Identification of Proteins and MicroRNAs with Prognostic Value for Assisted Reproduction Technology Outcomes in Follicular Fluid of Women with Endometriosis: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
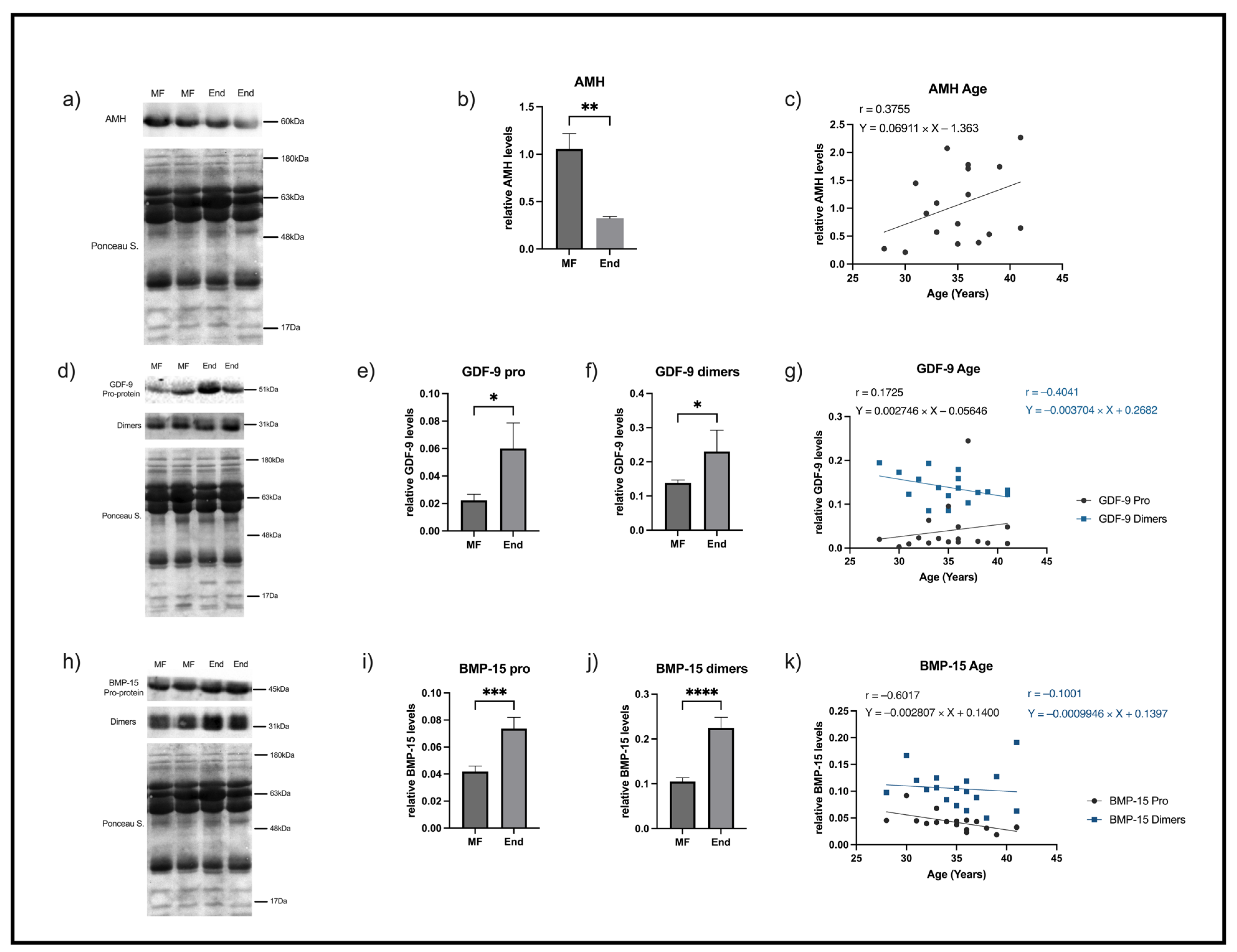
2.2. AMH Decreases and GDF-9 and BMP-15 Increases in FF Samples of Women with Endometriosis
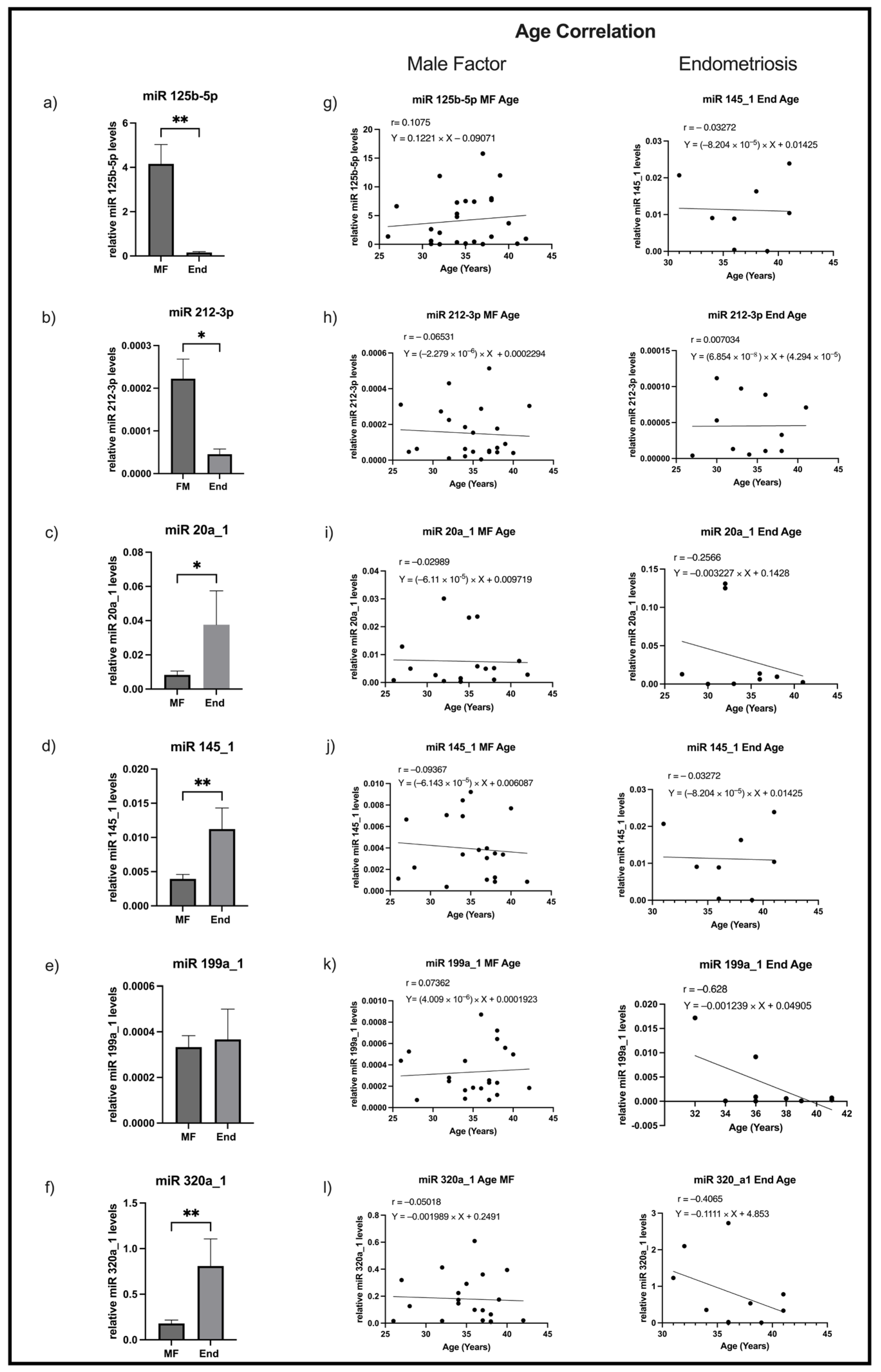
2.3. Levels of miR125b-5p and miR212-3p Decrease and of miR20a_1, miR145_1, and miR320a_1 Increase in FF Samples of Women with Endometriosis
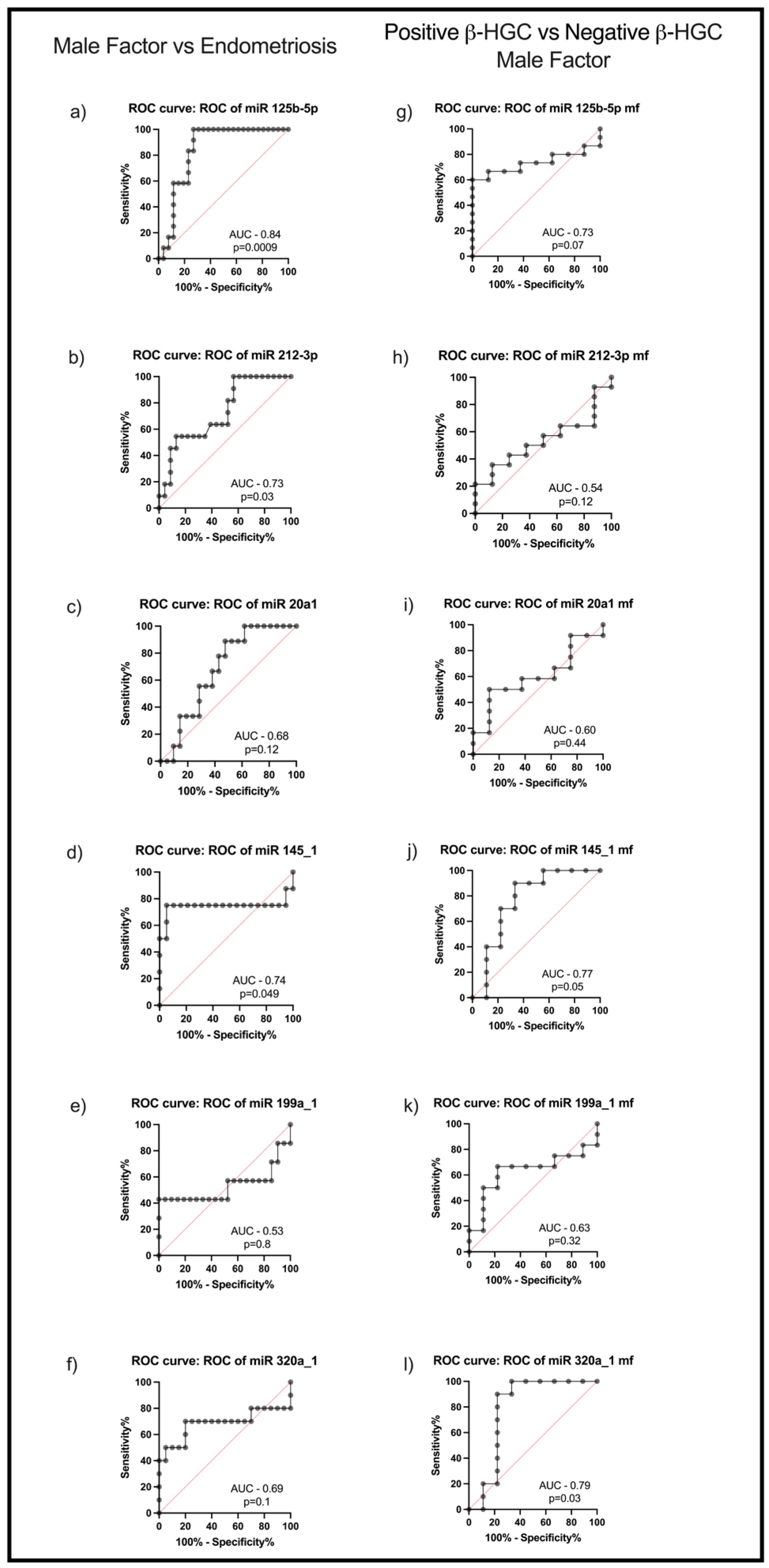
2.4. MiR125b-5p, miR212-3p, and miR145_1 Levels in FF Are Putatively Predictors of Endometriosis and miR145_1 and miR320a_1 of Biochemical Pregnancy
3. Discussion
4. Materials and Methods
4.1. Follicular Fluid Collection and Processing
4.2. Semi-Quantification of AMH, BMP-15, and GDF-9 in FF by Western Blotting
4.3. Reverse Transcription qPCR
4.4. Pregnancy Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| AMH | Anti-Mullerian hormone |
| ART | Assisted Reproductive Technology |
| BMP-15 | Bone morphogenetic protein-15 |
| BSA | Bovine Serum Albumin |
| CETI | Centro de Estudo e Tratamento de Infertilidade |
| END | Endometriosis |
| FF | Follicular fluid |
| GC | Granulosa cells |
| GDF-9 | Growth differentiation factor-9 |
| β-hCG | Human Chorionic Gonadotropin |
| ICSI | Intracytoplasmic Sperm Injection |
| IVF | In vitro fertilization |
| MF | Male Factor |
| miR | MicroRNA |
| MiRNA | MicroRNA |
| OSFs | Oocyte-Secreted Factors |
| ROC | Receiver Operating Characteristic |
| TBS | Tris-buffer Saline |
| TGF-β | Transforming Growth Factor β |
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Neto, A.C.; Botelho, M.; Rodrigues, A.R.; Lamas, S.; Araújo, B.; Guimarães, J.T.; Gouveia, A.M.; Almeida, H.; Neves, D. Metformin reverses infertility in a mouse model of endometriosis: Unveiling disease pathways and implications for future clinical approaches. Reprod. BioMed. Online 2024, 50, 104474. [Google Scholar] [CrossRef]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.E.; Batteux, F.; Borderie, D.; Chapron, C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef]
- Soares, S.R.; Martínez-Varea, A.; Hidalgo-Mora, J.J.; Pellicer, A. Pharmacologic therapies in endometriosis: A systematic review. Fertil. Steril. 2012, 98, 529–555. [Google Scholar] [CrossRef]
- Prefumo, F.; Rossi, A.C. Endometriosis, endometrioma, and ART results: Current understanding and recommended practices. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 34–40. [Google Scholar] [CrossRef]
- Freitas, C.; Neto, A.C.; Matos, L.; Silva, E.; Ribeiro, Â.; Silva-Carvalho, J.L.; Almeida, H. Follicular Fluid redox involvement for ovarian follicle growth. J. Ovarian Res. 2017, 10, 44. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef]
