Mechanistic Insights into Cytokinin-Regulated Leaf Senescence in Barley: Genotype-Specific Responses in Physiology and Protein Stability
Abstract
1. Introduction
2. Results
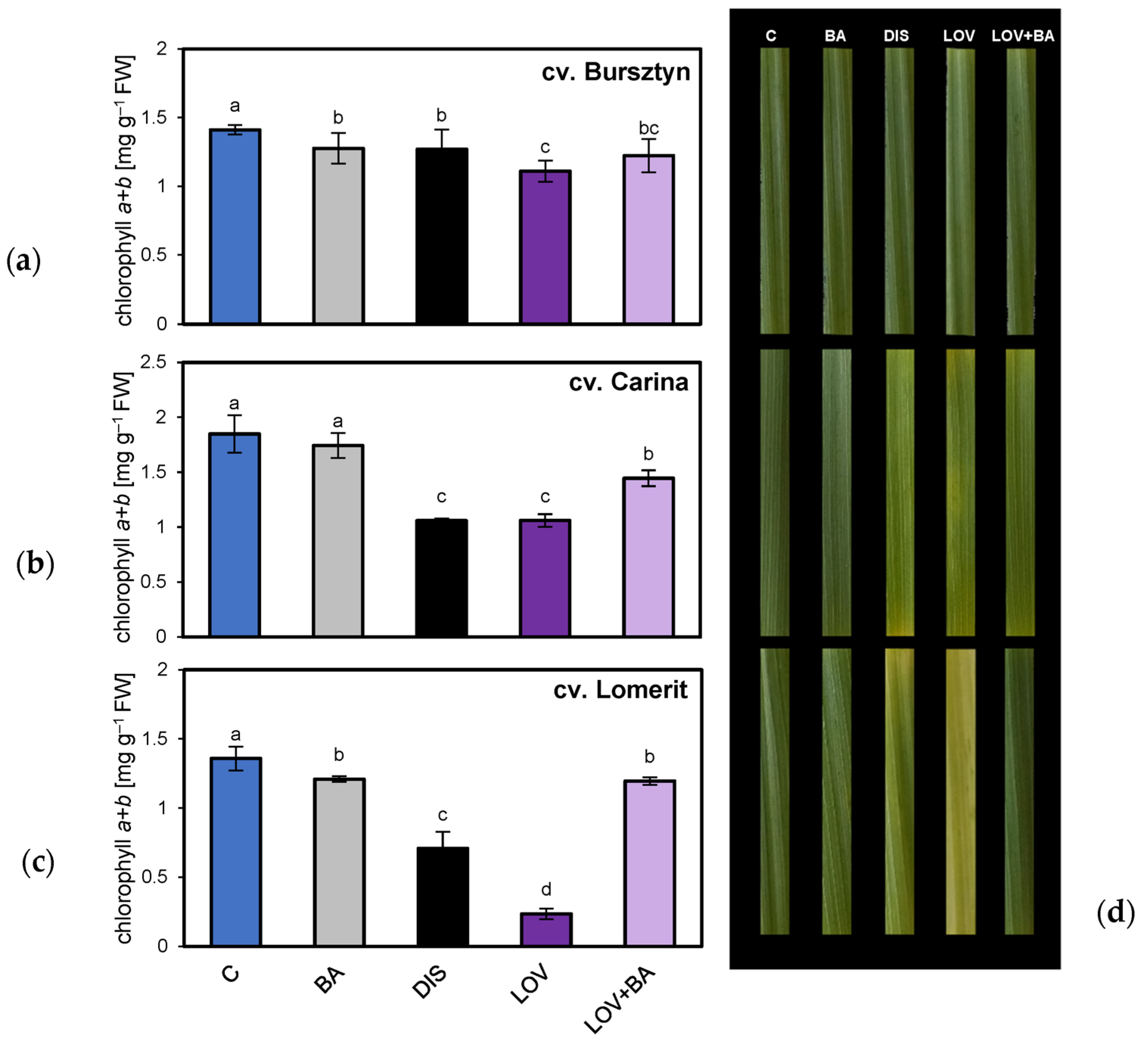
2.1. Phenotypic Characterization of the Stay-Green Trait in Barley Cultivars
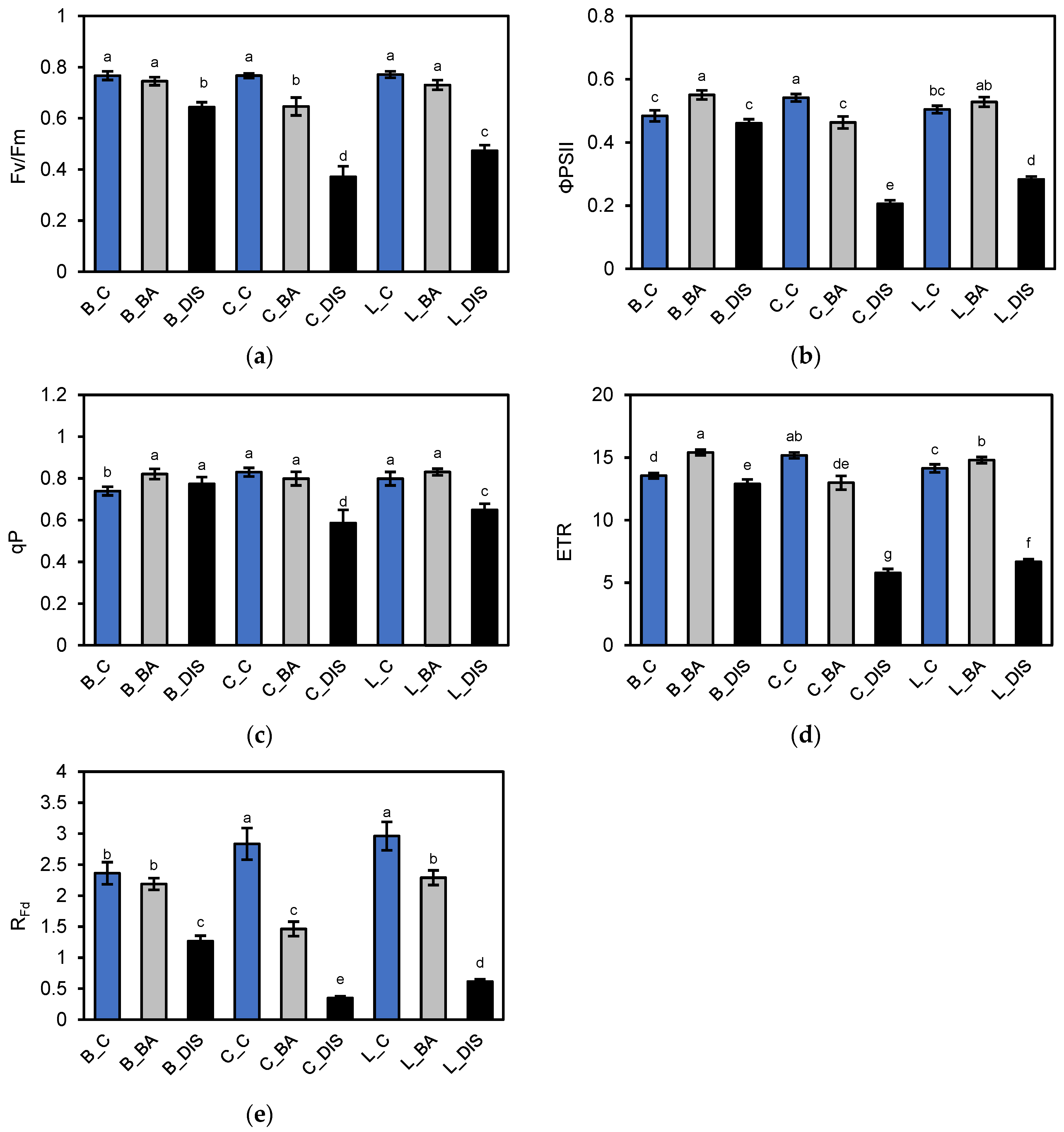
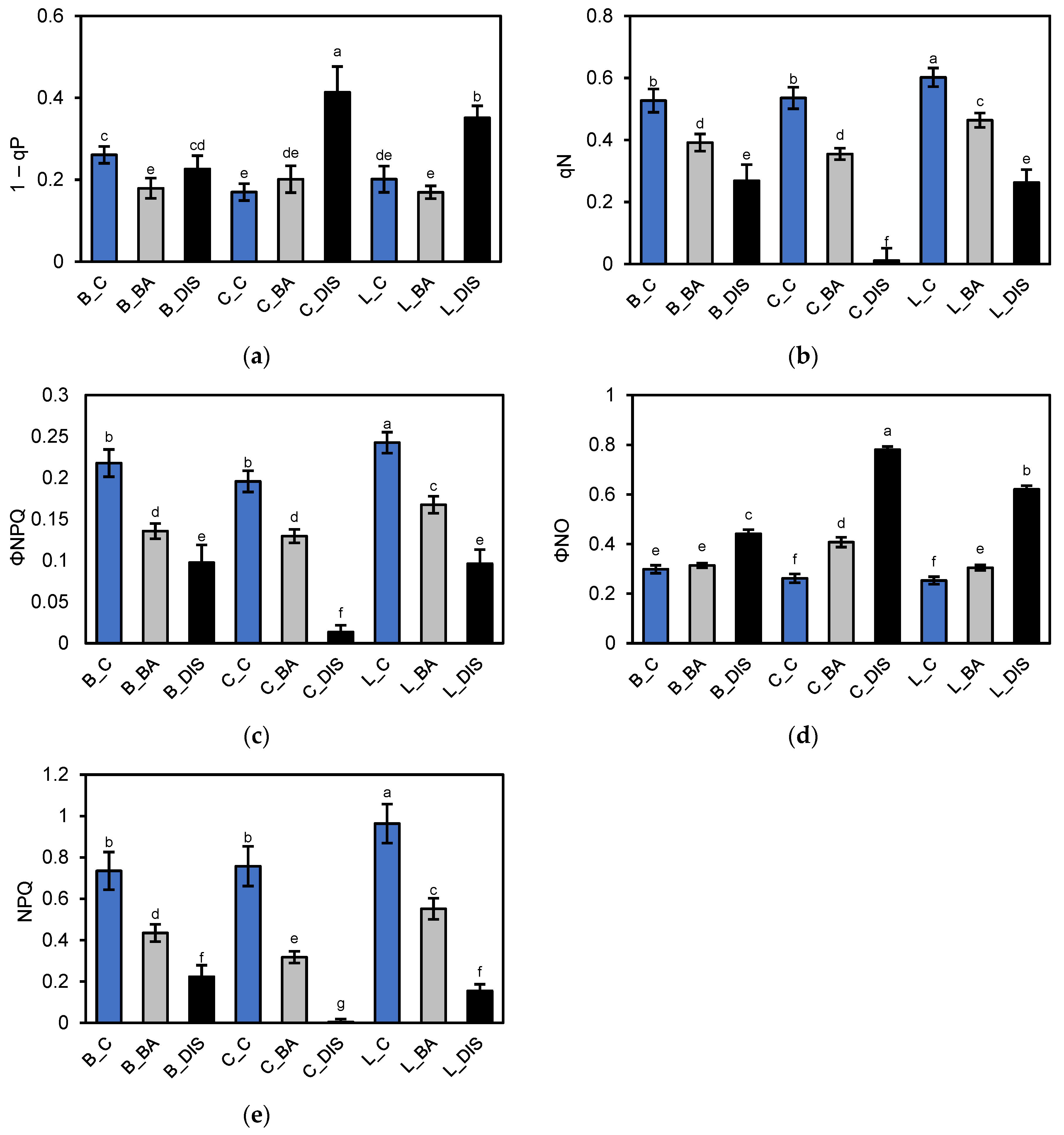
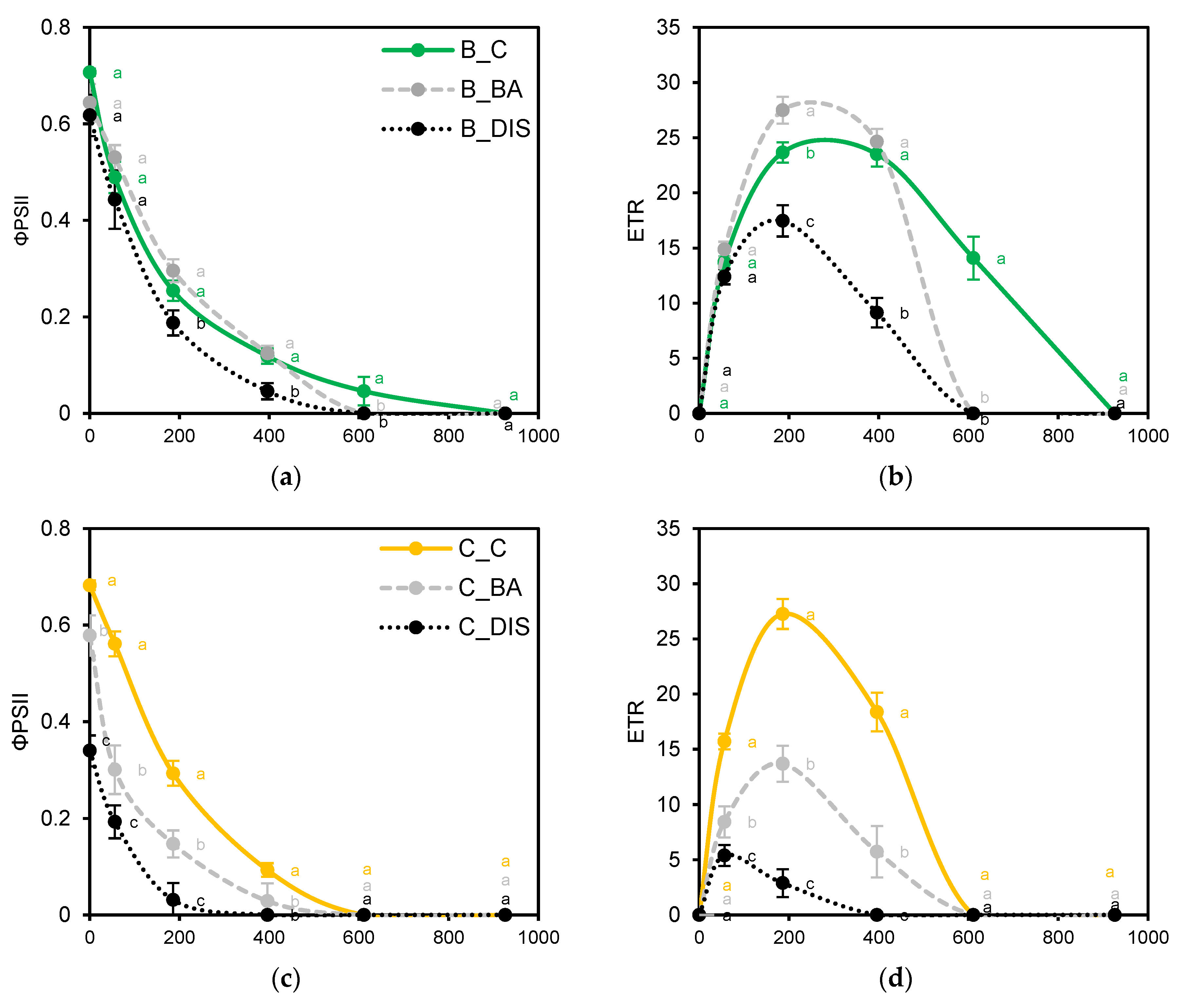
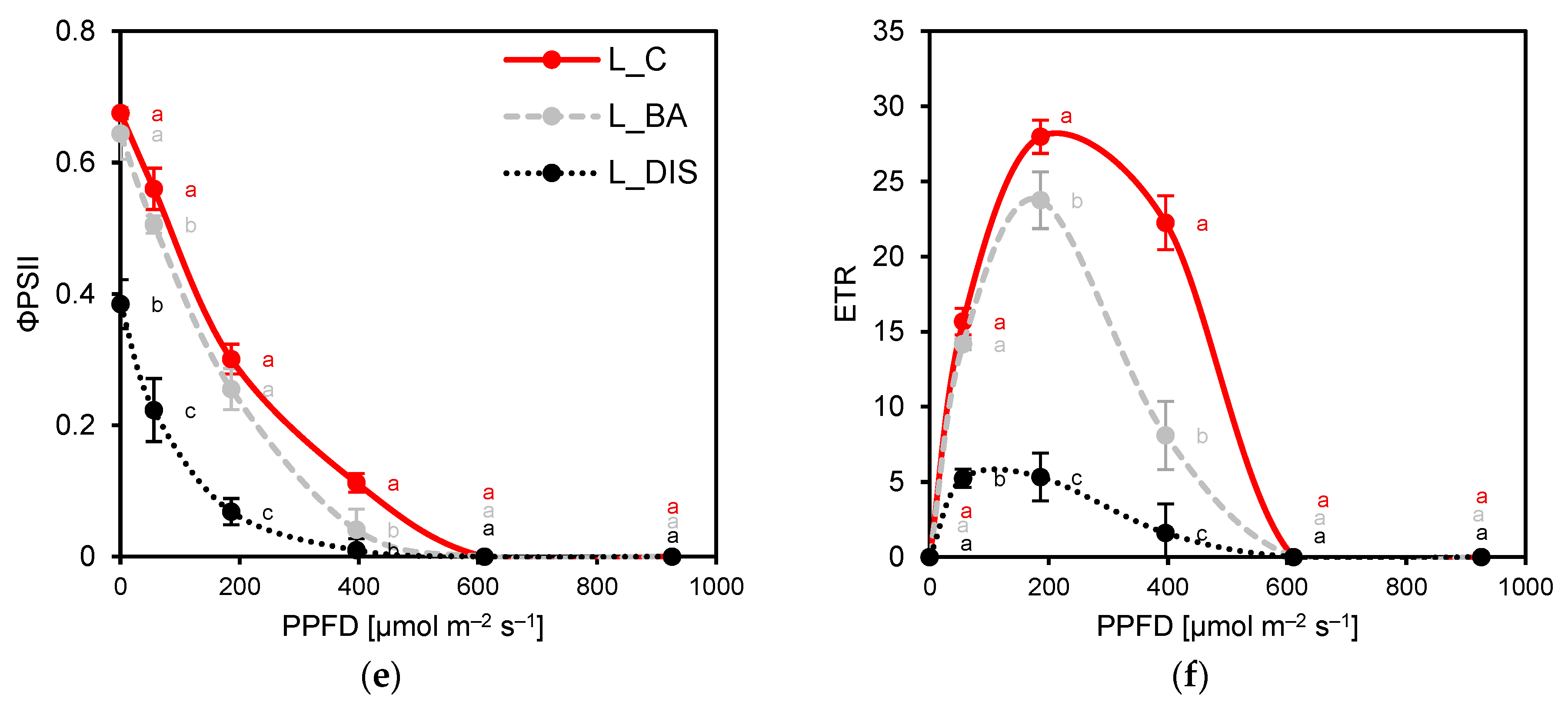
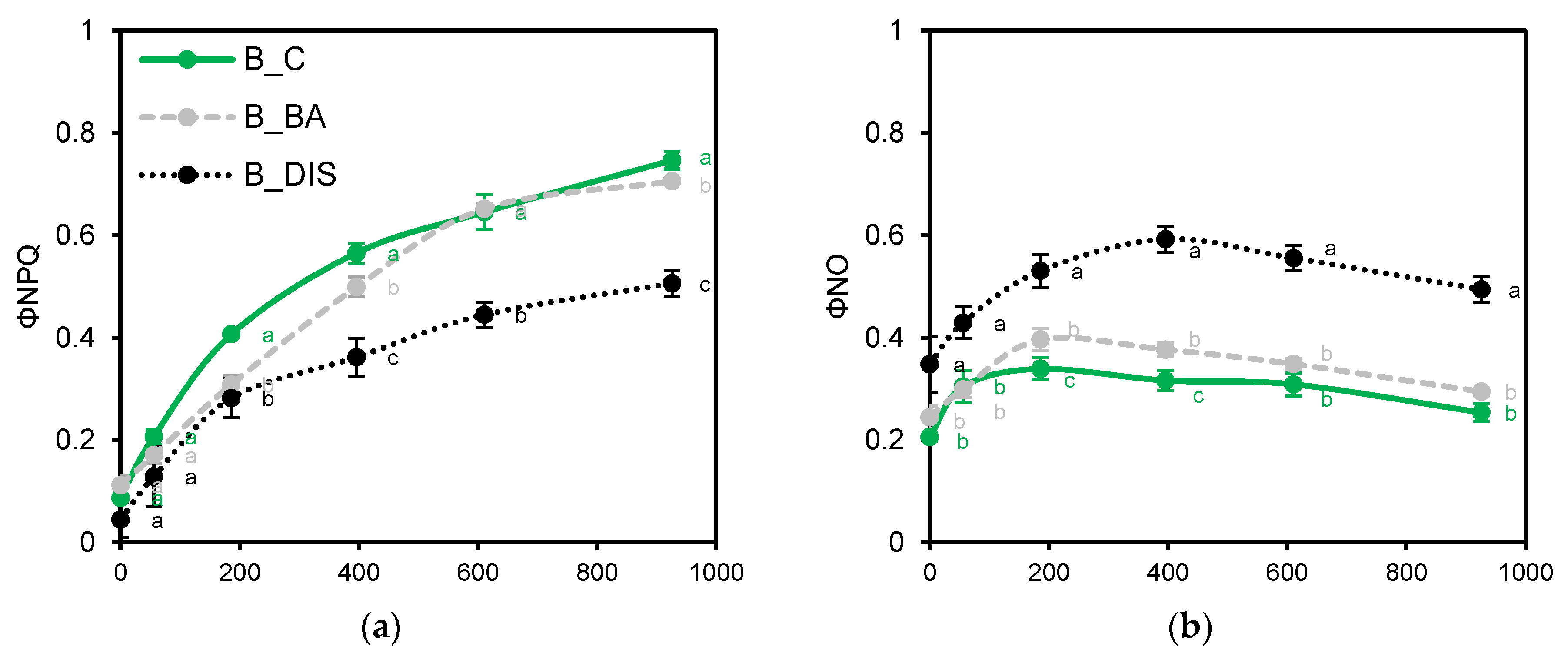
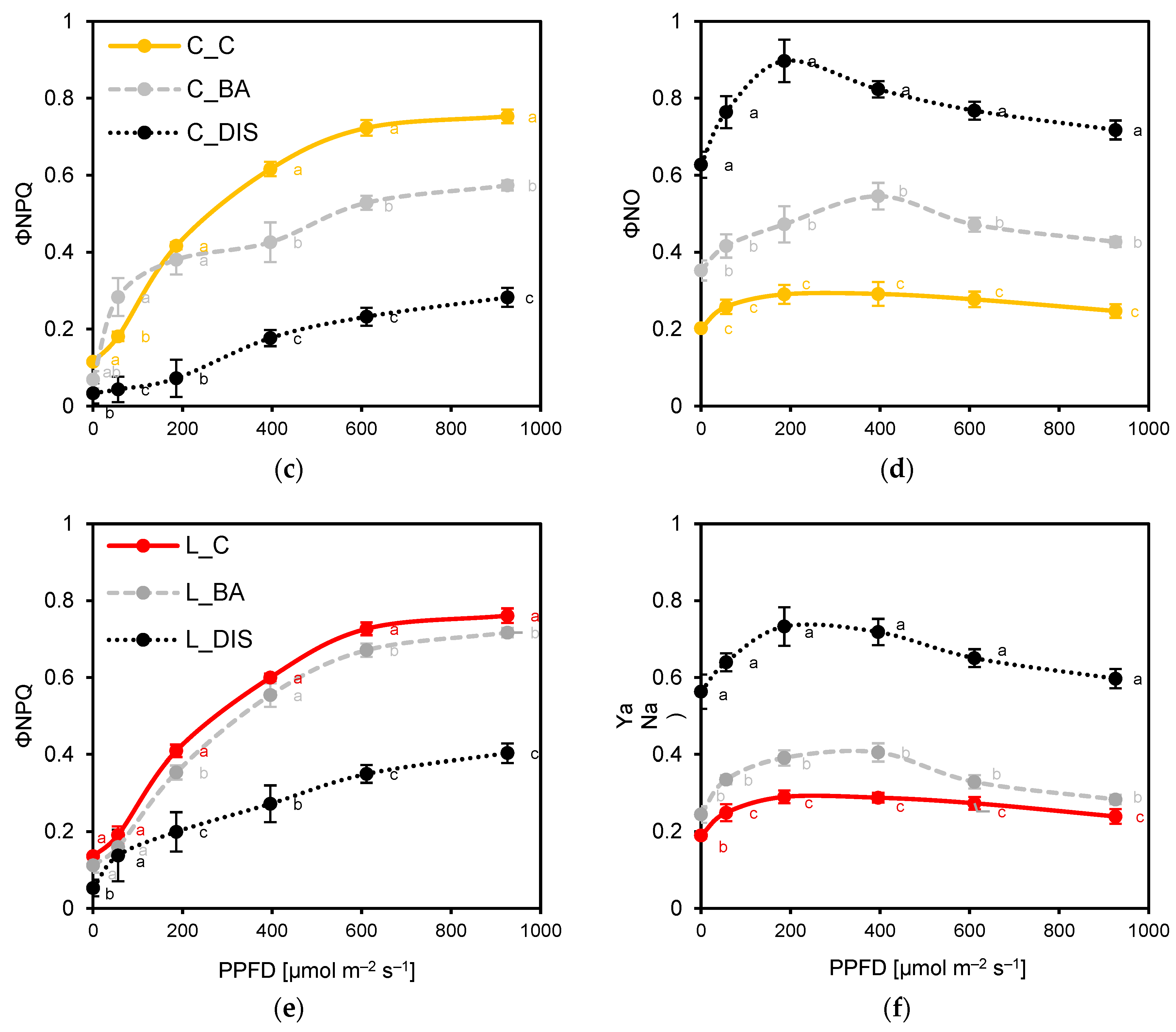
2.2. PSII Photochemical Efficiency Altered by Dark-Induced Senescence (DIS) and CK (BA) Treatment
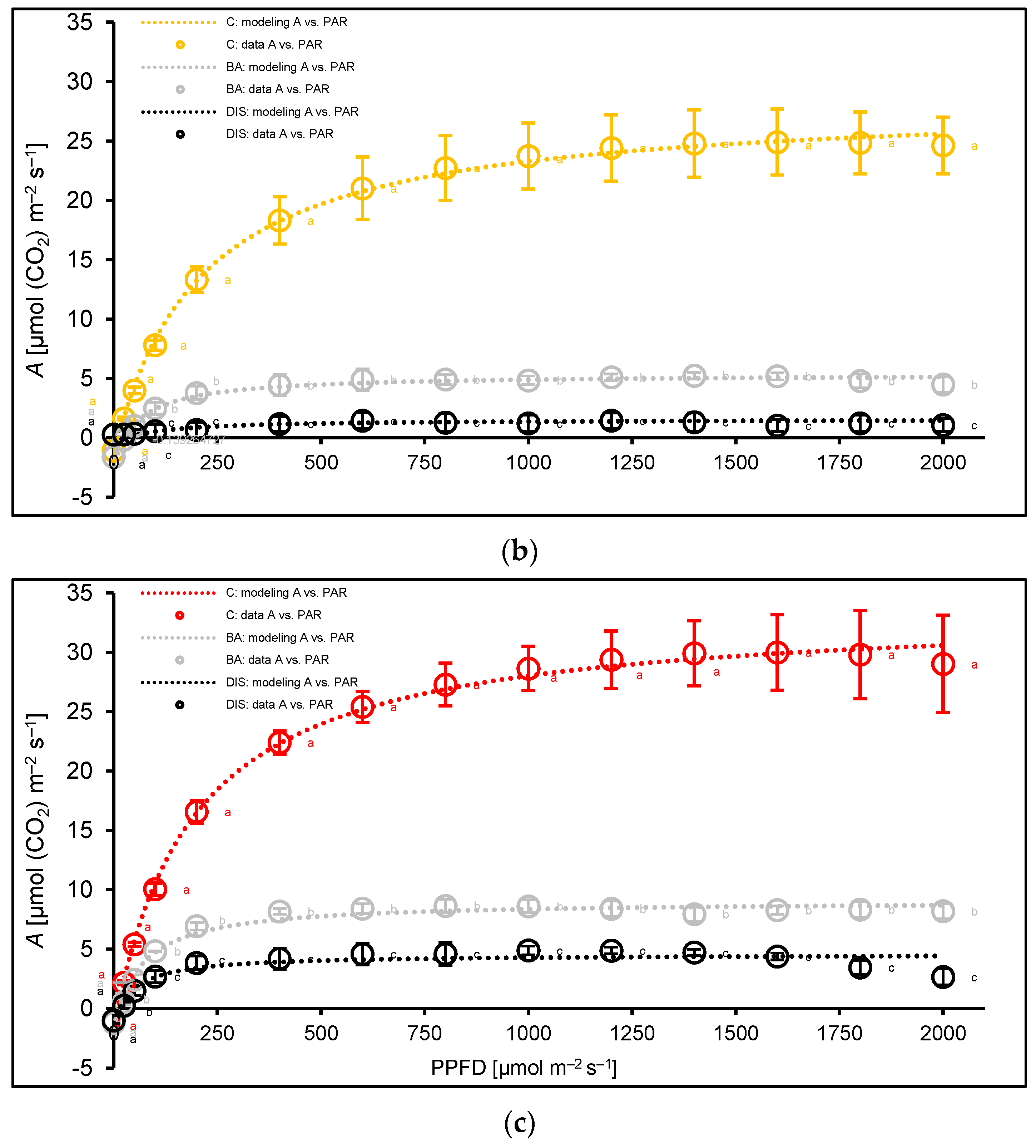
2.3. Analysis of Net Photosynthesis Rate and Its Modulation by Senescence and BA
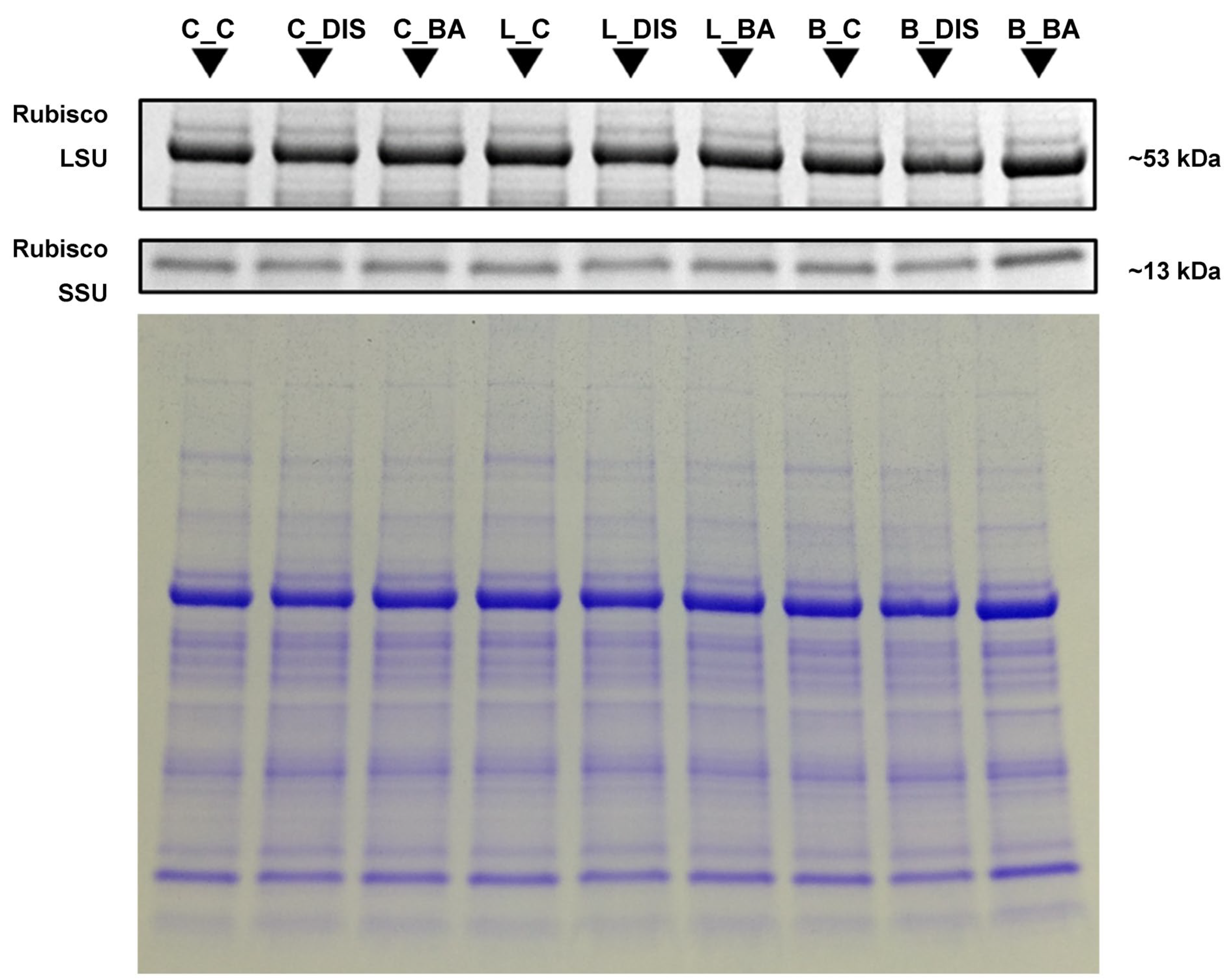
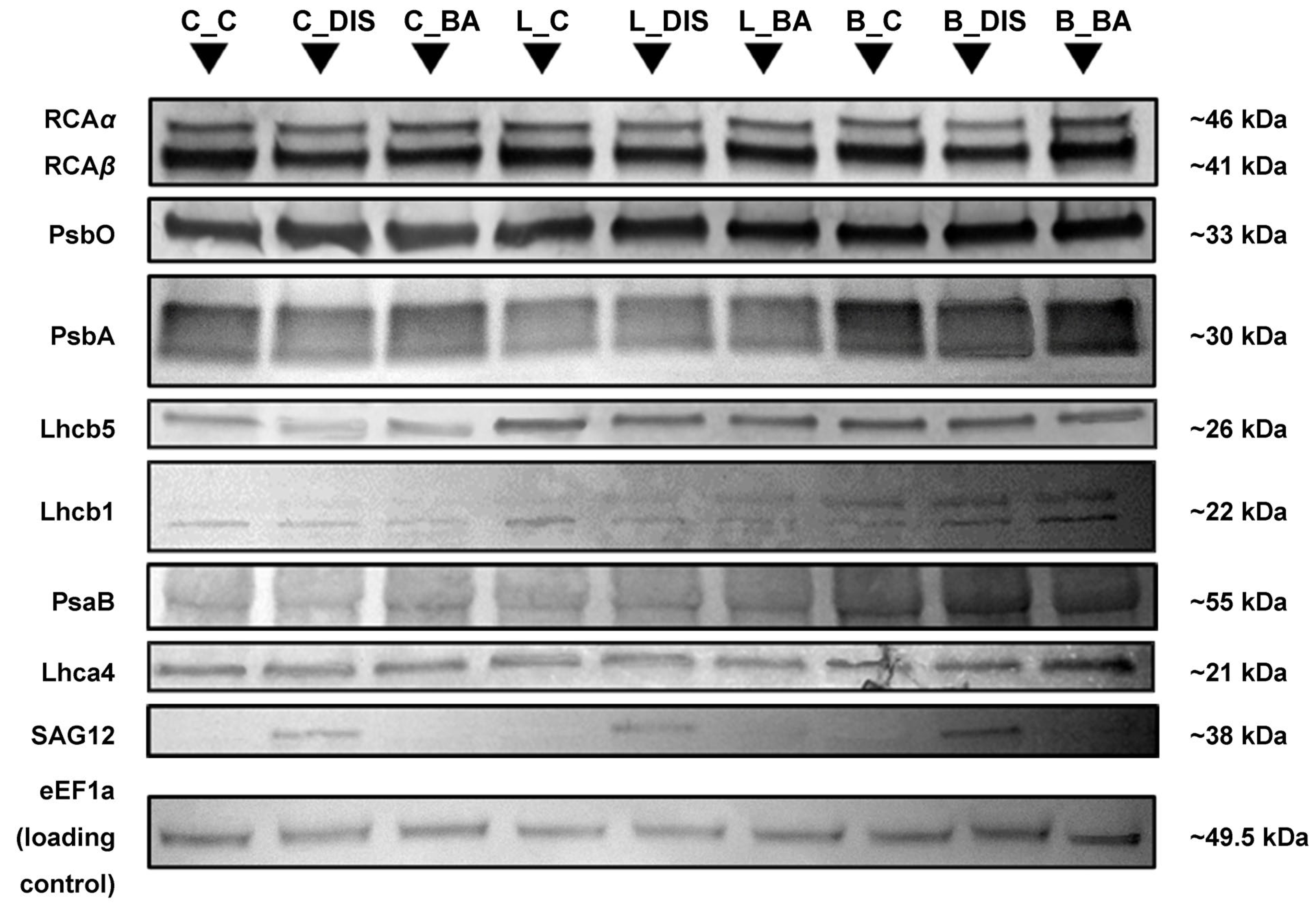
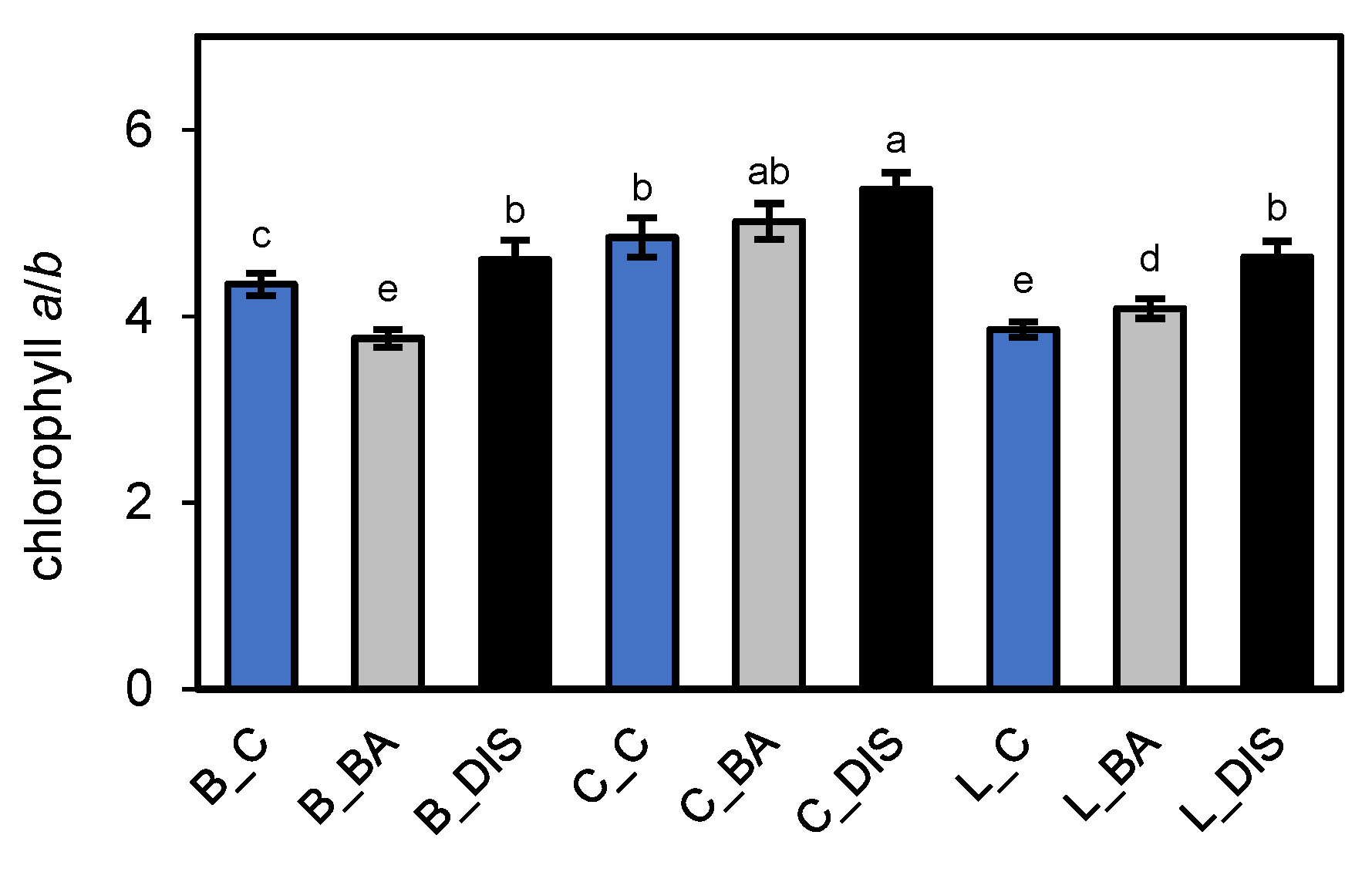
2.4. Changes in Photosynthetic Protein Levels, Chlorophyll a/b Ratio, and the Senescence Marker SAG12 During Senescence and BA Treatment
2.5. Effect of the CK Biosynthetic Pathway Inhibition on Leaf Senescence
2.6. Assessment of Oxidative Stress Induced by Senescence and BA Treatment
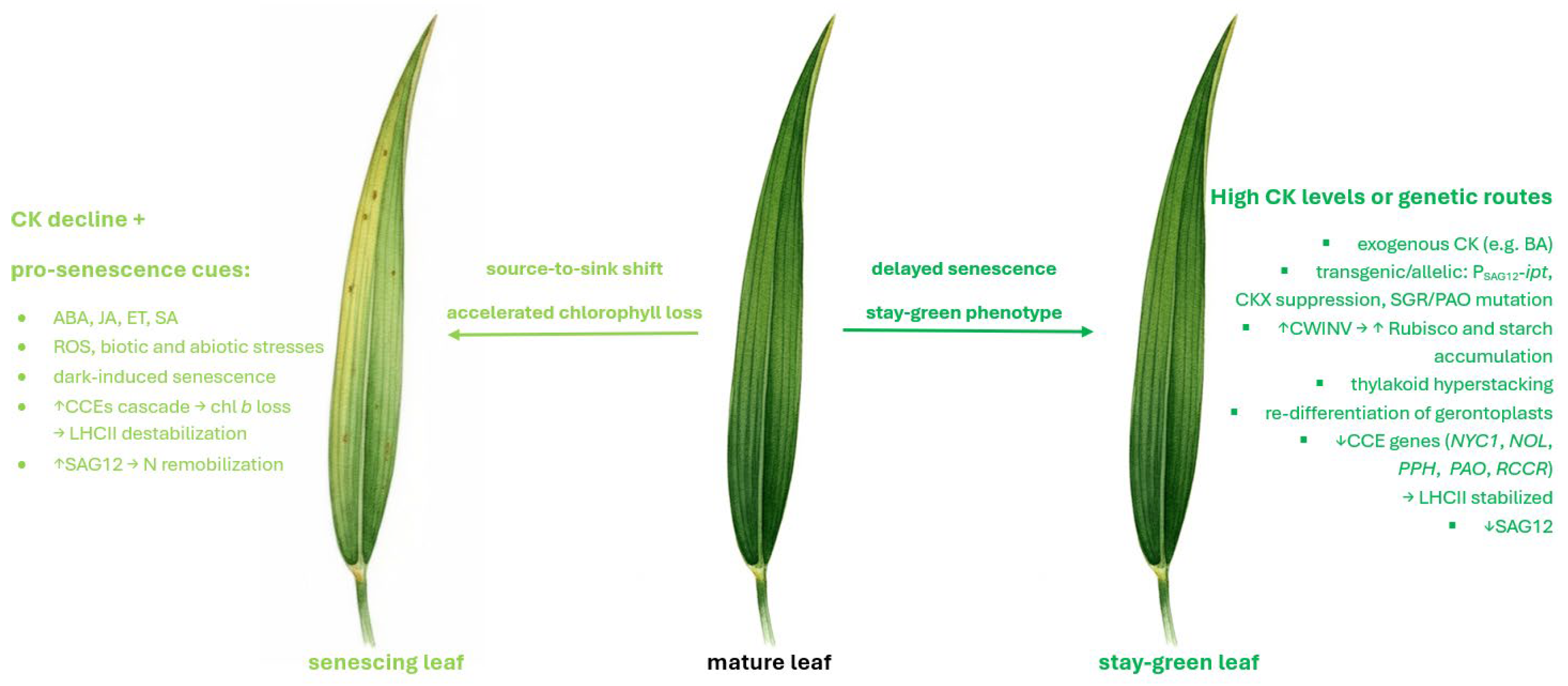
3. Discussion
3.1. Monitoring Barley Cultivars for Stay-Green Phenotype and Sensitivity to CK-Mediated Delay of Leaf Senescence
3.2. Chlorophyll a Fluorescence Analysis
3.3. Leaf Gas Exchange Under Dark-Induced Senescence and BA Treatment
3.4. Stability of Rubisco, Rubisco Activase, and Photosynthetic Proteins During Senescence and in Response to BA Treatment
3.5. Effect of BA on the Accumulation of the Senescence Marker SAG12 During DIS
