Bladder Dysfunction in Sickle Cell Disease Is Associated with Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Results
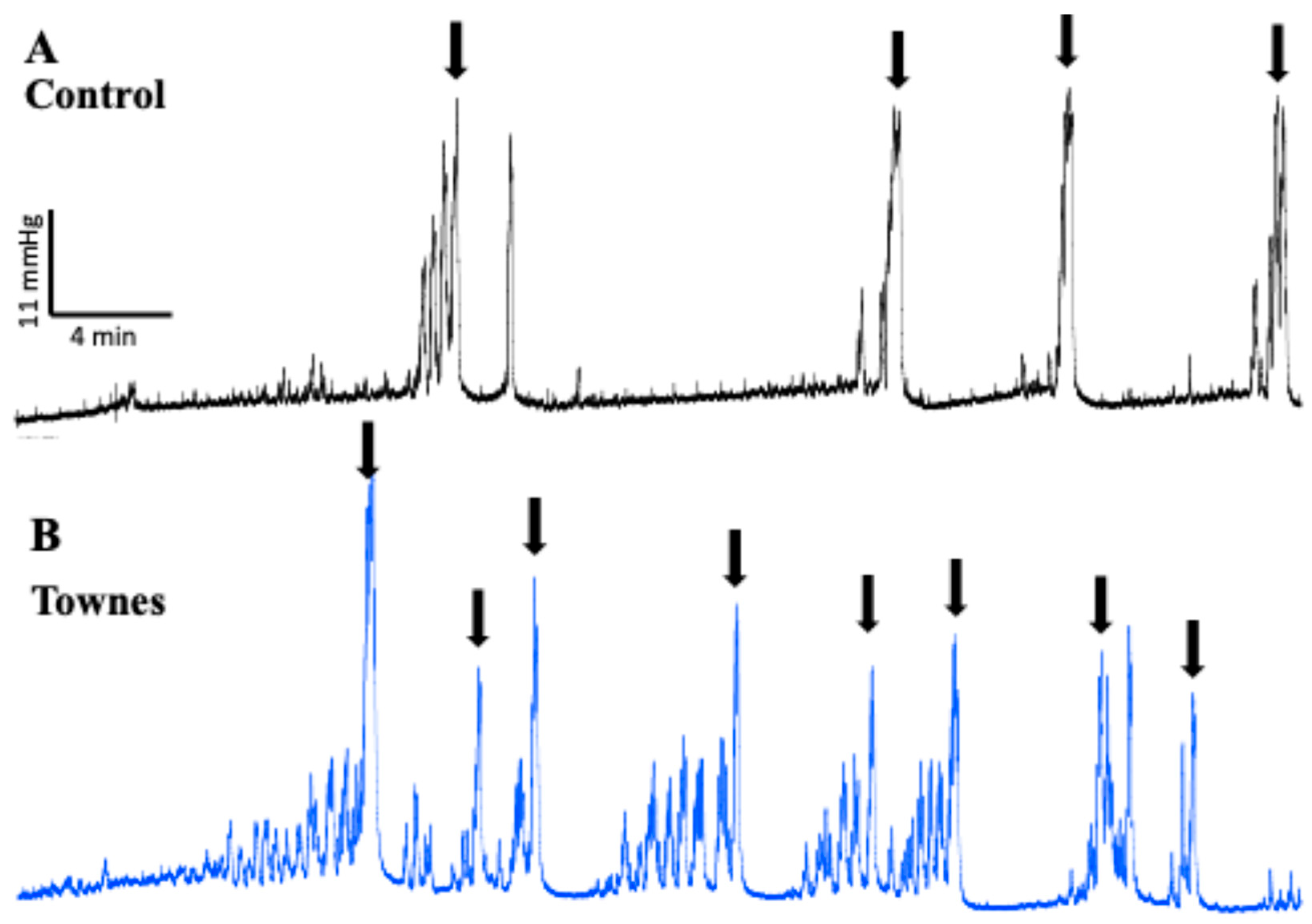
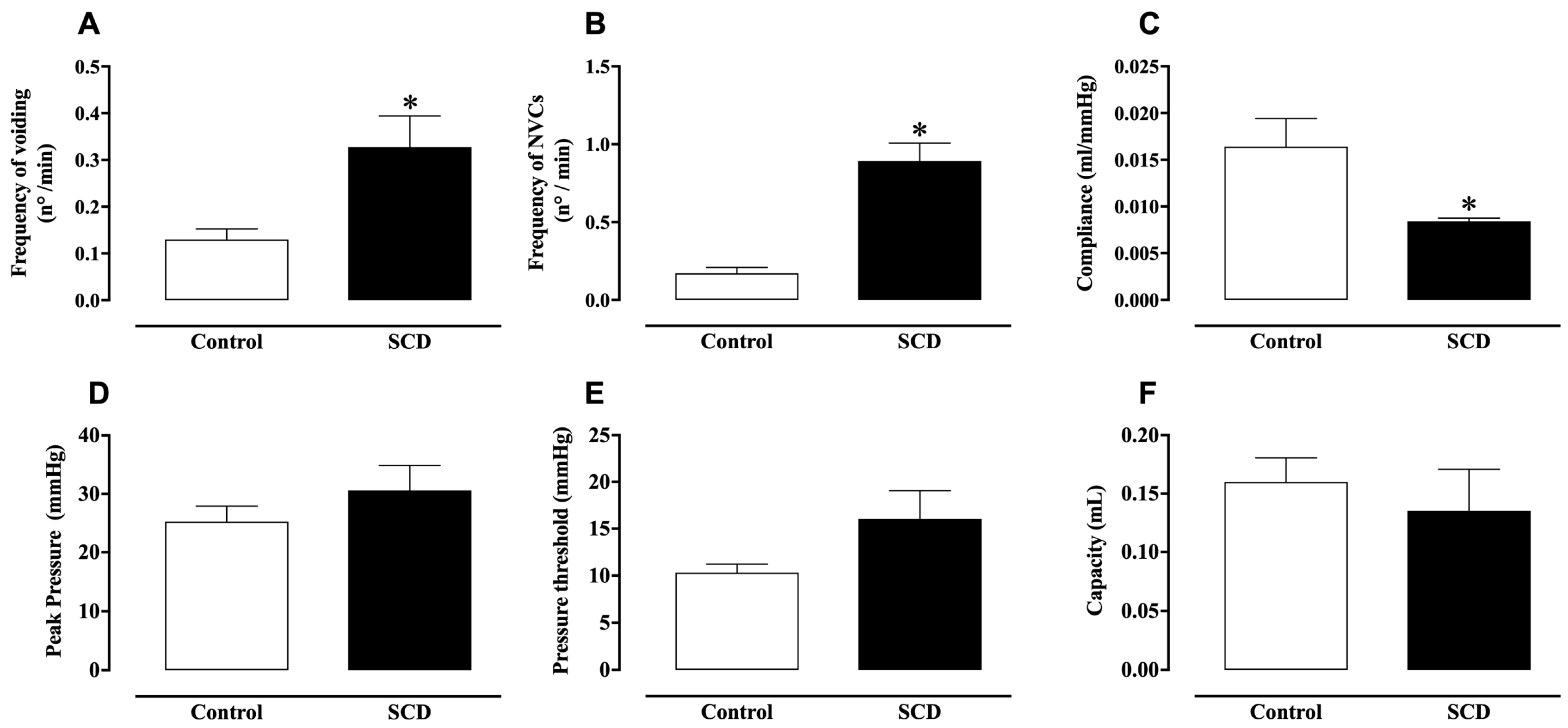
2.1. In Vivo Evaluation of Bladder Function
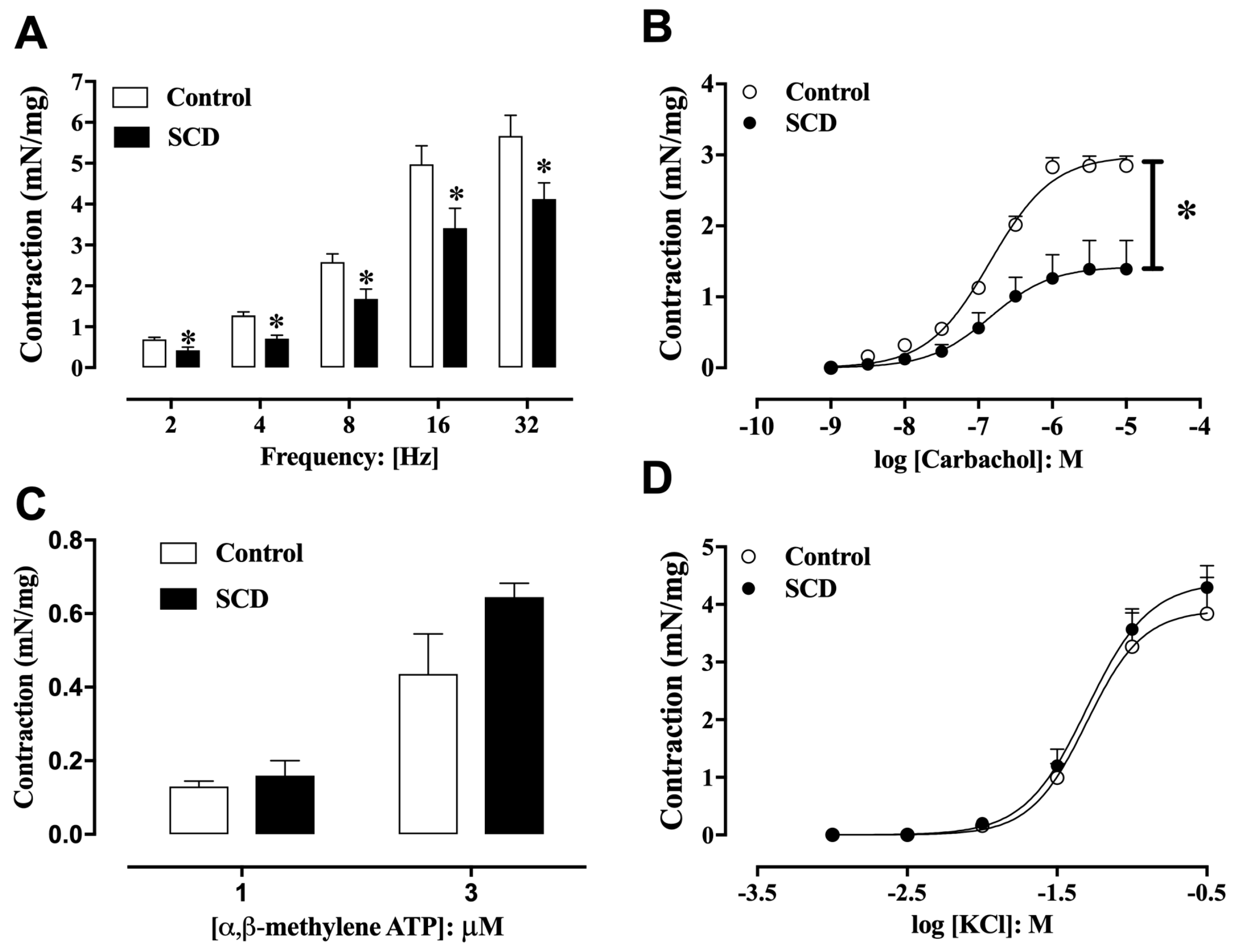
2.2. Functional Analysis of Detrusor Smooth Muscle Contractility
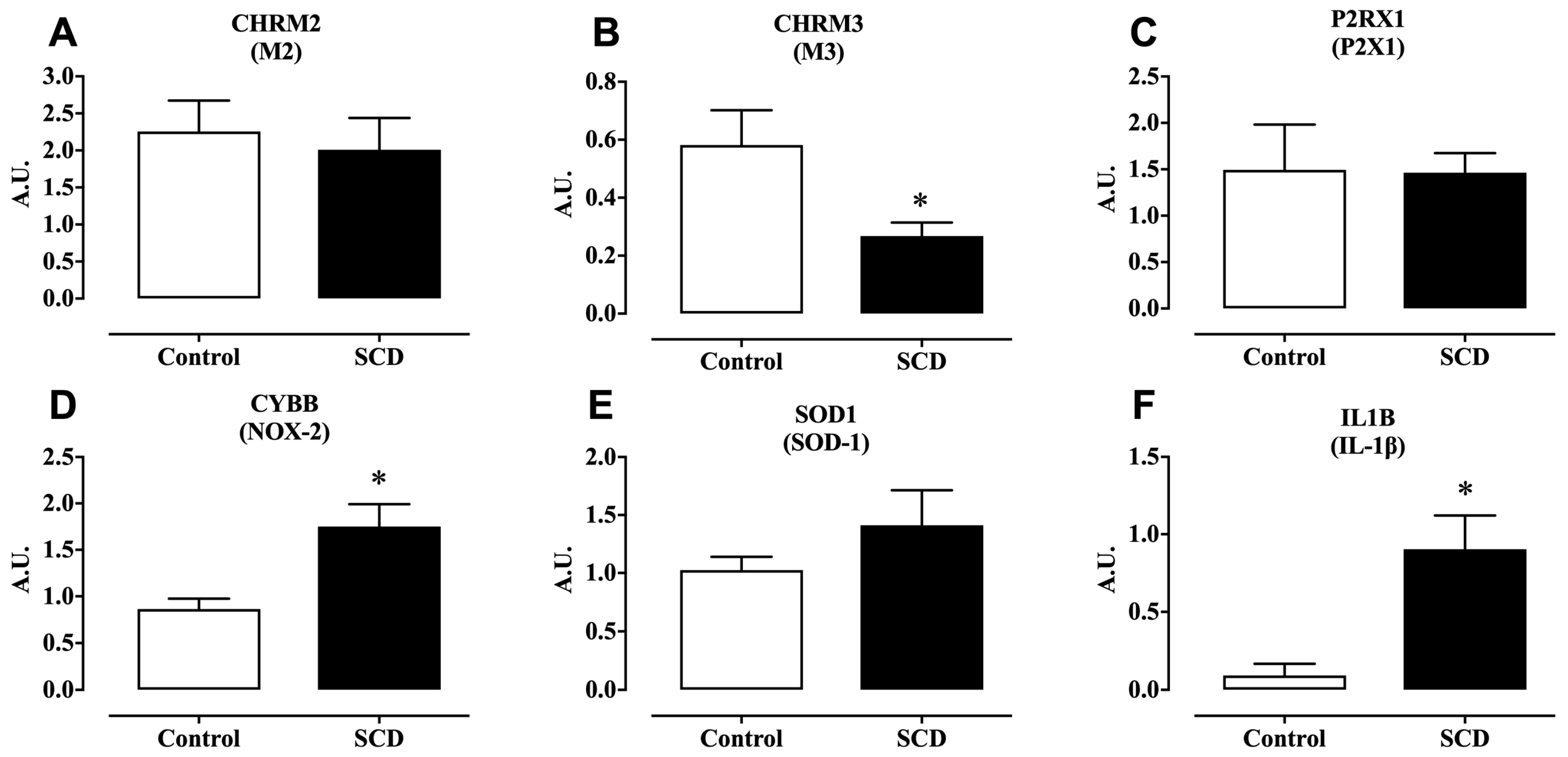
2.3. mRNA Expression of M2, M3, and P2X1 Receptors
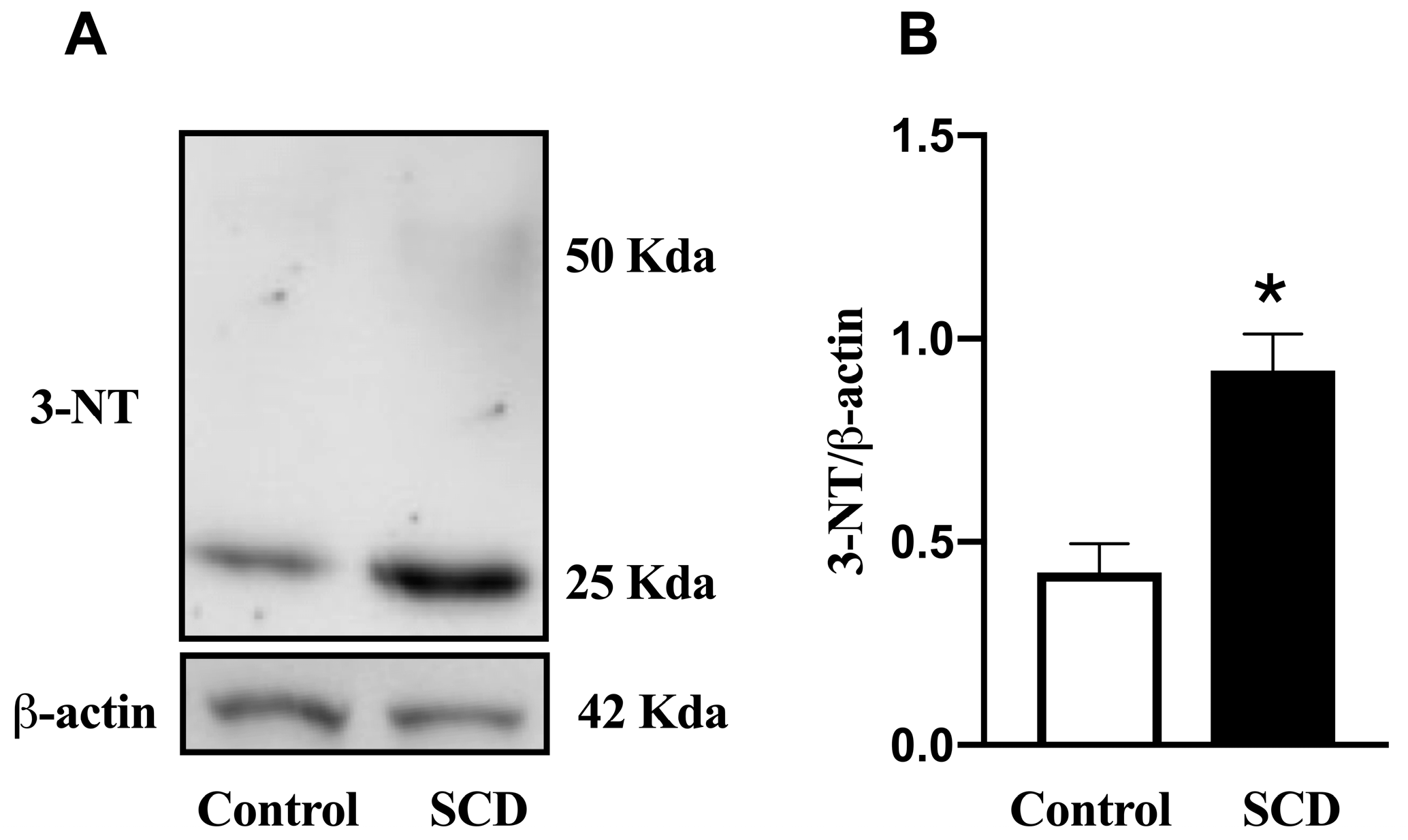
2.4. mRNA Expression of NOX-2 and SOD-1, and Protein Expression of 3-Nitrotyrosine
2.5. mRNA Expression of IL-1β
2.6. Myeloperoxidase Activity
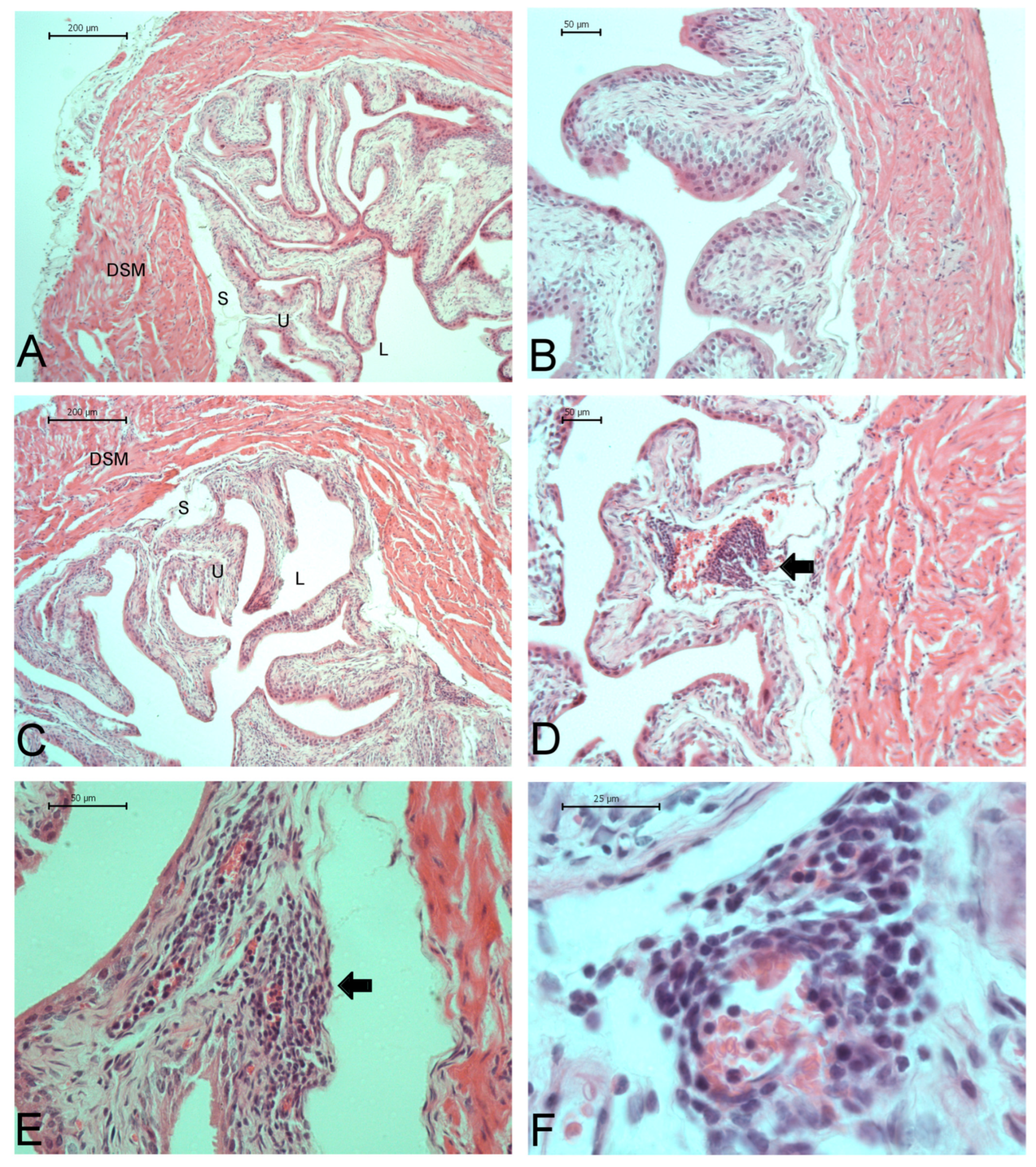
2.7. Bladder Characteristics and Histopathological Analysis
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Cystometry (In Vivo)
4.3. Functional Studies in Detrusor Smooth Muscle Strips and Concentration–Response Curves
4.4. Real-Time RT-PCR
4.5. Western Blotting
4.6. Histological Analysis
4.7. Myeloperoxidase Activity Assay
4.8. Drugs and Chemicals
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Primer 2018, 4, 18010. [Google Scholar] [CrossRef]
- Portocarrero, M.L.; Portocarrero, M.L.; Sobral, M.M.; Lyra, I.; Lordêlo, P.; Barroso, U. Prevalence of Enuresis and Daytime Urinary Incontinence in Children and Adolescents with Sickle Cell Disease. J. Urol. 2012, 187, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Anele, U.A.; Morrison, B.F.; Reid, M.E.; Madden, W.; Foster, S.; Burnett, A.L. Overactive Bladder in Adults with Sickle Cell Disease. Neurourol. Urodyn. 2016, 35, 642–646. [Google Scholar] [CrossRef]
