Mechanisms of Oncolytic Virus-Induced Multi-Modal Cell Death and Therapeutic Prospects
Abstract
1. Introduction
2. Oncolytic Virus
3. Oncolytic Virus and Apoptosis
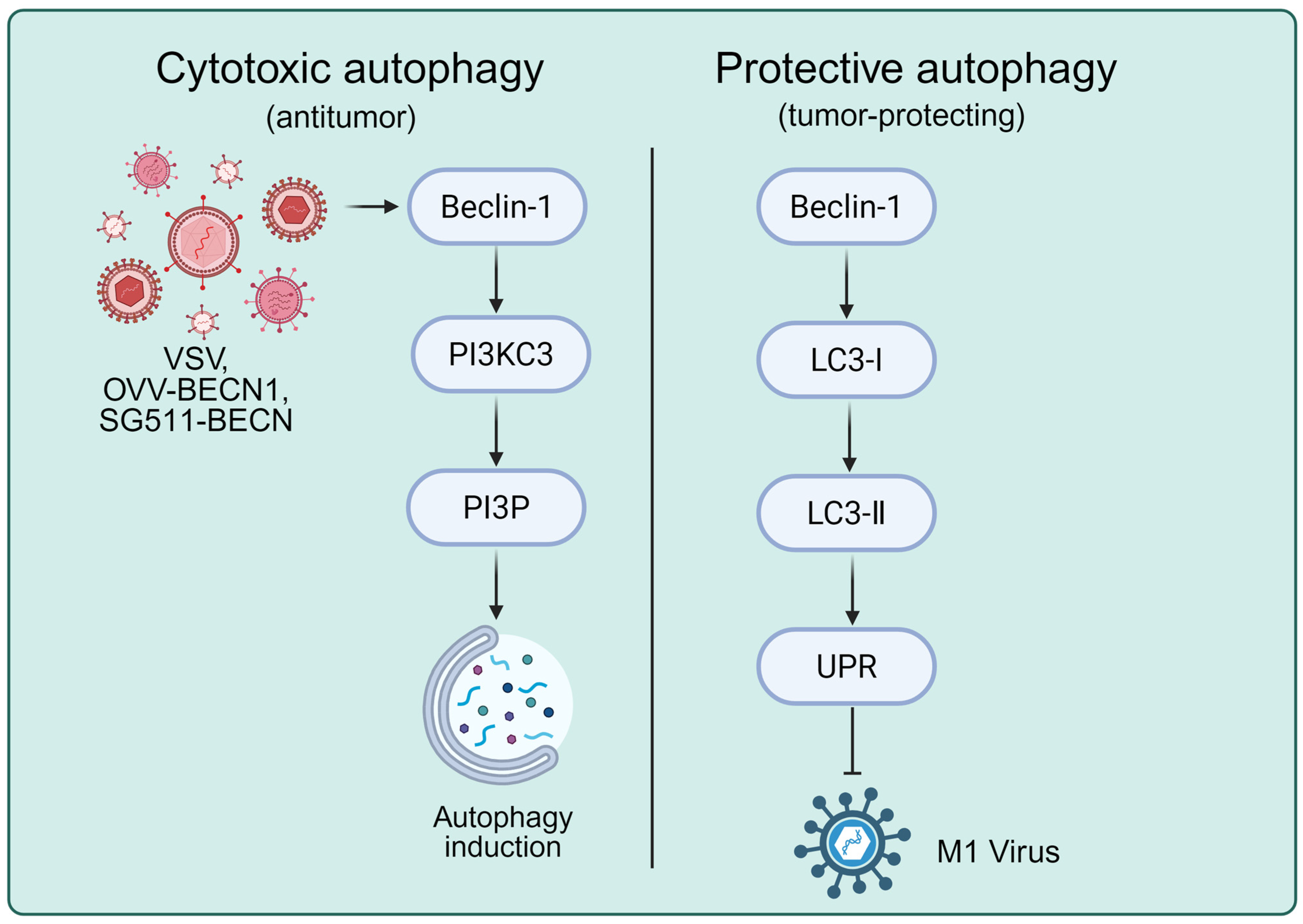
4. Oncolytic Virus and Autophagy
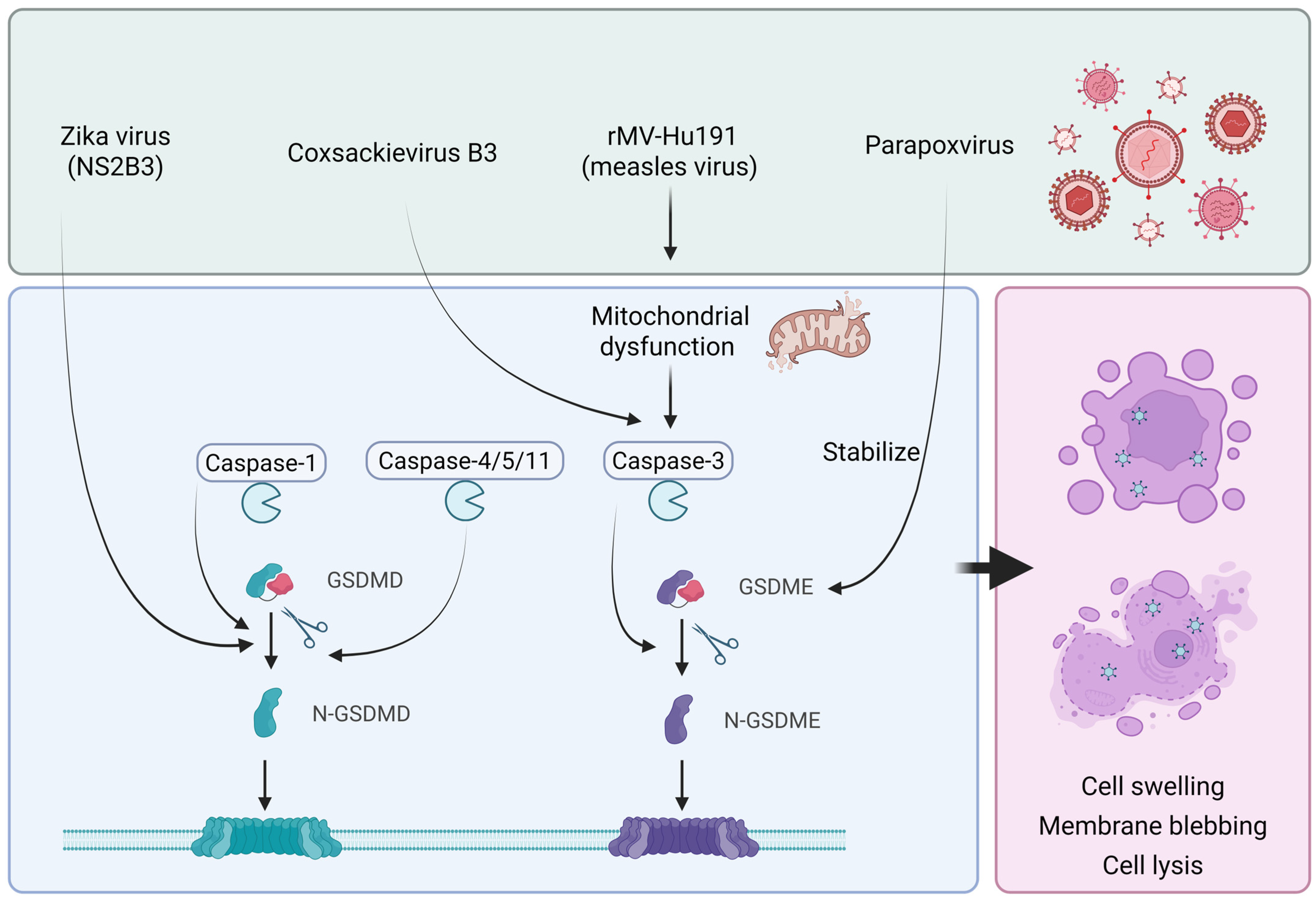
5. Oncolytic Virus and Pyroptosis
6. Oncolytic Viruses and Other Forms of Cell Death
7. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.A.-O.; Ferlay, J.; Siegel, R.A.-O.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pack, G.T. St. Peregrine, O.S.M.—The Patron Saint of Cancer Patients. CA Cancer J. Clin. 1967, 17, 183–184. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662, Erratum in Nat. Rev. Drug Discov. 2016, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Poh, A. First Oncolytic Viral Therapy for Melanoma. Cancer Discov. 2015, 6, 6. [Google Scholar] [PubMed]
- Todo, T.A.-O.; Ito, H.; Ino, Y.A.-O.; Ohtsu, H.A.-O.; Ota, Y.; Shibahara, J.; Tanaka, M.A.-O. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639, Erratum in Nat. Med. 2025, 31, 1365. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial. Nat. Med. 2023, 29, 450–457, Erratum in Nat. Med. 2023, 29, 3270. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Zhong, L.; Gan, L.; Wang, B.; Wu, T.; Yao, F.; Gong, W.; Peng, H.; Deng, Z.; Xiao, G.; Liu, X.; et al. Hyperacute rejection-engineered oncolytic virus for interventional clinical trial in refractory cancer patients. Cell 2025, 188, 1119–1136.e1123. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Bai, X.; Zhang, Q.; Liang, X.; Jin, X.; Zhao, Z.; Song, W.; Tan, Q.; Zhao, R.; Jia, W.; et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature 2025, 641, 503–511, Erratum in Nature 2025, 641, E5. [Google Scholar] [CrossRef] [PubMed]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M.; et al. Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378, Correction in Nat. Med. 2025, 31, 3204. [Google Scholar] [CrossRef]
- Hwang, T.H.; Moon, A.; Burke, J.; Ribas, A.; Stephenson, J.; Breitbach, C.J.; Daneshmand, M.; De Silva, N.; Parato, K.; Diallo, J.S.; et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. 2011, 19, 1913–1922. [Google Scholar] [CrossRef]
- Park, B.H.; Hwang, T.; Liu, T.C.; Sze, D.Y.; Kim, J.S.; Kwon, H.C.; Oh, S.Y.; Han, S.Y.; Yoon, J.H.; Hong, S.H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin. Cancer Res. 2018, 24, 4388–4398. [Google Scholar] [CrossRef]
