Circular RNAs in Cardiovascular Physiopathology: From Molecular Mechanisms to Therapeutic Opportunities
Abstract
1. Introduction
2. Biogenesis CircRNAs
2.1. Regulation of CircRNA Biogenesis
2.2. CircRNA Classification
- Exon circRNAs (ecircRNAs) represent the most abundant type and are formed exclusively through the back-splicing of exonic regions. Their sequence composition corresponds to that of their linear mRNA counterpart, although they lack the 5′ cap and 3′ poly-adenylated tail due to their covalently closed circular structure [28]. This process is often facilitated by complementary repetitive elements in flanking introns (e.g., Alu elements in mammals) or by the dimerization of RNA-binding proteins that bring splicing sites into proximity. EcircRNAs are predominately localized in the cytoplasm, present resistance to the RNase R-digestion, and are frequently involved in post-transcriptional regulatory functions (miRNA sponges, protein scaffolds, and, in some cases, translated into peptides [29]).
- Intronic circRNAs (ciRNAs) originate exclusively from intronic sequences and are generally less abundant and less stable than ecircRNAs. Their formation and stability rely on specific sequence motifs located near the branch point, such as a GU-rich element close to the 5′ site and a C-rich region near the branch point, which inhibit enzymatic debranching and facilitate the maintenance of the circular structure. ciRNAs predominantly accumulate in the nucleus, where they play a role in cis regulation of transcription [15].
- Exon-intron circRNAs (EIciRNAs) contain both exonic and intronic sequences [30]. These circRNAs are primarily localized in the nucleus where they interact with U1 small nuclear ribonucleoprotein (snRNP) and RNA polymerase II to promote cis regulatory transcription of their parental gene, participating in a transcriptional feedback mechanism [31].
- Read-through circRNAs (rt-circRNAs) result from the union of exons from two adjacent genes, which are typically oriented in the same transcriptional direction. This process, called transcription read-through, occurs when the RNA polymerase II enzyme fails to stop at the normal termination site of a gene and continues transcribing into the next downstream gene. Their resulting elongated RNA, which contains exons from the previous and subsequent genes without the intergenic region, is often more complex and difficult to annotate bioinformatically, because it contains two distinct genes. Their existence highlights the flexibility of RNA processing mechanisms and may have practical or diagnostic significance, as some rt-circRNAs are associated with tissue-specific expression patterns or relation with pathological conditions. These molecules are examples of how cells can use non-canonical splicing events to generate transcripts with potentially novel functions [32,33].
- Fusion-circRNAs (f-circRNAs) originate from chromosomal rearrangements such as translocations, inversions, and deletions, which are commonly observed in pathological conditions. They are generated by back-splicing of fusion gene transcripts, whereas exons, introns, or both from two non-adjacent genes are transcribed together. The back-splicing of these chimeric transcripts results in the formation of f-circRNAs, which are detectable only in cells harboring specific genomic rearrangements. Oncological studies have demonstrated that such rearrangements can give rise to f-circRNAs with distinct biological functions as represented by f-circM9 [34] whose tumorigenic properties have been experimentally tested in cellular and animal models [26].
3. Functions
3.1. miRNA Sponging
3.2. Binding Protein
3.3. Protein Scaffold
3.4. Protein Translation
3.5. Regulation of Gene Transcription
4. Methodologies for CircRNA Detection and Quantification
4.1. Detection of CircRNAs
- RNA sequencing (RNA-seq) represents an untargeted strategy to identify the whole RNA transcript, including the de novo identification of circRNAs [64]. However, due to the generally low expression levels of circRNAs, RNA-seq requires very high sequencing depth and coverage. Read lengths greater than 100 bp facilitate accurate alignment and detection of BSJs and are preferred for this type of investigation [69]. Due to the complexity and lack of standardization in bioinformatic pipelines for circRNA detection, well-structured and robust computational workflows are essential to ensure accurate and reproducible data analysis.
- Microarray assays represent a targeted methodology for profiling circRNA expression by using probes designed to specifically hybridize the known BSJs that are unique to each circRNA. This high-throughput approach provides greater sensitivity and more accurate detection of known circRNAs compared to sequencing approaches, because it specifically amplifies the signal for known targets, making it particularly indicated for low-abundance species. However, microarrays are limited only to previously discovered circRNAs and do not simultaneously quantify the linear transcripts, limiting the direct comparative analyses of circular vs. linear isoform quantity [72].
- 3.
- Long-read sequencing technologies, another important aspect to consider during circRNA investigation, is the length of sequencing reads, especially when multi-exonic circRNAs are present. Long-read sequencing technologies, such as Oxford Nanopore Technology (ONT), provide a suitable approach to overcome these limitations by enabling full-length circRNA sequencing and improving the resolution of complex splicing isoforms, including multi-exonic circRNAs [77,78]. In addition, this method facilitates the identification of novel circRNAs derived from gene fusions and read-through transcripts, providing a more comprehensive and accurate circRNA landscape [79].
- 4.
- Single-cell RNA sequencing and spatial transcriptomics: Recent advances in sequencing technologies, such as single-cell RNA sequencing and spatial transcriptomics, enable the profiling of circRNA expression at single-cell resolution and subcellular resolution within tissue architecture, respectively. These approaches overcome limitations inherent to bulk RNA-seq which analyzes tissue samples, often composed of heterogeneous cell populations and cell-type-specific circRNA expression patterns. Both techniques allow precise identification of circRNA distribution across subcellular compartments (including the nucleus, cytoplasm, and mitochondria), providing detailed spatial and temporal information on circRNA expression [68,80,81]. However, their high cost and technical complexity currently limit their widespread use.
| Methodology | Cost | Pros | Cons | Refs |
|---|---|---|---|---|
| Genome-Wide Approaches | ||||
| Microarray assay | +++ | Wide characterization High identification efficiency Standardized bioinformatic analysis | No de novo identification No differential expression Prone to false positive (reverse transcription + amplification) | [69,73,74,75,76] |
| RNA-sequencing | +++ | Genome-wide characterization De novo identification Differential expression analysis | High sequencing depth required Complex Prone to false positives (reverse transcription + amplification) | [72,73,74,75,76] |
| Long-read sequencing | +++ | Full-length sequencing Ideal for multiexonic and splicing isoform detection Fusion- and read-through-derived circRNA detection | Complex Specific technology | [77,78,79] |
| Single-cell sequencing Spatial transcriptomics | ++++ | Overcome RNA-seq biases Single cell/subcellular compartment resolution Spatial and temporal data | Complex High expertise Specific technology | [68,80,81] |
| Locus-specific approaches: PCR-based | ||||
| RT-PCR | + | Splicing isoform detection BSJ sequence verification | Qualitative detection Intronic circRNAs undetectable Prone to false positives (retrotranscription + amplification) | [73,74,75,76,82] |
| RT-qPCR | ++ | Quantitative assessment Relative quantification (circ to linear ratio) Splicing isoform detection | Intronic circRNAs undetectable Prone to false positives (retrotranscription + amplification) | [83,84] |
| ddPCR | ++ | Absolute quantification Less susceptible to artifacts Ideal for low-concentration samples (e.g., plasma/serum) | Intronic circRNAs undetectable Specific technology | [85,86] |
| Locus-specific approaches: Hybridization-based | ||||
| Northern blot | + | PCR-free method Enzyme-free detection | High level of circRNA expression required Large quantity of starting material Time-consuming | [87,88] |
| NanoString nCounter | ++ | PCR-free method Single molecule counting Ideal for low-quality samples (e.g., FFPE) | Specific technology Large quantity of starting material Time-consuming | [89,90,91] |
| In situ hybridization | ++ | PCR-free method Subcellular circRNA localization | High level of circRNA expression required Large quantity of starting material Time-consuming | [92,93] |
4.2. Challenges in Data Analysis for CircRNA Detection
- Alternative splicing and exon selection can generate multiple isoforms from a single gene locus. Consequently, linear isoforms and circRNAs can share sequence regions, making it difficult to computationally distinguish between them [77].
- CircRNAs can also have multiple isoform variants produced by differential exon selection or alternative BSJs, making it relevant to identify the exon composition of each circular isoform [94,95]. A simple counting of BSJ-spanning reads can misestimate circRNA abundances due to isoform overlap and shared sequences.
- If a gene, or parts of it, containing splice sites is duplicated in other genomic locations, sequencing reads may align ambiguously. This can create apparent “jumps” between homologous regions that mimic BSJs [74,96]. Additionally, transcripts from inverted or repeated regions can also produce signals resembling BSJs because of reverse-oriented splicing [96].
- Technical artifacts may further complicate analysis. For example, template-switching during reverse transcription can generate chimeric cDNA molecules that falsely appear as back-splicing events, especially when homologous regions are in proximity [73,97]. It has been estimated that 35–55% of computationally detected isoforms for a gene result from this artifact [98].
- Ultimately, sequencing errors, particularly at exon boundaries and in genes with high sequence similarity, can also introduce false splice signals (e.g., erroneous GU/AG) and need to be carefully considered [99].
4.3. Computational Identification of CircRNAs
4.3.1. Combination of Methods Limit Tool Variability
4.3.2. Machine Learning for CircRNA Prediction
4.3.3. Reconstruction of Full-Length CircRNAs
4.3.4. CircRNA Databases
4.4. Validation and Quantification: Locus-Specific Approach
4.4.1. PCR-Based Methods
- Qualitative detection of circRNAs and their splicing isoforms. In this approach reverse transcription PCR (RT-PCR) is followed by gel electrophoresis and Sanger sequencing to confirm probe specificity to the BSJ sequence [82].
- Quantitative assessment through real-time quantitative PCR (RT-qPCR) allows relative quantification of circRNAs and optionally, linear transcripts by combining a BSJ –specific assay with additional primers targeting linear exon junctions. Melting curve analysis and gel electrophoresis can also identify alternative splicing isoforms [83,84,145].PCR-based methods involving reverse transcription can induce template-switching artifacts, generating false positives. Additionally, they cannot assess intronic circRNAs since primers target exon junctions specifically. Furthermore, rolling circle amplification (concatemer formation) and trans-splicing events, might complicate quantification [76,84,89].
- Digital droplet PCR (ddPCR) offers absolute quantification with improved specificity and sensitivity. Even though it is based on PCR technology, it is less susceptible to artifacts introduced during reverse transcription and is particularly indicated for samples with low circRNAs quantity, such as plasma or serum [85,86].
4.4.2. Hybridization-Based Methods
- NanoString nCounter, which requires specific instrumentation, utilizes fluorescent probes targeting BSJ sequences, and through streptavidin-biotin interactions enables detection down to the single-molecule level. This technology is convenient for low-quality samples, like FFPE (formalin-fixed, paraffin-embedded) tissues [89,90,91].
