Characterization of α-L-Rhamnosidase and β-D-Glucosidase Subunits of Naringinase Immobilized on a Magnetic Polysaccharide Carrier
Abstract
1. Introduction
2. Results and Discussion
2.1. Immobilization Yield
2.2. Activity of α-L-rhamnosidase and β-D-glucosidase
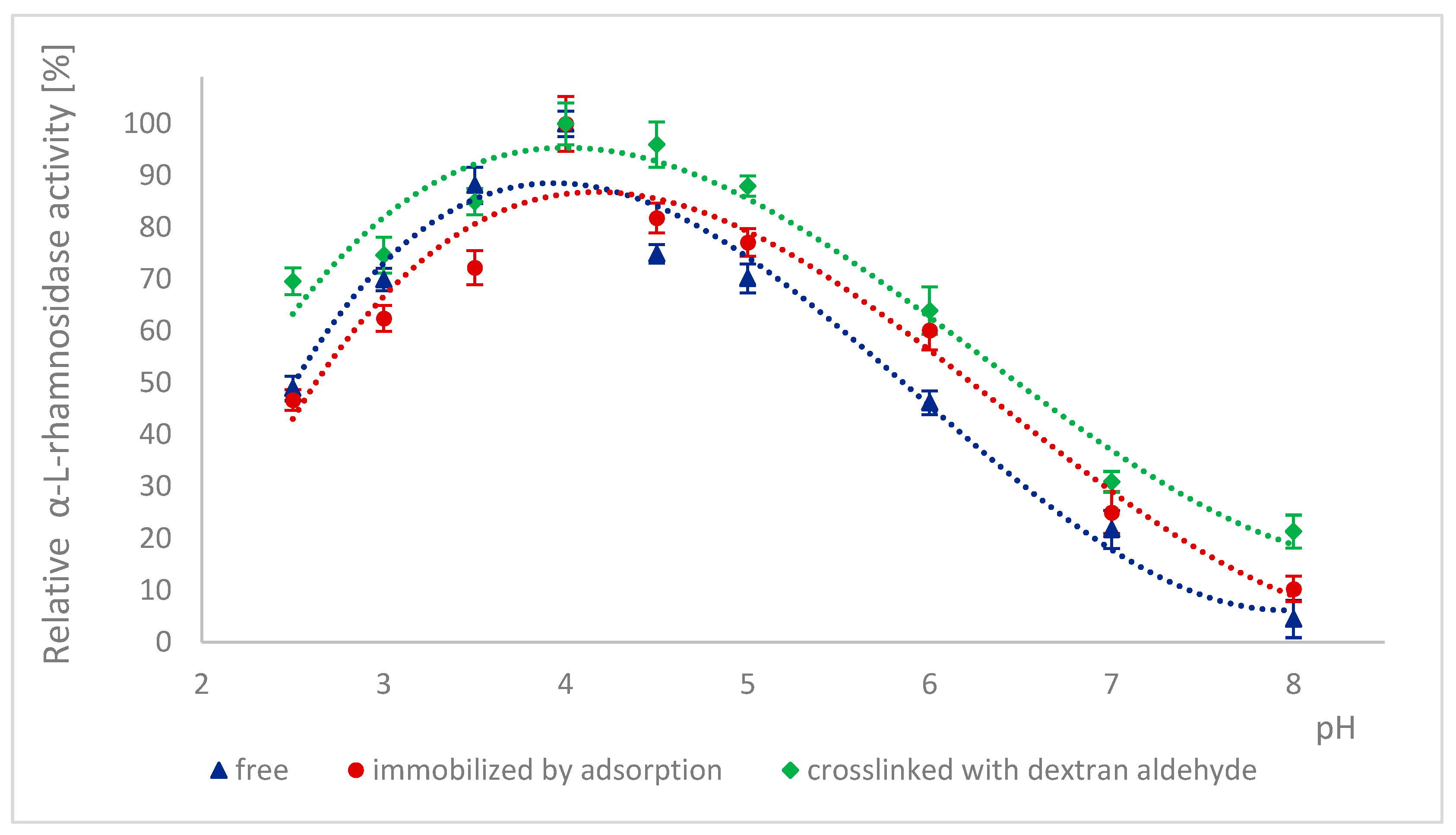
