Heart Failure Biomarkers—Pathophysiology, Diagnosis, Prognosis and Clinical Relevance
Abstract
1. Introduction
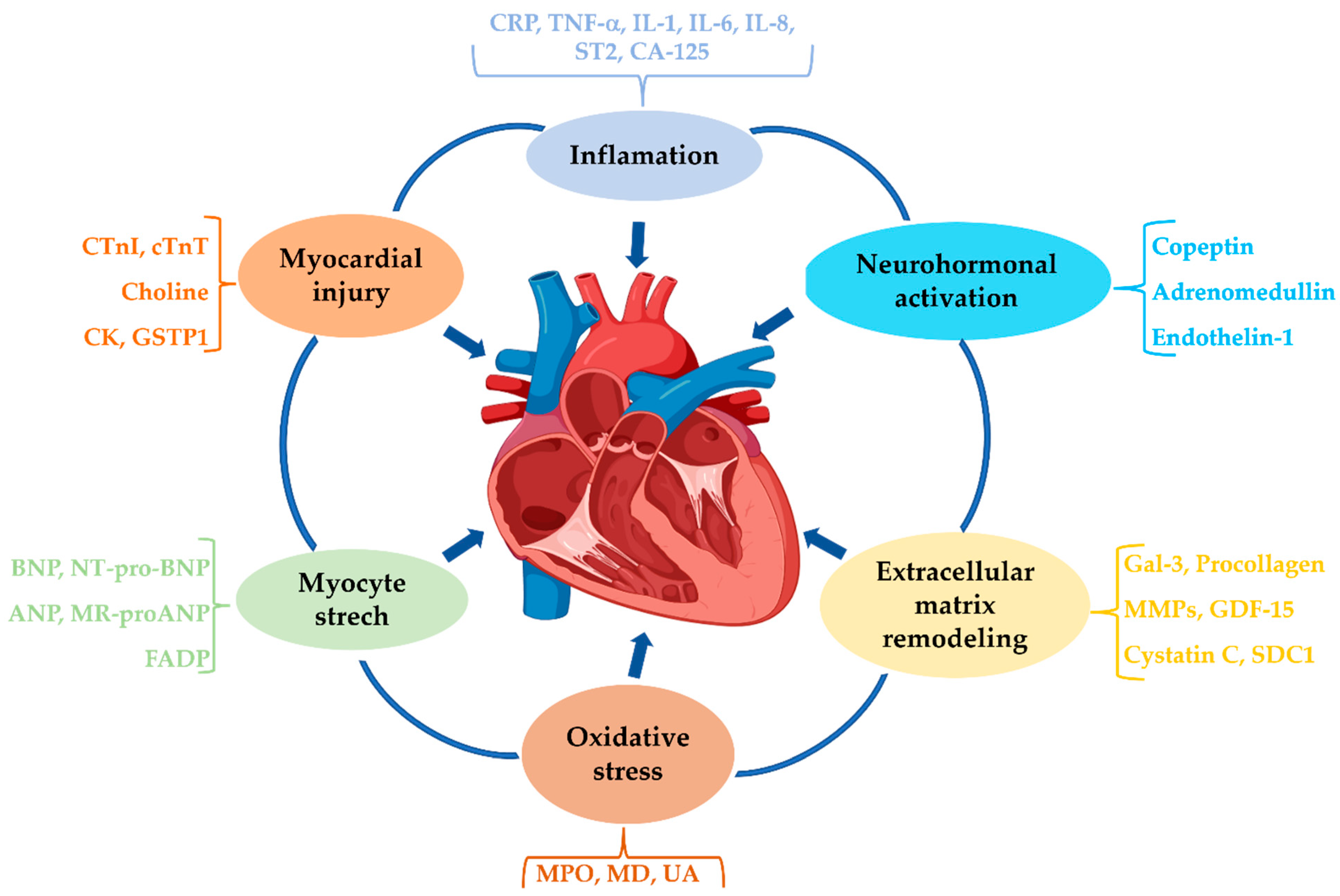
2. Pathophysiology of Heart Failure
2.1. Inflammation
2.2. Neurohormonal Activation
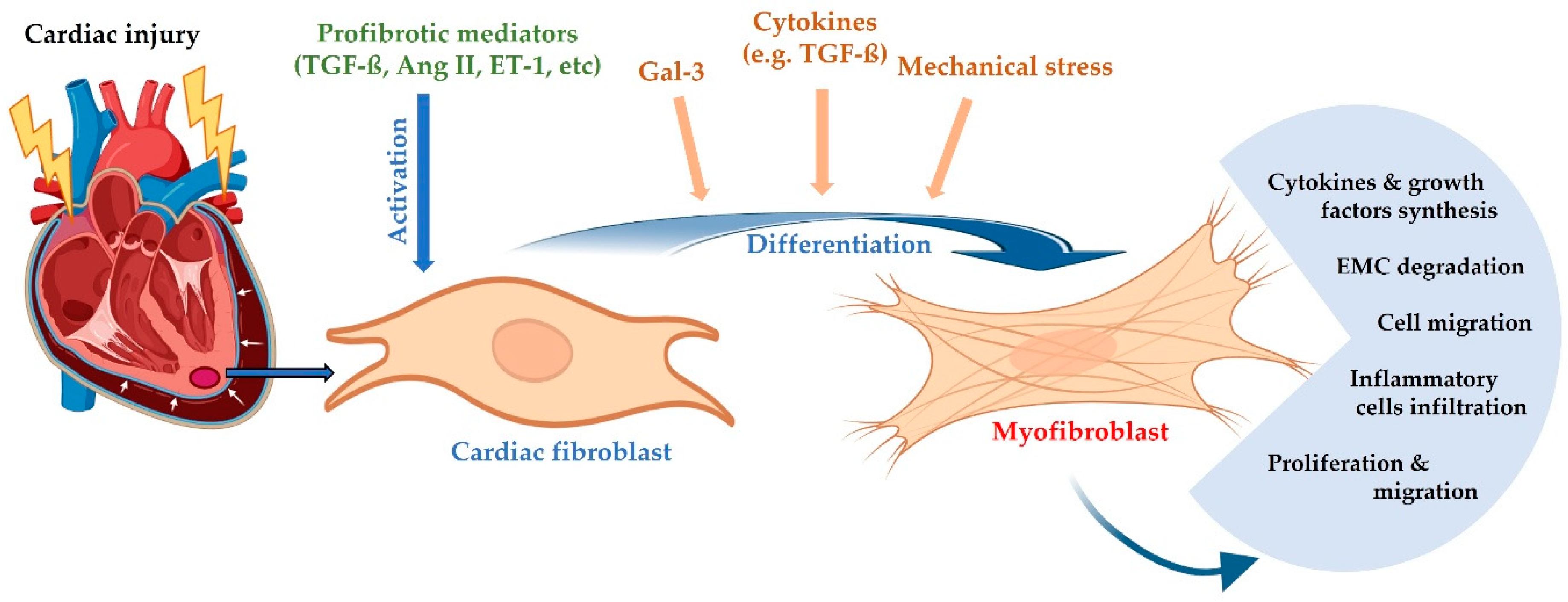
2.3. Cardiac Remodeling
2.4. Oxidative Stress
2.5. Genetic and Environmental Factors
3. Diagnostic and Prognostic Biomarkers
3.1. Inflammatory Biomarkers
3.2. Oxidative Stress Biomarkers
3.3. Cardiac Remodeling Biomarkers
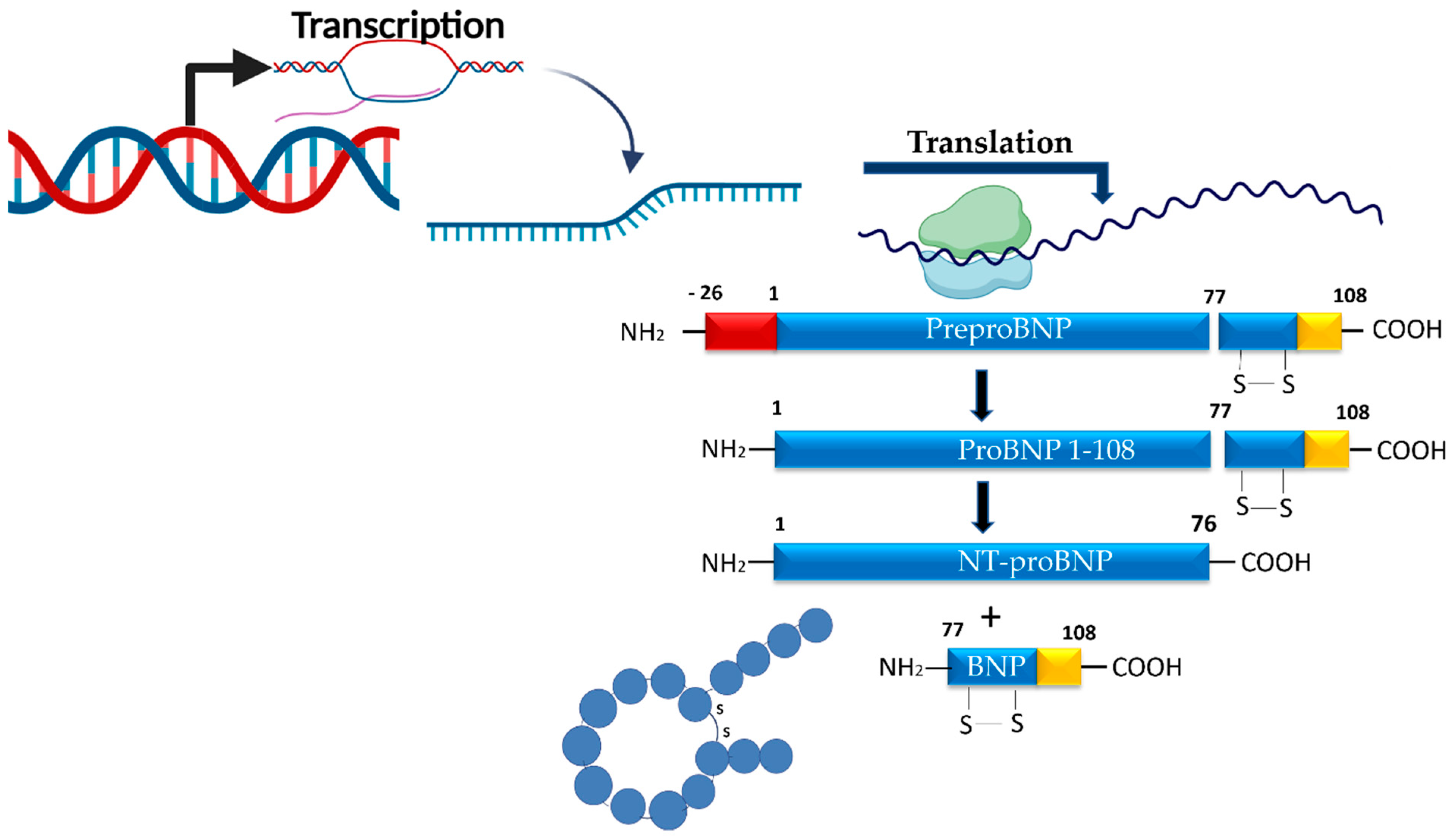
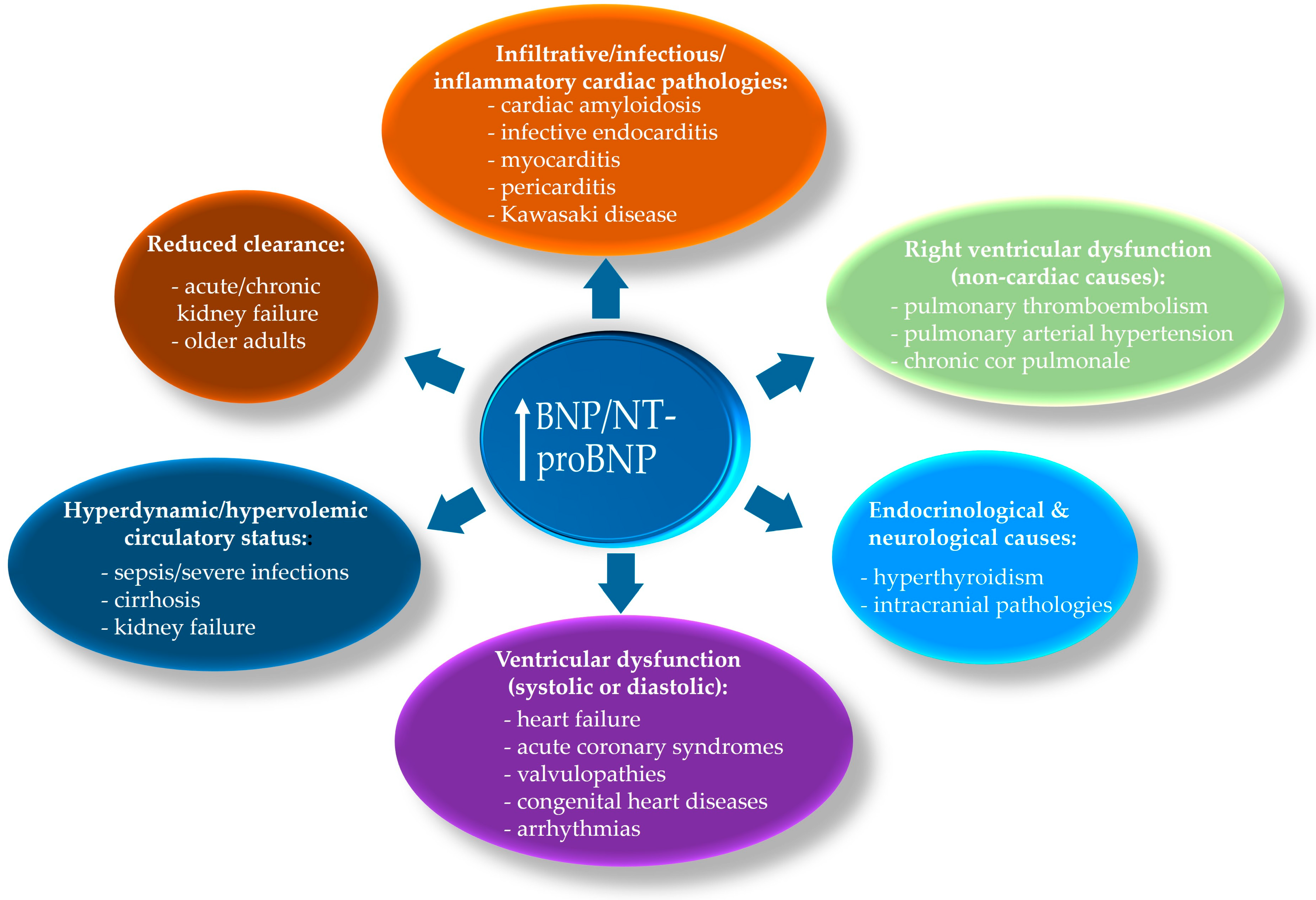
3.4. Myocardial Stress/Hemodynamic Biomarkers
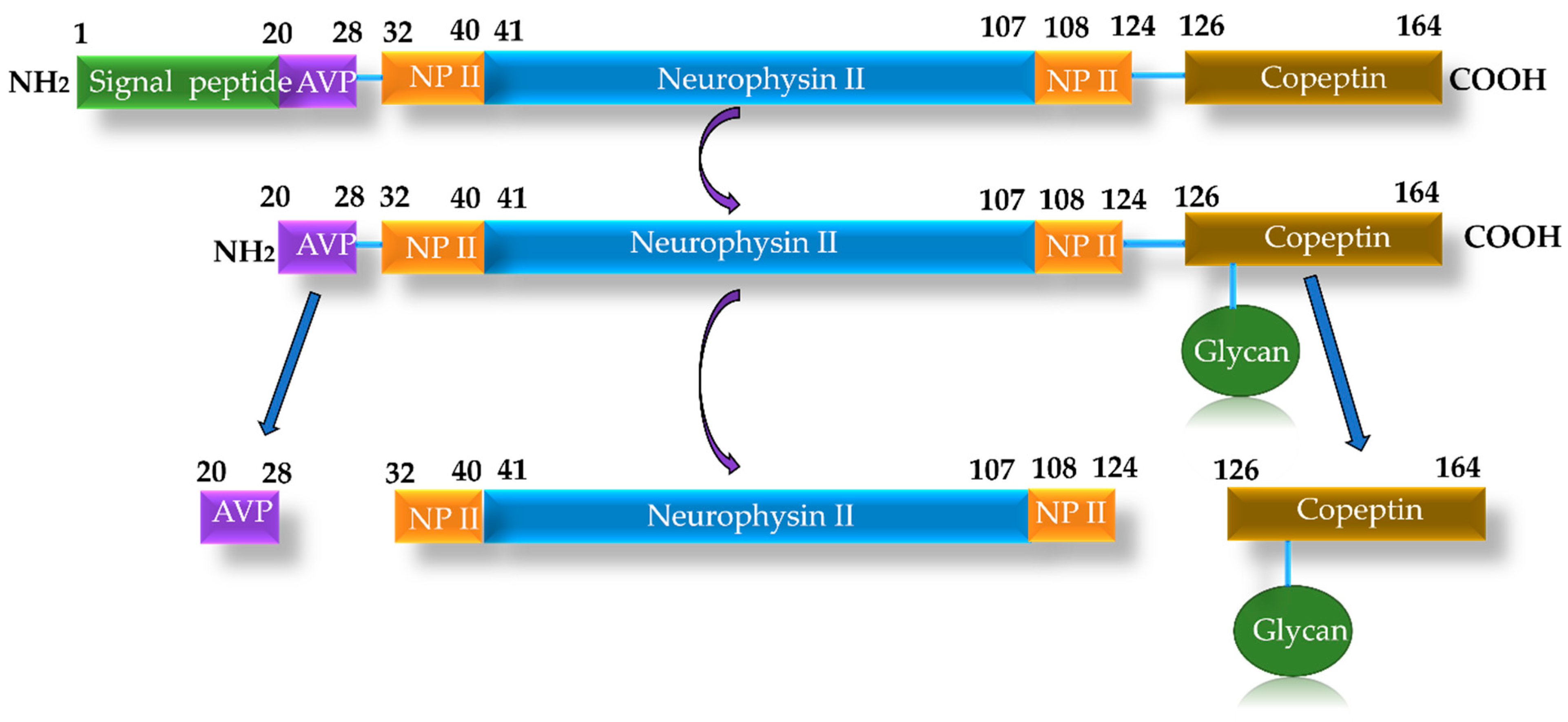
3.5. Biomarkers of Neurohormonal Activation
3.6. Biomarkers of Cardiomyocyte Injury
3.7. Other Types of Biomarkers
3.8. Biomarker Relevance in HFpEF Versus HFrEF
4. The Multimarker Approach in Heart Failure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noll, N.A.; Lal, H.; Merryman, W.D. Mouse models of heart failure with preserved or reduced ejection fraction. Am. J. Pathol. 2020, 190, 1596–1608. [Google Scholar] [CrossRef]
- van Ham, W.B.; Kessler, E.L.; Oerlemans, M.I.F.J.; Handoko, L.M.; Sluijter, J.P.G.; van Veen, T.A.B.; den Ruijter, H.M.; de Jager, S.C.A. Clinical phenotypes of heart failure with preserved ejection fraction to select preclinical animal models. JACC Basic Transl. Sci. 2022, 7, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bai, J.; Sun, Y.; Zhen, D.; Fu, D.; Wang, Y.; Wei, C. Oxymatrine attenuated isoproterenol-induced heart failure via the TLR4/NF-κB and MAPK pathways in vivo and in vitro. Eur. J. Pharmacol. 2023, 941, 175500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Magaye, R.; Kaye, D.M.; Wang, B.H. Heart failure with preserved ejection fraction: The role of inflammation. Eur. J. Pharmacol. 2024, 980, 176858. [Google Scholar] [CrossRef]
- Ponzoni, M.; Coles, J.G.; Maynes, J.T. Rodent models of dilated cardiomyopathy and heart failure for translational investigations and therapeutic discovery. Int. J. Mol. Sci. 2023, 24, 3162. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.M.; Lund, L.H.; Coats, A.J.S.; Savarese, G. An update on global epidemiology in heart failure. Eur. Heart J. 2022, 43, 3005–3007. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global burden of 371 diseases and in-juries in 204 countries and territories, 1990–2021: A systematic analysis for the Global Burden of Disease sSudy 2021. Lancet 2024, 403, 2000–2055. [Google Scholar] [CrossRef]
- Ran, X.; Guo, X.; Zhang, X.; Wu, C.; Lin, J.; Chen, L.; Li, Y. Global, regional, and national burdens of heart failure and its risk factors, 1990-2021: Results from the Global Burden of Disease Study 2021. Biomark. Res. 2025, 13, 18. [Google Scholar] [CrossRef]
- Bastos, J.M.; Colaço, B.; Baptista, R.; Gavina, C.; Vitorino, R. Innovations in heart failure management: The role of cutting-edge biomarkers and multi-omics integration. J. Mol. Cardiol. Cell. Plus 2025, 11, 100290. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Abdin, A.; Böhm, M.; Shahim, B.; Karlström, P.; Kulenthiran, S.; Skouri, H.; Lund, L.H. Heart failure with preserved ejection fraction epidemiology, pathophysiology, diagnosis and treatment strategies. Int. J. Cardiol. 2024, 412, 132304. [Google Scholar] [CrossRef] [PubMed]
- Kodur, N.; Tang, W.H.W. Management of heart failure with improved ejection fraction current evidence and controversies. JACC Heart Fail. 2025, 13, 537–553. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Saura, M.; Zamorano, J.L.; Zaragoza, C. Preclinical models of congestive heart failure, advantages, and limitations for application in clinical practice. Front. Physiol. 2022, 13, 850301, Erratum in Front. Physiol. 2022, 13, 1045550. https://doi.org/10.3389/fphys.2022.1045550. [Google Scholar] [CrossRef]
