Abstract
The advent of effective antiretroviral therapy in the mid-1990s, which successfully prevented the progression to AIDS in people living with HIV (PLWH), was associated with the appearance of the so-called HIV-associated lipodystrophy. This condition involved subcutaneous fat atrophy; abdominal fat hypertrophy; and, in some cases, lipomatosis. It was also associated with systemic metabolic disturbances, primarily insulin resistance and dyslipidemia. Following the replacement of certain antiretroviral drugs, particularly the thymidine-analog reverse transcriptase inhibitors stavudine and zidovudine, with less toxic alternatives, the incidences of lipoatrophy and lipomatosis significantly declined. However, lipodystrophy resulting from first-generation antiretroviral therapy does not always resolve after switching to newer agents. Although the widespread use of modern antiretroviral drugs—especially integrase strand transfer inhibitors and non-lipoatrophic reverse transcriptase inhibitors such as tenofovir alafenamide—has reduced the incidences of severe forms of lipodystrophy, these regimens are not entirely free of adipose tissue-related effects. Notably, they are associated with weight gain that resembles common obesity and can have adverse cardiometabolic consequences. Recent evidence also suggests the hypertrophy of specific fat depots, such as epicardial and perivascular adipose tissue, in PLWH on last-generation treatments, potentially contributing to increased cardiovascular risk. This evolving landscape underscores the persistent vulnerability of PLWH to adipose tissue alterations. While these morphological changes may not be as pronounced as those seen in classic HIV-associated lipodystrophy, they can still pose significant health risks. The continued optimization of treatment regimens and the vigilant monitoring of adipose tissue alterations and metabolic status remain essential strategies to improve the health of PLWH.
1. Introduction
The development of effective treatments for human immunodeficiency virus (HIV) infection has been one of the most significant therapeutic successes of recent decades. The expansion of the HIV epidemic in the 1980s led to high mortality rates associated with the immunodeficiency caused by the virus, known as acquired immunodeficiency syndrome (AIDS). This could only be mitigated after the mid-1990s through the development of combined antiretroviral drugs that effectively prevented the progression to AIDS and transformed the disease into a chronic condition. However, it was soon observed that, despite the success in preventing AIDS in treated patients, a complex syndrome involving alterations in the amount and distribution of adipose tissue developed, which came to be called HIV-associated lipodystrophy.
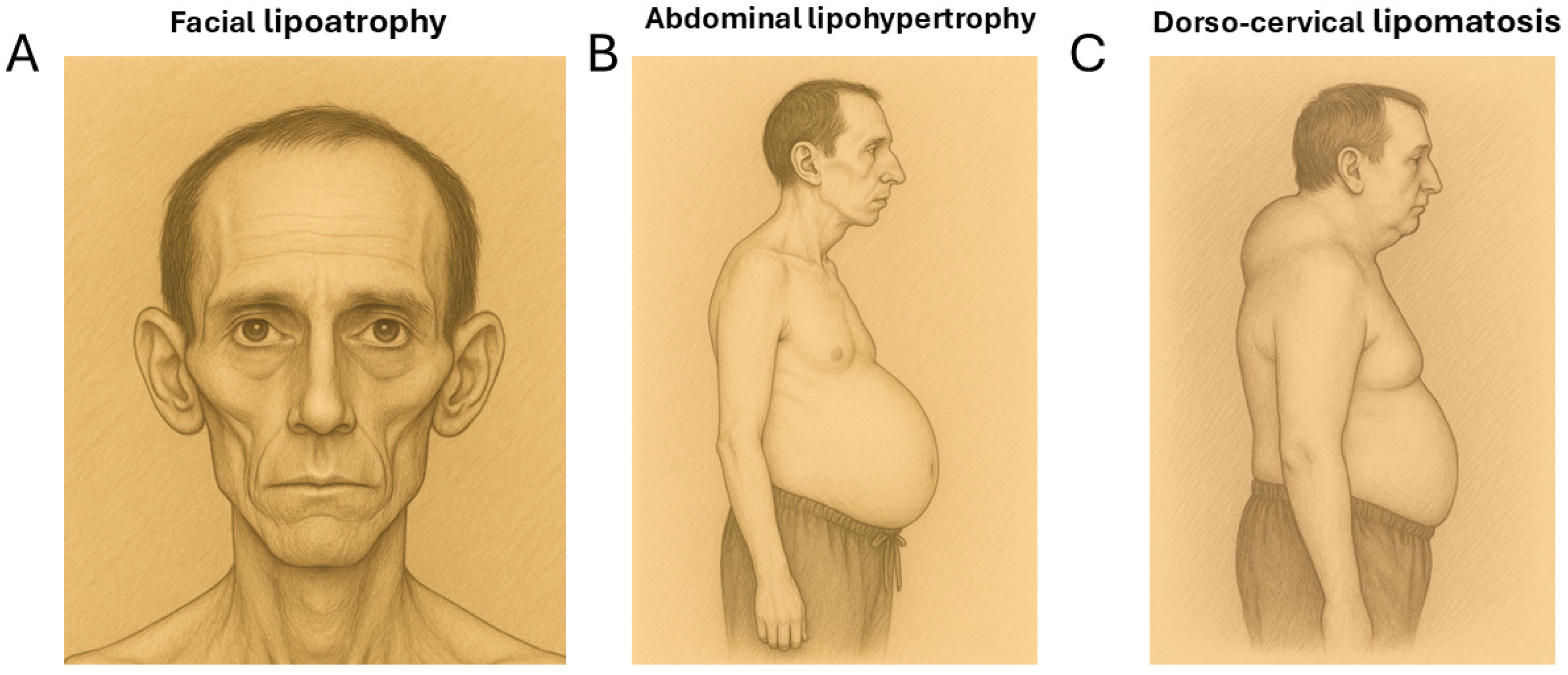
The lipodystrophy observed in people living with HIV (PLWH) is a partial type that has complex manifestations. Firstly, there is subcutaneous fat loss (lipoatrophy) in the face, arms, and legs. Secondly, there is also fat hypertrophy in the abdominal area, the reminiscent of visceral obesity. Lastly, less frequently than the previous phenomena, lipomatous accumulations appear in the dorso-cervical area (commonly called “buffalo humps”) and sometimes appear in other regions. All these changes can occur simultaneously in the same individual, or, at times, patients may predominantly manifest only one type of alteration [1,2,3] (Figure 1).
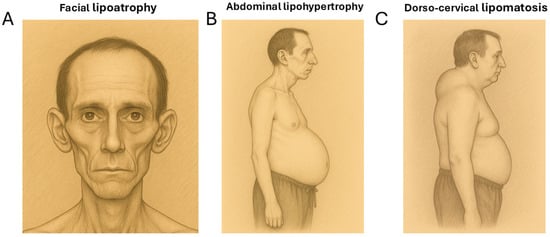
Figure 1.
The pictorial representations of the main features of HIV-associated lipodystrophy. (A) Facial lipoatrophy. (B) Abdominal lipohypertrophy. (C) A lipomatous “buffalo hump” adipose tissue accumulation in the dorso-cervical region.
Morphological changes in the amount and distribution of fat in PLWH are associated with systemic metabolic alterations partly resembling those observed in the genetic forms of partial lipodystrophy. These include insulin resistance, type 2 diabetes, dyslipidemia, hepatic steatosis, and elevated cardiovascular risk [3,4]. In fact, these metabolic disturbances are among the most significant health issues linked to HIV-associated lipodystrophy. However, other factors—such as the stigmatizing effects of facial lipoatrophy—also greatly affect the quality of life of PLWH [5].
HIV-associated lipodystrophy showed a remarkable prevalence until the early 2020s, although the available data on the global prevalence of lipodystrophy (including lipoatrophy and/or lipohypertrophy) vary widely. In studies based on the clinical diagnoses of lipodystrophy, the reported prevalence of HIV-associated lipodystrophy in PLWH receiving combined antiretroviral therapy (cART) that included first-generation drugs ranged from 23% to 70%. This wide variation in prevalence was attributed to differences in study design and the length of follow-up across studies [6]. Subsequently, in the 2020s, thanks to the development of highly effective antiretroviral drugs with minimal toxicity and treatments starting in the early stages post-infection, the occurrence of novel cases of overt lipodystrophy in PLWH has decreased dramatically. However, the persistence of relevant adipose tissue alterations that are not as obvious as overt lipoatrophy or lipomatosis in PLWH and the continued presence of lipodystrophy in PLWH who have been on treatment for many years (see later) remain significant challenges for maintaining a healthy status in PLWH.
