From Diabetes to Degenerative Diseases: The Multifaceted Action of Metformin
Abstract
1. Introduction
2. Metformin in T2DM: A Longstanding Therapeutic Application
3. Multifaceted Metformin: Exploring Its Role in Age-Related Diseases
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
| Preclinical Studies | |||
|---|---|---|---|
| Model | Metformin Dose/Concentration and Duration | Treatment Effects | Reference |
| In vitro. MPP+-treated SH-SY5Y cells In vivo. MPTP/p PD mouse model | 2 mM for 1 h 5 mg/mL in drinking water for 5 weeks | Protection against apoptosis ↓ the proportion of dysfunctional mitochondria and ROS generation Improvement in motor deficits ↑ dopamine levels ↓ DA neuron degeneration and SNCA accumulation ↓ NLRP3 inflammasome activity, ↓ IL-1 production, ↓ TNF-α, IL-6 mRNAs, ↑ IL-10 mRNA levels, no effect on IL-4 and TGF-β mRNA levels | [140] |
| In vitro. Tetracycline-treated SH-SY5Y cells In vitro. Transfected human SNCA HeLa cells In vivo. WT C57BL/6 mice | 0.5, 1.0, or 2.5 mM for 16 h or 24 h 0.5, 1.0, or 2.5 mM for 16 h 5 g/kg in food pellets for 1 month or 5 g/L in drinking water for 6 months | ↓ phospho-Ser129 SNCA levels ↓ phospho-Ser129 SNCA levels ↓ phosphorylated SNCA protein | [145] |
| In vitro. MPP+-treated SH-SY5Y cells In vivo. MPTP PD mouse model | 0.1, 0.25, or 0.5 mM for 48 h 200 or 400 mg/kg in drinking water for 14 days | ↑ mitochondrial marker proteins (SDHA, PDHA, VDAC, and HSP60) ↑ PGC-1α expression ↑ SOD1, SOD2, GPX1, CAT1, NRF1, TFAM, and UCP2 mRNAs ↑ mitochondrial marker proteins (SDHA, PDHA, VDAC, and COXIV) ↑ PGC-1α expression Protection of dopaminergic neurons Improvement in motor behaviour | [142] |
| In vitro. BV2 cells treated with LPS In vitro. BV2 cells treated with IL-4 In vivo. LPS-induced PD rat model | 1 mM for 12 h Two daily oral doses (150 mg/kg) dissolved in tap water for 7 days | ↓ microglial activation (↓ IL-1β mRNA levels, no effect on iNOS and TNF-α mRNAs) ↓ ROS production ↓ NLRP3 inflammasome activation ↓ microglial activation (↓ arginase mRNA levels, no effect on IL-10 mRNA levels) ↓ number of activated microglial cells ↓ TNF-α, IL-1β, and IL-6 mRNA levels; ↑ MCP-1, CD200, and CX3CR1 mRNA levels No effect on dopaminergic neuronal loss protection | [136] |
| In vitro. SH-SY5Y cells treated with rotenone | 10 μM, 100 μM, 1 mM, or 10 mM for 2 h, 3 h, 6 h | ↓ cellular death and 3/7 caspase activation ↓ ROS production ↑ GSH and SOD levels Upregulation of Nrf2/HO-1 pathway ↑ PGC-1α levels | [150] |
| In vitro. MPP+-treated SH-SY5Y cells In vivo. MPTP PD mouse model | 0.5 mM for 4 h Once daily intraperitoneal injection (200 mg/kg) for 7 days | Neuroprotection is partially mediated by the BDNF/TrkB signaling pathway ↓ Caspase-3 ↑ Dopamine and DOPAC levels Improvement in motor deficits ↓ Astroglial activation ↓ SNCA levels ↑ BDNF, AKT and ERK1/2 | [143] |
| In vitro. MPP+ -N27 dopaminergic cell line derived from rat ventral mesencephalon In vivo. MPTP PD mouse model | 0.1 mM or 1 mM for 24 h Two daily doses (150 mg/kg) for 7 days | ↓ ATP production ↑ ROS production ↓ TNF-α, IL-1β, and iNOS mRNAs levels No effect on dopaminergic neuronal loss ↓ DOPAC | [138] |
| In vivo. 6-OHDA PD mouse model | Once daily oral doses (100 mg/kg or 200 mg/kg) for 4 weeks | Improved motor impairments No protective effect against dopaminergic cell death ↑ AMPK, AKT, GSK3b, CREB phosphorylation, and BDNF levels ↓ Astrocyte activation | [146] |
| In vivo. MPTP PD mouse model | 500 mg/kg orally for 21 days | Improved locomotor and muscular activities ↑ SOD, CAT, and GSH levels ↓ Lipid peroxidation ↑ BDNF levels | [137] |
| In vivo. Haloperidol-induced catalepsy PD mouse model | Once daily oral doses (20, 50, or 100 mg/kg) for 21 days | No effect on motor coordination, but improvement in memory deficit ↓ Duration of catalepsy score ↓ MDA and nitric oxide levels, and ↑ GSH and CAT activity No effect on SOD activity | [139] |
| In vivo. MPTP AMPK WT and KO PD mouse model | 100 mg/kg dissolved in water for 27 days | Attenuation in the loss of neuron number and volume, as well as an increase in gliosis ↓ DOPAC:DA ratio | [141] |
| In vivo. MDMA PD mouse model | 200–400 mg/kg orally 11 h intervals for 48 h and 7 days | Attenuation of TH-positive neuronal loss | [144] |
| In vivo. Rotenone PD mouse model | Once daily oral doses (100 or 200 mg/kg) for 18 days administered through a gastric gavage tube | Improvement in animals’ motor function ↓ MDA, ↑ GSH, HO-1, and dopamine levels ↑ Nrf2, thioredoxin, AMPK, and FOXO3 mRNA levels ↑ Nrf2, HO-1, AMPK, FOXO3, and thioredoxin protein expression ↓ Cleaved-caspase 3 and VEGF levels ↑ the number of TH-positive neurons | [147] |
| In vivo. Rotenone PD mouse model | Once daily intraperitoneal injection (300 mg/kg) for 10 days | No difference in motor behaviour Partial attenuation of dopaminergic neuronal loss ↓ Caspase-3 ↓ SNCA accumulation ↓ 4-HNE and MDA levels | [149] |
| In vivo. 6-OHDA PD C. elegans strain BZ555 model | Oral doses (5 mM or 10 mM) for 72 h | Increase in lifespan ↓ Dopaminergic neurons degeneration ↓ SNCA aggregation ↑ TH gene cat3 and antioxidant gene sod3 | [148] |
| In vivo. RNAi-mediated knockdown of C. elegans bcat-1 PD model | 50 μM for 5 days | ↑ the number of dopaminergic cell bodies Improvement in neurite morphologies of dopaminergic neurons Restoration of normal mitochondrial activity levels | [177] |
| Clinical Studies | |||
| Study Method | Subjects | Treatment Effects | Reference |
| Retrospective cohort study | n = 1879 T2DM patients treated with metformin only for 11 years n = 3431 T2DM patients treated with sulfonylureas only for 11 years n = 6420 T2DM patients treated with metformin + sulfonylureas for 11 years | Increased PD risk in T2DM patients treated with sulfonylureas (HR = 1.57, 95% CI = 1.15–2.13) No effect on PD risk in T2DM patients treated with metformin (HR = 0.95, 95% CI = 0.53–1.71) and with sulfonylureas plus metformin (HR = 0.78, 95% CI = 0.61–1.01) | [151] |
| Retrospective cohort study | n = 4651 T2DM patients treated with metformin for at least 90 days n = 4651 T2DM patients with no metformin therapy | Higher PD risk (HR = 2.27, 95% CI = 1.68–3.07) in the metformin group Higher PD risk in patients with T2DM receiving metformin therapy for 300–399 days (aHR = 2.20, 95% CI = 1.47–3.28) and ≥400 days (aHR = 4.49, 95% CI = 3.06–6.58) PD risk increased from 1.58 (95% CI = 1.02–2.44) in patients receiving average doses of metformin ≤ 130 g per year to 3.54 in patients receiving average doses of >385 g per year (95% CI = 2.41–5.20) | [152] |
| Retrospective cohort study | n = 8396 T2DM patients treated with glitazones for 10 years n = 94,349 T2DM patients treated with metformin for 10 years | Lower PD risk in patients with T2DM receiving glitazone drug compared to the metformin group (HR = 0.72, 95% CI = 0.54–0.94) | [153] |
| Cross-sectional study | n = 384,716 T2DM patients treated with metformin | Higher ORs for PD in patients with T2DM with increased cDDD of metformin after 3 years: <300 (OR = 0.88, 95% CI = 0.83–0.94), 300–500 (OR = 1.09, 95% CI = 0.72–1.65), and >500 (OR = 2.59, 95% CI = 0.83–8.03) g per year Higher ORs for PD in patients with T2DM with increased intensity of metformin use (DDD/month) after 3 years: <10 (OR = 0.87, 95% CI = 0.81–0.93), 10–25 (OR = 0.92, 95% CI = 0.83–1.02), and ≥25 (OR = 1.17, 95% CI = 0.80–1.72) g per month Higher ORs for PD in patients with T2DM with increased cDDD of metformin after 5 years: <300 (OR = 0.94, 95% CI = 0.90–0.98), 300–500 (OR = 1.01, 95% CI = 0.75–1.35), and >500 (OR = 1.24, 95% CI = 0.40–3.83) g per year Higher ORs for PD in patients with T2DM with increased intensity of metformin use (DDD/month) after 5 years: <10 (OR = 0.93, 95% CI = 0.89–0.98), 10–25 (OR = 0.97, 95% CI = 0.90–1.04), and ≥25 (OR = 1.02, 95% CI = 0.77–1.35) g per month | [154] |
| Retrospective longitudinal cohort study | n = 2756 T2DM patients without metformin treatment n = 849 T2DM patients treated with metformin for less than 1 year n = 513 T2DM patients treated with metformin for 1–2 years n = 710 T2DM patients treated with metformin for 2–4 years n = 700 T2DM patients treated with metformin for more than 4 years | Lower risk of PD in elderly patients with T2DM after more than 4 years of metformin exposure (aHR = 0.04, 95% CI 0.00–0.37) | [71] |
3.3. Cardiovascular Disease
3.4. Age-Related Macular Degeneration
3.5. Osteoporosis
3.5.1. Effects on Osteoblasts
3.5.2. Effects on Osteoclasts
3.5.3. Clinical Insights into the Impact of Metformin on Bone Health in Older Adults
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kulkarni, A. Metformin: Past, Present, and Future. Curr. Diab. Rep. 2024, 24, 119–130. [Google Scholar] [CrossRef]
- Alrouji, M.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Ashour, N.A.; Jabir, M.S.; Negm, W.A.; Batiha, G.E.-S. Metformin Role in Parkinson’s Disease: A Double-Sword Effect. Mol. Cell. Biochem. 2024, 479, 975–991. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the Glucoregulatory Mechanisms of Metformin in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-Dose Metformin Targets the Lysosomal AMPK Pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-Eating and Self-Killing: Crosstalk between Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- He, L.; Wondisford, F.E. Metformin Action: Concentrations Matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Halabitska, I.; Petakh, P.; Lushchak, O.; Kamyshna, I.; Oksenych, V.; Kamyshnyi, O. Metformin in Antiviral Therapy: Evidence and Perspectives. Viruses 2024, 16, 1938. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef] [PubMed]
- Buczyńska, A.; Sidorkiewicz, I.; Krętowski, A.J.; Adamska, A. Examining the Clinical Relevance of Metformin as an Antioxidant Intervention. Front. Pharmacol. 2024, 15, 1330797. [Google Scholar] [CrossRef] [PubMed]
- Galal, M.A.; Al-Rimawi, M.; Hajeer, A.; Dahman, H.; Alouch, S.; Aljada, A. Metformin: A Dual-Role Player in Cancer Treatment and Prevention. Int. J. Mol. Sci. 2024, 25, 4083. [Google Scholar] [CrossRef]
- Ruan, G.; Wu, F.; Shi, D.; Sun, H.; Wang, F.; Xu, C. Metformin: Update on Mechanisms of Action on Liver Diseases. Front. Nutr. 2023, 10, 1327814. [Google Scholar] [CrossRef]
- Cao, G.; Gong, T.; Du, Y.; Wang, Y.; Ge, T.; Liu, J. Mechanism of Metformin Regulation in Central Nervous System: Progression and Future Perspectives. Biomed. Pharmacother. 2022, 156, 113686. [Google Scholar] [CrossRef]
- Song, A.; Zhang, C.; Meng, X. Mechanism and Application of Metformin in Kidney Diseases: An Update. Biomed. Pharmacother. 2021, 138, 111454. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.M.; Almouteri, M.M.; Alnakhli, F.T. Role of Metformin in Polycystic Ovary Syndrome (PCOS)-Related Infertility. Cureus 2023, 15, e44493. [Google Scholar] [CrossRef]
- Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Mabhida, S.E.; Jack, B.U.; Nyambuya, T.M.; Nkambule, B.B.; Basson, A.K.; Tiano, L.; Dludla, P.V. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int. J. Mol. Sci. 2023, 24, 2227. [Google Scholar] [CrossRef]
- Lupi, R.; Del Guerra, S.; Tellini, C.; Giannarelli, R.; Coppelli, A.; Lorenzetti, M.; Carmellini, M.; Mosca, F.; Navalesi, R.; Marchetti, P. The Biguanide Compound Metformin Prevents Desensitization of Human Pancreatic Islets Induced by High Glucose. Eur. J. Pharmacol. 1999, 364, 205–209. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Cheng, M.; Ren, L.; Jia, X.; Wang, J.; Cong, B. Understanding the Action Mechanisms of Metformin in the Gastrointestinal Tract. Front. Pharmacol. 2024, 15, 1347047. [Google Scholar] [CrossRef]
- Giusti, L.; Tesi, M.; Ciregia, F.; Marselli, L.; Zallocco, L.; Suleiman, M.; De Luca, C.; Del Guerra, S.; Zuccarini, M.; Trerotola, M.; et al. The Protective Action of Metformin against Pro-Inflammatory Cytokine-Induced Human Islet Cell Damage and the Mechanisms Involved. Cells 2022, 11, 2465. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gan, D.; Lin, S.; Zhong, Y.; Chen, M.; Zou, X.; Shao, Z.; Xiao, G. Metformin in Aging and Aging-Related Diseases: Clinical Applications and Relevant Mechanisms. Theranostics 2022, 12, 2722–2740. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.M.; Corum, D.; Beeson, C.C.; Schnellmann, R.G. Mitochondrial Biogenesis as a Pharmacological Target: A New Approach to Acute and Chronic Diseases. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 229–249. [Google Scholar] [CrossRef]
- Soukas, A.A.; Hao, H.; Wu, L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol. Metab. 2019, 30, 745–755. [Google Scholar] [CrossRef]
- Karnewar, S.; Neeli, P.K.; Panuganti, D.; Kotagiri, S.; Mallappa, S.; Jain, N.; Jerald, M.K.; Kotamraju, S. Metformin Regulates Mitochondrial Biogenesis and Senescence through AMPK Mediated H3K79 Methylation: Relevance in Age-Associated Vascular Dysfunction. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 1115–1128. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y. Targeting Autophagy in Aging and Aging-Related Cardiovascular Diseases. Trends Pharmacol. Sci. 2018, 39, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Niedernhofer, L.; Robbins, P.D.; Aroda, V.R.; Espeland, M.A.; Kritchevsky, S.B.; Kuchel, G.A.; Barzilai, N. Development of Clinical Trials to Extend Healthy Lifespan. Cardiovasc. Endocrinol. Metab. 2018, 7, 80–83. [Google Scholar] [CrossRef]
- Garber, A.J.; Duncan, T.G.; Goodman, A.M.; Mills, D.J.; Rohlf, J.L. Efficacy of Metformin in Type II Diabetes. Am. J. Med. 1997, 103, 491–497. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Goodman, A.M. Efficacy of Metformin in Patients with Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic Durability of Rosiglitazone, Metformin, or Glyburide Monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443, Correction in N. Engl. J. Med. 2007, 356, 1387–1388. [Google Scholar] [CrossRef]
- Charpentier, G.; Fleury, F.; Kabir, M.; Vaur, L.; Halimi, S. Improved Glycaemic Control by Addition of Glimepiride to Metformin Monotherapy in Type 2 Diabetic Patients. Diabet. Med. 2001, 18, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Wulffelé, M.G.; Kooy, A.; Lehert, P.; Bets, D.; Ogterop, J.C.; Borger Van Der Burg, B.; Donker, A.J.M.; Stehouwer, C.D.A. Combination of Insulin and Metformin in the Treatment of Type 2 Diabetes. Diabetes Care 2002, 25, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H.; Ryysy, L.; Nikkilä, K.; Tulokas, T.; Vanamo, R.; Heikkilä, M. Comparison of Bedtime Insulin Regimens in Patients with Type 2 Diabetes Mellitus. A Randomized, Controlled Trial. Ann. Intern. Med. 1999, 130, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Shen, S.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; et al. Safety and Effectiveness of Metformin plus Lifestyle Intervention Compared with Lifestyle Intervention Alone in Preventing Progression to Diabetes in a Chinese Population with Impaired Glucose Regulation: A Multicentre, Open-Label, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577, Correction in Lancet Diabetes Endocrinol. 2023, 11, E13. [Google Scholar] [CrossRef]
- Beysel, S.; Unsal, I.O.; Kizilgul, M.; Caliskan, M.; Ucan, B.; Cakal, E. The Effects of Metformin in Type 1 Diabetes Mellitus. BMC Endocr. Disord. 2018, 18, 1. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; DePinho, R.A.; Montminy, M.; Cantley, L.C. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single Phosphorylation Sites in Acc1 and Acc2 Regulate Lipid Homeostasis and the Insulin-Sensitizing Effects of Metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin Inhibits Hepatic Gluconeogenesis in Mice Independently of the LKB1/AMPK Pathway via a Decrease in Hepatic Energy State. J. Clin. Invest. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Kawoosa, F.; Shah, Z.A.; Masoodi, S.R.; Amin, A.; Rasool, R.; Fazili, K.M.; Dar, A.H.; Lone, A.; Ul Bashir, S. Role of Human Organic Cation Transporter-1 (OCT-1/SLC22A1) in Modulating the Response to Metformin in Patients with Type 2 Diabetes. BMC Endocr. Disord. 2022, 22, 140. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and Molecular Mechanisms of Metformin: An Overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sabet, A.; Djedjos, S.; Miller, R.; Sun, X.; Hussain, M.A.; Radovick, S.; Wondisford, F.E. Metformin and Insulin Suppress Hepatic Gluconeogenesis through Phosphorylation of CREB Binding Protein. Cell 2009, 137, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB Regulates Hepatic Gluconeogenesis through the Coactivator PGC-1. Nature 2001, 413, 179–183. [Google Scholar] [CrossRef]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin Reduces Liver Glucose Production by Inhibition of Fructose-1-6-Bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides Suppress Hepatic Glucagon Signalling by Decreasing Production of Cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef]
- Gunton, J.E.; Delhanty, P.J.D.; Takahashi, S.-I.; Baxter, R.C. Metformin Rapidly Increases Insulin Receptor Activation in Human Liver and Signals Preferentially through Insulin-Receptor Substrate-2. J. Clin. Endocrinol. Metab. 2003, 88, 1323–1332. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Funaki, M.; DiFransico, L.; Janmey, P.A. PI 4,5-P2 Stimulates Glucose Transport Activity of GLUT4 in the Plasma Membrane of 3T3-L1 Adipocytes. Biochim. Biophys. Acta BBA Mol. Cell Res. 2006, 1763, 889–899. [Google Scholar] [CrossRef]
- Froldi, G. View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences. Pharmaceuticals 2024, 17, 478. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Kamyshnyi, A. Effects of Metformin on the Gut Microbiota: A Systematic Review. Mol. Metab. 2023, 77, 101805. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, T.; Lv, N.; Liu, S.; Yuan, T.; Fu, Y.; Zhao, W.; Zhu, B. Metformin-Induced Changes of the Gut Microbiota in Patients with Type 2 Diabetes Mellitus: Results from a Prospective Cohort Study. Endocrine 2024, 85, 1178–1192. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Cong, B. Advances in the Mechanism of Metformin with Wide-Ranging Effects on Regulation of the Intestinal Microbiota. Front. Microbiol. 2024, 15, 1396031. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut Microbiota and Intestinal FXR Mediate the Clinical Benefits of Metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Abdulla, F.; Reljic, T.; Alnuaimi, S.; Serdarevic, F.; Asimi, Z.V.; Kumar, A.; Semiz, S. Effects of Combined Treatment of Probiotics and Metformin in Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2023, 202, 110806. [Google Scholar] [CrossRef]
- García-Lluch, G.; Marseglia, A.; Royo, L.M.; Albiach, J.P.; Garcia-Zamora, M.; Baquero, M.; Peña-Bautista, C.; Álvarez, L.; Westman, E.; Cháfer-Pericás, C. Associations between Antidiabetic Medications and Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2025, 103, 758–774. [Google Scholar] [CrossRef]
- Kaur, D.P.; Bucholc, M.; Finn, D.P.; Todd, S.; Wong-Lin, K.F.; McClean, P.L. Impact of Different Diagnostic Measures on Drug Class Association with Dementia Progression Risk: A Longitudinal Prospective Cohort Study. J. Alzheimer’s Dis. 2024, 100, 631–644. [Google Scholar] [CrossRef]
- Weinberg, M.S.; He, Y.; Kivisäkk, P.; Arnold, S.E.; Das, S. Effect of Metformin on Plasma and Cerebrospinal Fluid Biomarkers in Non-Diabetic Older Adults with Mild Cognitive Impairment Related to Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 99, S355–S365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, M.; Walker, V.; Yuan, J.; Korologou-Linden, R.; Robinson, J.; Huang, P.; Burgess, S.; Au Yeung, S.L.; Luo, S.; et al. Evaluating the Efficacy and Mechanism of Metformin Targets on Reducing Alzheimer’s Disease Risk in the General Population: A Mendelian Randomisation Study. Diabetologia 2022, 65, 1664–1675. [Google Scholar] [CrossRef]
- Pomilio, C.; Pérez, N.G.; Calandri, I.; Crivelli, L.; Allegri, R.; The ADNI Alzheimer’s Disease Neuroimaging Initiative; Sevlever, G.; Saravia, F. Diabetic Patients Treated with Metformin during Early Stages of Alzheimer’s Disease Show a Better Integral Performance: Data from ADNI Study. GeroScience 2022, 44, 1791–1805. [Google Scholar] [CrossRef]
- Secnik, J.; Xu, H.; Schwertner, E.; Hammar, N.; Alvarsson, M.; Winblad, B.; Eriksdotter, M.; Garcia-Ptacek, S.; Religa, D. The Association of Antidiabetic Medications and Mini-Mental State Examination Scores in Patients with Diabetes and Dementia. Alz. Res. Ther. 2021, 13, 197. [Google Scholar] [CrossRef]
- Wu, C.; Ouk, M.; Wong, Y.Y.; Anita, N.Z.; Edwards, J.D.; Yang, P.; Shah, B.R.; Herrmann, N.; Lanctôt, K.L.; Kapral, M.K.; et al. Relationships between Memory Decline and the Use of Metformin or DPP4 Inhibitors in People with Type 2 Diabetes with Normal Cognition or Alzheimer’s Disease, and the Role APOE Carrier Status. Alzheimer’s Dement. 2020, 16, 1663–1673. [Google Scholar] [CrossRef]
- Akimoto, H.; Negishi, A.; Oshima, S.; Wakiyama, H.; Okita, M.; Horii, N.; Inoue, N.; Ohshima, S.; Kobayashi, D. Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317519899546. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Kim, L.; Lee, J.; Moon, M.K. Taking Metformin and Cognitive Function Change in Older Patients with Diabetes. Geriatr. Gerontol. Int. 2019, 19, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Liu, S.; Fonseca, V.A.; Thethi, T.K.; Shi, L. Effect of Metformin on Neurodegenerative Disease among Elderly Adult US Veterans with Type 2 Diabetes Mellitus. BMJ Open 2019, 9, e024954. [Google Scholar] [CrossRef]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-Controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Perez, T.; Chang, H.; Mehta, P.; Steffener, J.; Pradabhan, G.; Ichise, M.; Manly, J.; Devanand, D.P.; Bagiella, E. Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J. Alzheimer’s Dis. 2016, 51, 501–514. [Google Scholar] [CrossRef]