3.6. The Role of Inhibited Endogenous CK Synthesis in Cultivars with Differing Senescence Dynamics
3.7. Oxidative Stress Intensity Following DIS and BA Treatment
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Leaf Senescence Induction and Chemical Treatment
4.3. Determination of Photosynthetic Pigment Content and Chlorophyll Stability Index
4.4. Chlorophyll Fluorescence Kinetics and Light Response Curve
4.5. Leaf Gas Exchange Measurements
4.6. Leaf Protein Extraction and Densitometric Quantification
4.7. Immunodetection and Densitometric Analysis of Selected Proteins
4.8. Determination of Hydrogen Peroxide, Superoxide Dismutase Activity and Lipid Peroxidation Levels
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojciechowska, N.; Sobieszczuk-Nowicka, E.; Bagniewska-Zadworna, A. Plant organ senescence–regulation by manifold pathways. Plant Biol. 2018, 20, 167–181. [Google Scholar] [CrossRef]
- Lim, P.O.; Woo, H.R.; Nam, H.G. Molecular genetics of leaf senescence in Arabidopsis. Trends Plant Sci. 2003, 8, 272–278. [Google Scholar] [CrossRef]
- Sarwat, M.; Naqvi, A.R.; Ahmad, P.; Ashraf, M.; Akram, N.A. Phytohormones and microRNAs as sensors and regulators of leaf senescence: Assigning macro roles to small molecules. Biotechnol. Adv. 2013, 31, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; Del Duca, S. Polyamines are common players in different facets of plant programmed cell death. Amino Acids 2015, 47, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ryu, H.; Hong, S.H.; Woo, H.R.; Lim, P.O.; Lee, I.C.; Sheen, J.; Nam, H.G. Cytokinin-Mediated