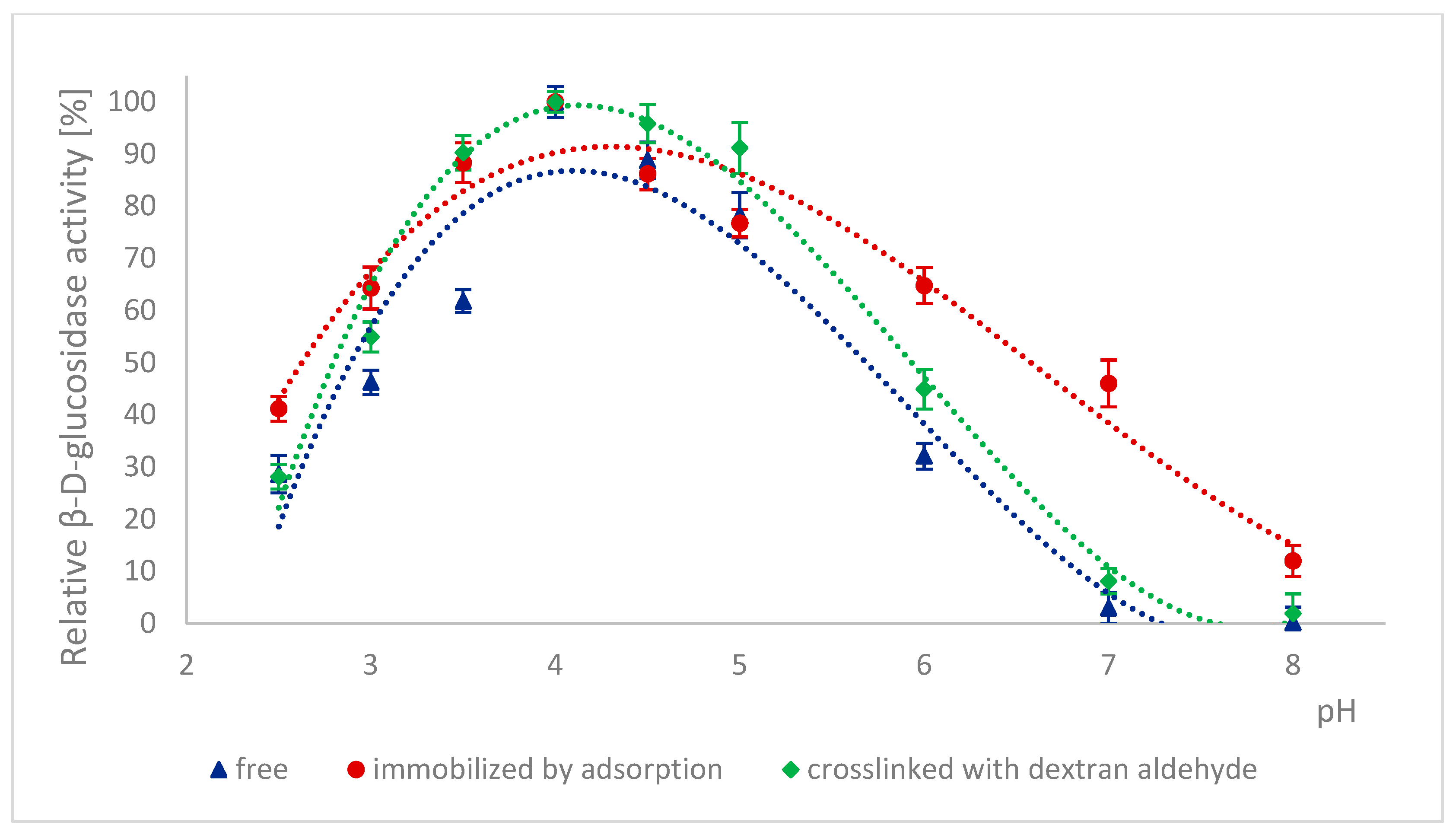
2.3. Effect of pH on the Activity of Naringinase Subunits
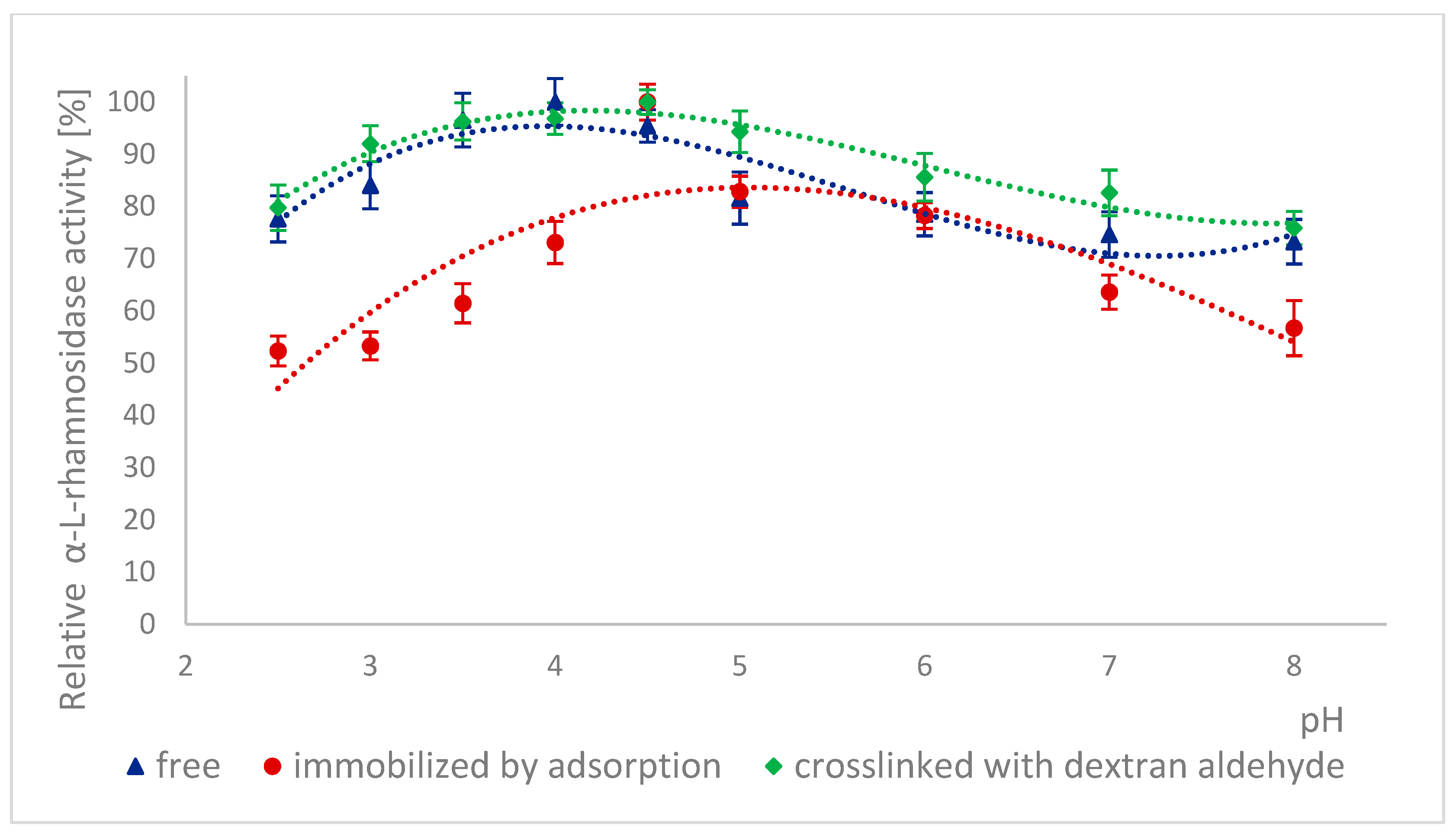
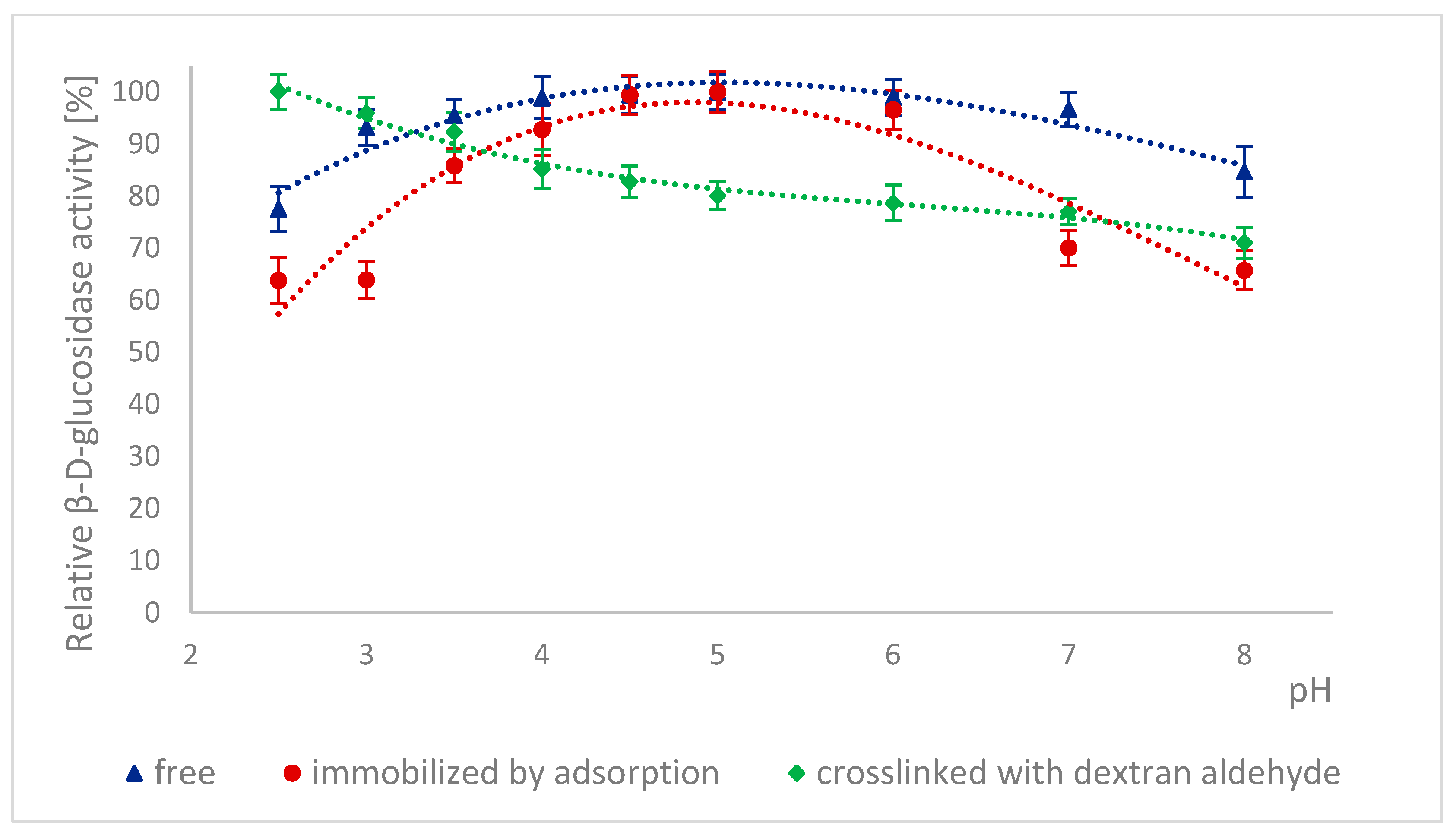
2.4. Effect of Incubation in Buffers with Different pH on the Activity of Naringinase Subunits
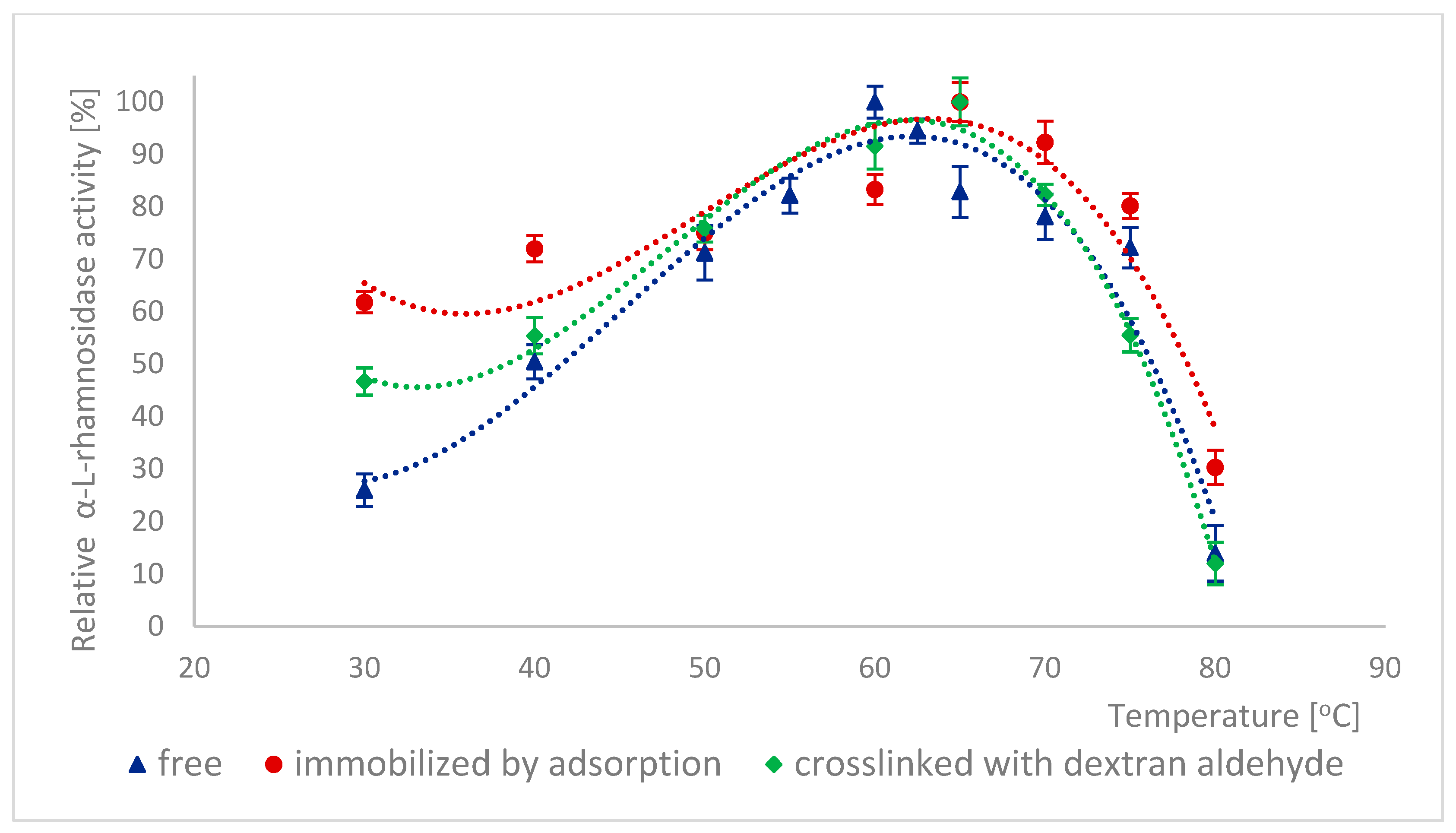
