Ezh2 Loss-of-Function Alters Zebrafish Cerebellum Development
Abstract
1. Introduction
2. Results
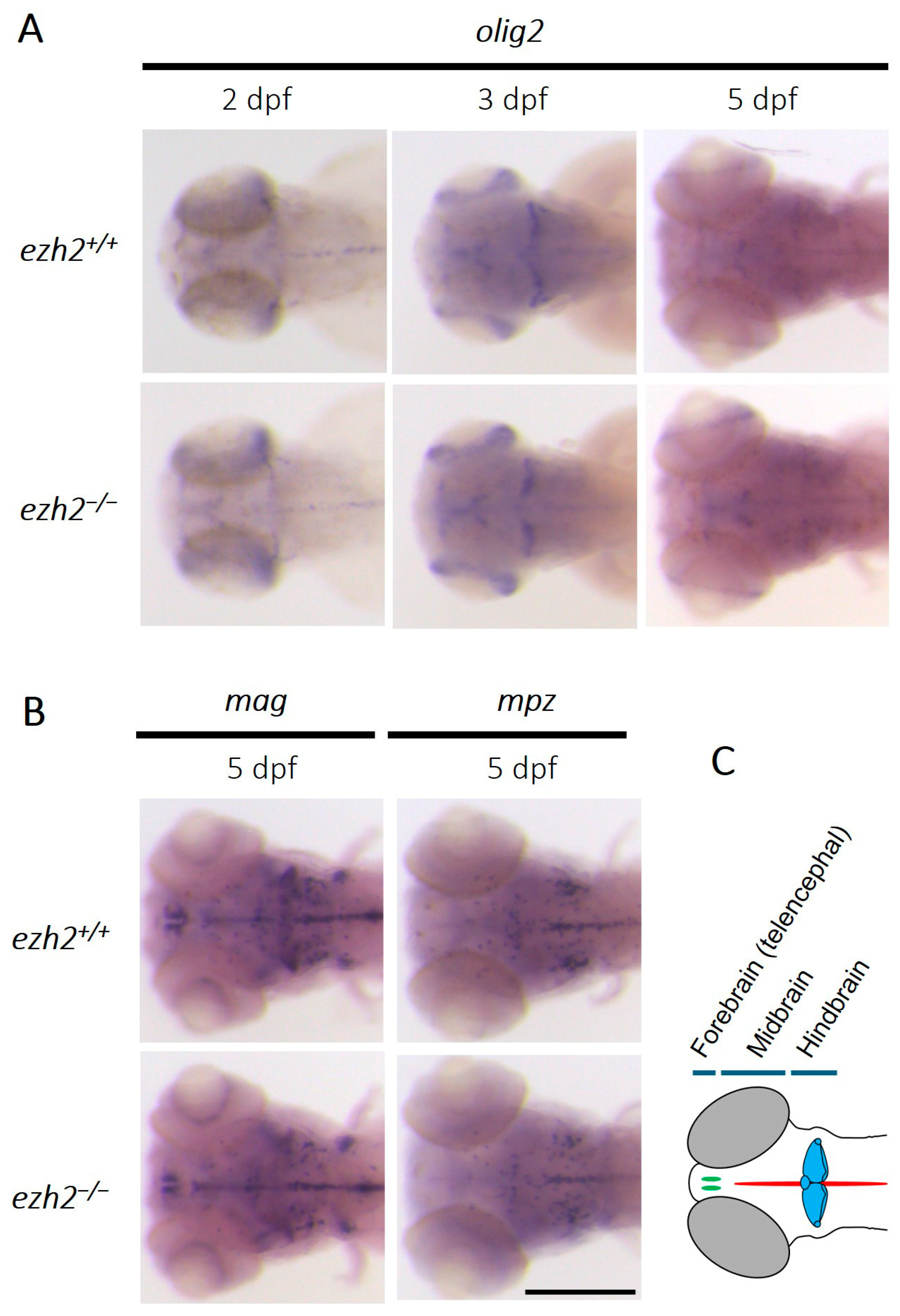
2.1. Role of Ezh2 in Oligodendrocyte Development
2.2. Loss of Ezh2 Function Selectively Impairs Cerebellar Progenitor Proliferation
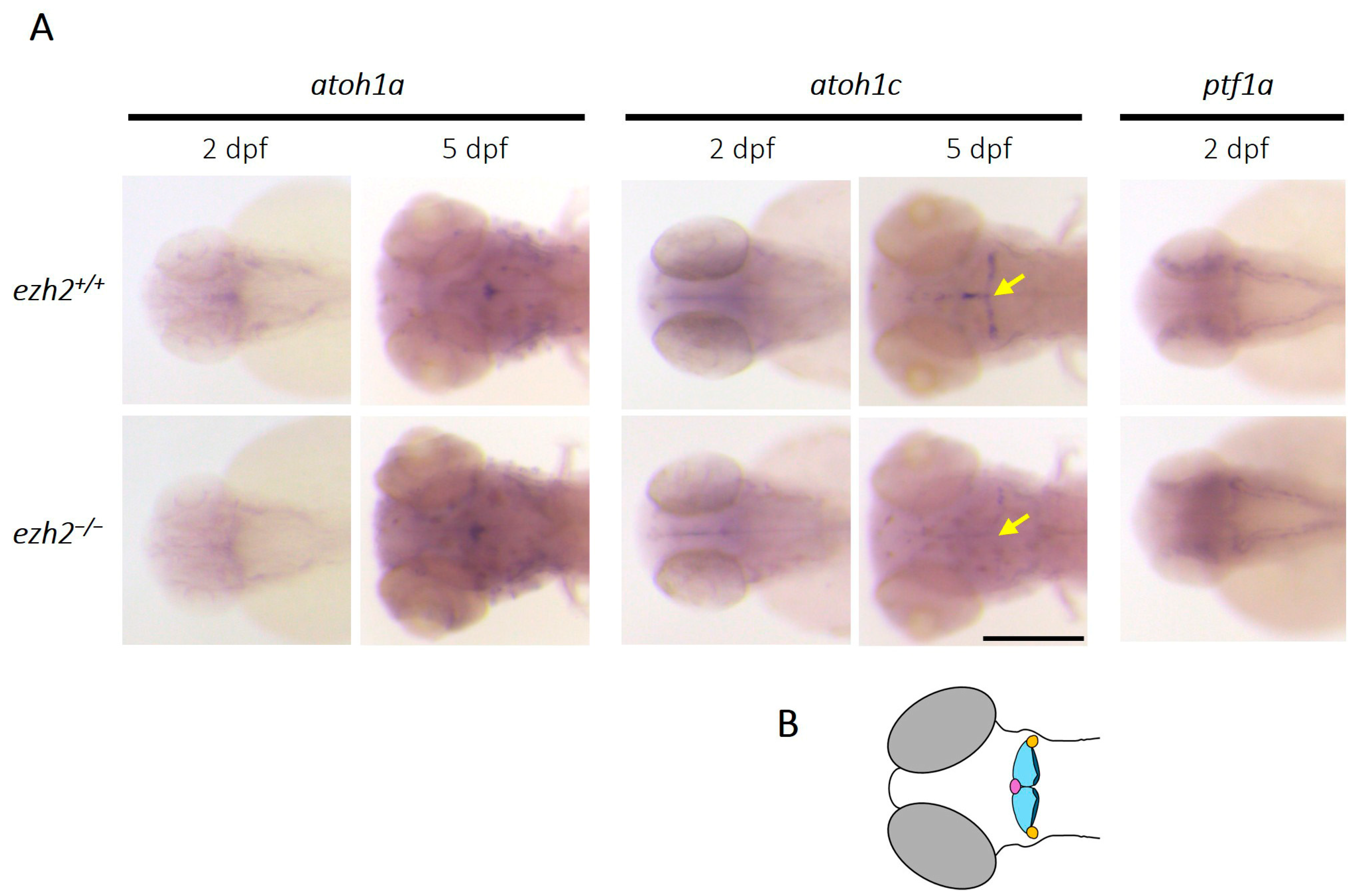
2.3. Loss of Ezh2 Function Selectively Affects Atoh1c-Expressing Cerebellar Progenitors
2.4. Loss of Ezh2 Function Impairs the Differentiation of Cerebellar Granule and Purkinje Cells
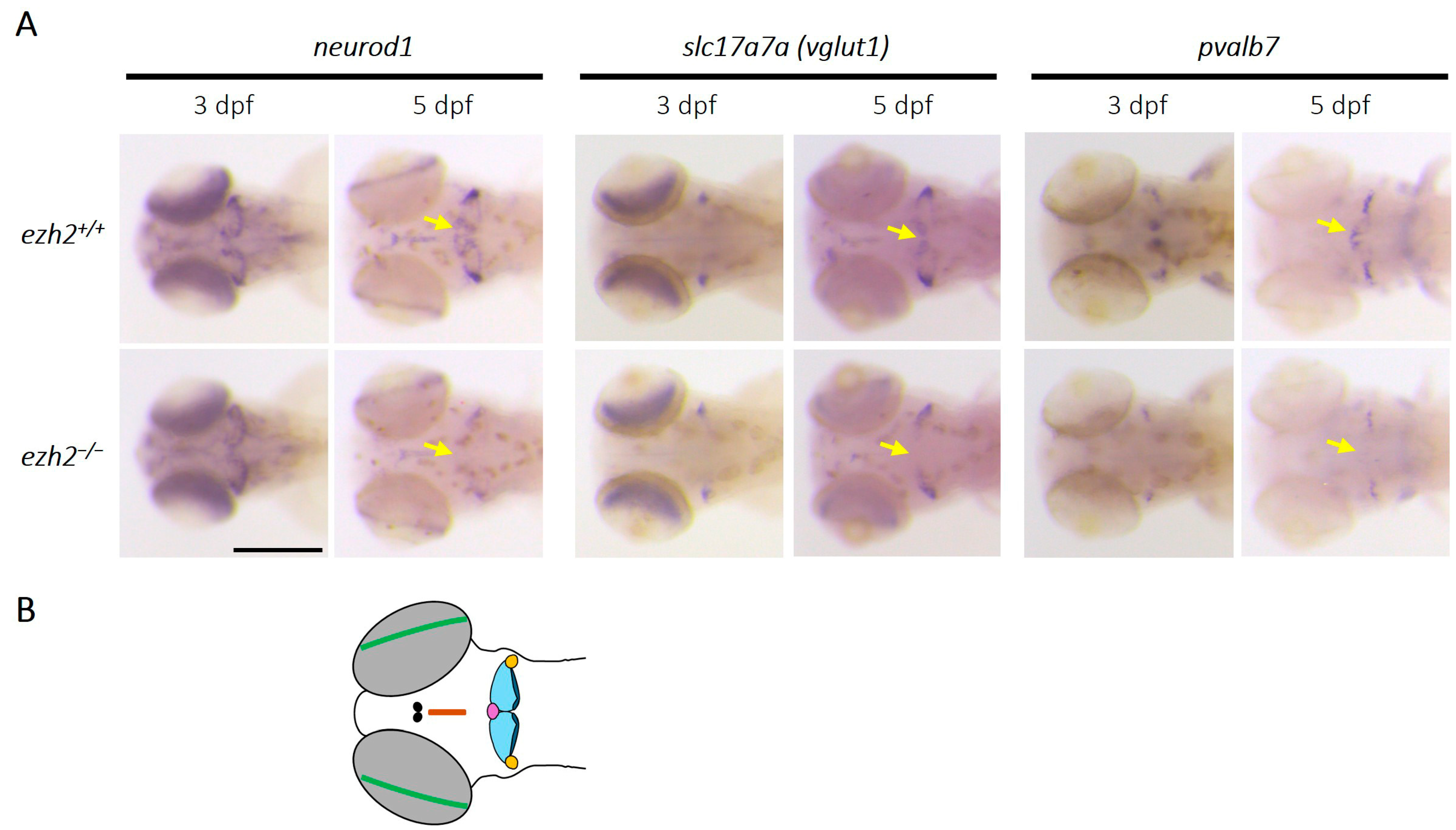
2.5. Loss of Ezh2 Function Does Not Affect the Development of Most Neurotransmitter-Specific Neuronal Populations
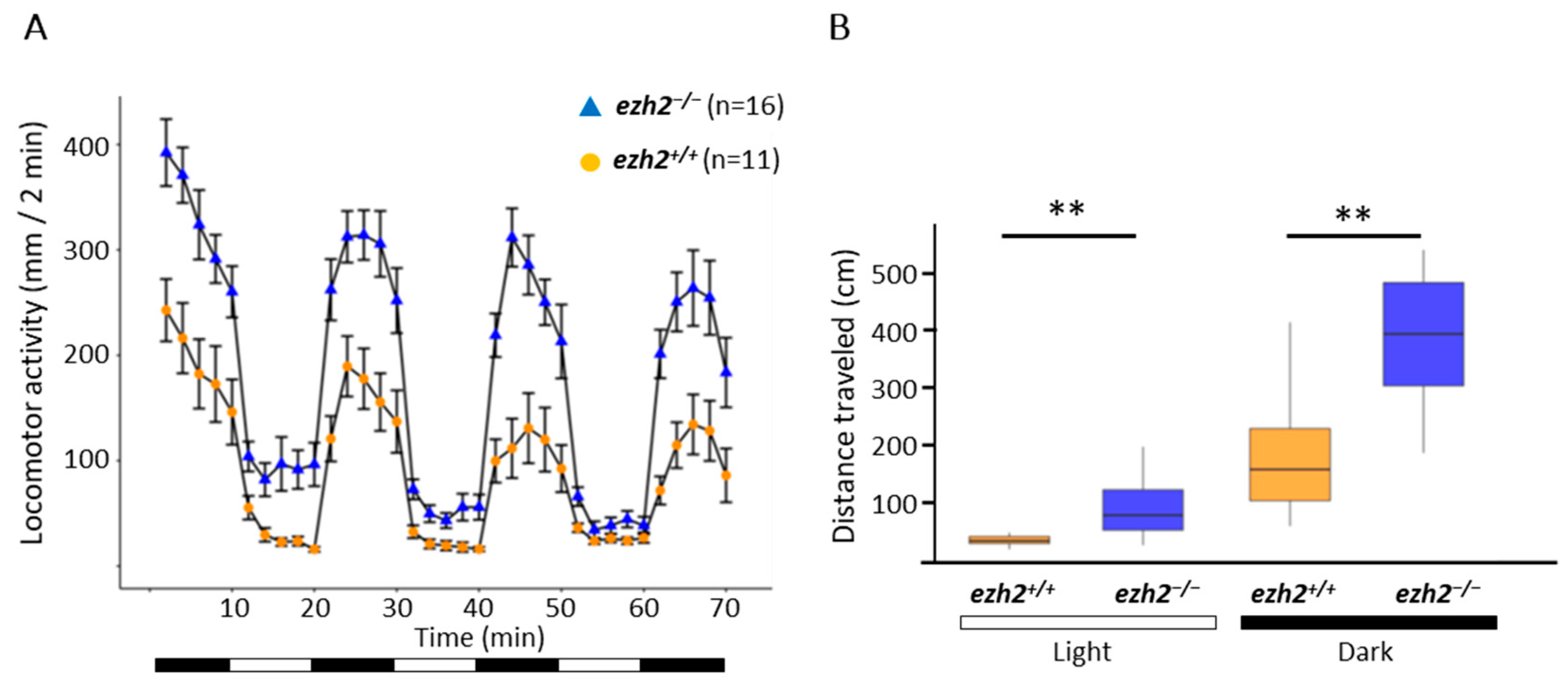
2.6. Loss of Ezh2 Function Alters Locomotor Activity
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance and Embryo Preparation
4.2. Whole-Mount In Situ Hybridization
- ISH_olig2_F: TAATACGACTCACTATAGGGATGGACTCTGACACGAGC
- ISH_olig2_R: GATTTAGGTGACACTATAGGGGCTGAGGAAGGTTTGCCAT
- ISH_mag_F: TAATACGACTCACTATAGGGCCGTGAGGGTGTTCAGTGTGTGT
- ISH_mag_R: GATTTAGGTGACACTATAGCGTCTCCCGTGCCTTCCTCT
- ISH_mpz_F: TAATACGACTCACTATAGGGGTGGTGCTCTTGGGCATAGCCTCTC
- ISH_mpz_R: GATTTAGGTGACACTATAGGGAGCCCGTTATCACACCAGCC
- ISH_pcna_F: TAATACGACTCACTATAGGGGGCAACATCAAGCTCTCACA
- ISH_pcna_R: GATTTAGGTGACACTATAGAAATCCCACAGATGACAGGC
- ISH_ccna2_F: TAATACGACTCACTATAGGGGGAAGGATGTCAACACAAGGAAG
- ISH_ccna2_R: GATTTAGGTGACACTATAGGAGAGAACTGTCAGCACCAGATG
- ISH_atoh1a_F: TAATACGACTCACTATAGGGCCAACGTCGTGCAGAAA
- ISH_atoh1a_R: GATTTAGGTGACACTATAGAACCCATTACAAAGCCCAGATA
- ISH_atoh1c_F: TAATACGACTCACTATAGGGTTTCTCAGCGCACACGACCCT
- ISH_atoh1c_R: GATTTAGGTGACACTATAGTTTGGTCTCTTCGGTCATAGGCAAC
- ISH_ptf1a_F: TAATACGACTCACTATAGGGCACAGGCTTAGACTCTTTCTCC
- ISH_ptf1a_R: GATTTAGGTGACACTATAGCCCGTAGTCTGGGTCATTTG
