Combined Treatment of Sodium Butyrate and Bromelain Enhanced Anticancer Effects in Colorectal Cancer Cell Lines: A Promising Therapeutic Approach
Abstract
1. Introduction
2. Results
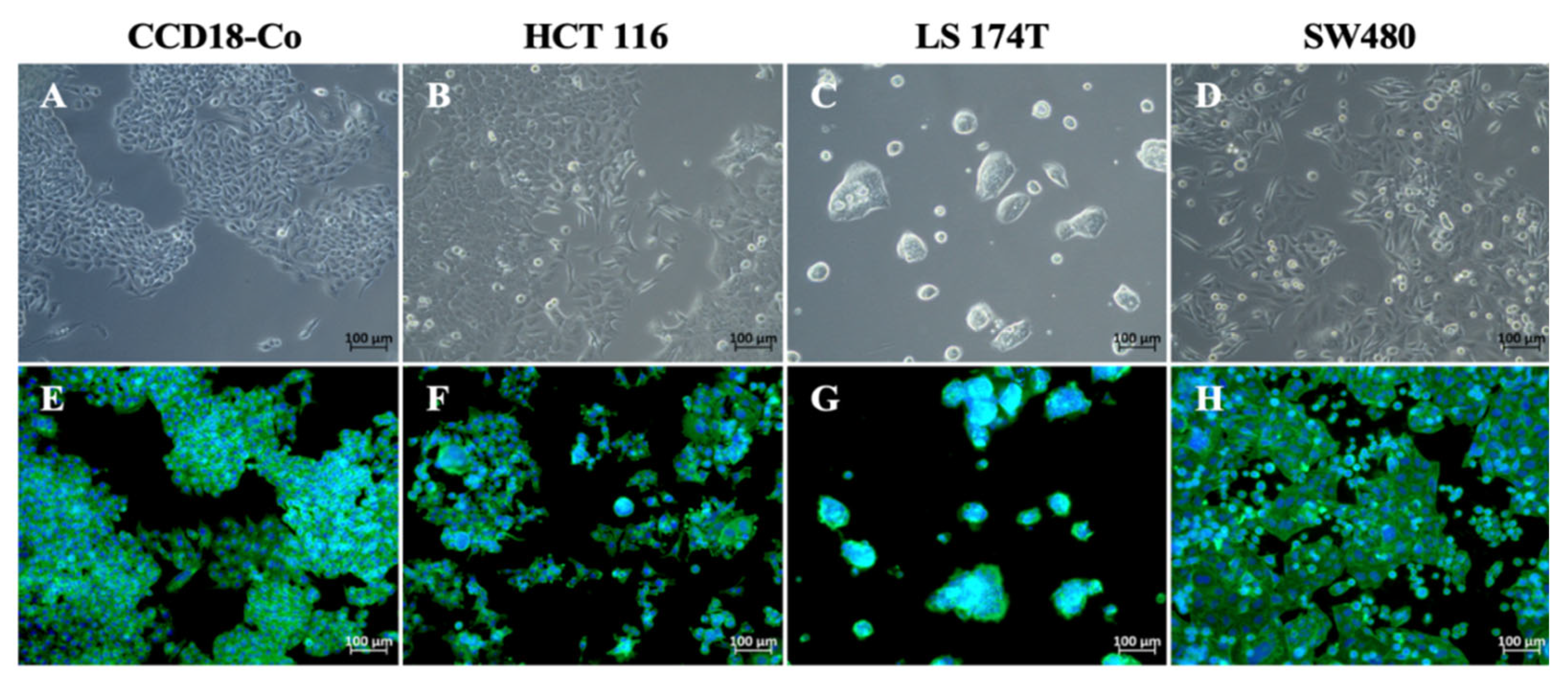
2.1. Analysis of Cell Morphology
2.2. Analysis of Cell Proliferation
2.3. Evaluation of Synergy Between Sodium Butyrate and Bromelain in Colorectal Cancer Cells
2.3.1. Dose–Response Curves and IC25 Calculation
2.3.2. Combined Treatment with IC25 Sodium Butyrate and IC25 Bromelain
- Morphological analysis after combination treatment

- Analysis of Necrosis/Apoptosis Changes Following Combination Treatment
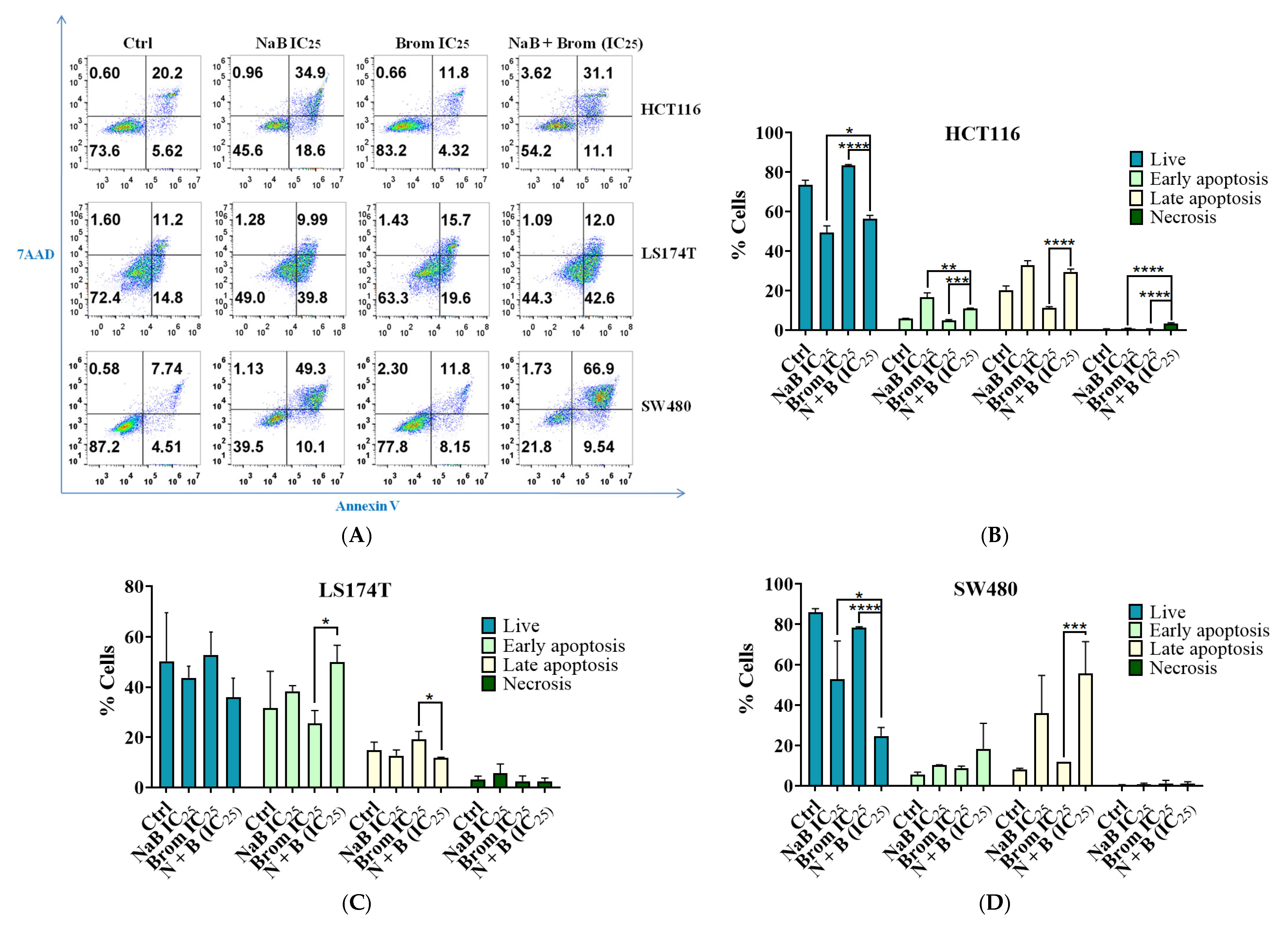
- Analysis of Cell Cycle Changes Following Combination Treatment

- Gene expression changes after treatment

2.3.3. Evaluation of Synergic Doses
2.4. Therapeutic Window-Based Optimal Dose Determination
2.5. Biosafety Assessment in Normal Cells
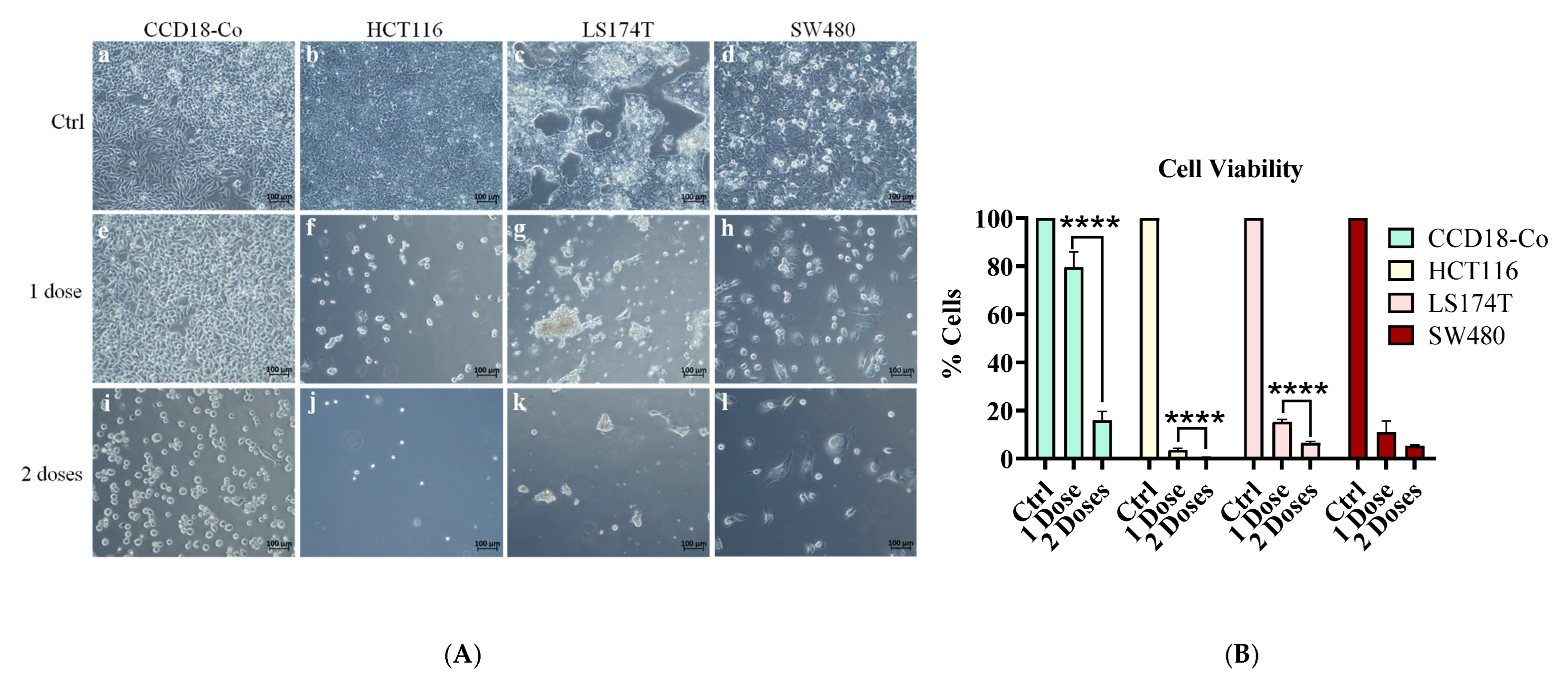
2.6. Effect of Sequential Treatment on Cell Viability in Normal and Tumor Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Dose–Response Curve and IC25 Calculation
4.3. Combination of IC25 Treatments
4.4. Cell Viability Assay
4.5. Flow Cytometry Analysis of Apoptosis, Necrosis, and Cell Cycle
4.6. Analysis of Gene Expression
4.7. Synergy Analysis Between Sodium Butyrate and Bromelain
4.8. Selective Cytotoxicity and Post-Treatment Recovery
4.9. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising Incidence of Early-Onset Colorectal Cancer—A Call to Action. Nat. Rev. Clin. Oncol. 2021, 18, 230–243. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shaham, S.H.; Vij, P.; Tripathi, M.K. Advances in Targeted and Chemotherapeutic Strategies for Colorectal Cancer: Current Insights and Future Directions. Biomedicines 2025, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Gavrilas, L.I.; Cruceriu, D.; Mocan, A.; Loghin, F.; Miere, D.; Balacescu, O. Plant-Derived Bioactive Compounds in Colorectal Cancer: Insights from Combined Regimens with Conventional Chemotherapy to Overcome Drug-Resistance. Biomedicines 2022, 10, 1948. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The Epigenetic Effects of Butyrate: Potential Therapeutic Implications for Clinical Practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate Generated by Gut Microbiota and Its Therapeutic Role in Metabolic Syndrome. Pharmacol. Res. 2020, 160, 105174. [Google Scholar] [CrossRef]