2. Causal Agents of Lipodystrophy in PLWH
The causal agents of lipodystrophy in PLWH are multiple and complex, involving both the effects of antiretroviral treatments and the consequences of the underlying HIV infection. Additionally, it appears that the different manifestations of lipodystrophy (lipoatrophy, lipohypertrophy, and lipomatosis) may have distinct causal factors during their development.
2.1. Lipoatrophy
Regarding lipoatrophy, it historically manifested from the use of the initial antiretroviral therapy, which was based on a combination of three drugs, a protease inhibitor (PI) and two nucleoside reverse transcriptase inhibitors (NRTIs), which were, in most cases, thymidine analogs such as stavudine or zidovudine. It was soon observed that the successful maintenance of CD4 T cell levels in people with HIV was accompanied by marked subcutaneous fat loss, which in many cases could involve losing up to 30% of the fat in the extremities and a noticeable facial fat reduction [7]. It was noted that the presence of thymidine-analog NRTIs (stavudine and zidovudine) during treatment was preferentially associated with the appearance of lipoatrophy [8]. This was later corroborated after the dramatic reduction in lipoatrophy seen when thymidine-analog NRTIs were replaced with other antiretroviral drugs that were developed more recently and have lower toxicity [9]. By the late 2000s, it was recommended to avoid using zidovudine as an NRTI during treatment to prevent lipoatrophy, and the use of tenofovir difumarate (TDF), a newer nucleotide analog inhibiting HIV reverse transcriptase, became widespread. This drug has less lipoatrophic action (but not none) [10,11]. More recently, TDF has been replaced by tenofovir alafenamide (TAF), an analog of TDF devoid of lipoatrophic effects [12]. Additionally, some other antiretroviral drugs previously used to treat PLWH, such as efavirenz, a non-nucleoside inhibitor of reverse transcriptase (NNRTI), have also been shown to exert lipoatrophic actions [12]. There are currently significantly fewer cases of lipoatrophy in PLWH under the current patterns of antiretroviral treatments, although individuals that had been treated with thymidine-analog NRTIs and were later switched to newer treatments have not always achieved the complete reversal of this phenomenon (see more below). Apart from the key role of thymidine-analog NRTIs, various factors influence the propensity to develop lipoatrophy; it occurs more frequently in men than in women, and the infection-related status (low CD4 levels and/or high HIV-1 RNA levels) is also associated with a higher likelihood of developing lipoatrophy [6].
2.2. Lipohypertrophy
Lipohypertrophy in the abdominal area is another common manifestation in PLWH, and it concerns intra-abdominal visceral adipose tissue accumulation. In principle, it is unclear to what extent such alterations have any differential features distinct from abdominal obesity occurring in the general population. Trunk lipohypertrophy was primarily attributed to the presence of PIs as part of the combinatorial antiretroviral treatment patterns. Thus, in the absence of thymidine-based NRTI drugs, the presence of PIs in antiretroviral treatment patterns was shown to increase trunk adiposity and associated metabolic alterations in PLWH, similar to what happens in common visceral obesity [13,14].
2.3. Lipomatosis
A third phenomenon characteristic of HIV-associated lipodystrophy is lipomatosis in anatomical areas such as the dorso-cervical region, the pubic area, or other locations [15,16]. The fat accumulation in these regions is entirely different from visceral lipohypertrophy, as it occurs in subcutaneous tissue rather than in visceral areas. Additionally, this accumulation results from a lipomatous process caused by cellular alterations in adipocytes that acquire proliferative properties, shown by the enhanced levels of the “proliferating cell nuclear antigen” (PCNA) and decreased telomere length [17]. In addition to cellular and molecular studies demonstrating these proliferative characteristics, the lipomatous features of these fat depots became particularly evident when the use of enlarged dorso-cervical fat to refill facial lipoatrophy had to be discontinued due to persistent adipose expansion in the cheeks [18]. Moreover, dorso-cervical lipomatosis is associated with a distortion of the white/brown phenotype of adipocytes characterized by the expression of typical brown adipose tissue marker genes such as uncoupling protein-1 (UCP1); however, adipose tissue in lipomatous areas does not acquire functional thermogenic properties [19,20].
2.4. Systemic Alterations in HIV-Associated Lipodystrophy
Lipodystrophy in PLWH is associated with important systemic alterations, often including insulin resistance, type 2 diabetes, dyslipidemia, hepatic steatosis, and elevated cardiovascular risk. Various processes associated with HIV-related lipodystrophy contribute to this scenario. Both subcutaneous adipose tissue atrophy and visceral hypertrophy generate local and systemic pro-inflammatory responses [21]. Studies on common obesity have widely indicated that a sustained local pro-inflammatory state in adipose tissue leads to systemic metabolic alterations, such as insulin resistance [22], which is also observed in PLWH affected by lipodystrophy. Alterations in adipose tissue also lead to the disrupted secretion of regulatory adipokines, and abnormally low levels of circulating adiponectin, an anti-inflammatory and insulin-sensitizing factor [23], have long been observed in PLWH [24]. Several in vitro studies have confirmed that first-generation transcriptase inhibitors and PI drugs induce secretion of inflammatory cytokines and suppress the secretion of adiponectin by adipocytes [25,26,27,28]. Various alterations in the leptin levels have been reported in PLWH with lipodystrophy, ranging from abnormally low levels to significantly high levels [29]. This is most likely due to the variable impact on the overall adipose tissue level resulting from the combination of lipoatrophy in subcutaneous areas and lipohypertrophy at the visceral level in each individual.
On the other hand, PLWH with lipodystrophy often exhibit, within the same individual, the harmful consequences of lipotoxicity arising from both the hypotrophy of subcutaneous adipose tissue and the hypertrophy of visceral adipose tissue [30,31]. As is known from the genetic forms of lipoatrophy, an inability to adequately store lipids in adipose tissue promotes ectopic lipid deposition in multiple tissues, impairing their function. A parallel phenomenon occurs as a result of visceral fat hypertrophy; once its expandability limits are reached, lipid spillover into other tissues is also promoted. Thus, an increase in the lipid content in the skeletal muscle of PLWH has been reported, a phenomenon known to contribute to insulin resistance [32]. Hepatic steatosis also frequently appears in PLWH affected by lipodystrophy [33] and may evolve to metabolic dysfunction-associated steatotic liver disease (MASLD). Myocardial lipid accumulation has also been reported in PLWH, even in the absence of abdominal lipohypertrophy [34].
Microbiota dysbiosis, defined as an imbalance in the composition of the gut microbiota, is a well-established contributor to systemic chronic inflammation in obesity, and emerging evidence suggests that similar mechanisms may be present in PLWH. Several studies have reported intestinal dysbiosis and reductions in bacterial alpha diversity in PLWH [35,36]. Notably, bacterial alpha diversity has been shown to correlate with the visceral adipose tissue volume in this population [37]. Furthermore, PLWH exhibit a lower abundance of butyrate-producing bacteria [38], which may impact adipose tissue function, as butyrate is a key short-chain fatty acid known to influence this tissue. Although existing studies are still limited, the specific role of microbiota alterations in driving adipose tissue dysfunction in PLWH and to what extent they affect lipoatrophic and lipohypertrophic alterations in distinct adipose depots remain active areas of investigation.
3. Mechanisms Underlying HIV-Associated Lipodystrophy
3.1. Antiretroviral Treatment-Driven Effects
The cellular and molecular mechanisms underlying lipoatrophy in PLWH have been extensively studied. In affected patients, reductions in the size and accumulation of fat in subcutaneous adipocytes in lipodystrophic areas are observed, along with apoptosis and cell death [39]. This is accompanied by the infiltration of macrophages and other inflammatory cells [40,41].
