Expansion of the Phenotypic Spectrum of MNGIE: Lipodystrophy and Metabolic Alterations Associated with a p.Arg393_Val400dup TYMP Variant
Abstract
1. Introduction
2. Results
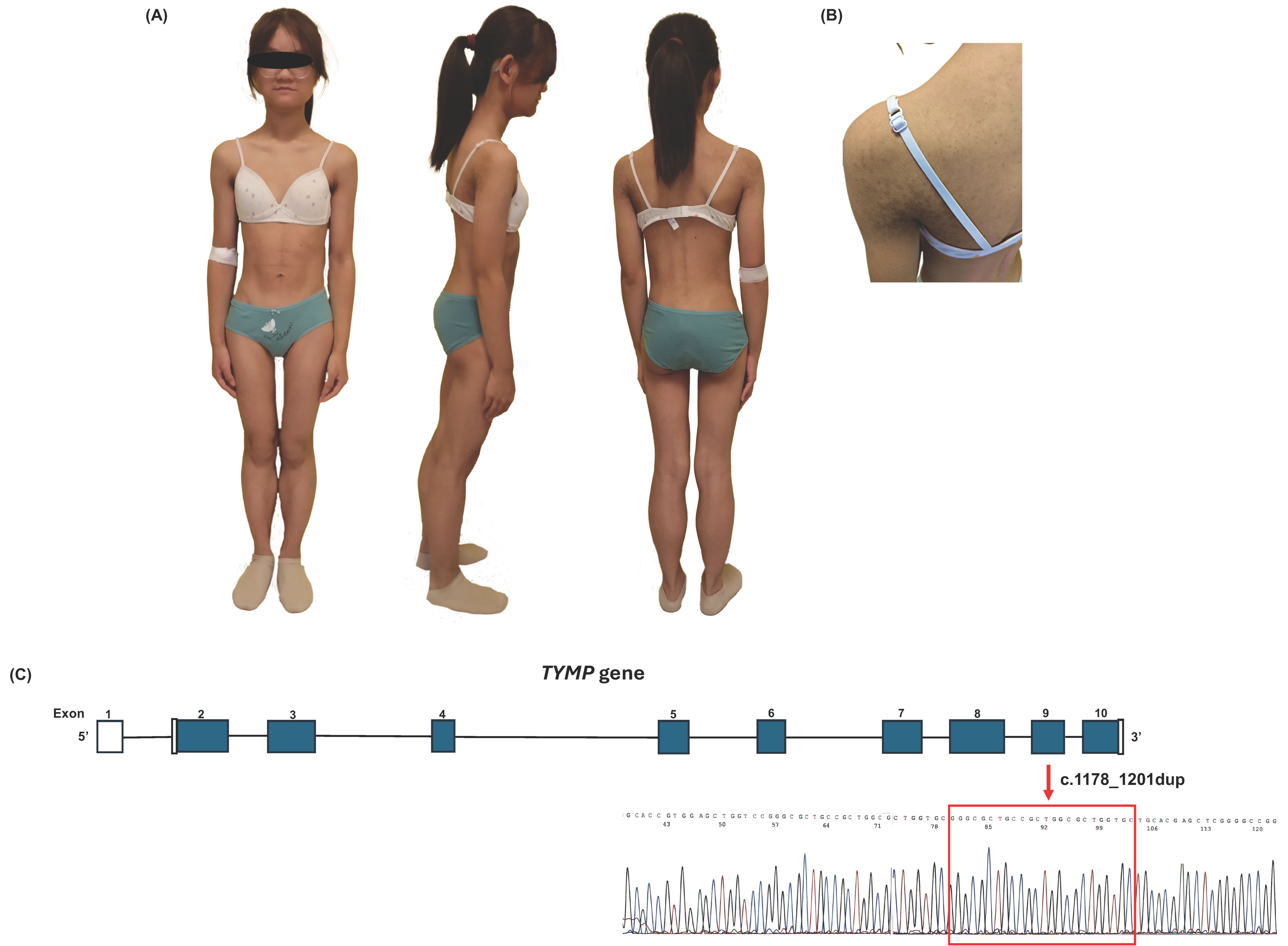
2.1. Case Description
2.2. Genetic Analysis
3. Discussion
4. Materials and Methods
4.1. Anthropometric Measurements
4.2. Biochemistry and Hormones
4.3. Hepatic and Cardiac Evaluation
4.4. Genetic Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacitti, D.; Levene, M.; Garone, C.; Nirmalananthan, N.; Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Into the Fourth Decade, What We Have Learned So Far. Front. Genet. 2018, 9, 669. [Google Scholar] [CrossRef]
- Spinazzola, A.; Marti, R.; Nishino, I.; Andreu, A.L.; Naini, A.; Tadesse, S.; Pela, I.; Zammarchi, E.; Donati, M.A.; Oliver, J.A.; et al. Altered thymidine metabolism due to defects of thymidine phosphorylase. J. Biol. Chem. 2002, 277, 4128–4133. [Google Scholar] [CrossRef]
- González-Vioque, E.; Torres-Torronteras, J.; Andreu, A.L.; Martí, R. Limited dCTP availability accounts for mitochondrial DNA depletion in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). PLoS Genet. 2011, 7, e1002035. [Google Scholar] [CrossRef]
- Hirano, M.; Carelli, V.; De Giorgio, R.; Pironi, L.; Accarino, A.; Cenacchi, G.; D’Alessandro, R.; Filosto, M.; Martí, R.; Nonino, F.; et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Position paper on diagnosis, prognosis, and treatment by the MNGIE International Network. J. Inherit. Metab. Dis. 2021, 44, 376–387. [Google Scholar] [CrossRef]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T.; Dunger, D.; Garg, A.; Jack, M.; Mungai, L.; Oral, E.A.; Patni, N.; Rother, K.I.; et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Santini, F. Diagnosis and treatment of lipodystrophy: A step-by-step approach. J. Endocrinol. Invest. 2019, 42, 61–73. [Google Scholar] [CrossRef]
- Garg, A. Acquired and inherited lipodystrophies. N. Engl. J. Med. 2004, 350, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Jéru, I. Genetics of lipodystrophy syndromes. Presse Méd. 2021, 50, 104074. [Google Scholar] [CrossRef]
- Sawyer, S.L.; Cheuk-Him Ng, A.; Innes, A.M.; Wagner, J.D.; Dyment, D.A.; Tetreault, M.; Care4Rare Canada Consortium; Majewski, J.; Boycott, K.M.; Screaton, R.A.; et al. Homozygous mutations in MFN2 cause multiple symmetric lipomatosis associated with neuropathy. Hum. Mol. Genet. 2015, 24, 5109–5114. [Google Scholar] [CrossRef]
- Elouej, S.; Harhouri, K.; Le Mao, M.; Baujat, G.; Nampoothiri, S.; Kayserili, H.; Al Menabawy, N.; Selim, L.; Paneque, A.L.; Kubisch, C.; et al. Loss of MTX2 causes mandibuloacral dysplasia and links mitochondrial dysfunction to altered nuclear morphology. Nat. Commun. 2020, 11, 4589, Erratum in Nat. Commun. 2020, 11, 5349. [Google Scholar] [CrossRef]