- Kozman, K.; Ferrannini, G.; Benson, L.; Dahlström, U.; Hage, C.; Savarese, G.; Shahim, B.; Lund, L.H. Etiology of heart failure across the ejection fraction spectrum and association with prognosis. JACC Heart Fail. 2025, 13, 102491. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, Z.; Lin, W.; Zhang, C.; Wang, X. Exploration of serum biomarkers in heart failure patients with preserved and reduced ejection fractions through analysis of heterogeneity. Biochem. Biophys. Rep. 2025, 43, 102183. [Google Scholar] [CrossRef] [PubMed]
- Pagel, P.S.; Tawil, J.N.; Boettcher, B.; Izquierdo, D.A.; Lazicki, T.J.; Crystal, G.J.; Freed, J.K. Heart failure with preserved ejection fraction: A comprehensive review and update of diagnosis, pathophysiology, treatment, and perioperative implications. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1839–1859. [Google Scholar] [CrossRef]
- Correale, M.; Salzano, A.; Tricarico, L.; Cittadini, A.; Crisci, G.; Dattilo, G.; Maria Inciardi, R.; Fiori, E.; Paolillo, S.; Ruocco, G.; et al. Non cardiovascular comorbidities in heart failure. An updated position paper from the Heart Failure Working Group of the Italian Society of Cardiology (SIC). Eur. J. Intern. Med. 2025, 140, 106457. [Google Scholar] [CrossRef]
- Swedberg, K. Heart failure subtypes: Pathophysiology and definitions. Diabetes Res. Clin. Pract. 2021, 175, 108815. [Google Scholar] [CrossRef]
- Ahmed, F.; Kahlon, T.; Mohamed, T.M.A.; Ghafghazi, S.; Settles, D. Literature review: Pathophysiology of heart failure with preserved ejection fraction. Curr. Probl. Cardiol. 2023, 48, 101745. [Google Scholar] [CrossRef]
- McBeath, K.; Cowie, M.R. Heart failure: Classification and pathophysiology. Medicine 2022, 50, 471–478. [Google Scholar] [CrossRef]
- Rist, A.; Sevre, K.; Wachtell, K.; Devereux, R.B.; Aurigemma, G.P.; Smiseth, O.A.; Kjeldsen, S.E.; Julius, S.; Pitt, B.; Burnier, M.; et al. The current best drug treatment for hypertensive heart failure with preserved ejection fraction. Eur. J. Intern. Med. 2024, 120, 3–10. [Google Scholar] [CrossRef]
- Haghighat, L.; DeJong, C.; Teerlink, J.R. New and future heart failure drugs. Nat. Cardiovasc. Res. 2024, 3, 1389–1407. [Google Scholar] [CrossRef]
- Lavalle, C.; Mariani, M.V.; Severino, P.; Palombi, M.; Trivigno, S.; D’Amato, A.; Silvetti, G.; Pierucci, N.; Di Lullo, L.; Chimenti, C.; et al. Efficacy of modern therapies for heart failure with reduced ejection fraction in specific population subgroups: A systematic review and network meta-analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar] [CrossRef]
- Biegus, J.; Niewinski, P.; Josiak, K.; Kuleja, K.; Ponikowskac, B.; Nowak, K.; Zymlinski, R.; Ponikowski, P. Pathophysiology of advanced heart failure what knowledge is needed for clinical management? Heart Fail. Clin. 2021, 17, 519–531. [Google Scholar] [CrossRef]
- Parichatikanond, W.; Luangmonkong, T.; Mangmool, S.; Kurose, H. Therapeutic targets for the treatment of cardiac fibrosis and cancer: Focusing on TGF-b signaling. Front. Cardiovasc. Med. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Ravassa, S.; Lopez, B.; Treibel, T.A.; San Jose, G.; Losada-Fuentenebro, B.; Tapia, L.; Bayes-Genís, A.; Díez, J.; Gonzalez, A. Cardiac fibrosis in heart failure: Focus on non-invasive diagnosis and emerging therapeutic strategies. Mol. Aspects Med. 2023, 93, 101194. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in heart failure. JACC State-of-the-Art Review. JACC 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Liu, D. The role of the MAPK signaling pathway in cardiovascular disease: Pathophysiological mechanisms and clinical therapy. Int. J. Mol. Sci. 2025, 26, 2667. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K. Transforming growth factor-ß. Governing the transition from inflammation to fibrosis in heart failure with preserved left ventricular function. Circ. Heart. Fail. 2011, 4, 5–7. [Google Scholar] [CrossRef]