First-generation NRTI drugs, especially thymidine analogs (e.g., zidovudine and stavudine), play a key role in lipoatrophy in PLWH. This is not the first case in medicine where a lipoatrophic reaction to a pharmacological treatment has been observed. However, the reported cases of subcutaneous lipoatrophy associated with treatments using insulin, growth hormone analogs, or corticosteroids are typically localized at the injection sites and tend to resolve spontaneously [42]. In contrast, the lipoatrophic effect of thymidine-analog NRTIs is systemic and, as previously mentioned, affects multiple subcutaneous adipose tissue depots across different anatomical sites.
The primary action of zidovudine and stavudine as lipoatrophic agents is attributed to the so-called mitochondrial toxicity. These drugs, along with their ability to inhibit HIV reverse transcriptase, have the collateral effect of inhibiting DNA polymerase-gamma, the enzyme responsible for mitochondrial DNA (mtDNA) replication. Treatment with these NRTIs results in the depletion of mtDNA levels in multiple tissues and organs at a range that is lower than that seen in genetic mtDNA depletion diseases but still biologically relevant [43]. The basis for the tissue-specific susceptibility of adipose tissue to this depletion is not well understood. Mitochondrial dysfunction caused by mtDNA depletion leads to energy deficits, the increased production of reactive oxygen species, and the abnormal proliferation of nonfunctional mitochondria, potentially causing adipocyte death. In vitro studies also demonstrate that these NRTIs inhibit the differentiation of preadipocytes into mature adipocytes by blocking the expression of key adipogenesis genes such as peroxisome proliferator-activated-γ (PPARγ) and others [26,44,45]. As a result, subcutaneous adipose tissue experiences dysfunction in replacing dead adipocytes with new ones, creating the context for lipoatrophy. The importance of mtDNA alterations in lipodystrophy has also been evidenced by the observation of different susceptibilities to lipodystrophy among individuals with different mitochondrial DNA haplogroups [46]. The basis for the particular susceptibility of subcutaneous adipose tissue to develop lipodystrophy remains poorly understood. Although studies on this matter are scarce, it has been reported that mtDNA depletion is similar in subcutaneous and visceral adipose tissues from PLWH, despite the latter not showing atrophy [47].
The precise mechanisms of the other main feature of HIV lipodystrophy, abdominal lipohypertrophy, also have not been fully delineated. The well-known distinct properties of visceral and subcutaneous adipose tissue may involve differential responsiveness to HIV infection and antiretroviral treatment insults. It has been reported that visceral and subcutaneous adipocytes from humans show differential sensitivity to the metabolic alterations elicited by PIs [48]. On the other hand, it cannot be ruled out that a relevant component of fat accumulation in visceral adipose tissue may be secondary to lipoatrophy in peripheral subcutaneous adipose tissue [49]. The impairment in the fat storage capacity in subcutaneous adipose tissue could lead to a redistribution of fat to the visceral compartment, which, for unknown reasons, does not exhibit atrophy in response to mitochondrial toxicity caused by drugs.
As mentioned above, mtDNA depletion cannot explain the differential behavior of visceral and subcutaneous fat in HIV-associated lipodystrophy, but unaffected adipogenesis and a milder induction of pro-inflammatory signaling in visceral relative to subcutaneous fat have been reported [48], which may explain the prevention of fat wasting in visceral adipose tissue.
The exact causes of lipomatosis are also not fully understood. However, the reduction in lipoatrophy associated with avoiding the use of thymidine-analog NRTIs has also led to a decreased occurrence of this phenomenon in PLWH [50]. Importantly, the lipomatous accumulations in the dorso-cervical area and other sites show similar signs of mtDNA depletion as lipoatrophic subcutaneous adipose tissue sites [17,19]. However, other alterations occurring in adipose depots affected by HIV-associated lipodystrophy, such as impaired adipogenesis and enhanced inflammation, do not occur in the enlarged adipose tissues in the dorso-cervical region and other areas [20]. In contrast, lipomatous adipose tissue in PLWH shows the accumulation of the toxic, non-processed form of lamin A, a phenomenon previously reported to take place in genetically determined lipomatosis [51] and in lipoatrophic areas in PLWH [20], so it does not appear to account for the differential behavior of HIV-associated lipodystrophy and lipoatrophic and lipomatous areas.
Subcutaneous adipose tissue in PLWH with lipodystrophy also shows the reduced expression of the micro-RNA-processing enzyme DICER, particularly in the lipomatous dorso-cervical area. This phenomenon is associated with the decreased expression of brown/beige adipose marker genes [52]. DICER dysregulation is associated with the duration of antiretroviral use and, interestingly, correlates with alterations in circulating miRNAs from small extracellular vesicles (enhanced miR-20a-3p and depleted miR-186 and miR-324-5p), which could also contribute to acquired lipodystrophy and associated metabolic and inflammatory perturbations in PLWH [53].
3.2. HIV Infection-Related Effects
In addition to antiretroviral treatments, various molecular and cellular events associated with HIV infection also contribute to the development of HIV-associated lipodystrophy. Although HIV-1 does not significantly infect adipocytes [54], it does target immune cells infiltrating adipose tissue, primarily macrophages and lymphocytes [55,56]. Adipose tissue is, in fact, considered a reservoir for HIV-1 in the body, largely due to the infection of these immune cells. One consequence of HIV-1 infection in adipose tissue is the release of inflammatory cytokines. This contributes to local alterations in adipose tissue and can lead to systemic metabolic abnormalities, such as insulin resistance and dyslipidemia [57], features similar to those observed in obesity, a condition characterized by increased local inflammation in adipose tissue. Furthermore, HIV-infected cells secrete extracellular viral-encoded proteins such as Vpr, Tat, and Nef, which can interfere with adipocyte differentiation; promote the expression of pro-inflammatory cytokines; and induce cellular senescence by inhibiting the expression of target genes of PPAR γ, the master regulator of adipogenesis, among other actions [58,59,60,61]. In this way, several alterations in adipogenic and mitochondrial gene expression are already present in the adipose tissue of untreated (naïve) HIV patients [62]. Thus, HIV infection of adipose tissue appears to prime the tissue for increased susceptibility to the structural and functional alterations characteristic of HIV-associated lipodystrophy that occur in response to antiretroviral therapy.