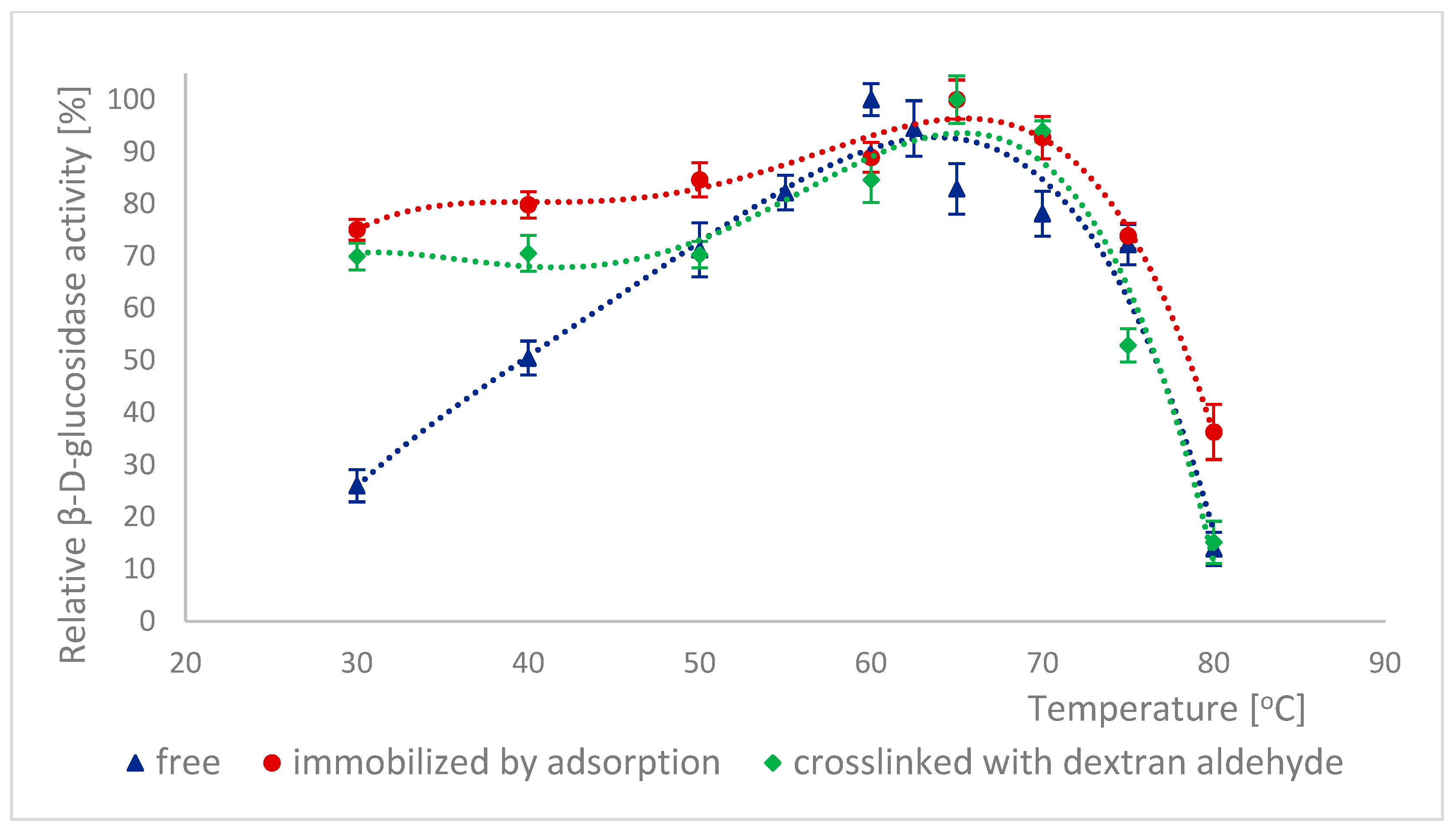
2.5. Effect of Temperature on the Activity of Naringinase Subunits
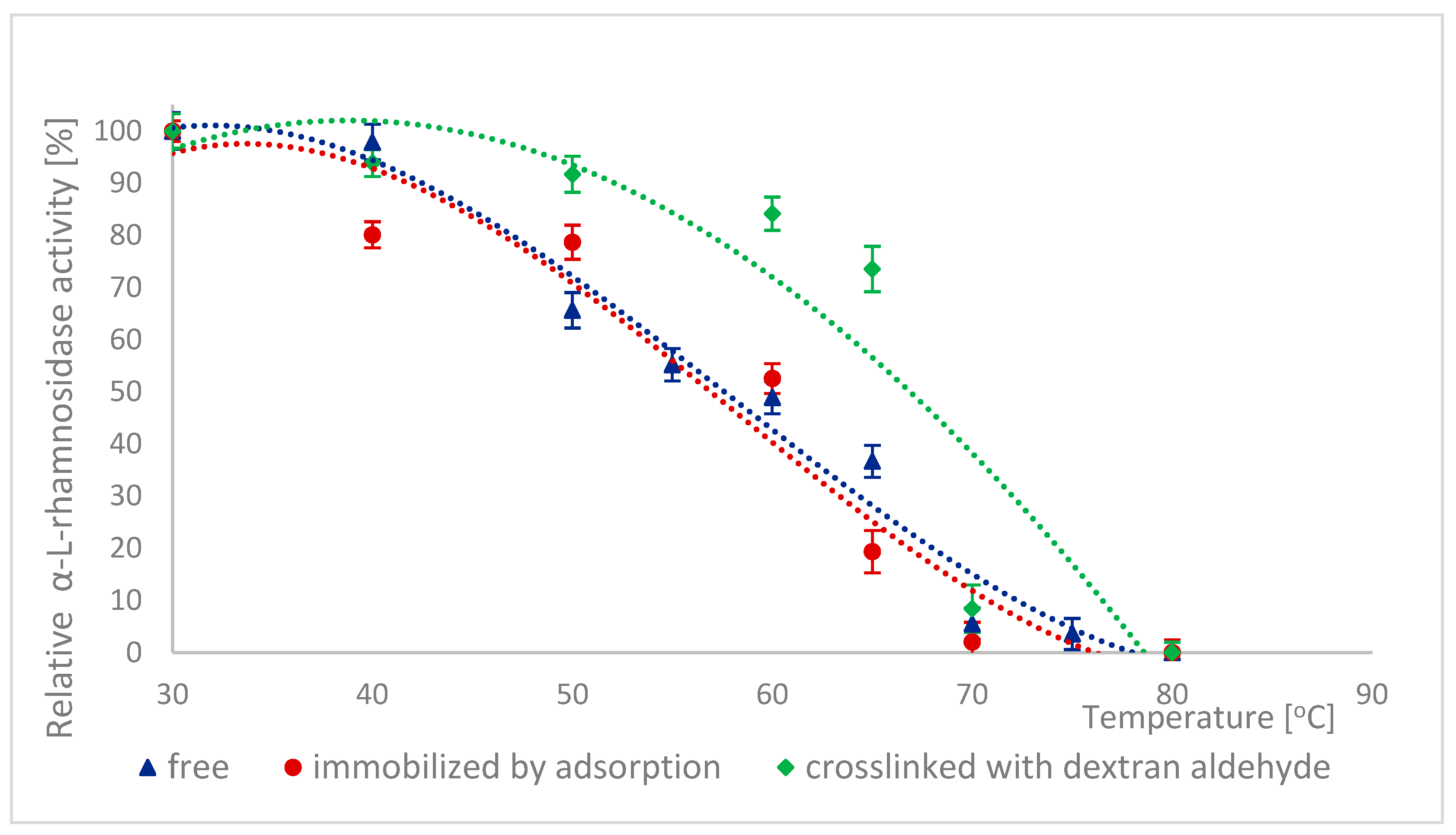
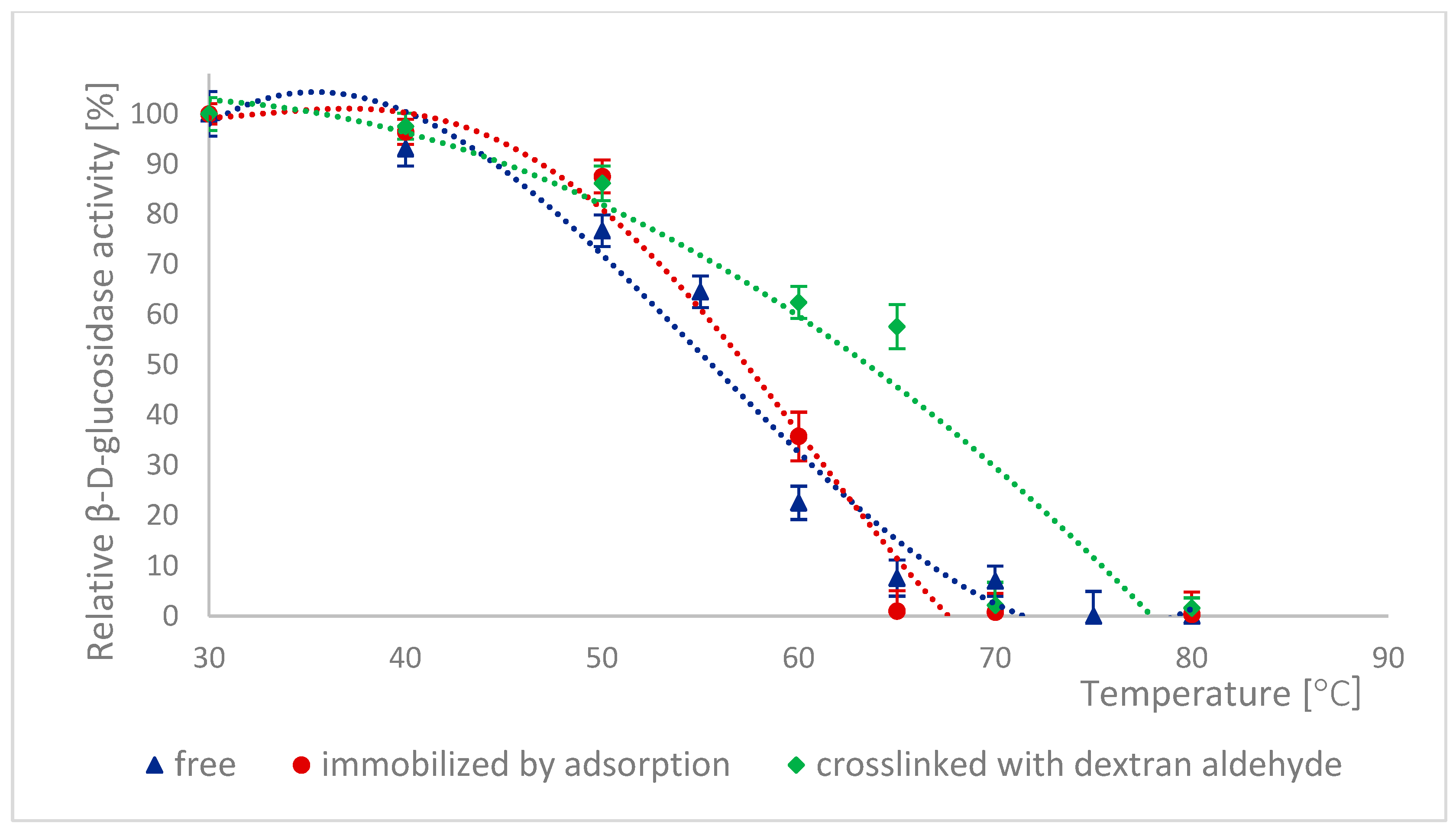
2.6. Thermal Stability of Naringinase Subunits
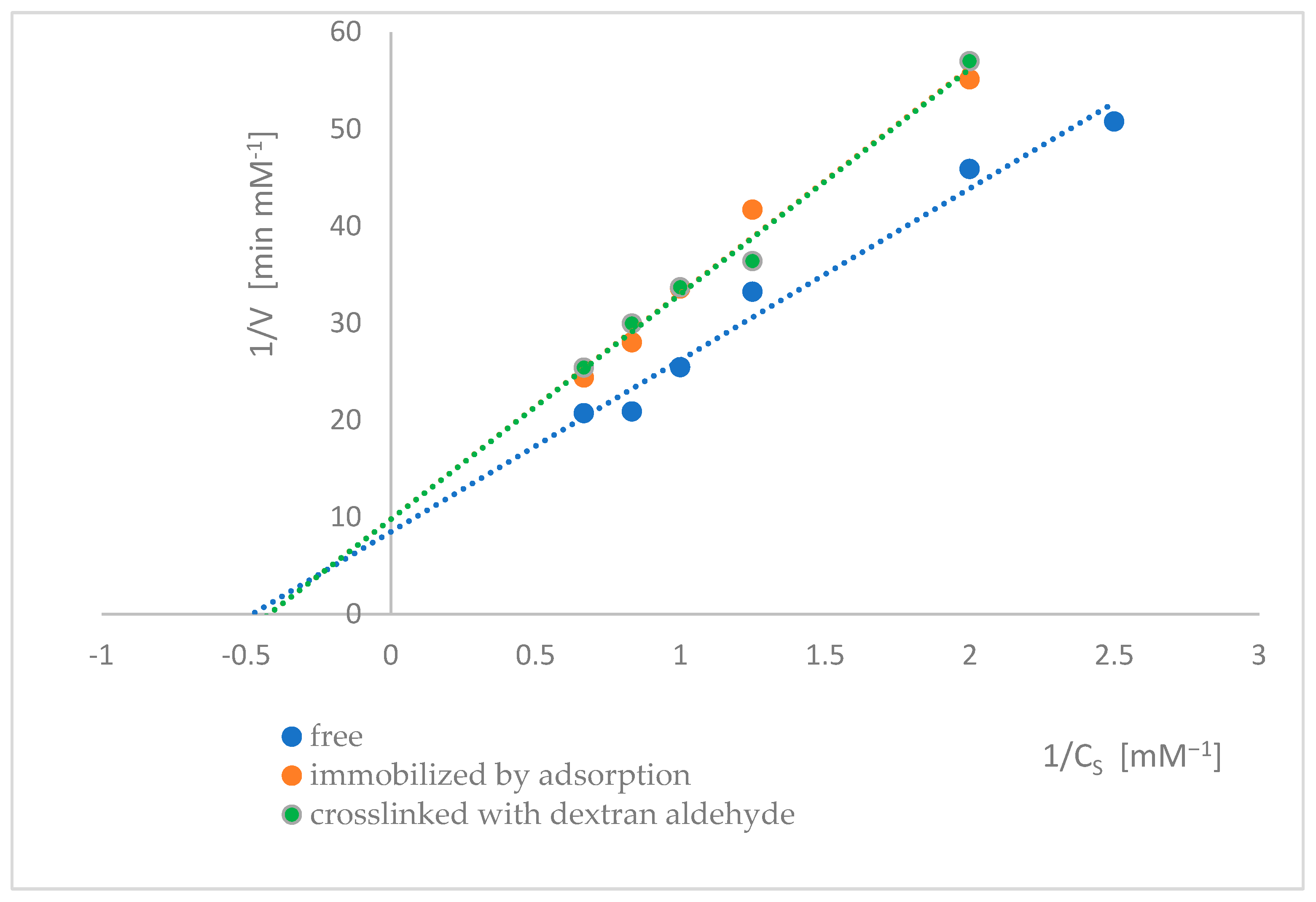
