D.; Lima, L.S.; Yshii, L.M.; Avellar, M.C.; Curi, R.; Mattson, M.P.; Scavone, C. Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J. Neurosci. Res. 2008, 86, 845–860. [Google Scholar] [CrossRef]
- Xu, H.; Tian, X.; Wang, Y.; Lin, J.; Zhu, B.; Zhao, C.; Wang, B.; Zhang, X.; Sun, Y.; Li, N.; et al. Exercise Promotes Hippocampal Neurogenesis in T2DM Mice via Irisin/TLR4/MyD88/NF-kappaB-Mediated Neuroinflammation Pathway. Biology 2024, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Bertan, F.; Wischhof, L.; Sosulina, L.; Mittag, M.; Dalugge, D.; Fornarelli, A.; Gardoni, F.; Marcello, E.; Di Luca, M.; Fuhrmann, M.; et al. Loss of Ryanodine Receptor 2 impairs neuronal activity-dependent remodeling of dendritic spines and triggers compensatory neuronal hyperexcitability. Cell Death Differ. 2020, 27, 3354–3373. [Google Scholar] [CrossRef] [PubMed]
- Paula-Lima, A.C.; Adasme, T.; SanMartin, C.; Sebollela, A.; Hetz, C.; Carrasco, M.A.; Ferreira, S.T.; Hidalgo, C. Amyloid beta-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid. Antioxid. Redox Signal. 2011, 14, 1209–1223. [Google Scholar] [CrossRef]
- Del Prete, D.; Checler, F.; Chami, M. Ryanodine receptors: Physiological function and deregulation in Alzheimer disease. Mol. Neurodegener. 2014, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Vervliet, T. Ryanodine Receptors in Autophagy: Implications for Neurodegenerative Diseases? Front. Cell Neurosci. 2018, 12, 89. [Google Scholar] [CrossRef]
- Hefter, D.; Ludewig, S.; Draguhn, A.; Korte, M. Amyloid, APP, and Electrical Activity of the Brain. Neuroscientist 2020, 26, 231–251. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Thompson, R.; Zhang, H.; Xu, H. APP processing in Alzheimer’s disease. Mol. Brain 2011, 4, 3. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Bhatnagar, A.; Armour, E.M.; Beaver, M.; Zhang, H.; Elefant, F. Amyloid-beta Peptide Impact on Synaptic Function and Neuroepigenetic Gene Control Reveal New Therapeutic Strategies for Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 577622. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, J.; Zhang, X.; Wang, S.; Viola, K.L.; Chow, F.E.; Zhang, Y.; Lippa, C.; Klein, W.L.; Gong, Y. Amyloid Beta Oligomers Target to Extracellular and Intracellular Neuronal Synaptic Proteins in Alzheimer’s Disease. Front. Neurol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Quintela-Lopez, T.; Ortiz-Sanz, C.; Serrano-Regal, M.P.; Gaminde-Blasco, A.; Valero, J.; Baleriola, J.; Sanchez-Gomez, M.V.; Matute, C.; Alberdi, E. Abeta oligomers promote oligodendrocyte differentiation and maturation via integrin beta1 and Fyn kinase signaling. Cell Death Dis. 2019, 10, 445. [Google Scholar] [CrossRef]
- Hoe, H.S.; Lee, H.K.; Pak, D.T. The upside of APP at synapses. CNS Neurosci. Ther. 2012, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Goh, M.Q.L.; Koo, E.H. Transcriptional regulation of APP by apoE: To boldly go where no isoform has gone before: ApoE, APP transcription and AD: Hypothesised mechanisms and existing knowledge gaps. Bioessays 2017, 39, 1700062. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.K.; Wurtman, R.J. Regulation of APP synthesis and secretion by neuroimmunophilin ligands and cyclooxygenase inhibitors. Ann. N. Y. Acad. Sci. 2000, 920, 261–268. [Google Scholar] [CrossRef]
- Sato, K.; Takayama, K.I.; Hashimoto, M.; Inoue, S. Transcriptional and Post-Transcriptional Regulations of Amyloid-beta Precursor Protein (APP) mRNA. Front. Aging 2021, 2, 721579. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Shen, J.; Kang, J. Regulation of gene expression by the APP family in the adult cerebral cortex. Sci. Rep. 2022, 12, 66. [Google Scholar] [CrossRef]
