Lychee Seed Extract Targets Proliferation, Differentiation, and Cell Cycle Proteins to Suppress Human Colorectal Tumor Growth in Xenograft Models
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization of Litchi chinensis Seed Extract
2.2. LCSE Exhibits No Overt Toxicity in Male and Female Mice
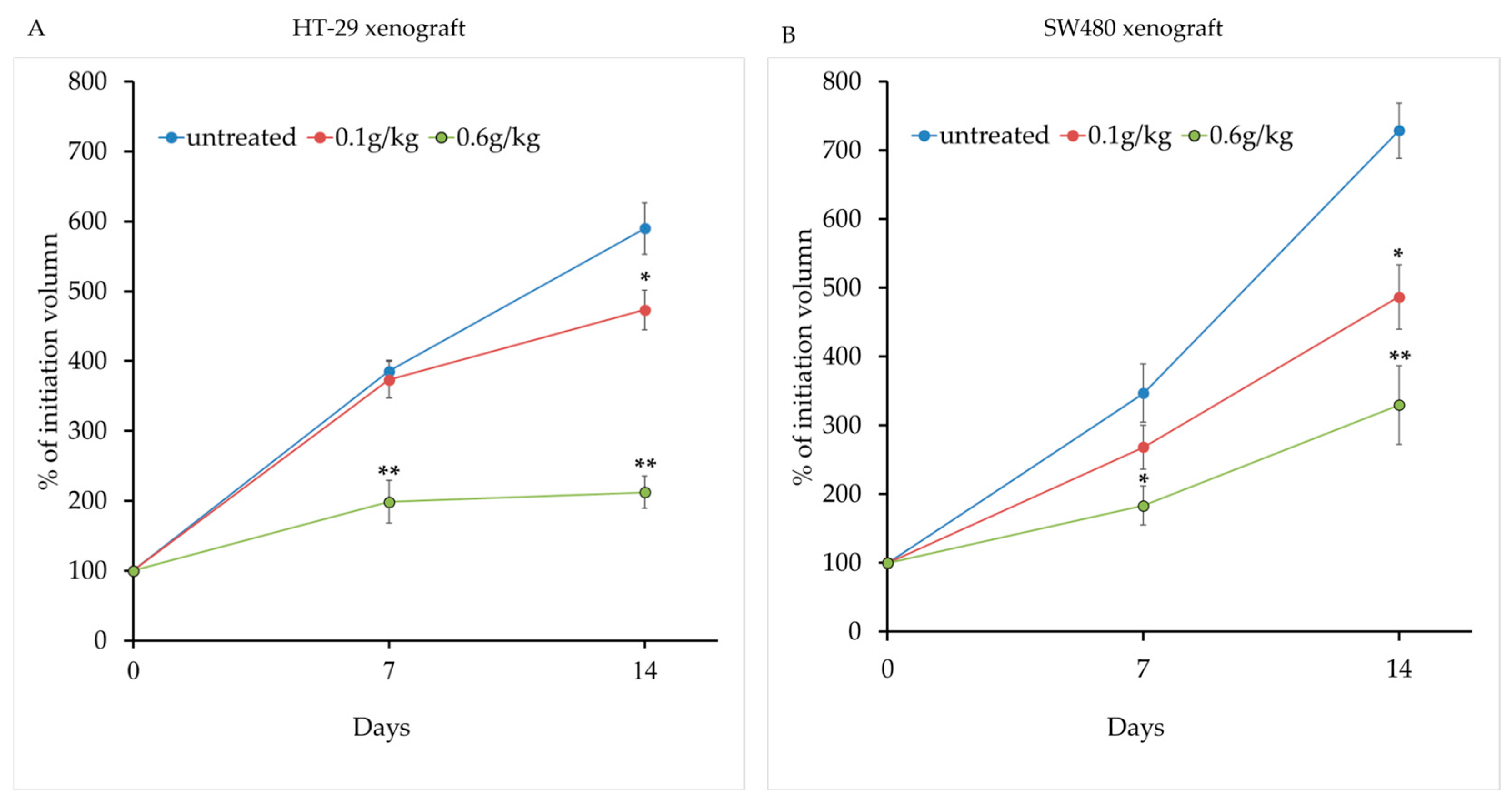
2.3. LCSE Inhibits Tumor Growth in a Xenograft Model of CRC
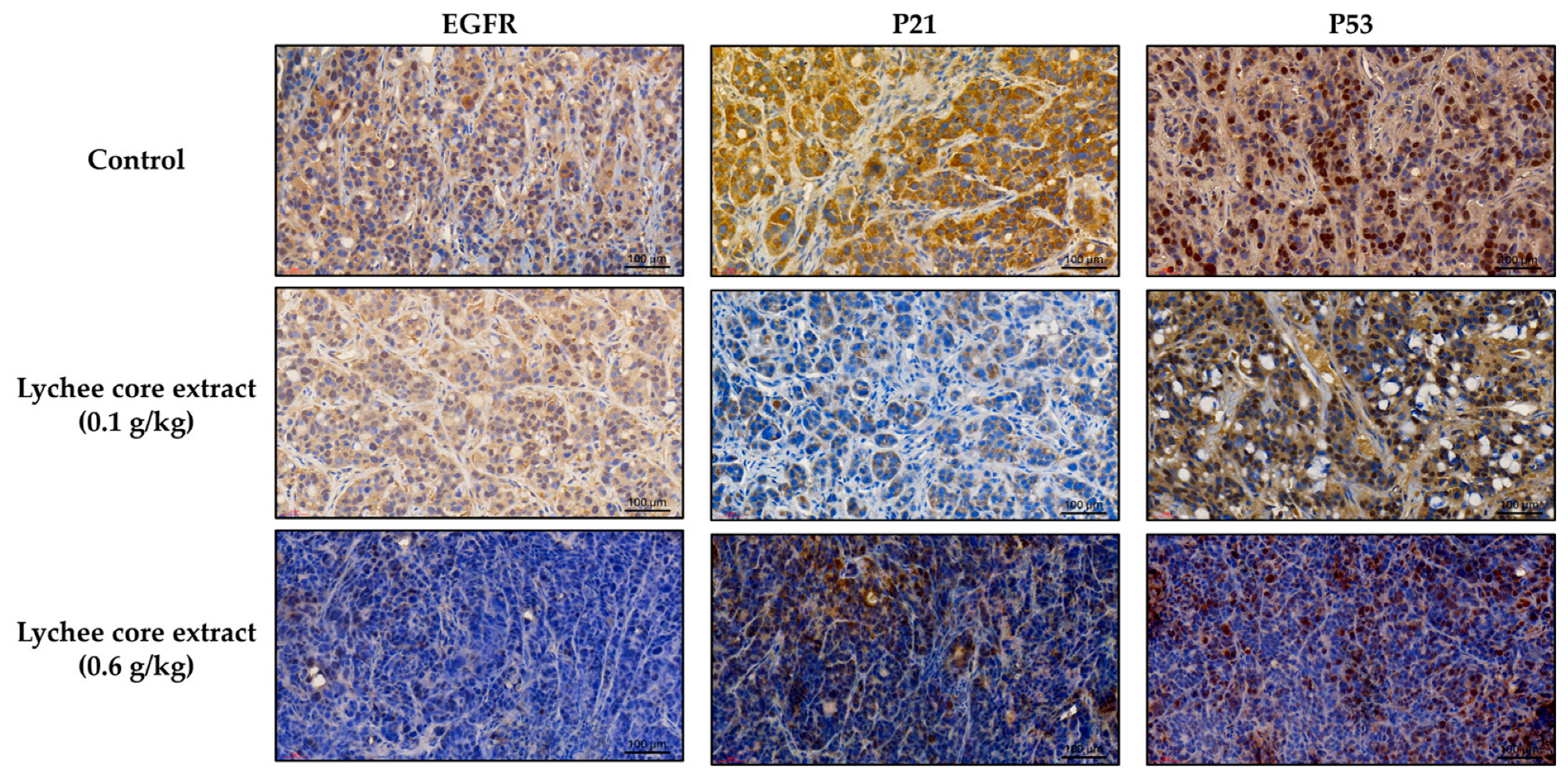
2.4. LCSE Modulates EGFR, p21, and p53 Expression in HT-29 CRC Xenografts
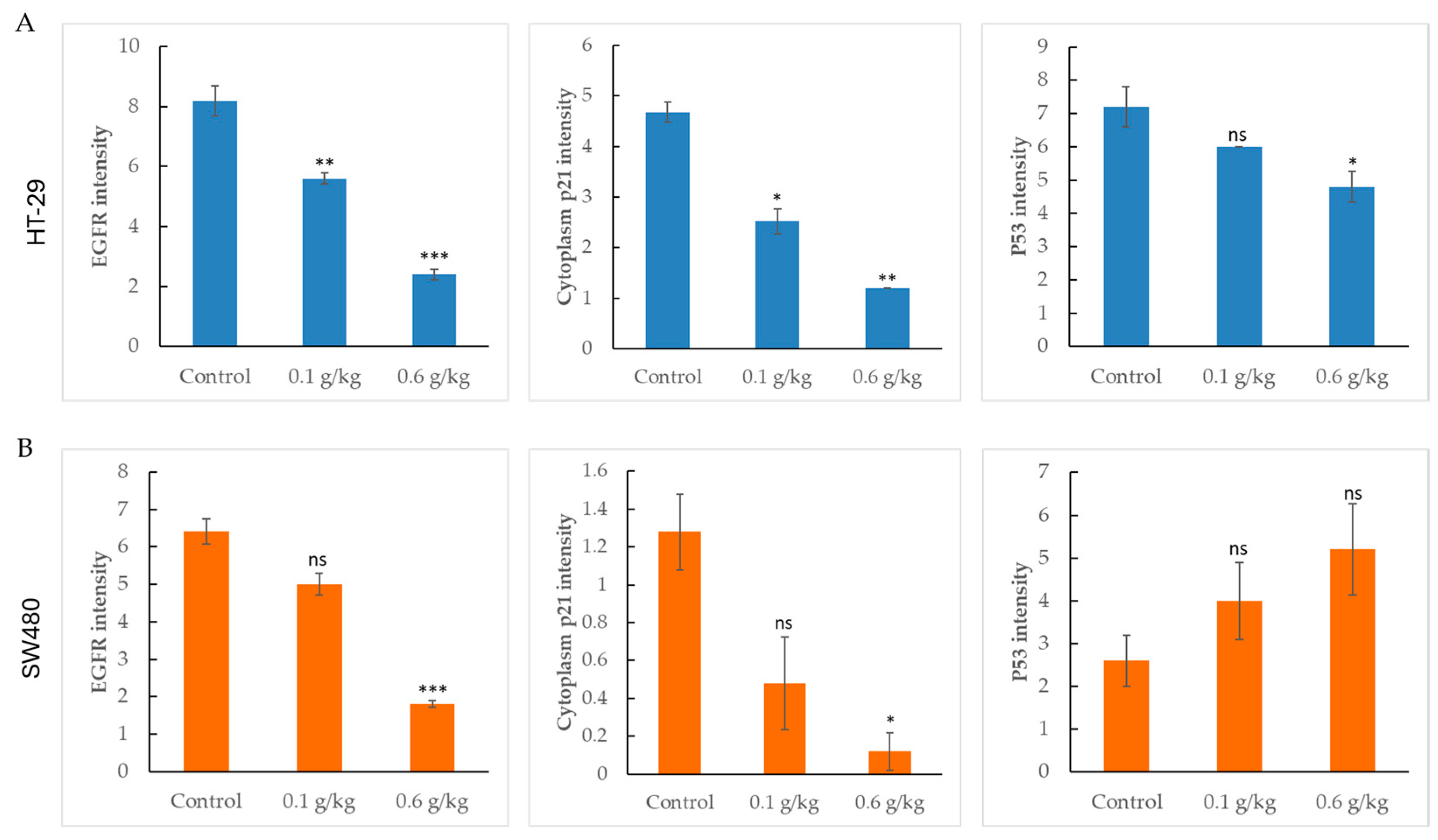
2.5. LCSE Reduces EGFR, p21, and p53 Expression in HT-29 and SW480 Xenograft Tumors
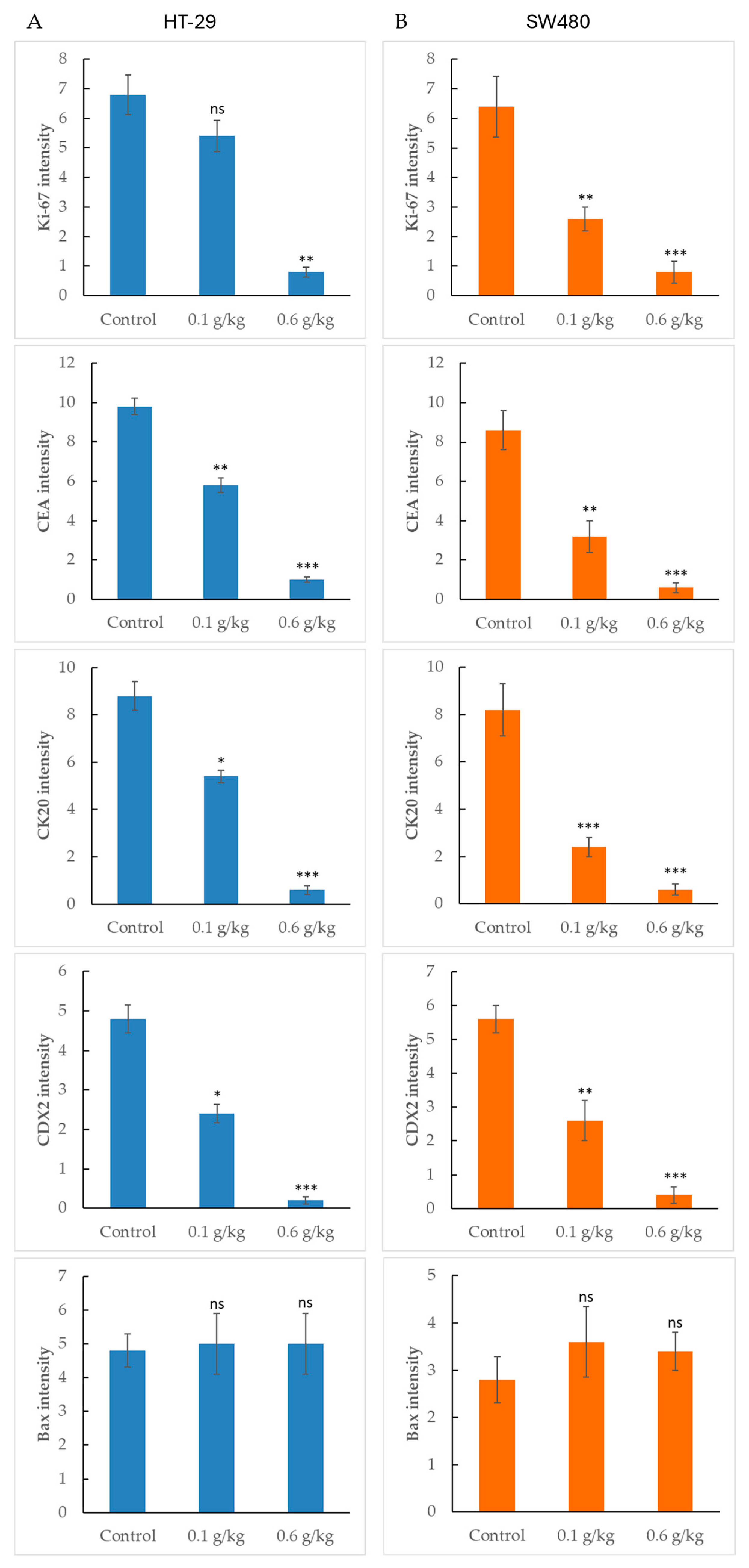
2.6. Dose-Dependent Suppression of Proliferation and Differentiation Markers by LCSE in HT-29 and SW480 Xenografts
3. Discussion
4. Materials and Methods
4.1. Preparation of LCSE
4.2. Phytochemical Characterization of LCSE
4.3. Cell Culture of HT-29 and SW480
4.4. Tumor Xenograft Establishment in Nude Mice
4.5. Immunohistochemistry Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J.; Park, B.H.; Kinzler, K.W.; Vogelstein, B. Role of BAX in the Apoptotic Response to Anticancer Agents. Science 2000, 290, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef]
- Werling, R.W.; Yaziji, H.; Bacchi, C.E.; Gown, A.M. CDX2, a Highly Sensitive and Specific Marker of Adenocarcinomas of Intestinal Origin. Am. J. Surg. Pathol. 2003, 27, 303–310. [Google Scholar] [CrossRef]
