Exposure to Fluoride During Pregnancy and Lactation Induces Metabolic Imbalance in Pancreas: A Toxicological Insight Using the Rat Model
Abstract
1. Introduction
2. Results
2.1. Analysis of Fluoride Ion Concentrations in the Tissues and Serum of Fluoride-Exposed Rats Compared with Controls
2.2. Analysis of the Concentration of the Hormones Insulin, Glucagon, and Somatostatin in the Serum of Rats Exposed to Fluoride and Control Group
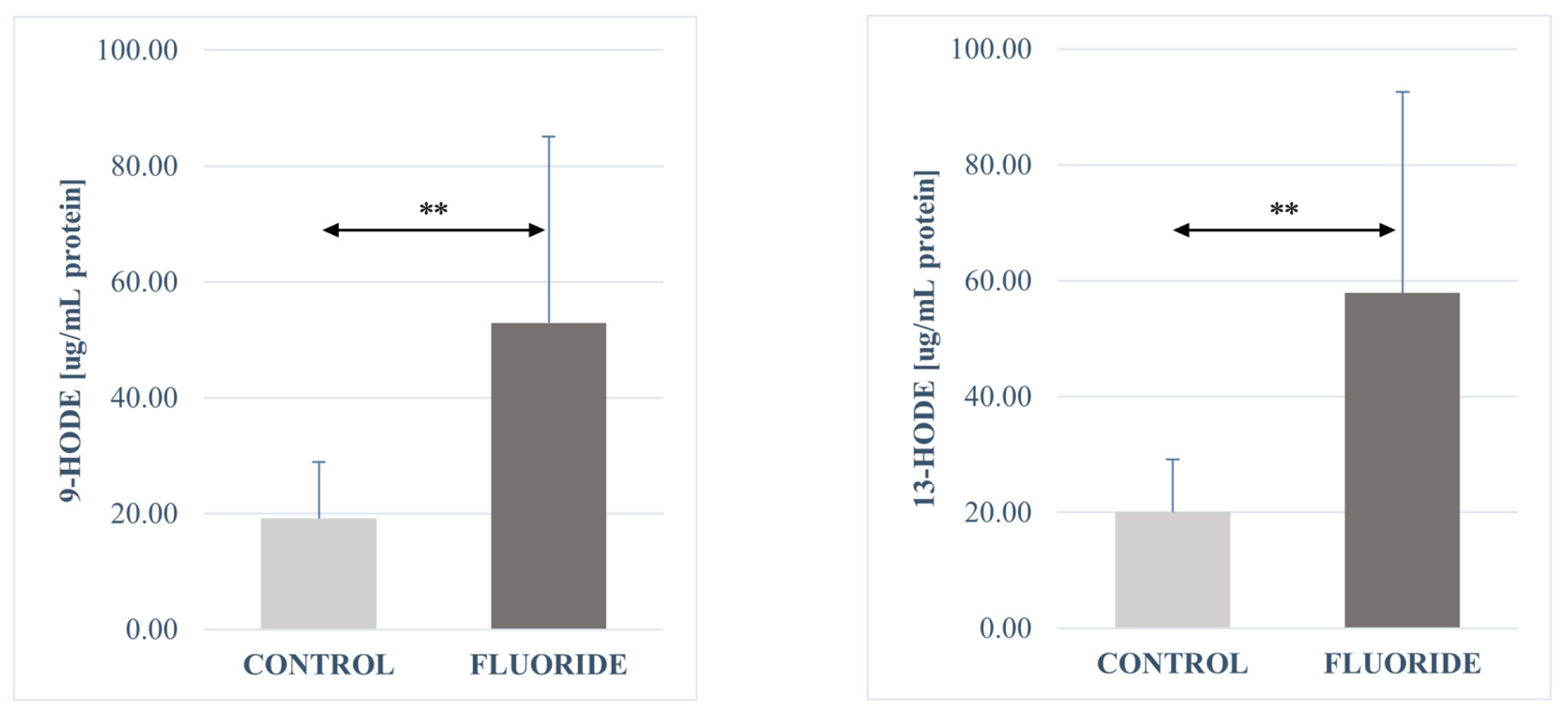
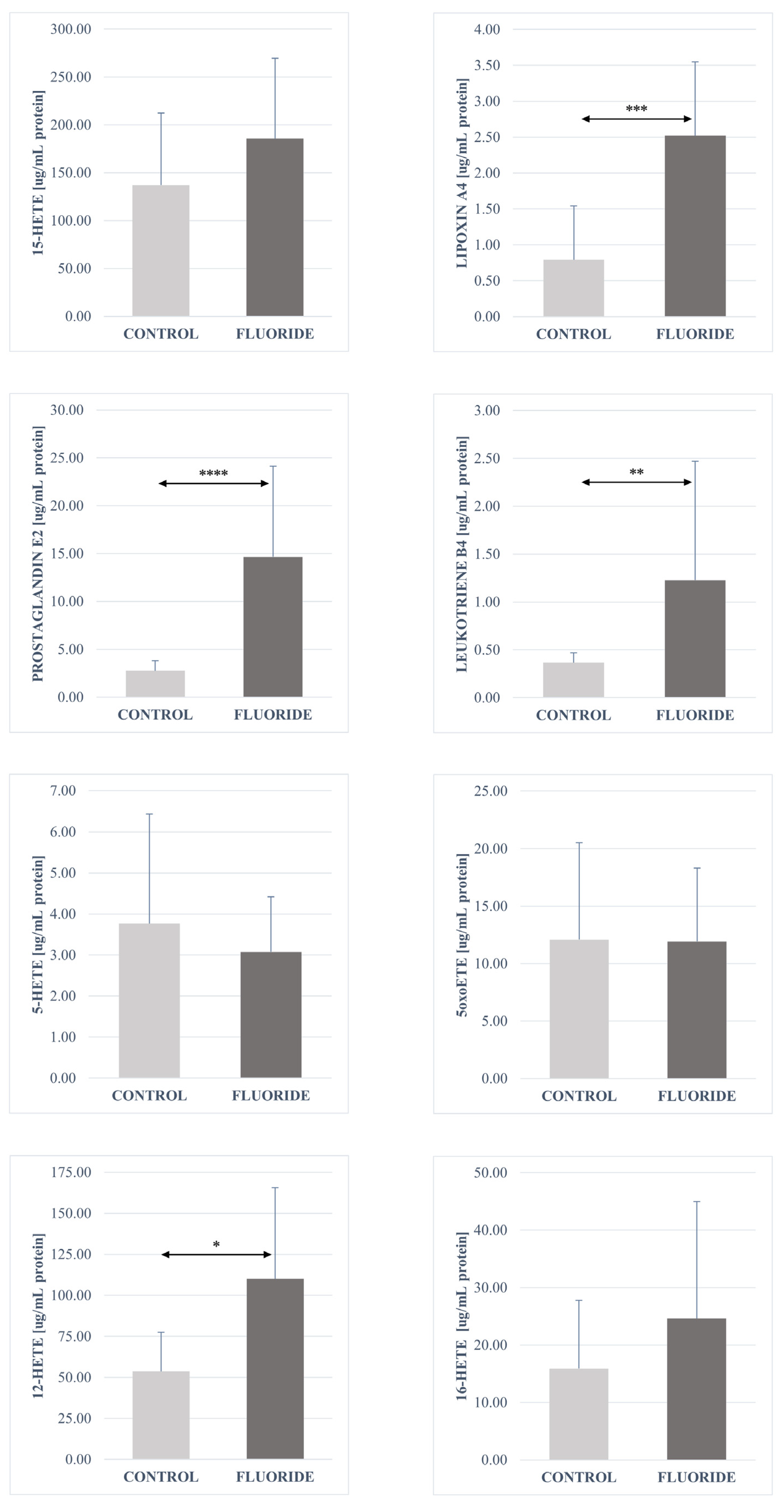
2.3. Analysis of the Fatty Acid Derivatives Concentrations in Pancreas Tissues of Rats Exposed to Fluoride and Control Group
3. Discussion
3.1. Fluoride Accumulation in the Rat Body
3.2. Concentration of Fatty Acid Derivatives in the Pancreas
3.3. Influence of Fluoride on Pancreatic Hormone Concentrations
4. Materials and Methods
4.1. Fluoride Toxicity Model In Vivo
4.2. Determination of the Concentration of Fluoride Ions in Tissue and Serum Homogenates Using the Potentiometric Method
4.3. Determination of Fatty Acid Derivative Concentrations
4.4. Measurement of Insulin, Glucagon, and Somatostatin in Serum
4.5. Measurement of Protein Concentration
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dec, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Pilutin, A.; Maciejewska, D.; Skonieczna-Żydecka, K.; Derkacz, R.; Goschorska, M.; Wąsik, A.; Rębacz-Maron, E.; et al. Pre- and postnatal exposition to fluorides induce changes in rats liver morphology by impairment of antioxidant defense mechanisms and COX induction. Chemosphere 2018, 211, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, A.; Verma, K.; Paliwal, S.; Sharma, S.; Dwivedi, J. Fluoride: A review of pre-clinical and clinical studies. Environ. Toxicol. Pharmacol. 2017, 56, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Chen, L.; Kong, M.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Polkowska, Ż.; Diduch, M.; Namieśnik, J. Determination of fluoride ions in drinking water samples collected from the area of the town of Malbork. Ecol. Chem. Eng. S 2010, 17, 3. [Google Scholar]
- Chlubek, D. Fluoride and oxidative stress. Fluoride 2003, 36, 217–228. [Google Scholar]
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of fluoride on endocrine tissues and their secretory functions—Review. Chemosphere 2020, 260, 127565. [Google Scholar] [CrossRef] [PubMed]
- Brook, C.; Marshall, N. Essential Endocrinology; Blackwell Science: Oxford, UK, 1996. [Google Scholar]
- Rodriguez-Diaz, R.; Tamayo, A.; Hara, M.; Caicedo, A. The Local Paracrine Actions of the Pancreatic α-Cell. Diabetes 2020, 69, 550–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dec, K.; Łukomska, A.; Maciejewska, D.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Chlubek, D.; Wąsik, A.; Gutowska, I. The Influence of Fluorine on the Disturbances of Homeostasis in the Central Nervous System. Biol. Trace Element Res. 2017, 177, 224–234. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Du, X.; Zhao, Y.; Wang, J.; Wang, J. Fluoride Can Damage the Spleen of Mice by Perturbing Th1/Th2 Cell Balance. Biol. Trace Element Res. 2020, 199, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S.J.S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523, Erratum in Nat. Rev. Immunol. 2015, 15, 724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, P.; Wang, M.H. Eicosanoids, β-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011, 95, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robertson, R.P. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes 1998, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Bleich, D.; Chen, S.; Gu, J.L.; Nadler, J.L. The role of 12-lipoxygenase in pancreatic-cells (Review). Int. J. Mol. Med. 1998, 1, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P. Eicosanoids as pluripotential modulators of pancreatic islet function. Diabetes 1988, 37, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Mateu, A.; Ramudo, L.; Manso, M.; Closa, D.; De Dios, I. Acinar inflammatory response to lipid derivatives generated in necrotic fat during acute pancreatitis. Biochim. Biophys. Acta 2014, 1842, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Seth, A.K.; Gupta, A.; Gavane, A.G. Transplacental passage of fluorides. J. Pediatr. 1993, 123, 139–141. [Google Scholar] [CrossRef]
- Shimonovitz, S.; Patz, D.; Ever-Hadani, P.; Singer, L.; Zacut, D.; Kidroni, G.; Ron, M. Umbilical cord fluoride serum levels may not reflect fetal fluoride status. J. Perinat. Med. 1995, 23, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Tewari, A.; Chawla, H.S.; Gauba, K.; Dhall, K. Placental transfer of fluoride in pregnant women consuming optimum fluoride in drinking water. J. Indian Soc. Pedod. Prev. Dent. 1993, 11, 1–3. [Google Scholar]
- Dhar, V.; Bhatnagar, M. Physiology and toxicity of fluoride. Indian J. Dent. Res. 2009, 20, 350–355. [Google Scholar] [CrossRef]
- Knaus, R.M.; Dost, F.N.; Johnson, D.E.; Wang, C.H. Fluoride distribution in rats during and after continuous infusion of Na18F. Toxicol. Appl. Pharmacol. 1976, 38, 335–343. [Google Scholar] [CrossRef]
- Ortiz-Placín, C.; Castillejo-Rufo, A.; Estarás, M.; González, A. Membrane Lipid Derivatives: Roles of Arachidonic Acid and Its Metabolites in Pancreatic Physiology and Pathophysiology. Molecules 2023, 28, 4316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, W.; Schlosser, S.; Ramadani, M.; Gansauge, F.; Gansauge, S.; Beger, H.G. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas 2002, 25, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Closa, D.; Rosello-Catafau, J.; Hotter, G.; Bulbena, O.; Fernandez-Cruz, L.; Gelpi, E. Cyclooxygenase and lipoxygenase metabolism in sodium taurocholate induced acute hemorrhagic pancreatitis in rats. Prostaglandins 1993, 45, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Taylor-Fishwick, D.A.