Control of Leaf Longevity by AHK3 through Phosphorylation of ARR2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 814–819. [Google Scholar] [CrossRef]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- van Doorn, W.G. Is the onset of senescence in leaf cells of intact plants due to low or high sugar levels? J. Exp. Bot. 2008, 59, 1963–1972. [Google Scholar] [CrossRef]
- Cortleven, A.; Noben, J.P.; Valcke, R. Analysis of the photosynthetic apparatus in transgenic tobacco plants with altered endogenous cytokinin content: A proteomic study. Proteome Sci. 2011, 9, 33. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 1995, 270, 1986–1988. [Google Scholar] [CrossRef]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef]
- Talla, S.K.; Panigrahy, M.; Kappara, S.; Nirosha, P.; Neelamraju, S.; Ramanan, R. Cytokinin delays dark-induced senescence in rice by maintaining the chlorophyll cycle and photosynthetic complexes. J. Exp. Bot. 2016, 67, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sasaki, D.; Noguchi, K.; Fujinuma, D.; Komatsu, H.; Kobayashi, M.; Sato, M.; Toyooka, K.; Sugimoto, K.; Niyogi, K.K.; et al. Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors. Plant Cell Physiol. 2013, 54, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Brownhill, E.; Pourtau, N. Mechanisms of the light-dependent induction of cell death in tobacco plants with delayed senescence. J. Exp. Bot. 2005, 56, 2897–2905. [Google Scholar] [CrossRef]
- Pilarska, M.; Skowron, E.; Pietraś, R.; Krupinska, K.; Niewiadomska, E. Changes in lipid peroxidation in stay-green leaves of tobacco with senescence-induced synthesis of cytokinins. Plant Physiol. Biochem. 2017, 118, 161–167. [Google Scholar] [CrossRef]