- Alkadhi, K.A.; Dao, A.T. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 86, 25–29. [Google Scholar] [CrossRef]
- Bartra, C.; Jager, L.A.; Alcarraz, A.; Meza-Ramos, A.; Sanguesa, G.; Corpas, R.; Guasch, E.; Batlle, M.; Sanfeliu, C. Antioxidant Molecular Brain Changes Parallel Adaptive Cardiovascular Response to Forced Running in Mice. Antioxidants 2022, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.; Yu, L.; Cui, R.; Zhang, Y.; Ding, H.; Yan, G. Treadmill Exercise Attenuates Cerebral Ischemia-Reperfusion Injury by Promoting Activation of M2 Microglia via Upregulation of Interleukin-4. Front. Cardiovasc. Med. 2021, 8, 735485. [Google Scholar] [CrossRef]
- Kwak, Y.D.; Dantuma, E.; Merchant, S.; Bushnev, S.; Sugaya, K. Amyloid-beta precursor protein induces glial differentiation of neural progenitor cells by activation of the IL-6/gp130 signaling pathway. Neurotox. Res. 2010, 18, 328–338. [Google Scholar] [CrossRef]
- Sontag, E.; Nunbhakdi-Craig, V.; Sontag, J.M.; Diaz-Arrastia, R.; Ogris, E.; Dayal, S.; Lentz, S.R.; Arning, E.; Bottiglieri, T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J. Neurosci. 2007, 27, 2751–2759. [Google Scholar] [CrossRef]
- Jian, Y.; Yuan, S.; Yang, J.; Lei, Y.; Li, X.; Liu, W. Aerobic Exercise Alleviates Abnormal Autophagy in Brain Cells of APP/PS1 Mice by Upregulating AdipoR1 Levels. Int. J. Mol. Sci. 2022, 23, 9921. [Google Scholar] [CrossRef]
- Jahabardeen, A.; Nirenjen, S.; Narayanan, J.; Chitra, V. A Review on the Role of SNCA Gene in Neurodegenerative Diseases. Cureus 2024, 16, e69450. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Yang, Y.P.; Chiang, T.; Yen, C. The alpha-MG exhibits neuroprotective potential by reducing amyloid beta peptide-induced inflammation, oxidative stress, and tau aggregation in human neural stem cells. Brain Res. 2025, 1852, 149506. [Google Scholar] [CrossRef]
- Massaad, C.A.; Washington, T.M.; Pautler, R.G.; Klann, E. Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13576–13581. [Google Scholar] [CrossRef]
- Carrazoni, G.S.; Mello-Carpes, P.B. Maternal exercise during pregnancy: Sex-specific impacts on offspring memory and maternal deprivation effects. Neurosci. Lett. 2025, 856–858, 138252. [Google Scholar] [CrossRef] [PubMed]
- Ogunro, O.B.; Karigidi, M.E.; Gyebi, G.A.; Turkistani, A.; Almehmadi, A.H. Tangeretin offers neuroprotection against colchicine-induced memory impairment in Wistar rats by modulating the antioxidant milieu, inflammatory mediators and oxidative stress in the brain tissue. BMC Complement. Med. Ther. 2025, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Marciniuk, K.; Taschuk, R.; Napper, S. Evidence for prion-like mechanisms in several neurodegenerative diseases: Potential implications for immunotherapy. Clin. Dev. Immunol. 2013, 2013, 473706. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Brandner, S. Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 522–545. [Google Scholar] [CrossRef]
- Cabrera, A.C.; Melo, E.; Roth, D.; Topp, A.; Delobel, F.; Stucki, C.; Chen, C.Y.; Jakob, P.; Banfai, B.; Dunkley, T.; et al. HtrA1 activation is driven by an allosteric mechanism of inter-monomer communication. Sci. Rep. 2017, 7, 14804. [Google Scholar] [CrossRef]