- Aktar, M.N.; Islam, M.M.; Raza, M.S.; Azhar, B.S.; Moni, Z.R.; Rahman, M.M.; Rahman, A.T.; Tang, M.A. Phytochemical characteristics and antioxidant potential of litchi chinensis sonn. seeds of bangladesh. Bangladesh J. Agric. 2022, 47, 16–26. [Google Scholar] [CrossRef]
- Kilari, E.; Putta, S. Biological and phytopharmacological descriptions of Litchi chinensis. Pharmacogn. Rev. 2016, 10, 60. [Google Scholar] [CrossRef]
- Hsu, C.P.; Lin, C.C.; Huang, C.C.; Lin, Y.H.; Chou, J.C.; Tsia, Y.T.; Su, J.R.; Chung, Y.C. Induction of Apoptosis and Cell Cycle Arrest in Human Colorectal Carcinoma by Litchi Seed Extract. J. Biomed. Biotechnol. 2012, 2012, 341479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, D.; An, X.; Duan, L.; Duan, Y.; Lian, F. Lychee Seed as a Potential Hypoglycemic Agent, and Exploration of its Underlying Mechanisms. Front. Pharmacol. 2021, 12, 737803. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Luo, H.; Yuan, H.; Xia, Y.; Shu, P.; Huang, X.; Lu, Y.; Liu, X.; Keller, E.T.; Sun, D.; et al. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3β signaling. Sci. Rep. 2017, 7, 41656, Correction in Sci Rep. 2021, 11, 15201. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Fabbri, A.; Del Cornò, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent updates on anticancer mechanisms of polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef]
- Li, W.; Liang, H.; Zhang, M.W.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Zhang, Y.; Tang, X.J. Phenolic Profiles and Antioxidant Activity of Litchi (Litchi Chinensis Sonn.) Fruit Pericarp from Different Commercially Available Cultivars. Molecules 2012, 17, 14954–14967. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
