Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy
Abstract
1. Introduction
2. Biology of Bone Marrow Adipocytes
2.1. Origin and Differentiation
2.2. Spatial Heterogeneity
3. Mechanistic Crosstalk Between Bone Marrow Adipocytes and Tumor Cells
3.1. Metabolic Coupling
3.2. Adipokine Signaling
3.3. Immunologic Crosstalk
3.4. Bone-Remodeling Signaling
3.5. Extracellular-Vesicle–Mediated Cargo Transfer
4. Comparative Mechanisms Across Cancer Types and Tumor-Specific Features
4.1. Breast Cancer
4.2. Prostate Cancer
4.3. Lung Cancer
4.4. Myeloma and Hematologic Malignancies
| Cancer Type | Effect of BMAs on Tumor Cells | Mechanism | References |
|---|---|---|---|
| Breast cancer | Supports osteolytic lesion formation | Mice treated with PPARγ showed a marked decrease in osteolytic lesions | [105] |
| Prostate cancer | Enhance spread | Transport fatty acids to tumor cells and upregulates IL-1β, HMOX1, and FABP4 | [25] |
| Promote bone remodeling | Release chemokines that attract osteoclasts | [14] | |
| Influence metabolism | Promote HIF-1α activation | [110] | |
| Lung cancer | Assist in tumor cell growth and angiogenesis | S100A8/9 or TLR4 interactions upregulate IL-6 release from BMAs | [72,111,112] |
| Promote tumor cell migration and survival | Leptin-mediated induction of EMT and inhibition of apoptosis | [113,114] | |
| Promote angiogenesis and osteolysis | Adiponectin-mediated enhancement of VEGF expression and promotion of the RANK/RANKL and JAK/STAT pathways | [115,116] | |
| Promote osteoclast activation, inhibit osteoblast activity, enhance microenvironment inflammation, and promote angiogenesis | Resistin-mediated activation of NF-κB, JAK/STAT, and PI3K/AKT pathways | [117,118,119,120] | |
| Myeloma and hematologic malignancies | Chemotherapy resistance | Leptin and adipsin-mediated induction of autophagy | [122] |
| Sequestrating and metabolizing daunorubicin within BMAs | [123] |
5. BMAs as Drivers of Therapeutic Resistance
6. Experimental Models
6.1. In-Vitro Models
6.1.1. D Co-Cultures
6.1.2. D Adipocyte–Bone Organoids
6.1.3. Microfluidic Bone-on-a-Chip
6.2. In-Vivo Models
6.2.1. High Marrow Fat Murine Models
Irradiation
Ovariectomy
PPARγ Agonists (e.g., Pioglitazone)
6.2.2. Patient-Derived Xenografts into Adipocyte-Rich Tibiae
6.2.3. Analytical Techniques
7. Clinical Evidence to Date
7.1. Key Clinical Observations
7.2. Limitations and Gaps
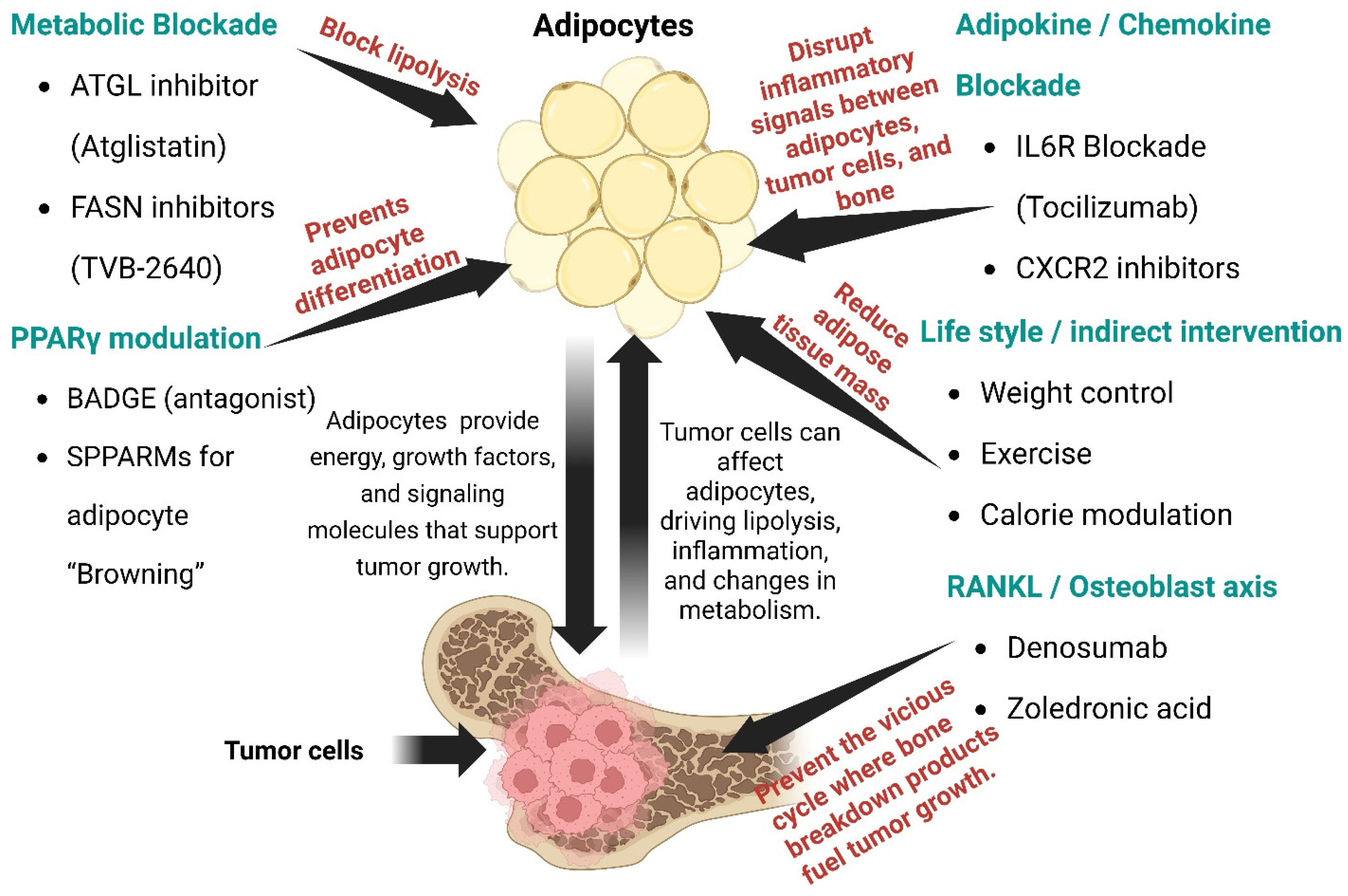
8. Therapeutic Targeting Strategies
8.1. Metabolic Blockade of BMA
8.2. PPARγ Modulation
8.3. RANKL Pathway
8.4. Adipokine/Chemokine Blockade
8.5. Lifestyle Modifications
9. Knowledge Gaps and Future Directions
9.1. Cellular Heterogeneity
9.2. Dynamic Lipidomics
9.3. Immunometabolic Interface
9.4. Standardized Biomarkers
9.5. Therapeutic Translation
9.6. Pediatric and Sex Differences
10. Conclusions
Funding
Conflicts of Interest
References
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the Bone in Cancer Metastasis. J. Bone Miner. Res. 2018, 33, 2099–2113. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Gajdosik, M.S.; Josic, D.; Clifton, J.G.; Logothetis, C.; Yu-Lee, L.-Y.; Gallick, G.E.; Maity, S.N.; Lin, S.-H. Secretome Analysis of an Osteogenic Prostate Tumor Identifies Complex Signaling Networks Mediating Cross-Talk of Cancer and Stromal Cells within the Tumor Microenvironment. Mol. Cell. Proteom. 2015, 14, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Lee, Y.-C.; Yu, G.; Cheng, C.-J.; Zhou, X.; Chu, K.; Murshed, M.; Le, N.-T.; Baseler, L.; Abe, J.-I.; et al. Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Dev. Cell 2017, 41, 467–480.e3. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Cheng, C.-J.; Bilen, M.A.; Lu, J.-F.; Satcher, R.L.; Yu-Lee, L.-Y.; Gallick, G.E.; Maity, S.N.; Lin, S.-H. BMP4 Promotes Prostate Tumor Growth in Bone through Osteogenesis. Cancer Res. 2011, 71, 5194–5203. [Google Scholar] [CrossRef]
- Delea, T.; Langer, C.; McKiernan, J.; Liss, M.; Edelsberg, J.; Brandman, J.; Sung, J.; Raut, M.; Oster, G. The Cost of Treatment of Skeletal-Related Events in Patients with Bone Metastases from Lung Cancer. Oncology 2004, 67, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.; Lamerato, L.; Glass, A.G.; Richert-Boe, K.E.; Lopez, A.; Chung, K.; Richhariya, A.; Dodge, T.; Wolff, G.G.; Balakumaran, A.; et al. Natural History of Skeletal-Related Events in Patients with Breast, Lung, or Prostate Cancer and Metastases to Bone: A 15-Year Study in Two Large US Health Systems. Support. Care Cancer 2013, 21, 3279–3286. [Google Scholar] [CrossRef]
- Giuliani, N.; Ferretti, M.; Bolzoni, M.; Storti, P.; Lazzaretti, M.; Dalla Palma, B.; Bonomini, S.; Martella, E.; Agnelli, L.; Neri, A.; et al. Increased Osteocyte Death in Multiple Myeloma Patients: Role in Myeloma-Induced Osteoclast Formation. Leukemia 2012, 26, 1391–1401. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple Myeloma: 2024 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2024, 99, 1802–1824. [Google Scholar] [CrossRef]
- Wiedmeier-Nutor, J.E.; Fonseca, R. Hyperdiploid Myeloma: The Silent Majority. Br. J. Haematol. 2025, 207, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; He, Y.; Yu, X. Bone Marrow Adipocyte: An Intimate Partner With Tumor Cells in Bone Metastasis. Front. Endocrinol. 2018, 9, 339. [Google Scholar] [CrossRef]
- Sato, S.; Hiruma, T.; Koizumi, M.; Yoshihara, M.; Nakamura, Y.; Tadokoro, H.; Motomatsu, S.; Yamanaka, T.; Washimi, K.; Okubo, Y.; et al. Bone Marrow Adipocytes Induce Cancer-Associated Fibroblasts and Immune Evasion, Enhancing Invasion and Drug Resistance. Cancer Sci. 2023, 114, 2674–2688. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Errani, C.; Sartori, M.; Borsari, V.; Giavaresi, G. Role of Bone Marrow Adipocytes in Bone Metastasis Development and Progression: A Systematic Review. Front. Endocrinol. 2023, 14, 1207416. [Google Scholar] [CrossRef]
- Shin, E.; Koo, J.S. The Role of Adipokines and Bone Marrow Adipocytes in Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2020, 21, 4967. [Google Scholar] [CrossRef]
- Zheng, Y.; Basel, D.; Chow, S.-O.; Fong-Yee, C.; Kim, S.; Buttgereit, F.; Dunstan, C.R.; Zhou, H.; Seibel, M.J. Targeting IL-6 and RANKL Signaling Inhibits Prostate Cancer Growth in Bone. Clin. Exp. Metastasis 2014, 31, 921–933. [Google Scholar] [CrossRef]