- Qasemi, M.; Amidi, F. Extracellular microRNA profiling in human follicular fluid: New biomarkers in female reproductive potential. J. Assist. Reprod. Genet. 2020, 37, 1769–1780. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Q.; Wigglesworth, K.; Rangarajan, A.; Kattamuri, C.; Peterson, R.T.; Eppig, J.J.; Thompson, T.B.; Matzuk, M.M. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. USA 2013, 110, E776–E785. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, P.; DeMayo, J.; DeMayo, F.J.; Elvin, J.A.; Carino, C.; Prasad, S.V.; Skinner, S.S.; Dunbar, B.S.; Dube, J.L.; et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol. 2001, 15, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Wu, X.; O’Brien, M.J.; Pendola, F.L.; Denegre, J.N.; Matzuk, M.M.; Eppig, J.J. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: Genetic evidence for an oocyte-granulosa cell regulatory loop. Dev. Biol. 2004, 276, 64–73. [Google Scholar] [CrossRef]
- Craig, J.; Orisaka, M.; Wang, H.; Orisaka, S.; Thompson, W.; Zhu, C.; Kotsuji, F.; Tsang, B.K. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628–3639. [Google Scholar] [CrossRef]
- Wei, L.N.; Liang, X.Y.; Fang, C.; Zhang, M.F. Abnormal expression of growth differentiation factor 9 and bone morphogenetic protein 15 in stimulated oocytes during maturation from women with polycystic ovary syndrome. Fertil. Steril. 2011, 96, 464–468. [Google Scholar] [CrossRef]
- La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C.; Stabile, G.; Volpe, A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum. Reprod. Update 2010, 16, 113–130. [Google Scholar] [CrossRef]
- van Rooij, I.A.; Broekmans, F.J.; Scheffer, G.J.; Looman, C.W.; Habbema, J.D.; de Jong, F.H.; Fauser, B.J.; Themmen, A.P.; te Velde, E.R. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: A longitudinal study. Fertil. Steril. 2005, 83, 979–987. [Google Scholar] [CrossRef]
- Kitajima, M.; Matsumoto, K.; Murakami, N.; Kajimura, I.; Harada, A.; Kitajima, Y.; Masuzaki, H.; Miura, K. AMH Concentrations in Peritoneal Fluids of Women With and Without Endometriosis. Front. Surg. 2020, 7, 600202. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Faruq, O.; Vecchione, A. microRNA: Diagnostic Perspective. Front. Med. 2015, 2, 51. [Google Scholar] [CrossRef]
- Agrawal, S.; Tapmeier, T.T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.T.; Becker, C.M. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef]
- Nematian, S.E.; Mamillapalli, R.; Kadakia, T.S.; Majidi Zolbin, M.; Moustafa, S.; Taylor, H.S. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J. Clin. Endocrinol. Metab. 2018, 103, 64–74. [Google Scholar] [CrossRef]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946.e2. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Liang, L.; Racowsky, C.; Dioni, L.; Mansur, A.; Adir, M.; Bollati, V.; Baccarelli, A.A.; Hauser, R.; Machtinger, R. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci. Rep. 2018, 8, 17036. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.L.; Bhatti, S.; Abbas, S.; Kaloglu, C.; Isa, A.M.; Younas, H.; Ziders, R.; Khan, Y.L.; Hassan, Z.; Turhan, B.O.; et al. Extracellular microRNAs: Key players to explore the outcomes of in vitro fertilization. Reprod. Biol. Endocrinol. 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef]