- Imfeld, P.; Bodmer, M.; Jick, S.S.; Meier, C.R. Metformin, Other Antidiabetic Drugs, and Risk of Alzheimer’s Disease: A Population-Based Case–Control Study. J. Am. Geriatr. Soc. 2012, 60, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Grau-Jurado, P.; Mostafaei, S.; Xu, H.; Mo, M.; Petek, B.; Kalar, I.; Naia, L.; Kele, J.; Maioli, S.; Pereira, J.B.; et al. Medications and Cognitive Decline in Alzheimer’s Disease: Cohort Cluster Analysis of 15,428 Patients. J. Alzheimer’s Dis. 2025, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Guo, J.; Shaaban, C.E.; Feng, Z.; Wu, Y.; Magoc, T.; Hu, X.; Donahoo, W.T.; DeKosky, S.T.; Bian, J. Heterogeneous Treatment Effects of Metformin on Risk of Dementia in Patients with Type 2 Diabetes: A Longitudinal Observational Study. Alzheimer’s Dement. 2024, 20, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, C.; Saskin, R.; Shah, B.R.; Kapral, M.K.; Lanctôt, K.L.; Herrmann, N.; Cogo-Moreira, H.; MacIntosh, B.J.; Edwards, J.D.; et al. No Association between Metformin Initiation and Incident Dementia in Older Adults Newly Diagnosed with Diabetes. J. Intern. Med. 2024, 295, 68–78. [Google Scholar] [CrossRef]
- Ríos, J.A.; Bórquez, J.C.; Godoy, J.A.; Zolezzi, J.M.; Furrianca, M.C.; Inestrosa, N.C. Emerging Role of Metformin in Alzheimer’s Disease: A Translational View. Ageing Res. Rev. 2024, 100, 102439. [Google Scholar] [CrossRef]
- Cai, C.; Gu, C.; Meng, C.; He, S.; Thashi, L.; Deji, D.; Zheng, Z.; Qiu, Q. Therapeutic Effects of Metformin on Central Nervous System Diseases: A Focus on Protection of Neurovascular Unit. Pharm. Res. 2024, 41, 1907–1920. [Google Scholar] [CrossRef]
- Xu, F.; Shi, J. Insulin Signaling and Oxidative Stress: Bridging the Gap between Type 2 Diabetes Mellitus and Alzheimer’s Disease. J. Alzheimer’s Dis. 2025, 103, 994–1004. [Google Scholar] [CrossRef]
- Tong, B.; Ba, Y.; Li, Z.; Yang, C.; Su, K.; Qi, H.; Zhang, D.; Liu, X.; Wu, Y.; Chen, Y.; et al. Targeting Dysregulated Lipid Metabolism for the Treatment of Alzheimer’s Disease and Parkinson’s Disease: Current Advancements and Future Prospects. Neurobiol. Dis. 2024, 196, 106505. [Google Scholar] [CrossRef]
- Hong, S.; Nagayach, A.; Lu, Y.; Peng, H.; Duong, Q.A.; Pham, N.B.; Vuong, C.A.; Bazan, N.G. A High Fat, Sugar, and Salt Western Diet Induces Motor-muscular and Sensory Dysfunctions and Neurodegeneration in Mice during Aging: Ameliorative Action of Metformin. CNS Neurosci. Ther. 2021, 27, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Chakrabarti, A.; Sarma, P.; Modi, M.; Banerjee, D.; Radotra, B.D.; Bhatia, A.; Medhi, B. Novel Therapeutic Mechanism of Action of Metformin and Its Nanoformulation in Alzheimer’s Disease and Role of AKT/ERK/GSK Pathway. Eur. J. Pharm. Sci. 2023, 181, 106348. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Lv, C.; Geng, P.; Fu, M.; Zhou, W.; Xiong, M.; Li, T. Novel Targets and Therapies of Metformin in Dementia: Old Drug, New Insights. Front. Pharmacol. 2024, 15, 1415740. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Liu, X.; Guo, X. Hypoglycemic Medicines in the Treatment of Alzheimer’s Disease: Pathophysiological Links between AD and Glucose Metabolism. Front. Pharmacol. 2023, 14, 1138499. [Google Scholar] [CrossRef]
- Torrandell-Haro, G.; Branigan, G.L.; Brinton, R.D.; Rodgers, K.E. Association Between Specific Type 2 Diabetes Therapies and Risk of Alzheimer’s Disease and Related Dementias in Propensity-Score Matched Type 2 Diabetic Patients. Front. Aging Neurosci. 2022, 14, 878304. [Google Scholar] [CrossRef] [PubMed]
- Trisal, A.; Singh, A.K. Mechanisms and Early Efficacy Data of Caloric Restriction and Caloric Restriction Mimetics in Neurodegenerative Disease. Neuroscience 2025, 567, 235–248. [Google Scholar] [CrossRef]
- Trisal, A.; Singh, A.K. Clinical Insights on Caloric Restriction Mimetics for Mitigating Brain Aging and Related Neurodegeneration. Cell. Mol. Neurobiol 2024, 44, 67. [Google Scholar] [CrossRef]
- Qiu-Yue, X.; Tian-Yuan, Y.; Xiao-Long, W.; Dong-Mei, Q.; Xiao-Rui, C. Effects of Metformin on Modulating the Expression of Brain-Related Genesof APP/PS1 Transgenic Mice Based on Single Cell Sequencing. Curr. Alzheimer Res. 2022, 19, 754–771. [Google Scholar] [CrossRef]
- Taheri, M.; Roghani, M.; Sedaghat, R. Metformin Mitigates Trimethyltin-Induced Cognition Impairment and Hippocampal Neurodegeneration. Cell. Mol. Neurobiol. 2024, 44, 70. [Google Scholar] [CrossRef]
- Oliveira, W.H.; Braga, C.F.; Lós, D.B.; Araújo, S.M.R.; França, M.R.; Duarte-Silva, E.; Rodrigues, G.B.; Rocha, S.W.S.; Peixoto, C.A. Metformin Prevents P-Tau and Amyloid Plaque Deposition and Memory Impairment in Diabetic Mice. Exp. Brain Res. 2021, 239, 2821–2839. [Google Scholar] [CrossRef]
- Rabieipoor, S.; Zare, M.; Ettcheto, M.; Camins, A.; Javan, M. Metformin Restores Cognitive Dysfunction and Histopathological Deficits in an Animal Model of Sporadic Alzheimer’s Disease. Heliyon 2023, 9, e17873. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi-Mehr, M.; Delshad, A.-A.; Shafie-Damavandi, S.; Roghani, M. Metformin Mitigates Amyloid Β1-40-Induced Cognitive Decline via Attenuation of Oxidative/Nitrosative Stress and Neuroinflammation. Metab. Brain Dis. 2023, 38, 1127–1142. [Google Scholar] [CrossRef]
- Aksoz, E.; Akyol, B.A.; Korkut, O. The Role of the Cholinergic System in the Memory-Protecting Effects of Metformin in a Model of Scopolamine-Induced Memory Impairment in Aged Rats. Behav. Brain Res. 2024, 466, 114978. [Google Scholar] [CrossRef]
- Abosharaf, H.A.; Elsonbaty, Y.; Tousson, E.; Mohamed, T.M. Metformin Effectively Alleviates the Symptoms of Alzheimer in Rats by Lowering Amyloid β Deposition and Enhancing the Insulin Signal. Metab. Brain Dis. 2024, 40, 41. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Roesler, E.; Niehoff, M.L.; Roby, D.A.; McKee, A.; Morley, J.E. Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, S.; Fan, Z.; Li, Z.; Zhu, Y.; Shen, T.; Li, K.; Yan, Y.; Tian, J.; Liu, Z.; et al. Metformin Attenuates Plaque-Associated Tau Pathology and Reduces Amyloid-β Burden in APP/PS1 Mice. Alzheimer’s Res. Ther. 2021, 13, 40. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Cen, X.; Shan, B.; Zhao, Q.; Xie, T.; Wang, Z.; Hou, T.; Xue, Y.; Zhang, M.; et al. Metformin Activates Chaperone-Mediated Autophagy and Improves Disease Pathologies in an Alzheimer Disease Mouse Model. Protein Cell 2021, 12, 769–787. [Google Scholar] [CrossRef]
- Pilipenko, V.; Narbute, K.; Pupure, J.; Langrate, I.K.; Muceniece, R.; Kluša, V. Neuroprotective Potential of Antihyperglycemic Drug Metformin in Streptozocin-Induced Rat Model of Sporadic Alzheimer’s Disease. Eur. J. Pharmacol. 2020, 881, 173290. [Google Scholar] [CrossRef]
- Ponce-Lopez, T.; González Álvarez Tostado, J.A.; Dias, F.; Montiel Maltez, K.H. Metformin Prevents NDEA-Induced Memory Impairments Associated with Attenuating Beta-Amyloid, Tumor Necrosis Factor-Alpha, and Interleukin-6 Levels in the Hippocampus of Rats. Biomolecules 2023, 13, 1289. [Google Scholar] [CrossRef]
- Aksoz, E.; Gocmez, S.S.; Sahin, T.D.; Aksit, D.; Aksit, H.; Utkan, T. The Protective Effect of Metformin in Scopolamine-Induced Learning and Memory Impairment in Rats. Pharmacol. Rep. 2019, 71, 818–825. [Google Scholar] [CrossRef]
- Li, Z.; Lin, C.; Cai, X.; Lv, F.; Yang, W.; Ji, L. Anti-Diabetic Agents and the Risks of Dementia in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Observational Studies and Randomized Controlled Trials. Alzheimer’s Res. Ther. 2024, 16, 272. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Zhang, X.-Y.; Sun, Y.-Q.; Lv, R.-H.; Chen, M.; Li, M. Metformin Use Is Associated with a Reduced Risk of Cognitive Impairment in Adults with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Neurosci. 2022, 16, 984559. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, Y.; Park, J.; Choi, C.-Y.; Shin, S.; Choi, Y.J. Risk of Dementia and Alzheimer’s Disease Associated With Antidiabetics: A Bayesian Network Meta-Analysis. Am. J. Prev. Med. 2024, 67, 434–443. [Google Scholar] [CrossRef]
- Madhu, L.; Kodali, M.; Shetty, A. Promise of Metformin for Preventing Age-Related Cognitive Dysfunction. Neural Regen. Res. 2022, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Kim, E.W.; Park, S.J.; Phillips, B.U.; Jeong, J.; Kim, H.; Heath, C.J.; Kim, D.; Jang, Y.; López-Cruz, L.; et al. Reconsidering Repurposing: Long-Term Metformin Treatment Impairs Cognition in Alzheimer’s Model Mice. Transl. Psychiatry 2024, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Brichmann, E.; Rühlmann, C.; Thiele, R.; Meuth, L.; Vollmar, B. Metformin Therapy Aggravates Neurodegenerative Processes in ApoE –/– Mice. J. Alzheimer’s Dis. 2019, 68, 1415–1427. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, X.; Wang, F.; Cai, H.; Lin, Y.; Kang, D.; Fang, W. Sex Differences in Cognition, Anxiety-Phenotype and Therapeutic Effect of Metformin in the Aged apoE-TR Mice. Biol. Sex Differ. 2025, 16, 3. [Google Scholar] [CrossRef]
- Kuate Defo, A.; Bakula, V.; Pisaturo, A.; Labos, C.; Wing, S.S.; Daskalopoulou, S.S. Diabetes, Antidiabetic Medications and Risk of Dementia: A Systematic Umbrella Review and Meta-analysis. Diabetes Obes. Metab. 2024, 26, 441–462. [Google Scholar] [CrossRef]
- Ji, S.; Zhao, X.; Zhu, R.; Dong, Y.; Huang, L.; Zhang, T. Metformin and the Risk of Dementia Based on an Analysis of 396,332 Participants. Ther. Adv. Chronic Dis. 2022, 13, 20406223221109454. [Google Scholar] [CrossRef]
- Ha, J.; Choi, D.-W.; Kim, K.J.; Cho, S.Y.; Kim, H.; Kim, K.Y.; Koh, Y.; Nam, C.M.; Kim, E. Association of Metformin Use with Alzheimer’s Disease in Patients with Newly Diagnosed Type 2 Diabetes: A Population-Based Nested Case–Control Study. Sci. Rep. 2021, 11, 24069. [Google Scholar] [CrossRef]
- Loan, A.; Syal, C.; Lui, M.; He, L.; Wang, J. Promising Use of Metformin in Treating Neurological Disorders: Biomarker-Guided Therapies. Neural Regen. Res. 2024, 19, 1045–1055. [Google Scholar] [CrossRef]
- Ang, S.F.; Low, S.; Ng, T.P.; Tan, C.S.H.; Ang, K.; Lim, Z.; Tang, W.E.; Subramaniam, T.; Sum, C.F.; Lim, S.C. Ethnic-Specific Type 2 Diabetes Risk Factor PAX4 R192H Is Associated with Attention-Specific Cognitive Impairment in Chinese with Type 2 Diabetes. J. Alzheimer’s Dis. 2022, 88, 241–249. [Google Scholar] [CrossRef]