- Grau, S.; Baldi, A.; Bussani, R.; Tian, X.; Stefanescu, R.; Przybylski, M.; Richards, P.; Jones, S.A.; Shridhar, V.; Clausen, T.; et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 2005, 102, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Kavanagh, T.; Pires, G.; Marta-Ariza, M.; Kanshin, E.; Nayak, S.; Faustin, A.; Berdah, V.; Ueberheide, B.; Wisniewski, T. The amyloid plaque proteome in early onset Alzheimer’s disease and Down syndrome. Acta Neuropathol. Commun. 2022, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.H.; Tan, L.; Cao, X.; Yu, J.T.; Tan, L. Clearance of Amyloid Beta and Tau in Alzheimer’s Disease: From Mechanisms to Therapy. Neurotox. Res. 2018, 34, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Kogishi, K.; Wang, J.; Xia, C.; Matsushita, T.; Miyazaki, J.; Saito, I.; Hosokawa, M.; Higuchi, K. Mouse senile amyloid deposition is suppressed by adenovirus-mediated overexpression of amyloid-resistant apolipoprotein A-II. Am. J. Pathol. 1999, 155, 1319–1326. [Google Scholar] [CrossRef]
- Ge, F.; Yao, J.; Fu, X.; Guo, Z.; Yan, J.; Zhang, B.; Zhang, H.; Tomozawa, H.; Miyazaki, J.; Sawashita, J.; et al. Amyloidosis in transgenic mice expressing murine amyloidogenic apolipoprotein A-II (Apoa2c). Lab. Investig. 2007, 87, 633–643. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, K.J.; Lee, C.W.; Shiesh, S.C.; Chen, W.T.; Pai, M.C.; Kuo, Y.M. Apolipoprotein C-III is an amyloid-beta-binding protein and an early marker for Alzheimer’s disease. J. Alzheimers Dis. 2014, 41, 855–865. [Google Scholar] [CrossRef]
- Sacks, F.M.; Furtado, J.D.; Jensen, M.K. Protein-based HDL subspecies: Rationale and association with cardiovascular disease, diabetes, stroke, and dementia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159182. [Google Scholar] [CrossRef]
- Song, Y.; Shi, X.; Gao, Z.; Li, R.; Tian, J.; Cao, X.; Yang, B.; Zhao, S.; Yang, Y. Acupoint Catgut Embedding Improves Lipid Metabolism in Exercise-Induced Fatigue Rats via the PPAR Signaling Pathway. Animals 2023, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, L.; Xu, D. Aerobic exercise reduces triglycerides by targeting apolipoprotein C3 in patients with coronary heart disease. Clin. Cardiol. 2019, 42, 56–61. [Google Scholar] [CrossRef]
- Festa, B.P.; Barbosa, A.D.; Rob, M.; Rubinsztein, D.C. The pleiotropic roles of autophagy in Alzheimer’s disease: From pathophysiology to therapy. Curr. Opin. Pharmacol. 2021, 60, 149–157. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhu, X.X.; Tang, D.S.; Lu, J.H. Targeting autophagy in Alzheimer’s disease: Animal models and mechanisms. Zool. Res. 2023, 44, 1132–1145. [Google Scholar] [CrossRef]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K.; et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell 2013, 12, 487–496. [Google Scholar] [CrossRef]
- Nishitsuji, K.; Tomiyama, T.; Ishibashi, K.; Ito, K.; Teraoka, R.; Lambert, M.P.; Klein, W.L.; Mori, H. The E693Delta mutation in amyloid precursor protein increases intracellular accumulation of amyloid beta oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am. J. Pathol. 2009, 174, 957–969. [Google Scholar] [CrossRef]
- Brazil, M.I.; Chung, H.; Maxfield, F.R. Effects of incorporation of immunoglobulin G and complement component C1q on uptake and degradation of Alzheimer’s disease amyloid fibrils by microglia. J. Biol. Chem. 2000, 275, 16941–16947. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Graham, L.C.; Richter, H.J.; Simeone, S.N.; Radell, J.E.; Grabowska, W.; Funkhouser, W.K.; Howell, M.C.; Howell, G.R. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol. 2015, 13, e1002279. [Google Scholar] [CrossRef]