- Machtinger, R.; Rodosthenous, R.S.; Adir, M.; Mansour, A.; Racowsky, C.; Baccarelli, A.A.; Hauser, R. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: An exploratory study. J. Assist. Reprod. Genet. 2017, 34, 525–533. [Google Scholar] [CrossRef]
- Feng, R.; Sang, Q.; Zhu, Y.; Fu, W.; Liu, M.; Xu, Y.; Shi, H.; Xu, Y.; Qu, R.; Chai, R.; et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci. Rep. 2015, 5, 8689. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, K.-J.; Sun, Q.; Chen, A.-Z.; Shen, W.-L.; Zhao, Z.-H.; Zheng, X.-F.; Yang, X. Functional screening for miRNAs targeting Smad4 identified miR-199a as a negative regulator of TGF-β signalling pathway. Nucleic Acids Res. 2012, 40, 9286–9297. [Google Scholar] [CrossRef]
- Fu, X.; Qie, J.; Fu, Q.; Chen, J.; Jin, Y.; Ding, Z. miR-20a-5p/TGFBR2 Axis Affects Pro-inflammatory Macrophages and Aggravates Liver Fibrosis. Front. Oncol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Melling, G.E.; Flannery, S.E.; Abidin, S.A.; Clemmens, H.; Prajapati, P.; Hinsley, E.E.; Hunt, S.; Catto, J.W.F.; Coletta, R.D.; Mellone, M.; et al. A miRNA-145/TGF-β1 negative feedback loop regulates the cancer-associated fibroblast phenotype. Carcinogenesis 2018, 39, 798–807. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.Y. Small RNA molecules in endometriosis: Pathogenesis and therapeutic aspects. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 183, 83–88. [Google Scholar] [CrossRef]
- Jia, S.Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. 2013, 28, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H.S. Circulating microRNAs as potential biomarkers for endometriosis. Fertil. Steril. 2015, 103, 1252–1260.e1. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson Teague, E.M.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, L.; Fang, T.; Zhang, Q.; Wu, S.; Jiang, Y.; Sun, H.; Hu, Y. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 2012, 586, 3263–3270. [Google Scholar] [CrossRef]
- Adammek, M.; Greve, B.; Kässens, N.; Schneider, C.; Brüggemann, K.; Schüring, A.N.; Starzinski-Powitz, A.; Kiesel, L.; Götte, M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil. Steril. 2013, 99, 1346–1355.e5. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hsieh, T.H.; Tsai, C.F.; Tsai, H.P.; Chen, H.S.; Chang, Y.; Chuang, H.Y.; Lee, J.N.; Hsu, Y.L.; Tsai, E.M. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J. Pathol. 2014, 232, 330–343. [Google Scholar] [CrossRef]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int. J. Gynaecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef]
- Chen, G.; Guo, J.; Li, W.; Zheng, R.; Shang, H.; Wang, Y. Diagnostic value of the combination of circulating serum miRNAs and CA125 in endometriosis. Medicine 2023, 102, e36339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef] [PubMed]
- Dancey, C.P.; Reidy, J. Statistics Without Maths for Psychology: Using SPSS for Windows; Prentice Hall: Saddle River, NJ, USA, 2004. [Google Scholar]