- Paluch-Lubawa, E.; Stolarska, E.; Sobieszczuk-Nowicka, E. Dark-Induced Barley Leaf Senescence—A Crop System for Studying Senescence and Autophagy Mechanisms. Front. Plant Sci. 2021, 12, 635619. [Google Scholar] [CrossRef]
- Rudy, E.; Tanwar, U.K.; Szlachtowska, Z.; Grabsztunowicz, M.; Arasimowicz-Jelonek, M.; Sobieszczuk-Nowicka, E. Unveiling the role of epigenetics in leaf senescence: A comparative study to identify different epigenetic regulations of senescence types in barley leaves. BMC Plant Biol. 2024, 24, 863. [Google Scholar] [CrossRef]
- Avila-Ospina, L.; Clément, G.; Masclaux-Daubresse, C. Metabolite profiling for leaf senescence in barley reveals decreases in amino acids and glycolysis intermediates. Agronomy 2017, 7, 15. [Google Scholar] [CrossRef]
- Lara, M.E.B.; Garcia, M.-C.G.; Fatima, T.; Ehneβ, R.; Lee, T.K.; Proels, R.; Tanner, W.; Roitsch, T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 2004, 16, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Schmülling, T. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta-Mancera, H.A.; Thomas, B.J.; Thomas, H.; Scott, I.M. Regreening of senescent nicotiana leaves. II. Redifferentiation of plastids. J. Exp. Bot. 1999, 50, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Park, S.Y.; Paek, N.-C. The Divergent Roles of STAYGREEN (SGR) Homologs in Chlorophyll Degradation. Mol. Cells 2015, 38, 390–395. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Zhang, X.; Li, W.; Chang, Y.; Pang, D.; Xu, X.; Li, Y.; Wang, Z. Interactions between cytokinin and nitrogen contribute to grain mass in wheat cultivars by regulating the flag leaf senescence process. Crop J. 2018, 6, 538–551. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Early anthesis and delayed but fast leaf senescence contribute to individual grain dry matter and water accumulation in wheat. Field Crops Res. 2016, 187, 24–34. [Google Scholar] [CrossRef]
- Krupinska, K.; Mulisch, M.; Hollmann, J.; Tokarz, K.; Zschiesche, W.; Kage, H.; Humbeck, K.; Bilger, W. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Physiol. Plant. 2012, 144, 189–200. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trösch, M.; Krupinska, K. Generation of reactive oxygen species in thylakoids from senescing flag leaves of the barley varieties Lomerit and Carina. Planta 2015, 241, 1497–1508. [Google Scholar] [CrossRef]
- Humbeck, K.; Quast, S.; Krupinska, K. Functional and molecular changes in the photosynthetic apparatus during senescence of flag leaves from field-grown barley plants. Plant Cell Environ. 1996, 19, 337–344. [Google Scholar] [CrossRef]
- Vlčková, A.; Špundová, M.; Kotabová, E.; Novotný, R.; Doležal, K.; Nauš, J. Protective cytokinin action switches to damaging during senescence of detached wheat leaves in continuous light. Physiol. Plant. 2006, 126, 257–267. [Google Scholar] [CrossRef]
- Heinz Walz GmbH. IMAGING-PAM M-Sries Chlorophyll Fluorometer Instrument Description and Information for Users. Heinz Walz GmbH: Effeltrich, Germany. Available online: https://www.walz.com/downloads/manuals/imaging-pam_ms/imag-m-series0e_3Dg.pdf (accessed on 13 August 2025).