- Mees, L.M.; Coulter, M.M.; Chrenek, M.A.; Motz, C.T.; Landis, E.G.; Boatright, J.H.; Pardue, M.T. Low-Intensity Exercise in Mice Is Sufficient to Protect Retinal Function During Light-Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Stoica, B.A.; Wu, J.; Sabirzhanov, B.; Zhao, Z.; Cabatbat, R.; Loane, D.J.; Faden, A.I. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Dis. 2013, 54, 252–263. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Rothschild-Rodriguez, D.; Causer, A.J.; Brown, F.F.; Collier-Bain, H.D.; Moore, S.; Murray, J.; Turner, J.E.; Campbell, J.P. The effects of exercise on complement system proteins in humans: A systematic scoping review. Exerc. Immunol. Rev. 2022, 28, 1–35. [Google Scholar]
- Kim, S.; Lee, D.; Song, J.C.; Cho, S.J.; Yun, S.M.; Koh, Y.H.; Song, J.; Johnson, G.V.; Jo, C. NDP52 associates with phosphorylated tau in brains of an Alzheimer disease mouse model. Biochem. Biophys. Res. Commun. 2014, 454, 196–201. [Google Scholar] [CrossRef]
- Zimbone, S.; Di Rosa, M.C.; Chiechio, S.; Giuffrida, M.L. Exploring the Role of Hsp60 in Alzheimer’s Disease and Type 2 Diabetes: Suggestion for Common Drug Targeting. Int. J. Mol. Sci. 2023, 24, 12456. [Google Scholar] [CrossRef]
- Cui, K.; Li, C.; Fang, G. Aerobic Exercise Delays Alzheimer’s Disease by Regulating Mitochondrial Proteostasis in the Cerebral Cortex and Hippocampus. Life 2023, 13, 1204. [Google Scholar] [CrossRef]
- Valle-Medina, A.; Calzada-Mendoza, C.C.; Ocharan-Hernandez, M.E.; Jimenez-Zamarripa, C.A.; Juarez-Cedillo, T. Heat shock protein 70 in Alzheimer’s disease and other dementias: A possible alternative therapeutic. J. Alzheimers Dis. Rep. 2025, 9, 25424823241307021. [Google Scholar] [CrossRef]
- Schirmer, C.; Lepvrier, E.; Duchesne, L.; Decaux, O.; Thomas, D.; Delamarche, C.; Garnier, C. Hsp90 directly interacts, in vitro, with amyloid structures and modulates their assembly and disassembly. Biochim. Biophys. Acta 2016, 1860, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Reichel, T.; Zeilinger, C. Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J. Appl. Physiol. (1985) 2019, 126, 916–927. [Google Scholar] [CrossRef]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 04. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, G.A.; Wei, Z.; Vandermey, M.; Jo, D.G.; Xin, O.; Mattson, M.P.; Chan, S.L. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: Implications for Alzheimer disease pathogenesis. J. Biol. Chem. 2008, 283, 25492–25502. [Google Scholar] [CrossRef]
- Brugger, M.; Lauri, A.; Zhen, Y.; Gramegna, L.L.; Zott, B.; Sekulic, N.; Fasano, G.; Kopajtich, R.; Cordeddu, V.; Radio, F.C.; et al. Bi-allelic variants in SNF8 cause a disease spectrum ranging from severe developmental and epileptic encephalopathy to syndromic optic atrophy. Am. J. Hum. Genet. 2024, 111, 594–613. [Google Scholar] [CrossRef] [PubMed]
- Fruhmann, G.; Marchal, C.; Vignaud, H.; Verduyckt, M.; Talarek, N.; De Virgilio, C.; Winderickx, J.; Cullin, C. The Impact of ESCRT on Abeta(1-42) Induced Membrane Lesions in a Yeast Model for Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, R.; He, Y.; Zhang, B.; Rui, X.; Yang, X.; Wang, J.Z.; Zeng, J.; Li, G.; Li, X.; et al. Overexpression of TECPR1 improved cognitive function of P301S-tau mice via activation of autophagy in the early and late process. Aging Cell 2025, 24, e14404. [Google Scholar] [CrossRef] [PubMed]
- Parrington, J.; Arnoult, C.; Fissore, R.A. The eggstraordinary story of how life begins. Mol. Reprod. Dev. 2019, 86, 4–19. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Khoonsari, P.E.; Haggmark, A.; Lonnberg, M.; Mikus, M.; Kilander, L.; Lannfelt, L.; Bergquist, J.; Ingelsson, M.; Nilsson, P.; Kultima, K.; et al. Analysis of the Cerebrospinal Fluid Proteome in Alzheimer’s Disease. PLoS ONE 2016, 11, e0150672. [Google Scholar] [CrossRef]