- Ning, D.; Yang, X.; Wang, T.; Jiang, Q.; Yu, J.; Wang, D. Atorvastatin treatment ameliorates cardiac function and remodeling induced by isoproterenol attack through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 2021, 574, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bazgi, F.; Nau, J.; Nakhaei-Rad, S.; Amin, E.; Wolf, M.J.; Saucerman, J.J.; Lorenz, K.; Ahmadian, M.R. The microenvironment of the pathogenesis of cardiac hypertrophy. Cells 2023, 12, 1780. [Google Scholar] [CrossRef]
- Haley, K.; Almas, T.; Shoar, S.; Shaikh, S.; Azhar, M.; Cheema, F.H.; Hameed, A. The role of anti-inflammatory drugs and nanoparticle-based drug delivery models in the management of ischemia-induced heart failure. Biomed. Pharmacother. 2021, 142, 112014. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Lazzarini, G.; Spezzini, J.; Pirone, A.; Calderone, V.; Testai, L.; Miragliotta, V. The isoproterenol-induced myocardial fibrosis: A biochemical and histological investigation. Biomed. Pharmacother. 2024, 174, 116534. [Google Scholar] [CrossRef]
- Stefanov, G.; Briyal, S.; Pais, G.; Puppala, B.; Gulati, A. Relationship between oxidative stress markers and endothelin-1 levels in newborns of different gestational ages. Front. Pediatr. 2020, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Nazanin, Z.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.K. Selenium supplementation and oxidative stress: A review. Pharma. Nutr. 2021, 17, 100263. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Dixit, P.; Khanom, N.; Sanghera, G.; McGurk, K.A. The genetic factors influencing cardiomyopathies and heart failure across the allele frequency spectrum. J. Cardiovasc. Transl. Res. 2024, 17, 1119–1139. [Google Scholar] [CrossRef]
- Shah, S.; Henry, A.; Roselli, C.; Lin, H.; Sveinbjörnsson, G.; Fatemifar, G.; Hedman, Å.K.; Wilk, J.B.; Morley, M.P.; Chaffin, M.D.; et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat. Commun. 2020, 11, 163. [Google Scholar] [CrossRef]
- Münzel, T.; Sørensen, M.; Lelieveld, J.; Landrigan, P.J.; Kuntic, M.; Nieuwenhuijsen, M.; Miller, M.R.; Schneider, A.; Daiber, A. A comprehensive review/expert statement on environmental risk factors of cardiovascular disease. Cardiovasc. Res. 2025, 121, 1653–1678. [Google Scholar] [CrossRef]
- Palmieri, G.; D’Ambrosio, M.F.; Correale, M.; Brunetti, N.D.; Santacroce, R.; Iacoviello, M.; Margaglione, M. The role of genetics in the management of heart failure patients. Int. J. Mol. Sci. 2023, 24, 15221. [Google Scholar] [CrossRef]
- Vondrakova, D.; Malek, F.; Ostadal, P.; Kruger, A.; Neuzil, P. New biomarkers and heart failure. Cor et Vasa 2013, 55, e345–e354. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef]
- Clerico, A.; Ripoli, A.; Zaninotto, M.; Masotti, S.; Passino, C. Biological variability and reference change value of NT-proBNP in healthy individuals. Circ. Heart Fail. 2022, 15, e009427. [Google Scholar] [CrossRef]
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Novel biomarkers of heart failure. Adv. Clin. Chem. 2017, 79, 93–150. [Google Scholar] [CrossRef]
- Liquori, M.E.; Christenson, R.H.; Collinson, P.O.; deFilippi, C.R. Cardiac biomarkers in heart failure. Clin. Biochem. 2014, 47, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cabrera, J.L.; Ankeny, J.; Fernández-Montero, A.; Kales, S.N.; Smith, D.L. A systematic review and meta-analysis of advanced biomarkers for predicting incident cardiovascular disease among asymptomatic middle-aged adults. Int. J. Mol. Sci. 2022, 23, 13540. [Google Scholar] [CrossRef]