4.5. Functional Characterization
- Loss-of-function studies primarily use RNA interference techniques such as siRNAs or antisense oligonucleotide (ASOs) specifically designed to target the back-splice junction (BSJ) sequence unique to circRNAs [69]. Targeting the BSJ allows for selective knockdown of circRNAs without affecting linear isoforms. Given the possibility of off-target effects in RNA interference experiments, it is recommended to design multiple siRNAs targeting different sequences of the BSJ and to include appropriate controls such as scrambled siRNAs. To accurately verify the knockdown efficiency and ensure phenotype specificity, the circRNA to linear RNA ratio should be consistently monitored through the experiment [147].Additionally, CRISPR-Cas-based genome editing tools are increasingly employed for circRNA depletion with Cas9 targeting DNA genomic loci [148], and Cas13 acting directly on RNA through sequence specific cleavage [149]. The CRISPR-Cas9 system allows circRNA depletion by deleting the entire gene locus [150], an approach applicable when the corresponding linear RNA is minimally or not co-expressed, or by removing specific DNA elements that promote circRNA biogenesis [2,151]. These elements include splice sites and repetitive sequences, crucial for back-splicing (see Section 2). Accurate design is essential to minimize off-target effects and to preserve expression of the linear mRNA counterpart. Efficient depletion is confirmed by PCR based methods, verifying an approximately 50% reduction in circRNA expression, consistent with allele-specific deletion. An alternative method to deplete circRNA expression, which acts directly on RNA instead of DNA and is less drastic than genome editing, is RNA-directed RNA-cleavage, obtained by using the CRISPR-Cas13 system [149,152]. The guide RNA in this system is designed to overlap the BSJ of the circRNAs, driving specific RNA recognition and cleavage of the circRNA molecule. However, optimization of the guide RNA design and targeting is recommended to minimize potential off-target effects such as cleavage of the corresponding linear RNA. Nevertheless, RNA-directed RNA-cleavage has been proved to be more specific compared to RNAi-mediated depletion [153,154].CircRNA depletion typically leads to observable phenotypic changes that reflect its functional role, including alterations in gene expression profiles, cellular behaviors, or developmental processes. For example, in vivo knockdown studies have shown that circRNA depletion causes transcriptome changes, impacting specific biological pathways or phenotypes, such as altered neuronal activity, sensory functions, or tumor-related traits. Thus, these methods allow the assessment of phenotypic outcomes directly attributable to circRNA loss, providing insights into their biological and translational significance [147].
- Gain-of-function experiments (rescue experiments), used to determine circRNA’s role in a biological process, are performed following circRNA inhibition to confirm that observed cellular phenotypes are directly linked to circRNA depletion. These approaches involve the exogenous expression of circRNAs resistant to RNAi or CRISPR-Cas13 cleavage, often delivered via plasmids or viral vectors. [28,155]. With these overexpression methods, circRNAs can also be investigated to study their effect on cells. circRNAs can also be synthetized in vitro (IVT) using different methods [156,157] and subsequently transfected into cells. The advantages of the IVT circRNAs are related to the fact that they offer precise control over length, circ-to-linear ratio and overall quantity. However, co-transcriptional modification such as m6A methylation and interaction with RNA-binding proteins (RBPs) may be absent in IVT circRNAs, potentially affecting their biological role [158]. To ensure specificity, IVT circRNAs should be treated with RNase R and highly purified before transfection. In contrast, in vivo expression systems use cellular splicing machinery to generate the circRNAs. Functional sequence elements, including splice sites and intronic inverted repeated sequences (natural or artificial), are incorporated into expression plasmid [11,159,160]. These plasmids undergo back-splicing to circularize the transcript. This method is usually less efficient than IVT, often producing linear transcripts, read-through, or concatemeric RNA species alongside circRNAs. Therefore, it is important to verify the circularity using RNase R treatments, monitor circ-to-linear ratio and confirm the correct BSJ formation throughout overexpression experiments [161,162,163,164]. Delivery of circRNA-overexpressing plasmids is typically achieved using viral vectors, such as adenovirus-associated virus (AAV) [165].
4.5.1. CircRNA-miRNA Interaction
4.5.2. CircRNA–Protein Interaction
- In the circRNA-centric approach, IP is performed using specific antisense oligonucleotides complementary to the BSJ sequence [45,174]. The proteins associated with the precipitated complex are then isolated and analyzed by Western blotting or mass spectrometry, the latter being particularly useful for unbiased protein identification [175].
- In the protein-centric approach, the target protein within the ribonucleoprotein (RNP) complex is immunoprecipitated, followed by detection and quantification of associated circRNAs through RNA sequencing or direct quantification methods as described previously [176].
4.5.3. CircRNA Translation
5. Role of CircRNAs in Cardiovascular Diseases
| CircRNA | Samples | Pathological Context | Mechanism of Action | Biological Effects | Refs |
|---|---|---|---|---|---|
| Apoptosis | |||||
| circNCX1/Slc8a1 | animal model: TAC mouse; sample: mouse CMs | CH/HF | miRNA sponge: miR-133a/ CTGF-SRF-ADRB1-PRKACB-ADCY6 | Induces CH | [205] |
| circNCX1/Slc8a1 | animal model: MI mouse; sample: Rat CMs | MI/I-R injury | miRNA sponge: miR-133a-3p/ CDIP1 | Induces CM apoptosis | [171] |
| circNfix | animal model: MI mouse/rat; sample: mouse/rat CMs | MI/I-R injury | miRNA sponge: miR-214/Gsk3β; protein scaffold: Ybx1-Nedd4l | Inhibits angiogenesis and CMs proliferation | [204] |
| circNfix | animal model: TAC mouse; sample: Ang II-treated CMs, human plasma | CH/HF | miRNA sponge: miR-145-5p/ATF3 | Reduces CH | [203] |
| HRCR (circ_012559) | animal model: ISO-injected mouse, TAC mouse; sample: mouse heart | CH/HF | miRNA sponge: miR-223/ARC | Reduces CH | [202] |
| CDR1as | animal model: MI mouse; sample: mouse CMs | MI/I-R injury | miRNA sponge: miR-7a/ PARP-SP1 | Induces CM apoptosis | [201] |
| CDR1as | sample: Human CMs, AC16 cell line | HF | miRNA sponge: miR-135(a/b)/ HMOX1 | Inhibits CM apoptosis | [230] |
| CDR1as | animal model: DM mouse; sample: D-glucose-treated CMs | DM | protein sponge: MST1/ LATS2-YAP | Induces CM apoptosis | [200] |
| circCDR1as | animal model: AMI pig; sample: porcine heart | MI/I-R injury | miRNA sponge: miR-7a | Inhibits CM apoptosis | [199] |
| ACR | sample: hypoxia-treated AC16 cells, CHF patient plasma | HF | unknown: miR-532 promoter methylation | Inhibits CM apoptosis | [193] |
| MFACR (circ_016597) | animal model: MI mouse; sample: human plasma, AC16 cell line, mouse heart | MI/I-R injury | unknown: miR-125 gene methylation | Induces CM apoptosis | [197] |
| circMAP3K5 | animal model: DM rat; sample: DCM rat hearts; high glucose-induced H9c2 | DM | miRNA sponge: miR-22-3p/ DAPK2 | Induces CM apoptosis | [196] |
| circZNF609 | animal model: I/R injury mouse; sample: mouse CMs, NRCMs | MI/I-R injury | protein sponge: YTHDF3-YAP | Induces CM apoptosis | [195] |
| circHIPK3 | animal model: TAC mouse; sample: mouse heart, NMCMs, HEK-293T cell line | CH | miRNA sponge: miR-185-3p/ CASR | Induces CH | [194] |
| Apoptosis-autophagy | |||||
| ACR | animal model: I/R injury mouse; sample: mouse CMs, CHF patient plasma | MI/I-R injury /HF | protein sponge: DNMT3B/ Pink1-FAM65B | Reduces CM autophagy and apoptosis | [198] |
| circHIPK2 | sample: H2O2-injured mouse CMs | M injury | miRNA sponge: miR-485-5p/ ATG101 | Induces CM autophagy and apoptosis | [192] |
| circPAN3 | animal model: I/R injury mouse; sample: human CMs, HEK293 cell line | M I-R injury | miRNA sponge: miR-421/ PINK1 | Reduces CM autophagy and apoptosis | [191] |
| circHIPK3 | animal model: MI/I-R injury mouse; sample: mouse heart, NMVCMs | MI/I-R injury | miRNA sponge: miR-20b-5p/ ATG7 | Induces CM autophagyand apoptosis | [42] |
| Apoptosis—mitochondrial Dynamics and Function | |||||
| MFACR (circ_016597) | animal model: I/R injury mouse; sample: mouse CMs | M I-R injury | miRNA sponge: miR-652-3p/MTP18 | Induces mitochondrial fission and CM apoptosis | [208] |
| circZNF609 | animal model: DOX-induced CT mouse; sample: mouse CFs/CMs, NRCMs | DOX-induced CT | protein sponge: FTO | Induces CM apoptosis, ROS production, mitochondrial iron overload | [209] |
| circSAMD4 | animal model: MI mouse; sample: mouse CMs | MI/I-R injury | unknown: VCP mitochondrial translocation/VDAC1 | Reduces CM apoptosis and mitochondria oxidative damage | [207] |
| Apoptosis-proliferation | |||||
| circANRIL | sample: HEK-293 cell line, human vascular tissue, human PBMCs, human primary ECs/SMCs/adventitial FBs | AS | protein sponge: PES1/nucleolar stress-p53 | Induces apoptosis and inhibits proliferation | [47] |
| circHIPK3 | sample: OGD/R-treated HCMs | MI/I-R injury | miRNA sponge: miR-124-3p/BAX-BCL2 | Induces CM apoptosis and inhibits proliferation | [189] |
| Apoptosis-inflammation | |||||
| circFOXO3 | animal model: AMI rats; sample: rat heart, H9c2 cell line | MI/I-R injury | protein sponge: KAT7/HMGB1 | Reduces CM autophagy and inflammation | [206] |
| circSAMD4 | animal model: AMI mouse; sample: rat CMs, H9c2 CMs | MI/I-R injury | miRNA sponge: miR-138-5p | Induces CM apoptosis and inflammation | [190] |
| Differentiation | |||||
| circSAMD4 | animal model: intravascular calcification mouse; sample: mouse aorta, HAECs, CAC patient plasma | VC | biomarker | Inversely correlates with vascular calcification | [224] |