- ISH_neurod1_F: TAATACGACTCACTATAGGGTCGAGACGCTCCGACTAGCCAA
- ISH_neurod1_R: GATTTAGGTGACACTATAGGCGTCGAGCCCGCGTAAAGA
- ISH_vglut1_F: TAATACGACTCACTATAGGGTGCCAGGGACTTGTGGAGGG
- ISH_vglut1_R: GATTTAGGTGACACTATAGCTGGCGTAGCGTGGTGCGA
- ISH_pvalb7_F: TAATACGACTCACTATAGGGTTATCCGTCTCTCACCTCCAGCCA
- ISH_pvalb7_R: GATTTAGGTGACACTATAGCGTGTTCGGTGGCTCTATCACAA
- ISH_gad1b_F: TAATACGACTCACTATAGGGTGAGCGGCATTGAGAGGGCA
- ISH_gad1b_R: GATTTAGGTGACACTATAGCGTAGGCGACCACTGAGCC
- ISH_slc18a2_F: TAATACGACTCACTATAGGGGCACTGGGAGGACTAGCAATGGG
- ISH_slc18a2_R: GATTTAGGTGACACTATAGGTTGGCGGGAGGATTTCGCAG
- ISH_tph2_F: TAATACGACTCACTATAGGGCGGACACCTGCCATGAACTGCTT
- ISH_tph2_R: GATTTAGGTGACACTATAGTGAGTAAGTCGATGCTCTGCGTGT
- ISH_th_F: TAATACGACTCACTATAGGGCCTGTCGGATGTTAGCACGCTGG
- ISH_th_R: GATTTAGGTGACACTATAGGGCCTCAACTGAAATCCTGTGCGT
- ISH_pax2a_F: TAATACGACTCACTATAGGGACACTGGAGCAGACGCAACCA
- ISH_pax2a_R: GATTTAGGTGACACTATAGAGGTCGCCGTCTCGCCTTGA.
4.3. Genotype Analyses
4.4. Locomotor Activity Assays
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMG | Diffuse midline glioma |
| dpf | Days post fertilization |
| EG | Eminentia granularis |
| ESC | Embryonic stem cell |
| hpf | Hours post fertilization |
| LCa | Lobus caudalis cerebelli |
| NSC | Neuronal stem cell |
| OPC | Oligodendrocyte precursor cell |
| pMN | Progenitor domain of motor neurons |
| PRC2 | Polycomb repressive complex 2 |
| URL | Upper rhombic lip |
| Va | Valvula cerebelli |
| CCe | Corpus Cerebelli |
References
- Liu, P.P.; Tang, G.B.; Xu, Y.J.; Zeng, Y.Q.; Zhang, S.F.; Du, H.Z.; Teng, Z.Q.; Liu, C.M. MiR-203 Interplays with Polycomb Repressive Complexes to Regulate the Proliferation of Neural Stem/Progenitor Cells. Stem Cell Rep. 2017, 9, 190–202. [Google Scholar] [CrossRef]
