Synthesis and Biological Profile of Omaveloxolone: The Cornerstone for Friedreich Ataxia Treatment
Abstract
1. Introduction
2. Chemistry of Omaveloxolone and Its Precursors
2.1. Oleanolic Acid Extraction, Characterization and Biological Activity
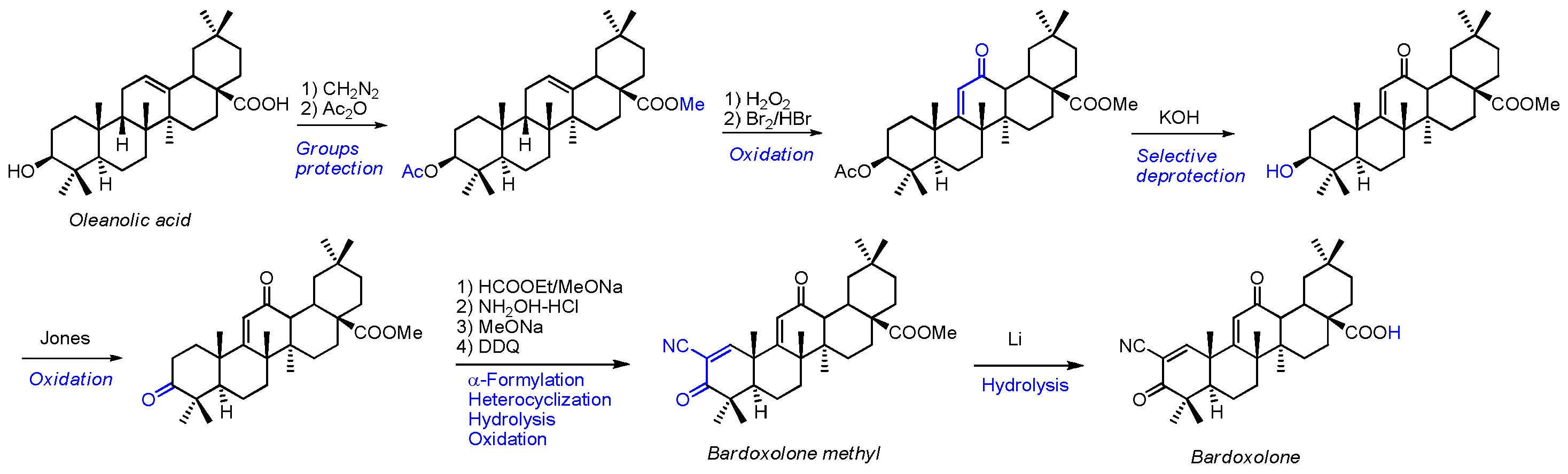
2.2. Bardoxolone Synthesis
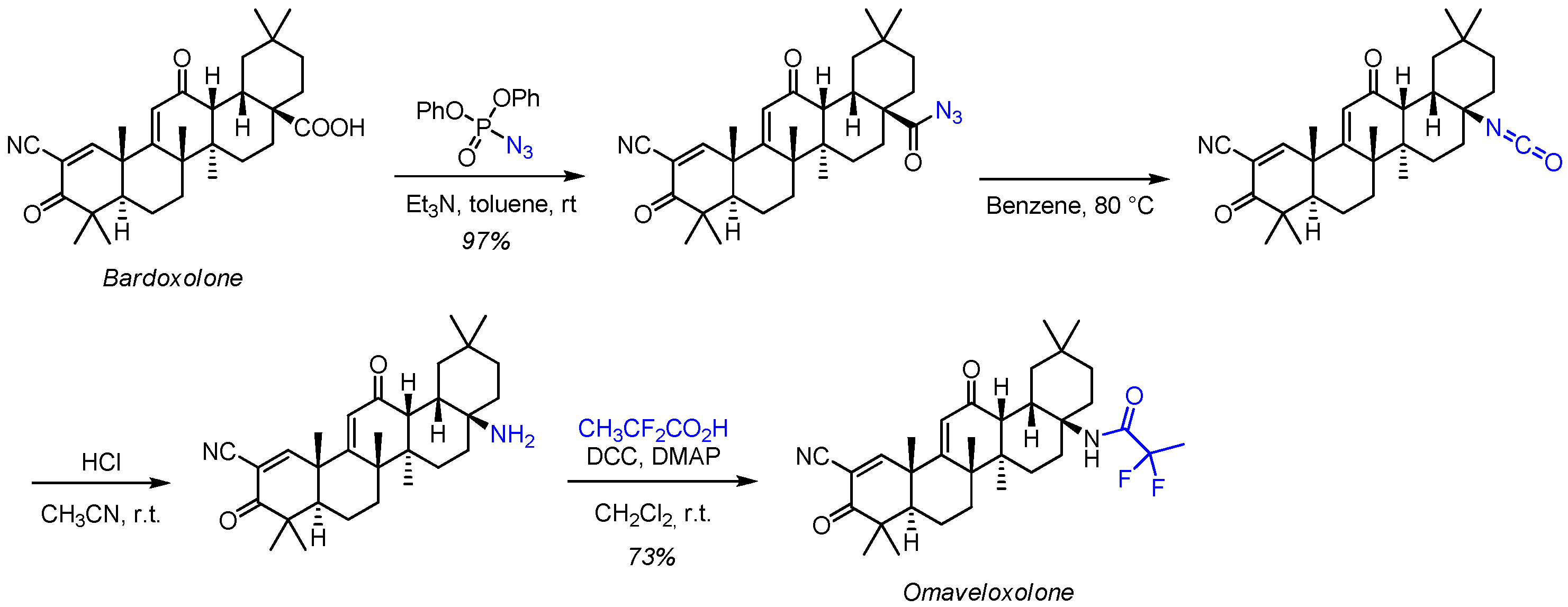
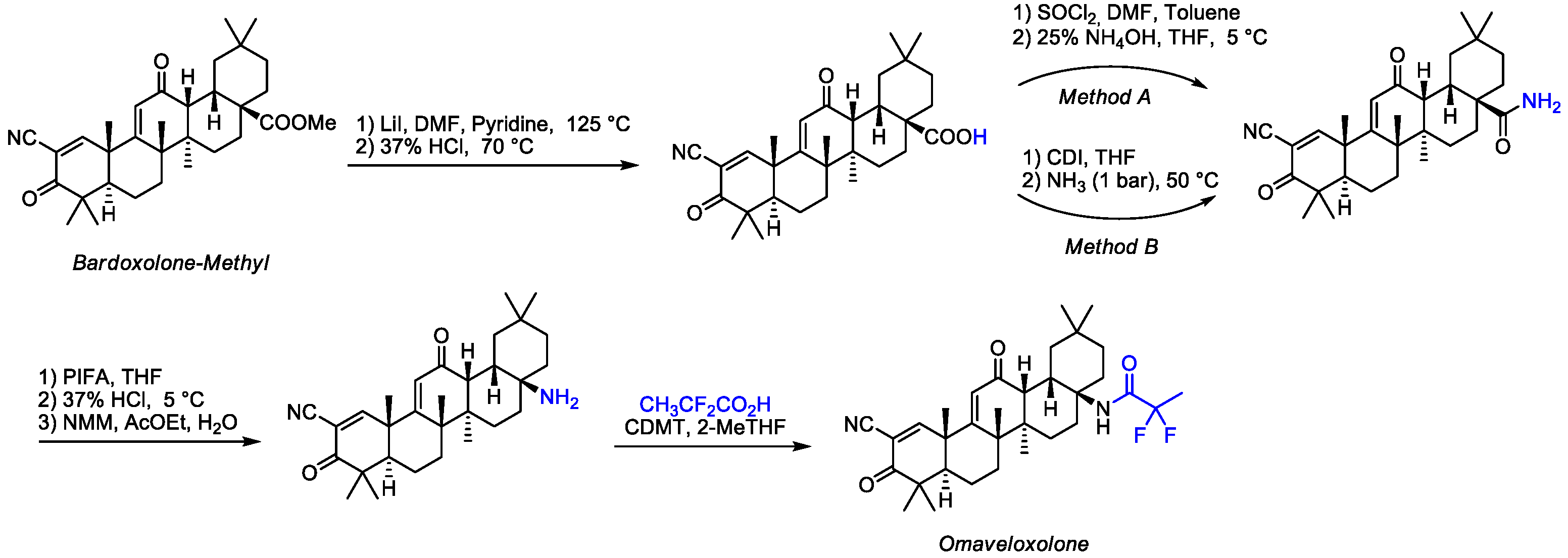
2.3. Chemistry of Omaveloxolone
3. Drug Delivery Systems of Omaveloxolone and Its Precursors
4. Computational and Statistical Techniques
4.1. Model-Informed Drug Development (MIDD)
4.2. Physiologically Based Biopharmaceutics Modeling (PBBM)
4.3. Wearable Devices and Machine Learning
4.4. Future Directions
- Multi-Omics Integration: Combining genomic, transcriptomic, and proteomic data with computational models to uncover novel biomarkers and therapeutic targets.
- Digital Twins: Developing patient-specific digital twins to simulate disease progression and predict individual treatment responses.