| CDR1as | sample: Human PASMCs | PH | miRNA sponge: miR-7-5p/ CNN3-CAMK2D | Induces osteogenic SMC differentiation and calcification | [225] |
| circARHGAP10 | animal model: aortic valve calcification mouse; samples: mouse aortic valve, human primary VICs, HEK-293T and COS7 cell lines | VC | miRNA sponge: miR-355-3p/RUNX2 | Induces VIC osteogenic differentiation and calcification | [226] |
| Extracellular Crosstalk-proliferation | |||||
| circHIPK3 | animal model: DM mouse; sample: mouse aorta, MAECs, high glucose-induced mouse VSMCs | DM | miRNA sponge: miR-106a-5p/FOXO1-VCAM1 | Induces VSMC proliferation and inhibits apoptosis | [211] |
| circHIPK3 | animal model: mouse; sample: hypoxia-pretreated NMCMs, CMECs | MI/I-R injury | miRNA sponge: miR-29a/VEGFA | Induces CMVEC proliferation, migration, and tube formation | [212] |
| Extracellular Crosstalk-survival | |||||
| circHIPK3 | animal model: mouse; sample: hypoxia-pretreated NMCMs, CMVECs | MI/I-R injury | miRNA sponge: miR-29a/IGF-1 | Induces CMVEC survival | [221] |
| Proliferation | |||||
| circ5078 | sample: HPAECs, BOECs, PAH patient PBMCs | PAH | Protein sponge: Caprin-1 | Reduces HPAEC proliferation, stress granule formation, and mitoribosomal protein translation | [220] |
| circFOXO3 | sample: mouse CF cell lines | non pat. | protein scaffold: CDK2-p21 | Inhibits CM proliferation | [174] |
| circRNA_000203 | animal model: Ang-II induced CH mouse; sample: mouse heart, Ang-II-treated NMVCs | CH/HF | miRNA sponge: miR-26b-5p-miR-140-3p/GATA4 | Induces CH | [219] |
| circCHFR | sample: ox-LDL treated human VSMCs | AS | miRNA sponge: miR-370/ FOXO1-Cyclin D1 | Induces VSMC proliferation and migration | [210] |
| circNFIB | animal model: MI mouse; sample: mouse CFs | CF | miRNA sponge: miR-433/AZIN1-JNK1 | Inhibits CF proliferation | [213] |
| circHIPK3 | animal model: MI mouse; sample: NMCMs | HF post MI/CF | miRNA sponge: miR-17-3p/ADCY6 | Induces CF proliferation and migration | [214] |
| circHIPK3 | animal model: Ang-II induced CF mouse; sample: mouse CFs | CF/HF | miRNA sponge: miR-29b-3p/a-SMA-COL1A1-COL3A1 | Induces CF proliferation and migration | [215] |
| circHIPK3 | animal model: MI mouse; sample: NMCMs, mouse CMs/ECs/CFs, HCAECs | MI/I-R injury | miRNA sponge: miR-133a/CTGF; protein sponge: NOTCH1 stabilization | Induces CM proliferation (NOTCH1), and HCAEC proliferation, migration, and tube formation (miR-133a) | [216] |
| circHIPK3 | animal model: mouse; samples: hypoxia-treated primary CFs | CF | miRNA sponge: miR-152-3p/TGF-β2 | Induces CF proliferation, migration, and phenotypic switching | [217] |
| circCHFR | sample: ox-LDL treated human VSMCs, AS patient serum | AS | miRNA sponge: miR-214-3p/Wnt3-β catenin | Induces VSMC proliferation, migration, and inflammation | [218] |
| Senescence | |||||
| circGNAQ | animal model: AS mouse; sample: mouse aorta, mouse heart, HUVECs and HCAEC cell lines | AS | miRNA sponge: miR-146a-5p/PLK2 | Prevents EC senescence | [227] |
| ciPVT1 | sample: HUVECs and HCAEC cell lines | AS | miRNA sponge: miR-24-3p/CDK4-pRb | Prevents EC senescence | [228] |
| circFOXO3 | animal model: CM mouse; sample: mouse CFs/CMs | CM | protein sponge: ID-1/E2F1-FAK | Promotes CM senescence | [229] |
| Survival | |||||
| circFNDC3B | animal model: MI mouse; sample: mouse CMs, mouse cardiac ECs | MI/I-R injury | protein sponge: FUS/VEGF-A | Reduces CM apoptosis and promotes neovascularization | [222] |
| CNEACR | animal model: I/R injury mouse; sample: NMCs, mouse heart | MI/I-R injury | protein sponge: HDAC7/Foxa2-RIPK3 | Induces CM survival | [223] |
| circAMOTL1 | animal model: DOX-induced CM mouse; sample: mouse CMs, Rat YPEN, and mouse CF cell lines | CM | protein scaffold: PDK1-AKT1 | Induces CM survival | [50] |
5.1. Myocardial Infarction (MI) and Ischemia/Reperfusion (I/R) Injury
5.2. Cardiac Hypertrophy (CH), Cardiac Fibrosis (CF), and Heart Failure (HF)
5.3. Vascular Pathologies: Atherosclerosis (AS) and Vascular Calcification (VC)
5.4. Pulmonary Hypertension (PH)
5.5. Diabetes Mellitus (DM) and Diabetic Cardiomyopathy (DCM)
6. Clinical Applications
6.1. Useful Molecular and Functional Features of Circular RNAs
6.2. Diagnostic Potential: Circulating Biomarkers
6.3. CircRNA-Based Therapies
6.3.1. miRNA Interaction
6.3.2. RNA Interaction
6.3.3. Protein Interaction
6.3.4. Modulation of CircRNA Expression
6.3.5. Genome Editing
6.3.6. Protein Production
7. Current Challenges in Clinical Translation in CVDs
7.1. Challenges of Clinical Applications
7.2. Challenges in Cardiovascular Research
8. Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Palazzo, A.F.; Lee, E.S. Non-Coding RNA: What Is Functional and What Is Junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, W.; Li, X.; Zhang, J.; Chen, S.; Zhang, J.L.; Yang, L.; Chen, L.L. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016, 15, 611–624. [Google Scholar] [CrossRef]
- Xiao, M.S.; Ai, Y.; Wilusz, J.E. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids Are Single Stranded Covalently Closed Circular RNA Molecules Existing as Highly Base Paired Rod like Structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef]
- Hsu, M.T.; Coca-Prados, M. Electron Microscopic Evidence for the Circular Form of RNA in the Cytoplasm of Eukaryotic Cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Zheng, Y.; Wen, S.; Jiang, S.; He, S.; Qiao, W.; Liu, Y.; Yang, W.; Zhou, J.; Wang, B.; Li, D.; et al. CircRNA/LncRNA–MiRNA–MRNA Network and Gene Landscape in Calcific Aortic Valve Disease. BMC Genom. 2023, 24, 419. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Piao, H.; Li, B.; Huang, M.; Zhu, Z.; Li, D.; Wang, T.; Xu, R.; Liu, K. Circular RNAs as Potential Biomarkers and Therapeutics for Cardiovascular Disease. PeerJ 2019, 7, e6831. [Google Scholar] [CrossRef]
- Zhang, X.O.; Wang, H.B.; Zhang, Y.; Lu, X.; Chen, L.L.; Yang, L. Complementary Sequence-Mediated Exon Circularization. Cell 2014, 159, 134–147. [Google Scholar] [CrossRef]
- Liang, D.; Tatomer, D.C.; Luo, Z.; Wu, H.; Yang, L.; Chen, L.L.; Cherry, S.; Wilusz, J.E. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-MRNA Processing Machinery Is Limiting. Mol. Cell 2017, 68, 940–954.e3. [Google Scholar] [CrossRef]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.H.; Bindereif, A. Exon Circularization Requires Canonical Splice Signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. CircRNA Biogenesis Competes with Pre-MRNA Splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, L. Regulation of CircRNA Biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, T.; Ilik, I.A.; Maticzka, D.; Bhardwaj, V.; Pessoa Rodrigues, C.; Mittler, G.; Manke, T.; Backofen, R.; Akhtar, A. DHX9 Suppresses RNA Processing Defects Originating from the Alu Invasion of the Human Genome. Nature 2017, 544, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Greenman, C.; Cook, P.R.; Papantonis, A. Exon Skipping Is Correlated with Exon Circularization. J. Mol. Biol. 2015, 427, 2414–2417. [Google Scholar] [CrossRef]
- Talhouarne, G.J.S.; Gall, J.G. Lariat Intronic RNAs in the Cytoplasm of Vertebrate Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E7970–E7977. [Google Scholar] [CrossRef]
- Enuka, Y.; Lauriola, M.; Feldman, M.E.; Sas-Chen, A.; Ulitsky, I.; Yarden, Y. Circular RNAs Are Long-Lived and Display Only Minimal Early Alterations in Response to a Growth Factor. Nucleic Acids Res. 2016, 44, 1370–1383. [Google Scholar] [CrossRef]
- Ferreira, H.J.; Davalos, V.; de Moura, M.C.; Soler, M.; Perez-Salvia, M.; Bueno-Costa, A.; Setien, F.; Moran, S.; Villanueva, A.; Esteller, M. Circular RNA CpG Island Hypermethylation-Associated Silencing in Human Cancer. Oncotarget 2018, 9, 29208–29219. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Okholm, T.L.H.; Venø, M.T.; Kjems, J. Circular RNAs Are Abundantly Expressed and Upregulated during Human Epidermal Stem Cell Differentiation. RNA Biol. 2018, 15, 280–291. [Google Scholar] [CrossRef]
- Shukla, S.; Kavak, E.; Gregory, M.; Imashimizu, M.; Shutinoski, B.; Kashlev, M.; Oberdoerffer, P.; Sandberg, R.; Oberdoerffer, S. CTCF-Promoted RNA Polymerase II Pausing Links DNA Methylation to Splicing. Nature 2011, 479, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.L. Coupling MRNA Processing with Transcription in Time and Space. Nat. Rev. Genet. 2014, 15, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.C.; Liang, D.; Tatomer, D.C.; Gold, B.; March, Z.M.; Cherry, S.; Wilusz, J.E. Combinatorial Control of Drosophila Circular RNA Expression by Intronic Repeats, HnRNPs, and SR Proteins. Genes. Dev. 2015, 29, 2168–2182. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.R.; Xing, L.; Kleiman, L.; Myong, S. Repetitive RNA Unwinding by RNA Helicase A Facilitates RNA Annealing. Nucleic Acids Res. 2014, 42, 8556–8564. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.X.; Xue, W.; Zhang, Y.; Jiang, S.; Yin, Q.F.; Wei, J.; Yao, R.W.; Yang, L.; Chen, L.L. Coordinated CircRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol. Cell 2017, 67, 214–227.e7. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zheng, Y.; Zhang, J.; Chen, S.; Zhao, F. Comprehensive Identification of Internal Structure and Alternative Splicing Events in Circular RNAs. Nat. Commun. 2016, 7, 12060. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient MicroRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Y.; Chen, L. EIciRNAs in Focus: Current Understanding and Future Perspectives. RNA Biol. 2024, 22, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhao, F. CIRI: An Efficient and Unbiased Algorithm for de Novo Circular RNA Identification. Genome Biol. 2015, 16, 4. [Google Scholar] [CrossRef]
- Vidal, A.F. Read-through Circular RNAs Reveal the Plasticity of RNA Processing Mechanisms in Human Cells. RNA Biol. 2020, 17, 1823–1826. [Google Scholar] [CrossRef]
- Guarnerio, J.; Bezzi, M.; Jeong, J.C.; Paffenholz, S.V.; Berry, K.; Naldini, M.M.; Lo-Coco, F.; Tay, Y.; Beck, A.H.; Pandolfi, P.P. Oncogenic Role of Fusion-CircRNAs Derived from Cancer-Associated Chromosomal Translocations. Cell 2016, 165, 289–302. [Google Scholar] [CrossRef]