- Ronan, J.L.; Wu, W.; Crabtree, G.R. From neural development to cognition: Unexpected roles for chromatin. Nat. Rev. Genet. 2013, 14, 347–359. [Google Scholar] [CrossRef]
- Feng, X.; Juan, A.H.; Wang, H.A.; Ko, K.D.; Zare, H.; Sartorelli, V. Polycomb Ezh2 controls the fate of GABAergic neurons in the embryonic cerebellum. Development 2016, 143, 1971–1980. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Mehrabi, A.; Gholami, M.H.; Zabolian, A.; Ranjbar, E.; Saleki, H.; Ranjbar, A.; Hashemi, M.; Ertas, Y.N.; Hushmandi, K.; et al. EZH2 as a new therapeutic target in brain tumors: Molecular landscape, therapeutic targeting and future prospects. Biomed. Pharmacother. 2022, 146, 112532. [Google Scholar] [CrossRef]
- Lewis, P.W.; Müller, M.M.; Koletsky, M.S.; Cordero, F.; Lin, S.; Banaszynski, L.A.; Garcia, B.A.; Muir, T.W.; Becher, O.J.; Allis, C.D. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 2013, 340, 857–861. [Google Scholar] [CrossRef]
- Jain, S.U.; Do, T.J.; Lund, P.J.; Rashoff, A.Q.; Diehl, K.L.; Cieslik, M.; Bajic, A.; Juretic, N.; Deshmukh, S.; Venneti, S.; et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat. Commun. 2019, 10, 2146. [Google Scholar] [CrossRef] [PubMed]
- Cassim, A.; Dun, M.D.; Gallego-Ortega, D.; Valdes-Mora, F. EZHIP’s role in diffuse midline glioma: Echoes of oncohistones? Trends Cancer 2024, 10, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.B.; Kasper, L.H.; Fan, Y.; Jin, H.; Wu, G.; Shaw, T.I.; Zhu, X.; Larson, J.D.; Easton, J.; Shao, Y.; et al. H3.3 K27M depletion increases differentiation and extends latency of diffuse intrinsic pontine glioma growth in vivo. Acta Neuropathol. 2019, 137, 637–655, Erratum in Acta Neuropathol. 2019, 137, 1021. [Google Scholar] [CrossRef]