- Ali, S.K.; Ali, R.H. Effects of Antidiabetic Agents on Alzheimer’s Disease Biomarkers in Experimentally Induced Hyperglycemic Rat Model by Streptozocin. PLoS ONE 2022, 17, e0271138. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Ports, K.D.; Corrada, M.M.; Odegaard, A.O.; O’Connell, J.; Jiang, L. Metformin and Dementia Risk: A Systematic Review with Respect to Time Related Biases. J. Alzheimer’s Dis. Rep. 2022, 6, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Kazkayasi, I.; Telli, G.; Nemutlu, E.; Uma, S. Intranasal Metformin Treatment Ameliorates Cognitive Functions via Insulin Signaling Pathway in ICV-STZ-Induced Mice Model of Alzheimer’s Disease. Life Sci. 2022, 299, 120538. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ren, T.; Wang, Y.; Jin, H.; Shi, D.; Tan, X.; Ge, D.; Hou, Z.; Jin, X.; Yang, L. Aβ-Responsive Metformin-Based Supramolecular Synergistic Nanodrugs for Alzheimer’s Disease via Enhancing Microglial Aβ Clearance. Biomaterials 2022, 283, 121452. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Zou, Y.; Wang, S. Romidepsin and Metformin Nanomaterials Delivery on Streptozocin for the Treatment of Alzheimer’s Disease in Animal Model. Biomed. Pharmacother. 2021, 141, 111864. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, B.; Chen, Y.; Jia, Z.; Yuan, X.; Zhang, L.; Liu, J.; Liu, Y. Multifunctional Mesoporous Nanoselenium Delivery of Metformin Breaks the Vicious Cycle of Neuroinflammation and ROS, Promotes Microglia Regulation and Alleviates Alzheimer’s Disease. Colloids Surf. B Biointerfaces 2025, 245, 114300. [Google Scholar] [CrossRef]
- Wen, H.; Tian, H.; Liu, C.; Zhang, X.; Peng, Y.; Yang, X.; Chen, F.; Li, J. Metformin and Cyanidin 3-O-Galactoside from Aronia Melanocarpa Synergistically Alleviate Cognitive Impairment in SAMP8 Mice. Food Funct. 2021, 12, 10994–11008. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Liu, J.; Shi, J.-S. Dendrobium Nobile Lindl Alkaloid and Metformin Ameliorate Cognitive Dysfunction in Senescence-Accelerated Mice via Suppression of Endoplasmic Reticulum Stress. Brain Res. 2020, 1741, 146871. [Google Scholar] [CrossRef]
- Ram, K.; Kumar, K.; Singh, D.; Chopra, D.; Mani, V.; Jaggi, A.S.; Singh, N. Beneficial Effect of Lupeol and Metformin in Mouse Model of Intracerebroventricular Streptozotocin Induced Dementia. Metab. Brain Dis. 2024, 39, 661–678. [Google Scholar] [CrossRef]
- Demirkılıç, O.; Eski, İ.; Çiftçi Öztürk, E.; Yasun, Ö.; Aydın, B.; Birkan, C.; Özsoy, A.; Şen, S. Association Between Dipeptidyl Peptidase-4 Inhibitor Use and Cognitive Functions, Brain-Derived Neurotrophic Factor, and Pentraxin-3 Levels in Patients With Type 2 Diabetes. Cureus 2024, 16, e54440. [Google Scholar] [CrossRef]
- Barbera, M.; Lehtisalo, J.; Perera, D.; Aspö, M.; Cross, M.; De Jager Loots, C.A.; Falaschetti, E.; Friel, N.; Luchsinger, J.A.; Gavelin, H.M.; et al. A Multimodal Precision-Prevention Approach Combining Lifestyle Intervention with Metformin Repurposing to Prevent Cognitive Impairment and Disability: The MET-FINGER Randomised Controlled Trial Protocol. Alzheimer’s Res. Ther. 2024, 16, 23. [Google Scholar] [CrossRef]
- Padhy, D.S.; Aggarwal, P.; Velayutham, R.; Banerjee, S. Aerobic Exercise and Metformin Attenuate the Cognitive Impairment in an Experimental Model of Type 2 Diabetes Mellitus: Focus on Neuroinflammation and Adult Hippocampal Neurogenesis. Metab. Brain Dis. 2025, 40, 92. [Google Scholar] [CrossRef]
- Xue, Y.; Xie, X. The Association between Metformin Use and Risk of Developing Severe Dementia among AD Patients with Type 2 Diabetes. Biomedicines 2023, 11, 2935. [Google Scholar] [CrossRef]
- Luo, A.; Ning, P.; Lu, H.; Huang, H.; Shen, Q.; Zhang, D.; Xu, F.; Yang, L.; Xu, Y. Association Between Metformin and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Clinical Observational Studies. J. Alzheimer’s Dis. 2022, 88, 1311–1323. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Devanand, D.; Goldberg, T.E.; Cammack, S.; Hernández-Santiago, G.; Oishi, K.; Jagust, W.; Baker, S.; Landau, S.; Yenokyan, G.; et al. Protocol for a Randomized Phase II/III Double-Blind Placebo-Controlled Trial to Evaluate the Safety and Efficacy of Extended-Release Metformin in Amnestic Mild Cognitive Impairment: Metformin in Alzheimer Dementia Prevention (MAP). Alzheimer Dis. Assoc. Disord. 2025, 39, 123–133. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 666737. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Shen, Y.; Jiang, H.; Han, C.; Zhang, G.; Liu, L.; Xu, X.; Li, J.; Lin, Z.; et al. HMGB1 Mediates Autophagy Dysfunction via Perturbing Beclin1-Vps34 Complex in Dopaminergic Cell Model. Front. Mol. Neurosci. 2017, 10, 13. [Google Scholar] [CrossRef]
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging Therapeutic Strategies for Parkinson’s Disease and Future Prospects: A 2021 Update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, Y.-Q.; Xu, L.-L.; Qiao, C.; Sun, X.-L.; Ding, J.-H.; Lu, M.; Hu, G. Metabolic Inflammation Exacerbates Dopaminergic Neuronal Degeneration in Response to Acute MPTP Challenge in Type 2 Diabetes Mice. Exp. Neurol. 2014, 251, 22–29. [Google Scholar] [CrossRef]
- Yue, X.; Li, H.; Yan, H.; Zhang, P.; Chang, L.; Li, T. Risk of Parkinson Disease in Diabetes Mellitus: An Updated Meta-Analysis of Population-Based Cohort Studies. Medicine 2016, 95, e3549. [Google Scholar] [CrossRef]
- Tayara, K.; Espinosa-Oliva, A.M.; García-Domínguez, I.; Ismaiel, A.A.; Boza-Serrano, A.; Deierborg, T.; Machado, A.; Herrera, A.J.; Venero, J.L.; De Pablos, R.M. Divergent Effects of Metformin on an Inflammatory Model of Parkinson’s Disease. Front. Cell. Neurosci. 2018, 12, 440. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective Effect of Metformin in MPTP-Induced Parkinson’s Disease in Mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef]
- Ismaiel, A.A.K.; Espinosa-Oliva, A.M.; Santiago, M.; García-Quintanilla, A.; Oliva-Martín, M.J.; Herrera, A.J.; Venero, J.L.; De Pablos, R.M. Metformin, besides Exhibiting Strong in Vivo Anti-Inflammatory Properties, Increases Mptp-Induced Damage to the Nigrostriatal Dopaminergic System. Toxicol. Appl. Pharmacol. 2016, 298, 19–30. [Google Scholar] [CrossRef]
- Adedeji, H.A.; Ishola, I.O.; Adeyemi, O.O. Novel Action of Metformin in the Prevention of Haloperidol-Induced Catalepsy in Mice: Potential in the Treatment of Parkinson’s Disease? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 245–251. [Google Scholar] [CrossRef]
- Lu, M.; Su, C.; Qiao, C.; Bian, Y.; Ding, J.; Hu, G. Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int. J. Neuropsychopharmacol. 2016, 19, pyw047. [Google Scholar] [CrossRef]
- Bayliss, J.A.; Lemus, M.B.; Santos, V.V.; Deo, M.; Davies, J.S.; Kemp, B.E.; Elsworth, J.D.; Andrews, Z.B. Metformin Prevents Nigrostriatal Dopamine Degeneration Independent of AMPK Activation in Dopamine Neurons. PLoS ONE 2016, 11, e0159381. [Google Scholar] [CrossRef]
- Kang, H.; Khang, R.; Ham, S.; Jeong, G.R.; Kim, H.; Jo, M.; Lee, B.D.; Lee, Y.I.; Jo, A.; Park, C.; et al. Activation of the ATF2/CREB-PGC-1α Pathway by Metformin Leads to Dopaminergic Neuroprotection. Oncotarget 2017, 8, 48603–48618. [Google Scholar] [CrossRef]
- Katila, N.; Bhurtel, S.; Shadfar, S.; Srivastav, S.; Neupane, S.; Ojha, U.; Jeong, G.-S.; Choi, D.-Y. Metformin Lowers α-Synuclein Phosphorylation and Upregulates Neurotrophic Factor in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2017, 125, 396–407. [Google Scholar] [CrossRef]
- Porceddu, P.F.; Ishola, I.O.; Contu, L.; Morelli, M. Metformin Prevented Dopaminergic Neurotoxicity Induced by 3,4-Methylenedioxymethamphetamine Administration. Neurotox. Res. 2016, 30, 101–109. [Google Scholar] [CrossRef]
- Pérez-Revuelta, B.I.; Hettich, M.M.; Ciociaro, A.; Rotermund, C.; Kahle, P.J.; Krauss, S.; Di Monte, D.A. Metformin Lowers Ser-129 Phosphorylated α-Synuclein Levels via mTOR-Dependent Protein Phosphatase 2A Activation. Cell Death Dis. 2014, 5, e1209. [Google Scholar] [CrossRef]
- Ryu, Y.-K.; Go, J.; Park, H.-Y.; Choi, Y.-K.; Seo, Y.J.; Choi, J.H.; Rhee, M.; Lee, T.G.; Lee, C.-H.; Kim, K.-S. Metformin Regulates Astrocyte Reactivity in Parkinson’s Disease and Normal Aging. Neuropharmacology 2020, 175, 108173. [Google Scholar] [CrossRef] [PubMed]
- El-Ghaiesh, S.H.; Bahr, H.I.; Ibrahiem, A.T.; Ghorab, D.; Alomar, S.Y.; Farag, N.E.; Zaitone, S.A. Metformin Protects from Rotenone–Induced Nigrostriatal Neuronal Death in Adult Mice by Activating AMPK-FOXO3 Signaling and Mitigation of Angiogenesis. Front. Mol. Neurosci. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Saewanee, N.; Praputpittaya, T.; Malaiwong, N.; Chalorak, P.; Meemon, K. Neuroprotective Effect of Metformin on Dopaminergic Neurodegeneration and α-Synuclein Aggregation in C. Elegans Model of Parkinson’s Disease. Neurosci. Res. 2021, 162, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, G.; Nemutlu-Samur, D.; Parlak, H.; Yildirim, S.; Aslan, M.; Tanriover, G.; Agar, A. Metformin Protects Rotenone-Induced Dopaminergic Neurodegeneration by Reducing Lipid Peroxidation. Pharmacol. Rep. 2020, 72, 1397–1406. [Google Scholar] [CrossRef]
- Katila, N.; Bhurtel, S.; Park, P.-H.; Choi, D.-Y. Metformin Attenuates Rotenone-Induced Oxidative Stress and Mitochondrial Damage via the AKT/Nrf2 Pathway. Neurochem. Int. 2021, 148, 105120. [Google Scholar] [CrossRef]
- Wahlqvist, M.L.; Lee, M.-S.; Hsu, C.-C.; Chuang, S.-Y.; Lee, J.-T.; Tsai, H.-N. Metformin-Inclusive Sulfonylurea Therapy Reduces the Risk of Parkinson’s Disease Occurring with Type 2 Diabetes in a Taiwanese Population Cohort. Park. Relat. Disord. 2012, 18, 753–758. [Google Scholar] [CrossRef]
- Kuan, Y.-C.; Huang, K.-W.; Lin, C.-L.; Hu, C.-J.; Kao, C.-H. Effects of Metformin Exposure on Neurodegenerative Diseases in Elderly Patients with Type 2 Diabetes Mellitus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 77–83. [Google Scholar] [CrossRef]
- Brakedal, B.; Flønes, I.; Reiter, S.F.; Torkildsen, Ø.; Dölle, C.; Assmus, J.; Haugarvoll, K.; Tzoulis, C. Glitazone Use Associated with Reduced Risk of Parkinson’s Disease. Mov. Disord. 2017, 32, 1594–1599. [Google Scholar] [CrossRef]
- Huang, K.-H.; Chang, Y.-L.; Gau, S.-Y.; Tsai, T.-H.; Lee, C.-Y. Dose–Response Association of Metformin with Parkinson’s Disease Odds in Type 2 Diabetes Mellitus. Pharmaceutics 2022, 14, 946. [Google Scholar] [CrossRef]
- Curry, D.W.; Stutz, B.; Andrews, Z.B.; Elsworth, J.D. Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson’s Disease. J. Park. Dis. 2018, 8, 161–181. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, Y.; Qing, H. The Phosphorylation of A-synuclein: Development and Implication for the Mechanism and Therapy of the Parkinson’s Disease. J. Neurochem. 2015, 135, 4–18. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.; Park, E.S.; Oh, S.; Yan, R.; Zhang, J.; Beach, T.G.; Adler, C.H.; Voronkov, M.; Braithwaite, S.P.; et al. Dysregulation of Protein Phosphatase 2A in Parkinson Disease and Dementia with Lewy Bodies. Ann. Clin. Transl. Neurol. 2016, 3, 769–780. [Google Scholar] [CrossRef]
- Webb, J.L.; Ravikumar, B.; Atkins, J.; Skepper, J.N.; Rubinsztein, D.C. α-Synuclein Is Degraded by Both Autophagy and the Proteasome. J. Biol. Chem. 2003, 278, 25009–25013. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Beal, M.F. Mitochondrial Dysfunction in Parkinson’s Disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural. Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1 α, A Potential Therapeutic Target for Early Intervention in Parkinson’s Disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of Dopamine in the Pathophysiology of Parkinson’s Disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxidative Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef] [PubMed]