- Powell, L.C.; Szabo, S.M.; Walker, D.; Gooch, K. The Economic Burden of Overactive Bladder in the United States: A Systematic Literature Review. Neurourol. Urodyn. 2018, 37, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.-E. Muscarinic Acetylcholine Receptors in the Urinary Tract. Handb. Exp. Pharmacol. 2011, 319–344. [Google Scholar] [CrossRef]
- de Groat, W.C.; Yoshimura, N. Anatomy and Physiology of the Lower Urinary Tract. Handb. Clin. Neurol. 2015, 130, 61–108. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.J.; Liu, L.; Mitchelson, F.J.; Moore, K.H.; Millard, R.J.; Burcher, E. Muscarinic Receptor Subtypes in Human Bladder Detrusor and Mucosa, Studied by Radioligand Binding and Quantitative Competitive RT-PCR: Changes in Ageing. Br. J. Pharmacol. 2005, 144, 1089–1099. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Arner, A. Urinary Bladder Contraction and Relaxation: Physiology and Pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Claudino, M.A.; Leiria, L.O.S.; da Silva, F.H.; Alexandre, E.C.; Renno, A.; Mónica, F.Z.; de Nucci, G.; Fertrin, K.Y.; Antunes, E.; Costa, F.F.; et al. Urinary Bladder Dysfunction in Transgenic Sickle Cell Disease Mice. PLoS ONE 2015, 10, e0133996. [Google Scholar] [CrossRef]
- Karakus, S.; Anele, U.A.; Silva, F.H.; Musicki, B.; Burnett, A.L. Urinary Dysfunction in Transgenic Sickle Cell Mice: Model of Idiopathic Overactive Bladder Syndrome. Am. J. Physiol. Renal Physiol. 2019, 317, F540–F546. [Google Scholar] [CrossRef]
- Karakus, S.; Musicki, B.; Navati, M.S.; Friedman, J.M.; Davies, K.P.; Burnett, A.L. NO-Releasing Nanoparticles Ameliorate Detrusor Overactivity in Transgenic Sickle Cell Mice via Restored NO/ROCK Signaling. J. Pharmacol. Exp. Ther. 2020, 373, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Anele, U.A.; Campbell, J.D.; Karakus, S.; Shiva, S.; Silva, F.H.; Burnett, A.L. Dysregulated NO/PDE5 Signaling in the Sickle Cell Mouse Lower Urinary Tract: Reversal by Oral Nitrate Therapy. Life Sci. 2019, 238, 116922. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, M.; Sagawa, K.; Yazaki, J.; Takahashi, N.; Kushida, N.; Haga, N.; Aikawa, K.; Matsui, T.; Oka, M.; Fukui, T.; et al. Increased Bladder Activity Is Associated with Elevated Oxidative Stress Markers and Proinflammatory Cytokines in a Rat Model of Atherosclerosis-Induced Chronic Bladder Ischemia. Neurourol. Urodyn. 2012, 31, 185–189. [Google Scholar] [CrossRef]
- Alexandre, E.C.; Calmasini, F.B.; de Oliveira, M.G.; Silva, F.H.; da Silva, C.P.V.; André, D.M.; Leonardo, F.C.; Delbin, M.A.; Antunes, E. Chronic Treatment with Resveratrol Improves Overactive Bladder in Obese Mice via Antioxidant Activity. Eur. J. Pharmacol. 2016, 788, 29–36. [Google Scholar] [CrossRef]
- Akakpo, W.; Musicki, B.; Burnett, A.L. cAMP-Dependent Regulation of RhoA/Rho-Kinase Attenuates Detrusor Overactivity in a Novel Mouse Experimental Model. BJU Int. 2017, 120, 143–151. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.; Monica, F.Z.; Passos, G.R.; Victorio, J.A.; Davel, A.P.; Oliveira, A.L.L.; Parada, C.A.; D’Ancona, C.A.L.; Hill, W.G.; Antunes, E. Selective Pharmacological Inhibition of NOX2 by GSK2795039 Improves Bladder Dysfunction in Cyclophosphamide-Induced Cystitis in Mice. Antioxidants 2022, 12, 92. [Google Scholar] [CrossRef]
- Silveira, T.H.R.E.; Pereira, D.A.; Pereira, D.A.; Calmasini, F.B.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Impact of Intravascular Hemolysis on Functional and Molecular Alterations in the Urinary Bladder: Implications for an Overactive Bladder in Sickle Cell Disease. Front. Physiol. 2024, 15, 1369120. [Google Scholar] [CrossRef]