- Robinson, G.; Parker, M.; Kranenburg, T.A.; Lu, C.; Chen, X.; Ding, L.; Phoenix, T.N.; Hedlund, E.; Wei, L.; Zhu, X.; et al. Novel mutations target distinct subgroups of medulloblastoma. Nature 2012, 488, 43–48. [Google Scholar] [CrossRef]
- Gabriel, N.; Balaji, K.; Jayachandran, K.; Inkman, M.; Zhang, J.; Dahiya, S.; Goldstein, M. Loss of H3K27 Trimethylation Promotes Radiotherapy Resistance in Medulloblastoma and Induces an Actionable Vulnerability to BET Inhibition. Cancer Res. 2022, 82, 2019–2030. [Google Scholar] [CrossRef]
- Rougeot, J.; Chrispijn, N.D.; Aben, M.; Elurbe, D.M.; Andralojc, K.M.; Murphy, P.J.; Jansen, P.W.T.C.; Vermeulen, M.; Cairns, B.R.; Kamminga, L.M. Maintenance of spatial gene expression by Polycomb-mediated repression after formation of a vertebrate body plan. Development 2019, 146, dev178590. [Google Scholar] [CrossRef]
- Hanot, M.; Raby, L.; Völkel, P.; Le Bourhis, X.; Angrand, P.-O. The Contribution of the Zebrafish Model to the Understanding of Polycomb Repression in Vertebrates. Int. J. Mol. Sci. 2023, 24, 2322. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Erhardt, S.; Pagani, M.; Barton, S.C.; Surani, M.A.; Jenuwein, T. The polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell Biol. 2001, 21, 4330–4336. [Google Scholar] [CrossRef]
- San, B.; Chrispijn, N.D.; Wittkopp, N.; van Heeringen, S.J.; Lagendijk, A.K.; Aben, M.; Bakkers, J.; Ketting, R.F.; Kamminga, L.M. Normal formation of a vertebrate body plan and loss of tissue maintenance in the absence of ezh2. Sci. Rep. 2016, 6, 24658. [Google Scholar] [CrossRef]