- Turturici, G.; Sconzo, G.; Geraci, F. Hsp70 and its molecular role in nervous system diseases. Biochem. Res. Int. 2011, 2011, 618127. [Google Scholar] [CrossRef]
- Fontaine, S.N.; Martin, M.D.; Dickey, C.A. Neurodegeneration and the Heat Shock Protein 70 Machinery: Implications for Therapeutic Development. Curr. Top. Med. Chem. 2016, 16, 2741–2752. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Bavisotto, C.C.; Barone, R.; de Macario, E.C.; Macario, A.J. Alzheimer’s Disease and Molecular Chaperones: Current Knowledge and the Future of Chaperonotherapy. Curr. Pharm. Des. 2016, 22, 4040–4049. [Google Scholar] [CrossRef]
- Inda, C.; Bolaender, A.; Wang, T.; Gandu, S.R.; Koren, J., 3rd. Stressing Out Hsp90 in Neurotoxic Proteinopathies. Curr. Top. Med. Chem. 2016, 16, 2829–2838. [Google Scholar] [CrossRef]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; Pace, A.; Caruso Bavisotto, C.; Marzullo, P.; Marino Gammazza, A.; Buscemi, S.; Palumbo Piccionello, A. Heat Shock Proteins in Alzheimer’s Disease: Role and Targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.R.; Chen, S. Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med. 2017, 14, 5267–5274. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Time | Metrics | ||
|---|---|---|---|---|
| Mean | Upper | Lower | ||
| DALYs (Disability-Adjusted Life Years) | 2021 (2025) | 36′332′686.74 (40′363′865.03) | 76′873′276.22 (85′077′305.86) | 17′237′624.04 (19′031′052.83) |
| YLDs (Years Lived with Disability) | 2021 (2025) | 11′582′108.01 (13′150′993.85) | 15′296′793.45 (17′401′164.78) | 7′961′941.52 (8′999′781.94) |
| Incidence | 2021 (2025) | 56′856′688.21 (64′409′200.27) | 64′977′511.92 (73′637′607.71) | 49′382′064.01 (56′004′781.73) |
| Prevalence | 2021 (2025) | 56′856′688.21 (64′409′200.27) | 64′977′511.92 (73′637′607.71) | 49′382′064.01 (56′004′781.73) |
| α-secretase | |
| Down-regulated genes: | ANAPC10, COCH, EGFR, HKDC1, NHEJ1, NUP98, PIK3CA, SNRPD2, TBC1D8 |
| Up-regulated genes: | LTA4H, PRPF3 |
| β-secretase | |
| Down-regulated genes: | ARF1, CIT, CRELD1, DIAPH1, ITIH3, MRPL53, PRKCB, PRRT3, RAC3, RPGR, RYR2 |
| Up-regulated genes: | ARHGDIB, ECHDC3, FLNC, PRKCA, RHOC, SH3BGRL3, STX2, TECR |
| γ-secretase | |
| Down-regulated genes: | APBB1, APBB2, BRSK2, CIB1, COG3, CRHBP, DAP3, DNER, EIF4A3, FAF1, FBXW9, MAP2, MLLT11, MT-ATP8, NECTIN1, NFKB1, PDXP, PJA2, RALBP1, SERPINI1, SF3B5, SYAP1, TNPO3, U2AF2 |
| Up-regulated genes: | CHMP4B, LRP1B, MVP, RIPK2, SORT1, SOX10, SRI, SUN2 |
| Gene | Role in the Context of Aβ | Role in the Context of Physical Exercise |
|---|---|---|
| APBB1 | Adaptor for APP intracellular domain as transcriptional activator; FE65–APP signaling affects memory [52,53]. | Not reported |
| APBB2 | Regulates synaptic development via APP, the precursor of Aβ [54]. | Down-regulated by high and moderate physical activity [55]. |
| ARF1 | Regulates BACE1 transport and Aβ production [56]. | Up-regulated by endurance exercise [57]. |
| CIB1 | Inhibits γ-secretase, lowering Aβ production in neurons from early AD patients [58]. | Not reported |
| LRP1B | Reduces Aβ production [56] and is a substrate of γ-secretase [59]. | Not reported |
| MAP2 | Aβ1-42 reduces MAP2 expression in central nervous system (CNS) [60,61]. | Physical activity contributes to MAP2 expression up-regulation [62]. |
| NECTIN1 | PS1-dependent cleavage links adhesion to Aβ control [63]. | Not reported |
| NFKB1 | Increases both β- and γ-secretase activity, accelerating Aβ production [64]. | Transiently elevated in adipose after exercise [65]. |