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 466–475. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ(T): A chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Thimann, K.V.; Tetley, R.M.; Krivak, B.M. Metabolism of oat leaves during senescence: V. Senescence in light. Plant Physiol. 1977, 59, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Outlaw, W.H.; Manchester, J. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 1979, 64, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Satler, S.O.; Thimann, K.V. Relation between respiration and senescence in oat leaves. Plant Physiol. 1983, 72, 540–546. [Google Scholar] [CrossRef]
- Malik, N.S.; Thimann, K.V. Metabolism of oat leaves during senescence: VI. Changes in ATP levels. Plant Physiol. 1980, 65, 855–859. [Google Scholar] [CrossRef]
- Sulpice, R.; Pyl, E.T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C.; et al. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef]
- Santelia, D.; Lunn, J.E. Transitory starch metabolism in guard cells: Unique features for a unique function. Plant Physiol. 2017, 174, 539–549. [Google Scholar] [CrossRef]
- Pourtau, N.; Jennings, R.; Pelzer, E.; Pallas, J.; Wingler, A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 2006, 224, 556–568. [Google Scholar] [CrossRef]
- Wingler, A.; Masclaux-Daubresse, C.; Fischer, A.M. Sugars, senescence, and ageing in plants and heterotrophic organisms. J. Exp. Bot. 2009, 60, 1063–1066. [Google Scholar] [CrossRef]
- Izumi, M.; Ishida, H. The changes of leaf carbohydrate contents as a regulator of autophagic degradation of chloroplasts via Rubisco-containing bodies during leaf senescence. Plant Signal. Behav. 2011, 6, 685–687. [Google Scholar] [CrossRef]
- Bi, H.; Liu, P.; Jiang, Z.; Ai, X. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol. Plant 2017, 161, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Phee, B.K.; Jeong, S.; Lee, S.Y.; Tateno, Y.; Allakhverdiev, S.I.; Lee, C.H.; Nam, H.G. Age-dependent changes in the functions and compositions of photosynthetic complexes in the thylakoid membranes of Arabidopsis thaliana. Photosynth. Res. 2013, 117, 547–556. [Google Scholar] [CrossRef]
- Kucharewicz, W.; Distelfeld, A.; Bilger, W.; Müller, M.; Munné-Bosch, S.; Hensel, G.; Krupinska, K. Acceleration of leaf senescence is slowed down in transgenic barley plants deficient in the DNA/RNA-binding protein WHIRLY1. J. Exp. Bot. 2017, 68, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Miersch, I.; Heise, J.; Zelmer, I.; Humbeck, K. Differential degradation of the photosynthetic apparatus during leaf senescence in barley (Hordeum vulgare L.). Plant Biol. 2000, 2, 618–623. [Google Scholar] [CrossRef]
- Janečková, H.; Husičková, A.; Lazár, D.; Ferretti, U.; Pospíšil, P.; Špundová, M. Exogenous application of cytokinin during dark senescence eliminates the acceleration of photosystem II impairment caused by chlorophyll b deficiency in barley. Plant Physiol. Biochem. 2019, 136, 43–51. [Google Scholar] [CrossRef]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-Hydroxymethyl Chlorophyll a Reductase of the Chlorophyll Cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef]
- Sacharz, J.; Giovagnetti, V.; Ungerer, P.; Mastroianni, G.; Ruban, A.V. The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat. Plants 2017, 3, 16225. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Phil. Trans. R. Soc. B 2014, 369, 20130225. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef]
- Cortleven, A.; Nitschke, S.; Klaumünzer, M.; AbdElgawad, H.; Asard, H.; Grimm, B.; Riefler, M.; Schmülling, T. A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase 2 and 3 receptors. Plant Physiol. 2014, 164, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk-Nowicka, E.; Wrzesiński, T.; Bagniewska-Zadworna, A.; Kubala, S.; Rucińska-Sobkowiak, R.; Polcyn, W.; Misztal, L.; Mattoo, A.K. Physio-genetic dissection of dark-induced leaf senescence and timing its reversal in barley. Plant Physiol. 2018, 178, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant. 2016, 158, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Fischer, A.M. Cathepsin B- and L-like Protease Activities Are Induced During Developmental Barley Leaf Senescence. Plants 2024, 13, 3009. [Google Scholar] [CrossRef]
- Swartzberg, D.; Dai, N.; Gan, S.; Amasino, R.; Granot, D. Effects of cytokinin production under two SAG promoters on senescence and development of tomato plants. Plant Biol. 2006, 8, 579–586. [Google Scholar] [CrossRef]
- Fischer-Kilbienski, I.; Miao, Y.; Roitsch, T.; Zschiesche, W.; Humbeck, K.; Krupinska, K. Nuclear targeted AtS40 modulates senescence-associated gene expression in Arabidopsis thaliana during natural development and in darkness. Plant Mol. Biol. 2010, 73, 379–390. [Google Scholar] [CrossRef]
- Masferrer, A.; Arró, M.; Manzano, D.; Schaller, H.; Fernández-Busquets, X.; Moncaleán, P.; Fernández, B.; Cunillera, N.; Boronat, A.; Ferrer, A. Overexpression of Arabidopsis thaliana farnesyl diphosphate synthase (FPS1S) in transgenic Arabidopsis induces a cell death/senescence-like response and reduced cytokinin levels. Plant J. 2002, 30, 123–132. [Google Scholar] [CrossRef]
- Liu, Z.; Marella, C.B.N.; Hartmann, A.; Hajirezaei, M.R.; von Wirén, N. An Age-Dependent Sequence of Physiological Processes Defines Developmental Root Senescence. Plant Physiol. 2019, 181, 993–1007. [Google Scholar] [CrossRef]
- Veliz, C.G.; Criado, M.V.; Galotta, M.F.; Roberts, I.N.; Caputo, C. Regulation of senescence-associated protease genes by sulphur availability according to barley (Hordeum vulgare L.) phenological stage. Ann. Bot. 2020, 126, 435–444. [Google Scholar] [CrossRef]
- Han, Y.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 39155. [Google Scholar] [CrossRef]
- Zmienko, A.; Samelak-Czajka, A.; Goralski, M.; Sobieszczuk-Nowicka, E.; Kozlowski, P.; Figlerowicz, M. Selection of reference genes for qPCR- and ddPCR-based analyses of gene expression in senescing barley leaves. PLoS ONE 2015, 10, e0118226. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Hwang, I.; Sakakibara, H. Cytokinin biosynthesis and perception. Physiol. Plant. 2006, 126, 528–538. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book Am. Soc. Plant Biol. 2014, 12, e0168. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Glanz-Idan, N.; Lach, M.; Tarkowski, P.; Vrobel, O.; Wolf, S. Delayed Leaf Senescence by Upregulation of Cytokinin Biosynthesis Specifically in Tomato Roots. Front. Plant Sci. 2022, 13, 922106. [Google Scholar] [CrossRef]