- Mohan, I.K.; Baba, K.S.S.S.; Iyyapu, R.; Thirumalasetty, S.; Satish, O.S. Advances in congestive heart failure biomarkers. Adv. Clin. Chem. 2023, 112, 205–248. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, R.B.; Oktafia, P.; Saputra, P.B.T.; Purwati, D.D.; Saputra, M.E.; Maghfirah, I.; Faizah, N.N.; Oktaviono, Y.H.; Alkaff, F.F. The roles of C-reactive protein-albumin ratio as a novel prognostic biomarker in heart failure patients: A systematic review. Curr. Probl. Cardiol. 2024, 49, 102475. [Google Scholar] [CrossRef]
- Lichtenaue, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Joury, A.; Ventura, H.; Krim, S.R. Biomarkers in heart failure: Relevance in the clinical practice. Int. J. Cardiol. 2022, 363, 196–201. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L. Established and emerging roles of biomarkers in heart failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; D’Assante, R.; Israr, M.Z.; Eltayeb, M.; D’Agostino, A.; Bernieh, D.; De Luca, M.; Rega, S.; Ranieri, B.; Mauro, C.; et al. Biomarkers in heart failure: Clinical insights. Heart Fail. Clin. 2021, 17, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Lotierzo, M.; Dupuy, A.M.; Kalmanovich, E.; Roubille, F.; Cristol, J.P. sST2 as a value-added biomarker in heart failure. Clin. Chim. Acta 2020, 501, 120–130. [Google Scholar] [CrossRef]
- Marinescu, M.C.; Oprea, V.D.; Munteanu, S.N.; Nechita, A.; Tutunaru, D.; Nechita, L.C.; Romila, A. Carbohydrate antigen 125 (CA 125): A novel biomarker in acute heart failure. Diagnostics 2024, 14, 795. [Google Scholar] [CrossRef]
- Llàcer, P.; Núñez, J.; Manzano, L.; Cepeda Rodrigo, J.M.; Salamanca Bautista, P.; Guzmán García, M.; Trullás Vila, J.C.; Quirós López, R.; López Reboiro, M.L.; Montero-Pérez-Barquero, M. Carbohydrate antigen 125 (CA125) as a prognostic marker in the elderly with acute heart failure and preserved ejection fraction. Med. Clin. 2022, 159, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Colluoglu, T.; Kapansahin, T.; Akın, Y. Admission Ca-125 to identify diuretic response in hospitalized patients with acute decompensated heart failure. Heart Lung 2025, 74, 90–95. [Google Scholar] [CrossRef]
- de la Espriella, R.; Bayes-Genís, A.; Llacer, P.; Palau, P.; Minana, G.; Santas, E.; Pellicer, M.; Gonzalez, M.; Gorriz, J.L.; Bodi, V.; et al. Prognostic value of NT-proBNP and CA125 across glomerular filtration rate categories in acute heart failure. Eur. J. Intern. Med. 2022, 95, 67–73. [Google Scholar] [CrossRef]
- Zoltani, C.K. Cardiovascular toxicity biomarkers (cap 12). In Biomarkers in Toxicology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 209–228. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Carnes, J.; Gordon, G. Biomarkers in heart failure with preserved ejection fraction: An update on progress and future challenges. Heart Lung Circ. 2020, 29, 62–68. [Google Scholar] [CrossRef]
- Kott, K.A.; Bishop, M.; Yang, C.H.J.; Plasto, T.M.; Cheng, D.C.; Kaplan, A.I.; Cullen, L.; Celermajer, D.S.; Meikle, P.J.; Vernon, S.T.; et al. Biomarker development in cardiology: Reviewing the past to inform the future. Cells 2022, 11, 588. [Google Scholar] [CrossRef]
- Kim, I.C.; Yoo, B.S. Multidimensional approach of heart failure diagnosis and prognostication utilizing cardiac imaging with biomarkers. Diagnostics 2022, 12, 1366. [Google Scholar] [CrossRef]
- Singh, G.; Bamba, H.; Inban, P.; Chandrasekaran, S.H.; Priyatha, V.; John, J.; Prajjwal, P. The role of biomarkers in the prognosis and risk stratification in heart failure: A systematic review. Dis. Mon. 2024, 70, 101782. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.S.; Yanek, L.R.; Yang, W.; Butcher, B.; Norgard, S.; Marine, J.E.; Kolandaivelu, A.; Chrispin, J.; Fedarko, N.S.; Calkins, H.; et al. Growth differentiation factor-15 predicts mortality and heart failure exacerbation but not ventricular arrhythmias in patients with cardiomyopathy. Am. Heart Assoc. 2023, 12, e8023. [Google Scholar] [CrossRef] [PubMed]