- Real-World Evidence (RWE): Expanding the use of real-world data from wearable devices and electronic health records to enhance the generalizability of research findings.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Bayani, U.; Ajay, V.S.; Paolo, Z.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Ren, Y.; Kinghorn, A.D. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Medica 2019, 85, 802–814. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Triaa, N.; Znati, M.; Ben Jannet, H.; Bouajila, J. Biological Activities of Novel Oleanolic Acid Derivatives from Bioconversion and Semi-Synthesis. Molecules 2024, 29, 3091. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Jannus, F.; Sainz, J.; Reyes-Zurita, F.J. Principal Bioactive Properties of Oleanolic Acid, Its Derivatives, and Analogues. Molecules 2024, 29, 3291. [Google Scholar] [CrossRef]
- Schiavoni, V.; Di Crescenzo, T.; Membrino, V.; Alia, S.; Fantone, S.; Salvolini, E.; Vignini, A. Bardoxolone Methyl: A Comprehensive Review of Its Role as a Nrf2 Activator in Anticancer Therapeutic Applications. Pharmaceuticals 2025, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New Synthetic Triterpenoids: Potent Agents for Prevention and Treatment of Tissue Injury Caused by Inflammatory and Oxidative Stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.R.; Mandal, A.; Bhatia, D.; Siveen, K.S.; Sethi, G.; Bishayee, A. Oleanane triterpenoids in the prevention and therapy of breast cancer: Current evidence and future perspectives. Phytochem. Rev. 2014, 13, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Yin-Xue, Y.; Hong, Z.; Zhi-Xu, H.; Zhou, S.-F. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Dev. Ther. 2014, 8, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
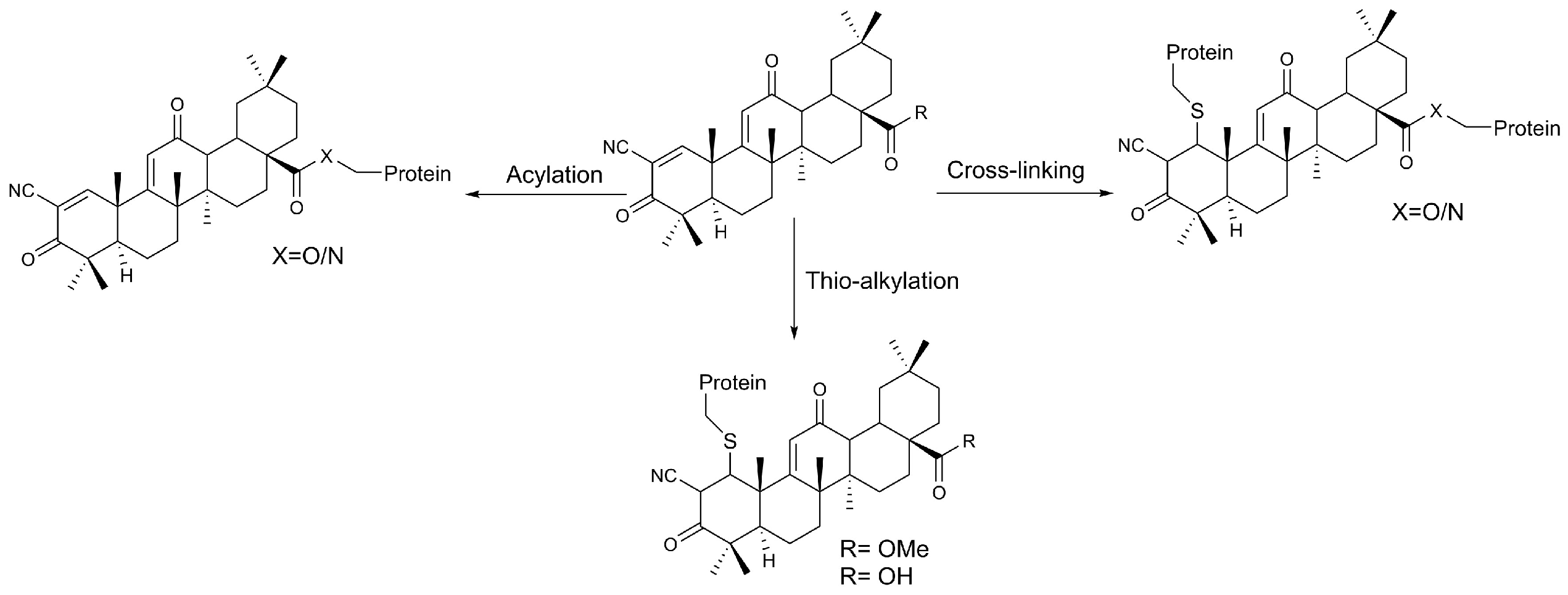
- Wong, M.H.L.; Bryan, H.K.; Copple, I.M.; Jenkins, R.E.; Chiu, P.H.; Bibby, J.; Berry, N.G.; Kitteringham, N.R.; Goldring, C.E.; O’Neill, P.M.; et al. Design and Synthesis of Irreversible Analogues of Bardoxolone Methyl for the Identification of Pharmacologically Relevant Targets and Interaction Sites. J. Med. Chem. 2016, 59, 2396–2409. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Raghuvanshi, D.S.; Singh, R.V. Recent advances in the chemistry and biology of oleanolic acid and its derivatives. Eur. J. Med. Chem. 2024, 276, 116619. [Google Scholar] [CrossRef] [PubMed]
- Probst, B.L.; Trevino, I.; McCauley, L.; Bumeister, R.; Dulubova, I.; Wigley, W.C.; Ferguson, D.A. RTA 408, A Novel Synthetic Triterpenoid with Broad Anticancer and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0122942. [Google Scholar] [CrossRef]
- Wei, H.-J.; Pareek, T.K.; Liu, Q.; Letterio, J.J. A unique tolerizing dendritic cell phenotype induced by the synthetic triterpenoid CDDO-DFPA (RTA-408) is protective against EAE. Sci. Rep. 2017, 7, 9886. [Google Scholar] [CrossRef]
- Reisman, S.A.; Lee, C.-Y.I.; Meyer, C.J.; Proksch, J.W.; Ward, K.W. Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin. Arch. Dermatol. Res. 2014, 306, 447–454. [Google Scholar] [CrossRef]
- Jiang, Z.; Qi, G.; Lu, W.; Wang, H.; Li, D.; Chen, W.; Ding, L.; Yang, X.; Yuan, H.; Zeng, Q. Omaveloxolone inhibits IL-1β-induced chondrocyte apoptosis through the Nrf2/ARE and NF-κB signalling pathways in vitro and attenuates osteoarthritis in vivo. Front. Pharmacol. 2022, 13, 952950. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Omaveloxolone (Skyclarys™) for patients with Friedreich’s ataxia. Trends Pharmacol. Sci. 2023, 44, 394–395. [Google Scholar]