- Funahashi, Y.; Oguchi, T.; Goins, W.F.; Gotoh, M.; Tyagi, P.; Goss, J.R.; Glorioso, J.C.; Yoshimura, N. Herpes Simplex Virus Vector Mediated Gene Therapy of Tumor Necrosis Factor-α Blockade for Bladder Overactivity and Nociception in Rats. J. Urol. 2013, 189, 366–373. [Google Scholar] [CrossRef]
- Nasrin, S.; Masuda, E.; Kugaya, H.; Ito, Y.; Yamada, S. Improvement by Phytotherapeutic Agent of Detrusor Overactivity, down-Regulation of Pharmacological Receptors and Urinary Cytokines in Rats with Cyclophosphamide Induced Cystitis. J. Urol. 2013, 189, 1123–1129. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lee, C.-L.; Kuo, H.-C. Urothelial Dysfunction, Suburothelial Inflammation and Altered Sensory Protein Expression in Men with Bladder Outlet Obstruction and Various Bladder Dysfunctions: Correlation with Urodynamics. J. Urol. 2016, 196, 831–837. [Google Scholar] [CrossRef]
- Wang, C.-C.; Kuo, H.-C. Urothelial Dysfunction and Chronic Inflammation in Diabetic Patients with Overactive Bladder. Low. Urin. Tract Symptoms 2017, 9, 151–156. [Google Scholar] [CrossRef]
- Hughes, F.M.; Allkanjari, A.; Odom, M.R.; Jin, H.; Purves, J.T. Diabetic Bladder Dysfunction Progresses from an Overactive to an Underactive Phenotype in a Type-1 Diabetic Mouse Model (Akita Female Mouse) and Is Dependent on NLRP3. Life Sci. 2022, 299, 120528. [Google Scholar] [CrossRef]
- He, Q.; Wu, L.; Deng, C.; He, J.; Wen, J.; Wei, C.; You, Z. Diabetes Mellitus, Systemic Inflammation and Overactive Bladder. Front. Endocrinol. 2024, 15, 1386639. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-C.; Sun, C.-W.; Ryan, T.M.; Pawlik, K.M.; Ren, J.; Townes, T.M. Correction of Sickle Cell Disease by Homologous Recombination in Embryonic Stem Cells. Blood 2006, 108, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Pászty, C.; Brion, C.M.; Manci, E.; Witkowska, H.E.; Stevens, M.E.; Mohandas, N.; Rubin, E.M. Transgenic Knockout Mice with Exclusively Human Sickle Hemoglobin and Sickle Cell Disease. Science 1997, 278, 876–878. [Google Scholar] [CrossRef]