- Wang, P.L.; Bao, Y.; Yee, M.C.; Barrett, S.P.; Hogan, G.J.; Olsen, M.N.; Dinneny, J.R.; Brown, P.O.; Salzman, J. Circular RNA Is Expressed across the Eukaryotic Tree of Life. PLoS ONE 2014, 9, e90859. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rodriguez, G.; Voineagu, I.; Weatheritt, R.J. Evolutionary Dynamics of Circular Rnas in Primates. eLife 2021, 10, e69148. [Google Scholar] [CrossRef] [PubMed]
- Cocquerelle, C.; Mascrez, B.; Hétuin, D.; Bailleul, B. Mis-Splicing Yields Circular RNA Molecules. FASEB J. 1993, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Denzler, R.; Agarwal, V.; Stefano, J.; Bartel, D.P.; Stoffel, M. Assessing the CeRNA Hypothesis with Quantitative Measurements of MiRNA and Target Abundance. Mol. Cell 2014, 54, 766–776. [Google Scholar] [CrossRef]
- Aufiero, S.; Reckman, Y.J.; Pinto, Y.M.; Creemers, E.E. Circular RNAs Open a New Chapter in Cardiovascular Biology. Nat. Rev. Cardiol. 2019, 16, 503–514. [Google Scholar] [CrossRef]
- Hansen, T.B.; Wiklund, E.D.; Bramsen, J.B.; Villadsen, S.B.; Statham, A.L.; Clark, S.J.; Kjems, J. MiRNA-Dependent Gene Silencing Involving Ago2-Mediated Cleavage of a Circular Antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Liu, W.; Li, C.; Zhao, R.; Long, X.; Rong, J.; Deng, W.; Shen, C.; Yuan, J.; et al. CircHIPK3 Regulates the Autophagy and Apoptosis of Hypoxia/Reoxygenation-Stimulated Cardiomyocytes via the MiR-20b-5p/ATG7 Axis. Cell Death Discov. 2021, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Yu, W.; Wang, S. CircNCX1/MiR-133a: A Potential Novel Biomarker and Risk Factor Predictor for Myocardial Ischemia-Reperfusion Injury. Int. J. Cardiol. 2020, 299, 256. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Lu, D.; Xu, A. The Interaction of CircRNAs and RNA Binding Proteins: An Important Part of CircRNA Maintenance and Function. J. Neurosci. Res. 2020, 98, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR Target Circular RNAs Uncovers Suppression of PABPN1 Translation by CircPABPN1. RNA Biol. 2017, 14, 361–369. [Google Scholar] [CrossRef]
- Granneman, S.; Petfalski, E.; Tollervey, D. A Cluster of Ribosome Synthesis Factors Regulate Pre-RRNA Folding and 5.8S RRNA Maturation by the Rat1 Exonuclease. EMBO J. 2011, 30, 4006–4019. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular Non-Coding RNA ANRIL Modulates Ribosomal RNA Maturation and Atherosclerosis in Humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Wang, C.; Tan, S.; Li, J.; Liu, W.R.; Peng, Y.; Li, W. CircRNAs in Lung Cancer—Biogenesis, Function and Clinical Implication. Cancer Lett. 2020, 492, 106–115. [Google Scholar] [CrossRef]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of Tumor Apoptosis through a Circular RNA Enhancing Foxo3 Activity. Cell Death Differ. 2017, 24, 357–370. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A Circular RNA Binds to and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics 2017, 7, 3842–3855. [Google Scholar] [CrossRef]
- Yamamoto, H.; Unbehaun, A.; Spahn, C.M.T. Ribosomal Chamber Music: Toward an Understanding of IRES Mechanisms. Trends Biochem. Sci. 2017, 42, 655–668. [Google Scholar] [CrossRef]
- Abe, N.; Matsumoto, K.; Nishihara, M.; Nakano, Y.; Shibata, A.; Maruyama, H.; Shuto, S.; Matsuda, A.; Yoshida, M.; Ito, Y.; et al. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci. Rep. 2015, 5, 16435. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.W.; Wu, J.; Wang, X.; Guo, H.; Sun, S. Advances and Breakthroughs in IRES-Directed Translation and Replication of Picornaviruses. mBio 2023, 14, e0035823. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.; Lacerda, R.; Romão, L. Internal Ribosome Entry Site (IRES)-Mediated Translation and Its Potential for Novel MRNA-Based Therapy Development. Biomedicines 2022, 10, 1865. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Y.; Chen, C.; Wang, Z. Pervasive Translation of Circular RNAs Driven by Short IRES-like Elements. Nat. Commun. 2022, 13, 3751. [Google Scholar] [CrossRef]
- Kearse, M.G.; Wilusz, J.E. Non-AUG Translation: A New Start for Protein Synthesis in Eukaryotes. Genes. Dev. 2017, 31, 1717–1731. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA That Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e9. [Google Scholar] [CrossRef]
- Ouyang, X.; He, Z.; Fang, H.; Zhang, H.; Yin, Q.; Hu, L.; Gao, F.; Yin, H.; Hao, T.; Hou, Y.; et al. A Protein Encoded by Circular ZNF609 RNA Induces Acute Kidney Injury by Activating the AKT/MTOR-Autophagy Pathway. Mol. Ther. 2023, 31, 1722–1738. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Wang, Z.; Gong, Z.; Liu, Y.; Wang, Z. Emerging Roles of Circ-ZNF609 in Multiple Human Diseases. Front. Genet. 2022, 13, 837343. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The Emerging Roles of CircRNAs in Cancer and Oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Eidem, T.M.; Kugel, J.F.; Goodrich, J.A. Noncoding RNAs: Regulators of the Mammalian Transcription Machinery. J. Mol. Biol. 2016, 428, 2652–2659. [Google Scholar] [CrossRef]
- Lam, M.T.Y.; Li, W.; Rosenfeld, M.G.; Glass, C.K. Enhancer RNAs and Regulated Transcriptional Programs. Trends Biochem. Sci. 2014, 39, 170–182. [Google Scholar] [CrossRef]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A New Star of Noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Ji, P.; Wu, W.; Chen, S.; Zheng, Y.; Zhou, L.; Zhang, J.; Cheng, H.; Yan, J.; Zhang, S.; Yang, P.; et al. Expanded Expression Landscape and Prioritization of Circular RNAs in Mammals. Cell Rep. 2019, 26, 3444–3460.e5. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of Circular RNA Abundance with Proliferation–Exemplified with Colorectal and Ovarian Cancer, Idiopathic Lung Fibrosis, and Normal Human Tissues. Sci. Rep. 2015, 5, 8057. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, J.L.; Korsgaard, U.; Ahmadov, U.; Jarlstad Olesen, M.T.; Dietrich, K.G.; Hansen, E.B.; Vissing, S.M.; Ulhøi, B.P.; Dyrskjøt, L.; Sørensen, K.D.; et al. Spatial Profiling of Circular RNAs in Cancer Reveals High Expression in Muscle and Stromal Cells. Cancer Res. 2023, 83, 3340–3353. [Google Scholar] [CrossRef]
- Nielsen, A.F.; Bindereif, A.; Bozzoni, I.; Hanan, M.; Hansen, T.B.; Irimia, M.; Kadener, S.; Kristensen, L.S.; Legnini, I.; Morlando, M.; et al. Best Practice Standards for Circular RNA Research. Nat. Methods 2022, 19, 1208–1220. [Google Scholar] [CrossRef]
- Suzuki, H.; Tsukahara, T. A View of Pre-MRNA Splicing from RNase R Resistant RNAs. Int. J. Mol. Sci. 2014, 15, 9331–9342. [Google Scholar] [CrossRef]
- Xiao, M.S.; Wilusz, J.E. An Improved Method for Circular RNA Purification Using RNase R That Efficiently Removes Linear RNAs Containing G-Quadruplexes or Structured 3′ Ends. Nucleic Acids Res. 2019, 47, 8755–8769. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.-X.; Wei, W.-S.; Li, Y.-H.; Feng, Z.-H.; Tan, L.; Chen, J.-W.; Yuan, G.-J.; Chen, S.-L.; Guo, S.-J.; et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging MiR-30c to Induce Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2018, 24, 6319–6330. [Google Scholar] [CrossRef]
- Tang, C.; Yu, T.; Xie, Y.; Wang, Z.; McSwiggin, H.; Zhang, Y.; Zheng, H.; Yan, W. Template Switching Causes Artificial Junction Formation and False Identification of Circular RNAs. bioRxiv 2018, 259556. [Google Scholar] [CrossRef]
- Szabo, L.; Salzman, J. Detecting Circular RNAs: Bioinformatic and Experimental Challenges. Nat. Rev. Genet. 2016, 17, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-J.; Chen, Y.-J.; Chen, C.-Y.; Mai, T.-L.; Wang, Y.-D.; Yeh, C.-S.; Yang, M.-Y.; Hsiao, Y.-T.; Chang, T.-H.; Kuo, T.-C.; et al. Integrative Transcriptome Sequencing Reveals Extensive Alternative Trans-Splicing and Cis-Backsplicing in Human Cells. Nucleic Acids Res. 2018, 46, 3671–3691. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.; Conn, S.J. SplintQuant: A Method for Accurately Quantifying Circular RNA Transcript Abundance without Reverse Transcription Bias. RNA 2019, 25, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hou, L.; Zuo, Z.; Ji, P.; Zhang, X.; Xue, Y.; Zhao, F. Comprehensive Profiling of Circular RNAs with Nanopore Sequencing and CIRI-Long. Nat. Biotechnol. 2021, 39, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Tapial, J.; Ha, K.C.H.; Sterne-Weiler, T.; Gohr, A.; Braunschweig, U.; Hermoso-Pulido, A.; Quesnel-Vallières, M.; Permanyer, J.; Sodaei, R.; Marquez, Y.; et al. An Atlas of Alternative Splicing Profiles and Functional Associations Reveals New Regulatory Programs and Genes That Simultaneously Express Multiple Major Isoforms. Genome Res. 2017, 27, 1759–1768. [Google Scholar] [CrossRef]
- Ruan, H.; Wang, P.C.; Han, L. Characterization of Circular RNAs with Advanced Sequencing Technologies in Human Complex Diseases. Wiley Interdiscip. Rev. RNA 2023, 14, e1759. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Ebbesen, K.K.; Sokol, M.; Jakobsen, T.; Korsgaard, U.; Eriksen, A.C.; Hansen, T.B.; Kjems, J.; Hager, H. Spatial Expression Analyses of the Putative Oncogene CiRS-7 in Cancer Reshape the MicroRNA Sponge Theory. Nat. Commun. 2020, 11, 4551. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, J.; Cao, X.; Cai, Z.; Zhao, F. Exploring the Cellular Landscape of Circular RNAs Using Full-Length Single-Cell RNA Sequencing. Nat. Commun. 2022, 13, 3242. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Panda, A.; Gorospe, M. Detection and Analysis of Circular RNAs by RT-PCR. Bio Protoc. 2018, 8, e2775. [Google Scholar] [CrossRef]
- Yu, C.Y.; Liu, H.J.; Hung, L.Y.; Kuo, H.C.; Chuang, T.J. Is an Observed Non-Co-Linear RNA Product Spliced in Trans, in Cis or Just in Vitro? Nucleic Acids Res. 2014, 42, 9410–9423. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-F.; Zhang, L.-J.; Tan, K.; Jing, Q. Application of Droplet Digital PCR in Quantitative Detection of the Cell-Free Circulating CircRNAs. Biotechnol. Biotechnol. Equip. 2018, 32, 116–123. [Google Scholar] [CrossRef]
- Li, T.; Shao, Y.; Fu, L.; Xie, Y.; Zhu, L.; Sun, W.; Yu, R.; Xiao, B.; Guo, J. Plasma Circular RNA Profiling of Patients with Gastric Cancer and Their Droplet Digital RT-PCR Detection. J. Mol. Med. 2018, 96, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Schreiner, S.; Preußer, C.; Bindereif, A.; Rossbach, O. Northern Blot Analysis of Circular RNAs. In Circular RNAs; Humana: New York, NY, USA, 2018; pp. 119–133. [Google Scholar]