- Lynch, D.R.; Goldsberry, A.; Rummey, C.; Farmer, J.; Boesch, S.; Delatycki, M.B.; Giunti, P.; Hoyle, J.C.; Mariotti, C.; Mathews, K.D.; et al. Propensity matched comparison of omaveloxolone treatment to Friedreich ataxia natural history data. Ann. Clin. Transl. Neurol. 2024, 11, 4–16. [Google Scholar] [CrossRef]
- Lynch, D.R.; Schadt, K.; Kichula, E.; McCormack, S.; Lin, K.Y. Friedreich Ataxia: Multidisciplinary Clinical Care. J. Multidiscip. Healthc. 2021, 14, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.; Boesch, S.; Delatycki, M.; Giunti, P.; Goldsberry, A.; Hoyle, C.; Mathews, K.; Khan, S.; Meyer, C.; Murai, M.; et al. The MOXIe Trial of Omaveloxolone in Friedreich Ataxia: Exploring the Transient Nature of Treatment-emergent Adverse Events (P7-3.016). Neurology 2024, 102 (Suppl. 1), 3490. [Google Scholar] [CrossRef]
- Olatunde, O.Z.; Yong, J.; Tian, D.; Lu, C. Comprehensive review of plant-derived triterpenoid types, structures and cytotoxicity: An update from 2015 to 2024. Org. Biomol. Chem. 2025, 23, 5929–6051. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Dai, S.-Y.; Deng, F.-H.; Peng, L.-H.; Li, C.; Pei, Y.-H. Recent advances in medicinal chemistry of oleanolic acid derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef]
- Honda, T.; Rounds, B.V.; Bore, L.; Finlay, H.J.; Favaloro, F.G.; Suh, N.; Wang, Y.; Sporn, M.B.; Gribble, G.W. Synthetic Oleanane and Ursane Triterpenoids with Modified Rings A and C: A Series of Highly Active Inhibitors of Nitric Oxide Production in Mouse Macrophages. J. Med. Chem. 2000, 43, 4233–4246. [Google Scholar] [CrossRef]
- Honda, T.; Honda, Y.; Favaloro, F.G.; Gribble, G.W.; Suh, N.; Place, A.E.; Rendi, M.H.; Sporn, M.B. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg. Med. Chem. Lett. 2002, 12, 1027–1030. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Vinogradov, M.G.; Turova, O.V.; Zlotin, S.G. Recent advances in the asymmetric synthesis of pharmacology-relevant nitrogen heterocycles via stereoselective aza-Michael reactions. Org. Biomol. Chem. 2019, 17, 3670–3708. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Wang, B.-W.; Xu, X.-R.; Zhu, L.; Song, Y.; Li, H.-B. Microwave-Assisted Extraction of Oleanolic Acid and Ursolic Acid from Ligustrum lucidum Ait. Int. J. Mol. Sci. 2011, 12, 5319–5329. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Guinda, A.; Macías, L.; Santos-Lozano, J.M.; Lapetra, J.; Rada, M. Free radical scavenging and α-glucosidase inhibition, two potential mechanisms involved in the anti-diabetic activity of oleanolic acid. Grasas Y Aceites 2016, 67, e142. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Pang, Z.; Zhi-Yan, Z.; Wang, W.; Ma, Y.; Feng-Ju, N.; Zhang, X.; Han, C. The Advances in Research on the Pharmacological Effects of Fructus Ligustri Lucidi. BioMed Res. Int. 2015, 2015, 281873. [Google Scholar] [CrossRef]
- Ngubane, P.S.; Bubuya, M.; Musabayane, C.T. The Effects of Syzygium aromaticum-Derived Oleanolic Acid on Glycogenic Enzymes in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2011, 33, 434–439. [Google Scholar] [CrossRef]
- Sánchez, M.; Theoduloz, C.; Schmeda-Hirschmann, G.; Razmilic, I.; Yáñez, T.; Rodríguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro–in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef]
- Banik, R.M.; Pandey, D.K. Optimizing conditions for oleanolic acid extraction from Lantana camara roots using response surface methodology. Ind. Crops Prod. 2008, 27, 241–248. [Google Scholar] [CrossRef]