- Mell, L.K.; Brumund, K.T.; Daniels, G.A.; Advani, S.J.; Zakeri, K.; Wright, M.E.; Onyeama, S.J.; Weisman, R.A.; Sanghvi, P.R.; Martin, P.J.; et al. Phase I Trial of Intravenous Oncolytic Vaccinia Virus (GL-ONC1) with Cisplatin and Radiotherapy in Patients with Locoregionally Advanced Head and Neck Carcinoma. Clin. Cancer Res. 2017, 23, 5696–5702. [Google Scholar] [CrossRef]
- Alvarez-Breckenridge, C.; Kaur, B.; Chiocca, E.A. Pharmacologic and chemical adjuvants in tumor virotherapy. Chem. Rev. 2009, 109, 3125–3140. [Google Scholar] [CrossRef]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2022, 9, 122–139. [Google Scholar] [CrossRef]
- Schirrmacher, V. Cancer Vaccines and Oncolytic Viruses Exert Profoundly Lower Side Effects in Cancer Patients than Other Systemic Therapies: A Comparative Analysis. Biomedicines 2020, 8, 61. [Google Scholar] [CrossRef]
- Gujar, S.; Dielschneider, R.; Clements, D.; Helson, E.; Shmulevitz, M.; Marcato, P.; Pan, D.; Pan, L.-Z.; Ahn, D.-G.; Alawadhi, A.; et al. Multifaceted therapeutic targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation. Mol. Ther. 2013, 21, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Q.; Zhou, X.; Zhao, H.; Wang, K.; Niu, H.; Wang, Y. Gospel of malignant Glioma: Oncolytic virus therapy. Gene 2022, 818, 146217. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Mohajel, N.; Malekshahi, Z.V.; Jahangiri, S.; Najafi, M.; Farhood, B.; Mortezaee, K.; Negahdari, B.; Arashkia, A. Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J. Cell. Physiol. 2019, 23, 8636–8646. [Google Scholar] [CrossRef]
- Ghose, J.; Dona, A.; Murtadha, M.; Gunes, E.G.; Caserta, E.; Yoo, J.Y.; Russell, L.; Jaime-Ramirez, A.C.; Barwick, B.G.; Gupta, V.A.; et al. Oncolytic herpes simplex virus infects myeloma cells in vitro and in vivo. Mol. Ther. Oncolytics 2021, 20, 519–531. [Google Scholar] [CrossRef]
- Garant, K.A.; Shmulevitz, M.; Pan, L.; Daigle, R.M.; Ahn, D.G.; Gujar, S.A.; Lee, P.W. Oncolytic reovirus induces intracellular redistribution of Ras to promote apoptosis and progeny virus release. Oncogene 2016, 35, 771–782. [Google Scholar] [CrossRef]
- Kaneda, Y. The RIG-I/MAVS signaling pathway in cancer cell-selective apoptosis. OncoImmunology 2013, 2, e23566. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Liao, T.; Wang, C.; Han, J.; Yang, Z.; Lu, X.; Hu, Z.; Hu, J.; Wang, X.; et al. The HN protein of Newcastle disease virus induces cell apoptosis through the induction of lysosomal membrane permeabilization. PLoS Pathog. 2024, 20, e1011981. [Google Scholar] [CrossRef]
- Mansour, M.; Palese, P.; Zamarin, D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol. 2011, 85, 6015–6023. [Google Scholar] [CrossRef]