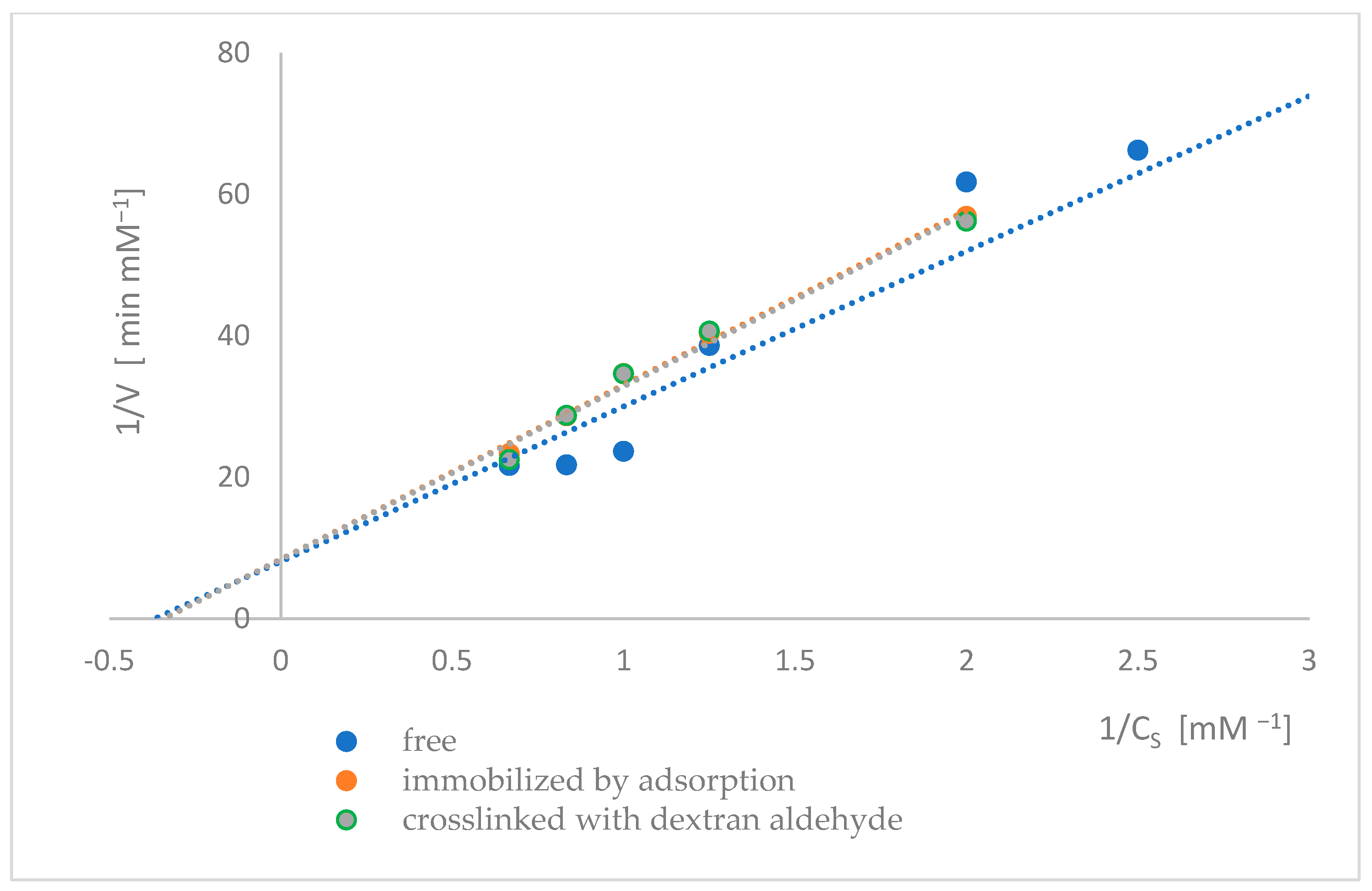
2.7. Determination of the Kinetic Parameters of Naringinase Subunits
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.2.1. Determination of α-L-rhamnosidase and β-D-glucosidase Activity from Free Naringinase
3.2.2. Determination of α-L-rhamnosidase and β-D-glucosidase Activity from Immobilized and Stabilized Naringinase
3.3. Immobilization of Naringinase
Immobilization Yield
- Xa—the difference between the total activity of the enzyme used for immobilization and the activity of the immobilized enzyme;
- Xa0—the total activity of the enzyme used for immobilization.