- Moraes, J.A.; Encarnação, C.; Franco, V.A.; Xavier Botelho, L.G.; Rodrigues, G.P.; Ramos-Andrade, I.; Barja-Fidalgo, C.; Renovato-Martins, M. Adipose Tissue-Derived Extracellular Vesicles and the Tumor Microenvironment: Revisiting the Hallmarks of Cancer. Cancers 2021, 13, 3328. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.-Q.; Da, M.-X.; Jin, W.-L.; Zhou, F.-H. Adipocyte-Derived Extracellular Vesicles: Bridging the Communications between Obesity and Tumor Microenvironment. Discov. Oncol. 2023, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Park, H.; Lim, E.H.; Lee, K.W. Exosomes from Breast Cancer Cells Can Convert Adipose Tissue-Derived Mesenchymal Stem Cells into Myofibroblast-like Cells. Int. J. Oncol. 2012, 40, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tratwal, J.; Rojas-Sutterlin, S.; Bataclan, C.; Blum, S.; Naveiras, O. Bone Marrow Adiposity and the Hematopoietic Niche: A Historical Perspective of Reciprocity, Heterogeneity, and Lineage Commitment. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101564. [Google Scholar] [CrossRef]
- Trotter, T.N.; Gibson, J.T.; Sherpa, T.L.; Gowda, P.S.; Peker, D.; Yang, Y. Adipocyte-Lineage Cells Support Growth and Dissemination of Multiple Myeloma in Bone. Am. J. Pathol. 2016, 186, 3054–3063. [Google Scholar] [CrossRef]
- Shafat, M.S.; Oellerich, T.; Mohr, S.; Robinson, S.D.; Edwards, D.R.; Marlein, C.R.; Piddock, R.E.; Fenech, M.; Zaitseva, L.; Abdul-Aziz, A.; et al. Leukemic Blasts Program Bone Marrow Adipocytes to Generate a Protumoral Microenvironment. Blood 2017, 129, 1320–1332. [Google Scholar] [CrossRef]
- Jafari, A.; Fairfield, H.; Andersen, T.L.; Reagan, M.R. Myeloma-Bone Marrow Adipocyte Axis in Tumour Survival and Treatment Response. Br. J. Cancer 2021, 125, 775–777. [Google Scholar] [CrossRef]
- Scheller, E.L.; Doucette, C.R.; Learman, B.S.; Cawthorn, W.P.; Khandaker, S.; Schell, B.; Wu, B.; Ding, S.-Y.; Bredella, M.A.; Fazeli, P.K.; et al. Region-Specific Variation in the Properties of Skeletal Adipocytes Reveals Regulated and Constitutive Marrow Adipose Tissues. Nat. Commun. 2015, 6, 7808. [Google Scholar] [CrossRef]
- Herroon, M.K.; Rajagurubandara, E.; Hardaway, A.L.; Powell, K.; Turchick, A.; Feldmann, D.; Podgorski, I. Bone Marrow Adipocytes Promote Tumor Growth in Bone via FABP4-Dependent Mechanisms. Oncotarget 2013, 4, 2108–2123. [Google Scholar] [CrossRef]
- Craft, C.S.; Li, Z.; MacDougald, O.A.; Scheller, E.L. Molecular Differences between Subtypes of Bone Marrow Adipocytes. Curr. Mol. Biol. Rep. 2018, 4, 16–23. [Google Scholar] [CrossRef]
- Singhal, V.; Torre Flores, L.P.; Stanford, F.C.; Toth, A.T.; Carmine, B.; Misra, M.; Bredella, M.A. Differential Associations between Appendicular and Axial Marrow Adipose Tissue with Bone Microarchitecture in Adolescents and Young Adults with Obesity. Bone 2018, 116, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and Applications for Single-Cell and Spatial Multi-Omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Cabia, B.; Andrade, S.; Carreira, M.C.; Casanueva, F.F.; Crujeiras, A.B. A Role for Novel Adipose Tissue-Secreted Factors in Obesity-Related Carcinogenesis. Obes. Rev. 2016, 17, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Na, H.; Lee, S.E.; Kim, Y.M.; Moon, J.; Nam, T.W.; Ji, Y.; Jin, Y.; Park, J.H.; Cho, S.C.; et al. Dysfunctional Adipocytes Promote Tumor Progression through YAP/TAZ-Dependent Cancer-Associated Adipocyte Transformation. Nat. Commun. 2024, 15, 4052. [Google Scholar] [CrossRef]
- Panaroni, C.; Fulzele, K.; Mori, T.; Siu, K.T.; Onyewadume, C.; Maebius, A.; Raje, N. Multiple Myeloma Cells Induce Lipolysis in Adipocytes and Uptake Fatty Acids through Fatty Acid Transporter Proteins. Blood 2022, 139, 876–888. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-Based Mechanism for Breast Cancer Bone Metastasis and Related Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Fajol, A.; Komaba, H. Additional Evidence for the Role of Parathyroid Hormone in Adipose Tissue Browning. EBioMedicine 2019, 40, 3–4. [Google Scholar] [CrossRef]
- Mukherjee, S.; Aseer, K.R.; Yun, J.W. Roles of Macrophage Colony Stimulating Factor in White and Brown Adipocytes. Biotechnol. Bioprocess Eng. 2020, 25, 29–38. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Yu, X. Bone Marrow Adipocytes, Adipocytokines, and Breast Cancer Cells: Novel Implications in Bone Metastasis of Breast Cancer. Front. Oncol. 2020, 10, 561595. [Google Scholar] [CrossRef]
- Soni, S.; Torvund, M.; Mandal, C.C. Molecular Insights into the Interplay between Adiposity, Breast Cancer and Bone Metastasis. Clin. Exp. Metastasis 2021, 38, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Pestell, R.G.; Howell, A.; Tykocinski, M.L.; Nagajyothi, F.; Machado, F.S.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Energy Transfer in “Parasitic” Cancer Metabolism: Mitochondria Are the Powerhouse and Achilles’ Heel of Tumor Cells. Cell Cycle 2011, 10, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-Associated Adipocytes: Key Players in Breast Cancer Progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, D.; Hong, Z. Sarcopenia and Cachexia: Molecular Mechanisms and Therapeutic Interventions. MedComm (2020) 2025, 6, e70030. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Ngandiri, D.A.; Llerins Perez, M.; Wolf, A.; Wang, Y. The Molecular Brakes of Adipose Tissue Lipolysis. Front. Physiol. 2022, 13, 826314. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Z.; Yao, F.; Sun, K.; Li, Z.; Sun, S.; Li, C. Breast Cancer Cell-Derived Exosome-Delivered microRNA-155 Targets UBQLN1 in Adipocytes and Facilitates Cancer Cachexia-Related Fat Loss. Hum. Mol. Genet. 2023, 32, 2219–2228. [Google Scholar] [CrossRef]
- Librizzi, M.; Naselli, F.; Abruscato, G.; Luparello, C.; Caradonna, F. Parathyroid Hormone Related Protein (PTHrP)-Associated Molecular Signatures in Tissue Differentiation and Non-Tumoral Diseases. Biology 2023, 12, 950. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bilecz, A.J.; Lengyel, E. The Adipocyte Microenvironment and Cancer. Cancer Metastasis Rev. 2022, 41, 575–587. [Google Scholar] [CrossRef]
- Gyamfi, J.; Yeo, J.H.; Kwon, D.; Min, B.S.; Cha, Y.J.; Koo, J.S.; Jeong, J.; Lee, J.; Choi, J. Interaction between CD36 and FABP4 Modulates Adipocyte-Induced Fatty Acid Import and Metabolism in Breast Cancer. NPJ Breast Cancer 2021, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, L.; Wagner, R.; Heller, G.; Pils, D.; Grunt, T.W. Pharmacological Inhibition of Lipid Import and Transport Proteins in Ovarian Cancer. Cancers 2022, 14, 6004. [Google Scholar] [CrossRef]
- Zhao, G.; Cardenas, H.; Matei, D. Ovarian Cancer-Why Lipids Matter. Cancers 2019, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Niu, X.; Du, Y.; Chen, Y.; Liu, X.; Xu, L.; Iwakura, Y.; Ma, X.; Li, Y.; Yao, Z.; et al. IL-17A Promotes Fatty Acid Uptake through the IL-17A/IL-17RA/p-STAT3/FABP4 Axis to Fuel Ovarian Cancer Growth in an Adipocyte-Rich Microenvironment. Cancer Immunol. Immunother. 2020, 69, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Patti, G.J. The Warburg Effect: A Signature of Mitochondrial Overload. Trends Cell Biol. 2023, 33, 1014–1020. [Google Scholar] [CrossRef]