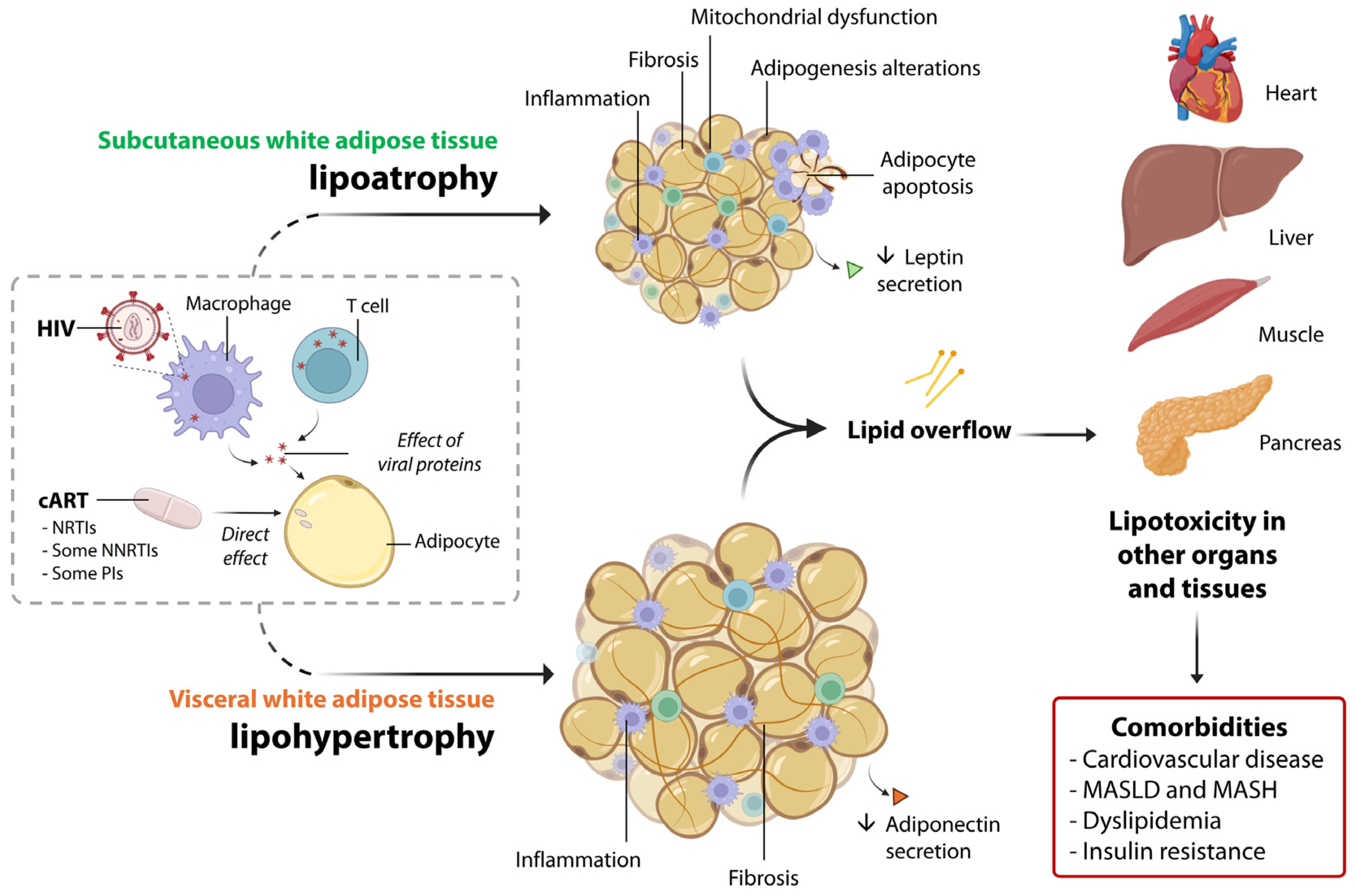
Figure 2 summarizes the main molecular actors eliciting lipoatrophy and lipohypertrophy in PLWH, as well as systemic alterations.
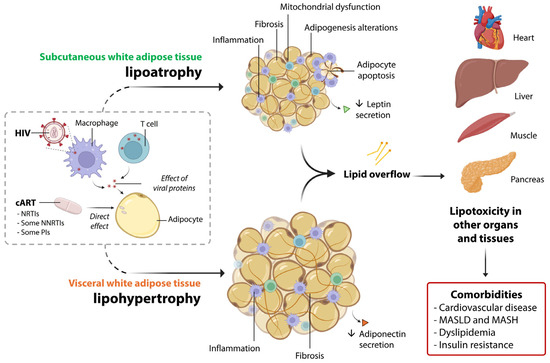
Figure 2.
A summary of the main pathophysiological alterations in subcutaneous and visceral white adipose tissue associated with lipodystrophy in PLWH. HIV is present within adipose-resident T cells and macrophages, and interactions between HIV proteins and adipocytes also occur. Additionally, certain drugs used in cART, such as NRTIs, NNRTIs, and PIs, directly affect the molecular functions of adipocytes. Together, these factors contribute to the pathological alterations observed in both the subcutaneous and visceral adipose tissue compartments, which in turn promote lipotoxicity in other organs. This ultimately leads to several systemic comorbidities, such as cardiovascular disease, MASLD, metabolic dysfunction-associated steatohepatitis (MASH), dyslipidemia, and insulin resistance.
5. Conclusions: Current Challenges for Management of Healthy Adipose Tissue in PLWH
The current clinical concerns related to HIV-associated lipodystrophy manifest in several ways. The avoidance of thymidine-based NRTIs worldwide has decreased the prevalence of lipoatrophy in PLWH. However, a significant number of PLWH who were treated with first-generation NRTIs and developed lipoatrophy did not recover after switching to a treatment based on last-generation antiretroviral drugs. Among the patients in the APROCO cohort previously treated with thymidine-analog NRTIs, 22% continue to experience mixed lipoatrophy/lipohypertrophy and elevated insulin resistance rates, even after transitioning to modern antiretroviral regimens [82]. Visceral lipohypertrophy often persists in PLWH who have had long-term exposure to thymidine-analog NRTIs. Each year of cumulative exposure to first-generation NRTIs is associated with an increase in visceral fat of 3.7 cm2 after the discontinuation of these drugs [83]. The persistent signs of lipodystrophy and associated metabolic alterations that occur even after the discontinuation of more lipodystrophy-prone drugs are especially common in PLWH who started treatment during childhood. Increased waist circumference is nearly twice as prevalent in adults with HIV who were exposed to thymidine NRTIs during childhood [84].
Second, although visceral obesity remains the primary adipose alteration in PLWH undergoing contemporary antiretroviral therapy, a recent report revealed that the subcutaneous fat transcriptome in patients with more visceral adipose tissue shows the increased expression of genes associated with the extracellular matrix and inflammation, along with the reduced expression of genes related to lipolysis and fatty acid metabolism [85]. This highlights the current persistence of complex alterations in different adipose depots in PLWH.
Recently, novel aspects relating adipose tissue dysfunction and conditions compromising health in PLWH are emerging. It has long been known that PLWH show an increased risk of diastolic dysfunction. Recent data indicate that epicardial adipose tissue, a fat depot closely associated with the myocardium and known to be involved in cardiac function [86], is enlarged in PLWH. This is associated with diastolic dysfunction, regardless of the HIV serostatus and viral suppression [87,88]. Increased pericoronary adipose density has been found to be independently associated with the prevalence and severity of coronary plaque in PLWH [89]. There are also data indicating that PLWH show signs of inflammation in perivascular fat, the type of adipose tissue coating vessels. This inflammation could be involved in the microvascular disease that often occurs in PLWH [90]. As such, the potential roles of altered epicardiac, pericoronary, and perivascular adipose tissue in cardiovascular function among PLWH remain speculative and are based on observed correlations and the more established impacts of these fat depots on cardiac and vascular pathophysiology in the general population. In any case, such findings provide further evidence of the influence of distorted adipose tissue on health conditions in PLWH.
Treatment strategies based on lifestyle changes are increasingly considered to be relevant for mitigating adipose tissue alterations and systemic cardiometabolic disturbances in PLWH. When overt lipodystrophy was highly prevalent, some data indicated that adhesion to a Mediterranean diet and supplementation with omega-3 polyunsaturated fatty acids ameliorated systemic parameters, including inflammation [91,92]. Notably, an intervention based on the Mediterranean diet led to a marked increase in the relative abundance of Bifidobacterium spp., which is linked to a microbiota profile indicative of beneficial immunometabolic outcomes [93]. In the current context of increased obesity in PLWH under treatments including INSTIs, even in the absence of accurate specific studies, clinical practices recommend lifestyle modifications (a decrease in caloric intake and an increase in exercise habits) as in the first-line treatment approach for common obesity.
On the other hand, several recent studies have shown that, in PLWH, GLP-1 receptor agonists commonly used to treat obesity and type 2 diabetes (e.g., semaglutide) produce a degree of weight loss similar to that observed in the general population [94], along with reductions in both visceral and subcutaneous adipose tissue [95]. A clinical trial investigating the effects of semaglutide in overweight PLWH found a significant reduction in abdominal visceral adipose tissue, which was the most pronounced effect [96]. These findings suggest that incretin analogs, currently used to treat common obesity and type 2 diabetes, may also hold promise as therapeutic agents for managing visceral lipohypertrophy and the associated metabolic dysregulation in PLWH [97].
In summary, recent data underscore the ongoing vulnerability of PLWH to changes in adipose tissue. Although the current alterations in adipose tissue among PLWH may not be as severe as traditional HIV-related lipodystrophy, they can still have significant health implications. To improve the well-being of PLWH, it is essential to continue refining antiretroviral treatment strategies and to monitor potential adipose tissue changes as part of comprehensive metabolic health management.