- di Candia, A.M.; de Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth differentiation factor-15, a novel systemic biomarker of oxidative stress, inflammation, and cellular aging: Potential role in cardiovascular diseases. Am. Heart J. Plus 2021, 9, 100046. [Google Scholar] [CrossRef]
- Sawalha, K.; Norgard, N.B.; Drees, B.M.; López-Candales, A. Growth differentiation factor 15 (GDF-15), a new biomarker in heart failure management. Curr. Heart Fail. Rep. 2023, 20, 287–299. [Google Scholar] [CrossRef]
- Monzo, L.; Jarolim, P.; Borlaug, B.A.; Benes, J.; Jurcova, I.; Jenca, D.; Kroupova, K.; Wohlfahrt, P.; Kotrc, M.; Melenovsky, V. Growth differentiation factor–15 is associated with congestion-related anorexia and weight loss in advanced heart failure. JACC Heart Fail. 2025, 13, 315–329. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Laribi, S.; Ishihara, S.; Vergaro, G.; Baudet, M.; Logeart, D.; Mebaza, A.; Gaya, E.; Vodovar, N.; Pascual-Figal, D.A.; et al. Prognostic markers of acute decompensated heart failure: The emerging roles of cardiac biomarkers and prognostic scores. Arch. Cardiovasc. Dis. 2015, 108, 64–74. [Google Scholar] [CrossRef]
- West, M.; Kirby, A.; Stewart, R.A.; Blankenberg, S.; Sullivan, D.; White, H.D.; Hunt, D.; Marschner, I.; Janus, E.; Kritharides, L.; et al. Circulating cystatin C is an independent risk marker for cardiovascular outcomes, development of renal impairment, and long-term mortality in patients with stable coronary heart disease: The lipid study. J Am. Heart Assoc. 2022, 11, e020745. [Google Scholar] [CrossRef] [PubMed]
- Hähnel, V.; Meretz, V.; Butter, C.; Paar, V.; Edlinger, C.; Lichtenauer, M.; Biemann, R.; Isermann, B.; Hoffmeister, M.; Haase, M.; et al. Novel and established biomarkers to complement risk scores in patients with acute decompensated heart failure—A pilot study. Am. Heart J. Plus 2025, 53, 100544. [Google Scholar] [CrossRef]
- Sakamoto, D.; Sotomi, Y.; Matsuoka, Y.; Nakatani, D.; Okada, K.; Sunaga, A.; Kida, H.; Sato, T.; Kitamura, T.; Seo, M.; et al. Prognostic utility and cutoff differences in NT-proBNP levels across subgroups in heart failure with preserved ejection fraction: Insights from the PURSUIT-HFpEF Registry. J. Card. Fail. 2025, 31, 771–780. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Barallat, J.; Galan, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Gastelurrutia, P.; Vila, J.; Penafiel, J.; Galvez-Monton, C.; et al. Multimarker strategy for heart failure prognostication. Value of neurohormonal biomarkers: Neprilysin vs NT-proBNP. Rev. Esp. Cardiol. 2015, 68, 1075–1084. [Google Scholar] [CrossRef][Green Version]
- Bayés-Genís, A.; Barallat, J.; Galán, A.; de Antonio, M.; Domingo, M.; Zamora, E.; Urrutia, A.; Lupón, J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Padmanabhan, G. Soluble neprilysin: A versatile biomarker for heart failure, cardiovascular diseases and diabetic complications, A systematic review. Indian Heart J. 2020, 72, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Iyer, S.R.; Redfield, M.M.; Reddy, Y.N.; Felker, G.M.; Cappola, T.P.; Hernandez, A.F.; Scott, C.G.; Burnett, J.C.; Pereira, N.L. Circulating neprilysin in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2020, 8, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Berliner, D.; Bavendiek, U.; Vodovar, N.; Lichtinghagen, R.; David, S.; Patecki, M.; Launay, J.M.; Bauersachs, J.; Haller, H.; et al. Soluble neprilysin, NT-proBNP, and growth differentiation factor-15 as biomarkers for heart failure in dialysis patients (SONGBIRD). Clin. Res. Cardiol. 2020, 109, 1035–1047. [Google Scholar] [CrossRef]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular biomarkers: Lessons of the past and prospects for the future. Int. J. Mol. Sci. 2022, 23, 5680. [Google Scholar] [CrossRef]
- Refardt, J.; Winzeler, B.; Christ-Crain, M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin. Endocrinol. 2019, 91, 22–32. [Google Scholar] [CrossRef]