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 2011, 50, 115–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, S.; Ishii, M.; Nakadate, T.; Nakaki, T.; Kato, R. Modulation of insulin secretion by lipoxygenase products of arachidonic acid. Relation to lipoxygenase activity of pancreatic islets. J. Biol. Chem. 1983, 258, 12149–12152. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.A. Feedback modulation of glucose-induced insulin secretion by arachidonic acid metabolites: Possible molecular mechanisms and relevance to diabetes mellitus. Prostaglandins Med. 1981, 7, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Dobrian, A.D.; Morris, M.A.; Taylor-Fishwick, D.A.; Nadler, J.L. Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia 2016, 59, 673–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lieb, D.C.; Brotman, J.J.; Hatcher, M.A.; Aye, M.S.; Cole, B.K.; Haynes, B.A.; Wohlgemuth, S.D.; Fontana, M.A.; Beydoun, H.; Nadler, J.L.; et al. Adipose tissue 12/15 lipoxygenase pathway in human obesity and diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1713–E1720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, K.; Nunemaker, C.S.; Wu, R.; Chakrabarti, S.K.; Taylor-Fishwick, D.A.; Nadler, J.L. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. J. Clin. Endocrinol. Metab. 2010, 95, 887–893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabinovitch, A.; Baquerizo, H.; Sumoski, W. Cytotoxic effects of cytokines on islet beta-cells: Evidence for involvement of eicosanoids. Endocrinology 1990, 126, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trauelsen, M.; Lückmann, M.; Frimurer, T.M.; Schwartz, T.W. The HETE Is on FFAR1 and Pancreatic Islet Cells. Cell Metab. 2018, 27, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Taketo, M.M.; Oshima, M. Destruction of pancreatic beta-cells by transgenic induction of prostaglandin E2 in the islets. J. Biol. Chem. 2006, 281, 29330–29336. [Google Scholar] [CrossRef] [PubMed]
- Mateu, A.; Ramudo, L.; Manso, M.; De Dios, I. Cross-talk between TLR4 and PPARγ pathways in the arachidonic acid-induced inflammatory response in pancreatic acini. Int. J. Biochem. Cell Biol. 2015, 69, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.O.T.; Gleason, C.E.; Robertson, R.P. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes 2002, 51, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Horwitz, M.; Hershko-Moshe, A.; Bronstein, S.; Ben-Dov, I.Z.; Melloul, D. Posttranscriptional regulation of the prostaglandin E receptor spliced-isoform EP3-γ and its implication in pancreatic β-cell failure. FASEB J. 2023, 37, e22958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Levine, B.A.; Olson, M.S. Lipid mediator production in acute and chronic pancreatitis in the rat. J. Surg. Res. 1994, 56, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Gregor, J.I.; Heukamp, I.; Wagner, C.; Walz, M.K.; Schimke, I.; Kristiansen, G.; Wenger, F.A. Early inhibition of prostaglandin