- Sakano, Y.; Okada, Y.; Matsunaga, A.; Suwama, T.; Kaneko, T.; Ito, K.; Noguchi, H.; Abe, I. Molecular cloning, expression, and characterization of adenylate isopentenyltransferase from hop (Humulus lupulus L.). Phytochemistry 2004, 65, 2439–2446. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Forés, O.; Martínez-García, J.F.; González, V.; Phillips, M.A.; Ferrer, A.; Boronat, A. Distinct light-mediated pathways regulate the biosynthesis and exchange of isoprenoid precursors during Arabidopsis seedling development. Plant Cell 2004, 16, 144–156. [Google Scholar] [CrossRef]
- Yang, D.; Du, X.; Liang, X.; Han, R.; Liang, Z.; Liu, Y.; Liu, F.; Zhao, J. Different roles of the mevalonate and methylerythritol phosphate pathways in cell growth and tanshinone production of Salvia miltiorrhiza hairy roots. PLoS ONE 2012, 7, e46797. [Google Scholar] [CrossRef]
- Hartig, K.; Beck, E. Assessment of lovastatin application as tool in probing cytokinin-mediated cell cycle regulation. Physiol. Plant. 2005, 125, 260–267. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Hoeffler, J.F.; Meyer, O.; Tritsch, D.; Kagan, I.A.; Grosdemange-Billiard, C.; Rohmer, M.; Bach, T.J. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 2003, 278, 26666–26676. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Tian, F.; Li, Q.; Wang, W. Cytokinin-regulated sucrose metabolism in stay-green wheat phenotype. PLoS ONE 2016, 11, e0161351. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Tian, F.; Li, Q.; Wang, W. The stay-green phenotype of wheat mutant tasg1 is associated with altered cytokinin metabolism. Plant Cell Rep. 2016, 35, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Crowell, D.N.; Salaz, M.S. Inhibition of growth of cultured tobacco cells at low concentrations of lovastatin is reversed by cytokinin. Plant Physiol. 1992, 100, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, T.; Matsubara, S.; Endo, A. Compactin (ML-236B) as a new growth inhibitor of plant callus. Agric. Biol. Chem. 1983, 47, 1401–1403. [Google Scholar] [CrossRef][Green Version]
- Węzigowska, P.; Trojak, M.; Skowron, E. The role of the cytokinin biosynthesis pathways in the rate of tobacco leaf senescence. Folia Biol. Oecol. 2024, 18, 39–47. [Google Scholar] [CrossRef]
- Rosenwasser, S.; Rot, I.; Sollner, E.; Meyer, A.J.; Smith, Y.; Leviatan, N.; Fluhr, R.; Friedman, H. Organelles contribute differentially to reactive oxygen species-related events during extended darkness. Plant Physiol. 2011, 156, 185–201. [Google Scholar] [CrossRef]
- Shirao, M.; Kuroki, S.; Kaneko, K.; Kinjo, Y.; Tsuyama, M.; Förster, B.; Takahashi, S.; Badger, M.R. Gymnosperms have increased capacity for electron leakage to oxygen (Mehler and PTOX reactions) in photosynthesis compared with angiosperms. Plant Cell Physiol. 2013, 54, 1152–1163. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Procházková, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Ferrante, A.; Carillo, P. Leaf senescence-associated genes and transcriptional changes under dark conditions. Ann. Bot. 2025, mcaf063, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Wilhelmová, N. Leaf senescence and activities of the antioxidant enzymes. Biol. Plant. 2007, 51, 401–406. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Noworolnik, K. Porównanie reakcji wielorzędowych i dwurzędowych odmian jęczmienia ozimego na poziom nawożenia azotem i termin siewu. Biul. Inst. Hodowli Aklim. Roślin 2005, 237, 39–49. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Noworolnik, K. Plonowanie jęczmienia ozimego dwurzędowego w zależności od gęstości i terminu siewu. Fragm. Agron. 2017, 34, 40–48. [Google Scholar]
- Yamburenko, M.V.; Zubo, Y.O.; Vanková, R.; Kusnetsov, V.V.; Kulaeva, O.N.; Börner, T. Abscisic acid represses the transcription of chloroplast genes. J. Exp. Bot. 2013, 64, 4491–4502. [Google Scholar] [CrossRef]
- Guo, F.Q.; Crawford, N.M. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 2005, 17, 3436–3450. [Google Scholar] [CrossRef]
- Roman, H.; Girault, T.; Barbier, F.; Péron, T.; Brouard, N.; Pěnčík, A.; Novák, O.; Vian, A.; Sakr, S.; Lothier, J.; et al. Cytokinins are initial targets of light in the control of bud outgrowth. Plant Physiol. 2020, 172, 489–509. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Pazos-Navarro, M.; Piñero, M.C.; Otálora-Alcón, G.; López-Marín, J.; del Amor, F.M. Regulation of the drought response of sweet pepper (Capsicum annuum L.) by foliar-applied hormones, in Mediterranean-climate greenhouse conditions. Plant Growth Regul. 2016, 80, 159–169. [Google Scholar] [CrossRef]
- Sasi, J.M.; Gupta, S.; Singh, A.; Kujur, A.; Agarwal, M.; Katiyar-Agarwal, S. Know when and how to die: Gaining insights into the molecular regulation of leaf senescence. Physiol. Mol. Biol. Plants 2022, 28, 1515–1534. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Terzi, R.; Kadioglu, A. Drought stress tolerance and the antioxidant enzyme system. Acta Biol. Cracov. Ser. Bot. 2006, 48, 89–96. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Dahal, K.; Martyn, G.D.; Alber, N.A.; Vanlerberghe, G.C. Coordinated regulation of photosynthetic and respiratory components is necessary to maintain chloroplast energy balance in varied growth conditions. J. Exp. Bot. 2017, 68, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From Leaf to Whole-Plant Water Use Efficiency (WUE) in Complex Canopies: Limitations of Leaf WUE as a Selection Target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Evans, J.R.; Santiago, L.S. PrometheusWiki Gold Leaf Protocol: Gas Exchange Using LI-COR 6400. Funct. Plant Biol. 2014, 41, 223–226. [Google Scholar] [CrossRef]
- Lobo, F.D.A.; de Barros, M.P.; Dalmagro, H.J.; Dalmolin, Â.C.; Pereira, W.E.; de Souza, É.C.; Vourlitis, G.L.; Rodríguez Ortíz, C.E. Fitting net photosynthetic light-response curves with Microsoft Excel—A critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Kaipiainen, E.L. Parameters of photosynthesis light curve in Salix dasyclados and their changes during the growth season. Russ. J. Plant Physiol. 2009, 56, 445–453. [Google Scholar] [CrossRef]
- Skowron, E.; Trojak, M. Effect of exogenously-applied abscisic acid, putrescine and hydrogen peroxide on drought tolerance of barley. Biologia 2021, 76, 453–468. [Google Scholar] [CrossRef]