- Dodbele, S.; Mutlu, N.; Wilusz, J.E. Best Practices to Ensure Robust Investigation of Circular RNAs: Pitfalls and Tips. EMBO Rep. 2021, 22, e52072. [Google Scholar] [CrossRef]
- Dahl, M.; Daugaard, I.; Andersen, M.S.; Hansen, T.B.; Grønbæk, K.; Kjems, J.; Kristensen, L.S. Enzyme-Free Digital Counting of Endogenous Circular RNA Molecules in B-Cell Malignancies. Lab. Investig. 2018, 98, 1657–1669. [Google Scholar] [CrossRef]
- Geiss, G.K.; Bumgarner, R.E.; Birditt, B.; Dahl, T.; Dowidar, N.; Dunaway, D.L.; Fell, H.P.; Ferree, S.; George, R.D.; Grogan, T.; et al. Direct Multiplexed Measurement of Gene Expression with Color-Coded Probe Pairs. Nat. Biotechnol. 2008, 26, 317–325. [Google Scholar] [CrossRef]
- Kristensen, L.S. Profiling of CircRNAs Using an Enzyme-Free Digital Counting Method. Methods 2021, 196, 11–16. [Google Scholar] [CrossRef]
- Baker, A.-M.; Huang, W.; Wang, X.-M.M.; Jansen, M.; Ma, X.-J.; Kim, J.; Anderson, C.M.; Wu, X.; Pan, L.; Su, N.; et al. Robust RNA-Based in Situ Mutation Detection Delineates Colorectal Cancer Subclonal Evolution. Nat. Commun. 2017, 8, 1998. [Google Scholar] [CrossRef] [PubMed]
- Erben, L.; He, M.-X.; Laeremans, A.; Park, E.; Buonanno, A. A Novel Ultrasensitive In Situ Hybridization Approach to Detect Short Sequences and Splice Variants with Cellular Resolution. Mol. Neurobiol. 2018, 55, 6169–6181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.O.; Dong, R.; Zhang, Y.; Zhang, J.L.; Luo, Z.; Zhang, J.; Chen, L.L.; Yang, L. Diverse Alternative Back-Splicing and Alternative Splicing Landscape of Circular RNAs. Genome Res. 2016, 26, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, S.R.; Meyer, I.M. CYCLeR—A Novel Tool for the Full Isoform Assembly and Quantification of CircRNAs. Nucleic Acids Res. 2023, 51, e10. [Google Scholar] [CrossRef]
- Jakobi, T.; Dieterich, C. Computational Approaches for Circular RNA Analysis. Wiley Interdiscip. Rev. RNA 2019, 10, e1528. [Google Scholar] [CrossRef]
- Cocquet, J.; Chong, A.; Zhang, G.; Veitia, R.A. Reverse Transcriptase Template Switching and False Alternative Transcripts. Genomics 2006, 88, 127–131. [Google Scholar] [CrossRef]
- Roy, C.K.; Olson, S.; Graveley, B.R.; Zamore, P.D.; Moore, M.J. Assessing Long-Distance RNA Sequence Connectivity via RNA-Templated DNA-DNA Ligation. eLife 2015, 4, e03700. [Google Scholar] [CrossRef]
- Salzman, J. RNA Isoform Discovery Through Goodness of Fit Diagnostics. In Statistical Analysis of Next Generation Sequencing Data; Springer International Publishing: Cham, Switzerland, 2014; pp. 261–276. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate Alignment of Transcriptomes in the Presence of Insertions, Deletions and Gene Fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Hoffmann, S.; Otto, C.; Doose, G.; Tanzer, A.; Langenberger, D.; Christ, S.; Kunz, M.; Holdt, L.M.; Teupser, D.; Hackermüller, J.; et al. A Multi-Split Mapping Algorithm for Circular RNA, Splicing, Trans-Splicing and Fusion Detection. Genome Biol. 2014, 15, R34. [Google Scholar] [CrossRef]
- Wang, K.; Singh, D.; Zeng, Z.; Coleman, S.J.; Huang, Y.; Savich, G.L.; He, X.; Mieczkowski, P.; Grimm, S.A.; Perou, C.M.; et al. MapSplice: Accurate Mapping of RNA-Seq Reads for Splice Junction Discovery. Nucleic Acids Res. 2010, 38, e178. [Google Scholar] [CrossRef]
- Westholm, J.O.; Miura, P.; Olson, S.; Shenker, S.; Joseph, B.; Sanfilippo, P.; Celniker, S.E.; Graveley, B.R.; Lai, E.C. Genome-Wide Analysis of Drosophila Circular RNAs Reveals Their Structural and Sequence Properties and Age-Dependent Neural Accumulation. Cell Rep. 2014, 9, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Zhao, F. Circular RNA Identification Based on Multiple Seed Matching. Brief. Bioinform. 2018, 19, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Metge, F.; Dieterich, C. Specific Identification and Quantification of Circular RNAs from Sequencing Data. Bioinformatics 2016, 32, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Morey, R.; Palpant, N.J.; Wang, P.L.; Afari, N.; Jiang, C.; Parast, M.M.; Murry, C.E.; Laurent, L.C.; Salzman, J. Statistically Based Splicing Detection Reveals Neural Enrichment and Tissue-Specific Induction of Circular RNA during Human Fetal Development. Genome Biol. 2015, 16, 126. [Google Scholar] [CrossRef]
- You, X.; Conrad, T.O. Acfs: Accurate CircRNA Identification and Quantification from RNA-Seq Data. Sci. Rep. 2016, 6, 38820. [Google Scholar] [CrossRef]
- Izuogu, O.G.; Alhasan, A.A.; Alafghani, H.M.; Santibanez-Koref, M.; Elliot, D.J.; Jackson, M.S. PTESFinder: A Computational Method to Identify Post-Transcriptional Exon Shuffling (PTES) Events. BMC Bioinform. 2016, 17, 31. [Google Scholar] [CrossRef]
- Song, X.; Zhang, N.; Han, P.; Moon, B.S.; Lai, R.K.; Wang, K.; Lu, W. Circular RNA Profile in Gliomas Revealed by Identification Tool UROBORUS. Nucleic Acids Res. 2016, 44, e87. [Google Scholar] [CrossRef]
- Gaffo, E.; Buratin, A.; Dal Molin, A.; Bortoluzzi, S. Sensitive, Reliable and Robust CircRNA Detection from RNA-Seq with CirComPara2. Brief. Bioinform. 2022, 23, bbab418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bu, D.; Zhao, Y. CircRNAwrap—A Flexible Pipeline for CircRNA Identification, Transcript Prediction, and Abundance Estimation. FEBS Lett. 2019, 593, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Digby, B.; Finn, S.P.; Ó Broin, P. Nf-Core/Circrna: A Portable Workflow for the Quantification, MiRNA Target Prediction and Differential Expression Analysis of Circular RNAs. BMC Bioinform. 2023, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ji, P.; Chen, S.; Hou, L.; Zhao, F. Reconstruction of Full-Length Circular RNAs Enables Isoform-Level Quantification. Genome Med. 2019, 11, 2. [Google Scholar] [CrossRef]
- Ma, X.K.; Wang, M.R.; Liu, C.X.; Dong, R.; Carmichael, G.G.; Chen, L.L.; Yang, L. CIRCexplorer3: A CLEAR Pipeline for Direct Comparison of Circular and Linear RNA Expression. Genom. Proteom. Bioinform. 2019, 17, 511–521. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Wang, C.; Cui, Y.; Xu, T.; Wang, C.; Wang, X.; Sha, J.; Jiang, B.; Wang, K.; et al. CircAST: Full-Length Assembly and Quantification of Alternatively Spliced Isoforms in Circular RNAs. Genom. Proteom. Bioinform. 2019, 17, 522–534. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, C.; Li, S.; Du, M.; Bai, Y.; Hu, X.; Li, Y.; Chen, J.; Yang, E. CircFL-Seq Reveals Full-Length Circular Rnas with Rolling Circular Reverse Transcription and Nanopore Sequencing. eLife 2021, 10, e69457. [Google Scholar] [CrossRef]
- Yu, K.H.O.; Shi, C.H.; Wang, B.; Chow, S.H.C.; Chung, G.T.Y.; Lung, R.W.M.; Tan, K.E.; Lim, Y.Y.; Tsang, A.C.M.; Lo, K.W.; et al. Quantifying Full-Length Circular RNAs in Cancer. Genome Res. 2021, 31, 2340–2353. [Google Scholar] [CrossRef]
- Xin, R.; Gao, Y.; Gao, Y.; Wang, R.; Kadash-Edmondson, K.E.; Liu, B.; Wang, Y.; Lin, L.; Xing, Y. IsoCirc Catalogs Full-Length Circular RNA Isoforms in Human Transcriptomes. Nat. Commun. 2021, 12, 266. [Google Scholar] [CrossRef]
- Pan, X.; Xiong, K. PredcircRNA: Computational Classification of Circular RNA from Other Long Non-Coding RNA Using Hybrid Features. Mol. Biosyst. 2015, 11, 2219–2226. [Google Scholar] [CrossRef]
- Chaabane, M.; Williams, R.M.; Stephens, A.T.; Park, J.W. CircDeep: Deep Learning Approach for Circular RNA Classification from Other Long Non-Coding RNA. Bioinformatics 2020, 36, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L. Deep Learning of the Back-Splicing Code for Circular RNA Formation. Bioinformatics 2019, 35, 5235–5242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Ju, C.J.T.; Hao, J.; Chen, M.; Wang, W. JEDI: Circular RNA Prediction Based on Junction Encoders and Deep Interaction among Splice Sites. Bioinformatics 2021, 37, i289–i298. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, C. CircCNNs, a Convolutional Neural Network Framework to Better Understand the Biogenesis of Exonic CircRNAs. Sci. Rep. 2024, 14, 18982. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, J.; Zheng, X.; Pan, Z.; Zhao, F.; Gao, Y. CIRI-Deep Enables Single-Cell and Spatial Transcriptomic Analysis of Circular RNAs with Deep Learning. Adv. Sci. 2024, 11, e2308115. [Google Scholar] [CrossRef]
- Hansen, T.B.; Venø, M.T.; Damgaard, C.K.; Kjems, J. Comparison of Circular RNA Prediction Tools. Nucleic Acids Res. 2015, 44, e58. [Google Scholar] [CrossRef]
- Hansen, T.B. Improved CircRNA Identification by Combining Prediction Algorithms. Front. Cell Dev. Biol. 2018, 6, 20. [Google Scholar] [CrossRef]
- Thomas, L.F.; Sætrom, P. Circular RNAs Are Depleted of Polymorphisms at MicroRNA Binding Sites. Bioinformatics 2014, 30, 2243–2246. [Google Scholar] [CrossRef]
- Glažar, P.; Papavasileiou, P.; Rajewsky, N. CircBase: A Database for Circular RNAs. RNA 2014, 20, 1666–1670. [Google Scholar] [CrossRef]
- Wu, W.; Ji, P.; Zhao, F. CircAtlas: An Integrated Resource of One Million Highly Accurate Circular RNAs from 1070 Vertebrate Transcriptomes. Genome Biol. 2020, 21, 101. [Google Scholar] [CrossRef]
- Dong, R.; Ma, X.K.; Li, G.W.; Yang, L. CIRCpedia v2: An Updated Database for Comprehensive Circular RNA Annotation and Expression Comparison. Genom. Proteom. Bioinform. 2018, 16, 226–233. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, L.; Tang, Y.; Jhong, J.H.; Wan, J.; Chang, J.; Cui, S.; Luo, Y.; Cai, X.; Li, W.; et al. CircNet 2.0: An Updated Database for Exploring Circular RNA Regulatory Networks in Cancers. Nucleic Acids Res. 2022, 50, D93–D101. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, F.; Zhang, J. CircAtlas 3.0: A Gateway to 3 Million Curated Vertebrate Circular RNAs Based on a Standardized Nomenclature Scheme. Nucleic Acids Res. 2024, 52, D52–D60. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Shen, J.; Yang, B.B.; Ding, X. Circbank: A Comprehensive Database for CircRNA with Standard Nomenclature. RNA Biol. 2019, 16, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. StarBase v2.0: Decoding MiRNA-CeRNA, MiRNA-NcRNA and Protein-RNA Interaction Networks from Large-Scale CLIP-Seq Data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed]