- Frey, R.S.; Ushio-Fukai, M.; Malik, A.B. NADPH Oxidase-Dependent Signaling in Endothelial Cells: Role in Physiology and Pathophysiology. Antioxid. Redox Signal. 2009, 11, 791–810. [Google Scholar] [CrossRef]
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Kennedy, C.; Tasker, P.N.; Gallacher, G.; Westfall, T.D. Identification of Atropine- and P2X1 Receptor Antagonist-Resistant, Neurogenic Contractions of the Urinary Bladder. J. Neurosci. 2007, 27, 845–851. [Google Scholar] [CrossRef]
- Arcaroli, J.; Yang, K.-Y.; Yum, H.-K.; Kupfner, J.; Pitts, T.M.; Park, J.S.; Strassheim, D.; Abraham, E. Effects of Catecholamines on Kinase Activation in Lung Neutrophils after Hemorrhage or Endotoxemia. J. Leukoc. Biol. 2002, 72, 571–579. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Weihrauch, D.; Jones, D.W.; Jing, X.; Shi, Y.; Gourlay, D.; Oldham, K.T.; Hillery, C.A.; Pritchard, K.A. Inhibition of Myeloperoxidase Decreases Vascular Oxidative Stress and Increases Vasodilatation in Sickle Cell Disease Mice. J. Lipid Res. 2013, 54, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Castilhos, L.G.; de Oliveira, J.S.; Adefegha, S.A.; Magni, L.P.; Doleski, P.H.; Abdalla, F.H.; de Andrade, C.M.; Leal, D.B.R. Increased Oxidative Stress Alters Nucleosides Metabolite Levels in Sickle Cell Anemia. Redox Rep. Commun. Free Radic. Res. 2017, 22, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Merrill, L.; Malley, S.; Vizzard, M.A. Repeated Variate Stress in Male Rats Induces Increased Voiding Frequency, Somatic Sensitivity, and Urinary Bladder Nerve Growth Factor Expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R147–R156. [Google Scholar] [CrossRef]