- Balling, L.; Gustafsson, F. Copeptin in heart failure. Adv. Clin. Chem. 2016, 73, 29–64. [Google Scholar] [CrossRef]
- Gherasim, L. Troponins in heart failure—A perpetual challenge. Maedica J. Clin. Med. 2019, 14, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Vasan, R.S. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc. Med. 2017, 27, 123–133. [Google Scholar] [CrossRef]
- Aimo, A.; Spitaleri, G.; Panichella, G.; Lupón, J.; Emdin, M.; Bayes-Genis, A. Pirfenidone as a novel cardiac protective treatment. Heart Fail. Rev. 2022, 27, 525–532. [Google Scholar] [CrossRef]
- Shaik, F.; Balderstone, M.J.M.; Arokiasamy, S.; Whiteford, J.R. Roles of syndecan-4 in cardiac injury and repair. Int. J. Biochem. Cell Biol. 2022, 146, 106196. [Google Scholar] [CrossRef] [PubMed]
- Asta, L.; Pisano, C.; Sbrigata, A.; Raffa, G.M.; Scola, L.; Balistreri, C.R. Biomarkers in heart failure: A review and a wish. Int. J. Mol. Sci. 2025, 26, 8046. [Google Scholar] [CrossRef]
- Hillen, A.E.J.; Burbach, P.H.; Hol, E.M. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog. Neurobiol. 2018, 165–167, 66–86. [Google Scholar] [CrossRef]
- Miftode, R.S.; Costache, I.I.; Constantinescu, D.; Mitu, O.; Timpau, A.S.; Hancianu, M.; Leca, D.A.; Miftode, I.L.; Gigoranu, R.A.; Oancea, A.F.; et al. Syndecan-1: From a promising novel cardiac biomarker to a surrogate early predictor of kidney and liver injury in patients with acute heart failure. Life 2023, 13, 898. [Google Scholar] [CrossRef]
- Miftode, R.S.; Şerban, I.L.; Timpau, A.S.; Miftode, I.L.; Ion, A.; Buburuz, A.M.; Costache, A.D.; Costache, I.I. Syndecan-1: A review on its role in heart failure and chronic liver disease patients’ assessment. Cardiol. Res. Pract. 2019, 2019, 4750580. [Google Scholar] [CrossRef]
- Tromp, J.; Khan, M.A.F.; Klip, I.J.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J. Am. Heart Assoc. 2017, 6, e003989. [Google Scholar] [CrossRef] [PubMed]
- Szyszkowska, A.; Olesiewicz, T.; Płonska-Korabiewska, I.; Tarasiuk, E.; Olesiewicz, B.; Knapp, M.; Sledziewski, R.; Sobkowicz, B.; Lisowska, A. The importance of lung ultrasound and IGFBP7 (insulin-like growth factor binding protein 7) assessment in diagnosing patients with heart failure. J. Clin. Med. 2024, 13, 2220. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Packer, M.; Sattar, N.; Butler, J.; González Maldonado, S.; Panova-Noeva, M.; Sumin, M.; Masson, S.; Pocock, S.J.; Anker, S.D.; et al. Insulin-like growth factor binding protein-7 concentrations in chronic heart failure: Results from the EMPEROR programme. Eur. J. Heart Fail. 2024, 26, 806–816. [Google Scholar] [CrossRef]
- Aboumsallem, J.P.; de Boer, R.A. IGFBP7: From senescence biomarker to a vaccine for heart failure. Circulation 2024, 150, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Adamson, C.; Welsh, P.; Docherty, K.F.; de Boer, R.A.; Diez, M.; Drożdż, J.; Dukát, A.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; et al. IGFBP-7 and outcomes in heart failure with reduced ejection fraction: Findings from DAPA-HF. JACC Heart Fail. 2023, 11, 291–304. [Google Scholar] [CrossRef]
- Yeoh, S.E.; Docherty, K.F.; Campbell, R.T.; Jhund, P.S.; Hammarstedt, A.; Heerspink, H.J.L.; Jarolim, P.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; et al. Endothelin-1, outcomes in patients with heart failure and reduced ejection fraction, and effects of dapagliflozin: Findings from DAPA-HF. Circulation 2023, 147, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Netala, V.R.; Hou, T.; Wang, Y.; Zhang, Z.; Teertam, S.K. Cardiovascular biomarkers: Tools for precision diagnosis and prognosis. Int. J. Mol. Sci. 2025, 26, 3218. [Google Scholar] [CrossRef]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Figueiredo, R.; Adão, R.; Leite-Moreira, A.F.; Mâncio, J.; Brás-Silva, C. Candidate microRNAs as prognostic biomarkers in heart failure: A systematic review. Rev. Port. Cardiol. 2022, 41, 865–885. [Google Scholar] [CrossRef]