- Templeton, A. Infertility and the establishment of pregnancy—Overview. Br. Med. Bull. 2000, 56, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Poto, L.; Farkas, N.; Koppan, M.; Varnagy, A.; Kovacs, K.; Papp, S.; Bohonyi, N.; Bodis, J. Follicular fluid progesterone concentration is associated with fertilization outcome after IVF: A systematic review and meta-analysis. Reprod. BioMed. Online 2019, 38, 871–882. [Google Scholar] [CrossRef]
- Yoo, J.H.; Cha, S.H.; Park, C.W.; Kim, J.Y.; Yang, K.M.; Song, I.O.; Koong, M.K.; Kang, I.S.; Kim, H.O. Serum anti-Müllerian hormone is a better predictor of ovarian response than FSH and age in IVF patients with endometriosis. Clin. Exp. Reprod. Med. 2011, 38, 222–227. [Google Scholar] [CrossRef]
- Lemos, N.A.; Arbo, E.; Scalco, R.; Weiler, E.; Rosa, V.; Cunha-Filho, J.S. Decreased anti-Mullerian hormone and altered ovarian follicular cohort in infertile patients with mild/minimal endometriosis. Fertil. Steril. 2008, 89, 1064–1068. [Google Scholar] [CrossRef]
- Belguith, I.; Dhieb, D.; Turki, M.; Yaich, S.; Chaabene, K.; Mnif, M.; Ayadi, F.; Keskes, L.A. Diagnostic value of miR-199a and miR-21 in the plasma of infertile women with dysregulated AMH levels. Hum. Fertil. 2022, 25, 154–165. [Google Scholar] [CrossRef]
- Lutful Kabir, F.; Ambalavanan, N.; Liu, G.; Li, P.; Solomon, G.M.; Lal, C.V.; Mazur, M.; Halloran, B.; Szul, T.; Gerthoffer, W.T.; et al. MicroRNA-145 Antagonism Reverses TGF-β Inhibition of F508del CFTR Correction in Airway Epithelia. Am. J. Respir. Crit. Care Med. 2018, 197, 632–643. [Google Scholar] [CrossRef]
- Zhao, N.; Koenig, S.N.; Trask, A.J.; Lin, C.H.; Hans, C.P.; Garg, V.; Lilly, B. MicroRNA miR145 regulates TGFBR2 expression and matrix synthesis in vascular smooth muscle cells. Circ. Res. 2015, 116, 23–34. [Google Scholar] [CrossRef] [PubMed]
- McNatty, K.P.; Moore, L.G.; Hudson, N.L.; Quirke, L.D.; Lawrence, S.B.; Reader, K.; Hanrahan, J.P.; Smith, P.; Groome, N.P.; Laitinen, M.; et al. The oocyte and its role in regulating ovulation rate: A new paradigm in reproductive biology. Reproduction 2004, 128, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.X.; Moore, R.K.; Otsuka, F.; Shimasaki, S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9. Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J. Biol. Chem. 2003, 278, 3713–3719. [Google Scholar] [CrossRef]
- Mottershead, D.G.; Sugimura, S.; Al-Musawi, S.L.; Li, J.J.; Richani, D.; White, M.A.; Martin, G.A.; Trotta, A.P.; Ritter, L.J.; Shi, J.; et al. Cumulin, an Oocyte-secreted Heterodimer of the Transforming Growth Factor-beta Family, Is a Potent Activator of Granulosa Cells and Improves Oocyte Quality. J. Biol. Chem. 2015, 290, 24007–24020. [Google Scholar] [CrossRef]
- Chatroudi, M.H.; Khalili, M.A.; Ashourzadeh, S.; Anbari, F.; Shahedi, A.; Safari, S. Growth differentiation factor 9 and cumulus cell supplementation in in vitro maturation culture media enhances the viability of human blastocysts. Clin. Exp. Reprod. Med. 2019, 46, 166–172. [Google Scholar] [CrossRef]
- Li, J.-J.; Sugimura, S.; Mueller, T.D.; White, M.A.; Martin, G.A.; Ritter, L.J.; Liang, X.-Y.; Gilchrist, R.B.; Mottershead, D.G. Modifications of human growth differentiation factor 9 to improve the generation of embryos from low competence oocytes. Mol. Endocrinol. 2015, 29, 40–52. [Google Scholar] [CrossRef]