- Wen, J.S.; Zhao, W.Z.; Liu, J.W.; Zhou, H.; Tao, J.P.; Yan, H.J.; Liang, Y.; Zhou, J.J.; Jiang, L.F. Genomic analysis of a Chinese isolate of Getah-like virus and its phylogenetic relationship with other Alphaviruses. Virus Genes 2007, 35, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; et al. Identification and characterization of alphavirus M1 as a selective oncolytic virus targeting ZAP-defective human cancers. Proc. Natl. Acad. Sci. USA 2014, 111, E4504–E4512. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 2011, 472, 481–485, Erratum in Nature 2015, 525, 144. [Google Scholar] [CrossRef]
- Zhang, H.A.-O.X.; Li, K.; Lin, Y.A.-O.; Xing, F.; Xiao, X.; Cai, J.; Zhu, W.; Liang, J.; Tan, Y.; Fu, L.; et al. Targeting VCP enhances anticancer activity of oncolytic virus M1 in hepatocellular carcinoma. Sci. Transl. Med. 2017, 9, eaam7996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Zhu, W.B.; Zhang, H.; Huang, W.T.; Liu, X.C.; Lin, Y.; Cai, J.; Yan, G.M.; Qiu, J.G.; et al. Suppression of CCDC6 sensitizes tumor to oncolytic virus M1. Neoplasia 2021, 23, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lin, Y.; Zhang, H.; Liang, J.; Tan, Y.; Cavenee, W.A.-O.; Yan, G. Selective replication of oncolytic virus M1 results in a bystander killing effect that is potentiated by Smac mimetics. Proc. Natl. Acad. Sci. USA 2017, 114, 6812–6817. [Google Scholar] [CrossRef]
- Tamura, K.; Wakimoto, H.; Agarwal, A.S.; Rabkin, S.D.; Bhere, D.; Martuza, R.L.; Kuroda, T.; Kasmieh, R.; Shah, K. Multimechanistic tumor targeted oncolytic virus overcomes resistance in brain tumors. Mol. Ther. 2013, 21, 68–77. [Google Scholar] [CrossRef]
- Lv, C.; Su, Q.; Liang, Y.; Hu, J.; Yuan, S. Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis. Biochem. Biophys. Res. Commun. 2016, 476, 21–28. [Google Scholar] [CrossRef]
- Jung, K.H.; Choi, I.K.; Lee, H.S.; Yan, H.H.; Son, M.K.; Ahn, H.M.; Hong, J.; Yun, C.O.; Hong, S.S. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer Lett. 2017, 396, 155–166, Erratum in Cancer Lett. 2017, 404, 93. [Google Scholar] [CrossRef]
- Zhang, L.F.; Tan, D.Q.; Jeyasekharan, A.D.; Hsieh, W.S.; Ho, A.S.; Ichiyama, K.; Ye, M.; Pang, B.; Ohba, K.; Liu, X.; et al. Combination of vaccine-strain measles and mumps virus synergistically kills a wide range of human hematological cancer cells: Special focus on acute myeloid leukemia. Cancer Lett. 2014, 354, 272–280. [Google Scholar] [CrossRef]