- Chu, F.M.; Dmochowski, R. Pathophysiology of Overactive Bladder. Am. J. Med. 2006, 119, 3–8. [Google Scholar] [CrossRef]
- Birder, L.A.; Andersson, K.-E.; Kanai, A.J.; Hanna-Mitchell, A.T.; Fry, C.H. Urothelial Mucosal Signaling and the Overactive Bladder-ICI-RS 2013. Neurourol. Urodyn. 2014, 33, 597–601. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, D.Y.; Cho, K.J.; Hashimoto, M.; Matsuoka, K.; Kamijo, T.; Wang, Z.; Karnup, S.; Robertson, A.M.; Tyagi, P.; et al. Pathophysiology of Overactive Bladder and Pharmacologic Treatments Including Β3-Adrenoceptor Agonists -Basic Research Perspectives. Int. Neurourol. J. 2024, 28, 12–33. [Google Scholar] [CrossRef]
- Silva, F.H.; Claudino, M.A.; Calmasini, F.B.; Alexandre, E.C.; Franco-Penteado, C.; Burnett, A.L.; Antunes, E.; Costa, F.F. Sympathetic Hyperactivity, Increased Tyrosine Hydroxylase and Exaggerated Corpus Cavernosum Relaxations Associated with Oxidative Stress Plays a Major Role in the Penis Dysfunction in Townes Sickle Cell Mouse. PLoS ONE 2016, 11, e0166291. [Google Scholar] [CrossRef]
- Vona, R.; Sposi, N.M.; Mattia, L.; Gambardella, L.; Straface, E.; Pietraforte, D. Sickle Cell Disease: Role of Oxidative Stress and Antioxidant Therapy. Antioxidants 2021, 10, 296. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Pereira, P.d.S.; Pereira, D.A.; Calmasini, F.B.; Reis, L.O.; Brinkman, N.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Haptoglobin Treatment Contributes to Regulating Nitric Oxide Signal and Reduces Oxidative Stress in the Penis: A Preventive Treatment for Priapism in Sickle Cell Disease. Front. Physiol. 