- Bourne, S.C.; Townsend, K.N.; Shyr, C.; Matthews, A.; Lear, S.A.; Attariwala, R.; Lehman, A.; Wasserman, W.W.; van Karnebeek, C.; Sinclair, G.; et al. Optic atrophy, cataracts, lipodystrophy/lipoatrophy, and peripheral neuropathy caused by a de novo OPA3 mutation. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001156. [Google Scholar] [CrossRef]
- Rocha, N.; Bulger, D.A.; Frontini, A.; Titheradge, H.; Gribsholt, S.B.; Knox, R.; Page, M.; Harris, J.; Payne, F.; Adams, C.; et al. Human biallelic MFN2 mutations induce mitochondrial dysfunction, upper body adipose hyperplasia, and suppression of leptin expression. eLife 2017, 6, e23813. [Google Scholar] [CrossRef]
- Capel, E.; Vatier, C.; Cervera, P.; Stojkovic, T.; Disse, E.; Cottereau, A.-S.; Auclair, M.; Verpont, M.-C.; Mosbah, H.; Gourdy, P.; et al. MFN2-associated lipomatosis: Clinical spectrum and impact on adipose tissue. J. Clin. Lipidol. 2018, 12, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Gautheron, J.; Lima, L.; Akinci, B.; Zammouri, J.; Auclair, M.; Ucar, S.K.; Ozen, S.; Altay, C.; Bax, B.E.; Nemazanyy, I.; et al. Loss of thymidine phosphorylase activity disrupts adipocyte differentiation and induces insulin-resistant lipoatrophic diabetes. BMC Med. 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Wang, J.; Wang, Y.L.; Fan, J.-J.; Mo, G.-L.; Gong, F.-Y.; Chai, Z.-M.; Zhang, J.; Meng, H.-X.; Li, C.-X.; et al. A novel thymidine phosphorylase mutation in a Chinese MNGIE patient. Acta Neurol. Belg. 2017, 117, 259–267. [Google Scholar] [CrossRef]
- Li, H.; Ji, C.Y.; Zong, X.N.; Zhang, Y.Q. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. 2009, 47, 487–492. [Google Scholar]
- Li, H.; Ji, C.Y.; Zong, X.N.; Zhang, Y.Q. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. 2009, 47, 493–498. [Google Scholar]
- Haque, W.A.; Shimomura, I.; Matsuzawa, Y.; Garg, A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 2002, 87, 2395. [Google Scholar] [CrossRef]
- Ceccarini, G.; Pelosini, C.; Paoli, M.; Tyutyusheva, N.; Magno, S.; Gilio, D.; Palladino, L.; Sessa, M.R.; Bertelloni, S.; Santini, F. Serum levels of adiponectin differentiate generalized lipodystrophies from anorexia nervosa. J. Endocrinol. Invest. 2024, 47, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Spinazzola, A.; Hirano, M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999, 283, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Garone, C.; Tadesse, S.; Hirano, M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain 2011, 134, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.; Pagan, C.; Hardy, G.; Besson, G.; Lombès, A. MyoNeuroGastroIntestinal Encephalopathy: Natural History and Means for Early Diagnosis. Gastroenterology 2019, 156, 1525–1527.e4. [Google Scholar] [CrossRef]