- Marchini, A.; Scott, E.M.; Rommelaere, J. Overcoming Barriers in Oncolytic Virotherapy with HDAC Inhibitors and Immune Checkpoint Blockade. Viruses 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Soleimanjahi, H.; Soleimani, M.; Arefian, E. Mesenchymal stem cells loaded with oncolytic reovirus enhances antitumor activity in mice models of colorectal cancer. Biochem. Pharmacol. 2021, 190, 114644. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, H.; Qiu, J.; Lin, Y.; Liang, J.; Xiao, X.; Fu, L.; Wang, F.; Cai, J.; Tan, Y.; et al. Activation of Cyclic Adenosine Monophosphate Pathway Increases the Sensitivity of Cancer Cells to the Oncolytic Virus M1. Mol. Ther. 2016, 24, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.A.-O.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential role for autophagy in life span extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Lenz, G.; Lesniak, M.S. Autophagy in glioma cells: An identity crisis with a clinical perspective. Cancer Lett. 2018, 428, 139–146. [Google Scholar] [CrossRef]
- Chen, N.; Debnath, J. Autophagy and tumorigenesis. FEBS Lett. 2010, 584, 1427–1435. [Google Scholar] [CrossRef]
- Zhirnov, O.P. Biochemical Variations in Cytolytic Activity of Ortho- and Paramyxoviruses in Human Lung Tumor Cell Culture. Biochemistry 2017, 82, 1048–1054. [Google Scholar]
- Wu, Y.Y.; Sun, T.K.; Chen, M.S.; Munir, M.; Liu, H.J. Oncolytic viruses-modulated immunogenic cell death, apoptosis and autophagy linking to virotherapy and cancer immune response. Front. Cell. Infect. Microbiol. 2023, 13, 1142172. [Google Scholar] [CrossRef]
- Abudoureyimu, M.; Lai, Y.; Tian, C.; Wang, T.; Wang, R.; Chu, X. Oncolytic Adenovirus-A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC. Front. Oncol. 2019, 9, 1182. [Google Scholar] [CrossRef]
- Jakubczak, J.L.; Ryan, P.; Gorziglia, M.; Clarke, L.; Hawkins, L.K.; Hay, C.; Huang, Y.; Kaloss, M.; Marinov, A.; Phipps, S.; et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: Dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003, 63, 1490–1499. [Google Scholar] [PubMed]
- Askari, F.S.; Mohebbi, A.A.-O.X.; Moradi, A.; Javid, N. The Role of Vesicular Stomatitis Virus Matrix Protein in Autophagy in the Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wang, S.; Xu, N.; Chen, Y.; Wu, G.; Zhang, A.; Chen, X.; Tong, Y.; Qian, W. Enhancing therapeutic efficacy of oncolytic vaccinia virus armed with Beclin-1, an autophagic Gene in leukemia and myeloma. Biomed. Pharmacother. 2020, 125, 110030. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; You, L.; Liu, H.; Li, L.; Meng, H.; Qian, Q.; Qian, W. Potent antitumor activity of oncolytic adenovirus expressing Beclin-1 via induction of autophagic cell death in leukemia. Oncotarget 2013, 4, 860. [Google Scholar] [CrossRef]