- Fairfield, H.; Karam, M.; Schimelman, A.; Qiang, Y.-W.; Reagan, M.R. Adipocytes and Metabolism: Contributions to Multiple Myeloma. J. Bone Oncol. 2024, 46, 100609. [Google Scholar] [CrossRef]
- Tabe, Y.; Konopleva, M.; Andreeff, M. Fatty Acid Metabolism, Bone Marrow Adipocytes, and AML. Front. Oncol. 2020, 10, 155. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yu, H.; Yue, R.; Zhao, Z.; Rios, J.J.; Naveiras, O.; Morrison, S.J. Bone Marrow Adipocytes Promote the Regeneration of Stem Cells and Haematopoiesis by Secreting SCF. Nat. Cell Biol. 2017, 19, 891–903. [Google Scholar] [CrossRef]
- Maroni, P. Leptin, Adiponectin, and Sam68 in Bone Metastasis from Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1051. [Google Scholar] [CrossRef]
- Gu, L.; Wang, C.-D.; Cao, C.; Cai, L.-R.; Li, D.-H.; Zheng, Y.-Z. Association of Serum Leptin with Breast Cancer: A Meta-Analysis. Medicine 2019, 98, e14094. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Roles of Omental and Bone Marrow Adipocytes in Tumor Biology. Adipocyte 2019, 8, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.V.; Edwards, C.M. Adipokines, Adiposity, and Bone Marrow Adipocytes: Dangerous Accomplices in Multiple Myeloma. J. Cell. Physiol. 2018, 233, 9159–9166. [Google Scholar] [CrossRef]
- Gorrab, A.; Pagano, A.; Ayed, K.; Chebil, M.; Derouiche, A.; Kovacic, H.; Gati, A. Leptin Promotes Prostate Cancer Proliferation and Migration by Stimulating STAT3 Pathway. Nutr. Cancer 2021, 73, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.W.; Rossi, E.L.; McDonell, S.B.; Doerstling, S.S.; Khatib, S.A.; Lineberger, C.G.; Albright, J.E.; Tang, X.; deGraffenried, L.A.; Hursting, S.D. Leptin Signaling Mediates Obesity-Associated CSC Enrichment and EMT in Preclinical TNBC Models. Mol. Cancer Res. 2018, 16, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Cruz, J.C.; Zuñiga-Eulogio, M.D.; Olea-Flores, M.; Castañeda-Saucedo, E.; Mendoza-Catalán, M.Á.; Ortuño-Pineda, C.; Moreno-Godínez, M.E.; Villegas-Comonfort, S.; Padilla-Benavides, T.; Navarro-Tito, N. Leptin Induces Cell Migration and Invasion in a FAK-Src-Dependent Manner in Breast Cancer Cells. Endocr. Connect. 2019, 8, 1539–1552. [Google Scholar] [CrossRef]
- Bader, J.E.; Wolf, M.M.; Lupica-Tondo, G.L.; Madden, M.Z.; Reinfeld, B.I.; Arner, E.N.; Hathaway, E.S.; Steiner, K.K.; Needle, G.A.; Hatem, Z.; et al. Obesity Induces PD-1 on Macrophages to Suppress Anti-Tumour Immunity. Nature 2024, 630, 968–975. [Google Scholar] [CrossRef]
- Tsai, C.-F.; Chen, J.-H.; Wu, C.-T.; Chang, P.-C.; Wang, S.-L.; Yeh, W.-L. Induction of Osteoclast-like Cell Formation by Leptin-Induced Soluble Intercellular Adhesion Molecule Secreted from Cancer Cells. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846806. [Google Scholar] [CrossRef]
- He, J.-Y.; Wei, X.-H.; Li, S.-J.; Liu, Y.; Hu, H.-L.; Li, Z.-Z.; Kuang, X.-H.; Wang, L.; Shi, X.; Yuan, S.-T.; et al. Adipocyte-Derived IL-6 and Leptin Promote Breast Cancer Metastasis via Upregulation of Lysyl Hydroxylase-2 Expression. Cell Commun. Signal. 2018, 16, 100. [Google Scholar] [CrossRef]
- Du, H.; Pang, M.; Hou, X.; Yuan, S.; Sun, L. PLOD2 in Cancer Research. Biomed. Pharmacother. 2017, 90, 670–676. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Mitsui, A.; Sumardika, I.W.; Yokoyama, Y.; Sakaguchi, M.; Kondo, E. PLOD2-Driven IL-6/STAT3 Signaling Promotes the Invasion and Metastasis of Oral Squamous Cell Carcinoma via Activation of Integrin Β1. Int. J. Oncol. 2021, 58, 29. [Google Scholar] [CrossRef]
- Viveiros, M.M.H.; de Melo Viveiros, M.E.; Silva, M.G.; Kaneno, R.; Avelino, N.P.; Rainho, C.A.; Schellini, S.A. Expression of Inflammatory Cytokines in Mesenchymal Stem Cells Derived from Proximal Humerus Fractures. Stem Cell Investig. 2022, 9, 3. [Google Scholar] [CrossRef]
- Morris, E.V.; Suchacki, K.J.; Hocking, J.; Cartwright, R.; Sowman, A.; Gamez, B.; Lea, R.; Drake, M.T.; Cawthorn, W.P.; Edwards, C.M. Myeloma Cells Down-Regulate Adiponectin in Bone Marrow Adipocytes Via TNF-Alpha. J. Bone Miner. Res. 2020, 35, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, J.; Shi, H.; Wang, S.; Yan, Y.; Xu, Q.; Zhou, S.; Zhao, Z.; Mu, Y.; Qian, C.; et al. Adiponectin Suppresses Tumor Growth of Nasopharyngeal Carcinoma through Activating AMPK Signaling Pathway. J. Transl. Med. 2022, 20, 89. [Google Scholar] [CrossRef]
- Nigro, E.; Daniele, A.; Salzillo, A.; Ragone, A.; Naviglio, S.; Sapio, L. AdipoRon and Other Adiponectin Receptor Agonists as Potential Candidates in Cancer Treatments. Int. J. Mol. Sci. 2021, 22, 5569. [Google Scholar] [CrossRef]
- Tulotta, C.; Ottewell, P. The Role of IL-1B in Breast Cancer Bone Metastasis. Endocr. Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Xie, Y.; Yu, X. Bone Marrow Adipocytes and Lung Cancer Bone Metastasis: Unraveling the Role of Adipokines in the Tumor Microenvironment. Front. Oncol. 2024, 14, 1360471. [Google Scholar] [CrossRef]
- Bilwani, F.A.; Knight, K.L. Adipocyte-Derived Soluble Factor(s) Inhibits Early Stages of B Lymphopoiesis. J. Immunol. 2012, 189, 4379–4386. [Google Scholar] [CrossRef]
- Kennedy, D.E.; Knight, K.L. Inhibition of B Lymphopoiesis by Adipocytes and IL-1-Producing Myeloid-Derived Suppressor Cells. J. Immunol. 2015, 195, 2666–2674. [Google Scholar] [CrossRef]
- Perico, M.E.; Maluta, T.; Conti, G.; Vella, A.; Provezza, L.; Cestari, T.; De Cao, G.; Segalla, L.; Tecchio, C.; Benedetti, F.; et al. The Cross-Talk between Myeloid and Mesenchymal Stem Cells of Human Bone Marrow Represents a Biomarker of Aging That Regulates Immune Response and Bone Reabsorption. Cells 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, P.C.; Ochoa, A.C. T Cell Dysfunction in Cancer: Role of Myeloid Cells and Tumor Cells Regulating Amino Acid Availability and Oxidative Stress. Semin. Cancer Biol. 2006, 16, 66–72. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Niu, M.; Mei, Q.; Wu, K. Myeloid-Derived Suppressor Cells: An Emerging Target for Anticancer Immunotherapy. Mol. Cancer 2022, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Z.; Hong, J.; Wu, J.; Huang, O.; He, J.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Targeting Cancer-Associated Adipocyte-Derived CXCL8 Inhibits Triple-Negative Breast Cancer Progression and Enhances the Efficacy of Anti-PD-1 Immunotherapy. Cell Death Dis. 2023, 14, 703. [Google Scholar] [CrossRef]