- Dupret, B.; Völkel, P.; Vennin, C.; Toillon, R.-A.; Le Bourhis, X.; Angrand, P.-O. The histone lysine methyltransferase Ezh2 is required for maintenance of the intestine integrity and for caudal fin regeneration in zebrafish. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 1079–1093. [Google Scholar] [CrossRef]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish—From embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef]
- Mohn, F.; Weber, M.; Rebhan, M.; Roloff, T.C.; Richter, J.; Stadler, M.B.; Bibel, M.; Schübeler, D. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol. Cell 2008, 30, 755–766. [Google Scholar] [CrossRef]
- Corley, M.; Kroll, K.L. The roles and regulation of Polycomb complexes in neural development. Cell Tissue Res. 2015, 359, 65–85. [Google Scholar] [CrossRef]
- Sher, F.; Rössler, R.; Brouwer, N.; Balasubramaniyan, V.; Boddeke, E.; Copray, S. Differentiation of neural stem cells into oligodendrocytes: Involvement of the polycomb group protein Ezh2. Stem Cells 2008, 26, 2875–2883. [Google Scholar] [CrossRef]
- Sher, F.; Boddeke, E.; Olah, M.; Copray, S. Dynamic changes in Ezh2 gene occupancy underlie its involvement in neural stem cell self-renewal and differentiation towards oligodendrocytes. PLoS ONE 2012, 7, e40399. [Google Scholar] [CrossRef]
- Wang, W.; Cho, H.; Kim, D.; Park, Y.; Moon, J.H.; Lim, S.J.; Yoon, S.M.; McCane, M.; Aicher, S.A.; Kim, S.; et al. PRC2 Acts as a Critical Timer That Drives Oligodendrocyte Fate over Astrocyte Identity by Repressing the Notch Pathway. Cell Rep. 2020, 32, 108147. [Google Scholar] [CrossRef]
- Ackerman, S.D.; Monk, K.R. The scales and tales of myelination: Using zebrafish and mouse to study myelinating glia. Brain Res. 2016, 1641, 79–91. [Google Scholar] [CrossRef]
- Emery, B.; Lu, Q.R. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2015, 7, a020461. [Google Scholar] [CrossRef]