- Li, L.; You, L.S.; Mao, L.P.; Jin, S.H.; Chen, X.H.; Qian, W.B. Combing oncolytic adenovirus expressing Beclin-1 with chemotherapy agent doxorubicin synergistically enhances cytotoxicity in human CML cells in vitro. Acta Pharmacol. Sin. 2017, 39, 251–260. [Google Scholar] [CrossRef]
- Jin, K.T.; Tao, X.H.; Fan, Y.B.; Wang, S.B. Crosstalk between oncolytic viruses and autophagy in cancer therapy. Biomed. Pharmacother. 2021, 134, 110932. [Google Scholar] [CrossRef]
- Tazawa, H.; Yano, S.; Yoshida, R.; Yamasaki, Y.; Sasaki, T.; Hashimoto, Y.; Kuroda, S.; Ouchi, M.; Onishi, T.; Uno, F.; et al. Genetically engineered oncolytic adenovirus induces autophagic cell death through an E2F1-microRNA-7-epidermal growth factor receptor axis. Int. J. Cancer 2012, 131, 2939–2950. [Google Scholar] [CrossRef]
- Wu, A.A.-O.; Li, Z.; Wang, Y.; Chen, Y.; Peng, J.; Zhu, M.; Li, Y.; Song, H.; Zhou, D.; Zhang, C.; et al. Recombinant measles virus vaccine rMV-Hu191 exerts an oncolytic effect on esophageal squamous cell carcinoma via caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023, 9, 171. [Google Scholar] [CrossRef]
- Li, K.; Hu, C.; Xing, F.; Gao, M.; Liang, J.; Xiao, X.; Cai, J.; Tan, Y.; Hu, J.; Zhu, W.; et al. Deficiency of the IRE1α-Autophagy Axis Enhances the Antitumor Effects of the Oncolytic Virus M1. J. Virol. 2018, 92, e01331-17. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Nyström, S.; Antoine, D.J.; Lundbäck, P.; Lock, J.G.; Nita, A.F.; Högstrand, K.; Grandien, A.; Erlandsson-Harris, H.; Andersson, U.; Applequist, S.E. TLR activation regulates damage-associated molecular pattern isoforms released during pyroptosis. EMBO J. 2013, 32, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, S.; Zhao, K.; Gao, F.; Wang, R.; Li, Q.; Zhou, Y.; Zhang, J.; Li, Y.; Wang, X.; et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat. Commun. 2023, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, T.; Tian, H.; Wu, J.; Yu, X.; Zeng, L.; Liu, F.; Liu, Q.; Huang, X. Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis. Cancers 2022, 14, 6206. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhu, Y.; Li, N.; Li, W.; Shang, C.; Song, G.; Li, S.; Cong, J.; Li, T.; et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci. 2022, 18, 717–730. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Liu, Y.; Zhu, W.-B.; Li, S.-H.; Wei, S.; Cai, J.; Lin, Y.; Liang, J.-K.; Yan, G.-M.; Guo, L.; et al. Arming oncolytic M1 virus with gasdermin E enhances antitumor efficacy in breast cancer. iScience 2024, 27, 111148. [Google Scholar] [CrossRef]