- Sudiman, J.; Sutton-McDowall, M.L.; Ritter, L.J.; White, M.A.; Mottershead, D.G.; Thompson, J.G.; Gilchrist, R.B. Bone morphogenetic protein 15 in the pro-mature complex form enhances bovine oocyte developmental competence. PLoS ONE 2014, 9, e103563. [Google Scholar] [CrossRef]
- Sudiman, J.; Ritter, L.J.; Feil, D.K.; Wang, X.; Chan, K.; Mottershead, D.G.; Robertson, D.M.; Thompson, J.G.; Gilchrist, R.B. Effects of differing oocyte-secreted factors during mouse in vitro maturation on subsequent embryo and fetal development. J. Assist. Reprod. Genet. 2014, 31, 295–306. [Google Scholar] [CrossRef]
- Yeo, C.X.; Gilchrist, R.B.; Thompson, J.G.; Lane, M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum. Reprod. 2008, 23, 67–73. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.-Q.; Ou, S.-B.; Zhang, N.-F.; Ren, L.; Wei, L.-N.; Zhang, Q.-X.; Yang, D.-Z. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod. Biol. Endocrinol. 2014, 12, 81. [Google Scholar] [CrossRef]
- Wu, Y.T.; Wang, T.T.; Chen, X.J.; Zhu, X.M.; Dong, M.Y.; Sheng, J.Z.; Xu, C.M.; Huang, H.F. Bone morphogenetic protein-15 in follicle fluid combined with age may differentiate between successful and unsuccessful poor ovarian responders. Reprod. Biol. Endocrinol. 2012, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, E.; Beck-Peccoz, P.; Persani, L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am. J. Hum. Genet. 2004, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, E.; Rossetti, R.; Marozzi, A.; Bodega, B.; Borgato, S.; Cavallo, L.; Einaudi, S.; Radetti, G.; Russo, G.; Sacco, M.; et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J. Clin. Endocrinol. Metab. 2006, 91, 1976–1979. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef]
- Guéripel, X.; Brun, V.; Gougeon, A. Oocyte Bone Morphogenetic Protein 15, but not Growth Differentiation Factor 9, Is Increased During Gonadotropin-Induced Follicular Development in the Immature Mouse and Is Associated with Cumulus Oophorus Expansion1. Biol. Reprod. 2006, 75, 836–843. [Google Scholar] [CrossRef]
- Ribeiro, A.; Freitas, C.; Matos, L.; Gouveia, A.; Gomes, F.; Silva Carvalho, J.L.; Almeida, H. Age-related expression of TGF beta family receptors in human cumulus oophorus cells. J. Assist. Reprod. Genet. 2017, 34, 1121–1129. [Google Scholar] [CrossRef]
- Hendarto, H.; Prabowo, P.; Moeloek, F.A.; Soetjipto, S. Growth differentiation factor 9 concentration in the follicular fluid of infertile women with endometriosis. Fertil. Steril. 2010, 94, 758–760. [Google Scholar] [CrossRef]
- Kawabe, S.; Yamashita, Y.; Saito, N.; Kokunai, K.; Hayashi, A.; Hayashi, M.; Terai, Y.; Miyazaki, K.; Ohmichi, M. The effect of moderate to severe endometriosis on expression of growth differentiation factor-9 mRNA in human granulosa cells under controlled ovarian hyperstimulation. Reprod. Med. Biol. 2015, 14, 179–184. [Google Scholar] [CrossRef]
- Rasulzade, A.; Akalın, M.; Demirdağ, E.; Erdem, M.; Erdem, A. Comparing serum and follicle fluid GDF-9 and BMP-15 parameters in IVF patients with ovarian endometrioma. Reprod. BioMed. Online 2023, 47, 103459. [Google Scholar] [CrossRef]