- Paliga, M.; Novello, Z.; Dallago, R.M.; Scapinello, J.; Magro, J.D.; Di Luccio, M.; Tres, M.V.; Oliveira, J.V. Extraction, chemical characterization and antioxidant activity of Litchi chinensis Sonn. and Avena sativa L. seeds extracts obtained from pressurized n-butane. J. Food Sci. Technol. 2017, 54, 846–851. [Google Scholar] [CrossRef]
- Dasharath, O.; Singh, K.; Narayan, B. Characterization of different parts of litchi fruit using UHPLC-QExactive Orbitrap. J. Food Sci. Technol. 2022, 59, 4889–4906. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, H.; Liu, W.; Fan, C.; Jin, F.; Xiang, X. Metabolite Differences of Polyphenols in Different Litchi Cultivars (Litchi chinensis Sonn.) Based Extensive Target. Metabonom. Mol. 2021, 26, 1181. [Google Scholar] [CrossRef]
- Shu, B.; Wang, J.; Wu, G.; Cao, X.; Huang, F.; Dong, L.; Zhang, R.; Liu, H.; Su, D. Newly generated and increased bound phenolic in lychee pulp during heat-pump drying detected by UPLC–ESI-triple-TOF-MS/MS. J. Sci. Food Agric. 2021, 102, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Lima de Oliveira, J.P.; Franco Carneiro, W.; Duarte da Silva, K.C.; Silvestre de Azevedo Martins, M.; Lucinda Machado, G.G.; Abrahão Nogueira, L.; Varaschin, M.S.; de Barros Vilas Boas, E.V.; Solis Murgas, L.D.; Carvalho, E.E. Anti-Obesogenic and Antioxidant Potential of Lychee Seed Flour in Zebrafish Fed a High-Fat Diet. J. Am. Nutr. Assoc. 2025, 44, 454–467. [Google Scholar] [CrossRef]