Author Contributions
Conceptualization, M.G., P.D. and F.V.; writing—original draft preparation, M.G. and F.V.; writing—review and editing, M.G., P.D., T.Q.-L., R.C. and F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the State Agency of Research (AEI) of the Spanish Ministry of Science (MCIN) (grant PID2023-1467810-I00 funded by MICIN/AEI/10.13039/50110 0011033 and FEDER, UE) and the Instituto de Salud Carlos III (ISCIII), Spain (grants PI20/00106 and PI20/00137), and it was co-financed by the European Regional Development Fund (ERDF). T.Q-L. was awarded a “Juan de la Cierva-Incorporación” researcher contract (grant IJC2020-043380-I funded by MCIN/AEI/10.13039/501100011033 and the European Union Next Generation (EU/PRTR)).
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT 4o from OpenAI for the purpose of generating pictorial representations of lipodystrophy features in PLWH. The authors have reviewed the output and take full responsibility for the content.
Conflicts of Interest
M.G., T.Q.-L., R.C. and F.V. declare no conflicts of interest. P.D. reports honoraria for advice or public speaking from Gilead, MSD, Janssen, and ViiV Healthcare.
Abbreviations
The following abbreviations are used in this manuscript:
| AIDS | acquired immunodeficiency syndrome |
| cART | combined antiretroviral therapy |
| GLP-1 | glucagon-like peptide-1 |
| HIV | human immunodeficiency virus |
| INSTI | integrase strand transfer inhibitor |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MASH | metabolic dysfunction-associated steatohepatitis |
| MC4R | melanocortin-4 receptor |
| mtDNA | mitochondrial DNA |
| NRTI | nucleotide analog reverse transcriptase inhibitor |
| NNRTI | non-nucleoside analog reverse transcriptase inhibitor |
| PCNA | proliferating cell nuclear antigen |
| PI | protease inhibitor |
| PLWH | people living with HIV |
| PPARγ | peroxisome proliferator-activated-γ |
| TAF | tenofovir alafenamide |
| TDF | tenofovir difumarate |
| UCP1 | uncoupling protein-1 |
References
- Giralt, M.; Domingo, P.; Villarroya, F. Adipose tissue biology and HIV-infection. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Lagathu, C.; Lake, J.E.; Domingo, P.; Calmy, A.; Falutz, J.; Brown, T.T.; Capeau, J. HIV and antiretroviral therapy-related fat alterations. Nat. Rev. Dis. Primers 2020, 6, 48. [Google Scholar] [CrossRef]
- Ramirez Bustamante, C.E.; Agarwal, N.; Cox, A.R.; Hartig, S.M.; Lake, J.E.; Balasubramanyam, A. Adipose Tissue Dysfunction and Energy Balance Paradigms in People Living with HIV. Endocr. Rev. 2024, 45, 190–209. [Google Scholar] [CrossRef]
- Capeau, J.; Lagathu, C.; Béréziat, V.; Fève, B. Recent data on adipose tissue, insulin resistance, diabetes and dyslipidaemia in antiretroviral therapy controlled HIV-infected persons. Curr. Opin. HIV AIDS 2021, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Tilhet, L.; Rouanet, M.; Henry, A.S.; Pop, A.; Claudic, Y.; Saraoui, W.; Nachaoui, H.; Hu, W. Current status and perspectives in the treatment of facial lipoatrophy in HIV-positive patients in 2024. Ann. Chir. Plast. Esthet. 2025, 70, 192–205. [Google Scholar] [CrossRef]
- Domingo, P.; Estrada, V.; López-Aldeguer, J.; Villaroya, F.; Martínez, E. Fat redistribution syndromes associated with HIV-1 infection and combination antiretroviral therapy. AIDS Rev. 2012, 14, 112–123. [Google Scholar] [PubMed]
- Carr, A.; Law, M. HIV Lipodystrophy Case Definition Study Group. An objective lipodystrophy severity grading scale derived from the lipodystrophy case definition score. J. Acquir. Immune Defic. Syndr. 2003, 33, 571–576. [Google Scholar] [CrossRef]
- Mallon, P.W.; Miller, J.; Cooper, D.A.; Carr, A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS 2003, 17, 971–979. [Google Scholar] [CrossRef]
- Martin, A.; Mallon, P.W. Therapeutic approaches to combating lipoatrophy: Do they work? J. Antimicrob. Chemother. 2005, 55, 612–615. [Google Scholar] [CrossRef]
- Glidden, D.V.; Mulligan, K.; McMahan, V.; Anderson, P.L.; Guanira, J.; Chariyalertsak, S.; Buchbinder, S.P.; Bekker, L.G.; Schechter, M.; Grinsztejn, B.; et al. Metabolic Effects of Preexposure Prophylaxis with Coformulated Tenofovir Disoproxil Fumarate and Emtricitabine. Clin. Infect. Dis. 2018, 67, 411–419. [Google Scholar] [CrossRef]
- Martínez, E.; Ribera, E.; Clotet, B.; Estrada, V.; Sanz, J.; Berenguer, J.; Rubio, R.; Pulido, F.; Larrousse, M.; Curran, A.; et al. Switching from zidovudine/lamivudine to tenofovir/emtricitabine improves fat distribution as measured by fat mass ratio. HIV Med. 2015, 16, 370–374. [Google Scholar] [CrossRef]
- Capeau, J.; Lagathu, C.; Béréziat, V. Recent data on the role of antiretroviral therapy in weight gain and obesity in persons living with HIV. Curr. Opin. HIV AIDS 2024, 19, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.; Navarro, A.; Curto, J.; Medina, P.; Rozas, N.; Barrera, G.; Saumoy, M.; Tiraboschi, J.M.; Gomez, C.; Podzamczer, D. Long-term fat redistribution in ARV-naïve HIV+ patients initiating a non-thymidine containing regimen in clinical practice. J. Int. AIDS Soc. 2014, 17 (Suppl. 3), 19553. [Google Scholar] [CrossRef]
- Grant, P.M.; Kitch, D.; McComsey, G.A.; Collier, A.C.; Bartali, B.; Koletar, S.L.; Erlandson, K.M.; Lake, J.E.; Yin, M.T.; Melbourne, K.; et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016, 30, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Mulligan, K.; Tai, V.W.; Algren, H.; Schambelan, M. “Buffalo hump” in men with HIV-1 infection. Lancet 1998, 351, 867–870. [Google Scholar] [CrossRef]
- Guaraldi, G.; Orlando, G.; Squillace, N.; Roverato, A.; De Fazio, D.; Vandelli, M.; Nardini, G.; Beghetto, B.; De Paola, M.; Esposito, R.; et al. Prevalence of and risk factors for pubic lipoma development in HIV-infected persons. J. Acquir. Immune Defic. Syndr. 2007, 45, 72–76. [Google Scholar] [CrossRef]
- Cereijo, R.; Gallego-Escuredo, J.M.; Moure, R.; Villarroya, J.; Domingo, J.C.; Fontdevila, J.; Martínez, E.; Gutiérrez, M.M.; Mateo, M.G.; Giralt, M.; et al. The Molecular Signature of HIV-1-Associated Lipomatosis Reveals Differential Involvement of Brown and Beige/Brite Adipocyte Cell Lineages. PLoS ONE 2015, 10, e0136571. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; De Fazio, D.; Orlando, G.; Murri, R.; Wu, A.; Guaraldi, P.; Esposito, R. Facial lipohypertrophy in HIV-infected subjects who underwent autologous fat tissue transplantation. Clin. Infect. Dis. 2005, 40, e13–e15. [Google Scholar] [CrossRef]
- Guallar, J.P.; Gallego-Escuredo, J.M.; Domingo, J.C.; Alegre, M.; Fontdevila, J.; Martínez, E.; Hammond, E.L.; Domingo, P.; Giralt, M.; Villarroya, F. Differential gene expression indicates that ‘buffalo hump’ is a distinct adipose tissue disturbance in HIV-1-associated lipodystrophy. AIDS 2008, 22, 575–584. [Google Scholar] [CrossRef]