- Upadhya, V.; Ankad, G.M.; Pai, S.R.; Hegde, H.V.; Kholkute, S.D. Accumulation and trends in distribution of three triterpenoids in various parts of Achyranthes coynei determined using RP-UFLC analysis. Pharmacogn. Mag. 2014, 10, 398–401. [Google Scholar] [CrossRef]
- Gohari, A.; Saeidnia, S.; Hadjiakhoondi, A.; Abdoullahi, M.; Nezafati, M. Isolation and Quantificative Analysis of Oleanolic Acid from Satureja mutica Fisch. & C. A. Mey. J. Med. Plants 2009, 8, 65–69. [Google Scholar]
- Okello, D.; Yang, S.; Komakech, R.; Rahmat, E.; Chung, Y.; Gang, R.; Kim, Y.-G.; Omujal, F.; Kang, Y. An in vitro Propagation of Aspilia africana (Pers.) C. D. Adams, and Evaluation of Its Anatomy and Physiology of Acclimatized Plants. Front. Plant Sci. 2021, 12, 704896. [Google Scholar] [CrossRef]
- Khan, M.I.; Shah, S.; Faisal, S.; Gul, S.; Khan, S.; Abdullah; Shah, S.A.; Shah, W.A. Monotheca buxifolia Driven Synthesis of Zinc Oxide Nano Material Its Characterization and Biomedical Applications. Micromachines 2022, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Shabnam Javed, S.J.; Oise, I.; Lutfun Nahar, L.N.; Ismail, F.; Zaid Mahmood, Z.M.; Sarker, S. Isolation, identification and antiproliferative activity of triterpenes from the genus Monotheca A. DC. Rec. Nat. Prod. 2016, 10, 782–787. [Google Scholar]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef]
- Fu, L.; Gribble, G.W. Efficient and Scalable Synthesis of Bardoxolone Methyl (CDDO-methyl Ester). Org. Lett. 2013, 15, 1622–1625. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Yang, P.-C.; Zhang, Y.-F.; Sun, J.-F. Synthesis and clinical application of new drugs approved by FDA in 2023. Eur. J. Med. Chem. 2024, 265, 116124. [Google Scholar] [CrossRef]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. Biomed. Res. Int. 2020, 2020, 1308749. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Decker, A.; Liu, X. 2,2-difluoropropionamide Derivatives of Bardoxolone Methyl, Polymorphic Forms and Methods of Use Thereof. Patent WO 2013/163344 A l, 31 October 2013. [Google Scholar]
- BiIjanl, T.; Sagud, I.; Pavlicic, D.; Matanovic Skugor, M. Process and Intermediates for Preparation of Omaveloxolone and Salts Thereof. Patent EP22197004.9, 21 September 2023. [Google Scholar]
- De Stefani, C.; Lodovichi, J.; Albonetti, L.; Salvatici, M.C.; Quintela, J.C.; Bilia, A.R.; Bergonzi, M.C. Solubility and Permeability Enhancement of Oleanolic Acid by Solid Dispersion in Poloxamers and γ-CD. Molecules 2022, 27, 3042. [Google Scholar] [CrossRef]
- Oliva, R.; Ginestra, G.; Piperno, A.; Mazzaglia, A.; Nostro, A.; Scala, A. Harnessing the power of PLA-PEG nanoparticles for Linezolid delivery against methicillin-resistant Staphylococcus aureus. Int. J. Pharm. 2023, 642, 123067. [Google Scholar] [CrossRef]
- Oliva, R.; Torcasio, S.M.; Coulembier, O.; Piperno, A.; Mazzaglia, A.; Scalese, S.; Rossi, A.; Bassi, G.; Panseri, S.; Montesi, M.; et al. RGD-tagging of star-shaped PLA-PEG micellar nanoassemblies enhances doxorubicin efficacy against osteosarcoma. Int. J. Pharm. 2024, 657, 124183. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Scala, A.; Celesti, C.; Lambertsen Larsen, K.; Genovese, F.; Bongiorno, C.; Leggio, L.; Iraci, N.; Iraci, N.; Mazzaglia, A.; et al. Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study. Molecules 2023, 28, 3023. [Google Scholar] [CrossRef] [PubMed]
- Torcasio, S.M.; Oliva, R.; Montesi, M.; Panseri, S.; Bassi, G.; Mazzaglia, A.; Piperno, A.; Coulembier, O.; Scala, A. Three-armed RGD-decorated starPLA-PEG nanoshuttle for docetaxel delivery. Biomater. Adv. 2022, 140, 213043. [Google Scholar] [CrossRef] [PubMed]