- 100% yield was defined as the total activity of naringinase (Xa0) immobilized.
3.4. Characteristics of Free, Immobilized and Stabilized Biocatalyst
3.4.1. Effect of pH on the Activity of α-L-rhamnosidase and β-D-glucosidase
3.4.2. Effect of Incubation in Buffers with Different pH on the Activity of α-L-rhamnosidase, and β-D-glucosidase
3.4.3. Effect of Temperature on the Activity of α-L-rhamnosidase and β-D-glucosidase
3.4.4. Thermal Stability of α-L-rhamnosidase and β-D-glucosidase
3.4.5. Determination of Kinetic Parameters
- —enzyme reaction rate [mM·min−1];
- max—maximum reaction rate [mM·min−1];
- KM—Michaelis constant [mM];
- Cs—substrate concentration [mM].
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Hes-7-G | hesperetin 7-O-glucoside |
| pNPR | 4-nitrophenyl α-L-rhamnopyranoside |
| pNPG | 4-nitrophenyl β-D-glucopyranoside |
| max | maximum reaction rate |
| KM | Michaelis constant |
References
- Puri, M. Updates on naringinase: Structural and biotechnological aspects. Appl. Microbiol. Biotechnol. 2012, 93, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Golgeri, M.D.B.; Mulla, S.I.; Bagewadi, Z.K.; Faniband, B.; Mishra, P.; Bankole, P.O.; Sharma, S.; Américo-Pinheiro, J.H.P.; Bharagava, R.N.; Romanholo Ferreira, L.F. Microbial naringinase: From microbial source to its current applications in various fields. Biologia 2025, 80, 977–991. [Google Scholar] [CrossRef]
- Cui, Z.; Maruyama, Y.; Mikami, B.; Hashimoto, W.; Murata, K. Crystal Structure of Glycoside Hydrolase Family 78 α-L-Rhamnosidase from Bacillus sp. GL1. J. Mol. Biol. 2007, 374, 384–398. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, H.; Xi, M.; Xu, L.; Wu, S.; Li, X. Purification and characterization of a naringinase from a newly isolated strain of Bacillus amyloliquefaciens 11568 suitable for the transformation of flavonoids. Food Chem. 2017, 214, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Ni, H.; Xiao, A.-F.; Wang, Y.Q.; Chen, F.; Cai, H.-N.; Su, W.-J. Development and evaluation of an HPLC method for accurate determinations of enzyme activities of naringinase complex. J. Agric. Food Chem. 2013, 61, 10026–10032. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, H.; Chen, F.; Cai, H.; Li, L.; Su, W. Purification and characterization of a naringinase from Aspergillus aculeatus JMUdb058. J. Agric. Food Chem. 2013, 61, 931–938. [Google Scholar] [CrossRef]
- Ni, H.; Chen, F.; Cai, H.; Xiao, A.; You, Q.; Lu, Y. Characterization and preparation of Aspergillus niger naringinase for debittering citrus juice. J. Food Sci. 2012, 77, C1–C7. [Google Scholar] [CrossRef]
- Soria, F.; Ellenrieder, G.; Grasselli, M.; Navarro Del Cañizo, A.A.; Cascone, O. Fractionation of the naringinase complex from Aspergillus terreus by dye affinity chromatography. Biotechnol. Lett. 2004, 26, 1265–1268. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Use of Naringinase to Modify the Sensory Quality of Foods and Increase the Bioavailability of Flavonoids: A Systematic Review. Molecules 2025, 30, 2376. [Google Scholar] [CrossRef]
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883–1895. [Google Scholar] [CrossRef]
- Li, B.; Wu, B.; Hou, X.; Ding, G. Substrate Selectivities of GH78 α -L-Rhamnosidases from Human Gut Bacteria on Dietary Flavonoid Glycosides. Molecules 2025, 30, 980. [Google Scholar] [CrossRef]
- RoitNer, M.; Schalkhammer, T.; Pittner, F. Preparation of prunin with the help of immobilized naringinase pretreated with alkaline buffer. Appl. Biochem. Biotechnol. 1984, 9, 483–488. [Google Scholar] [CrossRef]
- Rajendran, D.S.; Chidambaram, A.; Kumar, P.S.; Venkataraman, S.; Muthusamy, S.; Vo, D.-V.N.; Rangasamy, G.; Vaithyanathan, V.K.; Vaidyanathan, V.K. Three-phase partitioning for the separation of proteins, enzymes, biopolymers, oils and pigments: A review. Environ. Chem. Lett. 2023, 21, 911–934. [Google Scholar] [CrossRef]
- Mazzaferro, L.; Piñuel, L.; Minig, M.; Breccia, J.D. Extracellular monoenzyme deglycosylation system of 7-O-linked X avonoid-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria. Arch. Microbiol. 2010, 192, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Huh, J.Y.; Nam, S.H.; Moon, S.K.; Lee, S.B. Enzymatic bioconversion of citrus hesperidin by Aspergillus sojae naringinase: Enhanced solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase, and growth of Helicobacter pylori. Food Chem. 2012, 135, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xin, X.; Xu, H.; Yuan, H.; Li, X.; Yu, Y.; Zhao, G. Highly efficient bioconversion of flavonoid glycosides from citrus-processing wastes in solvent-buffer systems. Green Chem. 2020, 22, 3196–3207. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Bronze, M.R.; Calado, A.R.T.; Ribeiro, M.H.L. Enzymatic Synthesis of the Flavone Glucosides, Prunin and Isoquercetin, and the Aglycones, Naringenin and Quercetin, with Selective α-L-Rhamnosidase and β-D-Glucosidase Activities of Naringinase. Enzym. Res. 2011, 2011, 692618. [Google Scholar] [CrossRef]
- Li, L.J.; Liu, X.Q.; Du, X.P.; Wu, L.; Jiang, Z.D.; Ni, H.; Li, Q.B.; Chen, F. Preparation of isoquercitrin by biotransformation of rutin using α-L-rhamnosidase from Aspergillus niger JMU-TS528 and HSCCC purification. Prep. Biochem. Biotechnol. 2020, 50, 1–9. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Torres, C.; Guerrero, C.; Wilson, L. Amination of naringinase to improve citrus juice debittering using a catalyst immobilized on glyoxyl-agarose. Food Chem. 2024, 452, 139600. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Aspergillus niger on a Magnetic Polysaccharide Carrier. Molecules 2020, 25, 2731. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Ribeiro, I.A.C.; Ribeiro, M.H.L. Kinetic modelling of naringin hydrolysis using a bitter sweet alfa-rhamnopyranosidase immobilized in k-carrageenan. J. Mol. Catal. B Enzym. 2008, 51, 10–18. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilizacja naringinazy z Penicillium decumbens na magnetycznych polisacharydowych nośnikach. Pr. Nauk. Uniw. Ekon. We Wrocławiu 2019, 524, 9–24. [Google Scholar] [CrossRef]