- Engin, A.B. Message Transmission Between Adipocyte and Macrophage in Obesity. Adv. Exp. Med. Biol. 2024, 1460, 273–295. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Zgutka, K.; Tkacz, M.; Tarnowski, M. Physical Activity as a Modern Intervention in the Fight against Obesity-Related Inflammation in Type 2 Diabetes Mellitus and Gestational Diabetes. Antioxidants 2023, 12, 1488. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Kim, J.-H.; Han, I.-H.; Ryu, J.-S. Polarization of M2 Macrophages by Interaction between Prostate Cancer Cells Treated with Trichomonas Vaginalis and Adipocytes. Korean J. Parasitol. 2020, 58, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zeng, N.; Zhou, X.; Tan, Y.; Wang, Y.; Zhang, J.; Wu, Y.; Zhang, Q. CAA-Derived IL-6 Induced M2 Macrophage Polarization by Activating STAT3. BMC Cancer 2023, 23, 392. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, J.; Liu, Y.; Dong, W.; Dai, X.; Du, X.; Gu, D. LINC01119 Encapsulated by Cancer-Associated Adipocytes-Derived Exosomes Promotes M2 Polarization of Macrophages to Induce Immune Escape in Ovarian Cancer in a 3D Co-Culture Cell-Based Model. Clin. Transl. Oncol. 2023, 25, 3174–3187. [Google Scholar] [CrossRef]
- He, Y.; Luo, W.; Liu, Y.; Wang, Y.; Ma, C.; Wu, Q.; Tian, P.; He, D.; Jia, Z.; Lv, X.; et al. IL-20RB Mediates Tumoral Response to Osteoclastic Niches and Promotes Bone Metastasis of Lung Cancer. J. Clin. Investig. 2022, 132, e157917. [Google Scholar] [CrossRef]
- Yu, W.; Zhong, L.; Yao, L.; Wei, Y.; Gui, T.; Li, Z.; Kim, H.; Holdreith, N.; Jiang, X.; Tong, W.; et al. Bone Marrow Adipogenic Lineage Precursors Promote Osteoclastogenesis in Bone Remodeling and Pathologic Bone Loss. J. Clin. Investig. 2021, 131, e140214. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.-I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Clabaut, A.; Grare, C.; Rolland-Valognes, G.; Letarouilly, J.-G.; Bourrier, C.; Andersen, T.L.; Sikjær, T.; Rejnmark, L.; Ejersted, C.; Pastoureau, P.; et al. Adipocyte-Induced Transdifferentiation of Osteoblasts and Its Potential Role in Age-Related Bone Loss. PLoS ONE 2021, 16, e0245014. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Rosen, C.J. Bone Marrow Adipocytes as Novel Regulators of Metabolic Homeostasis: Clinical Consequences of Bone Marrow Adiposity. Curr. Obes. Rep. 2025, 14, 9. [Google Scholar] [CrossRef]
- Deepika, F.; Bathina, S.; Armamento-Villareal, R. Novel Adipokines and Their Role in Bone Metabolism: A Narrative Review. Biomedicines 2023, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, L.-M.; Bucher, C.H.; Löffler, J.; Rinne, C.; Duda, G.N.; Geissler, S.; Schulz, T.J.; Schmidt-Bleek, K. The Benefits of Adipocyte Metabolism in Bone Health and Regeneration. Front. Cell Dev. Biol. 2023, 11, 1104709. [Google Scholar] [CrossRef] [PubMed]
- Abuna, R.P.F.; Almeida, L.O.; Souza, A.T.P.; Fernandes, R.R.; Sverzut, T.F.V.; Rosa, A.L.; Beloti, M.M. Osteoporosis and Osteoblasts Cocultured with Adipocytes Inhibit Osteoblast Differentiation by Downregulating Histone Acetylation. J. Cell. Physiol. 2021, 236, 3906–3917. [Google Scholar] [CrossRef]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037275. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Patil, K.; Ramm, G.A.; Ochiya, T.; Soekmadji, C. Extracellular Vesicles in the Development of Organ-Specific Metastasis. J. Extracell. Vesicles 2021, 10, e12125. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating miRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Flaherty, S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A. A Lipase-Independent Pathway of Lipid Release and Immune Modulation by Adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Valenzuela Alvarez, M.; Gutierrez, L.M.; Correa, A.; Lazarowski, A.; Bolontrade, M.F. Metastatic Niches and the Modulatory Contribution of Mesenchymal Stem Cells and Its Exosomes. Int. J. Mol. Sci. 2019, 20, 1946. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from Human Adipose-Derived Mesenchymal Stem Cells Promote Migration through Wnt Signaling Pathway in a Breast Cancer Cell Model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Clement, E.; Lazar, I.; Attané, C.; Carrié, L.; Dauvillier, S.; Ducoux-Petit, M.; Esteve, D.; Menneteau, T.; Moutahir, M.; Le Gonidec, S.; et al. Adipocyte Extracellular Vesicles Carry Enzymes and Fatty Acids That Stimulate Mitochondrial Metabolism and Remodeling in Tumor Cells. EMBO J. 2020, 39, e102525. [Google Scholar] [CrossRef]
- Giordano, C.; La Camera, G.; Gelsomino, L.; Barone, I.; Bonofiglio, D.; Andò, S.; Catalano, S. The Biology of Exosomes in Breast Cancer Progression: Dissemination, Immune Evasion and Metastatic Colonization. Cancers 2020, 12, 2179. [Google Scholar] [CrossRef]
- Reza, A.M.M.T.; Choi, Y.-J.; Yasuda, H.; Kim, J.-H. Human Adipose Mesenchymal Stem Cell-Derived Exosomal-miRNAs Are Critical Factors for Inducing Anti-Proliferation Signalling to A2780 and SKOV-3 Ovarian Cancer Cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef]
- Yang, E.; Wang, X.; Gong, Z.; Yu, M.; Wu, H.; Zhang, D. Exosome-Mediated Metabolic Reprogramming: The Emerging Role in Tumor Microenvironment Remodeling and Its Influence on Cancer Progression. Signal Transduct. Target. Ther. 2020, 5, 242. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Deng, T.; Liu, R.; Ning, T.; Bai, M.; Ying, G.; Zhang, H.; Ba, Y. Exosomal miR-155 from Gastric Cancer Induces Cancer-Associated Cachexia by Suppressing Adipogenesis and Promoting Brown Adipose Differentiation via C/EPBβ. Cancer Biol. Med. 2022, 19, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, H.; Zhu, L.; Li, X.; Li, J.; Yang, S.; Liu, D.; Song, X.; Yokota, H.; Zhang, P. Mechanical Loading Attenuates Breast Cancer-Associated Bone Metastasis in Obese Mice by Regulating the Bone Marrow Microenvironment. J. Cell. Physiol. 2021, 236, 6391–6406. [Google Scholar] [CrossRef]
- Ren, H.; Mao, K.; Yuan, X.; Mu, Y.; Zhao, S.; Fan, X.; Zhu, L.; Ye, Z.; Lan, J. AN698/40746067 Suppresses Bone Marrow Adiposity to Ameliorate Hyperlipidemia-Induced Osteoporosis through Targeted Inhibition of ENTR1. Biomed. Pharmacother. 2024, 176, 116843. [Google Scholar] [CrossRef]
- Gaculenko, A.; Gregoric, G.; Popp, V.; Seyler, L.; Ringer, M.; Kachler, K.; Wu, Z.; Kisel, W.; Hofbauer, C.; Hofbauer, L.C.; et al. Systemic PPARγ Antagonism Reduces Metastatic Tumor Progression in Adipocyte-Rich Bone in Excess Weight Male Rodents. J. Bone Miner. Res. 2021, 36, 2440–2452. [Google Scholar] [CrossRef]