- Bultman, S.J. Molecular Pathways: Gene-Environment Interactions Regulating Dietary Fiber Induction of Proliferation and Apoptosis via Butyrate for Cancer Prevention. Clin. Cancer Res. 2014, 20, 799–803. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, J. Unraveling the Gut Health Puzzle: Exploring the Mechanisms of Butyrate and the Potential of High-Amylose Maize Starch Butyrate (HAMSB) in Alleviating Colorectal Disturbances. Front. Nutr. 2024, 11, 1285169. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Li, Y.; Xue, K.; Kan, J. Use of Dietary Fibers in Reducing the Risk of Several Cancer Types: An Umbrella Review. Nutrients 2023, 15, 2545. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Połom, K. Sodium Butyrate in Both Prevention and Supportive Treatment of Colorectal Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef]
- Oncel, S.; Safratowich, B.D.; Lindlauf, J.E.; Liu, Z.; Palmer, D.G.; Briske-Anderson, M.; Zeng, H. Efficacy of Butyrate to Inhibit Colonic Cancer Cell Growth Is Cell Type-Specific and Apoptosis-Dependent. Nutrients 2024, 16, 529. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Luo, S.; Li, Z.; Mao, L.; Chen, S.; Sun, S. Sodium Butyrate Induces Autophagy in Colorectal Cancer Cells through LKB1/AMPK Signaling. J. Physiol. Biochem. 2019, 75, 53–63. [Google Scholar] [CrossRef]
- Schwartz, B.; Avivi-Green, C.; Polak-Charcon, S. Sodium Butyrate Induces Retinoblastoma Protein Dephosphorylation, P16 Expression and Growth Arrest of Colon Cancer Cells. Mol. Cell. Biochem. 1998, 188, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shannar, A.A.F.; Wu, R.; Chou, P.; Sarwar, M.S.; Kuo, H.-C.; Peter, R.M.; Wang, Y.; Su, X.; Kong, A.-N. Butyrate Drives Metabolic Rewiring and Epigenetic Reprogramming in Human Colon Cancer Cells. Mol. Nutr. Food Res. 2022, 66, e2200028. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, D.; Zhang, H.; Xue, J.; Wangchuk, D.; Du, J.; Jiang, L. Sodium Butyrate Selectively Kills Cancer Cells and Inhibits Migration in Colorectal Cancer by Targeting Thioredoxin-1. OncoTargets Ther. 2020, 13, 4691–4704. [Google Scholar] [CrossRef]