- Khattar, D.; Khaliq, F.; Vaney, N.; Madhu, S.V. Is Metformin-Induced Vitamin B12 Deficiency Responsible for Cognitive Decline in Type 2 Diabetes? Indian J. Psychol. Med. 2016, 38, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive Impairment and Vitamin B12: A Review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sadkowska, A.; Huttunen, K.M.; Podsiedlik, M.; Mikiciuk-Olasik, E.; Sikora, J. An Investigation into the Pleiotropic Activity of Metformin. A Glimpse of Haemostasis. Eur. J. Pharmacol. 2020, 872, 172984. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.-S.A. Metformin: A Review of Its Therapeutic Efficacy and Adverse Effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Li, H.; Chen, G.; Qi, G.; Ma, X.; Jin, Y. Role of Homocysteine in the Development and Progression of Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Streck, E.L.; Matté, C.; Vieira, P.S.; Calcagnotto, T.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T.S. Impairment of Energy Metabolism in Hippocampus of Rats Subjected to Chemically-Induced Hyperhomocysteinemia. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2003, 1637, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of Hyperhomocysteinemia Models Vascular Dementia by Induction of Cerebral Microhemorrhages and Neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef]
- McCarter, S.J.; Teigen, L.M.; McCarter, A.R.; Benarroch, E.E.; St. Louis, E.K.; Savica, R. Low Vitamin B12 and Parkinson Disease. Mayo Clin. Proc. 2019, 94, 757–762. [Google Scholar] [CrossRef]
- Christine, C.W.; Auinger, P.; Joslin, A.; Yelpaala, Y.; Green, R.; the Parkinson Study Group—DATATOP Investigators. Vitamin B12 and Homocysteine Levels Predict Different Outcomes in Early Parkinson’s Disease. Mov. Disord. 2018, 33, 762–770. [Google Scholar] [CrossRef]
- Mor, D.E.; Sohrabi, S.; Kaletsky, R.; Keyes, W.; Tartici, A.; Kalia, V.; Miller, G.W.; Murphy, C.T. Metformin Rescues Parkinson’s Disease Phenotypes Caused by Hyperactive Mitochondria. Proc. Natl. Acad. Sci. USA 2020, 117, 26438–26447. [Google Scholar] [CrossRef]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Moxon, J.V.; Krishna, S.M.; Singh, T.P.; Golledge, J. Abdominal Aortic Aneurysm Pathology and Progress Towards a Medical Therapy. In Mechanisms of Vascular Disease; Fitridge, R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 263–291. ISBN 978-3-030-43682-7. [Google Scholar]
- Dahlöf, B. Cardiovascular Disease Risk Factors: Epidemiology and Risk Assessment. Am. J. Cardiol. 2010, 105, 3A–9A. [Google Scholar] [CrossRef]
- Netala, V.R.; Teertam, S.K.; Li, H.; Zhang, Z. A Comprehensive Review of Cardiovascular Disease Management: Cardiac Biomarkers, Imaging Modalities, Pharmacotherapy, Surgical Interventions, and Herbal Remedies. Cells 2024, 13, 1471. [Google Scholar] [CrossRef]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is It a Drug for All Reasons and Diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef]
- Luo, T. Treatment with Metformin Prevents Myocardial Ischemia-Reperfusion Injury via STEAP4 Signaling Pathway. Anatol. J. Cardiol. 2019, 21, 261–271. [Google Scholar] [CrossRef]
- Shi, Y.; Hou, S.-A. Protective Effects of Metformin against Myocardial Ischemia-reperfusion Injury via AMPK-dependent Suppression of NOX4. Mol. Med. Rep. 2021, 24, 712. [Google Scholar] [CrossRef]
- Driver, C.; Bamitale, K.D.S.; Kazi, A.; Olla, M.; Nyane, N.A.; Owira, P.M.O. Cardioprotective Effects of Metformin. J. Cardiovasc. Pharmacol. 2018, 72, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zhang, L.; Li, X.; Zhou, Z.; Jiao, L.; Shao, Y.; Li, M.; Leng, B.; Zhou, Y.; et al. Metformin Protects against H2O2-Induced Cardiomyocyte Injury by Inhibiting the miR-1a-3p/GRP94 Pathway. Mol. Ther. Nucleic Acids 2018, 13, 189–197. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Karnewar, S.; Kanugula, A.K.; Thatipalli, A.R.; Kumar, J.M.; Kotamraju, S. Metformin Inhibits Monocyte-to-Macrophage Differentiation via AMPK-Mediated Inhibition of STAT3 Activation: Potential Role in Atherosclerosis. Diabetes 2015, 64, 2028–2041. [Google Scholar] [CrossRef]
- Seneviratne, A.; Cave, L.; Hyde, G.; Moestrup, S.K.; Carling, D.; Mason, J.C.; Haskard, D.O.; Boyle, J.J. Metformin Directly Suppresses Atherosclerosis in Normoglycaemic Mice via Haematopoietic Adenosine Monophosphate-Activated Protein Kinase. Cardiovasc. Res. 2021, 117, 1295–1308. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, S.; Rasheed, A.; Pietrangelo, A.; Doyoung Kim, A.; Boucher, D.M.; Emerton, C.; Vijithakumar, V.; Gharibeh, L.; Fairman, G.; Mak, E.; et al. Autophagy Is Differentially Regulated in Leukocyte and Nonleukocyte Foam Cells During Atherosclerosis. Circ. Res. 2022, 130, 831–847. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.-M.; Cline, G.W.; Nakahara, K.; et al. Metformin, Phenformin, and Galegine Inhibit Complex IV Activity and Reduce Glycerol-Derived Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef] [PubMed]
- Soberanes, S.; Misharin, A.V.; Jairaman, A.; Morales-Nebreda, L.; McQuattie-Pimentel, A.C.; Cho, T.; Hamanaka, R.B.; Meliton, A.Y.; Reyfman, P.A.; Walter, J.M.; et al. Metformin Targets Mitochondrial Electron Transport to Reduce Air-Pollution-Induced Thrombosis. Cell Metab. 2019, 29, 335–347.e5. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.-F.; Wang, N.-Y.; Wang, X.-M.; Liang, S.-T.; Zheng, W.; Lu, Y.-B.; Zhao, X.; Hao, D.-L.; Zhang, Z.-Q.; et al. SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Tabima, D.M.; Dube, J.J.; Hughan, K.S.; Vanderpool, R.R.; Goncharov, D.A.; St. Croix, C.M.; Garcia-Ocaña, A.; Goncharova, E.A.; Tofovic, S.P.; et al. SIRT3–AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated with Heart Failure with Preserved Ejection Fraction. Circulation 2016, 133, 717–731. [Google Scholar] [CrossRef]
- Gao, P.; You, M.; Li, L.; Zhang, Q.; Fang, X.; Wei, X.; Zhou, Q.; Zhang, H.; Wang, M.; Lu, Z.; et al. Salt-Induced Hepatic Inflammatory Memory Contributes to Cardiovascular Damage Through Epigenetic Modulation of SIRT3. Circulation 2022, 145, 375–391. [Google Scholar] [CrossRef]
- Xie, Z.; Lau, K.; Eby, B.; Lozano, P.; He, C.; Pennington, B.; Li, H.; Rathi, S.; Dong, Y.; Tian, R.; et al. Improvement of Cardiac Functions by Chronic Metformin Treatment Is Associated with Enhanced Cardiac Autophagy in Diabetic OVE26 Mice. Diabetes 2011, 60, 1770–1778. [Google Scholar] [CrossRef]
- Efentakis, P.; Kremastiotis, G.; Varela, A.; Nikolaou, P.-E.; Papanagnou, E.-D.; Davos, C.H.; Tsoumani, M.; Agrogiannis, G.; Konstantinidou, A.; Kastritis, E.; et al. Molecular Mechanisms of Carfilzomib-Induced Cardiotoxicity in Mice and the Emerging Cardioprotective Role of Metformin. Blood 2019, 133, 710–723. [Google Scholar] [CrossRef]
- Papanagnou, E.; Gumeni, S.; Sklirou, A.D.; Rafeletou, A.; Terpos, E.; Keklikoglou, K.; Kastritis, E.; Stamatelopoulos, K.; Sykiotis, G.P.; Dimopoulos, M.A.; et al. Autophagy Activation Can Partially Rescue Proteasome Dysfunction-mediated Cardiac Toxicity. Aging Cell 2022, 21, e13715. [Google Scholar] [CrossRef]
- Jadhav, S.; Ferrell, W.; Greer, I.A.; Petrie, J.R.; Cobbe, S.M.; Sattar, N. Effects of Metformin on Microvascular Function and Exercise Tolerance in Women with Angina and Normal Coronary Arteries. J. Am. Coll. Cardiol. 2006, 48, 956–963. [Google Scholar] [CrossRef]
- Preiss, D.; Lloyd, S.M.; Ford, I.; McMurray, J.J.; Holman, R.R.; Welsh, P.; Fisher, M.; Packard, C.J.; Sattar, N. Metformin for Non-Diabetic Patients with Coronary Heart Disease (the CAMERA Study): A Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2014, 2, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B.; Aroda, V.R.; Bluemke, D.A.; Barrett-Connor, E.; Budoff, M.; Crandall, J.P.; Dabelea, D.; Horton, E.S.; Mather, K.J.; Orchard, T.J.; et al. Effect of Long-Term Metformin and Lifestyle in the Diabetes Prevention Program and Its Outcome Study on Coronary Artery Calcium. Circulation 2017, 136, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kelly, S.; Ajodha, S.; Sahdev, A.; Bestwick, J.P.; Gabrovska, P.; Akanle, O.; Ajjan, R.; Kola, B.; Stadler, M.; et al. Metformin to Reduce Metabolic Complications and Inflammation in Patients on Systemic Glucocorticoid Therapy: A Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept, Phase 2 Trial. Lancet Diabetes Endocrinol. 2020, 8, 278–291. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and Metabolic Effects of Metformin in Patients with Type 1 Diabetes (REMOVAL): A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609, Correction in Lancet Diabetes Endocrinol. 2017, 5, E7. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Schäfer, M.; Truong, U.; Cree-Green, M.; Pyle, L.; Baumgartner, A.; Garcia Reyes, Y.; Maniatis, A.; Nayak, S.; Wadwa, R.P.; et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus: Randomized Controlled Trial. Circulation 2018, 138, 2895–2907. [Google Scholar] [CrossRef]
- Benes, J.; Kotrc, M.; Kroupova, K.; Wohlfahrt, P.; Kovar, J.; Franekova, J.; Hegarova, M.; Hoskova, L.; Hoskova, E.; Pelikanova, T.; et al. Metformin Treatment Is Associated with Improved Outcome in Patients with Diabetes and Advanced Heart Failure (HFrEF). Sci. Rep. 2022, 12, 13038. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Bhatt, D.L.; McGuire, D.K.; Cahn, A.; Mosenzon, O.; Steg, G.; Im, K.; Kanevsky, E.; Gurmu, Y.; Raz, I.; et al. Metformin Use and Clinical Outcomes Among Patients with Diabetes Mellitus With or Without Heart Failure or Kidney Dysfunction: Observations From the SAVOR-TIMI 53 Trial. Circulation 2019, 140, 1004–1014. [Google Scholar] [CrossRef]
- Larsen, A.H.; Jessen, N.; Nørrelund, H.; Tolbod, L.P.; Harms, H.J.; Feddersen, S.; Nielsen, F.; Brøsen, K.; Hansson, N.H.; Frøkiær, J.; et al. A Randomised, Double-blind, Placebo-controlled Trial of Metformin on Myocardial Efficiency in Insulin-resistant Chronic Heart Failure Patients without Diabetes. Eur. J. Heart Fail. 2020, 22, 1628–1637. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A Randomized Controlled Trial of Metformin on Left Ventricular Hypertrophy in Patients with Coronary Artery Disease without Diabetes: The MET-REMODEL Trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef]
- Ladeiras-Lopes, R.; Sampaio, F.; Leite, S.; Santos-Ferreira, D.; Vilela, E.; Leite-Moreira, A.; Bettencourt, N.; Gama, V.; Braga, P.; Fontes-Carvalho, R. Metformin in Non-Diabetic Patients with Metabolic Syndrome and Diastolic Dysfunction: The MET-DIME Randomized Trial. Endocrine 2021, 72, 699–710. [Google Scholar] [CrossRef]