| PJA2 | Lowers APP mRNA via P2X receptor regulation [66]. | Not reported |
| PRKCA | PKCα activity mediates Aβ-induced synaptic depression [67]. | Not reported |
| RYR2 | Ca2+ release via RYR2 enhances β-secretase activity [68], and Aβ promotes RYR2 Ca2+ release [69]. | RYR2 expression is up-regulated by exercise [70]. |
| SORT1 | Regulates APP/Aβ trafficking, accumulates in plaques [71]. | Not reported |
| SRI | It counters Aβ and Tau related toxicity [72]. | Not reported |
| APP Metabolism and Expression Regulation | |
| Down-regulated genes: | APBB1, PSAP, RFC2, RFC4, RFC5, SNCA, SOD2, TOR1A, WDR12 |
| Up-regulated genes: | CAT, GPX1, JAK1, LAMP2, PPP2CA, WBP11 |
| Gene | Role in the Context of Aβ | Role in the Context of Physical Exercise |
|---|---|---|
| CAT | May indirectly regulate APP expression by modulation of ROS, and the subsequent regulation of the ROS-induced activation of NF-κB/AP-1 [88]. | Physical activity elevates CAT expression in murine models [91]. |
| GPX1 | GPX1 also indirectly regulates APP expression by acting on ROS concentration [88]. | Not reported |
| JAK1 | Activated by Aβ via IL-6/JAK1/STAT3, promoting gliosis and neuroinflammation [92]. | Exercise stimulates IL-4/JAK1/STAT6, driving anti-inflammatory microglial polarization and neuroprotection [93]. |
| PPP2CA | Component of PP2A. It is associated with decreased concentration of Aβ peptides, due to its modifications on APP [94]. | Exercise in murine AD models significantly increases PP2A, likely exerting positive effects in such models [95]. |
| SNCA | Participates in synaptic dysfunction and Lewy body formation in presence of Aβ and tau aggregates [96]. | Not reported |
| SOD2 | Down-regulated in Aβ-exposed neural stem cells, increases oxidative damage. Its increase seemingly mitigates Aβ plaque burden in Murine models [97,98]. | Not reported |
| Aβ Clearance | |
| Down-regulated genes: | APOA2, APOC3, ATG101, BSN, COMMD9, COX4I1, CTSF, GABARAP, MADD, MAP1LC3A, NUMB, NUMBL, SNF8, TAOK2, TECPR1, UBE2H, VPS36, ZFYVE20 |
| Up-regulated genes: | ANKFY1, ANP32A, APPL2, AUP1, C1QA, C1QB, C1S, CALCOCO2, CD99, CNPY4, FHL5, MAP2K6, MAVS, TAOK3 |
| Gene | Role in the Context of Aβ | Role in the Context of Physical Exercise |
|---|---|---|
| APOA2 | In an experimental model, APOA2(b) suppresses Aβ fibril extension [107]. APOA2(c) promotes systemic and spontaneous Aβ deposition in transgenic mice [108]. | Not reported |
| APOC3 | APOC3 has been shown to bind Aβ, and low levels of this protein are associated with AD risk [109,110]. | In murine experimental models it was observed that treadmill exercise increases APOC3 [111]; however, another study highlighted a reduction in APOC3 after aerobic exercise [112]. |
| ATG101 | ATG101 is an essential component of the ULK1–ATG13–FIP200 initiation complex, which is important for autophagy [113]. Autophagy has been implicated in proper APP and Aβ turnover [114]. Its down-regulation in AD brains likely contributes to the autophagy defects that exacerbate Aβ accumulation. | Not reported |
| AUP1 | While no direct correlation of Aβ degradation has been observed, this protein is an important component of the HRD1-SEL1L complex, essential in the degradation of misfolded proteins of the plasmatic reticulum, where the highly amyloidogenic Aβ1-42, is generated and accumulated [115,116]. | Not reported |
| C1QA | Opsonizes Aβ fibrils for microglial uptake, potentially promoting plaque clearance, even though the process is slow [117]. It is also involved in neuroinflammation [117]. | Exercise seemingly decreases C1QA+ microglia in murine models [118,119]. |