synthesis by n-3 fatty acids determinates histologic severity of necrotizing pancreatitis. Pancreas 2009, 38, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.O.T.; Gleason, C.E.; Poitout, V.; Robertson, R.P. Prostaglandin E(2) mediates inhibition of insulin secretion by interleukin-1beta. J. Biol. Chem. 1999, 274, 31245–31248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henkel, J.; Neuschäfer-Rube, F.; Pathe-Neuschäfer-Rube, A.; Püschel, G.P. Aggravation by prostaglandin E2 of interleukin-6-dependent insulin resistance in hepatocytes. Hepatology 2009, 50, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Pek, S.; Tai, T.Y.; Fajans, S.; Elster, A. Stimulation by prostaglandin E2 of glucagon and insulin release from isolated rat pancreas. Prostaglandins 1975, 10, 493–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaworek, J.; Bonior, J.; Tomaszewska, R.; Jachimczak, B.; Kot, M.; Bielański, W.; Pawlik, W.W.; Sendur, R.; Stachura, J.; Konturek, P.C.; et al. Involvement of cyclooxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide. J. Physiol. Pharmacol. 2001, 52, 107–126. [Google Scholar] [PubMed][Green Version]
- Coelle, E.F.; Adham, N.; Elashoff, J.; Lewin, K.; Taylor, I.L. Effects of prostaglandin and indomethacin on diet-induced acute pancreatitis in mice. Gastroenterology 1983, 85, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Vitamin C for Type 2 Diabetes Mellitus and Hypertension. Arch. Med. Res. 2019, 50, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol. In Vitro 2015, 29, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Baranowska-Bosiacka, I.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Telesiński, A.; Siennicka, A.; Droździk, M.; Chlubek, D.; Stachowska, E. Fluoride in low concentration modifies expression and activity of 15 lipoxygenase in human PBMC differentiated monocyte/macrophage. Toxicology 2012, 295, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ridley, W.; Matsuoka, M. Fluoride-induced cyclooxygenase-2 expression and prostaglandin E2 production in A549 pulmonary epithelial cells. Toxicol. Lett. 2009, 188, 180–185. [Google Scholar] [CrossRef]
- Bellenger, J.; Bellenger, S.; Bataille, A.; Massey, K.A.; Nicolaou, A.; Rialland, M.; Tessier, C.; Kang, J.X.; Narce, M. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: Inflammatory pathway inhibition. Diabetes 2011, 60, 1090–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, T.; Yang, H.; Wang, H.; Zhi, L.; Liu, T.; Cui, L.; Liu, W.; Wang, Y.; Zhang, M.; Liu, Y.; et al. Inhibition of voltage-gated K+ channels mediates docosahexaenoic acid-stimulated insulin secretion in rat pancreatic β-cells. Food Funct. 2020, 11, 8893–8904. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Lim, J.W.; Kim, H. Docosahexaenoic Acid Inhibits Cytokine Expression by Reducing Reactive Oxygen Species in Pancreatic Stellate Cells. J. Cancer Prev. 2021, 26, 195–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, D.; Wang, F.-Y.; Liu, Z.-Y.; Liu, Y. Resolvin D1 Reduces Cerulein and Lipopolysaccharide-induced Severe Acute Pancreatitis in Mice Fracture Patients. Sichuan Da Xue Xue Bao Yi Xue Ban 2019, 50, 215–218. (In Chinese) [Google Scholar] [PubMed]