- Dewangga, R.; Winston, K.; Ilhami, L.G.; Indriani, S.; Siddiq, T.; Adiarto, S. Association of Metformin Use with Abdominal Aortic Aneurysm: A Systematic Review and Meta-Analysis. Asian Cardiovasc. Thorac. Ann. 2024, 32, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Shabil, M.; Bushi, G.; Yadav, A.; Ahmed, M.; Kishore, J.; Lekamwasam, S.; Joshi, A. Effect of Metformin on Cardiovascular Outcomes: A Systematic Review and Meta Analysis of Observational Studies and RCTs. Evid. J. 2023, 1, 23–34. [Google Scholar] [CrossRef]
- American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2002, 25, s28–s32. [Google Scholar] [CrossRef]
- Rena, G.; Lang, C.C. Repurposing Metformin for Cardiovascular Disease. Circulation 2018, 137, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Moir, J.; Hyman, M.J.; Kaufmann, G.T.; Flores, A.; Hariprasad, S.M.; Skondra, D. Metformin Use and Age-Related Macular Degeneration in Patients Without Diabetes. JAMA Ophthalmol. 2024, 142, 53. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Mousavi, M. Overview of Risk Factors for Age-Related Macular Degeneration (AMD). J. Stem Cells 2015, 10, 171–191. [Google Scholar]
- Taylor, T.R.P.; Menten, M.J.; Rueckert, D.; Sivaprasad, S.; Lotery, A.J. The Role of the Retinal Vasculature in Age-Related Macular Degeneration: A Spotlight on OCTA. Eye 2024, 38, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, M.; Hassan, Y.; Bakka Vemana, P.P.S.; Bellary Pattanashetty, M.S.; Abdin, Z.U.; Siddiqui, H.F. Age-Related Macular Degeneration: An Exponentially Emerging Imminent Threat of Visual Impairment and Irreversible Blindness. Cureus 2023, 15, e39624. [Google Scholar] [CrossRef]
- Blasiak, J.; Sobczuk, P.; Pawlowska, E.; Kaarniranta, K. Interplay between Aging and Other Factors of the Pathogenesis of Age-Related Macular Degeneration. Ageing Res. Rev. 2022, 81, 101735. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef]
- Huh, M.D.; Le, S.N.; O’Brien, K.S.; Keenan, J.D.; Stewart, J.M. Potential Efficacy of Metformin for Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Ophthalmol. Sci. 2025, 5, 100741. [Google Scholar] [CrossRef]
- Li, Y.; Gappy, S.; Liu, X.; Sassalos, T.; Zhou, T.; Hsu, A.; Zhang, A.; Edwards, P.A.; Gao, H.; Qiao, X. Metformin Suppresses Pro-Inflammatory Cytokines in Vitreous of Diabetes Patients and Human Retinal Vascular Endothelium. PLoS ONE 2022, 17, e0268451. [Google Scholar] [CrossRef]
- Xu, L.; Kong, L.; Wang, J.; Ash, J.D. Stimulation of AMPK Prevents Degeneration of Photoreceptors and the Retinal Pigment Epithelium. Proc. Natl. Acad. Sci. USA 2018, 115, 10475–10480. [Google Scholar] [CrossRef]
- Xiao, J.F.; Luo, W.; Mani, A.; Barba, H.; Solanki, A.; Droho, S.; Lavine, J.A.; Skondra, D. Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration. Int. J. Mol. Sci. 2024, 25, 11357. [Google Scholar] [CrossRef]
- Li, X.; Leng, Y.; Jiang, Q.; Wang, Z.; Luo, P.; Zhang, C.; Chen, L.; Wang, Y.; Wang, H.; Yue, X.; et al. Eye Drops of Metformin Prevents Fibrosis After Glaucoma Filtration Surgery in Rats via Activating AMPK/Nrf2 Signaling Pathway. Front. Pharmacol. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Romdhoniyyah, D.F.; Harding, S.P.; Cheyne, C.P.; Beare, N.A.V. Metformin, A Potential Role in Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Ophthalmol. Ther. 2021, 10, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Stephenson, M.D.; De Courten, B.; Chapman, I.; Bellman, S.M.; Aromataris, E. Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2018, 65, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Keenan, J.D.; Chahal, N.; Taha, A.T.; Saroya, J.; Ma, C.J.; Sun, M.; Yang, D.; Psaras, C.; Callander, J.; et al. METformin for the MINimization of Geographic Atrophy Progression (METforMIN): A Randomized Trial. Ophthalmol. Sci. 2024, 4, 100440. [Google Scholar] [CrossRef]
- Seddon, J.M.; Silver, R.E.; Kwong, M.; Rosner, B. Risk Prediction for Progression of Macular Degeneration: 10 Common and Rare Genetic Variants, Demographic, Environmental, and Macular Covariates. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2192. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.; Hyman, M.J.; Gonnah, R.; Flores, A.; Hariprasad, S.M.; Skondra, D. The Association Between Metformin Use and New-Onset ICD Coding of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 23. [Google Scholar] [CrossRef]
- Chen, X.; Rong, S.S.; Xu, Q.; Tang, F.Y.; Liu, Y.; Gu, H.; Tam, P.O.S.; Chen, L.J.; Brelén, M.E.; Pang, C.P.; et al. Diabetes Mellitus and Risk of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e108196. [Google Scholar] [CrossRef]
- He, M.-S.; Chang, F.-L.; Lin, H.-Z.; Wu, J.-L.; Hsieh, T.-C.; Lee, Y.-C. The Association Between Diabetes and Age-Related Macular Degeneration Among the Elderly in Taiwan. Diabetes Care 2018, 41, 2202–2211. [Google Scholar] [CrossRef]
- Blitzer, A.L.; Ham, S.A.; Colby, K.A.; Skondra, D. Association of Metformin Use With Age-Related Macular Degeneration: A Case-Control Study. JAMA Ophthalmol. 2021, 139, 302. [Google Scholar] [CrossRef]
- Brown, E.E.; Ball, J.D.; Chen, Z.; Khurshid, G.S.; Prosperi, M.; Ash, J.D. The Common Antidiabetic Drug Metformin Reduces Odds of Developing Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1470. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Shen, Y.-C.; Lai, Y.-J.; Wang, C.-Y.; Lin, K.-H.; Feng, S.-C.; Liang, C.-Y.; Wei, L.-C.; Chou, P. Association between Metformin and a Lower Risk of Age-Related Macular Degeneration in Patients with Type 2 Diabetes. J. Ophthalmol. 2019, 2019, 1649156. [Google Scholar] [CrossRef] [PubMed]
- Eton, E.A.; Wubben, T.J.; Besirli, C.G.; Hua, P.; McGeehan, B.; VanderBeek, B.L. Association of Metformin and Development of Dry Age-Related Macular Degeneration in a U.S. Insurance Claims Database. Eur. J. Ophthalmol. 2022, 32, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, K.M.; Adderley, N.J.; Subramanian, A.; Lee, W.H.; Han, D.; Coker, J.; Braithwaite, T.; Denniston, A.K.; Keane, P.A.; Nirantharakumar, K. Metformin and Risk of Age-Related Macular Degeneration in Individuals with Type 2 Diabetes: A Retrospective Cohort Study. Br. J. Ophthalmol. 2023, 107, 980–986. [Google Scholar] [CrossRef]
- Huang, K.-H.; Chang, Y.-L.; Lee, C.B.; Gau, S.-Y.; Tsai, T.-H.; Chung, N.-J.; Lee, C.-Y. Dose-Response Association of Metformin Use and Risk of Age-Related Macular Degeneration among Patients with Type 2 Diabetes Mellitus: A Population-Based Study. Front. Pharmacol. 2023, 14, 1275095. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Zhang, H.; Yuan, W.; Zhao, T.; Wang, N.; Fan, G.; Zheng, D.; Wang, Z. Association between Metformin Use and the Risk of Age-Related Macular Degeneration in Patients with Type 2 Diabetes: A Retrospective Study. BMJ Open 2022, 12, e054420. [Google Scholar] [CrossRef]
- Tseng, C.-H. The Risk of Age-Related Macular Degeneration Is Reduced in Type 2 Diabetes Patients Who Use Metformin. Pharmaceuticals 2023, 16, 224. [Google Scholar] [CrossRef]
- Dehkordi, A.H.; Abbaszadeh, A.; Mir, S.; Hasanvand, A. Metformin and Its Anti-Inflammatory and Anti-Oxidative Effects; New Concepts. J. Renal. Inj. Prev. 2018, 8, 54–61. [Google Scholar] [CrossRef]
- Feng, Q.; Ruan, X.; Lu, M.; Bu, S.; Zhang, Y. Metformin Protects Retinal Pigment Epithelium Cells against H2O2-Induced Oxidative Stress and Inflammation via the Nrf2 Signaling Cascade. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 1519–1530. [Google Scholar] [CrossRef]
- Toppila, M.; Ranta-aho, S.; Kaarniranta, K.; Hytti, M.; Kauppinen, A. Metformin Alleviates Inflammation and Induces Mitophagy in Human Retinal Pigment Epithelium Cells Suffering from Mitochondrial Damage. Cells 2024, 13, 1433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; Jiang, Y.; Silva, M.; Zhen, X.; Zheng, W. Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2524174. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Y.; Liu, X.; Zhou, T.; Sun, H.; Edwards, P.; Gao, H.; Yu, F.-S.; Qiao, X. Metformin Suppresses Retinal Angiogenesis and Inflammation in Vitro and in Vivo. PLoS ONE 2018, 13, e0193031. [Google Scholar] [CrossRef]
- Yagasaki, R.; Morita, A.; Mori, A.; Sakamoto, K.; Nakahara, T. The Anti-Diabetic Drug Metformin Suppresses Pathological Retinal Angiogenesis via Blocking the mTORC1 Signaling Pathway in Mice (Metformin Suppresses Pathological Angiogenesis). Curr. Eye Res. 2024, 49, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.T.; Luu, I.Y.; Keenan, J.D.; Stewart, J.M. Long-Term Metformin Use and Reduced Risk of Age-Related Macular Degeneration. Ophthalmol. Retin. 2025; S2468653025003549in press. [Google Scholar] [CrossRef]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Zamani, M.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of Osteoporosis with the World Health Organization Diagnostic Criteria in the Eastern Mediterranean Region: A Systematic Review and Meta-Analysis. Arch. Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic Burden of Osteoporosis in the World: A Systematic Review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar] [CrossRef]
- Dimai, H.P.; Redlich, K.; Peretz, M.; Borgström, F.; Siebert, U.; Mahlich, J. Economic Burden of Osteoporotic Fractures in Austria. Health Econ. Rev. 2012, 2, 12. [Google Scholar] [CrossRef]
- Nih Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Ortendahl, J.D.; Vanderpuye-Orgle, J.; Grauer, A.; Arellano, J.; Lemay, J.; Harmon, A.L.; Broder, M.S.; Singer, A.J. Healthcare Policy Changes in Osteoporosis Can Improve Outcomes and Reduce Costs in the United States. JBMR Plus 2019, 3, e10192. [Google Scholar] [CrossRef]
- Salari, N.; Darvishi, N.; Bartina, Y.; Larti, M.; Kiaei, A.; Hemmati, M.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Osteoporosis among the World Older Adults: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 669. [Google Scholar] [CrossRef]
- Özmen, S.; Kurt, S.; Timur, H.T.; Yavuz, O.; Kula, H.; Demir, A.Y.; Balcı, A. Prevalence and Risk Factors of Osteoporosis: A Cross-Sectional Study in a Tertiary Center. Medicina 2024, 60, 2109. [Google Scholar] [CrossRef]
- Blümel, J.E.; Arteaga, E.; Aedo, S.; Arriola-Montenegro, J.; López, M.; Martino, M.; Miranda, C.; Miranda, O.; Mostajo, D.; Ñañez, M.; et al. Metformin Use Is Associated with a Lower Risk of Osteoporosis in Adult Women Independent of Type 2 Diabetes Mellitus and Obesity. REDLINC IX Study. Gynecol. Endocrinol. 2020, 36, 421–425. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Q.; He, H.; Jiang, L.; Lee, K.O.; Li, D.; Ma, J. Metformin Treatment Is Associated with an Increase in Bone Mineral Density in Type 2 Diabetes Mellitus Patients in China: A Retrospective Single Center Study. Diabetes Metab. 2022, 48, 101350. [Google Scholar] [CrossRef]
- Cai, Y.; Jun, G.; Zhuang, X. Metformin Treatment Reduces the Incidence of Osteoporosis: A Two-Sample Mendelian Randomized Study. Osteoporos. Int. 2024, 35, 1089–1098. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Chen, P.-B.; Ju, L.-L.; Deng, G.-H. Causal Association of Metformin and Osteoporosis: A 2-Sample Mendelian Randomization Study. Medicine 2023, 102, e35191. [Google Scholar] [CrossRef]