- Kao, Y.T.; Wang, H.I.; Shie, C.T.; Lin, C.F.; Lai, M.M.C.; Yu, C.Y. Zika virus cleaves GSDMD to disseminate prognosticable and controllable oncolysis in a human glioblastoma cell model. Mol. Ther. Oncolytics 2023, 28, 104–117. [Google Scholar] [CrossRef]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 2021, 40, 4783–4795. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, Y.L.; Lin, H.W.; Chang, C.F.; Huang, B.S.; Sun, W.Z.; Cheng, W.F. Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and DAMPs. Cancer Lett. 2021, 523, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.A.-O.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. Medcomm 2022, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- de Andrea, C.A.-O.; Ochoa, M.C.; Villalba-Esparza, M.; Teijeira, Á.; Schalper, K.A.; Abengozar-Muela, M.; Eguren-Santamaría, I.; Sainz, C.; Sánchez-Gregorio, S.; Garasa, S.; et al. Heterogenous presence of neutrophil extracellular traps in human solid tumours is partially dependent on IL-8. J. Pathol. 2021, 255, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Tian, R.; Yu, L.; Bian, S.A.-O.; Chen, Y.; Yin, B.; Luan, Y.; Chen, S.; Fan, Z.; Yan, R.; et al. Overcoming therapeutic resistance in oncolytic herpes virotherapy by targeting IGF2BP3-induced NETosis in malignant glioma. Nat. Commun. 2024, 15, 131. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Kan, X.; Yin, Y.; Song, C.; Tan, L.; Qiu, X.; Liao, Y.; Liu, W.; Meng, S.; Sun, Y.; Ding, C. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience 2021, 24, 102837. [Google Scholar] [CrossRef]

| Name | Classification | Size (nm) | Capsid Coating | Extracellular Receptor |
|---|---|---|---|---|
| Adenovirus | dsDNA | 70–90 | No | CAR |
| Vaccinia virus | dsDNA | 70–100 | Yes | Unknown |
| Herpesvirus | dsDNA | 200 | Yes | HVEM, nectin1, nectin2 |
| Reovirus | dsRNA | 75 | No | Unknown |
| Coxsackievirus | ssRNA | 28 | No | CAR, DAF, ICAM-1 |
| Measles virus | ss(-)RNA | 100–200 | Yes | CD46, CD150, SLAM |
| Newcastle disease virus | ss(-)RNA | 100–400 | Yes | sialic acid receptors |
| Viral Name | Viral Type | Interventions | Indication | Clinical Phase | Identifier. | Clinical Responses |
|---|---|---|---|---|---|---|
| T-VEC | HSV | T-VEC | IIIB to IV melanoma | Phase III | NCT00769704 [7] | Median OS was 23.3 months (19.5–29.6 months) with T-VEC and 18.9 months (16.0–23.7 months) with GM-CSF |
| T-VEC | HSV | T-VEC plus NAC | nonmetastatic triple-negative breast cancer | Phase II | NCT02779855 [8] | RCB0 rate = 45.9% and RCB0–1 descriptive rate = 65%. |