- Xi, Y.; Jing, Z.; Wei, W.; Chun, Z.; Quan, Q.; Qing, Z.; Jiamin, X.; Shuwen, H. Inhibitory Effect of Sodium Butyrate on Colorectal Cancer Cells and Construction of the Related Molecular Network. BMC Cancer 2021, 21, 127. [Google Scholar] [CrossRef]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Liauw, W.; Morris, D.L. Depletion of Mucin in Mucin-Producing Human Gastrointestinal Carcinoma: Results from In Vitro and In Vivo Studies with Bromelain and N-Acetylcysteine. Oncotarget 2015, 6, 33329–33344. [Google Scholar] [CrossRef]
- Mekkawy, A.H.; Pillai, K.; Badar, S.; Akhter, J.; Ke, K.; Valle, S.J.; Morris, D.L. Addition of Bromelain and Acetylcysteine to Gemcitabine Potentiates Tumor Inhibition In Vivo in Human Colon Cancer Cell Line LS174T. Am. J. Cancer Res. 2021, 11, 2252–2263. [Google Scholar]
- Pezzani, R.; Jiménez-Garcia, M.; Capó, X.; Sönmez Gürer, E.; Sharopov, F.; Rachel, T.Y.L.; Ntieche Woutouoba, D.; Rescigno, A.; Peddio, S.; Zucca, P.; et al. Anticancer Properties of Bromelain: State-of-the-Art and Recent Trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B. Enzyme-Based Anti-Inflammatory Therapeutics for Inflammatory Diseases. Pharmaceutics 2025, 17, 606. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase Activity and Stability of Natural Bromelain Preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Shamsuri, A.S.; Sim, E.U.-H. In Silico Prediction of the Action of Bromelain on PI3K/Akt Signalling Pathway to Arrest Nasopharyngeal Cancer Oncogenesis by Targeting Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha Protein. BMC Res. Notes 2024, 17, 346. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wei, P.-L.; Makondi, P.T.; Chen, W.-T.; Huang, C.-Y.; Chang, Y.-J. Bromelain Inhibits the Ability of Colorectal Cancer Cells to Proliferate via Activation of ROS Production and Autophagy. PLoS ONE 2019, 14, e0210274. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Zhou, L.; Chen, H.; Zhou, Z. From Intestinal Epithelial Homeostasis to Colorectal Cancer: Autophagy Regulation in Cellular Stress. Antioxidants 2022, 11, 1308. [Google Scholar] [CrossRef]
- Takahama, Y.; Kadoi, A.; Tauchi, Y.; Kasahara, S.; Ishii, K.; Yano, T. Regulation of Human Colorectal Cancer Cells in Tumor Spheroids by Sodium Butyrate. Biol. Pharm. Bull. 2025, 48, 872–877. [Google Scholar] [CrossRef]