- Mitura, P.; Paja, W.; Klebowski, B.; Wronecki, L.; Godzisz, M.; Sudoł, D.; Bar, K.; Depciuch, J. FTIR Markers of Prostate Cancer Tissue and Their Correlation With Medical Parameters of Tumor Aggressiveness. J. Biophotonics 2025, 18, e70046. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Wrobel, T.P.; Panek, A.; Kwiatek, W.M. High-Definition FT-IR Reveals a Synergistic Effect on Lipid Accumulation in Prostate Cancer Cells Induced by a Combination of X-Rays and Radiosensitizing Drugs. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159468. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; D’Amelio, P.; Gorassini, E.; Grimaldi, A.; Bonello, L.; Fiori, C.; Delsedime, L.; Tizzani, A.; De Libero, A.; Isaia, G.; et al. Osteoclasts Are Active in Bone Forming Metastases of Prostate Cancer Patients. PLoS ONE 2008, 3, e3627. [Google Scholar] [CrossRef]
- Yu, L.; Sui, B.; Fan, W.; Lei, L.; Zhou, L.; Yang, L.; Diao, Y.; Zhang, Y.; Li, Z.; Liu, J.; et al. Exosomes Derived from Osteogenic Tumor Activate Osteoclast Differentiation and Concurrently Inhibit Osteogenesis by Transferring COL1A1-Targeting miRNA-92a-1-5p. J. Extracell. Vesicles 2021, 10, e12056. [Google Scholar] [CrossRef]
- Diedrich, J.D.; Rajagurubandara, E.; Herroon, M.K.; Mahapatra, G.; Hüttemann, M.; Podgorski, I. Bone Marrow Adipocytes Promote the Warburg Phenotype in Metastatic Prostate tumorsviaHIF-1α Activation. Oncotarget 2016, 7, 64854–64877. [Google Scholar] [CrossRef]
- Luo, G.; Tang, M.; Zhao, Q.; Lu, L.; Xie, Y.; Li, Y.; Liu, C.; Tian, L.; Chen, X.; Yu, X. Bone Marrow Adipocytes Enhance Osteolytic Bone Destruction by Activating 1q21.3(S100A7/8/9-IL6R)-TLR4 Pathway in Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 2241–2253. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting Interleukin-6 in Inflammatory Autoimmune Diseases and Cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Xu, M.; Cao, F.-L.; Li, N.; Gao, X.; Su, X.; Jiang, X. Leptin Induces Epithelial-to-Mesenchymal Transition via Activation of the ERK Signaling Pathway in Lung Cancer Cells. Oncol. Lett. 2018, 16, 4782–4788. [Google Scholar] [CrossRef]
- Philp, L.K.; Rockstroh, A.; Sadowski, M.C.; Taherian Fard, A.; Lehman, M.; Tevz, G.; Libério, M.S.; Bidgood, C.L.; Gunter, J.H.; McPherson, S.; et al. Leptin Antagonism Inhibits Prostate Cancer Xenograft Growth and Progression. Endocr. Relat. Cancer 2021, 28, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Luzzati, A.; Perrucchini, G.; Cannavò, L.; Bendinelli, P. Leptin, Leptin Receptor, KHDRBS1 (KH RNA Binding Domain Containing, Signal Transduction Associated 1), and Adiponectin in Bone Metastasis from Breast Carcinoma: An Immunohistochemical Study. Biomedicines 2020, 8, 510. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Wang, L.-J.; Liu, S.-H.; Li, J.; Xiao, L.-G.; Yang, G.-T. Adiponectin Regulates Bone Mass in AIS Osteopenia via RANKL/OPG and IL6 Pathway. J. Transl. Med. 2019, 17, 64. [Google Scholar] [CrossRef]
- Sudan, S.K.; Deshmukh, S.K.; Poosarla, T.; Holliday, N.P.; Dyess, D.L.; Singh, A.P.; Singh, S. Resistin: An Inflammatory Cytokine with Multi-Faceted Roles in Cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188419. [Google Scholar] [CrossRef]
- Muruganandan, S.; Ionescu, A.M.; Sinal, C.J. At the Crossroads of the Adipocyte and Osteoclast Differentiation Programs: Future Therapeutic Perspectives. Int. J. Mol. Sci. 2020, 21, 2277. [Google Scholar] [CrossRef] [PubMed]
- Tzanavari, T.; Tasoulas, J.; Vakaki, C.; Mihailidou, C.; Tsourouflis, G.; Theocharis, S. The Role of Adipokines in the Establishment and Progression of Head and Neck Neoplasms. Curr. Med. Chem. 2019, 26, 4726–4748. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-S.; Tang, C.-H.; Chie, M.-J.; Tsai, C.-H.; Fong, Y.-C.; Lu, Y.-C.; Chen, W.-C.; Lai, C.-T.; Wei, C.-Y.; Tai, H.-C.; et al. Resistin Facilitates VEGF-A-Dependent Angiogenesis by Inhibiting miR-16-5p in Human Chondrosarcoma Cells. Cell Death Dis. 2019, 10, 31. [Google Scholar] [CrossRef]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Chatterjee Bhowmick, D.; Murano, I.; Cohen, P.; et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; He, J.; Liu, H.; Lin, P.; Wan, X.; Navone, N.M.; Tong, Q.; Kwak, L.W.; Orlowski, R.Z.; et al. Mature Adipocytes in Bone Marrow Protect Myeloma Cells against Chemotherapy through Autophagy Activation. Oncotarget 2015, 6, 34329–34341. [Google Scholar] [CrossRef]
- Sheng, X.; Parmentier, J.-H.; Tucci, J.; Pei, H.; Cortez-Toledo, O.; Dieli-Conwright, C.M.; Oberley, M.J.; Neely, M.; Orgel, E.; Louie, S.G.; et al. Adipocytes Sequester and Metabolize the Chemotherapeutic Daunorubicin. Mol. Cancer Res. 2017, 15, 1704–1713. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2019, 35, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.Y.; Nassar, Z.D.; Swinnen, J.V.; Butler, L.M. Lipogenic Effects of Androgen Signaling in Normal and Malignant Prostate. Asian J. Urol. 2020, 7, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Tabe, Y.; Yamamoto, S.; Saitoh, K.; Sekihara, K.; Monma, N.; Ikeo, K.; Mogushi, K.; Shikami, M.; Ruvolo, V.; Ishizawa, J.; et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res. 2017, 77, 1453–1464. [Google Scholar] [CrossRef]
- Foo, B.J.-A.; Eu, J.Q.; Hirpara, J.L.; Pervaiz, S. Interplay between Mitochondrial Metabolism and Cellular Redox State Dictates Cancer Cell Survival. Oxid. Med. Cell. Longev. 2021, 2021, 1341604. [Google Scholar] [CrossRef]
- Lee, I. Regulation of Cytochrome c Oxidase by Natural Compounds Resveratrol, (-)-Epicatechin, and Betaine. Cells 2021, 10, 1346. [Google Scholar] [CrossRef] [PubMed]
- Hamabe-Horiike, T.; Harada, S.-I.; Yoshida, K.; Kinoshita, J.; Yamaguchi, T.; Fushida, S. Adipocytes Contribute to Tumor Progression and Invasion of Peritoneal Metastasis by Interacting with Gastric Cancer Cells as Cancer Associated Fibroblasts. Cancer Rep. 2023, 6, e1647. [Google Scholar] [CrossRef]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in Cancer: Unity in Heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef]
- Pang, J.; Shi, Q.; Liu, Z.; He, J.; Liu, H.; Lin, P.; Cui, J.; Yang, J. Resistin Induces Multidrug Resistance in Myeloma by Inhibiting Cell Death and Upregulating ABC Transporter Expression. Haematologica 2017, 102, 1273–1280. [Google Scholar] [CrossRef]
- Lehuédé, C.; Li, X.; Dauvillier, S.; Vaysse, C.; Franchet, C.; Clement, E.; Esteve, D.; Longué, M.; Chaltiel, L.; Le Gonidec, S.; et al. Adipocytes Promote Breast Cancer Resistance to Chemotherapy, a Process Amplified by Obesity: Role of the Major Vault Protein (MVP). Breast Cancer Res. 2019, 21, 7. [Google Scholar] [CrossRef]
- Tuncer, C.; Hacioglu, C. Notch1 and Major Vault Proteins Modulate Temozolomide Resistance in Glioblastoma. J. Cell. Mol. Med. 2025, 29, e70474. [Google Scholar] [CrossRef] [PubMed]