- Valentino, M.L.; Martí, R.; Tadesse, S.; López, L.C.; Manes, J.L.; Lyzak, J.; Hahn, A.; Carelli, V.; Hirano, M. Thymidine and deoxyuridine accumulate in tissues of patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). FEBS Lett. 2007, 581, 3410–3414. [Google Scholar] [CrossRef]
- Martí, R.; Nishigaki, Y.; Hirano, M. Elevated plasma deoxyuridine in patients with thymidine phosphorylase deficiency. Biochem. Biophys. Res. Commun. 2003, 303, 14–18. [Google Scholar] [CrossRef]
- Ferraro, P.; Pontarin, G.; Crocco, L.; Fabris, S.; Reichard, P.; Bianchi, V. Mitochondrial deoxynucleotide pools in quiescent fibroblasts: A possible model for mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). J. Biol. Chem. 2005, 280, 24472–24480. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E. Mitochondrial neurogastrointestinal encephalomyopathy: Approaches to diagnosis and treatment. J. Transl. Genet. Genom. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Giordano, C.; Sebastiani, M.; De Giorgio, R.; Travaglini, C.; Tancredi, A.; Valentino, M.L.; Bellan, M.; Cossarizza, A.; Hirano, M.; d’Amati, G. Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am. J. Pathol. 2008, 173, 1120–1128. [Google Scholar] [CrossRef]
- Hirano, M.; Silvestri, G.; Blake, D.M.; Lombes, A.; Minetti, C.; Bonilla, E.; Hays, A.P.; Lovelace, R.E.; Butler, I.; Bertorini, T.E.; et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology 1994, 44, 721–727. [Google Scholar] [CrossRef]
- Martí, R.; Verschuuren, J.J.; Buchman, A.; Hirano, I.; Tadesse, S.; van Kuilenburg, A.B.P.; van Gennip, A.H.; Poorthuis, B.J.H.M.; Hirano, M. Late-onset MNGIE due to partial loss of thymidine phosphorylase activity. Ann. Neurol. 2005, 58, 649–652. [Google Scholar] [CrossRef]
- Mazat, J.P.; Rossignol, R.; Malgat, M.; Rocher, C.; Faustin, B.; Letellier, T. What do mitochondrial diseases teach us about normal mitochondrial functions...that we already knew: Threshold expression of mitochondrial defects. Biochim. Biophys. Acta. 2001, 1504, 20–30. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Martí, R.; Copeland, W.C.; Hirano, M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J. Clin. Invest. 2003, 111, 1913–1921. [Google Scholar] [CrossRef]
- Demaria, F.; De Crescenzo, F.; Caramadre, A.M.; D’Amico, A.; Diamanti, A.; Fattori, F.; Casini, M.P.; Vicari, S. Mitochondrial Neurogastrointestinal Encephalomyopathy Presenting as Anorexia Nervosa. J. Adolesc. Health 2016, 59, 729–731. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Wang, F.; Wu, D.; Qian, J.; Kang, J.; Li, H.; Ma, E. Nutrition Therapy for Mitochondrial Neurogastrointestinal Encephalopathy with Homozygous Mutation of the TYMP Gene. Clin. Nutr. Res. 2015, 4, 132–136. [Google Scholar] [CrossRef]