- Bathina, S.; Das, U.N. PUFAs, BDNF and lipoxin A4 inhibit chemical-induced cytotoxicity of RIN5F cells in vitro and streptozotocin-induced type 2 diabetes mellitus in vivo. Lipids Health Dis. 2019, 18, 214. [Google Scholar] [CrossRef]
- Lund, T.; Mangsbo, S.M.; Scholz, H.; Gjorstrup, P.; Tötterman, T.H.; Korsgren, O.; Foss, A. Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro. Exp. Clin. Endocrinol. Diabetes 2010, 118, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is There a Role for Bioactive Lipids in the Pathobiology of Diabetes Mellitus? Front. Endocrinol. 2017, 8, 182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutowska, I.; Baranowska-Bosiacka, I.; Baśkiewicz, M.; Milo, B.; Siennicka, A.; Marchlewicz, M.; Wiszniewska, B.; Machaliński, B.; Stachowska, E. Fluoride as a pro-inflammatory factor and inhibitor of ATP bioavailability in differentiated human THP1 monocytic cells. Toxicol. Lett. 2010, 196, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Izabela, G.; Irena, B.-B.; Aldona, S.; Magdalena, B.; Bogusław, M.; Ewa, S.; Dariusz, C. Fluoride and generation of pro-inflammatory factors in human macrophages. Fluoride 2011, 44, 125–134. [Google Scholar]
- Jouvet, N.; Estall, J.L. The pancreas: Bandmaster of glucose homeostasis. Exp. Cell Res. 2017, 360, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.; Buzalaf, M.A.R.; Rigalli, A. Wpływ wody fluorowanej na poziom insuliny w osoczu i homeostazę glukozy u szczurów z niewydolnością nerek. Biol. Trace Elem. Res. 2011, 140, 198–207. [Google Scholar] [CrossRef]
- Hu, C.Y.; Ren, L.Q.; Li, X.N.; Wu, N.; Li, G.S.; Liu, Q.Y.; Xu, H. Effect of fluoride on insulin level of rats and insulin receptor expression in the MC3T3-E1 cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef]
- Rigalli, A.; Alloatti, R.; Menoyo, I.; Puche, R.C. Comparative study of the effect of sodium fluoride and sodium monofluorophosphate on glucose homeostasis in the rat. Arzneimittelforschung 1995, 45, 289–292. [Google Scholar] [PubMed]
- Menoyo, I.; Rigalli, A.; Puche, R.C. Effect of fluoride on the secretion of insulin in the rat. Arzneimittelforschung 2005, 55, 455–460. [Google Scholar] [CrossRef] [PubMed]
- García-Montalvo, E.A.; Reyes-Pérez, H.; Del Razo, L.M. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology 2009, 263, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rigalli, A.; Ballina, J.C.; Roveri, E.; Puche, R.C. Inhibitory effect of fluoride on the secretion of insulin. Calcif. Tissue Int. 1990, 46, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.A.; Li, J.X.; Wang, M.; Xu, Z.R. Effects of fluoride on growth and thyroid function in young pigs. Fluoride 2006, 39, 95–100. [Google Scholar]