2022, 13, 961534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Chueh, K.-S.; Chuang, S.-M.; Long, C.-Y.; Lu, J.-H.; Juan, Y.-S. Bladder Hyperactivity Induced by Oxidative Stress and Bladder Ischemia: A Review of Treatment Strategies with Antioxidants. Int. J. Mol. Sci. 2021, 22, 6014. [Google Scholar] [CrossRef]
- Janssen, D.A.W.; Schalken, J.A.; Heesakkers, J.P.F.A. Urothelium Update: How the Bladder Mucosa Measures Bladder Filling. Acta Physiol. Oxf. Engl. 2017, 220, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Buttyan, R.; Chen, M.W.; Levin, R.M. Animal Models of Bladder Outlet Obstruction and Molecular Insights into the Basis for the Development of Bladder Dysfunction. Eur. Urol. 1997, 32 (Suppl. S1), 32–39. [Google Scholar] [PubMed]
- Fusco, F.; Creta, M.; De Nunzio, C.; Iacovelli, V.; Mangiapia, F.; Li Marzi, V.; Finazzi Agrò, E. Progressive Bladder Remodeling Due to Bladder Outlet Obstruction: A Systematic Review of Morphological and Molecular Evidences in Humans. BMC Urol. 2018, 18, 15. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]

| Parameters | Control | SCD |
|---|---|---|
| Body weight (g) | 33.0 ± 1.06 | 31.59 ± 2.24 |
| Bladder weight (mg) | 29.98 ± 1.06 | 41.9 ± 1.32 * |
| Bladder weight: body weight | 0.90 ± 0.02 | 1.34 ± 0.12 * |
| Bladder wall thickness (μm) | 283.0 ± 13.36 | 335.2 ± 15.70 * |
| Urothelium thickness (μm) | 45.93 ± 2.31 | 36.65 ± 0.51 ** |
| Gene | Primer Sequence | Concentration |
|---|---|---|
| CHRM2–F | 5′-ACACGGTTTCCCACTTCCCTG-3′ | 150 nM |
| CHRM2–R | 5′-TGCATGCGTCACCCTTTTG-3′ | |
| CHRM3–F | 5′-CCCACAGGCAGTTCTCGAA-3′ | 150 nM |
| CHRM3–R | 5′-CCTCCTAGATGACCGTTTCGT-3′ | |
| P2RX1–F | 5′-ATTCGCTTTGATATCCTTGTGG-3′ | 150 nM |
| P2RX1–R | 5′-GCCGATGGTAGTCATAGTAGGG-3′ | |