- Shamsa, A.; Gilchrist, R.B.; Robertson, D.M.; Rodgers, R.J.; Donoghoe, M.W.; Ledger, W.L.; Abbott, J.A.; Riepsamen, A.H. Oocyte-Secreted Serum Biomarkers GDF9 and BMP15 in Women with Endometriosis. Reprod. Sci. 2023, 30, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Quan, Y.; Zhan, M.; Liao, H.; Li, Y.; Lu, L. miR-125b-5p inhibits cell proliferation, migration, and invasion in hepatocellular carcinoma via targeting TXNRD1. Cancer Cell Int. 2019, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Fan, X.; Zhang, P.; Wang, P.; Cheng, S.; Zhang, J. MicroRNA-125b as a tumor suppressor by targeting MMP11 in breast cancer. Thorac. Cancer 2020, 11, 1613–1620. [Google Scholar] [CrossRef]
- Tang, C.; Wu, Y.; Wang, X.; Chen, K.; Tang, Z.; Guo, X. LncRNA MAFG-AS1 regulates miR-125b-5p/SphK1 axis to promote the proliferation, migration, and invasion of bladder cancer cells. Hum. Cell 2021, 34, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 565–592. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.L.; Li, X.F.; Tang, Y.C.; Zhao, X. miR-212-3p reduced proliferation, and promoted apoptosis of fibroblast-like synoviocytes via down-regulating SOX5 in rheumatoid arthritis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 461–471. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Zhang, P.; Li, Q.; Liang, X.; Liu, J. MiR-212-3p inhibits LPS-induced inflammatory response through targeting HMGB1 in murine macrophages. Exp. Cell Res. 2017, 350, 318–326. [Google Scholar] [CrossRef]
- Ohlsson Teague, E.M.C.; Print, C.G.; Hull, M.L. The role of microRNAs in endometriosis and associated reproductive conditions. Hum. Reprod. Update 2009, 16, 142–165. [Google Scholar] [CrossRef]
- Vanhie, A.; Caron, E.; Vermeersch, E.; O, D.; Tomassetti, C.; Meuleman, C.; Mestdagh, P.; D’Hooghe, T.M. Circulating microRNAs as Non-Invasive Biomarkers in Endometriosis Diagnosis—A Systematic Review. Biomedicines 2024, 12, 888. [Google Scholar] [CrossRef]
- Andreas, E.; Pandey, H.O.; Hoelker, M.; Salilew-Wondim, D.; Gebremedhn, S.; Schellander, K.; Tesfaye, D. The regulatory role of miR-20a in bovine cumulus cells and its contribution to oocyte maturation. Zygote 2021, 29, 435–444. [Google Scholar] [CrossRef]
- Andreas, E.; Hoelker, M.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D.; Salilew-Wondim, D. MicroRNA 17-92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting PTEN and BMPR2 genes. Cell Tissue Res. 2016, 366, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Han, Y.; Zhu, D.; Li, Z.; Shan, S.; Jin, W.; Lu, Q.; Ren, T. miR-145 and miR-497 suppress TGF-β-induced epithelial–mesenchymal transition of non-small cell lung cancer by targeting MTDH. Cancer Cell Int. 2018, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Zou, Y.; Dai, D.Q. MicroRNA-320a suppresses tumor progression by targeting PBX3 in gastric cancer and is downregulated by DNA methylation. World J. Gastrointest. Oncol. 2019, 11, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Sharma, U.; Barwal, T.S.; Seam, R.K.; Gupta, M.; Rana, M.K.; Vasquez, K.M.; Jain, A. Circulating miR-320a Acts as a Tumor Suppressor and Prognostic Factor in Non-small Cell Lung Cancer. Front. Oncol. 2021, 11, 645475. [Google Scholar] [CrossRef]