- Ferreira, L.; Afonso, C.; Vila-real, H.; Alfaia, A. Evaluation of the Effect of High Pressure on Naringin Hydrolysis in Grapefruit Juice with Naringinase Immobilised in Calcium Alginate Beads. Food Technol. Biotechnol. 2008, 46, 146–150. [Google Scholar]
- Norouzian, D.; Hosseinzadeh, A.; Inanlou, D.N.; Moazami, N. Various techniques used to immobilize naringinase produced by Penicillium decombens PTCC 5248. World, J. Microbiol. Biotechnol. 1999, 15, 501–502. [Google Scholar] [CrossRef]
- Awad, G.E.A.; Abd El Aty, A.A.; Shehata, A.N.; Hassan, M.E.; Elnashar, M.M. Covalent immobilization of microbial naringinase using novel thermally stable biopolymer for hydrolysis of naringin. 3 Biotech 2016, 6, 14. [Google Scholar] [CrossRef]
- Garzón-Alonso, K.E.; Bohorquez-Peña, M.J.; Vargas-Suaza, B.; Rendón-Londoño, J.C.; Mesa, M.; Franco-Tobón, Y.N.; Martínez-Galán, J.P. Hydrolysis of flavanones from orange peel and evaluation of anticancer potential using naringinase immobilized on corn cob powder. Food Biosci. 2025, 69, 106752. [Google Scholar] [CrossRef]
- Serra, I.; Serra, C.D.; Rocchietti, S.; Ubiali, D.; Terreni, M. Stabilization of thymidine phosphorylase from Escherichia coli by immobilization and post immobilization techniques. Enzym. Microb. Technol. 2011, 49, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zheng, P.; Chen, P.; Wu, D. Immobilization of alpha-L-rhamnosidase on a magnetic metal-organic framework to effectively improve its reusability in the hydrolysis of rutin. Bioresour. Technol. 2021, 323, 124611. [Google Scholar] [CrossRef]
- Wan, W.; Xia, N.; Zhu, S.; Liu, Q.; Gao, Y. A Novel and High-Effective Biosynthesis Pathway of Hesperetin-7-O-Glucoside Based on the Construction of Immobilized Rhamnosidase Reaction Platform. Front. Bioeng. Biotechnol. 2020, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Liu, F.; Liu, X.; Cheng, L.; Yuan, Q.; Gao, H.; Liang, H. Enhancing stability and by-product tolerance of β-glucuronidase based on magnetic cross-linked enzyme aggregates. Colloids Surf. B Biointerfaces 2022, 210, 112241. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, M.; Habibi, A. Development of Magnetic Immobilized Cellulase Biocatalysts for Saccharification of Paper Waste. Catal. Lett. 2024, 154, 5791–5805. [Google Scholar] [CrossRef]
- He, G.; Liu, H.; Yang, C.; Hu, K.; Zhai, X.; Fang, B.; Liu, K.; Zulekha; Li, D. A comparison of dual-enzyme immobilization by magnetic nanoparticles and magnetic enzyme aggregates for cascade enzyme reactions. Biochem. Eng. J. 2024, 204, 109207. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Dari, D.N.; Aires, F.I.d.S.; de Castro, E.C.; dos Santos, K.M.; dos Santos, J.C.S. Advancements in enzyme immobilization on magnetic nanomaterials: Toward sustainable industrial applications. RSC Adv. 2024, 14, 17946–17988. [Google Scholar] [CrossRef]
- Yu, C.; Li, Q.; Tian, J.; Zhan, H.; Zheng, X.; Wang, S.; Sun, X.; Sun, X. A facile preparation of immobilized naringinase on polyethyleneimine-modified Fe3O4 magnetic nanomaterials with high activity. RSC Adv. 2021, 11, 14568–14577. [Google Scholar] [CrossRef]
- Ladole, M.R.; Pokale, P.B.; Varude, V.R.; Belokar, P.G.; Pandit, A.B. One pot clarification and debittering of grapefruit juice using co-immobilized enzymes@chitosanMNPs. Int. J. Biol. Macromol. 2021, 167, 1297–1307. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Henríquez, A.; Torres, C.; Wilson, L.; Flores, M.; Pio, E.; Jullian, D.; Urbano, B.; Braun-Galleani, S.; Ottone, C.; et al. Immobilization of Naringinase onto Polydopamine-Coated Magnetic Iron Oxide Nanoparticles for Juice Debittering Applications. Polymers 2024, 16, 3279. [Google Scholar] [CrossRef]
- Luo, J.; Li, Q.; Sun, X.; Tian, J.; Fei, X.; Shi, F.; Zhang, N.; Liu, X. The study of the characteristics and hydrolysis properties of naringinase immobilized by porous silica material. RSC Adv. 2019, 9, 4514–4520. [Google Scholar] [CrossRef]
- Liu, R.; Chen, D.; Fu, H.; Lv, P.; Zhang, D.; He, Y. A facile preparation process of magnetic aldehyde-functionalized Ni0.5Zn0.5Fe2O4@SiO2 nanocomposites for immobilization of Penicillin G Acylase (PGA). J. Nanosci. Nanotechnol. 2017, 17, 893–899. [Google Scholar] [CrossRef]
- Busto, M.D.; Meza, V.; Ortega, N.; Perez-Mateos, M. Immobilization of naringinase from Aspergillus niger CECT 2088 in poly(vinyl alcohol) cryogels for the debittering of juices. Food Chem. 2007, 104, 1177–1182. [Google Scholar] [CrossRef]
- Puri, M.; Marwaha, S.S.; Kothari, R.M. Studies on the applicability of alginate-entrapped naringinase for the debittering of kinnow juice. Enzym. Microb. Technol. 1996, 18, 281–285. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Rosa, M.E.; Calado, A.R.; Ribeiro, M.H.L. An innovative sol-gel naringinase bioencapsulation process for glycosides hydrolysis. Process Biochem. 2010, 45, 841–850. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.-X.; Xiao, Q.; Chen, J.; Chen, F.-Q.; Yang, Q.-M.; Weng, H.-F.; Fang, B.-S.; Zhang, Y.-H.; Xiao, A.-F. Artificial naringinase system for cooperative enzymatic synthesis of naringenin. Biochem. Eng. J. 2022, 178, 108277. [Google Scholar] [CrossRef]