- Bariş, Z.; Eminoğlu, T.; Dalgiç, B.; Tümer, L.; Hasanoğlu, A. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Case report with a new mutation. Eur. J. Pediatr. 2010, 169, 1375–1378. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Schranz, M.; Bösch, S.; Karall, D.; Bürgi, S.S.; Ensinger, C.; Drach, M.; Mayr, J.A.; Janecke, A.R.; Vogel, W.; et al. MNGIE Syndrome: Liver Cirrhosis Should Be Ruled Out Prior to Bone Marrow Transplantation. JIMD Rep. 2013, 10, 41–44. [Google Scholar] [CrossRef]
- Suh, B.C.; Jeong, H.N.; Yoon, B.S.; Park, J.H.; Kim, H.J.; Park, S.W.; Hwang, J.H.; Choi, B.-O.; Chung, K.W. Compound heterozygous mutations of TYMP as underlying causes of mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). Mol. Med. Rep. 2013, 8, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hanbali, A.; Rasheed, W.; Peedikayil, M.C.; Boholega, S.; Alzahrani, H.A. Mitochondrial Neurogastrointestinal Encephalomyopathy Syndrome Treated with Stem Cell Transplant: A Case Series and Literature Review. Exp. Clin. Transplant. 2018, 16, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Slama, A.; Lacroix, C.; Plante-Bordeneuve, V.; Lombès, A.; Conti, M.; Reimund, J.M.; Auxenfants, E.; Crenn, P.; Laforêt, P.; Joannard, A.; et al. Thymidine phosphorylase gene mutations in patients with mitochondrial neurogastrointestinal encephalomyopathy syndrome. Mol. Genet. Metab. 2005, 84, 326–331. [Google Scholar] [CrossRef]
- Filosto, M.; Scarpelli, M.; Tonin, P.; Testi, S.; Cotelli, M.S.; Rossi, M.; Salvi, A.; Grottolo, A.; Vielmi, V.; Todeschini, A.; et al. Pitfalls in diagnosing mitochondrial neurogastrointestinal encephalomyopathy. J. Inherit. Metab. Dis. 2011, 34, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, Y.; Marti, R.; Hirano, M. ND5 is a hot-spot for multiple atypical mitochondrial DNA deletions in mitochondrial neurogastrointestinal encephalomyopathy. Hum. Mol. Genet. 2004, 13, 91–101. [Google Scholar] [CrossRef]
- Carrière, A.; Carmona, M.C.; Fernandez, Y.; Rigoulet, M.; Wenger, R.H.; Pénicaud, L.; Casteilla, L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: A mechanism for hypoxia-dependent effect. J. Biol. Chem. 2004, 279, 40462–40469. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chande, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Leloup, C.; Casteilla, L.; Carrière, A.; Galinier, A.; Benani, A.; Carneiro, L.; Pénicaud, L. Balancing mitochondrial redox signaling: A key point in metabolic regulation. Antioxid. Redox Signal. 2011, 14, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Monickaraj, F.; Aravind, S.; Nandhini, P.; Prabu, P.; Sathishkumar, C.; Mohan, V.; Balasubramanyam, M. Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. J. Biosci. 2013, 38, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Halter, J.P.; Michael, W.; Schüpbach, M.; Mandel, H.; Casali, C.; Orchard, K.; Collin, M.; Valcarcel, D.; Rovelli, A.; Filosto, M.; et al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain 2015, 138, 2847–2858. [Google Scholar] [CrossRef]
- Zaidman, I.; Elhasid, R.; Gefen, A.; Dovrat, A.Y.; Mutaz, S.; Shaoul, R.; Adiv, O.E.; Mandel, H.; Tal, G. Hematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalopathy: A single-center experience underscoring the multiple factors involved in the prognosis. Pediatr. Blood Cancer 2021, 68, e28926. [Google Scholar] [CrossRef]
- Paisiou, A.; Rogalidou, M.; Pons, R.; Ioannidou, E.; Dimakou, K.; Papadopoulou, A.; Vaz, F.M.; Vessalas, G.; Goorden, S.M.I.; Roelofsen, J.; et al. Mitochondrial neurogastrointestinal encephalomyopathy: Clinical and biochemical impact of allogeneic stem cell transplantation in a Greek patient with one novel TYMP mutation. Mol. Genet. Metab. Rep. 2022, 30, 100829. [Google Scholar] [CrossRef]
- D’Angelo, R.; Boschetti, E.; Amore, G.; Costa, R.; Pugliese, A.; Caporali, L.; Gramegna, L.L.; Papa, V.; Vizioli, L.; Capristo, M.; et al. Liver transplantation in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Clinical long-term follow-up and pathogenic implications. J. Neurol. 2020, 267, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Pironi, L.; Rinaldi, R.; Boschetti, E.; Caporali, L.; Capristo, M.; Casali, C.; Cenacchi, G.; Contin, M.; D’Angelo, R.; et al. Liver transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Ann. Neurol. 2016, 80, 448–455. [Google Scholar] [CrossRef]
- Kripps, K.; Nakayuenyongsuk, W.; Shayota, B.J.; Berquist, W.; Gomez-Ospina, N.; Esquivel, C.O.; Concepcion, W.; Sampson, J.B.; Cristin, D.J.; Jackson, W.E.; et al. Successful liver transplantation in mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). Mol. Gene.t Metab. 2020, 130, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Filosto, M.; Scarpelli, M.; Tonin, P.; Lucchini, G.; Pavan, F.; Santus, F.; Parini, R.; Donati, M.A.; Cotelli, M.S.; Vielmi, V.; et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J. Neurol. 2012, 259, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, L.L.; Pisano, A.; Testa, C.; Manners, D.N.; D’Angelo, R.; Boschetti, E.; Giancola, F.; Pironi, L.; Caporali, L.; Capristo, M.; et al. Cerebral Mitochondrial Microangiopathy Leads to Leukoencephalopathy in Mitochondrial Neurogastrointestinal Encephalopathy. Am. J. Neuroradiol. 2018, 39, 427–434. [Google Scholar] [CrossRef]