- Herroon, M.K.; Diedrich, J.D.; Rajagurubandara, E.; Martin, C.; Maddipati, K.R.; Kim, S.; Heath, E.I.; Granneman, J.; Podgorski, I. Prostate Tumor Cell-Derived IL1β Induces an Inflammatory Phenotype in Bone Marrow Adipocytes and Reduces Sensitivity to Docetaxel via Lipolysis-Dependent Mechanisms. Mol. Cancer Res. 2019, 17, 2508–2521. [Google Scholar] [CrossRef]
- Kumar, B.; Orellana, M.; Brooks, J.; Madabushi, S.S.; Vishwasrao, P.; Parra, L.E.; Sanchez, J.; Salhotra, A.; Stein, A.; Chen, C.-C.; et al. Exosomes-Driven Lipolysis and Bone Marrow Niche Remodeling Supports Leukemia Expansion. Haematologica 2020, 106, 1484–1488. [Google Scholar] [CrossRef]
- Heuer, T.S.; Ventura, R.; Mordec, K.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G. FASN Inhibition and Taxane Treatment Combine to Enhance Anti-Tumor Efficacy in Diverse Xenograft Tumor Models through Disruption of Tubulin Palmitoylation and Microtubule Organization and FASN Inhibition-Mediated Effects on Oncogenic Signaling and Gene Expression. EBioMedicine 2017, 16, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H.A.; Bao, L.; Cheng, X.; Qin, Z.; Liu, C.-J.; Heth, J.A.; Udager, A.M.; Soellner, M.B.; Merajver, S.D.; Morikawa, A.; et al. Targeting Fatty Acid Synthase in Preclinical Models of TNBC Brain Metastases Synergizes with SN-38 and Impairs Invasion. NPJ Breast Cancer 2024, 10, 43. [Google Scholar] [CrossRef]
- Falchook, G.; Infante, J.; Arkenau, H.-T.; Patel, M.R.; Dean, E.; Borazanci, E.; Brenner, A.; Cook, N.; Lopez, J.; Pant, S.; et al. First-in-Human Study of the Safety, Pharmacokinetics, and Pharmacodynamics of First-in-Class Fatty Acid Synthase Inhibitor TVB-2640 Alone and with a Taxane in Advanced Tumors. EClinicalMedicine 2021, 34, 100797. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Sun, K.; Shi, Q.; Wang, B.; Huang, Y.; Ren, T.; Tang, X. Tocilizumab (Monoclonal Anti-IL-6R Antibody) Reverses Anlotinib Resistance in Osteosarcoma. Front. Oncol. 2023, 13, 1192472. [Google Scholar] [CrossRef]
- Yang, H.; Karl, M.N.; Wang, W.; Starich, B.; Tan, H.; Kiemen, A.; Pucsek, A.B.; Kuo, Y.-H.; Russo, G.C.; Pan, T.; et al. Engineered Bispecific Antibodies Targeting the Interleukin-6 and -8 Receptors Potently Inhibit Cancer Cell Migration and Tumor Metastasis. Mol. Ther. 2022, 30, 3430–3449. [Google Scholar] [CrossRef] [PubMed]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow Adipocyte-Derived CXCL1 and CXCL2 Contribute to Osteolysis in Metastatic Prostate Cancer. Clin. Exp. Metastasis 2015, 32, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, X.; Yan, F.; Jiang, Z.; Li, Y.; Fang, C.; Shen, J. Effect of Zoledronic Acid on Vertebral Marrow Adiposity in Postmenopausal Osteoporosis Assessed by MR Spectroscopy. Skeletal Radiol. 2015, 44, 1499–1505. [Google Scholar] [CrossRef]
- Li, G.-W.; Chang, S.-X.; Fan, J.-Z.; Tian, Y.-N.; Xu, Z.; He, Y.-M. Marrow Adiposity Recovery after Early Zoledronic Acid Treatment of Glucocorticoid-Induced Bone Loss in Rabbits Assessed by Magnetic Resonance Spectroscopy. Bone 2013, 52, 668–675. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Coleman, R.; Shore, N.; Fizazi, K.; Tombal, B.; Miller, K.; Sieber, P.; Karsh, L.; Damião, R.; et al. Denosumab and Bone-Metastasis-Free Survival in Men with Castration-Resistant Prostate Cancer: Results of a Phase 3, Randomised, Placebo-Controlled Trial. Lancet 2012, 379, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Finkelstein, D.M.; Barrios, C.; Martin, M.; Iwata, H.; Hegg, R.; Glaspy, J.; Periañez, A.M.; Tonkin, K.; Deleu, I.; et al. Adjuvant Denosumab in Early Breast Cancer (D-CARE): An International, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 60–72. [Google Scholar] [CrossRef]
- Bessot, A.; Gunter, J.; McGovern, J.; Bock, N. Bone Marrow Adipocytes in Cancer: Mechanisms, Models, and Therapeutic Implications. Biomaterials 2025, 322, 123341. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Bone Marrow Fat: Linking Adipocyte-Induced Inflammation with Skeletal Metastases. Cancer Metastasis Rev. 2014, 33, 527–543. [Google Scholar] [CrossRef]
- Reagan, M.R.; Rosen, C.J. Navigating the Bone Marrow Niche: Translational Insights and Cancer-Driven Dysfunction. Nat. Rev. Rheumatol. 2016, 12, 154–168. [Google Scholar] [CrossRef]
- Fairfield, H.; Falank, C.; Farrell, M.; Vary, C.; Boucher, J.M.; Driscoll, H.; Liaw, L.; Rosen, C.J.; Reagan, M.R. Development of a 3D Bone Marrow Adipose Tissue Model. Bone 2019, 118, 77–88. [Google Scholar] [CrossRef]
- Ottewell, P.D.; O’Donnell, L.; Holen, I. Molecular Alterations That Drive Breast Cancer Metastasis to Bone. BoneKEy Rep. 2015, 4, 643. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Czernik, B.; Stechschulte, L.A. Bone and Fat: A Relationship of Different Shades. Arch. Biochem. Biophys. 2014, 561, 124–129. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-Derived Xenograft Models in Cancer Therapy: Technologies and Applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. The Role of Bone Marrow Adipocytes in Bone Metastasis. J. Bone Oncol. 2016, 5, 121–123. [Google Scholar] [CrossRef]
- Karampinos, D.C.; Ruschke, S.; Dieckmeyer, M.; Diefenbach, M.; Franz, D.; Gersing, A.S.; Krug, R.; Baum, T. Quantitative MRI and Spectroscopy of Bone Marrow. Magn. Reson. Imaging 2018, 47, 332–353. [Google Scholar] [CrossRef]
- Agazzi, G.M.; Di Meo, N.; Rondi, P.; Saeli, C.; Dalla Volta, A.; Vezzoli, M.; Berruti, A.; Borghesi, A.; Maroldi, R.; Ravanelli, M.; et al. Fat Fraction Extracted from Whole-Body Magnetic Resonance (WB-MR) in Bone Metastatic Prostate Cancer: Intra- and Inter-Reader Agreement of Single-Slice and Volumetric Measurements. Tomography 2024, 10, 1014–1023. [Google Scholar] [CrossRef]
- Faubert, B.; Tasdogan, A. Approaches to Stable Isotope Tracing and in Vivo Metabolomics in the Cancer Clinic. EMBO J. 2025, 44, 3303–3306. [Google Scholar] [CrossRef]
- Cristini, N.; Tavakoli, M.; Sanati, M.; Yavari, S.A. Exploring Bone-Tumor Interactions through 3D in Vitro Models: Implications for Primary and Metastatic Cancers. J. Bone Oncol. 2025, 53, 100698. [Google Scholar] [CrossRef]
- Morris, E.V.; Edwards, C.M. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front. Endocrinol. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Otley, M.O.C.; Sinal, C.J. Adipocyte-Cancer Cell Interactions in the Bone Microenvironment. Front. Endocrinol. 2022, 13, 903925. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, Q.; Zhang, H.; Wu, Y.; Wang, X.; Ding, S.; Chen, S.; Xu, S.; Duan, G.; Qiu, D.; et al. Radiomics Model Based on MRI to Differentiate Spinal Multiple Myeloma from Metastases: A Two-Center Study. J. Bone Oncol. 2024, 45, 100599. [Google Scholar] [CrossRef]