- Wasim, M.; Bergonzi, M.C. Unlocking the Potential of Oleanolic Acid: Integrating Pharmacological Insights and Advancements in Delivery Systems. Pharmaceutics 2024, 16, 692. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Xing, Z.; Cai, J.; Wang, L.; Zhao, C.; Wei, X.; Jiang, M.; Sun, H.; Zhou, L.; et al. Hepatocyte-targeted delivery using oleanolic acid-loaded liposomes for enhanced hepatocellular carcinoma therapy. Biomater. Sci. 2023, 11, 3952–3964. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, D.; Wang, Y.; Zhang, Z.; Lin, X.; Ji, Q.; Han, Y.; Yu, L.; Xu, J. Neutrophil-Mimetic oleanolic acid-loaded Liposomes targeted to alleviate oxidative stress for renal ischemia-reperfusion injury treatment. Int. J. Pharm. X 2025, 9, 100344. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, B.; Xu, Y.; Luo, F.; Chen, T.; Chen, L.; Wang, X.; Wu, D.; Hu, J. Oleanolic acid-based nanoparticles for the treatment of ulcerative colitis. Nanomedicine 2025, 20, 677–690. [Google Scholar] [CrossRef]
- He, J.-X.; Zhu, C.-Q.; Liang, G.-F.; Mao, H.-B.; Shen, W.-Y.; Hu, J.-B. Targeted-lung delivery of bardoxolone methyl using PECAM-1 antibody-conjugated nanostructure lipid carriers for the treatment of lung inflammation. Biomed. Pharmacother. 2024, 178, 116992. [Google Scholar] [CrossRef]
- Huang, Y.; Osouli, A.; Pham, J.; Mancino, V.; O’Grady, C.; Khan, T.; Chaudhuri, B.; Pastor-Soler, N.M.; Hallows, K.R.; Chung, E.J. Investigation of Basolateral Targeting Micelles for Drug Delivery Applications in Polycystic Kidney Disease. Biomacromolecules 2024, 25, 2749–2761. [Google Scholar] [CrossRef]
- Ye, Z.; Sheu, W.C.; Qu, H.; Peng, B.; Liu, J.; Zhang, L.; Yuan, F.; Wei, Y.; Zhou, J.; Chen, Q.; et al. Autocatalytic, Brain Tumor-Targeting Delivery of Bardoxolone Methyl Self-Assembled Nanoparticles for Glioblastoma Treatment. Small Sci. 2024, 4, 2400081. [Google Scholar] [CrossRef]
- Mitra, A.; Tania, N.; Ahmed, M.A.; Rayad, N.; Krishna, R.; Albusaysi, S.; Bakhaidar, R.; Shang, E.; Burian, M.; Martin-Pozo, M.; et al. New Horizons of Model Informed Drug Development in Rare Diseases Drug Development. Clin. Pharmacol. Ther. 2024, 116, 1398–1411. [Google Scholar] [CrossRef]
- Pepin, X.J.H.; Hynes, S.M.; Zahir, H.; Walker, D.; Semmens, L.Q.; Suarez-Sharp, S. Understanding the mechanisms of food effect on omaveloxolone pharmacokinetics through physiologically based biopharmaceutics modeling. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 1771–1783. [Google Scholar] [CrossRef]
- Kadirvelu, B.; Gavriel, C.; Nageshwaran, S.; Chan, J.P.K.; Nethisinghe, S.; Athanasopoulos, S.; Ricotti, V.; Voit, T.; Giunti, P.; Festenstein, R.; et al. A wearable motion capture suit and machine learning predict disease progression in Friedreich’s ataxia. Nat. Med. 2023, 29, 86–94. [Google Scholar] [CrossRef]
- Mishra, R.K.; Nunes, A.S.; Enriquez, A.; Profeta, V.R.; Wells, M.; Lynch, D.R.; Vaziri, A. At-home wearable-based monitoring predicts clinical measures and biological biomarkers of disease severity in Friedreich’s Ataxia. Commun. Med. 2024, 4, 217. [Google Scholar] [CrossRef]



| Plant Sources | Extraction (Yield) | Biological Activity | Ref. |
|---|---|---|---|
| Olea europaea | Maceration (90%) | Antitumor, antimicrobial, anti-diabetic | [32,33] |
| Ligustrum lucidum | Microwave assisted extraction (5.8 mg/gm) | Anti-hepatitis, anti-inflammatory, antioxidative, antitumor, antiprotozoal, antimutagenic | [30,34] |