- Tercé-Laforgue, T.; Mäck, G.; Hirel, B. New Insights Towards the Function of Glutamate Dehydrogenase Revealed During Source–Sink Transition of Tobacco (Nicotiana tabacum) Plants Grown under Different Nitrogen Regimes. Physiol. Plant. 2004, 120, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Barreto, P.; Yassitepe, J.E.C.T.; Wilson, Z.A.; Arruda, P. Mitochondrial uncoupling protein 1 overexpression increases yield in Nicotiana tabacum under drought stress by improving source and sink metabolism. Front. Plant Sci. 2017, 8, 1836. [Google Scholar] [CrossRef]
- Xu, P.L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of Long-Term Chilling on Ultrastructure and Antioxidant Activity in Leaves of Two Cucumber Cultivars under Low Light. Physiol. Plant. 2008, 132, 467–478. [Google Scholar] [CrossRef]




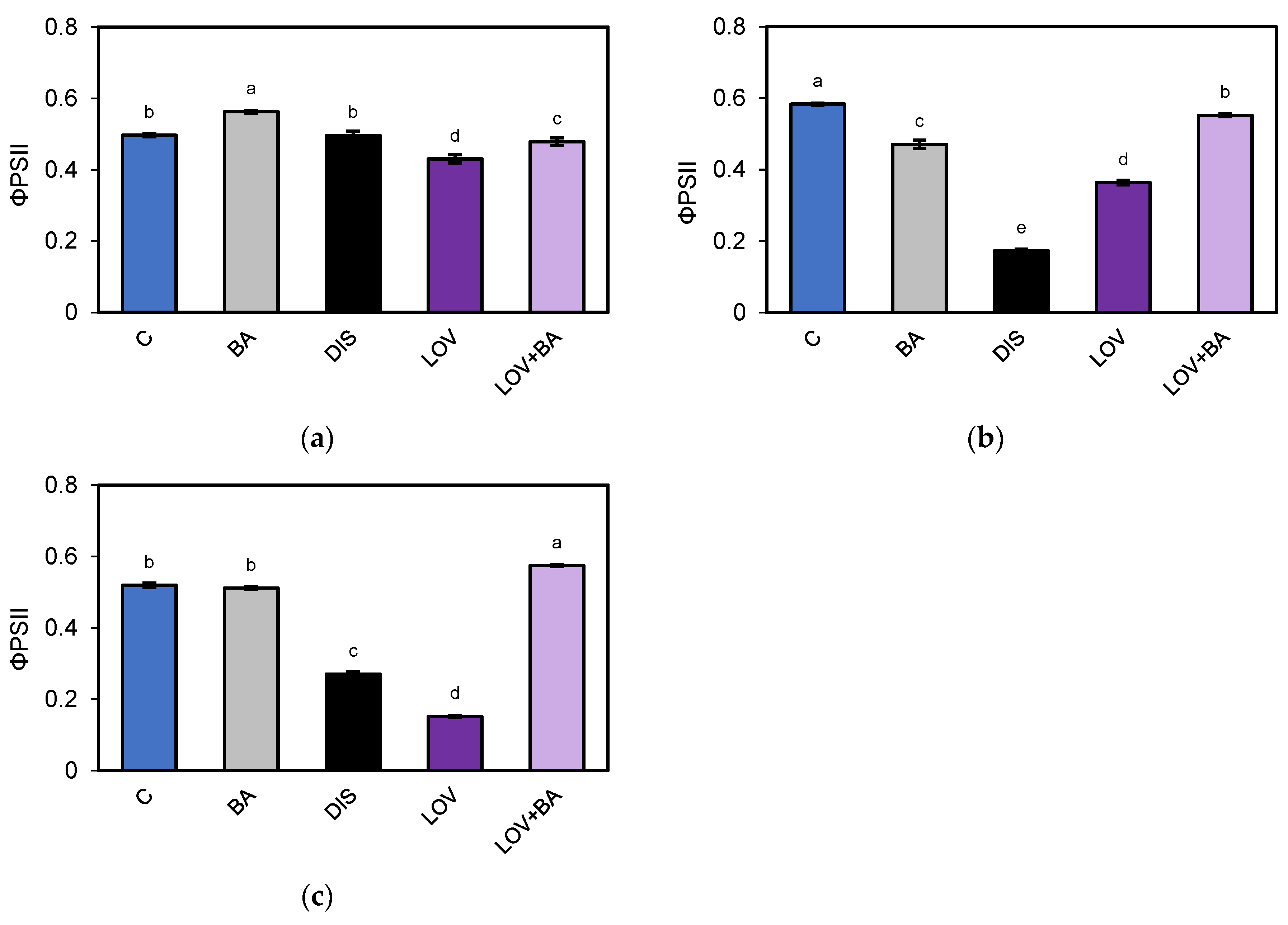
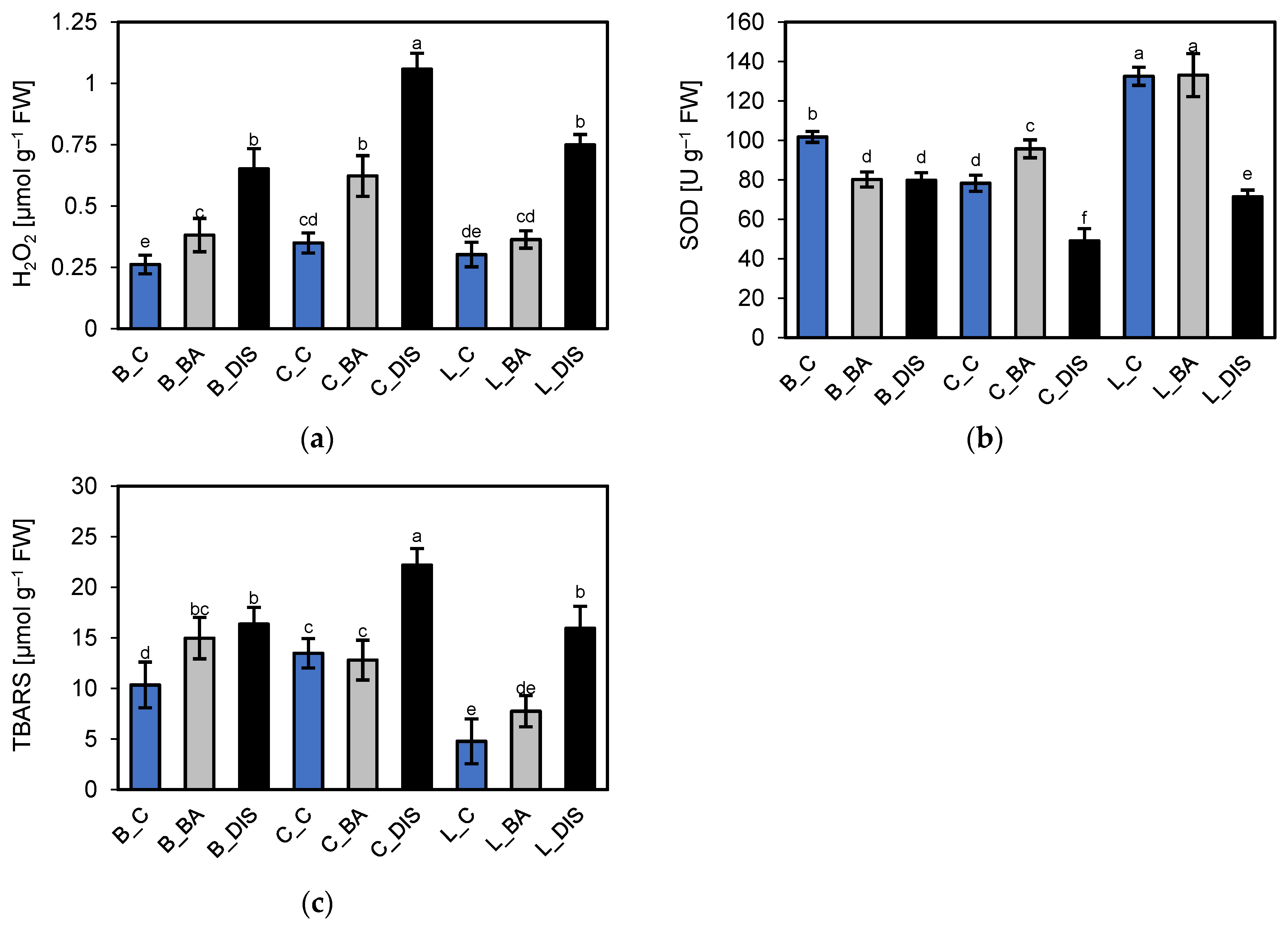
| Parameter | Cultivar and Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B_C | B_BA | B_DIS | C_C | C_BA | C_DIS | L_C | L_BA | L_DIS | |
| Pgmax [µmol(CO2) m−2 s−1] | 28.63 ± 3.16 b | 15.04 ± 1.87 c | 9.49 ± 1.11 d | 29.88 ± 3.34 b | 7.14 ± 0.68 e | 2.00 ± 0.32 g | 35.33 ± 3.95 a | 10.70 ± 1.14 d | 5.78 ± 0.84 f |
| Pnmax [µmol(CO2) m−2 s−1] | 25.07 ± 2.43 b | 12.60 ± 1.49 c | 7.68 ± 0.89 d | 25.52 ± 2.62 b | 5.12 ± 0.54 e | 1.46 ± 0.13 f | 30.50 ± 3.86 a | 8.68 ± 0.87 d | 4.41 ± 0.59 e |
| Rd [µmol(CO2) m−2 s−1] | 1.08 ± 0.14 c | 1.77 ± 0.11 a | 1.49 ± 0.24 b | 1.52 ± 0.12 b | 1.78 ± 0.21 a | 0.44 ± 0.12 d | 1.72 ± 0.23 a | 1.65 ± 0.27 ab | 1.22 ± 0.12 c |
| Icomp [µmol(photons) m−2 s−1] | 7.25 ± 0.76 e | 12.03 ± 1.43 c | 12.63 ± 1.47 c | 10.99 ± 1.24 d | 23.16 ± 2.56 b | 29.62 ± 3.65 a | 9.65 ± 1.17 d | 12.44 ± 1.45 c | 13.40 ± 1.28 c |
| ΦI0–Icomp [µmol(CO2) µmol(photons)–1] | 0.149 ± 0.02 b | 0.147 ± 0.03 b | 0.119 ± 0.02 c | 0.139 ± 0.024 b | 0.076 ± 0.013 d | 0.015 ± 0.004 e | 0.179 ±0.02 a | 0.133 ±0.02 b | 0.091 ± 0.011 d |
| ΦIcomp–I200 [µmol(CO2) µmol(photons)–1] | 0.068 ± 0.01 a | 0.041 ± 0.01 b | 0.026 ± 0.004 c | 0.067 ±0.013 a | 0.018 ± 0.004 d | 0.005 ± 0.001 e | 0.083 ± 0.012 a | 0.029 ± 0.01 c | 0.015 ± 0.003 d |
| Protein | Relative Abundance [AU] ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C_C | C_DIS | C_BA | L_C | L_DIS | L_BA | B_C | B_DIS | B_BA | |
| Rubisco LSU | 12.07 ± 0.30 c | 11.72 ± 0.22 d | 12.58 ± 0.14 b | 13.19 ± 0.55 a | 12.16 ± 0.15 c | 12.79 ± 0.41 b | 12.09 ± 0.17 c | 11.67 ± 0.41 d | 12.37 ± 0.30 b |
| Rubisco SSU | 3.00 ± 0.04 b | 2.67 ± 0.03 d | 2.82 ± 0.06 c | 2.92 ± 0.12 b | 2.76 ± 0.13 cd | 2.97 ± 0.08 b | 2.87 ± 0.13 bc | 2.64 ± 0.03 d | 3.62 ± 0.10 a |
| RCAtotal | 11.19 ± 0.20 bc | 8.75 ± 0.13 f | 10.57 ± 0.22 d | 11.66 ± 0.29 b | 10.05 ± 0.36 e | 10.44 ± 0.29 d | 10.95 ± 0.17 c | 8.10 ± 0.37 g | 12.63 ± 0.23 a |
| PsbO | 8.88 ± 0.39 b | 8.85 ± 0.34 b | 8.10 ± 0.20 cd | 9.73 ± 0.14 a | 8.43 ± 0.34 bc | 7.99 ± 0.27 d | 7.63 ± 0.30 e | 7.52 ± 0.09 e | 7.87 ± 0.20 d |
| PsbA | 9.24 ± 0.09 a | 6.98 ± 0.08 d | 8.63 ± 0.37 b | 5.25 ± 0.14 g | 5.59 ± 0.11 f | 5.61 ± 0.23 f | 7.70 ± 0.35 c | 6.30 ± 0.14 e | 7.63 ± 0.09 c |
| Lhcb5 | 2.33 ± 0.11 b | 1.47 ± 0.04 e | 1.78 ± 0.05 d | 2.81 ± 0.08 a | 1.96 ± 0.09 c | 1.97 ± 0.05 c | 1.86 ± 0.03 c | 1.91 ± 0.08 c | 1.68 ± 0.05 d |
| Lhcb1 | 14.26 ± 0.52 b | 5.75 ± 0.25 f | 7.44 ± 0.32 e | 13.89 ± 0.27 b | 11.76 ± 0.41 d | 12.74 ± 0.22 c | 12.44 ± 0.62 c | 11.76 ± 0.48 d | 15.58 ± 0.58 a |
| PsaB | 5.64 ± 0.06 b | 4.19 ± 0.06 e | 6.01 ± 0.23 a | 4.75 ± 0.06 d | 3.70 ± 0.04 f | 3.71 ± 0.11 f | 5.10 ± 0.25 c | 4.61 ± 0.06 d | 5.52 ± 0.19 b |
| Lhca4 | 2.28 ± 0.03 d | 2.07 ± 0.06 e | 2.56 ± 0.09 b | 2.12 ± 0.10 e | 2.17 ± 0.07 e | 2.44 ± 0.11 bc | 2.36 ± 0.12 cd | 2.23 ± 0.05 d | 2.85 ± 0.09 a |
| SAG12 | 0.00 ± 0.00 d | 18.91 ± 0.35 a | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 16.64 ± 0.34 b | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 15.65 ± 0.51 c | 0.00 ± 0.00 d |
| eEF1a | 3.73 ± 0.15 a | 3.86 ± 0.12 a | 3.88 ± 0.15a | 3.05 ± 0.06 bc | 2.82 ± 0.11 c | 2.94 ± 0.06 c | 2.96 ± 0.03 c | 3.16 ± 0.07 b | 3.01 ± 0.13 bc |
| Parameter | Equation | Definition | Reference |
|---|---|---|---|
| Fv/Fm | Fv/Fm = (Fm − Fo)/Fm | maximum quantum efficiency of PSII photochemistry after dark adaptation | [96] |
| ΦPSII | ΦPSII = (Fm′ − F)/Fm′ | effective quantum yield of PSII in light-adapted state * | [97] |
| qP | qP = (Fm′ − F)/(Fm′ − Fo′) | photochemical quenching coefficient of PSII based on the puddle model | [98] |
| ETR | ETR = ΦPSII × PAR × Abs × 0.5 | electron transport rate through PSII | [99] |
| RFd | RFd = (Fm – Fs **)/Fs | fluorescence decrease ratio (vitality index) | [94] |
| ΦNPQ | ΦNPQ = 1 − ΦPSII − 1/[NPQ + 1 + qL (Fm/Fo − 1)] | quantum yield of regulated non-photochemical energy dissipation | [98] |
| ΦNO | ΦNO = 1/[NPQ + 1 + qL (Fm/Fo − 1)] | quantum yield of non-regulated (constitutive) energy dissipation | [98] |
| NPQ | NPQ = (Fm − Fm′)/Fm′ | non-photochemical quenching in PSII | [100] |
| qN | qN = (Fm − Fm′)/(Fm − Fo′) | coefficient of non-photochemical quenching in PSII | [31] |
| 1 − qP | 1 − qP = 1 − [(Fm′ − F)/(Fm′ − Fo′)] | PSII excitation pressure based on photochemical quenching | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, E.; Trojak, M.; Szymkiewicz, J.; Nawrot, D. Mechanistic Insights into Cytokinin-Regulated Leaf Senescence in Barley: Genotype-Specific Responses in Physiology and Protein Stability. Int. J. Mol. Sci. 2025, 26, 9749. https://doi.org/10.3390/ijms26199749
Skowron E, Trojak M, Szymkiewicz J, Nawrot D. Mechanistic Insights into Cytokinin-Regulated Leaf Senescence in Barley: Genotype-Specific Responses in Physiology and Protein Stability. International Journal of Molecular Sciences. 2025; 26(19):9749. https://doi.org/10.3390/ijms26199749
Chicago/Turabian StyleSkowron, Ernest, Magdalena Trojak, Julia Szymkiewicz, and Dominika Nawrot. 2025. "Mechanistic Insights into Cytokinin-Regulated Leaf Senescence in Barley: Genotype-Specific Responses in Physiology and Protein Stability" International Journal of Molecular Sciences 26, no. 19: 9749. https://doi.org/10.3390/ijms26199749
APA StyleSkowron, E., Trojak, M., Szymkiewicz, J., & Nawrot, D. (2025). Mechanistic Insights into Cytokinin-Regulated Leaf Senescence in Barley: Genotype-Specific Responses in Physiology and Protein Stability. International Journal of Molecular Sciences, 26(19), 9749. https://doi.org/10.3390/ijms26199749