| T-VEC | HSV | T-VEC plus pembrolizumab | Advanced Melanoma | Phase III | NCT02263508 [9] | T-VEC-pembrolizumab did not significantly improve PFS or OS compared with placebo-pembrolizumab. |
| G47∆ | HSV | G47∆ | residual or recurrent, supratentorial glioblastoma | Phase II | UMIN000015995 [6] | 1-yr survival rate: 84.2% (60.4–96.6) |
| CAN-3110 | HSV | CAN-3110 | recurrent glioblastoma | Phase I | NCT03152318 [10] | median OS: 11.6 months (7.8–14.9 months) |
| NDV-GT | NDV | NDV-GT | advanced malignant solid tumors | Phase I | ChiCTR2000031980 [11] | disease control rate: 90% |
| VG161 | HSV | VG161 | refractory hepatocellular carcinoma | Phase I | NCT04806464 [12] | median PFS: 2.9 months (1.81–3.70) and OS: 12.4 months (7.10–20.10) |
| DNX-2401 | AD | DNX-2401 | diffuse intrinsic pontine glioma | Phase I | NCT03178032 [13] | median OS 17.8 months |
| DNX-2401 | AD | DNX-2401 plus pembrolizumab | recurrent glioblastoma | phase I/II | NCT02798406 [14] | Median OS: 12.5 months (10.7–13.5 months) |
| JX-594 | VV | JX-594 | Melanoma | phase I/II | NCT00429312 [15] | DOR and PFS were not assessable since most patients went off study within 6 weeks |
| JX-594 | VV | JX-594 | Neoplasms, liver | Phase I | NCT00629759 [16] | Median OS: 9 months |
| GL-ONC1 | VV | GL-ONC1 | Peritoneal Carcinomatosis | Phase I | NCT01443260 [17] | GL-ONC1 was well tolerated when administered into the peritoneal cavity of patients with advanced stage peritoneal carcinomatosis |
| GL-ONC1 | VV | GL-ONC1 | Locoregionally Advanced Head and Neck Carcinoma | Phase I | NCT01584284 [18] | 1-year (2-year) PFS and OS were 74.4% (64.1%) and 84.6% (69.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, C.; An, Y.; Sun, J.; Wang, S.; Xia, Q. Mechanisms of Oncolytic Virus-Induced Multi-Modal Cell Death and Therapeutic Prospects. Int. J. Mol. Sci. 2025, 26, 9770. https://doi.org/10.3390/ijms26199770
Xu J, Liu C, An Y, Sun J, Wang S, Xia Q. Mechanisms of Oncolytic Virus-Induced Multi-Modal Cell Death and Therapeutic Prospects. International Journal of Molecular Sciences. 2025; 26(19):9770. https://doi.org/10.3390/ijms26199770
Chicago/Turabian StyleXu, Jinzhou, Chenqian Liu, Ye An, Jianxuan Sun, Shaogang Wang, and Qidong Xia. 2025. "Mechanisms of Oncolytic Virus-Induced Multi-Modal Cell Death and Therapeutic Prospects" International Journal of Molecular Sciences 26, no. 19: 9770. https://doi.org/10.3390/ijms26199770
APA StyleXu, J., Liu, C., An, Y., Sun, J., Wang, S., & Xia, Q. (2025). Mechanisms of Oncolytic Virus-Induced Multi-Modal Cell Death and Therapeutic Prospects. International Journal of Molecular Sciences, 26(19), 9770. https://doi.org/10.3390/ijms26199770