- Koseki, T.; Mese, Y.; Nishibori, N.; Masaki, K.; Fujii, T.; Handa, T.; Yamane, Y.; Shiono, Y.; Murayama, T.; Iefuji, H. Characterization of an α-L-rhamnosidase from Aspergillus kawachii and its gene. Biochem. Relev. Enzym. Proteins 2008, 80, 1007. [Google Scholar] [CrossRef]
- Thammawat, K.; Pongtanya, P. Isolation, Preliminary Enzyme Characterization and Optimization of Culture Parameters for Production of Naringinase Isolated from Aspergillus niger BCC 25166. Nat. Sci. 2008, 42, 61–72. [Google Scholar]
- Gallego, M.V.; Pinaga, F.; Ramon, D.; Valles, S. Purification and characterization of an alpha-L-rhamnosidase from Aspergillus terreus of interest in winemaking. J. Food Sci. 2001, 66, 204–209. [Google Scholar] [CrossRef]
- Decker, C.H.; Visser, J.; Schreier, P. B-Glucosidases from Five Black Aspergillus Species: Study of Their Physico-Chemical and Biocatalytic Properties Keywords: J. Agric. Food Chem. 2000, 48, 4929–4936. [Google Scholar] [CrossRef]
- Muñoz, M.; Holtheuer, J.; Wilson, L.; Urrutia, P. Grapefruit Debittering by Simultaneous Naringin Hydrolysis and Limonin Adsorption Using Naringinase Immobilized in Agarose Supports. Molecules 2022, 27, 2867. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, H.; Xi, M.; Li, J.; Yang, L.; Li, X. Characterization of a naringinase from Aspergillus oryzae 11250 and its application in the debitterization of orange juice. Process Biochem. 2017, 62, 114–121. [Google Scholar] [CrossRef]
- Roitner, M.; Schalkhammer, T.; Pittner, F. Characterisation of naringinase from Aspergillus niger. Monatshefte Für Chem. Chem. Mon. 1984, 115, 1255–1267. [Google Scholar] [CrossRef]
- Manzanares, P.; Hetty, C.; Visser, J. Purification and Characterization of Two Different-alpha-L-Rhamnosidases, RhaA and RhaB, from Aspergillus aculeatus. Appl. Environ. Microbiol. 2001, 67, 2230–2234. [Google Scholar] [CrossRef]
- Abbate, E.; Palmeri, R.; Todaro, A.; Blanco, R.M. Production of a α-L-Rhamnosidase from Aspergillus terreus Using Citrus Solid Waste as Inducer for Application in Juice Industry. Chem. Eng. Trans. 2012, 27, 253–258. [Google Scholar]
- Chang, H.-Y.; Lee, Y.-B.; Bae, H.-A.; Huh, J.-Y.; Nam, S.-H.; Sohn, H.-S.; Lee, H.J.; Lee, S.-B. Purification and characterisation of Aspergillus sojae naringinase: The production of prunin exhibiting markedly enhanced solubility with in vitro inhibition of HMG-CoA reductase. Food Chem. 2011, 124, 234–241. [Google Scholar] [CrossRef]
- Zdarta, J.; Jędrzak, A.; Klapiszewski, Ł.; Jesionowski, T. Immobilization of Cellulase on a Functional Inorganic–Organic Hybrid Support: Stability and Kinetic Study. Catalysts 2017, 7, 374. [Google Scholar] [CrossRef]
- Spagna, G.; Barbagallo, R.N.; Casarini, D.; Pifferi, P.G. A novel chitosan derivative to immobilize alpha-L-rhamnopyranosidase from Aspergillus niger for application in beverage technologies. Enzym. Microb. Technol. 2001, 28, 427–438. [Google Scholar] [CrossRef]
- Keerti; Gupta, A.; Kumar, V.; Dubey, A.; Verma, A.K. Kinetic Characterization and Effect of Immobilized Thermostable beta -Glucosidase in Alginate Gel Beads on Sugarcane Juice. ISRN Biochem. 2014, 2014, 8. [CrossRef]
- Spagna, G.; Romagnoli, D.; Angela, M.; Bianchi, G.; Pifferi, P.G. A simple method for purifying glycosidases: Alpha-L-arabinofuranosidase and beta-D-glucopyranosidase from Aspergillus niger to increase the aroma of wine. Part, I. Enzym. Microb. Technol. 1998, 22, 298–304. [Google Scholar] [CrossRef]
| Enzyme | Free | Adsorbed | Adsorbed and Crosslinked |
|---|---|---|---|
| µmol·min−1 g−1 | µmol·min−1·g−1 of Carrier | µmol·min−1·g−1 of Carrier | |
| α-L-rhamnosidase | 887.17 ± 33 | 4.82 ±0.12 | 5.09 ± 0.13 |
| β-D-glucosidase | 273.75 ± 13 | 2.57 ± 0.06 | 3.02 ± 0.08 |
| Type of Enzyme | Vmax [mM·min−1] | KM [mM] |
|---|---|---|
| Free α-L-rhamnosidase | 0.1176 ± 0.0016 (a) | 2.0803 ± 0.0355 (c) |
| Adsorbed α-L-rhamnosidase | 0.1019 ± 0.0044 (b) | 2.3698 ± 0.1288 (d) |
| Adsorbed α-L-rhamnosidase crosslinked with dextran aldehyde | 0.1021 ± 0.0053 (b) | 2.3678 ± 0.1359 (d) |
| Type of Enzyme | Vmax [mM·min−1] | KM [mM] |
|---|---|---|
| Free β-D-glucosidase | 0.1240 ± 0.0032 (a) | 2.7213 ± 0.0780 (c) |
| Adsorbed β-D-glucosidase | 0.1188 ± 0.0026 (b) | 2.9280 ± 0.0780 (d) |
| Adsorbed β-D-glucosidase crosslinked with dextran aldehyde | 0.1195 ± 0.0019 (b) | 2.9277 ± 0.0565 (d) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodakowska-Boczniewicz, J.; Garncarek, Z. Characterization of α-L-Rhamnosidase and β-D-Glucosidase Subunits of Naringinase Immobilized on a Magnetic Polysaccharide Carrier. Int. J. Mol. Sci. 2025, 26, 9813. https://doi.org/10.3390/ijms26199813
Bodakowska-Boczniewicz J, Garncarek Z. Characterization of α-L-Rhamnosidase and β-D-Glucosidase Subunits of Naringinase Immobilized on a Magnetic Polysaccharide Carrier. International Journal of Molecular Sciences. 2025; 26(19):9813. https://doi.org/10.3390/ijms26199813
Chicago/Turabian StyleBodakowska-Boczniewicz, Joanna, and Zbigniew Garncarek. 2025. "Characterization of α-L-Rhamnosidase and β-D-Glucosidase Subunits of Naringinase Immobilized on a Magnetic Polysaccharide Carrier" International Journal of Molecular Sciences 26, no. 19: 9813. https://doi.org/10.3390/ijms26199813
APA StyleBodakowska-Boczniewicz, J., & Garncarek, Z. (2025). Characterization of α-L-Rhamnosidase and β-D-Glucosidase Subunits of Naringinase Immobilized on a Magnetic Polysaccharide Carrier. International Journal of Molecular Sciences, 26(19), 9813. https://doi.org/10.3390/ijms26199813