- Bertin, P.; Rector-Brooks, J.; Sharma, D.; Gaudelet, T.; Anighoro, A.; Gross, T.; Martínez-Peña, F.; Tang, E.L.; Suraj, M.S.; Regep, C.; et al. RECOVER Identifies Synergistic Drug Combinations In Vitro Through Sequential Model Optimization. Cell Rep. Methods 2023, 3, 100599. [Google Scholar] [CrossRef]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Aslan, E.S.; Akcali, N.; Yavas, C.; Eslamkhah, S.; Gur, S.; Karcioglu Batur, L. Enhancing the Chemosensitivity of MKN-45 Gastric Cancer Cells to Docetaxel via B7H6 Suppression: A Novel Therapeutic Strategy. Life 2024, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hung, M.; Pan, C.; Huang, S.; Jan, C.; Li, Y.; Chiu, S.; Cho, D. Pemetrexed Combined with Dual Immune Checkpoint Blockade Enhances Cytotoxic T Lymphocytes Against Lung Cancer. Cancer Sci. 2023, 114, 2761–2773. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, B.B.; Maurer, H.R.; Porwol, T.; Probst, B.; Bjerkvig, R.; Hoover, F. Bromelain Reversibly Inhibits Invasive Properties of Glioma Cells. Neoplasia 2001, 3, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H. P53 Protein Stability Plays a Crucial Role in NaB-Mediated Apoptosis in Colorectal Cancer Cells. Curr. Issues Mol. Biol. 2025, 47, 579. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Pawlowski, D.; Wittrup, K.D. Effect of Antigen Turnover Rate and Expression Level on Antibody Penetration into Tumor Spheroids. Mol. Cancer Ther. 2008, 7, 2233–2240. [Google Scholar] [CrossRef]
- Heydarian, R.; Divsalar, A.; Kouchesfehani, H.M.; Rasouli, M. Folic Acid-Targeted β-Lactoglobulin Nanocarriers for Enhanced Delivery of 5-Fluorouracil and Sodium Butyrate in Colorectal Cancer Treatment. Int. J. Pharm. 2025, 671, 125262. [Google Scholar] [CrossRef]
- Gorgich, E.A.C.; Heidari, Z.; Mahmoudzadeh-Sagheb, H. P16ink4a Subcellular Expression Patterns in Colorectal Adenocarcinoma, Adenoma and Non-Neoplastic Tissue Samples. Asian Pac. J. Cancer Prev. 2017, 18, 3049–3054. [Google Scholar] [CrossRef]
- Chen, J.-K.; Merrick, K.A.; Kong, Y.W.; Izrael-Tomasevic, A.; Eng, G.; Handly, E.D.; Patterson, J.C.; Cannell, I.G.; Suarez-Lopez, L.; Hosios, A.M.; et al. An RNA Damage Response Network Mediates the Lethality of 5-FU in Colorectal Cancer. Cell Rep. Med. 2024, 5, 101778. [Google Scholar] [CrossRef]
- Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Koessler, T.; van Beijnum, J.R.; Weiss, A.; Mieville, V.; Piersma, S.R.; de Haas, R.R.; Delucinge-Vivier, C.; et al. Optimized Low-dose Combinatorial Drug Treatment Boosts Selectivity and Efficacy of Colorectal Carcinoma Treatment. Mol. Oncol. 2020, 14, 2894–2919. [Google Scholar] [CrossRef]
- Folkesson, E.; Niederdorfer, B.; Nakstad, V.T.; Thommesen, L.; Klinkenberg, G.; Lægreid, A.; Flobak, Å. High-Throughput Screening Reveals Higher Synergistic Effect of MEK Inhibitor Combinations in Colon Cancer Spheroids. Sci. Rep. 2020, 10, 11574. [Google Scholar] [CrossRef] [PubMed]
- Rønneberg, L.; Kirk, P.D.W.; Zucknick, M. Dose–Response Prediction for In-Vitro Drug Combination Datasets: A Probabilistic Approach. BMC Bioinform. 2023, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, Y.; Li, D.; Dai, Y.; Wu, J.; Zhang, Z.; Pan, H.; Chen, W.; Li, R.; Hu, L. CDH1 Genetic Variants and Its Aberrant Expression Are the Risk Factors for Colorectal Cancer Metastasis. BMC Gastroenterol. 2025, 25, 214. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.T.; Wooten, D.J.; Paudel, B.B.; Bauer, J.; Hardeman, K.N.; Westover, D.; Lovly, C.M.; Harris, L.A.; Tyson, D.R.; Quaranta, V. Quantifying Drug Combination Synergy along Potency and Efficacy Axes. Cell Syst. 2019, 8, 97–108.E16. [Google Scholar] [CrossRef]
- Fernández-Hernández, S.; Hidalgo-León, M.Á.; Lacalle-González, C.; Olivera-Salazar, R.; Ochieng’ Otieno, M.; García-Foncillas, J.; Martinez-Useros, J. Dormancy in Colorectal Carcinoma: Detection and TherapeuticPotential. Biomolecules 2025, 15, 1119. [Google Scholar] [CrossRef]
- Hu, J.; Guo, F.; Han, C.; Zhao, Q.; Yi, H.; Xu, J.; Jia, H.; Wu, Y.; Dong, L.; Kao, X.; et al. CD169+ Macrophages Identify and Eliminate Tumor Cells in Colorectal Cancer Through CD169/CD43 Interaction and FasL-Driven Apoptosis. Cell Rep. 2025, 44, 116351. [Google Scholar] [CrossRef]
- Shuvayeva, G.; Tupychak, M.; Vovk, O.; Demash, D.; Chernyshuk, S.; Bobak, Y.; Prokopiv, A.; Pokhodylo, N.; Kunz-Schughart, L.A.; Fletcher, M.T.; et al. Extract of Indigofera Spicata Exerts Antiproliferative Effects on Human Colorectal and Ovarian Carcinoma Cells. Toxins 2025, 17, 431. [Google Scholar] [CrossRef]