| CYBB–F | 5′-TTGGGTCAGCACTGGCTCTG-3′ | 150 nM |
| CYBB–R | 5′-TGGCGGTGTGCAGTGCTATC-3′ | |
| SOD1–F | 5′-CAGCATGGGTTCCACGTCCA-3′ | 150 nM |
| SOD1–R | 5′-CACATTGGCCACACCGTCCT-3 | |
| IL1B–F | 5′-CAAGGAGACGGAATACAGGGC-3′ | 150 nM |
| IL1B–R | 5′-CCAGGTCACCTCGACGTTTG-3 | |
| GAPDH–F | 5′-TGCACCACCAACTGCTTA-3′ | 70 nM |
| GAPDH–R | 5′-GGATGCAGGGATGATGTTC-3′ | |
| ACTB–F | 5′-ACTGCCGCATCCTCTTCCT-3′ | 70 nM |
| ACTB–R | 5′-GAACCGCTCGTTGCCAATA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.A.; Calmasini, F.B.; Silveira, T.H.R.; Pereira, D.A.; de Oliveira, M.G.; Costa, F.F.; Silva, F.H. Bladder Dysfunction in Sickle Cell Disease Is Associated with Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 9776. https://doi.org/10.3390/ijms26199776
Pereira DA, Calmasini FB, Silveira THR, Pereira DA, de Oliveira MG, Costa FF, Silva FH. Bladder Dysfunction in Sickle Cell Disease Is Associated with Inflammation and Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(19):9776. https://doi.org/10.3390/ijms26199776
Chicago/Turabian StylePereira, Dalila Andrade, Fabiano Beraldi Calmasini, Tammyris Helena Rebecchi Silveira, Danillo Andrade Pereira, Mariana G. de Oliveira, Fernando Ferreira Costa, and Fábio Henrique Silva. 2025. "Bladder Dysfunction in Sickle Cell Disease Is Associated with Inflammation and Oxidative Stress" International Journal of Molecular Sciences 26, no. 19: 9776. https://doi.org/10.3390/ijms26199776
APA StylePereira, D. A., Calmasini, F. B., Silveira, T. H. R., Pereira, D. A., de Oliveira, M. G., Costa, F. F., & Silva, F. H. (2025). Bladder Dysfunction in Sickle Cell Disease Is Associated with Inflammation and Oxidative Stress. International Journal of Molecular Sciences, 26(19), 9776. https://doi.org/10.3390/ijms26199776