- Dudekula, D.B.; Panda, A.C.; Grammatikakis, I.; De, S.; Abdelmohsen, K.; Gorospe, M. Circinteractome: A Web Tool for Exploring Circular RNAs and Their Interacting Proteins and MicroRNAs. RNA Biol. 2016, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Das, S.; Sen, R.; Basak, P.; Chakrabarti, J. Circ2Traits: A Comprehensive Database for Circular RNA Potentially Associated with Disease and Traits. Front. Genet. 2013, 4, 283. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, L.; Zheng, M.; Sun, X.; Lu, Y.; Liu, P. Circ2Disease: A Manually Curated Database of Experimentally Validated CircRNAs in Human Disease. Sci. Rep. 2018, 8, 11018. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Yang, C.L.; Huang, L.J.; Mo, Z.C.; Zhang, K.N.; Fan, W.H.; Wang, K.Y.; Wu, F.; Wang, J.G.; Meng, F.L.; et al. CircRNADisease v2.0: An Updated Resource for High-Quality Experimentally Supported CircRNA-Disease Associations. Nucleic Acids Res. 2024, 52, D1193–D1200. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Y.; Zhang, C.; Cui, Q.; Tang, Y.D.; Ji, X.; Cui, C. LncRNADisease v3.0: An Updated Database of Long Non-Coding RNA-Associated Diseases. Nucleic Acids Res. 2024, 52, D1365–D1369. [Google Scholar] [CrossRef]
- Fan, C.; Lei, X.; Tie, J.; Zhang, Y.; Wu, F.X.; Pan, Y. CircR2Disease v2.0: An Updated Web Server for Experimentally Validated CircRNA–Disease Associations and Its Application. Genom. Proteom. Bioinform. 2022, 20, 435–445. [Google Scholar] [CrossRef]
- Chen, X.; Han, P.; Zhou, T.; Guo, X.; Song, X.; Li, Y. CircRNADb: A Comprehensive Database for Human Circular RNAs with Protein-Coding Annotations. Sci. Rep. 2016, 6, 34985. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bejugam, P.R.; Das, A.; Panda, A.C. Seeing Is Believing: Visualizing Circular RNAs. Noncoding RNA 2020, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Pamudurti, N.R.; Patop, I.L.; Krishnamoorthy, A.; Ashwal-Fluss, R.; Bartok, O.; Kadener, S. An in Vivo Strategy for Knockdown of Circular RNAs. Cell Discov. 2020, 6, 52. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA Targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef]
- Piwecka, M.; Glažar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P.; et al. Loss of a Mammalian Circular RNA Locus Causes MiRNA Deregulation and Affects Brain Function. Science 2017, 357, eaam8526. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA Profiling Reveals an Abundant CircHIPK3 That Regulates Cell Growth by Sponging Multiple MiRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Xue, W.; Zhang, L.; Yang, L.-Z.; Cao, S.-M.; Lei, Y.-N.; Liu, C.-X.; Guo, S.-K.; Shan, L.; et al. Screening for Functional Circular RNAs Using the CRISPR-Cas13 System. Nat. Methods 2021, 18, 51–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Nguyen, T.M.; Zhang, X.-O.; Wang, L.; Phan, T.; Clohessy, J.G.; Pandolfi, P.P. Optimized RNA-Targeting CRISPR/Cas13d Technology Outperforms ShRNA in Identifying Functional CircRNAs. Genome Biol. 2021, 22, 41. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, Cellular Roles, and Applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef]
- Petkovic, S.; Müller, S. RNA Circularization Strategies in Vivo and in Vitro. Nucleic Acids Res. 2015, 43, 2454–2465. [Google Scholar] [CrossRef]
- Puttaraju, M.; Been, M.D. Group I Permuted Intron-Exon (PIE) Sequences Self-Splice to Produce Circular Exons. Nucleic Acids Res. 1992, 20, 5357–5364. [Google Scholar] [CrossRef]
- Zhou, C.; Molinie, B.; Daneshvar, K.; Pondick, J.V.; Wang, J.; Van Wittenberghe, N.; Xing, Y.; Giallourakis, C.C.; Mullen, A.C. Genome-Wide Maps of M6A CircRNAs Identify Widespread and Cell-Type-Specific Methylation Patterns That Are Distinct from MRNAs. Cell Rep. 2017, 20, 2262–2276. [Google Scholar] [CrossRef]
- Liang, D.; Wilusz, J.E. Short Intronic Repeat Sequences Facilitate Circular RNA Production. Genes Dev. 2014, 28, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Meganck, R.M.; Liu, J.; Hale, A.E.; Simon, K.E.; Fanous, M.M.; Vincent, H.A.; Wilusz, J.E.; Moorman, N.J.; Marzluff, W.F.; Asokan, A. Engineering Highly Efficient Backsplicing and Translation of Synthetic CircRNAs. Mol. Ther. Nucleic Acids 2021, 23, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B. Characterization of Circular RNA Concatemers. In Circular RNAs; Humana: New York, NY, USA, 2018; Volume 1724, pp. 143–157. [Google Scholar] [CrossRef]
- Stagsted, L.V.W.; O’Leary, E.T.; Ebbesen, K.K.; Hansen, T.B. The RNA-Binding Protein SFPQ Preserves Long-Intron Splicing and Regulates CircRNA Biogenesis in Mammals. eLife 2021, 10, e63088. [Google Scholar] [CrossRef] [PubMed]
- Ho-Xuan, H.; Glažar, P.; Latini, C.; Heizler, K.; Haase, J.; Hett, R.; Anders, M.; Weichmann, F.; Bruckmann, A.; Van den Berg, D.; et al. Comprehensive Analysis of Translation from Overexpressed Circular RNAs Reveals Pervasive Translation from Linear Transcripts. Nucleic Acids Res. 2020, 48, 10368–10382. [Google Scholar] [CrossRef]
- Chu, J.; Robert, F.; Pelletier, J. Trans-Spliced MRNA Products Produced from CircRNA Expression Vectors. RNA 2021, 27, 676–682. [Google Scholar] [CrossRef]
- Meganck, R.M.; Borchardt, E.K.; Castellanos Rivera, R.M.; Scalabrino, M.L.; Wilusz, J.E.; Marzluff, W.F.; Asokan, A. Tissue-Dependent Expression and Translation of Circular RNAs with Recombinant AAV Vectors In Vivo. Mol. Ther. Nucleic Acids 2018, 13, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Jarlstad Olesen, M.T.; Kristensen, L.S. Circular RNAs as MicroRNA Sponges: Evidence and Controversies. Essays Biochem. 2021, 65, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The MicroRNA.Org Resource: Targets and Expression. Nucleic Acids Res. 2007, 36, D149–D153. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The Biochemical Basis of MicroRNA Targeting Efficacy. Science 2019, 366, 1470. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: MicroRNA Target Prediction Easy, Fast and Flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded Identification and Characterization of Mammalian Circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A Circular Transcript of Ncx1 Gene Mediates Ischemic Myocardial Injury by Targeting MiR-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous MicroRNA Sponges: Evidence and Controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Chen, Y.G.; Kim, M.V.; Chen, X.; Batista, P.J.; Aoyama, S.; Wilusz, J.E.; Iwasaki, A.; Chang, H.Y. Sensing Self and Foreign Circular RNAs by Intron Identity. Mol. Cell 2017, 67, 228–238.e5. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 Circular RNA Retards Cell Cycle Progression via Forming Ternary Complexes with P21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic Discovery of Xist RNA Binding Proteins. Cell 2015, 161, 404–416. [Google Scholar] [CrossRef]
- Schneider, T.; Hung, L.-H.; Schreiner, S.; Starke, S.; Eckhof, H.; Rossbach, O.; Reich, S.; Medenbach, J.; Bindereif, A. CircRNA-Protein Complexes: IMP3 Protein Component Defines Subfamily of CircRNPs. Sci. Rep. 2016, 6, 31313. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, N.; Yang, X.; Luo, J.; Yan, S.; Xiao, F.; Chen, W.; Gao, X.; Zhao, K.; Zhou, H.; et al. A Novel Protein Encoded by the Circular Form of the SHPRH Gene Suppresses Glioma Tumorigenesis. Oncogene 2018, 37, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, X.; Zhang, M.; Yan, S.; Sun, C.; Xiao, F.; Huang, N.; Yang, X.; Zhao, K.; Zhou, H.; et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. JNCI J. Natl. Cancer Inst. 2018, 110, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, K.; Xu, X.; Yang, Y.; Yan, S.; Wei, P.; Liu, H.; Xu, J.; Xiao, F.; Zhou, H.; et al. A Peptide Encoded by Circular Form of LINC-PINT Suppresses Oncogenic Transcriptional Elongation in Glioblastoma. Nat. Commun. 2018, 9, 4475. [Google Scholar] [CrossRef]
- Stagsted, L.V.; Nielsen, K.M.; Daugaard, I.; Hansen, T.B. Noncoding AUG CircRNAs Constitute an Abundant and Conserved Subclass of Circles. Life Sci. Alliance 2019, 2, e201900398. [Google Scholar] [CrossRef]
- Chen, C.-K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured Elements Drive Extensive Circular RNA Translation. Mol. Cell 2021, 81, 4300–4318.e13. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Gong, Z.; Yang, X.; Yang, M.; Zhang, S.; Xiong, F.; Xiang, B.; Zhou, M.; Liao, Q.; et al. Circular RNAs in Human Cancer. Mol. Cancer 2017, 16, 25. [Google Scholar] [CrossRef]
- Akhter, R. Circular RNA and Alzheimer’s Disease. In Circular RNAs; Springer: Singapore, 2018; Volume 1087, pp. 239–243. [Google Scholar] [CrossRef]
- Nozima, T.; Batyrkhankyzy, N.N.; Kadham, M.J.; Abdufattoevna, K.A.; Khatamov, A.; Khaydarova, P.S.; Bakhodir, I.; Alisherovna, R.N.; Kuvatovna, K.D.; Mukhlisa, K.; et al. Circular RNA Biomarkers in Cardiovascular Disease. Clin. Chim. Acta 2025, 576, 120424. [Google Scholar] [CrossRef]
- Bibi, A.; Bartekova, M.; Gandhi, S.; Greco, S.; Madè, A.; Sarkar, M.; Stopa, V.; Tastsoglou, S.; de Gonzalo-Calvo, D.; Devaux, Y.; et al. Circular RNA Regulatory Role in Pathological Cardiac Remodelling. Br. J. Pharmacol. 2025, 182, 316–339. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, C.; Cheng, Z.; Zhou, H.; Qin, W.; Feng, J.; Wan, M.; Zhang, Y.; Jia, C.; Shao, S.; et al. Research Trends and Hotspots of Circular RNA in Cardiovascular Disease: A Bibliometric Analysis. Noncoding RNA Res. 2024, 9, 930–944. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, S.; Peng, L.; Liu, Y.; Cheng, D.; Wang, Y.; Ni, C. CircZNF609 Regulates Pulmonary Fibrosis via MiR-145-5p/KLF4 Axis and Its Translation Function. Cell. Mol. Biol. Lett. 2023, 28, 105. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Pan, C.L.; Jiang, G.X.; Zhang, Y.M.; Zhang, Z. CircHIPK3 Aggravates Myocardial Ischemia-Reperfusion Injury by Binding to MiRNA-124-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10107–10114. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ma, R.; Cao, J.; Du, X.; Cai, X.; Fan, Y. CircSAMD4A Aggravates H/R-induced Cardiomyocyte Apoptosis and Inflammatory Response by Sponging MiR-138-5p. J. Cell. Mol. Med. 2022, 26, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Long, T.-Y.; Bi, S.-S.; Sheikh, S.-A.; Li, F. CircPAN3 Ameliorates Myocardial Ischaemia/Reperfusion Injury by Targeting MiR-421/Pink1 Axis-Mediated Autophagy Suppression. Lab. Investig. 2021, 101, 89–103. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Hu, H.; Wu, J.; Chen, H.; Feng, K.; Ma, L. Circ-HIPK2 Accelerates Cell Apoptosis and Autophagy in Myocardial Oxidative Injury by Sponging MiR-485-5p and Targeting ATG101. J. Cardiovasc. Pharmacol. 2020, 76, 427–436. [Google Scholar] [CrossRef]