| C1QB | Opsonizes Aβ fibrils for microglial uptake, potentially promoting plaque clearance, even though the process is slow [117]. It is also involved in neuroinflammation [117]. | Exercise reduces C1q+ microglia [118,120], even though some data indicate that acute exercise increases C1QB [120]. |
| C1S | Participates in the opsonization of Aβ fibrils for microglial uptake, potentially promoting plaque clearance, even though the process is slow [117,121]. | Up-regulated in response to ultra-endurance running and resistance training [122]. |
| CALCOCO2 | Up-regulated in AD mouse brain; binds intracellular Aβ in autophagic vesicles for lysosomal degradation [123]. | Not reported |
| Chaperon HSP60 | Inhibits Aβ1–40 and Aβ1–42 aggregation, reducing toxic oligomer formation and supporting mitochondrial function [124]. | In murine experimental models of AD exercise increased the expression of Chaperon HSP60 [125]. |
| Chaperon HSP70 | Prevents Aβ aggregation by promoting solubilization and degradation via autophagy and proteasomal pathways, protecting neurons from Aβ-induced toxicity [126]. | In murine experimental models of AD exercise increased the expression of Chaperon HSP70 [125]. |
| Chaperon HSP90 | Binds to Aβ fibrils and tau aggregates, modulating assembly/disassembly and contributing to Aβ pathology regulation [127]. | Appears to be up-regulated by exercise, despite it having variable responses to specific exercise types and modalities [128]. |
| MAP1LC3A | Multiple studies report decreased expression of LC3 proteins—including LC3A—in postmortem AD hippocampus and cortex, correlating with autophagic-vacuole accumulation and extracellular Aβ plaque buildup [129]. | Not reported |
| NUMB | Likely involved in the transport of APP and in the modulation of its accumulation [130]. | Not reported |
| SNF8 | SNF8 encodes the EAP30 subunit of the ESCRTII complex. Loss of its function impairs lysosome trafficking, promoting aberrant accumulation of Aβ and damage, as observe in yeast models of AD [131,132]. | Not reported |
| TECPR1 | Results down-regulated in murine models of AD. Here, the induced overexpression of TECPR1 seems able to restore autophagic flux, reducing abnormal proteins accumulation [133]. | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astorino, M.F.; Cipriano, G.L.; Anchesi, I.; Lui, M.; Raffaele, I.; Calabrò, M.; Crisafulli, C. Gene-Exercise Interactions in Amyloid Metabolism and Clearance: Implications for Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9816. https://doi.org/10.3390/ijms26199816
Astorino MF, Cipriano GL, Anchesi I, Lui M, Raffaele I, Calabrò M, Crisafulli C. Gene-Exercise Interactions in Amyloid Metabolism and Clearance: Implications for Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9816. https://doi.org/10.3390/ijms26199816
Chicago/Turabian StyleAstorino, Maria Francesca, Giovanni Luca Cipriano, Ivan Anchesi, Maria Lui, Ivana Raffaele, Marco Calabrò, and Concetta Crisafulli. 2025. "Gene-Exercise Interactions in Amyloid Metabolism and Clearance: Implications for Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 19: 9816. https://doi.org/10.3390/ijms26199816
APA StyleAstorino, M. F., Cipriano, G. L., Anchesi, I., Lui, M., Raffaele, I., Calabrò, M., & Crisafulli, C. (2025). Gene-Exercise Interactions in Amyloid Metabolism and Clearance: Implications for Alzheimer’s Disease. International Journal of Molecular Sciences, 26(19), 9816. https://doi.org/10.3390/ijms26199816