- Béréziat, V.; Cervera, P.; Le Dour, C.; Verpont, M.C.; Dumont, S.; Vantyghem, M.C.; Capeau, J.; Vigouroux, C.; Lipodystrophy Study Group. LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue. Am. J. Pathol. 2011, 179, 2443–2453. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Olivo, A.; Le Pelletier, L.; Capeau, J.; Lambotte, O.; Béréziat, V.; Lagathu, C. Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated with HIV Infection and Its Treatment. Front. Immunol. 2021, 12, 670566. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell. Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Sankal, J.L.; Hadigan, C.M.; Tan, G.; Rosenberg, E.S.; Kanki, P.J.; Grinspoon, S.K.; Hotamisligil, G.S. Regulation of adiponectin in human immunodeficiency virus-infected patients: Relationship to body composition and metabolic in-dices. J. Clin. Endocrinol. Metab. 2003, 88, 1559–1564. [Google Scholar] [CrossRef]
- Lagathu, C.; Bastard, J.P.; Auclair, M.; Maachi, M.; Kornprobst, M.; Capeau, J.; Caron, M. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir. Ther. 2004, 9, 911–920. [Google Scholar] [CrossRef]
- Lagathu, C.; Eustace, B.; Prot, M.; Frantz, D.; Gu, Y.; Bastard, J.P.; Maachi, M.; Azoulay, S.; Briggs, M.; Caron, M.; et al. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 2007, 12, 489–500. [Google Scholar] [CrossRef]
- Jones, S.P.; Janneh, O.; Back, D.J.; Pirmohamed, M. Altered adipokine response in murine 3T3-F442A adipocytes treated with protease inhibitors and nucleoside reverse transcriptase inhibitors. Antivir. Ther. 2005, 10, 207–213. [Google Scholar] [CrossRef]
- Gallego-Escuredo, J.M.; Del Mar Gutierrez, M.; Diaz-Delfin, J.; Domingo, J.C.; Mateo, M.G.; Domingo, P.; Giralt, M.; Villarroya, F. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr. HIV Res. 2010, 8, 545–553. [Google Scholar] [CrossRef]
- Tiliscan, C.; Aramă, V.; Mihăilescu, R.; Munteanu, D.I.; Streinu-Cercel, A.; Ion, D.A.; Rădulescu, M.A.; Popescu, C.; Lobodan, A.E.; Negru, A.R.; et al. Leptin expression in HIV-infected patients during antiretroviral therapy. Germs 2015, 5, 92–98. [Google Scholar] [CrossRef]
- Giralt, M.; Díaz-Delfín, J.; Gallego-Escuredo, J.M.; Villarroya, J.; Domingo, P.; Villarroya, F. Lipotoxicity on the basis of metabolic syndrome and lipodystrophy in HIV-1-infected patients under antiretroviral treatment. Curr. Pharm. Des. 2010, 16, 3371–3378. [Google Scholar] [CrossRef]
- Villarroya, F.; Domingo, P.; Giralt, M. Drug-induced lipotoxicity: Lipodystrophy associated with HIV-1 infection and antiretroviral treatment. Biochim. Biophys. Acta 2010, 1801, 392–399. [Google Scholar] [CrossRef]
- Torriani, M.; Thomas, B.J.; Barlow, R.B.; Librizzi, J.; Dolan, S.; Grinspoon, S. Increased intramyocellular lipid accumulation in HIV-infected women with fat redistribution. J. Appl. Physiol. 2006, 100, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, J.; Häkkinen, A.M.; Westerbacka, J.; Seppälä-Lindroos, A.; Vehkavaara, S.; Halavaara, J.; Järvinen, A.; Ristola, M.; Yki-Järvinen, H. Increased fat accumulation in the liver in HIV-infected patients with antiretroviral therapy-associated lipodystrophy. AIDS 2002, 16, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Nguyen, K.L.; Zaha, V.G.; Chew, K.W.; Morrison, L.; Ntusi, N.A.B.; Toribio, M.; Awadalla, M.; Drobni, Z.D.; Nelson, M.D.; et al. Myocardial Steatosis Among Antiretroviral Therapy-Treated People with Human Immunodeficiency Virus Participating in the REPRIEVE Trial. J. Infect. Dis. 2020, 222 (Suppl. 1), S63–S69. [Google Scholar] [CrossRef]
- Tuddenham, S.A.; Koay, W.L.A.; Zhao, N.; White, J.R.; Ghanem, K.G.; Sears, C.L.; HIV Microbiome Re-analysis Consortium. The Impact of Human Immunodeficiency Virus Infection on Gut Microbiota α-Diversity: An Individual-level Meta-analysis. Clin. Infect. Dis. 2020, 70, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Gogokhia, L.; Taur, Y.; Juluru, K.; Yagan, N.; Zhu, Y.S.; Pamer, E.; Glesby, M.J. Intestinal Dysbiosis and Markers of Systemic Inflammation in Viscerally and Generally Obese Persons Living with HIV. J. Acquir. Immune Defic. Syndr. 2020, 83, 81–89. [Google Scholar] [CrossRef]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.; Santiago, M.L.; Austin, G.L.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS 2017, 31, 511–521. [Google Scholar] [CrossRef]
- Lloreta, J.; Domingo, P.; Pujol, R.M.; Arroyo, J.A.; Baixeras, N.; Matias-Guiu, X.; Gilaberte, M.; Sambeat, M.A.; Serrano, S. Ultra-structural features of highly active antiretroviral therapy-associated partial lipodystrophy. Virchows Arch. 2002, 441, 599–604. [Google Scholar] [CrossRef]
- Jan, V.; Cervera, P.; Maachi, M.; Baudrimont, M.; Kim, M.; Vidal, H.; Girard, P.M.; Levan, P.; Rozenbaum, W.; Lombès, A.; et al. Altered fat differentiation and adipocytokine expression are interrelated and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir. Ther. 2004, 9, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, G.; Luzi, K.; Bellistrì, G.M.; Zona, S.; Domingues da Silva, A.R.; Bai, F.; Garlassi, E.; Marchetti, G.; Capeau, J.; Monforte, A.D. CD8 T-cell activation is associated with lipodystrophy and visceral fat accumulation in antiretroviral therapy-treated virologically suppressed HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2013, 64, 360–366. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F.; Araujo-Vilar, D. Lipodystrophies. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhyaniemi, I., Martini, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 482–495. [Google Scholar]
- Villarroya, F.; Domingo, P.; Giralt, M. Lipodystrophy associated with highly active anti-retroviral therapy for HIV infection: The adipocyte as a target of anti-retroviral-induced mitochondrial toxicity. Trends Pharmacol. Sci. 2005, 26, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Auclair, M.; Lagathu, C.; Lombès, A.; Walker, U.A.; Kornprobst, M.; Capeau, J. The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS 2004, 18, 2127–2136. [Google Scholar] [CrossRef]
- Stankov, M.V.; Lücke, T.; Das, A.M.; Schmidt, R.E.; Behrens, G.M. Mitochondrial DNA depletion and respiratory chain activity in primary human subcutaneous adipocytes treated with nucleoside analogue reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2010, 54, 280–287. [Google Scholar] [CrossRef]
- De Luca, A.; Nasi, M.; Di Giambenedetto, S.; Cozzi-Lepri, A.; Pinti, M.; Marzocchetti, A.; Mussini, C.; Fabbiani, M.; Bracciale, L.; Cauda, R.; et al. Mitochondrial DNA haplogroups and incidence of lipodystrophy in HIV-infected patients on long-term antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2012, 59, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Escuredo, J.M.; Villarroya, J.; Domingo, P.; Targarona, E.M.; Alegre, M.; Domingo, J.C.; Villarroya, F.; Giralt, M. Differentially altered molecular signature of visceral adipose tissue in HIV-1-associated lipodystrophy. J. Acquir. Immune Defic. Syndr. 2013, 64, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kovsan, J.; Osnis, A.; Maissel, A.; Mazor, L.; Tarnovscki, T.; Hollander, L.; Ovadia, S.; Meier, B.; Klein, J.; Bashan, N.; et al. Depot-specific adipocyte cell lines reveal differential drug-induced responses of white adipocytes--relevance for partial lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E315–E322. [Google Scholar] [CrossRef]