- Park, H.C.; Shin, J.; Roberts, R.K.; Appel, B. An olig2 reporter gene marks oligodendrocyte precursors in the postembryonic spinal cord of zebrafish. Dev. Dyn. 2007, 236, 3402–3407. [Google Scholar] [CrossRef]
- McFarland, K.A.; Topczewska, J.M.; Weidinger, G.; Dorsky, R.I.; Appel, B. Hh and Wnt signaling regulate formation of olig2+ neurons in the zebrafish cerebellum. Dev. Biol. 2008, 318, 162–171. [Google Scholar] [CrossRef]
- Bae, Y.K.; Kani, S.; Shimizu, T.; Tanabe, K.; Nojima, H.; Kimura, Y.; Higashijima, S.; Hibi, M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev. Biol. 2009, 330, 406–426. [Google Scholar] [CrossRef]
- Kani, S.; Bae, Y.K.; Shimizu, T.; Tanabe, K.; Satou, C.; Parsons, M.J.; Scott, E.; Higashijima, S.; Hibi, M. Proneural gene-linked neurogenesis in zebrafish cerebellum. Dev. Biol. 2010, 343, 1–17. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Knipp, S. Proliferation pattern changes in the zebrafish brain from embryonic through early postembryonic stages. Anat. Embryol. 2000, 202, 385–400. [Google Scholar] [CrossRef]
- Adolf, B.; Bellipanni, G.; Huber, V.; Bally-Cuif, L. atoh1.2 and beta3.1 are two new bHLH-encoding genes expressed in selective precursor cells of the zebrafish anterior hindbrain. Gene Expr. Patterns 2004, 5, 35–41. [Google Scholar] [CrossRef]
- Volkmann, K.; Rieger, S.; Babaryka, A.; Köster, R.W. The zebrafish cerebellar rhombic lip is spatially patterned in producing granule cell populations of different functional compartments. Dev. Biol. 2008, 313, 167–180. [Google Scholar] [CrossRef]
- Lüffe, T.M.; D’Orazio, A.; Bauer, M.; Gioga, Z.; Schoeffler, V.; Lesch, K.P.; Romanos, M.; Drepper, C.; Lillesaar, C. Increased locomotor activity via regulation of GABAergic signalling in foxp2 mutant zebrafish-implications for neurodevelopmental disorders. Transl. Psychiatry 2021, 11, 529. [Google Scholar] [CrossRef]