- Gargiulo, P.; Marzano, F.; Salvatore, M.; Basile, C.; Buonocore, D.; Parlati, A.L.M.; Nardi, E.; Asile, G.; Abbate, V.; Colella, A.; et al. MicroRNAs: Diagnostic, prognostic and therapeutic role in heart failure—A review. ESC Heart Fail. 2023, 10, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Rios, F.J.; Sevilla-Montero, J.; Montezano, A.C.; Touyz, R.M. Exosomes and the cardiovascular system: Role in cardiovascular health and disease. J. Physiol. 2023, 601, 4923–4936. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Fu, Q.; Fu, S.; Xiang, T. Exploring the landscape of exosomes in heart failure: A bibliometric analysis. Int. J. Surg. 2025, 111, 3356–3372. [Google Scholar] [CrossRef] [PubMed]
- Bernáth-Nagy, D.; Kalinyaprak, M.S.; Giannitsis, E.; Ábrahám, P.; Leuschner, F.; Frey, N.; Krohn, J.B. Circulating extracellular vesicles as biomarkers in the diagnosis, prognosis and therapy of cardiovascular diseases. Front. Cardiovasc. Med. 2024, 11, 1425159. [Google Scholar] [CrossRef] [PubMed]
- Gruson, D.; Carbone, V.; Feracin, B.; Ahn, S.A.; Rousseau, M.F. Incremental value of intact fibroblast growth factor 23 to natriuretic peptides for long-term risk estimation of heart failure patients. Clin. Biochem. 2018, 61, 47–49. [Google Scholar] [CrossRef]
- Hayek, S.S.; Tahhan, A.S.; Ko, Y.-A.; Alkhoder, A.; Zheng, S.; Bhimani, R.; Hartsfield, J.; Kim, J.; Wilson, P.; Shaw, L.; et al. Soluble urokinase plasminogen activator receptor levels and outcomes in patients with heart failure. J. Card. Fail. 2023, 29, 158–167. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Wang, C.-Y. Role of irisin in myocardial infarction, heart failure, and cardiac hypertrophy. Cells 2021, 10, 2103. [Google Scholar] [CrossRef]
- Fu, J.; Li, F.; Tang, Y.; Cai, L.; Zeng, C.; Yang, Y.; Yang, J. The emerging role of irisin in cardiovascular diseases. J. Am. Heart Assoc. 2021, 10, e022453. [Google Scholar] [CrossRef]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Luna-Ceron, E.; Gonzalez-Gil, A.M.; Casillas-Fikentscher, A.; Villarreal-Calderon, J.R.; Enriquez, C.; De la Pena-Almaguer, E.; Castillo, E.C.; Silva-Platas, C.; et al. Association of irisin levels with cardiac magnetic resonance, inflammatory, and biochemical parameters in patients with chronic heart failure versus controls. Magn. Reson. Imaging 2022, 93, 62–72. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Chandra, J.A.; Rajamani, K.; Silambanan, S. Biomarkers in the pathogenesis of heart failure with preserved as well as reduced ejection fraction: A crosssectional study. Int. J. Cardiovasc. Acad. 2025, 11, 47–57. [Google Scholar] [CrossRef]
- Takvorian, K.S.; Wang, D.; Courchesne, P.; Vasan, R.S.; Benjamin, E.J.; Cheng, S.; Larson, M.G.; Levy, D.; Ho, J.E. The association of protein biomarkers with incident HFpEF and HFrEF. Circ. Heart Fail. 2023, 16, e009446. [Google Scholar] [CrossRef]
- van der Stam, J.A.; Bouwmeester, S.; van Loon, S.L.M.; van Riel, N.A.W.; Dekker, L.R.; Boer, A.-K.; Houthuizen, P.; Scharnhorst, V. Prognostic value of combined biomarkers in patients with heart failure: The heartmarker score. Ann. Lab. Med. 2023, 43, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical phenogroups in heart failure with preserved ejection fraction: Detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020, 8, 172–184. [Google Scholar] [CrossRef]