- Veldhuis-Vlug, A.G.; Rosen, C.J. Clinical Implications of Bone Marrow Adiposity. J. Intern. Med. 2018, 283, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V. Marrow Fat and Bone: Review of Clinical Findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone Marrow Fat. Jt. Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef]
- El-Masri, B.M.; Leka, B.; Mustapha, F.; Gundesen, M.T.; Hinge, M.; Lund, T.; Andersen, T.L.; Diaz-delCastillo, M.; Jafari, A. Bone Marrow Adipocytes Provide Early Sign for Progression from MGUS to Multiple Myeloma. Oncotarget 2024, 15, 20–26. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal Stem Cell-Derived Exosome: A Tumor Regulator and Carrier for Targeted Tumor Therapy. Cancer Lett. 2022, 526, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Cao, P.H.A.; Pulliam, T.L.; Spradlin, M.; Subramani, E.; Tellman, T.V.; Ribeiro, C.F.; Muzzioli, R.; Jewell, B.E.; Pakula, H.; et al. Adipose Triglyceride Lipase Is a Therapeutic Target in Advanced Prostate Cancer That Promotes Metabolic Plasticity. Cancer Res. 2024, 84, 703–724. [Google Scholar] [CrossRef]
- Mayer, N.; Schweiger, M.; Romauch, M.; Grabner, G.F.; Eichmann, T.O.; Fuchs, E.; Ivkovic, J.; Heier, C.; Mrak, I.; Lass, A.; et al. Development of Small-Molecule Inhibitors Targeting Adipose Triglyceride Lipase. Nat. Chem. Biol. 2013, 9, 785–787. [Google Scholar] [CrossRef]
- Iglesias, J.; Lamontagne, J.; Erb, H.; Gezzar, S.; Zhao, S.; Joly, E.; Truong, V.L.; Skorey, K.; Crane, S.; Madiraju, S.R.M.; et al. Simplified Assays of Lipolysis Enzymes for Drug Discovery and Specificity Assessment of Known Inhibitors. J. Lipid Res. 2016, 57, 131–141. [Google Scholar] [CrossRef]
- Schweiger, M.; Romauch, M.; Schreiber, R.; Grabner, G.F.; Hütter, S.; Kotzbeck, P.; Benedikt, P.; Eichmann, T.O.; Yamada, S.; Knittelfelder, O.; et al. Pharmacological Inhibition of Adipose Triglyceride Lipase Corrects High-Fat Diet-Induced Insulin Resistance and Hepatosteatosis in Mice. Nat. Commun. 2017, 8, 14859. [Google Scholar] [CrossRef]
- Xie, H.; Heier, C.; Kien, B.; Vesely, P.W.; Tang, Z.; Sexl, V.; Schoiswohl, G.; Strießnig-Bina, I.; Hoefler, G.; Zechner, R.; et al. Adipose Triglyceride Lipase Activity Regulates Cancer Cell Proliferation via AMP-Kinase and mTOR Signaling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158737. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.J.; Park, B.-H.; Park, J.-H.; Han, J.; Lee, I.-K.; Park, J.W.; Koh, G.Y. Activation of PPAR Gamma Induces Profound Multilocularization of Adipocytes in Adult Mouse White Adipose Tissues. Exp. Mol. Med. 2009, 41, 880–895. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Liu, Q.; Postigo-Fernandez, J.; Ji, R.; Kon, N.; Larrea, D.; Namwanje, M.; Fan, L.; Chan, M.; Area-Gomez, E.; et al. PPARγ Deacetylation Dissociates Thiazolidinedione’s Metabolic Benefits from Its Adverse Effects. J. Clin. Investig. 2018, 128, 2600–2612. [Google Scholar] [CrossRef]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-Fat-Mediated Tumour Suppression by Cold-Altered Global Metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, Y. Cold Snap for Cancer: Cold-Induced Brown Fat Thermogenesis Starves Tumor Growth. Signal Transduct. Target. Ther. 2023, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.L.; Tometsko, M.; Miller, R.; Jones, J.C.; Dougall, W.C. RANK Expression on Breast Cancer Cells Promotes Skeletal Metastasis. Clin. Exp. Metastasis 2014, 31, 233–245. [Google Scholar] [CrossRef]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of Cancer Cell Migration and Bone Metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-N.; Zhang, F.; Tang, P.; Qi, X.-W.; Jiang, J. Hypoxia Induces RANK and RANKL Expression by Activating HIF-1α in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2011, 408, 411–416. [Google Scholar] [CrossRef]
- Vargas, G.; Bouchet, M.; Bouazza, L.; Reboul, P.; Boyault, C.; Gervais, M.; Kan, C.; Benetollo, C.; Brevet, M.; Croset, M.; et al. ERRα Promotes Breast Cancer Cell Dissemination to Bone by Increasing RANK Expression in Primary Breast Tumors. Oncogene 2019, 38, 950–964. [Google Scholar] [CrossRef]
- Onji, M.; Werschler, N.; Penninger, J. A Critical Relationship between Bone and Fat: The Role of Bone Marrow Adipose-Derived RANKL in Bone Metabolism. EMBO Rep. 2021, 22, e52986. [Google Scholar] [CrossRef]
- Beekman, K.M.; Zwaagstra, M.; Veldhuis-Vlug, A.G.; van Essen, H.W.; den Heijer, M.; Maas, M.; Kerckhofs, G.; Parac-Vogt, T.N.; Bisschop, P.H.; Bravenboer, N. Ovariectomy Increases RANKL Protein Expression in Bone Marrow Adipocytes of C3H/HeJ Mice. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1050–E1054. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Clemente, A.; Bravo-Cuellar, A.; González-Ochoa, S.; Santiago-Mercado, M.; Palafox-Mariscal, L.; Jave-Suárez, L.; Solorzano-Ibarra, F.; Villaseñor-García, M.; Ortiz-Lazareno, P.; Hernández-Flores, G. Dual STAT-3 and IL-6R Inhibition with Stattic and Tocilizumab Decreases Migration, Invasion and Proliferation of Prostate Cancer Cells by Targeting the IL-6/IL-6R/STAT-3 Axis. Oncol. Rep. 2022, 48, 138. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Tencerova, M.; Figeac, F.; Kassem, M.; Jafari, A. The Pathophysiology of Osteoporosis in Obesity and Type 2 Diabetes in Aging Women and Men: The Mechanisms and Roles of Increased Bone Marrow Adiposity. Front. Endocrinol. 2022, 13, 981487. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Schafer, A.L. Diabetes and Bone Marrow Adiposity. Curr. Osteoporos. Rep. 2016, 14, 337–344. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Chen, D.; Qian, S.; Mei, R.; Li, Q.; Yu, X.; He, X. PPARγ Inhibitors Enhance the Efficacy of Statin Therapy for Steroid-Induced Osteonecrosis of the Femoral Head by Directly Inhibiting Apoptosis and Indirectly Modulating Lipoprotein Subfractions. PLoS ONE 2025, 20, e0325190. [Google Scholar] [CrossRef]
- McGrath, C.; Sankaran, J.S.; Misaghian-Xanthos, N.; Sen, B.; Xie, Z.; Styner, M.A.; Zong, X.; Rubin, J.; Styner, M. Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT). J. Bone Miner. Res. 2019, 35, 106–115. [Google Scholar] [CrossRef]
- Rinne, C.; Soultoukis, G.A.; Oveisi, M.; Leer, M.; Schmidt-Bleek, O.; Burkhardt, L.-M.; Bucher, C.H.; Moussa, E.A.; Makhlouf, M.; Duda, G.N.; et al. Caloric Restriction Reduces Trabecular Bone Loss during Aging and Improves Bone Marrow Adipocyte Endocrine Function in Male Mice. Front. Endocrinol. 2024, 15, 1394263. [Google Scholar] [CrossRef]
- Lin, W.; Li, Y.; Qiu, C.; Zou, B.; Gong, Y.; Zhang, X.; Tian, D.; Sherman, W.; Sanchez, F.; Wu, D.; et al. Mapping the Spatial Atlas of the Human Bone Tissue Integrating Spatial and Single-Cell Transcriptomics. Nucleic Acids Res. 2025, 53, gkae1298. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Duffy, M.P.; Ahn, K.J.; Sussman, J.H.; Pang, M.; Smith, D.; Duncan, G.; Zhang, I.; Huang, J.; Lin, Y.; et al. Mapping the Cellular Biogeography of Human Bone Marrow Niches Using Single-Cell Transcriptomics and Proteomic Imaging. Cell 2024, 187, 3120–3140.e29. [Google Scholar] [CrossRef]