- Yan, H.; Du, D.; Wang, C.; Tian, M. Downregulation of Autophagy-Related Circular RNA (ACR) Is Correlated with Poor Survival of Patients with Chronic Heart Failure. Bioengineered 2022, 13, 13141–13149. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Wang, X. Silencing of CircHIPK3 Inhibits Pressure Overload-Induced Cardiac Hypertrophy and Dysfunction by Sponging MiR-185-3p. Drug Des. Devel. Ther. 2020, 14, 5699–5710. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Wang, J.; Xu, G.; Wang, T.; Feng, J.; Bei, Y.; Xu, J.; Wang, H.; Das, S.; et al. Downregulation of Circ-ZNF609 Promotes Heart Repair by Modulating RNA N6-Methyladenosine-Modified Yap Expression. Research 2022, 2022, 9825916. [Google Scholar] [CrossRef]
- Shen, M.; Wu, Y.; Li, L.; Zhang, L.; Liu, G.; Wang, R. CircMAP3K5 Promotes Cardiomyocyte Apoptosis in Diabetic Cardiomyopathy by Regulating MiR -22-3p/DAPK2 Axis. J. Diabetes 2024, 16, e13471. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Deng, W.; Jiang, M. CircRNA MFACR Is Upregulated in Myocardial Infarction and Downregulates MiR-125b to Promote Cardiomyocyte Apoptosis Induced by Hypoxia. J. Cardiovasc. Pharmacol. 2021, 78, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.-H.; Zhang, R.-C.; Liu, C.-Y.; Dong, Y.-H.; Wang, M.; et al. The Circular RNA ACR Attenuates Myocardial Ischemia/Reperfusion Injury by Suppressing Autophagy via Modulation of the Pink1/FAM65B Pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef] [PubMed]
- Mester-Tonczar, J.; Winkler, J.; Einzinger, P.; Hasimbegovic, E.; Kastner, N.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Traxler, D.; Batkai, S.; et al. Association between Circular RNA CDR1as and Post-Infarction Cardiac Function in Pig Ischemic Heart Failure: Influence of the Anti-Fibrotic Natural Compounds Bufalin and Lycorine. Biomolecules 2020, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, M.; Yu, Q.; Gong, M.; Wang, Y.; Yang, X.; Liu, L.; Liu, D.; Tan, Z.; Zhang, Y.; et al. CircRNA CDR1as Promotes Cardiomyocyte Apoptosis through Activating Hippo Signaling Pathway in Diabetic Cardiomyopathy. Eur. J. Pharmacol. 2022, 922, 174915. [Google Scholar] [CrossRef]
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of MiR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef]
- Wang, K.; Long, B.; Liu, F.; Wang, J.-X.; Liu, C.-Y.; Zhao, B.; Zhou, L.-Y.; Sun, T.; Wang, M.; Yu, T.; et al. A Circular RNA Protects the Heart from Pathological Hypertrophy and Heart Failure by Targeting MiR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Z.; Guo, G.; Xu, C.; Song, Z.; Li, K.; Zhong, K.; Wang, D. Circ_nuclear Factor I X (CircNfix) Attenuates Pressure Overload-Induced Cardiac Hypertrophy via Regulating MiR-145-5p/ATF3 Axis. Bioengineered 2021, 12, 5373–5385. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated CircRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.A.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R.S.-Y. Targeting the Highly Abundant Circular RNA CircSlc8a1 in Cardiomyocytes Attenuates Pressure Overload Induced Hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Shen, J.F.; Wei, X.F.; Qi, G.X. Circular RNA Foxo3 Relieves Myocardial Ischemia/Reperfusion Injury by Suppressing Autophagy via Inhibiting HMGB1 by Repressing KAT7 in Myocardial Infarction. J. Inflamm. Res. 2021, 14, 6397–6407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, S.; Wei, G.; Sun, Y.; Li, C.; Si, X.; Chen, Y.; Tang, Z.; Li, X.; Chen, Y.; et al. CircRNA Samd4 Induces Cardiac Repair after Myocardial Infarction by Blocking Mitochondria-Derived ROS Output. Mol. Ther. 2022, 30, 3477–3498. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, T.-Y.; Li, N.; Liu, C.-Y.; Zhou, L.-Y.; Gao, J.-N.; Chen, C.; Yan, K.-W.; Ponnusamy, M.; Zhang, Y.-H.; et al. Circular RNA Mediates Cardiomyocyte Death via MiRNA-Dependent Upregulation of MTP18 Expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef]
- Yu, P.; Wang, J.; Xu, G.-E.; Zhao, X.; Cui, X.; Feng, J.; Sun, J.; Wang, T.; Spanos, M.; Lehmann, H.I.; et al. RNA M6A-Regulated Circ-ZNF609 Suppression Ameliorates Doxorubicin-Induced Cardiotoxicity by Upregulating FTO. JACC Basic. Transl. Sci. 2023, 8, 677–698. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA CircCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via MiR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Li, J.; Zhang, Y.; Wang, W.; Xu, P.; Li, Y. Endothelial Cell-Derived Exosomal CircHIPK3 Promotes the Proliferation of Vascular Smooth Muscle Cells Induced by High Glucose via the MiR-106a-5p/Foxo1/Vcam1 Pathway. Aging 2021, 13, 25241–25255. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Shen, C.; Liu, W.; Yuan, J.; Li, C.; Deng, W.; Wang, Z.; Zhang, W.; Ge, J.; et al. Exosomal CircHIPK3 Released from Hypoxia-Induced Cardiomyocytes Regulates Cardiac Angiogenesis after Myocardial Infarction. Oxid. Med. Cell Longev. 2020, 2020, 8418407. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.; Yang, T.; Meng, X.; Jiang, Z.; Tao, L.; Wang, L. Upregulation of Circular RNA CircNFIB Attenuates Cardiac Fibrosis by Sponging MiR-433. Front. Genet. 2019, 10, 564. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, J.; Xie, G.; Zeng, X.; Li, H. Circ-HIPK3 Strengthens the Effects of Adrenaline in Heart Failure by MiR-17-3p–ADCY6 Axis. Int. J. Biol. Sci. 2019, 15, 2484–2496. [Google Scholar] [CrossRef]
- Ni, H.; Li, W.; Zhuge, Y.; Xu, S.; Wang, Y.; Chen, Y.; Shen, G.; Wang, F. Inhibition of CircHIPK3 Prevents Angiotensin II-Induced Cardiac Fibrosis by Sponging MiR-29b-3p. Int. J. Cardiol. 2019, 292, 188–196. [Google Scholar] [CrossRef]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. CircRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and MiR-133a. Mol. Ther. Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Qiu, Z.; Zhao, R.; Liu, Z.; Chen, W.; Ge, J.; Shi, B. CircHIPK3 Regulates Cardiac Fibroblast Proliferation, Migration and Phenotypic Switching through the MiR-152-3p/TGF-Β2 Axis under Hypoxia. PeerJ 2020, 8, e9796. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-B.; Li, T.; Hu, X.-M.; Ning, M.; Gao, W.-Q.; Lang, Y.-H.; Zheng, W.-F.; Wei, J. Circ_CHFR Expedites Cell Growth, Migration and Inflammation in Ox-LDL-Treated Human Vascular Smooth Muscle Cells via the MiR-214-3p/Wnt3/β-Catenin Pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3282–3292. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.-D.; Fang, X.-H.; Zhu, J.-N.; Yang, J.; Pan, R.; Yuan, S.-J.; Zeng, N.; Yang, Z.-Z.; Yang, H.; et al. Circular RNA CircRNA_000203 Aggravates Cardiac Hypertrophy via Suppressing MiR-26b-5p and MiR-140-3p Binding to Gata4. Cardiovasc. Res. 2020, 116, 1323–1334. [Google Scholar] [CrossRef]
- VandenBroek, M.M.; Sharp, M.C.; Thompson, P.; Fagbola, E.; Quilty, D.; Mewburn, J.D.; Theilmann, A.L.; Dunham-Snary, K.J.; Hemnes, A.R.; Cogan, J.D.; et al. Circular RNA Profiling Identifies Circ5078 as a BMPR2-Derived Regulator of Endothelial Proliferation and Stress Responses. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 1546–1561. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal CircHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the MiR-29a/IGF-1 Pathway. Oxid. Med. Cell Longev. 2019, 2019, 7954657. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b Modulates Cardiac Repair after Myocardial Infarction via FUS/VEGF-A Axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef]
- Gao, X.-Q.; Liu, C.-Y.; Zhang, Y.-H.; Wang, Y.-H.; Zhou, L.-Y.; Li, X.-M.; Wang, K.; Chen, X.-Z.; Wang, T.; Ju, J.; et al. The CircRNA CNEACR Regulates Necroptosis of Cardiomyocytes through Foxa2 Suppression. Cell Death Differ. 2022, 29, 527–539. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Xuan, S.; Jin, T.; Chen, K.; Wu, Z.; Su, W.; Chen, L.; Zong, G. CircSamd4: A Novel Biomarker for Predicting Vascular Calcification. J. Clin. Lab. Anal. 2022, 36, e24156. [Google Scholar] [CrossRef]
- Ma, C.; Gu, R.; Wang, X.; He, S.; Bai, J.; Zhang, L.; Zhang, J.; Li, Q.; Qu, L.; Xin, W.; et al. CircRNA CDR1as Promotes Pulmonary Artery Smooth Muscle Cell Calcification by Upregulating CAMK2D and CNN3 via Sponging MiR-7-5p. Mol. Ther. Nucleic Acids 2020, 22, 530–541. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, J.; Chen, Z.; Wang, W.; Cao, Z.; Chen, X.; Chen, J. CircRNA ARHGAP10 Promotes Osteogenic Differentiation through the MiR-335-3p/RUNX2 Pathway in Aortic Valve Calcification. J. Thorac. Dis. 2023, 15, 5971–5991. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, M.; Liu, D.; Min, X.; Shao, T.; Xu, Z.; Jing, X.; Cai, M.; Xu, S.; Liang, X.; et al. CircGNAQ, a Circular RNA Enriched in Vascular Endothelium, Inhibits Endothelial Cell Senescence and Atherosclerosis Progression. Mol. Ther. Nucleic Acids 2021, 26, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Cai, M.; Shao, T.; Xu, Z.; Liao, Z.; Liu, D.; Zhou, M.; Wu, W.; Zhou, Y.; Mo, M.; et al. A Circular Intronic RNA CiPVT1 Delays Endothelial Cell Senescence by Regulating the MiR-24-3p/CDK4/PRb Axis. Aging Cell 2022, 21, e13529. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 Circular RNA Promotes Cardiac Senescence by Modulating Multiple Factors Associated with Stress and Senescence Responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef]