- Hussen, B.M.; Abdullah, S.R.; Hidayat, H.J.; Glassy, M.C.; Safarzadeh, A.; Komaki, A.; Samsami, M.; Taheri, M. CRISPR/Cas as a Tool to Overcome Drug Resistance in Cancer: From Challenge to Opportunity. Mol. Cell. Probes 2025, 48, 102052. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Kong, Y.; Hou, Y.; Ma, C. Organoids: New Frontiers in Tumor Immune Microenvironment Research. Front. Immunol. 2024, 15, 1422031. [Google Scholar] [CrossRef]
- Jeong, S.-R.; Kang, M. Exploring Tumor-Immune Interactions in Co-Culture Models of T Cells and Tumor Organoids Derived from Patients. Int. J. Mol. Sci. 2023, 24, 14609. [Google Scholar] [CrossRef]
- Tawara, M.; Suzuki, H.; Ohishi, T.; Kaneko, M.K.; Kato, Y. Antitumor Activity by an Anti-CD44 Variant 9 Monoclonal Antibody in Gastric and Colorectal Cancer Xenograft Models. Int. J. Mol. Sci. 2025, 26, 9170. [Google Scholar] [CrossRef]
- Dunne, P.D.; Arends, M.J. Molecular Pathological Classification of Colorectal Cancer—An Update. Virchows Arch. 2024, 484, 273–285. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current Trends in Drug Metabolism and Pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D Cell Culture Models: Drug Pharmacokinetics, Safety Assessment, and Regulatory Consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Binienda, A.; Machelak, W.; Zielińska, M.; Fichna, J. Free Fatty Acid Receptors Type 2 and 4 Mediate the Anticancer Effects of Fatty Acids in Colorectal Cancer—In Vitro and In Vivo Studies. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167708. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Perez, J.; Kurzrock, R. Combining Targeted Therapies: Practical Issues to Consider at the Bench and Bedside. Oncologist 2010, 15, 37–50. [Google Scholar] [CrossRef]
- Spinozzi, G.; Tini, V.; Ferrari, A.; Gionfriddo, I.; Ranieri, R.; Milano, F.; Pierangeli, S.; Donnini, S.; Mezzasoma, F.; Silvestri, S.; et al. SiCoDEA: A Simple, Fast and Complete App for Analyzing the Effect of Individual Drugs and Their Combinations. Biomolecules 2022, 12, 904. [Google Scholar] [CrossRef]





| Gene | Gene Bank | Forward | Reverse |
|---|---|---|---|