- Villarroya, F.; Domingo, P.; Giralt, M. Lipodystrophy in HIV 1-infected patients: Lessons for obesity research. Int. J. Obes. 2007, 31, 1763–1776. [Google Scholar] [CrossRef]
- Srinivasa, S.; Grinspoon, S.K. Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur. J. Endocrinol. 2014, 170, R185–R202. [Google Scholar] [CrossRef]
- Shackleton, S.; Lloyd, D.J.; Jackson, S.N.; Evans, R.; Niermeijer, M.F.; Singh, B.M.; Schmidt, H.; Brabant, G.; Kumar, S.; Durrington, P.N.; et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat. Genet. 2000, 24, 153–156. [Google Scholar] [CrossRef]
- Torriani, M.; Srinivasa, S.; Fitch, K.V.; Thomou, T.; Wong, K.; Petrow, E.; Kahn, C.R.; Cypess, A.M.; Grinspoon, S.K. Dysfunctional Subcutaneous Fat with Reduced Dicer and Brown Adipose Tissue Gene Expression in HIV-Infected Patients. J. Clin. Endocrinol. Metab. 2016, 10, 1225–1234. [Google Scholar] [CrossRef]
- Srinivasa, S.; Garcia-Martin, R.; Torriani, M.; Fitch, K.V.; Carlson, A.R.; Kahn, C.R.; Grinspoon, S.K. Altered pattern of circulating miRNAs in HIV lipodystrophy perturbs key adipose differentiation and inflammation pathways. JCI Insight 2021, 6, e150399. [Google Scholar] [CrossRef]
- Munier, S.; Borjabad, A.; Lemaire, M.; Mariot, V.; Hazan, U. In vitro infection of human primary adipose cells with HIV-1: A reassessment. AIDS 2003, 17, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Suliburk, J.W.; Brown, J.M.; Luke, D.J.; Agarwal, N.; Yu, X.; Nguyen, C.; Iyer, D.; Kozinetz, C.A.; Overbeek, P.A.; et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Damouche, A.; Lazure, T.; Avettand-Fènoël, V.; Huot, N.; Dejucq-Rainsford, N.; Satie, A.P.; Mélard, A.; David, L.; Gommet, C.; Ghosn, J.; et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015, 11, e1005153. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Lewis, D.E. HIV Persistence in Adipose Tissue Reservoirs. Curr. HIV/AIDS Rep. 2018, 15, 60–71. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Fève, B.; Lambotte, O.; et al. Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Gorwood, J.; Ejlalmanesh, T.; Bourgeois, C.; Mantecon, M.; Rose, C.; Atlan, M.; Desjardins, D.; Le Grand, R.; Fève, B.; Lambotte, O.; et al. SIV Infection and the HIV Proteins Tat and Nef Induce Senescence in Adipose Tissue and Human Adipose Stem Cells, Resulting in Adipocyte Dysfunction. Cells 2020, 9, 854. [Google Scholar] [CrossRef]
- Agarwal, N.; Balasubramanyam, A. Viral mechanisms of adipose dysfunction: Lessons from HIV-1 Vpr. Adipocyte 2014, 4, 55–59. [Google Scholar] [CrossRef]
- Díaz-Delfín, J.; Domingo, P.; Wabitsch, M.; Giralt, M.; Villarroya, F. HIV-1 Tat protein impairs adipogenesis and induces the expression and secretion of proinflammatory cytokines in human SGBS adipocytes. Antivir. Ther. 2012, 17, 529–540. [Google Scholar] [CrossRef]
- Giralt, M.; Domingo, P.; Guallar, J.P.; Rodriguez de la Concepción, M.L.; Alegre, M.; Domingo, J.C.; Villarroya, F. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV- 1/HAART-associated lipodystrophy. Antivir. Ther. 2006, 11, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; Mccomsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Venter, W.D.F.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef]
- Grabar, S.; Potard, V.; Piroth, L.; Abgrall, S.; Bernard, L.; Allavena, C.; Caby, F.; de Truchis, P.; Duvivier, C.; Enel, P.; et al. Striking differences in weight gain after cART initiation depending on early or advanced presentation: Results from the ANRS CO4 FHDH cohort. J. Antimicrob. Chemother. 2023, 78, 757–768. [Google Scholar] [CrossRef]
- Lake, J.E.; Wu, K.; Bares, S.H.; Debroy, P.; Godfrey, C.; Koethe, J.R.; McComsey, G.A.; Palella, F.J.; Tassiopoulos, K.; Erlandson, K.M. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin. Infect. Dis. 2020, 71, e471–e477. [Google Scholar] [CrossRef]
- Bailin, S.S.; Koethe, J.R.; Rebeiro, P.F. The pathogenesis of obesity in people living with HIV. Curr. Opin. HIV AIDS 2024, 19, 6–13. [Google Scholar] [CrossRef]
- Domingo, P.; Villarroya, F.; Giralt, M.; Domingo, J.C. Potential role of the melanocortin signaling system interference in the excess weight gain associated to some antiretroviral drugs in people living with HIV. Int. J. Obes. 2020, 44, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Chandiwana, N.C.; Siedner, M.J.; Marconi, V.C.; Hill, A.; Ali, M.K.; Batterham, R.L.; Venter, W.D.F. Weight Gain After HIV Therapy Initiation: Pathophysiology and Implications. J. Clin. Endocrinol. Metab. 2024, 109, e478–e487. [Google Scholar] [CrossRef]
- Ngono Ayissi, K.; Gorwood, J.; Le Pelletier, L.; Bourgeois, C.; Beaupère, C.; Auclair, M.; Foresti, R.; Motterlini, R.; Atlan, M.; Bar-rail-Tran, A.; et al. Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells 2022, 11, 1841. [Google Scholar] [CrossRef]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef]
- Domingo, P.; Quesada-López, T.; Villarroya, J.; Cairó, M.; Gutierrez, M.D.M.; Mateo, M.G.; Mur, I.; Corbacho, N.; Domingo, J.C.; Villarroya, F.; et al. Differential effects of dolutegravir, bictegravir and raltegravir in adipokines and inflammation markers on human adipocytes. Life Sci. 2022, 308, 120948. [Google Scholar] [CrossRef]
- Quesada-López, T.; Cereijo, R.; Blasco-Roset, A.; Mestres-Arenas, A.; Prieto, P.; Domingo, J.C.; Villarroya, F.; Domingo, P.; Giralt, M. Divergent effects of the antiretroviral drugs, dolutegravir, tenofovir alafenamide, and tenofovir disoproxil fumarate, on human adipocyte function. Biochem. Pharmacol. 2024, 220, 116010. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Carleo, M.A.; Mascolo, S.; Guida, A.; Contieri, M.; Sellitto, C.; Hay, E.; De Blasiis, P.; Lucariello, A.; Guerra, G.; et al. Adipocyte differentiation of 3T3-L1 cells under tenofovir alafenamide, tenofovir disoproxil fumarate, and integrase strand transfer inhibitors selective challenge: An in-vitro model. AIDS 2023, 37, 561–570. [Google Scholar] [CrossRef]
- Hocqueloux, L.; Menard, A.; Arvieux, C.; Joly, V.; Becker, A.; Chéret, A.; Duvivier, C.; Cabié, A.; Delpierre, C.; Allavena, C.; et al. Weight gain following the single substitution of tenofovir disoproxil fumarate by tenofovir alafenamide in HIV-infected people from the French Dat’AIDS cohort: A propensity score-matched analysis. HIV Med. 2023, 24, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Dulion, B.; Olali, A.Z.; Patel, N.; Virdi, A.K.; Naqib, A.; Wallace, J.; Ross, R.D. Tenofovir alafenamide promotes weight gain and impairs fatty acid metabolism-related signaling pathways in visceral fat tissue compared to tenofovir disoproxil fumarate. Antivir. Res. 2025, 237, 106151. [Google Scholar] [CrossRef] [PubMed]