- Xie, X.; Hu, L.; Mi, B.; Xue, H.; Hu, Y.; Panayi, A.C.; Endo, Y.; Chen, L.; Yan, C.; Lin, Z.; et al. Metformin Alleviates Bone Loss in Ovariectomized Mice through Inhibition of Autophagy of Osteoclast Precursors Mediated by E2F1. Cell Commun. Signal 2022, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Cao, F.; Qiu, S.; Jiang, W.; Tao, L.; Zhu, Y. Metformin Promotes Differentiation and Attenuates H2O2-Induced Oxidative Damage of Osteoblasts via the PI3K/AKT/Nrf2/HO-1 Pathway. Front. Pharmacol. 2022, 13, 829830. [Google Scholar] [CrossRef]
- Shah, M.; Kola, B.; Bataveljic, A.; Arnett, T.R.; Viollet, B.; Saxon, L.; Korbonits, M.; Chenu, C. AMP-Activated Protein Kinase (AMPK) Activation Regulates in Vitro Bone Formation and Bone Mass. Bone 2010, 47, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, T.; Guo, D.; Hu, K.; Shu, Y.; Xu, H.H.K.; Schneider, A. Metformin Induces Osteoblastic Differentiation of Human Induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells. J. Tissue Eng. Regen. Med. 2018, 12, 437–446. [Google Scholar] [CrossRef]
- Jiating, L.; Buyun, J.; Yinchang, Z. Role of Metformin on Osteoblast Differentiation in Type 2 Diabetes. BioMed Res. Int. 2019, 2019, 9203934. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.G.; Kim, E.J.; Bae, I.-H.; Lee, K.-N.; Kim, Y.D.; Kim, D.-K.; Kim, S.-H.; Lee, C.-H.; Franceschi, R.T.; Choi, H.-S.; et al. Metformin Induces Osteoblast Differentiation via Orphan Nuclear Receptor SHP-Mediated Transactivation of Runx2. Bone 2011, 48, 885–893. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Sig. Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Lu, W.; Xiong, L. Metformin Activates Wnt/β-Catenin for the Treatment of Diabetic Osteoporosis. BMC Endocr. Disord. 2022, 22, 189. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.-L.; Hu, X.-T.; Wang, X.-T.; Chen, A.-M. Metformin Promotes Differentiation of Human Bone Marrow Derived Mesenchymal Stem Cells into Osteoblast via GSK3β Inhibition. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7962–7968. [Google Scholar] [CrossRef]
- Gu, Q.; Gu, Y.; Yang, H.; Shi, Q. Metformin Enhances Osteogenesis and Suppresses Adipogenesis of Human Chorionic Villous Mesenchymal Stem Cells. Tohoku J. Exp. Med. 2017, 241, 13–19. [Google Scholar] [CrossRef]
- Li, Y.; Su, J.; Sun, W.; Cai, L.; Deng, Z. AMP-Activated Protein Kinase Stimulates Osteoblast Differentiation and Mineralization through Autophagy Induction. Int. J. Mol. Med. 2018, 41, 2535–2544. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chowdhury, S.; Bhattacharyya, M. Effect of Metformin on Oxidative Stress, Nitrosative Stress and Inflammatory Biomarkers in Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 2011, 93, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shen, X.; Ye, J.; Xie, Y.; Yan, S. Metformin Alleviates Hyperglycemia-Induced Apoptosis and Differentiation Suppression in Osteoblasts through Inhibiting the TLR4 Signaling Pathway. Life Sci. 2019, 216, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, S.S.; Amsalem-Zafran, A.R.; Shapira, K.E.; Ehrlich, M.; Henis, Y.I. Competition between Type I Activin and BMP Receptors for Binding to ACVR2A Regulates Signaling to Distinct Smad Pathways. BMC Biol. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Puerto, M.C.; Iyengar, P.V.; García De Vinuesa, A.; Ten Dijke, P.; Sanchez-Duffhues, G. Bone Morphogenetic Protein Receptor Signal Transduction in Human Disease. J. Pathol. 2019, 247, 9–20. [Google Scholar] [CrossRef]
- Lin, H.; Shi, F.; Jiang, S.; Wang, Y.; Zou, J.; Ying, Y.; Huang, D.; Luo, L.; Yan, X.; Luo, Z. Metformin Attenuates Trauma-induced Heterotopic Ossification via Inhibition of Bone Morphogenetic Protein Signalling. J. Cell. Mol. Med. 2020, 24, 14491–14501. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Sheng, L.; Huang, Y. Metformin Suppresses Proliferation and Differentiation Induced by BMP9 via AMPK Signaling in Human Fetal Lung Fibroblast-1. Front. Pharmacol. 2022, 13, 984730. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.; Yao, Z.; Xing, L. Osteoclasts Have Multiple Roles in Bone in Addition to Bone Resorption. Crit. Rev. Eukar. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef]
- Zhen, D.; Chen, Y.; Tang, X. Metformin Reverses the Deleterious Effects of High Glucose on Osteoblast Function. J. Diabetes Complicat. 2010, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Mai, Q.-G.; Zhang, Z.-M.; Xu, S.; Lu, M.; Zhou, R.-P.; Zhao, L.; Jia, C.-H.; Wen, Z.-H.; Jin, D.-D.; Bai, X.-C. Metformin Stimulates Osteoprotegerin and Reduces RANKL Expression in Osteoblasts and Ovariectomized Rats. J. Cell. Biochem. 2011, 112, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, M.S.; Schurman, L.; McCarthy, A.D.; Cortizo, A.M.; Tolosa, M.J.; Gangoiti, M.V.; Arnol, V.; Sedlinsky, C. Effect of Metformin on Bone Marrow Progenitor Cell Differentiation: In Vivo and in Vitro Studies. J. Bone Miner. Res. 2010, 25, 211–221. [Google Scholar] [CrossRef]
- Shaik, A.R.; Singh, P.; Shaik, C.; Kohli, S.; Vohora, D.; Ferrari, S.L. Metformin: Is It the Well Wisher of Bone Beyond Glycemic Control in Diabetes Mellitus? Calcif. Tissue Int. 2021, 108, 693–707. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Hu, Y.; Peng, B. Protective Effect of Metformin on Periapical Lesions in Rats by Decreasing the Ratio of Receptor Activator of Nuclear Factor Kappa B Ligand/Osteoprotegerin. J. Endod. 2012, 38, 943–947. [Google Scholar] [CrossRef]
- Tao, L.Y.; Łagosz-Ćwik, K.B.; Hogervorst, J.M.A.; Schoenmaker, T.; Grabiec, A.M.; Forouzanfar, T.; Van Der Weijden, F.A.; De Vries, T.J. Diabetes Medication Metformin Inhibits Osteoclast Formation and Activity in In Vitro Models for Periodontitis. Front. Cell Dev. Biol. 2022, 9, 777450. [Google Scholar] [CrossRef]
- Halleen, J.M.; Tiitinen, S.L.; Ylipahkala, H.; Fagerlund, K.M.; Väänänen, H.K. Tartrate-Resistant Acid Phosphatase 5b (TRACP 5b) as a Marker of Bone Resorption. Clin. Lab. 2006, 52, 499–509. [Google Scholar]
- Janckila, A.J.; Yam, L.T. Biology and Clinical Significance of Tartrate-Resistant Acid Phosphatases: New Perspectives on an Old Enzyme. Calcif. Tissue Int. 2009, 85, 465–483. [Google Scholar] [CrossRef]
- Mira-Pascual, L.; Patlaka, C.; Desai, S.; Paulie, S.; Näreoja, T.; Lång, P.; Andersson, G. A Novel Sandwich ELISA for Tartrate-Resistant Acid Phosphatase 5a and 5b Protein Reveals That Both Isoforms Are Secreted by Differentiating Osteoclasts and Correlate to the Type I Collagen Degradation Marker CTX-I In Vivo and In Vitro. Calcif. Tissue Int. 2020, 106, 194–207. [Google Scholar] [CrossRef]
- Hie, M.; Shimono, M.; Fujii, K.; Tsukamoto, I. Increased Cathepsin K and Tartrate-Resistant Acid Phosphatase Expression in Bone of Streptozotocin-Induced Diabetic Rats. Bone 2007, 41, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-J.; Xie, J.-J.; Hong, R.-H.; Pan, H.-S.; Zhang, F.-G.; Liang, Y.-M. Puerarin Alleviates Streptozotocin (STZ)-Induced Osteoporosis in Rats through Suppressing Inflammation and Apoptosis via HDAC1/HDAC3 Signaling. Biomed. Pharmacother. 2019, 115, 108570. [Google Scholar] [CrossRef]
- Rao Sirasanagandla, S.; Ranganath Pai Karkala, S.; Potu, B.K.; Bhat, K.M.R. Beneficial Effect of Cissus Quadrangularis Linn. on Osteopenia Associated with Streptozotocin-Induced Type 1 Diabetes Mellitus in Male Wistar Rats. Adv. Pharmacol. Sci. 2014, 2014, 483051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Z.; Xie, C.; Ling, L.; Hu, H.; Cao, Y.; Huang, Y.; Zhu, Q.; Hua, Y. The Hyperglycemia and Hyperketonemia Impaired Bone Microstructures: A Pilot Study in Rats. Front. Endocrinol. 2020, 11, 590575. [Google Scholar] [CrossRef]
- Suzuki, K.; Ishida, H.; Takeshita, N.; Taguchi, Y.; Sugimoto, C.; Nosaka, K.; Seino, Y. Circulating Levels of Tartrate-Resistant Acid Phosphatase in Rat Models of Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Complicat. 1998, 12, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, W.J.; Olayaki, L.A.; Abdussalam, T.A.; Fabiyi, T.O.; Raji, T.L.; Adetunji, A.A.-R. Co-Administration of Omega-3 Fatty Acids and Metformin Showed More Desirable Effects than the Single Therapy on Indices of Bone Mineralisation but Not Gluco-Regulatory and Antioxidant Markers in Diabetic Rats. Biomed. Pharmacother. 2020, 121, 109631. [Google Scholar] [CrossRef]
- Park, S.-H.; Kang, M.-A.; Moon, Y.J.; Jang, K.Y.; Kim, J.R. Metformin Coordinates Osteoblast/Osteoclast Differentiation Associated with Ischemic Osteonecrosis. Aging 2020, 12, 4727–4741. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. 13. Older Adults: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S266–S282. [Google Scholar] [CrossRef]
- Lazarus, B.; Wu, A.; Shin, J.-I.; Sang, Y.; Alexander, G.C.; Secora, A.; Inker, L.A.; Coresh, J.; Chang, A.R.; Grams, M.E. Association of Metformin Use With Risk of Lactic Acidosis Across the Range of Kidney Function: A Community-Based Cohort Study. JAMA Intern. Med. 2018, 178, 903. [Google Scholar] [CrossRef]
- Orloff, J.; Min, J.Y.; Mushlin, A.; Flory, J. Safety and Effectiveness of Metformin in Patients with Reduced Renal Function: A Systematic Review. Diabetes Obes. Metab. 2021, 23, 2035–2047. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in Patients With Type 2 Diabetes and Kidney Disease: A Systematic Review. J. Am. Med. Assoc. 2014, 312, 2668. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Wang, G.-Y.; Su, N.; Ma, J.; Li, Y.-K. Effects of Different Doses of Metformin on Bone Mineral Density and Bone Metabolism in Elderly Male Patients with Type 2 Diabetes Mellitus. World J. Clin. Cases 2020, 8, 4010–4016. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Q.; Si, M. Dapagliflozin Combined with Metformin Improves Blood Glucose, Bone Metabolism and Bone Mineral Density in Elderly Patients with Type 2 Diabetes Mellitus Complicated with Osteoporosis. Kaohsiung J. Med. Sci. 2025, 41, e12937. [Google Scholar] [CrossRef]
- Chen, M.; Lin, S.; Chen, W.; Chen, X. Antidiabetic Drug Administration Prevents Bone Mineral Density Loss: Evidence from a Two-Sample Mendelian Randomization Study. PLoS ONE 2024, 19, e0300009. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, J.; Jin, M.; Jin, J.; Zhu, J. Effects of Metformin on Bone Mineral Density and Bone Turnover Markers: A Systematic Review and Meta-Analysis. BMJ Open 2023, 13, e072904. [Google Scholar] [CrossRef]
- Saadi, M.S.S.; Das, R.; Mullath Ullas, A.; Powell, D.E.; Wilson, E.; Myrtziou, I.; Rakieh, C.; Kanakis, I. Impact of Different Anti-Hyperglycaemic Treatments on Bone Turnover Markers and Bone Mineral Density in Type 2 Diabetes Mellitus Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 7988. [Google Scholar] [CrossRef]
- De Vries, T.J.; Kleemann, A.S.; Jin, J.; Schoenmaker, T. The Differential Effect of Metformin on Osteocytes, Osteoblasts, and Osteoclasts. Curr. Osteoporos. Rep. 2023, 21, 743–749. [Google Scholar] [CrossRef]