- Boschetti, E.; Caporali, L.; D’Angelo, R.; Malagelada, C.; Accarino, A.; Dotti, M.T.; Costa, R.; Cenacchi, G.; Pironi, L.; Rinaldi, R.; et al. Anatomical Laser Microdissection of the Ileum Reveals mtDNA Depletion Recovery in A Mitochondrial Neuro-Gastrointestinal Encephalomyopathy (MNGIE) Patient Receiving Liver Transplant. Int. J. Mol. Sci. 2022, 23, 8792. [Google Scholar] [CrossRef] [PubMed]

| Patient 1 (Index Case) | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Current Study | [14] | [14] | [14] | |
| Sex | F | F | M | F |
| TYMP variant | c.1178_1201dup, HO | c.647-1G>A, HO | c.647-1G>A, HO | c.392C>T, HO |
| Age at report (y) | 16 | Deceased at the age of 24 | 27 | 20 |
| Height (m) | 1.55 | 1.63 | 1.70 | 1.60 |
| Weight (kg) | 37 | 34 | 53 | 37.5 |
| BMI (kg/m2) | 15.4 | 12.8 | 18.3 | 14.6 |
| Lipodystrophy | Generalized | Generalized | Generalized | Generalized |
| Hypermuscular appearance | Yes | No | Yes | Yes |
| Acanthosis nigricans | Yes | Yes | Yes | Yes |
| Liver steatosis | Yes | Yes | Yes | Yes |
| Hypertriglyceridemia | Yes | Yes | Yes | Yes |
| Diabetes/IR | Yes | Yes | No | Yes |
| Hirsutism | Yes | Yes | NA | Yes |
| Menstrual abnormalities | Yes, hypermenorrhea | Yes, amenorrhea | NA | Yes, amenorrhea |
| Neurological signs | Yes, motor peripheral neuropathy, leukoencephalopathy | Yes, demyelinating sensory motor peripheral neuropathy, electromyogram abnormalities, leukoencephalopathy, ptosis, muscular atrophy | Yes, leukoencephalopathy | Yes, demyelinating sensory motor peripheral neuropathy, electromyogram abnormalities, leukoencephalopathy |
| Gastrointestinal signs | No | Yes, gastroparesis, abdominal pain | No | Yes, gastroparesis, abdominal pain, diarrhea |
| Whole body fat (%)-DXA | 16.9 | 9.6 | 8.4 | NR |
| Arm fat (%)-DXA | 21.4 | 9.9 | 6.95 | NR |
| Leg fat (%)-DXA | 19.4 | 8.7 | 5.5 | NR |
| Truncal fat (%)-DXA | 12.3 | 8.1 | 8.5 | NR |
| Patient 1 (Index Case) | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Current Study | [14] | [14] | [14] | |
| Fasting glucose, mmol/L | 3.11 (3.3–5.5) | 5.2 (4.1–6.1) | 4.2 (4.1–6.1) | 14.2 (4.1–6.1) |
| 2h-OGTT glucose, mmol/L | 7.94 (≤7.8) | 13 (≤7.8) | 10.9 (≤7.8) | NR |
| Fasting insulin, pmol/L | 37 (<160) | 358.3 (<70) | 519.4 (<70) | 530.6 (<70) |
| HbA1c, (%) | 5.2 (<6) | 5 (<6) | 4.9 (<6) | 8.4 (<6) |
| AST, IU/L | 27 (<40) | 142 (<40) | 100 (<40) | 50 (<40) |
| ALT, IU/L | 45 (<40) | 52 (<40) | 134 (<40) | 40 (<40) |
| γGT, IU/L | 33 (<36) | 77 (8–44) | 65 (8–44) | 142 (8–44) |
| Total cholesterol, mmol/L | 3.72 (<5.17) | NR | NR | NR |
| LDL-cholesterol, mmol/L | 2.17 (<2.97) | NR | NR | NR |
| HDL-cholesterol, mmol/L | 0.90 (>1.16) | 0.77 (>1) | 0.65 (>1) | 0.33 (>1) |
| Triglycerides, mmol/L | 1.95 (<1.69) | 7.6 (<1.70) | 2.2 (<1.70) | 28.1 (<1.70) |