- Milic, J.; Renzetti, S.; Ferrari, D.; Barbieri, S.; Menozzi, M.; Carli, F.; Dolci, G.; Ciusa, G.; Mussini, C.; Calza, S.; et al. Relationship between weight gain and insulin resistance in people living with HIV switching to integrase strand transfer inhibitors-based regimens. AIDS 2022, 36, 1643–1653. [Google Scholar] [CrossRef]
- González-Cordón, A.; Assoumou, L.; Moyle, G.; Waters, L.; Johnson, M.; Domingo, P.; Fox, J.; Stellbrink, H.J.; Guaraldi, G.; Masiá, M.; et al. Switching from boosted PIs to dolutegravir decreases soluble CD14 and adiponectin in high cardiovascular risk people living with HIV. J. Antimicrob. Chemother. 2021, 76, 2380–2393. [Google Scholar] [CrossRef]
- Summers, N.A.; Lahiri, C.D.; Angert, C.D.; Aldredge, A.; Mehta, C.C.; Ofotokun, I.; Kerchberger, A.M.; Gustafson, D.; Weiser, S.D.; Kassaye, S.; et al. Metabolic Changes Associated with the Use of Integrase Strand Transfer Inhibitors Among Virally Controlled Women. J. Acquir. Immune Defic. Syndr. 2020, 85, 355–362. [Google Scholar] [CrossRef]
- Neesgaard, B.; Greenberg, L.; Miró, J.M.; Grabmeier-Pfistershammer, K.; Wandeler, G.; Smith, C.; De Wit, S.; Wit, F.; Pelchen-Matthews, A.; Mussini, C.; et al. Associations between integrase strand-transfer inhibitors and cardiovascular disease in people living with HIV: A multicentre prospective study from the RESPOND cohort consortium. Lancet HIV 2022, 9, e474–e485. [Google Scholar] [CrossRef]
- Rebeiro, P.F.; Emond, B.; Rossi, C.; Bookhart, B.K.; Shah, A.; Caron-Lapointe, G.; Lafeuille, M.H.; Donga, P. Incidence of cardiometabolic outcomes among people living with HIV-1 initiated on integrase strand transfer inhibitor versus non-integrase strand transfer inhibitor antiretroviral therapies: A retrospective analysis of insurance claims in the United States. J. Int. AIDS Soc. 2023, 26, e26123. [Google Scholar] [CrossRef]
- Bastard, J.P.; Couffignal, C.; Fellahi, S.; Bard, J.M.; Mentre, F.; Salmon, D.; Katlama, C.; Raffi, F.; Leport, C.; Capeau, J.; et al. Diabetes and dyslipidaemia are associated with oxidative stress independently of inflammation in long-term antiretroviral-treated HIV-infected patients. Diabetes Metab. 2019, 45, 573–581. [Google Scholar] [CrossRef]
- Gelpi, M.; Afzal, S.; Lundgren, J.; Ronit, A.; Roen, A.; Mocroft, A.; Gerstoft, J.; Lebech, A.M.; Lindegaard, B.; Kofoed, K.F.; et al. Higher Risk of Abdominal Obesity, Elevated Low-Density Lipoprotein Cholesterol, and Hypertriglyceridemia, but not of Hypertension, in People Living with Human Immunodeficiency Virus (HIV): Results From the Copenhagen Comorbidity in HIV Infection Study. Clin. Infect. Dis. 2018, 67, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Arrive, E.; Viard, J.P.; Salanave, B.; Dollfus, C.; Matheron, S.; Reliquet, V.; Arezes, E.; Nailler, L.; Vigouroux, C.; Warszawski, J.; et al. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS ONE 2018, 13, e0206745. [Google Scholar] [CrossRef]
- Bailin, S.S.; Gabriel, C.L.; Gangula, R.D.; Hannah, L.; Nair, S.; Carr, J.J.; Terry, J.G.; Silver, H.J.; Simmons, J.D.; Mashayekhi, M.; et al. Single-Cell Analysis of Subcutaneous Fat Reveals Profibrotic Cells That Correlate with Visceral Adiposity in HIV. J. Clin. Endocrinol. Metab. 2024, 110, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Telders, C.; Eringa, E.C.; de Groot, J.R.; de Man, F.S.; Handoko, M.L. The role of epicardial adipose tissue remodelling in heart failure with preserved ejection fraction. Cardiovasc. Res. 2025, 16, cvaf056. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M.; Apornpong, T.; Tumkosit, M.; Avihingsanon, A.; Chattranukulchai, P. Epicardial fat tissue and diastolic dysfunction in both men and women with HIV. AIDS 2024, 38, 1893–1894. [Google Scholar] [CrossRef]
- Goldberg, R.L.; Peterson, T.E.; Haberlen, S.A.; Witt, M.D.; Palella, F.J.; Magnani, J.W.; Brown, T.T.; Lake, J.E.; Lima, J.A.C.; Budoff, M.J.; et al. Response to “Epicardial fat tissue and diastolic dysfunction in both men and women with HIV”. AIDS 2024, 38, 1895. [Google Scholar] [CrossRef]
- Foldyna, B.; Mayrhofer, T.; Zanni, M.V.; Lyass, A.; Barve, R.; Karady, J.; McCallum, S.; Burdo, T.H.; Fitch, K.V.; Paradis, K.; et al. Pericoronary Adipose Tissue Density, Inflammation, and Subclinical Coronary Artery Disease Among People with HIV in the REPRIEVE Cohort. Clin. Infect. Dis. 2023, 77, 1676–1686. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Herbert, C.; Wang, C.; Ma, Y.; Sun, P.; Li, T.; Verbesey, J.; Kumar, P.; Kassaye, S.; Welch, W.J.; et al. Signals From Inflamed Perivascular Adipose Tissue Contribute to Small-Vessel Dysfunction in Women with Human Immunodeficiency Virus. J. Infect. Dis. 2024, 230, 67–77. [Google Scholar] [CrossRef]
- Basta, D.; Latinovic, O.S.; Tagaya, Y.; Silvestri, G. Potential Advantages of a Well-balanced Nutrition Regimen for People Living with Human Immunodeficiency Virus Type-1. J. AIDS HIV Treat. 2024, 6, 11–27. [Google Scholar] [CrossRef]
- Domingo, P.; Fernández, I.; Gallego-Escuredo, J.M.; Torres, F.; Gutierrez, M.D.M.; Mateo, M.G.; Villarroya, J.; Giralt, M.; Vidal, F.; Villarroya, F.; et al. Effects of docosahexanoic acid on metabolic and fat parameters in HIV-infected patients on cART: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2018, 37, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Ibáñez, R.; Blanco-Heredia, J.; Etcheverry, F.; Sánchez-Palomino, S.; Díez-Fuertes, F.; Casas, R.; Navarrete-Muñoz, M.Á.; Castro-Barquero, S.; Lucero, C.; Fernández, I.; et al. Adherence to a Supplemented Mediterranean Diet Drives Changes in the Gut Microbiota of HIV-1-Infected Individuals. Nutrients 2021, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Haidar, L.; Crane, H.M.; Nance, R.M.; Webel, A.; Ruderman, S.A.; Whitney, B.M.; Willig, A.L.; Napravnik, S.; Mixson, L.S.; Leong, C.; et al. Weight loss associated with semaglutide treatment among people with HIV. AIDS 2024, 38, 531–535. [Google Scholar] [CrossRef]
- Lee, D.; Capeau, J. Is the GLP-1 receptor agonist, semaglutide, a good option for weight loss in persons with HIV? AIDS 2024, 38, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Eckard, A.R.; Wu, Q.; Sattar, A.; Ansari-Gilani, K.; Labbato, D.; Foster, T.; Fletcher, A.A.; Adekunle, R.O.; McComsey, G.A. Once-weekly semaglutide in people with HIV-associated lipohypertrophy: A randomised, double-blind, placebo-controlled phase 2b single-centre clinical trial. Lancet Diabetes Endocrinol. 2024, 12, 523–534. [Google Scholar] [CrossRef]
- Thomas, T.S.; Srinivasa, S. Weighing in: Glucagon-like Peptide-1 Receptor Agonism for Persons with HIV. Top. Antivir. Med. 2024, 32, 579–588. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).