- Matsuo, S.; Nakagawa, H.; Kiyomiya, K.-I.; Kurebe, M. Fluoride-induced ultrastructural changes in exocrine pancreas cells of rats: Fluoride disrupts the export of zymogens from the rough endoplasmic reticulum (rER). Arch. Toxicol. 2000, 73, 611–617. [Google Scholar] [CrossRef]
- Aziz, N.M.; Ragy, M.M.; Ahmed, S.M. Somatostatin analogue, Octreotide, improves restraint stress-induced liver injury by ameliorating oxidative stress, inflammatory response, and activation of hepatic stellate cells. Cell Stress Chaperones 2018, 23, 1237–1245. [Google Scholar] [CrossRef]
- Patarrão, R.S.; Lautt, W.W.; Macedo, M.P. Acute Glucagon Induces Postprandial Peripheral Insulin Resistance. PLoS ONE 2015, 10, e0127221. [Google Scholar] [CrossRef]
- Trevizol, J.S.; Dionizio, A.; Delgado, A.Q.; Ventura, T.M.O.; Ribeiro, C.F.d.S.; Ribeiro, L.; Buzalaf, N.R.; Cestari, T.M.; Magalhães, A.C.; Suzuki, M.; et al. Metabolic effect of low fluoride levels in the islets of NOD mice: Integrative morphological, immunohistochemical, and proteomic analyses. J. Appl. Oral Sci. 2023, 31, e20230036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trevizol, J.S.; Buzalaf, N.R.; Dionizio, A.; Delgado, A.Q.; Cestari, T.M.; Bosqueiro, J.R.; Magalhães, A.C.; Buzalaf, M.A.R. Effects of low-level fluoride exposure on glucose homeostasis in female NOD mice. Chemosphere 2020, 254, 126602. [Google Scholar] [CrossRef] [PubMed]
- Balaha, M.; Ahmed, N.; Geddawy, A.; Kandeel, S. Fraxetin prevented sodium fluoride-induced chronic pancreatitis in rats: Role of anti-inflammatory, antioxidant, antifibrotic and anti-apoptotic activities. Int. Immunopharmacol. 2021, 93, 107372. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Can, M.I.; Beyaz, S.; Gok, O.; Parlak, G.; Gundogdu, R.; Ozercan, I.H.; Erman, O. A new approach on the regulation of NF-κB and Bax protein signaling pathway activation by royal jelly in fluoride-induced pancreas damage in rats. Tissue Cell 2022, 79, 101913. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Rana, S.; Baltoo, R. Exploring fluoride’s role in diabetes development: A review. J. Trace Elem. Med. Biol. 2025, 89, 127635. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Thakur, N.; Thakur, R. Fluoride-Induced Alterations in the Pancreas of Mammals: A Meta-analysis. Biol. Trace Elem. Res. 2025, 203, 2654–2674. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.; Dionizio, A.S.; Fernandes, M.S. Fluoride intensifies hypercaloric diet induced ER oxidative stress and alters lipid metabolism. PLoS ONE 2016, 11, e0158121. [Google Scholar] [CrossRef] [PubMed]
- Flores-Méndez, M.; Ramírez, D.; Alamillo, N.; Hernández-Kelly, L.C.; Del Razo, L.M.; Ortega, A. Fluoride exposure regulates the elongation phase of protein synthesis in cultured Bergmann glia cells. Toxicol. Lett. 2014, 229, 126–133. [Google Scholar] [CrossRef] [PubMed]



| Fluoride Concentration | Control | Fluoride | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Serum [mg/L] | 0.177 | 0.041 | 0.191 | 0.105 | ns |