- De-Ugarte, L.; Balcells, S.; Guerri-Fernandez, R.; Grinberg, D.; Diez-Perez, A.; Nogues, X.; Garcia-Giralt, N. Effect of the Tumor Suppressor miR-320a on Viability and Functionality of Human Osteosarcoma Cell Lines Compared to Primary Osteoblasts. Appl. Sci. 2020, 10, 2852. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Keijser, R.; Repping, S.; Lambalk, C.B.; Afink, G.B.; Mastenbroek, S.; Hamer, G. High-quality human preimplantation embryos stimulate endometrial stromal cell migration via secretion of microRNA hsa-miR-320a. Hum. Reprod. 2020, 35, 1797–1807. [Google Scholar] [CrossRef]
- Han, M.; Park, S.B.; Park, B.J. Lower growth factor expression in follicular fluid undergone in-vitro fertilization. Clin. Exp. Reprod. Med. 2011, 38, 210–215. [Google Scholar] [CrossRef]
- Ong, J.; Woldhuis, R.R.; Boudewijn, I.M.; van den Berg, A.; Kluiver, J.; Kok, K.; Terpstra, M.M.; Guryev, V.; de Vries, M.; Vermeulen, C.J.; et al. Age-related gene and miRNA expression changes in airways of healthy individuals. Sci. Rep. 2019, 9, 3765. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H., 3rd; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T.; Yeste, M. The Expression of miRNAs in Human Ovaries, Oocytes, Extracellular Vesicles, and Early Embryos: A Systematic Review. Cells 2019, 8, 1564. [Google Scholar] [CrossRef]
- Abbara, A.; Vuong, L.N.; Ho, V.N.A.; Clarke, S.A.; Jeffers, L.; Comninos, A.N.; Salim, R.; Ho, T.M.; Kelsey, T.W.; Trew, G.H.; et al. Follicle Size on Day of Trigger Most Likely to Yield a Mature Oocyte. Front. Endocrinol. 2018, 9, 193. [Google Scholar] [CrossRef]
- Calhaz-Jorge, C. Orientações Técnicas em Medicina da Reprodução; Sociedade Portuguesa de Medicina da Reprodução: Coimbra, Portugal, 2012. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing--when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]
| miRNA | Role in Endometriosis-Related Infertility | Reference(s) |
|---|---|---|
| miR-125b-5p | Upregulated in endometriosis. Implicated in lesion invasion, inflammation, and alterations of the oocyte/follicular environment. Pathways: PI3K/Akt, MAPK, and inflammation-related signalling. | [10,23,24,40] |
| miR-212-3p | Higher levels are associated with improved embryo development. Pathways: Regulation of folliculogenesis and oocyte competence (specific targets not consistently identified). | [25,26] |
| miR-320a_1 | Upregulated in serum exosomes of endometriosis patients. Associated with oocyte and embryo quality and IVF outcomes. Lower or altered expression linked to poorer embryo development. Pathways: Regulation of energy metabolism, oxidative stress, and embryo development genes. | [26,29,44] |
| miR-199a_1 | Upregulated in serum of endometriosis patients. Considered in the EFI and related to disease severity. Lower levels correlate with worse EFI and poor fertility prognosis. Pathways: ECM remodelling, inflammation, and lesion invasiveness. | [40,41,42] |