- Baccin, C.; Al-Sabah, J.; Velten, L.; Helbling, P.M.; Grünschläger, F.; Hernández-Malmierca, P.; Nombela-Arrieta, C.; Steinmetz, L.M.; Trumpp, A.; Haas, S. Combined Single-Cell and Spatial Transcriptomics Reveal the Molecular, Cellular and Spatial Bone Marrow Niche Organization. Nat. Cell Biol. 2020, 22, 38–48. [Google Scholar] [CrossRef]
- Faubert, B.; Tasdogan, A.; Morrison, S.J.; Mathews, T.P.; DeBerardinis, R.J. Stable Isotope Tracing to Assess Tumor Metabolism in Vivo. Nat. Protoc. 2021, 16, 5123–5145. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Park, S.; Jang, J.; Wolfe, R.R. Quantifications of Lipid Kinetics In Vivo Using Stable Isotope Tracer Methodology. J. Lipid Atheroscler. 2020, 9, 110–123. [Google Scholar] [CrossRef]
- Miggitsch, C.; Meryk, A.; Naismith, E.; Pangrazzi, L.; Ejaz, A.; Jenewein, B.; Wagner, S.; Nägele, F.; Fenkart, G.; Trieb, K.; et al. Human Bone Marrow Adipocytes Display Distinct Immune Regulatory Properties. EBioMedicine 2019, 46, 387–398. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic Regulation of the Immune System in Health and Diseases: Mechanisms and Interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Philippoteaux, C.; Badr, S.; Lombardo, D.; Cailliau, E.; Ruschke, S.; Karampinos, D.C.; Cotten, A.; Paccou, J. Marrow Adiposity Content and Composition Are Not Associated With Incident Fragility Fractures in Postmenopausal Women: The ADIMOS Fracture Study. J. Endocr. Soc. 2025, 9, bvaf033. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Yildiz, A.E.; Karaosmanoglu, A.D.; Ozmen, M.N.; Akata, D.; Karcaaltincaba, M. Proton Density Fat Fraction: Magnetic Resonance Imaging Applications beyond the Liver. Diagn. Interv. Radiol. 2022, 28, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Dembitz, V.; James, S.C.; Gallipoli, P. Targeting Lipid Metabolism in Acute Myeloid Leukemia: Biological Insights and Therapeutic Opportunities. Leukemia 2025, 39, 1814–1823. [Google Scholar] [CrossRef]
- Samudio, I.; Harmancey, R.; Fiegl, M.; Kantarjian, H.; Konopleva, M.; Korchin, B.; Kaluarachchi, K.; Bornmann, W.; Duvvuri, S.; Taegtmeyer, H.; et al. Pharmacologic Inhibition of Fatty Acid Oxidation Sensitizes Human Leukemia Cells to Apoptosis Induction. J. Clin. Investig. 2010, 120, 142–156. [Google Scholar] [CrossRef]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus Zoledronic Acid in Bone Disease Treatment of Newly Diagnosed Multiple Myeloma: An International, Double-Blind, Double-Dummy, Randomised, Controlled, Phase 3 Study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Beekman, K.M.; Regenboog, M.; Nederveen, A.J.; Bravenboer, N.; den Heijer, M.; Bisschop, P.H.; Hollak, C.E.; Akkerman, E.M.; Maas, M. Gender- and Age-Associated Differences in Bone Marrow Adipose Tissue and Bone Marrow Fat Unsaturation Throughout the Skeleton, Quantified Using Chemical Shift Encoding-Based Water-Fat MRI. Front. Endocrinol. 2022, 13, 815835. [Google Scholar] [CrossRef]
- Serota, A.; D’Erasmo, G. Estrogen Exposure and Skeletal Health: Special Populations and Considerations. J. Pediatr. Soc. N. Am. 2024, 7, 100061. [Google Scholar] [CrossRef] [PubMed]

| Mechanism | Key Factors/Molecules | Tumor Types | Evidence | Potential Therapeutic Strategies |
|---|---|---|---|---|
| Metabolic Coupling | Free fatty acids, CD36, FABP4, PTHrP, lipolysis enzymes | Breast, prostate, multiple myeloma, leukemia | BMAs supply lipids to tumor cells → FAO/oxidative phosphorylation “OXPHOS” ↑, metabolic reprogramming [31,37,38,39,40,41,42,43,48,49,50] | ATGL inhibitors (Atglistatin), FASN inhibitors (TVB-2640), Fatty acid oxidation (FAO) inhibitors, metabolic flux monitoring [110,134,135,136,137,138,139] |
| Adipokine Signaling | Leptin, IL-6, TNF-α, adiponectin, IL-1β | Breast, prostate, lung, and myeloma | Promote EMT, survival, invasion, angiogenesis; suppress adiponectin’s anti-tumor effects [57,58,59,60,61,62,63,68,69,70] | IL-6R blockade (Tocilizumab), leptin receptor antagonists, anti-inflammatory strategies [140,141] |
| Immune Modulation | MDSCs, TAMs, arginase-I, cytokines (IL-6, IL-1β, TNF-α) | Breast, prostate, lung, melanoma | BMAs recruit suppressive immune cells → impaired B/T cell function [73,74,75,76,77,78,81,82,83] | CXCR2 inhibitors, immunotherapy combinations, targeting MDSC/TAM polarization [142] |
| Bone Remodeling | RANKL, osteoclast activation, osteolysis → growth factor release | Breast, prostate, myeloma | BMAs produce RANKL → osteoclastogenesis → bone resorption [85,86] | Denosumab (RANKL inhibitor), bisphosphonates (zoledronic acid), osteoclast-targeted therapy [143,144,145,146] |
| Extracellular Vesicles | ADEVs carrying lipids, enzymes, miRNAs (miR-155, miR-126, miR-144) | Breast, melanoma, ovarian, leukemia | EV-mediated metabolic reprogramming, cancer-associated adipocyte phenotype [94,95,96,97,98,99,101,102] | EV-targeted therapies, blocking vesicle uptake, lipid transfer inhibitors [17,18] |
| Therapy Resistance | Lipid-fueled oxidative metabolism, BCL-2/BCL-xL upregulation, drug sequestration | Leukemia, myeloma, breast cancer | BMAs confer chemotherapy resistance via autophagy, FAO, and drug sequestration [132,133] | Combination therapies (ATGL inhibitors + chemotherapy), ABC transporter inhibitors, metabolic targeting [131,132,133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabaliz, A.; Al Hakawati, M.N.; Alrashdan, N.; Alrashdan, S.; Bakir, M.; Mohammad, K.S. Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy. Int. J. Mol. Sci. 2025, 26, 9781. https://doi.org/10.3390/ijms26199781
Dabaliz A, Al Hakawati MN, Alrashdan N, Alrashdan S, Bakir M, Mohammad KS. Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy. International Journal of Molecular Sciences. 2025; 26(19):9781. https://doi.org/10.3390/ijms26199781
Chicago/Turabian StyleDabaliz, Alhomam, Mohammad Nawar Al Hakawati, Najmuddeen Alrashdan, Sarah Alrashdan, Mohamad Bakir, and Khalid S. Mohammad. 2025. "Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy" International Journal of Molecular Sciences 26, no. 19: 9781. https://doi.org/10.3390/ijms26199781
APA StyleDabaliz, A., Al Hakawati, M. N., Alrashdan, N., Alrashdan, S., Bakir, M., & Mohammad, K. S. (2025). Adipocyte–Tumor Interactions in the Bone Marrow Niche: Implications for Metastasis and Therapy. International Journal of Molecular Sciences, 26(19), 9781. https://doi.org/10.3390/ijms26199781