- Traxler, D.; Zimmermann, M.; Simader, E.; Veraar, C.M.; Moser, B.; Mueller, T.; Mildner, M.; Dannenberg, V.; Lainscak, M.; Jug, B.; et al. The inflammatory markers sST2, HSP27 and hsCRP as a prognostic biomarker panel in chronic heart failure patients. Clin. Chim. Acta 2020, 510, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, E.; Lund, L.H.; Hage, C.; Shah, S.J.; Voors, A.A.; Saraste, A.; Redfors, B.; Grove, E.L.; Barasa, A.; Richards, A.M.; et al. Myeloperoxidase inhibition reverses biomarker profiles associated with clinical outcomes in HFpEF. JACC Heart Fail. 2023, 11, 775–787. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Kuster, N.; Curinier, C.; Huet, F.; Plawecki, M.; Solecki, K.; Roubille, F.; Cristol, J.P. Exploring collagen remodeling and regulation as prognosis biomarkers in stable heart failure. Clin. Chim. Acta 2019, 490, 167–171. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, X.; Lu, J.; Zhang, L.; Yan, X.; Huang, X.; Dai, H.; Wang, Y.; Hou, L.; Wang, S.; et al. Prognostic value of multiple circulating biomarkers for 2-year death in acute heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2021, 8, 779282. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Sørensen, M.; Lelieveld, J.; Landrigan, P.J.; Kuntic, M.; Nieuwenhuijsen, M.; Miller, M.R.; Schneider, A.; Daiber, A. Multiple biomarker strategy for improved diagnosis of acute heart failure in older patients presenting to the emergency department. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 137–147. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Lupu, S.; Huțanu, A.; Oprica, M.; Opriș, D.R.; Stan, A.; Scurtu, A.-C.; Aniței, D.; Harpa, M.; Brînzaniuc, K.; et al. Natriuretic peptides and soluble ST2 improves echocardiographic diagnosis of elevated left ventricular filling pressures. Sci. Rep. 2024, 14, 22171. [Google Scholar] [CrossRef]
- Karuna, N.; Tonry, C.; Ledwidge, M.; Glezeva, N.; Gallagher, J.; McDonald, K.; Wat-son, C. Proteomic-based biomarker discovery reveals panels of diagnostic biomarkers for early identification of heart failure subtypes. J. Transl. Med. 2025, 23, 546. [Google Scholar] [CrossRef]
- del Rocio Lopez-Buenafe, G.; Alonso-Cabrera, J.A.; Marcuello, C.; Ortiz-Perez, M.; Beni-to-Lopez, F.; Colom, A.; Basabe-Desmonts, L.; Saez, J. Fabrication and characterization of PEDOT:PSS-based microstructured electrodes for in vitro cell culture. Adv. Mater. Interfaces 2025, 2500097. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Schiffer, M.; Carls, E.; Angeloni, M.; Koleśnik-Gray, M.; Schruefer, S.; Schubert, D.W.; Ferrazzi, F.; Krstić, V.; Fleischmann, B.K.; et al. Electrically conductive collagen-PEDOT:PSS hydrogel prevents post-infarct cardiac arrhythmia and supports hiPSC-cardiomyocyte function. Adv. Mater. 2024, 36, 2403642. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarker-based guideline-directed medical therapy of heart failure: The gap between guidelines and clinical practice. EMJ Cardiol. 2021, 9, 51–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Profire, B.-Ș.; Lupașcu, F.G.; Stătescu, C.; Șorodoc, V.; Sascău, R.-A.; Profire, L.; Șorodoc, L. Heart Failure Biomarkers—Pathophysiology, Diagnosis, Prognosis and Clinical Relevance. Int. J. Mol. Sci. 2025, 26, 9740. https://doi.org/10.3390/ijms26199740
Profire B-Ș, Lupașcu FG, Stătescu C, Șorodoc V, Sascău R-A, Profire L, Șorodoc L. Heart Failure Biomarkers—Pathophysiology, Diagnosis, Prognosis and Clinical Relevance. International Journal of Molecular Sciences. 2025; 26(19):9740. https://doi.org/10.3390/ijms26199740
Chicago/Turabian StyleProfire, Bianca-Ștefania, Florentina Geanina Lupașcu, Cristian Stătescu, Victorița Șorodoc, Radu-Andy Sascău, Lenuța Profire, and Laurențiu Șorodoc. 2025. "Heart Failure Biomarkers—Pathophysiology, Diagnosis, Prognosis and Clinical Relevance" International Journal of Molecular Sciences 26, no. 19: 9740. https://doi.org/10.3390/ijms26199740
APA StyleProfire, B.-Ș., Lupașcu, F. G., Stătescu, C., Șorodoc, V., Sascău, R.-A., Profire, L., & Șorodoc, L. (2025). Heart Failure Biomarkers—Pathophysiology, Diagnosis, Prognosis and Clinical Relevance. International Journal of Molecular Sciences, 26(19), 9740. https://doi.org/10.3390/ijms26199740