| Syzygium aromaticum/Clove | Soxhlet extraction (92%) | Antinociceptive, antioxidant, anti-inflammatory, antihypertensive | [35,36] |
| Lantana camara | Maceration in ethanol 1.74% dry weight of root | Anti-inflammatory, antioxidative, antiprotozoal | [37] |
| Achyranthes aspera | Microwave and ultrasonic assisted extraction, (1.1% to 1.9%) | Antimicrobial, anti-inflammatory | [38] |
| Satureja montana | Percolation with diethyl ether (1.9%) | Antitumor, antibacterial | [39] |
| Aspilia Africana | Cold maceration (56.78%) | Anti-inflammatory, Antioxidant | [40] |
| Monotheca buxifolia | Maceration (0.5% to 0.9%) | Antipyretic | [41,42] |
| Vitis vinifera | maceration (0.5% to 1%) | Antibacterial, antitumor | [43] |
| Fruits | Analyzed Part | OA Concentration |
|---|---|---|
| Olives | Skin | 3094–4356 µg/g fw |
| Pulp | 27–29 µg/g fw | |
| Bilberries | Whole fruit | 1679.2–2029.6 µg/g dw |
| Jujube | Pulp | 360 ± 10.7 µg/g dw |
| Pears | Skin | 164.3–3066.6 µg/g dw |
| Pulp | 34.0–156.0 µg/g dw | |
| Grapes | Peel | 176.2 µg/g dw |
| Pomegranate | Peel | 26.96 ± 0.93 µg/g dw |
| Seed | 1.12 ± 0.09 µg/g dw | |
| Apples | Pomace | 16 µg/g.dm |
| Skin | 28 µg/g.dm | |
| Lemon | Peel | 0.62 ± 0.01 µg/g dw |
| Patent | Reagents | Conditions |
|---|---|---|
| [50] | Step (a) Diphenyl phosphoryl azide (DPPA) Step (b) Benzene Step (c) Sodium sulfate, sodium bicarbonate Step (d) CH3CF2CO2H, DCC, DMAP | Solvents: Toluene, Triethylamine, Ethyl acetate, Chloroform Step a: 0 °C for 5 min, then r.t. overnight Step b: 80 °C with stirring for 2 h Step c: Acetonitrile, cooled to 0 °C Step d: Methylene chloride, Hexanes, Room temperature (25 °C) |
| [51] | Step (a) Br2/NaOH, Cl2/NaOH, NaOCl, NaOBr, Pb(OAc)4/Et3N, NBS/DBU, PIFA, PIDA Step (b) DCC or CDMT, DMAP or NMM | Solvents: Diethyl ether, THF, Me-THF, DMF, DMSO, etc. Step a: 40–180 °C depending on reagent PIFA/PIDA: 5–30 °C Step b: −10 to 15 °C |
| Parameters | OA | CDDO | OMA |
|---|---|---|---|
| Molecular Formula | C30H48O3 | C32H43NO4 | C33H44F2N2O3 |
| Molecular weight | 456.7 g/mol | 505.7 g/mol | 554.71 g/mol |
| Solubility | Insoluble in H2O, | Insoluble in H2O | Insoluble in H2O |
| 21 mg/mL in DMSO | 20 mg/mL in DMSO | ≥55.5 mg/mL in DMSO | |
| 7 mg/mL in Ethanol | 10 mg/mL Ethanol | ≥25.05 mg/mL in Ethanol | |
| pKa | 4.74 | - | 7.4 |
| Appearance | Solid | Solid Powder | Solid |
| Color | White | White to yellow | White to off-white |
| OA | ||
| Ref | Description | Comment |
| Wei et al. 2023 [58] | Galactosylated chitosan-modified liposomes (GC@Lipo) were loaded with OA to address the challenge of non-specific drug distribution in hepatocellular carcinoma (HCC) treatment. | GC@Lipo binds to the asialoglycoprotein receptor (ASGPR) on HCC cell surfaces, improving the antitumor efficacy of OA. In mouse Hepa1-6 cells, OA loaded GC@Lipo inhibited migration and proliferation, upregulating E-cadherin and downregulating N-cadherin, vimentin, and Anexelekto compared to free OA. |
| Chen at al 2025 [59] | Targeted hybrid liposomes were loaded with OA and fused with neutrophil membrane coating (N-OAL) to enhance the precision treatment of renal ischemia–reperfusion injury | N-OAL enhances the accumulation and retention at inflammatory sites associated with AKI through biomimetic chemotaxis mediated by neutrophil membranes specifically targeting damaged renal tubular epithelial cells. N-OAL exerts significant antioxidant, anti-inflammatory anti-apoptotic properties. The remarkable protective effect of N-OAL on oxidative-damaged renal tissue caused by AKI induction was confirmed in vivo. |