| miR-20a_1 | Dysregulated in endometriosis. Associated with disease recurrence and lesion biology. Influences implantation and follicular function, impacting fertility. Pathways: TGF-β signalling, cell proliferation, and angiogenesis. | [35,36,37] |
| miR-145_1 | Downregulated in endometriosis (serum and lesions). Functional studies show regulation of granulosa cell proliferation. Impacts follicular cell behaviour, potentially reducing oocyte quality and ovarian function. Pathways: Cell-cycle regulation and expression of cytoskeletal-related genes. | [38,39] |
| Age (Years) | Body Mass Index (Kg/m2) | Parity (Number of Live Births/% of Women) | Type A embryos at 3rd day (Average Number) | 3rd Day Transfer (% of Cases) | 5th Day Transfer (% of Cases) | |
|---|---|---|---|---|---|---|
| Male factor (n = 34) | 26–42 | 23.5 | 0/88 1/8 2/4 | 1.81 | 47.1 | 52.9 |
| Endometriosis (n = 15) | 27–41 | 21.9 | 0/67 1/33 | 2.63 | 46.7 | 53.3 |
| MiRNA | Reference |
|---|---|
| RNU5G | hsa_miR-RNU5G miRCURY LNA miRNA PCR Assay (YP00203908) |
| miR-125b-5p | hsa_miR-125b-5p miRCURY LNA miRNA PCR Assay (YP00205713) |
| miR-212-3p_1 | Mm_miR-212-3p_1 miScript Primer Assay (MS00024570) |
| miR-20a_1 | Hs_miR-20a_1 miScript Primer Assay (MS00003199) |
| miR-199a_1 | Hs_miR-199a_1 miScript Primer Assay (MS0006741) |
| miR-145_1 | Hs_miR-145_1 miScript Primer Assay (MS00003528) |
| miR-320a_1 | Hs_miR-320a_1 miScript Primer Assay (MS00014707) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.C.; Freitas, C.; Ribeiro, Â.; Rodrigues, A.R.; Silva-Carvalho, J.L.; Almeida, H.; Neves, D. Identification of Proteins and MicroRNAs with Prognostic Value for Assisted Reproduction Technology Outcomes in Follicular Fluid of Women with Endometriosis: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 9752. https://doi.org/10.3390/ijms26199752
Neto AC, Freitas C, Ribeiro Â, Rodrigues AR, Silva-Carvalho JL, Almeida H, Neves D. Identification of Proteins and MicroRNAs with Prognostic Value for Assisted Reproduction Technology Outcomes in Follicular Fluid of Women with Endometriosis: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(19):9752. https://doi.org/10.3390/ijms26199752
Chicago/Turabian StyleNeto, Ana Catarina, Cláudia Freitas, Ângela Ribeiro, Adriana R. Rodrigues, João L. Silva-Carvalho, Henrique Almeida, and Delminda Neves. 2025. "Identification of Proteins and MicroRNAs with Prognostic Value for Assisted Reproduction Technology Outcomes in Follicular Fluid of Women with Endometriosis: A Pilot Study" International Journal of Molecular Sciences 26, no. 19: 9752. https://doi.org/10.3390/ijms26199752
APA StyleNeto, A. C., Freitas, C., Ribeiro, Â., Rodrigues, A. R., Silva-Carvalho, J. L., Almeida, H., & Neves, D. (2025). Identification of Proteins and MicroRNAs with Prognostic Value for Assisted Reproduction Technology Outcomes in Follicular Fluid of Women with Endometriosis: A Pilot Study. International Journal of Molecular Sciences, 26(19), 9752. https://doi.org/10.3390/ijms26199752