| Pancreas [mg/kg d.w.] | 21.382 | 5.371 | 21.343 | 3.062 | ns |
| Hormone Concentration in Serum [pg/mL] | Control Group | Fluoride Group | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Insulin | 197.002 | 63.168 | 157.781 | 64.311 | *** |
| Glucagon | 150.527 | 62.936 | 166.247 | 92.166 | ns |
| Somatostatin | 1539.312 | 1090.287 | 1362.465 | 1062.393 | * |
| Eicosanoids in Pancreas [µg/mg Protein] | Control | Fluoride | p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Resolvin E1 | 0.9756 | 0.7943 | 0.7975 | 0.35186 | - |
| Prostaglandin E2 | 2.7551 | 1.0522 | 14.6683 | 9.44807 | **** |
| Resolvin D1 | 0.1577 | 0.13661 | 0.5086 | 0.65992 | * |
| LTX A4 | 0.7941 | 0.74794 | 2.5184 | 1.03137 | *** |
| Protectin DX | 1.425 | 0.4579 | 2.0717 | 1.05778 | * |
| Maresin 1 | 0.3799 | 0.12804 | 0.892 | 0.42921 | ** |
| Leukotriene B4 | 0.3671 | 0.10222 | 1.227 | 1.24162 | ** |
| 18-HEPE | 13.1204 | 7.19018 | 12.2518 | 6.12348 | - |
| 16-HETE | 15.9062 | 11.89287 | 24.6099 | 20.34443 | - |
| 13-HODE | 20.0553 | 9.06033 | 57.8731 | 34.67874 | ** |
| 9-HODE | 19.1437 | 9.78235 | 52.9248 | 32.2025 | ** |
| 15-HETE | 136.9282 | 75.35555 | 185.5246 | 83.83571 | - |
| 17-HDHA | 9.2439 | 4.72145 | 21.3315 | 14.84319 | * |
| 12-HETE | 53.6656 | 23.73025 | 110.0985 | 55.4523 | * |
| 5oxoETE | 12.0962 | 8.44525 | 11.9253 | 6.40004 | - |
| 5-HETE | 3.77 | 2.66293 | 3.0749 | 1.34329 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skórka-Majewicz, M.; Żwierełło, W.; Drozd, A.; Baranowska-Bosiacka, I.; Simińska, D.; Wszołek, A.; Gutowska, I. Exposure to Fluoride During Pregnancy and Lactation Induces Metabolic Imbalance in Pancreas: A Toxicological Insight Using the Rat Model. Int. J. Mol. Sci. 2025, 26, 9817. https://doi.org/10.3390/ijms26199817
Skórka-Majewicz M, Żwierełło W, Drozd A, Baranowska-Bosiacka I, Simińska D, Wszołek A, Gutowska I. Exposure to Fluoride During Pregnancy and Lactation Induces Metabolic Imbalance in Pancreas: A Toxicological Insight Using the Rat Model. International Journal of Molecular Sciences. 2025; 26(19):9817. https://doi.org/10.3390/ijms26199817
Chicago/Turabian StyleSkórka-Majewicz, Marta, Wojciech Żwierełło, Arleta Drozd, Irena Baranowska-Bosiacka, Donata Simińska, Agata Wszołek, and Izabela Gutowska. 2025. "Exposure to Fluoride During Pregnancy and Lactation Induces Metabolic Imbalance in Pancreas: A Toxicological Insight Using the Rat Model" International Journal of Molecular Sciences 26, no. 19: 9817. https://doi.org/10.3390/ijms26199817
APA StyleSkórka-Majewicz, M., Żwierełło, W., Drozd, A., Baranowska-Bosiacka, I., Simińska, D., Wszołek, A., & Gutowska, I. (2025). Exposure to Fluoride During Pregnancy and Lactation Induces Metabolic Imbalance in Pancreas: A Toxicological Insight Using the Rat Model. International Journal of Molecular Sciences, 26(19), 9817. https://doi.org/10.3390/ijms26199817