- Mu, W.; Liang, G.; Feng, Y.; Jiang, Y.; Qu, F. The Potential Therapeutic Role of Metformin in Diabetic and Non-Diabetic Bone Impairment. Pharmaceuticals 2022, 15, 1274. [Google Scholar] [CrossRef]
- Bahrambeigi, S.; Yousefi, B.; Rahimi, M.; Shafiei-Irannejad, V. Metformin; an Old Antidiabetic Drug with New Potentials in Bone Disorders. Biomed. Pharmacother. 2019, 109, 1593–1601. [Google Scholar] [CrossRef] [PubMed]


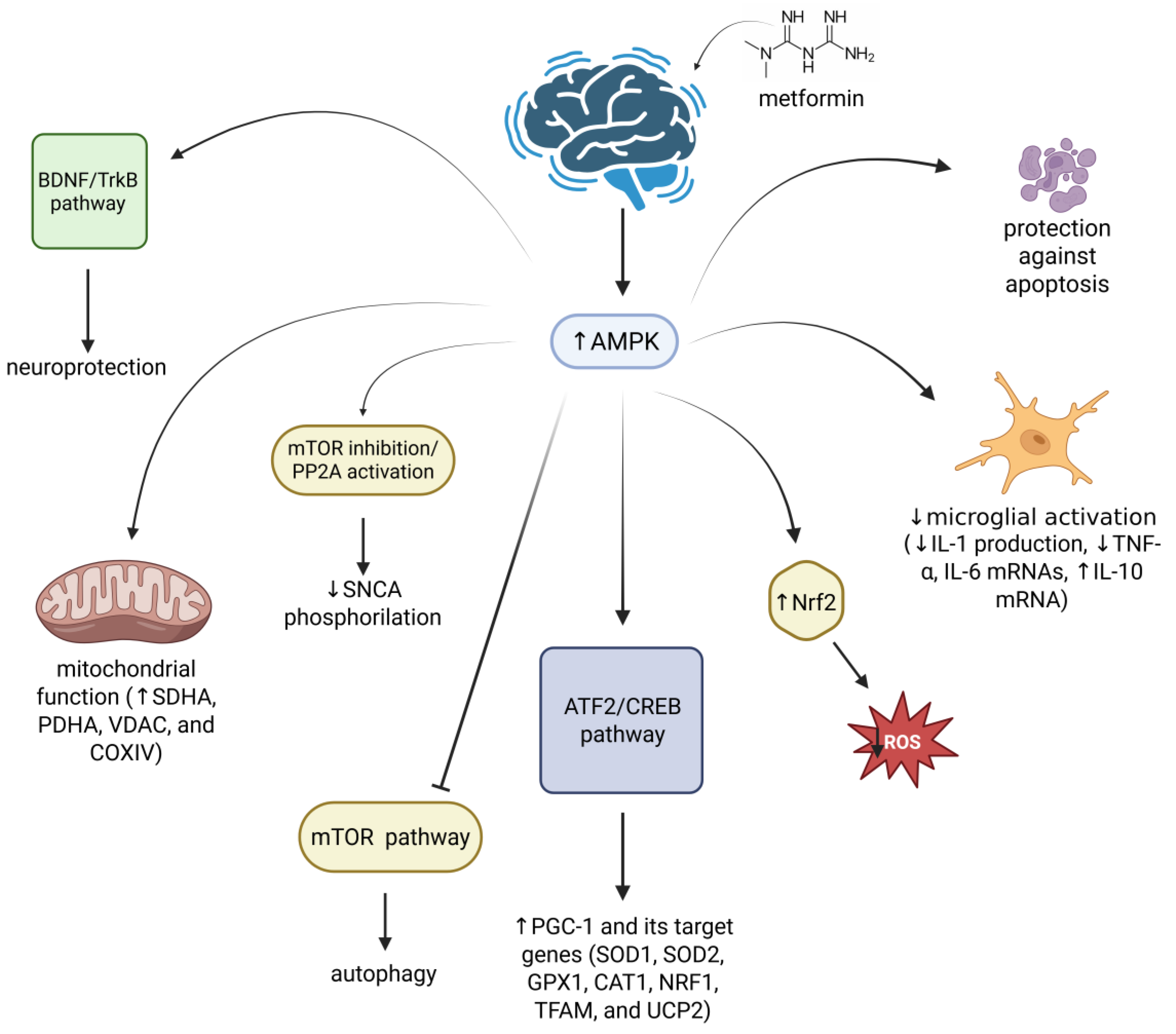
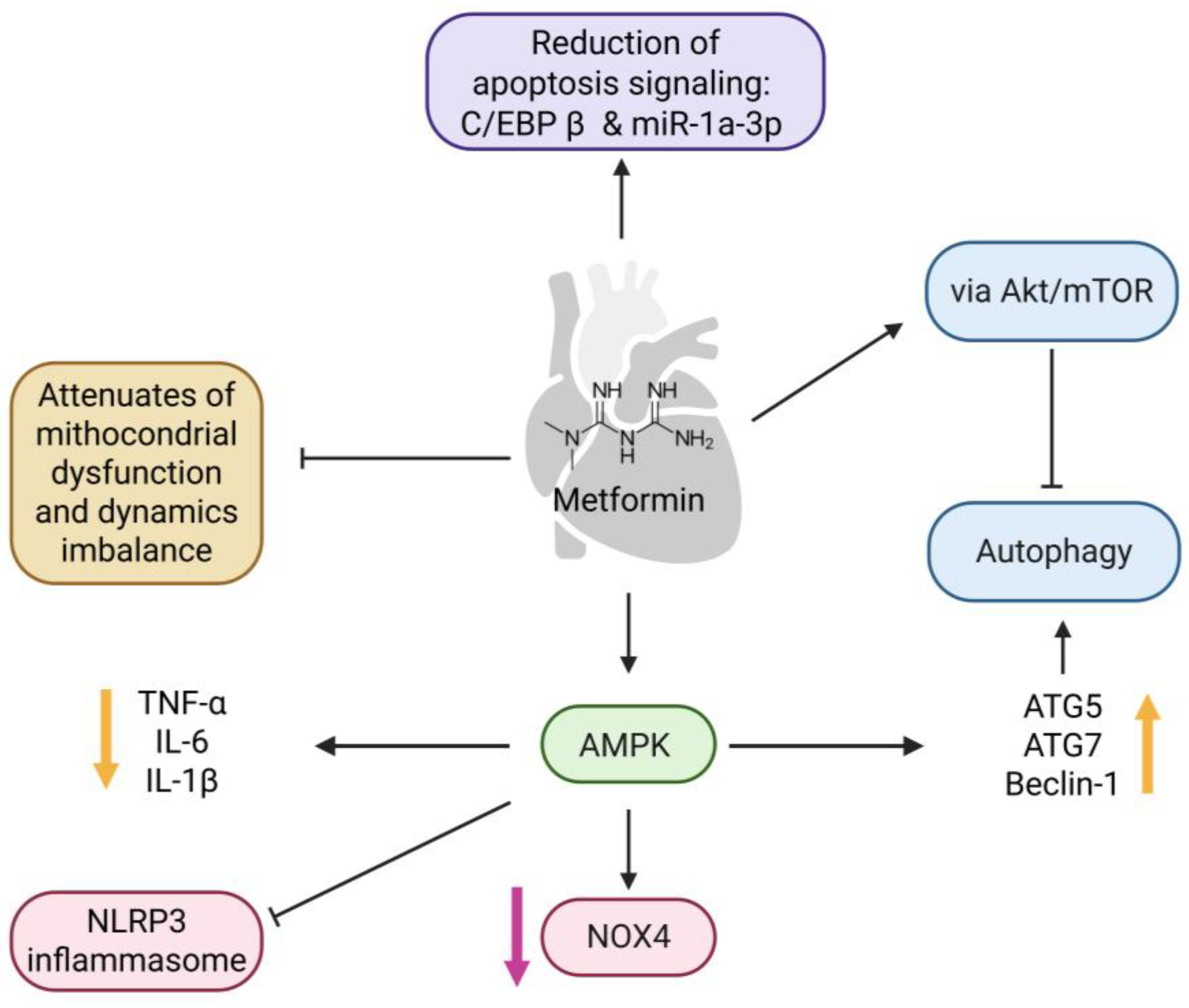
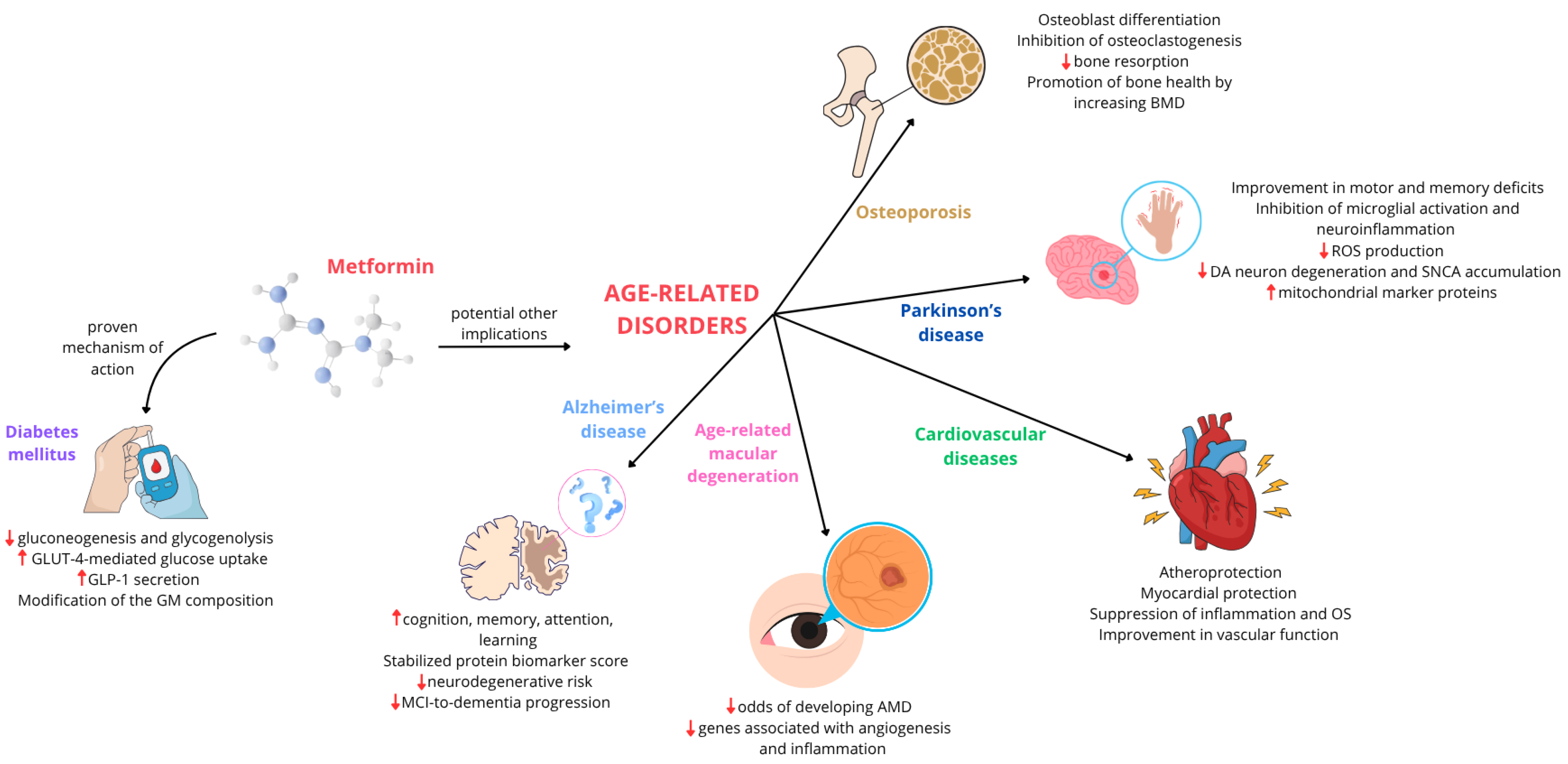
| Database/Centre/Study | Cohort | Main Outcome | Reference |
|---|---|---|---|
| VALCODIS Cohort | N = 213 T2DM (50–80 y.o.) | ↓ p-tau (CSF) | [62] |
| National Alzheimer’s Coordinating Center (NACC) | N = 48,605 MCI (>39 y.o.) | ↓ MCI-to-dementia progression | [63] |
| NCT01965756 | N = 48,605 MCI | Stabilized protein biomarker score (AZU1, CASP-3, CCL11, CCL20, IL32, PRTN3, and REG1A) (plasma + CSF) | [64] |
| N.A. | N = 527,138 (middle-aged) | ↓ mitochondrial complex I (brain cortex) | [65] |
| ADNI study | N = 6937 MCI-AD (55–90 y.o.) | ↑ Cognition, cortical thickness, hippocampal volume = glycemia, triglycerides, cholesterol | [66] |
| Korean National Health Insurance Service DM cohort | N= 10,050 T2DM (n = 1675 AD) (50–85 y.o.) | ↑ Cognition | [67] |
| National Alzheimer’s Coordinating Center database | N = 1999 T2DM (n = 807 AD) | ↑ Memory (in T2DM non-AD) | [68] |
| FAERS database | N = 66,085 (>64 y.o.) T2DM (n = 1250 AD) | No improvement | [69] |
| Korean cohort registry | N = 732 (>59 y.o.) | ↓ MMSE, verbal scores = daily activity index | [70] |
| US Veterans Affairs electronic medical record | N = 5528 T2DM (>49 y.o.) | ↓ Neurodegenerative risk | [71] |
| University of Pennsylvania Health System (UPHS) | N = 20 MCI (55–80 y.o.) | ↑ Execution performance, memory, attention, learning ↑/= orbitofrontal cerebral blood flow | [72] |
| Columbia University Medical Center | N = 80 MCI (55–90 y.o.) overweight/obese | ↑ Selective Reminding Test (SRT) | [73] |
| United Kingdom-based General Practice Research Database (GPRD) | N = 7086 AD (>65 y.o.) | ↑ AD risk | [74] |
| Swedish Registry for Cognitive/Dementia Disorders (SveDem) | N = 15,428 AD (77.29 mean y.o.) | ↑ Cognition | [75] |
| National Alzheimer’s Coordinating Center database | N = 1393 T2DM (>49 y.o.) | ↓ AD risk | [76] |
| ICES Database, Ontario, Canada | N = 34,700 T2DM (>65 y.o.) | = AD risk | [77] |
| Preclinical Studies | |||
|---|---|---|---|
| Mechanism | Model | Main Outcome | Reference |
| Activation of AMPK/autophagy axis (atheroprotection) | ApoE(−/−) mice | Attenuation of Ang-II-induced atheromatous plaque formation and aortic aneurysm partly | [187] |
| Normoglycemic Ldlr−/− hyperlipidaemic mice | Suppression of atherogenesis | [188] | |
| GFP-LC3 transgenic mice by PCSK9 | Improvement in autophagy-mediated cholesterol efflux | [189] | |
| Suppression of mitochondrial ROS-mediated pro-inflammatory pathway (atheroprotection) | Male Sprague–Dawley rats treated with glucose/metformin | Alteration in cellular redox state and reduction in glucose production by inhibiting complex IV | [190] |
| Mice exposed to PM and treated with metformin | Prevention in PM-induced generation of METC-ROS | [191] | |
| Activation of sirtuin signaling (myocardial protection) | Male C57BL/6J Sirt2 knockout mice | Activation of AMPK signaling Protection against cardiac hypertrophy | [192] |
| 2-Hit PH-HFpEF model in rats with multiple features of metabolic syndrome due to a double-leptin receptor defect (obese ZSF1) | Normalization of pulmonary hypertension associated with HF | [193] | |
| Salt-induced hepatic inflammation in mouse model | Amelioration of high-salt diet-induced hepatic inflammation and myocardial damage | [194] | |
| Activation of autophagy-dependent pathway (myocardial protection) | Diabetic mice | Prevention of cardiomyopathy | [195] |
| Carfilzomib-induced cardiotoxicity in mice | Protection against carfilzomib-induced cardiotoxicity | [196] | |
| Fly stocks | Activation of autophagy | [197] | |
| Clinical Studies | |||
| Database/Centre Cohort | Metformin Treatment | Main Outcome | Reference |
| n = 33 non-diabetic women with angina | n = 16 patients: 0.5 g twice daily for 8 weeks | Improvement in vascular function Decrease in myocardial ischemia | [198] |
| CAMERA study: n = 173 non-diabetic patients with coronary heart disease and on statin therapy | n = 86 patients: 850 mg twice daily for 18 months | No significant effect on cIMT Reduction in body weight, body fat, BMI, and waist circumference | [199] |
| DPPOS study: n = 3234 individuals with pre-diabetes | n = 926 patients: 850 mg twice daily (14 years of follow-up) | Reduction in CAC only in male subjects | [200] |
| CODYCE study: n = 258 propensity-matched patients with stable angina | n = 86 patients: 850 mg twice a day (6–12–24 months of follow-up) | Suppression of inflammation and oxidative stress | [201] |
| n = 849 non-diabetic patients with an inflammatory disease treated with continuous prednisolone | n = 26 patients: 850 mg/day for the first 5 days, 850 mg twice a day for the next 5 days, and 850 mg three times a day subsequently for 12 weeks | Reduction of metabolic complications, inflammation, LDL-cholesterol, and cIMT | [202] |
| REMOVAL study: n = 428 patients with T1DM (40 years and older) | n = 219 patients: 1 g twice daily for 3 years | Reduction in the maximal cIMT, body weight and blood LDL-cholesterol No adequate improvement in glycemic control | [203] |
| EMERALD study: n = 48 T1DM adolescents (12–21 years) | n = 25 patients: 2000 mg daily for 3 months | Reduction in cIMT, BMI, and fat mass Improvement in aortic dysfunction | [204] |
| n = 380 patients with diabetes and HF | n = 87 patients: 1000 mg (29 patients), lower than 1000 mg (18 patients), between 1000 and 2000 mg (14 patients), and 2000 mg (25 patients) daily or higher | Signs of more stable HF (lower BNP levels, reduced mitral and tricuspid regurgitation severity, improved left and right ventricular function, and decreased diuretic dosage) Increase in survival | [205] |
| SAVOR-TIMI 53 trial: n = 12,156 patients with T2DM and high cardiovascular risk with or without HF or kidney dysfunction | n = 8971 patients exposed to metformin | Lower risk of all-cause mortality and cardiovascular death No difference in risk of the composite endpoint of cardiovascular death, myocardial infarction, or ischemic stroke | [206] |
| n = 36 patients with HF | n = 19 patients: 1450 ± 550 mg/day for 3 months | Increase in relative efficiency Reduction in myocardial oxygen consumption Preservation of cardiac stroke work No effects on resting and exercise ejection fraction, global longitudinal strain, and exercise capacity | [207] |
| MET-REMODEL study: N = 68 patients without diabetes, but with CAD, LVH, IR, and/or pre-diabetes | n = 34 patients: 2000 mg daily for 12 months | Reduction in LVMI, LVM, body weight, subcutaneous adipose tissue, office systolic blood pressure, and in concentration of thiobarbituric acid reactive substances | [208] |
| MET-DIME trial: N = 54 non-diabetic patients with diastolic dysfunction | n = 27 patients: 1000 mg twice daily (2 years of follow-up) | Improvement in e-wave’ velocity Reduction in the HOMA-IR index No effect on LVMI, and hs-CRP and N-terminal -pro-BNP plasma levels | [209] |
| Type of Study, Follow-Up Periods (yrs), Patient Characteristics (Age Mean in Yrs (SD) N. Participants (Exposed/Nonexposed)) | Daily Metformin Dosage (mg) | AMD Type | Main Outcome | Reference |
|---|---|---|---|---|
| Case-control in the United States, 2 yrs, 74.9 yrs (10.3), 81,262/79,497 | Various doses, mean: 163 | N.A. | Metformin use was associated with reduced odds of developing AMD. This association was dose dependent, with low to moderate doses of metformin showing the greatest potential benefit. | [232] |
| Case-control in the United States, N.A, N.A, Control: 77 Case: 75.09 | N.A. | Dry and wet | Patients who had taken metformin had decreased odds of developing AMD, suggesting that metformin may have a therapeutic role in AMD development or progression in those who are at risk. | [233] |
| Retrospective cohort in Taiwan, 6.7 yrs, 56.1 yrs (12.6), 45,524/22,681 | Various doses (400–2100) | N.A. | Metformin use was associated with reduced odds of developing AMD. Also, the trend of a significantly lower AMD risk was found with a higher dose of metformin. | [234] |
| Retrospective cohort in United States, N.A, 67.5 yrs (8.9), 166,115/841,111 | Various doses, mean 1000 | Dry | There is not sufficient evidence to suggest that metformin has a meaningful impact on the development of AMD | [235] |
| Retrospective cohort in United Kingdom, 5.7 yrs (4.1), 62.8 yrs (11.6), 154,016/19,673 | N.A. | Dry and Wet | There is not sufficient evidence to suggest that metformin has a meaningful impact on the development of AMD | [236] |
| Retrospective cohort in Taiwan, 5 yrs, 62.06 yrs old (8.83), 377,873/350,825 | Various doses | N.A. | Metformin use is associated with a dose-dependent risk of AMD in patients with DM. Indeed, the greater benefit is observed at lowest dose, while the highest dose exhibited an increased risk of AMD. | [237] |
| Retrospective cohort in China, 6 months to 10 yrs, Median 67 yrs, 209/115 | More than 250 | Any type | Among patients with DM for ≥10 years, metformin users were less likely to develop any AMD and early AMD than non-users | [238] |
| Retrospective Cohort in Taiwan, 5.6 yrs, 65.04 yrs (8.3), 13,303/13,303 | Various doses | N.A. | The results of this study supported a lower risk of AMD in ever users of metformin when compared to never users | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagnoli, L.I.M.; Varesi, A.; Fahmideh, F.; Hakimizad, R.; Petkovic, P.; Barbieri, A.; Marchesi, N.; Pascale, A. From Diabetes to Degenerative Diseases: The Multifaceted Action of Metformin. Int. J. Mol. Sci. 2025, 26, 9748. https://doi.org/10.3390/ijms26199748
Campagnoli LIM, Varesi A, Fahmideh F, Hakimizad R, Petkovic P, Barbieri A, Marchesi N, Pascale A. From Diabetes to Degenerative Diseases: The Multifaceted Action of Metformin. International Journal of Molecular Sciences. 2025; 26(19):9748. https://doi.org/10.3390/ijms26199748
Chicago/Turabian StyleCampagnoli, Lucrezia Irene Maria, Angelica Varesi, Foroogh Fahmideh, Reza Hakimizad, Petra Petkovic, Annalisa Barbieri, Nicoletta Marchesi, and Alessia Pascale. 2025. "From Diabetes to Degenerative Diseases: The Multifaceted Action of Metformin" International Journal of Molecular Sciences 26, no. 19: 9748. https://doi.org/10.3390/ijms26199748
APA StyleCampagnoli, L. I. M., Varesi, A., Fahmideh, F., Hakimizad, R., Petkovic, P., Barbieri, A., Marchesi, N., & Pascale, A. (2025). From Diabetes to Degenerative Diseases: The Multifaceted Action of Metformin. International Journal of Molecular Sciences, 26(19), 9748. https://doi.org/10.3390/ijms26199748