- Wen, L.; Wei, W.; Gu, W.; Huang, P.; Ren, X.; Zhang, Z.; Zhu, Z.; Lin, S.; Zhang, B. Visualization of monoaminergic neurons and neurotoxicity of MPTP in live transgenic zebrafish. Dev. Biol. 2008, 314, 84–92. [Google Scholar] [CrossRef]
- Teraoka, H.; Russell, C.; Regan, J.; Chandrasekhar, A.; Concha, M.L.; Yokoyama, R.; Higashi, K.; Take-Uchi, M.; Dong, W.; Hiraga, T.; et al. Hedgehog and Fgf signaling pathways regulate the development of tphR-expressing serotonergic raphe neurons in zebrafish embryos. J. Neurobiol. 2004, 60, 275–288. [Google Scholar] [CrossRef]
- Pose-Méndez, S.; Schramm, P.; Valishetti, K.; Köster, R.W. Development, circuitry, and function of the zebrafish cerebellum. Cell Mol. Life Sci. 2023, 80, 227. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Batista, M.F.; Lewis, K.E. Pax2/8 act redundantly to specify glycinergic and GABAergic fates of multiple spinal interneurons. Dev. Biol. 2008, 323, 88–97. [Google Scholar] [CrossRef]
- Pose-Méndez, S.; Schramm, P.; Winter, B.; Meier, J.C.; Ampatzis, K.; Köster, R.W. Lifelong regeneration of cerebellar Purkinje cells after induced cell ablation in zebrafish. eLife 2023, 12, e79672. [Google Scholar] [CrossRef]
- Auer, F.; Nardone, K.; Matsuda, K.; Hibi, M.; Schoppik, D. Cerebellar Purkinje cells control posture in larval zebrafish (Danio rerio). eLife 2025, 13, RP97614. [Google Scholar] [CrossRef]
- Barth, P.G.; Aronica, E.; Fox, S.; Fluiter, K.; Weterman, M.A.J.; Poretti, A.; Miller, D.C.; Boltshauser, E.; Harding, B.; Santi, M.; et al. Deregulated expression of EZH2 in congenital brainstem disconnection. Neuropathol. Appl. Neurobiol. 2017, 43, 358–365. [Google Scholar] [CrossRef]