| LDH, IU/L | 228 (135–214) | 325 (120–246) | 256 (120–246) | 212 (98–192) |
| Leptin, ng/mL | 2.5 | 1.9 | 0.5 | 0.53 |
| Adiponectin, mg/L | 0.2 | <0.01 | 0.37 | NR |
| Criterion | Description (ACMG/AMP 2015) | Evidence in Our Case |
|---|---|---|
| PM2 | Absent from population databases | Variant not reported in gnomAD, dbSNP, or 1000 Genomes Project |
| PM4 | Protein length changes due to in-frame insertion/duplication in a non-repeat region | In-frame duplication within a conserved C-terminal region critical for thymidine phosphorylase function |
| PP1 | Co-segregation with disease in multiple affected family members in a gene definitively known to cause the disease | Variant found in heterozygous state in the mother (asymptomatic carrier); paternal testing not possible |
| PP3 | Multiple computational predictions support a deleterious effect | In silico analyses (PredictProtein in prior report [15]) indicate structural disruption of thymidine phosphorylase |
| PP4 | Patient’s phenotype highly specific for a disease with a single genetic etiology | Clinical and biochemical features fully consistent with TYMP-related disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilio, D.; Pelosini, C.; Magno, S.; Venanzi, J.M.; Daniotti, M.; Paoli, M.; Palladino, L.; Sessa, M.R.; Ricci, F.; Procopio, E.; et al. Expansion of the Phenotypic Spectrum of MNGIE: Lipodystrophy and Metabolic Alterations Associated with a p.Arg393_Val400dup TYMP Variant. Int. J. Mol. Sci. 2025, 26, 9751. https://doi.org/10.3390/ijms26199751
Gilio D, Pelosini C, Magno S, Venanzi JM, Daniotti M, Paoli M, Palladino L, Sessa MR, Ricci F, Procopio E, et al. Expansion of the Phenotypic Spectrum of MNGIE: Lipodystrophy and Metabolic Alterations Associated with a p.Arg393_Val400dup TYMP Variant. International Journal of Molecular Sciences. 2025; 26(19):9751. https://doi.org/10.3390/ijms26199751
Chicago/Turabian StyleGilio, Donatella, Caterina Pelosini, Silvia Magno, Jacopo Maria Venanzi, Marta Daniotti, Melania Paoli, Lavinia Palladino, Maria Rita Sessa, Franco Ricci, Elena Procopio, and et al. 2025. "Expansion of the Phenotypic Spectrum of MNGIE: Lipodystrophy and Metabolic Alterations Associated with a p.Arg393_Val400dup TYMP Variant" International Journal of Molecular Sciences 26, no. 19: 9751. https://doi.org/10.3390/ijms26199751
APA StyleGilio, D., Pelosini, C., Magno, S., Venanzi, J. M., Daniotti, M., Paoli, M., Palladino, L., Sessa, M. R., Ricci, F., Procopio, E., Ceccarini, G., & Santini, F. (2025). Expansion of the Phenotypic Spectrum of MNGIE: Lipodystrophy and Metabolic Alterations Associated with a p.Arg393_Val400dup TYMP Variant. International Journal of Molecular Sciences, 26(19), 9751. https://doi.org/10.3390/ijms26199751