| Zang 2025 [60] | OA nanoparticles (138.1 nm) were obtained by emulsion solvent evaporation method for the treatment of ulcerative colitis. Their anti-inflammatory effects and therapeutic efficacy were evaluated in vitro (RAW264.7 cells) and in vivo (DSS-induced UC mouse model). | OA NPs reduced oxidative stress and inflammation by downregulating TNF-α, IL-6, and IL-1β pro-inflammatory cytokines and promoting macrophage polarization from M1 to M2. Oral administration of OA NPs significantly alleviated colitis symptoms, reduced inflammation, and mitigated tissue damage, improved colon morphology, with minimal systemic toxicity. |
| CDDO | ||
| Ref | Description | Comment |
| He 2024 [61] | Nanostructured lipid carriers were loaded with CDDO and conjugated with anti-PECAM-1 antibody (PECAM@CDDO NLCs) to specifically bind pulmonary vascular endothelial cells that highly express PECAM-1 receptors for the treatment of acute lung injury (ALI). | PECAM@BM NLCs accumulated in the lungs and significantly alleviated the inflammation of ALI. PECAM@BM NLCs inhibited the assembly of NLRP3 inflammasome and pro-caspase-1 complex, thereby suppressing the induction of pyroptosis and resulting in the inhibition of N-terminal GSDMD expression without systemic toxicity. |
| Huang 2024 [62] | CDDO was incorporated into peptide amphiphile micelles containing RGD peptide to bind the basolateral surface of renal tubules via integrin receptors for treating polycystic kidney disease. Four drug combinations in RGD micelles were evaluated (CDDO, Octreotide, Salsalate, Pravastatin). | The highest synergistic effects were observed between CDDO and Salsalate in both 2D and 3D autosomal dominant polycystic kidney disease (ADPKD) in vitro models. Both combinations CDDO/Octreotide and CDDO/Salsalate in RGD micelles conferred greater therapeutic benefits in ADPKD mice. |
| Ye 2024 [63] | Targeted CDDO self-assembled nanoparticles conjugated with p28 peptide and loaded with lexiscan (LEX) (p28-LBM NPs) were designed for glioblastoma (GBM) treatment | p28-LBM NPs successfully penetrated brain tumors after intravenous administration, significantly inhibited GBM tumor growth and extended the survival of mice with tumors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordaro, M.; Neri, G.; Ansari, S.A.M.K.; Buccheri, R.; Scala, A.; Piperno, A. Synthesis and Biological Profile of Omaveloxolone: The Cornerstone for Friedreich Ataxia Treatment. Int. J. Mol. Sci. 2025, 26, 9747. https://doi.org/10.3390/ijms26199747
Cordaro M, Neri G, Ansari SAMK, Buccheri R, Scala A, Piperno A. Synthesis and Biological Profile of Omaveloxolone: The Cornerstone for Friedreich Ataxia Treatment. International Journal of Molecular Sciences. 2025; 26(19):9747. https://doi.org/10.3390/ijms26199747
Chicago/Turabian StyleCordaro, Massimiliano, Giulia Neri, Shoeb Anwar Mohammed Khawja Ansari, Rocco Buccheri, Angela Scala, and Anna Piperno. 2025. "Synthesis and Biological Profile of Omaveloxolone: The Cornerstone for Friedreich Ataxia Treatment" International Journal of Molecular Sciences 26, no. 19: 9747. https://doi.org/10.3390/ijms26199747
APA StyleCordaro, M., Neri, G., Ansari, S. A. M. K., Buccheri, R., Scala, A., & Piperno, A. (2025). Synthesis and Biological Profile of Omaveloxolone: The Cornerstone for Friedreich Ataxia Treatment. International Journal of Molecular Sciences, 26(19), 9747. https://doi.org/10.3390/ijms26199747