| BAX | NM_138761.4 | 5′–CCACCAGCTCTGAGCAGATC–3′ | 5′–ACTCGCTCAGCTTCTTGGTG–3′ |
| CDH1 | NM_004360.5 | 5′–AAAGGCCCATTTCCTAAAAACCT–3′ | 5′–TGCGTTCTCTATCCAGAGGCT–3′ |
| CDKN1A | NM_000389.5 | 5′–CCAACGCACCGAATAGTTACG–3′ | 5′–ACCAGCGTGTCCAGGAAG–3′ |
| CDKN2A | NM_000077.5 | 5′–ATGGAGCCTTCGGCTGACT–3′ | 5′–GTAACTATTCGGTGCGTTGGG–3′ |
| GAPDH | NM_002046.7 | 5′–GGTCACCAGGGCTGCTTTTA–3′ | 5′–TCGCCCCACTTGATTTTGGA–3′ |
| PTEN | NM_000314.8 | 5′–AGACCAGTGGCACTGTTGTT–3′ | 5′–TCACCACACACAGGTAACGG–3′ |
| TP53 | NM_000546.6 | 5′–TCTGACTGTACCACCATCCACTA–3′ | 5′–CAAACACGCACCTCAAAGC–3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera-Salazar, R.; Villarejo Campos, P.; Barrueco Gutiérrez, R.; Vega-Clemente, L.; Serrano, L.J.; García Gómez-Heras, S.; García-Olmo, D.; García-Arranz, M. Combined Treatment of Sodium Butyrate and Bromelain Enhanced Anticancer Effects in Colorectal Cancer Cell Lines: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2025, 26, 9803. https://doi.org/10.3390/ijms26199803
Olivera-Salazar R, Villarejo Campos P, Barrueco Gutiérrez R, Vega-Clemente L, Serrano LJ, García Gómez-Heras S, García-Olmo D, García-Arranz M. Combined Treatment of Sodium Butyrate and Bromelain Enhanced Anticancer Effects in Colorectal Cancer Cell Lines: A Promising Therapeutic Approach. International Journal of Molecular Sciences. 2025; 26(19):9803. https://doi.org/10.3390/ijms26199803
Chicago/Turabian StyleOlivera-Salazar, Rocío, Pedro Villarejo Campos, Rocío Barrueco Gutiérrez, Luz Vega-Clemente, Luis Javier Serrano, Soledad García Gómez-Heras, Damián García-Olmo, and Mariano García-Arranz. 2025. "Combined Treatment of Sodium Butyrate and Bromelain Enhanced Anticancer Effects in Colorectal Cancer Cell Lines: A Promising Therapeutic Approach" International Journal of Molecular Sciences 26, no. 19: 9803. https://doi.org/10.3390/ijms26199803
APA StyleOlivera-Salazar, R., Villarejo Campos, P., Barrueco Gutiérrez, R., Vega-Clemente, L., Serrano, L. J., García Gómez-Heras, S., García-Olmo, D., & García-Arranz, M. (2025). Combined Treatment of Sodium Butyrate and Bromelain Enhanced Anticancer Effects in Colorectal Cancer Cell Lines: A Promising Therapeutic Approach. International Journal of Molecular Sciences, 26(19), 9803. https://doi.org/10.3390/ijms26199803