- Sindhu, K.C.; Pandey, B.; Sistu, K.C.; Gurung, S.; Gautam, A. Anti-inflammatory, analgesic, and acute toxicity evaluation of Litchi chinensis seed extract in albino rat. Nat. Resour. Hum. Health 2021, 1, 30–35. [Google Scholar] [CrossRef]
- Emanuele, S.; Notaro, A.; Palumbo Piccionello, A.; Maggio, A.; Lauricella, M.; D’Anneo, A.; Cernigliaro, C.; Calvaruso, G.; Giuliano, M. Sicilian Litchi Fruit Extracts Induce Autophagy versus Apoptosis Switch in Human Colon Cancer Cells. Nutrients 2018, 10, 1490. [Google Scholar] [CrossRef]
- Subramanian, A.P.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E.; Muhamad, I.I. Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J. Gastroenterol. 2016, 22, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Daveri, E.; Adamo, A.M.; Alfine, E.; Zhu, W.; Oteiza, P.I. Hexameric procyanidins inhibit colorectal cancer cell growth through both redox and non-redox regulation of the epidermal growth factor signaling pathway. Redox Biol. 2020, 38, 101830. [Google Scholar] [CrossRef]
- Yao, Y.; Feng, S.; Li, X.; Liu, T.; Ye, S.; Ma, L.; Man, S. Litchi procyanidins inhibit colon cancer proliferation and metastasis by triggering gut-lung axis immunotherapy. Cell Death Dis. 2023, 14, 109. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Sreenesh, B.; Varghese, E.; Kubatka, P.; Samuel, S.M.; Büsselberg, D. Prebiotic Potential of Dietary Polyphenols in Colorectal Cancer Immunomodulation. Foods 2025, 14, 2392. [Google Scholar] [CrossRef]