- Anderson, J.L.; Muraleedharan, R.; Oatman, N.; Klotter, A.; Sengupta, S.; Waclaw, R.R.; Wu, J.; Drissi, R.; Miles, L.; Raabe, E.H.; et al. The transcription factor Olig2 is important for the biology of diffuse intrinsic pontine gliomas. Neuro Oncol. 2017, 19, 1068–1078. [Google Scholar] [CrossRef]
- Monje, M.; Mitra, S.S.; Freret, M.E.; Raveh, T.B.; Kim, J.; Masek, M.; Attema, J.L.; Li, G.; Haddix, T.; Edwards, M.S.; et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl. Acad. Sci. USA 2011, 108, 4453–4458. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R. Posit Software, Version 4.5.1; PBC: Boston, MA, USA, 2025. Available online: http://www.posit.co/ (accessed on 1 February 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanot, M.; Völkel, P.; Le Bourhis, X.; Lagadec, C.; Angrand, P.-O. Ezh2 Loss-of-Function Alters Zebrafish Cerebellum Development. Int. J. Mol. Sci. 2025, 26, 9736. https://doi.org/10.3390/ijms26199736
Hanot M, Völkel P, Le Bourhis X, Lagadec C, Angrand P-O. Ezh2 Loss-of-Function Alters Zebrafish Cerebellum Development. International Journal of Molecular Sciences. 2025; 26(19):9736. https://doi.org/10.3390/ijms26199736
Chicago/Turabian StyleHanot, Mariette, Pamela Völkel, Xuefen Le Bourhis, Chann Lagadec, and Pierre-Olivier Angrand. 2025. "Ezh2 Loss-of-Function Alters Zebrafish Cerebellum Development" International Journal of Molecular Sciences 26, no. 19: 9736. https://doi.org/10.3390/ijms26199736
APA StyleHanot, M., Völkel, P., Le Bourhis, X., Lagadec, C., & Angrand, P.-O. (2025). Ezh2 Loss-of-Function Alters Zebrafish Cerebellum Development. International Journal of Molecular Sciences, 26(19), 9736. https://doi.org/10.3390/ijms26199736