- Orlandi, G.; Luca Roncucci Carnevale, G.; Sena, P. Different Roles of Apoptosis and Autophagy in the Development of Human Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 10201. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.F.; Fan, Y.R.; Zhao, Y.H.; Liu, B. Emerging role of autophagy in colorectal cancer: Progress and prospects for clinical intervention. World J. Gastrointest. Oncol. 2023, 15, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yu, W.; Guo, M.; Zhu, N.; Chen, X.; Li, N.; Zhong, C.; Wang, G. Autophagy regulates apoptosis of colorectal cancer cells based on signaling pathways. Discov. Oncol. 2024, 15, 367. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, E.; Taneera, J.; Sulaiman, N.; Saber-Ayad, M. The role of autophagy in colorectal cancer: Impact on pathogenesis and implications in therapy. Front. Med. 2022, 9, 959348. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L. Autophagy is a double-edged sword in the therapy of colorectal cancer (Review). Oncol. Lett. 2021, 21, 378. [Google Scholar] [CrossRef]
- Gewirtz, D.A. Autophagy and senescence in cancer therapy. J. Cell. Physiol. 2013, 229, 6–9. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, B.; Wang, N.; Gu, J. Tumor immunomodulatory effects of polyphenols. Front. Immunol. 2022, 13, 1041138. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Xia, Y.; Liu, B. The mechanism and tumor inhibitory study of Lagopsis supine ethanol extract on colorectal cancer in nude mice. BMC Complement. Altern. Med. 2019, 19, 173. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-N.; Chang, Y.-P.; Yang, O.C.Y.; Wu, C.-S.; Huang, C.-C.; Chang, J.-F.; Liang, C.-M.; Dai, S.-T.; Chen, L.; Hsu, C.-P. Lychee Seed Extract Targets Proliferation, Differentiation, and Cell Cycle Proteins to Suppress Human Colorectal Tumor Growth in Xenograft Models. Int. J. Mol. Sci. 2025, 26, 9786. https://doi.org/10.3390/ijms26199786
Yang S-N, Chang Y-P, Yang OCY, Wu C-S, Huang C-C, Chang J-F, Liang C-M, Dai S-T, Chen L, Hsu C-P. Lychee Seed Extract Targets Proliferation, Differentiation, and Cell Cycle Proteins to Suppress Human Colorectal Tumor Growth in Xenograft Models. International Journal of Molecular Sciences. 2025; 26(19):9786. https://doi.org/10.3390/ijms26199786
Chicago/Turabian StyleYang, Szu-Nian, Yi-Ping Chang, Oscar C. Y. Yang, Chi-Sheng Wu, Chiu-Chen Huang, Jia-Feng Chang, Chia-Ming Liang, Shun-Tai Dai, Lung Chen, and Chih-Ping Hsu. 2025. "Lychee Seed Extract Targets Proliferation, Differentiation, and Cell Cycle Proteins to Suppress Human Colorectal Tumor Growth in Xenograft Models" International Journal of Molecular Sciences 26, no. 19: 9786. https://doi.org/10.3390/ijms26199786
APA StyleYang, S.-N., Chang, Y.-P., Yang, O. C. Y., Wu, C.-S., Huang, C.-C., Chang, J.-F., Liang, C.-M., Dai, S.-T., Chen, L., & Hsu, C.-P. (2025). Lychee Seed Extract Targets Proliferation, Differentiation, and Cell Cycle Proteins to Suppress Human Colorectal Tumor Growth in Xenograft Models. International Journal of Molecular Sciences, 26(19), 9786. https://doi.org/10.3390/ijms26199786