- Chen, C.; Shen, H.; Huang, Q.; Li, Q. The Circular RNA CDR1as Regulates the Proliferation and Apoptosis of Human Cardiomyocytes Through the MiR-135a/HMOX1 and MiR-135b/HMOX1 Axes. Genet. Test. Mol. Biomark. 2020, 24, 537–548. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in Ischemic Heart Disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef]
- Mishra, P.K.; Adameova, A.; Hill, J.A.; Baines, C.P.; Kang, P.M.; Downey, J.M.; Narula, J.; Takahashi, M.; Abbate, A.; Piristine, H.C.; et al. Guidelines for Evaluating Myocardial Cell Death. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H891–H922. [Google Scholar] [CrossRef]
- Michiels, C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Gabriel-Costa, D. The Pathophysiology of Myocardial Infarction-Induced Heart Failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef]
- Franceschelli, S.; De Cecco, F.; Pesce, M.; Ripari, P.; Guagnano, M.T.; Nuevo, A.B.; Grilli, A.; Sancilio, S.; Speranza, L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. Int. J. Mol. Sci. 2023, 24, 2057. [Google Scholar] [CrossRef] [PubMed]
- Lamprea-Montealegre, J.A.; Otto, C.M. Health Behaviors and Calcific Aortic Valve Disease. J. Am. Heart Assoc. 2018, 7, e008385. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwon, D.-H.; Choe, N.; Shin, S.; Jeong, G.; Lim, Y.-H.; Kim, J.; Park, W.J.; Kook, H.; Kim, Y.-K. Characterization of Circular RNAs in Vascular Smooth Muscle Cells with Vascular Calcification. Mol. Ther. Nucleic Acids 2020, 19, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of Disease: Pulmonary Arterial Hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Palevsky, H.I.; Schloo, B.L.; Pietra, G.G.; Weber, K.T.; Janicki, J.S.; Rubin, E.; Fishman, A.P. Primary Pulmonary Hypertension. Vascular Structure, Morphometry, and Responsiveness to Vasodilator Agents. Circulation 1989, 80, 1207–1221. [Google Scholar] [CrossRef]
- Konduracka, E.; Cieslik, G.; Galicka-Latala, D.; Rostoff, P.; Pietrucha, A.; Latacz, P.; Gajos, G.; Malecki, M.T.; Nessler, J. Myocardial Dysfunction and Chronic Heart Failure in Patients with Long-Lasting Type 1 Diabetes: A 7-Year Prospective Cohort Study. Acta Diabetol. 2013, 50, 597–606. [Google Scholar] [CrossRef]
- Poornima, I.G.; Parikh, P.; Shannon, R.P. Diabetic Cardiomyopathy: The Search for a Unifying Hypothesis. Circ. Res. 2006, 98, 596–605. [Google Scholar] [CrossRef]
- Ward, Z.; Pearson, J.; Schmeier, S.; Cameron, V.; Pilbrow, A. Insights into Circular RNAs: Their Biogenesis, Detection, and Emerging Role in Cardiovascular Disease. RNA Biol. 2021, 18, 2055–2072. [Google Scholar] [CrossRef]
- Ding, C.; Zhou, Y. Insights into Circular RNAs: Biogenesis, Function and Their Regulatory Roles in Cardiovascular Disease. J. Cell. Mol. Med. 2023, 27, 1299–1314. [Google Scholar] [CrossRef]
- Wen, Z.J.; Xin, H.; Wang, Y.C.; Liu, H.W.; Gao, Y.Y.; Zhang, Y.F. Emerging Roles of CircRNAs in the Pathological Process of Myocardial Infarction. Mol. Ther. Nucleic Acids 2021, 26, 828–848. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A Genotype Is Strongly Associated with Human Longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Willcox, B.J.; Tranah, G.J.; Chen, R.; Morris, B.J.; Masaki, K.H.; He, Q.; Willcox, D.C.; Allsopp, R.C.; Moisyadi, S.; Poon, L.W.; et al. The FoxO3 Gene and Cause-specific Mortality. Aging Cell 2016, 15, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, H.-Y.; Zhang, X.-B.; Gao, X.-L.; Peng, W.-P.; Zhou, Y.; Zhao, W.-H.; Yang, H.-F. LncRNA ANRIL Regulates Cell Proliferation and Migration via Sponging MiR-339-5p and Regulating FRS2 Expression in Atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Pardi, N. MRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering Circular RNA for Potent and Stable Translation in Eukaryotic Cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- O’Leary, E.; Jiang, Y.; Kristensen, L.S.; Hansen, T.B.; Kjems, J. The Therapeutic Potential of Circular RNAs. Nat. Rev. Genet. 2025, 26, 230–244. [Google Scholar] [CrossRef]
- Lasda, E.; Parker, R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for CircRNA Clearance. PLoS ONE 2016, 11, e0148407. [Google Scholar] [CrossRef]
- Preußer, C.; Hung, L.-H.; Schneider, T.; Schreiner, S.; Hardt, M.; Moebus, A.; Santoso, S.; Bindereif, A. Selective Release of CircRNAs in Platelet-Derived Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1424473. [Google Scholar] [CrossRef]
- Tai, J.; Chen, Y.G. Differences in the Immunogenicity of Engineered Circular RNAs. J. Mol. Cell Biol. 2023, 15, mjad002. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520.e4. [Google Scholar] [CrossRef]
- Chen, Y.G.; Chen, R.; Ahmad, S.; Verma, R.; Kasturi, S.P.; Amaya, L.; Broughton, J.P.; Kim, J.; Cadena, C.; Pulendran, B.; et al. N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol. Cell 2019, 76, 96–109.e9. [Google Scholar] [CrossRef]
- Liu, C.-X.; Guo, S.-K.; Nan, F.; Xu, Y.-F.; Yang, L.; Chen, L.-L. RNA Circles with Minimized Immunogenicity as Potent PKR Inhibitors. Mol. Cell 2022, 82, 420–434.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huo, S.T.; Wu, Z.; Chen, L.; Wen, C.; Chen, H.; Du, W.W.; Wu, N.; Guan, D.; Lian, S.; et al. Rapid Development of Targeting CircRNAs in Cardiovascular Diseases. Mol. Ther. Nucleic Acids 2020, 21, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA Is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- E, S.; Costa, M.C.; Kurc, S.; Drożdż, A.; Cortez-Dias, N.; Enguita, F.J. The Circulating Non-Coding RNA Landscape for Biomarker Research: Lessons and Prospects from Cardiovascular Diseases. Acta Pharmacol. Sin. 2018, 39, 1085–1099. [Google Scholar] [CrossRef]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef]
- Salgado-Somoza, A.; Zhang, L.; Vausort, M.; Devaux, Y. The Circular RNA MICRA for Risk Stratification after Myocardial Infarction. IJC Heart Vasc. 2017, 17, 33–36. [Google Scholar] [CrossRef]
- Burd, C.E.; Jeck, W.R.; Liu, Y.; Sanoff, H.K.; Wang, Z.; Sharpless, N.E. Expression of Linear and Novel Circular Forms of an INK4/ARF-Associated Non-Coding RNA Correlates with Atherosclerosis Risk. PLoS Genet. 2010, 6, e1001233. [Google Scholar] [CrossRef]
- Fasolo, F.; Winski, G.; Li, Z.; Wu, Z.; Winter, H.; Ritzer, J.; Glukha, N.; Roy, J.; Hultgren, R.; Pauli, J.; et al. The Circular RNA Ataxia Telangiectasia Mutated Regulates Oxidative Stress in Smooth Muscle Cells in Expanding Abdominal Aortic Aneurysms. Mol. Ther. Nucleic Acids 2023, 33, 848–865. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Wang, Y.; Zou, T.; Zhu, H.; Lu, X.; Li, L.; Yang, B.; Chen, J.; Chen, S.; et al. Identification of Circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as Novel Biomarkers for Coronary Artery Disease. Atherosclerosis 2019, 286, 88–96. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Hu, L.; Yu, H.; Xu, F.; Yang, B.; Zhang, R.; Zhang, Y.; An, Y. Circular RNA-Expression Profiling Reveals a Potential Role of Hsa_circ_0097435 in Heart Failure via Sponging Multiple MicroRNAs. Front. Genet. 2020, 11, 212. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, X.; Lv, Y.; Liang, X.; Zhao, B.; Bian, W.; Zhang, D.; Jiang, J.; Zhang, C. Circular RNA Expression Profiles in Plasma from Patients with Heart Failure Related to Platelet Activity. Biomolecules 2020, 10, 187. [Google Scholar] [CrossRef]
- Schulte, C.; Barwari, T.; Joshi, A.; Zeller, T.; Mayr, M. Noncoding RNAs versus Protein Biomarkers in Cardiovascular Disease. Trends Mol. Med. 2020, 26, 583–596. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Vea, A.; Bär, C.; Fiedler, J.; Couch, L.S.; Brotons, C.; Llorente-Cortes, V.; Thum, T. Circulating Non-Coding RNAs in Biomarker-Guided Cardiovascular Therapy: A Novel Tool for Personalized Medicine? Eur. Heart J. 2019, 40, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-K.; Kafert-Kasting, S.; Thum, T. Preclinical and Clinical Development of Noncoding RNA Therapeutics for Cardiovascular Disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef]
- Hollensen, A.K.; Andersen, S.; Hjorth, K.; Bak, R.O.; Hansen, T.B.; Kjems, J.; Aagaard, L.; Damgaard, C.K.; Mikkelsen, J.G. Enhanced Tailored MicroRNA Sponge Activity of RNA Pol II-Transcribed TuD Hairpins Relative to Ectopically Expressed CiRS7-Derived CircRNAs. Mol. Ther. Nucleic Acids 2018, 13, 365–375. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The MiRNA-212/132 Family Regulates Both Cardiac Hypertrophy and Cardiomyocyte Autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Lavenniah, A.; Luu, T.D.A.; Li, Y.P.; Lim, T.B.; Jiang, J.; Ackers-Johnson, M.; Foo, R.S.-Y. Engineered Circular RNA Sponges Act as MiRNA Inhibitors to Attenuate Pressure Overload-Induced Cardiac Hypertrophy. Mol. Ther. 2020, 28, 1506–1517. [Google Scholar] [CrossRef]
- Ren, S.; Huang, M.; Bai, R.; Chen, L.; Yang, J.; Zhang, J.; Guo, W.; Ji, W.; Chen, Y. Efficient Modulation of Exon Skipping via Antisense Circular RNAs. Research 2023, 6, 0045. [Google Scholar] [CrossRef]
- Pfafenrot, C.; Schneider, T.; Müller, C.; Hung, L.-H.; Schreiner, S.; Ziebuhr, J.; Bindereif, A. Inhibition of SARS-CoV-2 Coronavirus Proliferation by Designer Antisense-CircRNAs. Nucleic Acids Res. 2021, 49, 12502–12516. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, J.; Zhu, J.; Du, Y.; Tan, Y.; Wei, L.; Zhao, Y.; Hou, Q.; Zhang, Y.; Sun, Z.; et al. Circular MRNA Encoded PROTAC (RiboPROTAC) as a New Platform for the Degradation of Intracellular Therapeutic Targets. bioRxiv 2022, 489232. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Lv, J.; Huang, H.; Wang, J.; Zhuo, M.; Tan, Z.; Huang, G.; Liu, J.; Liu, Y.; et al. Engineered Circular Guide RNAs Boost CRISPR/Cas12a- and CRISPR/Cas13d-Based DNA and RNA Editing. Genome Biol. 2023, 24, 145. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; He, Z.; Zhao, K.T.; Zhu, H.; Hu, J.; Liu, G.; Gao, Q.; Liu, M.; Zhang, R.; Qiu, J.-L.; et al. Prime Editing Using CRISPR-Cas12a and Circular RNAs in Human Cells. Nat. Biotechnol. 2024, 42, 1867–1875. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Li, J.; Zhao, D.; Li, S.; Jiang, G.; Wang, J.; Chen, X.; Bi, C.; Zhang, X. Circular Guide RNA for Improved Stability and CRISPR-Cas9 Editing Efficiency in Vitro and in Bacteria. ACS Synth. Biol. 2023, 12, 350–359. [Google Scholar] [CrossRef]
- Katrekar, D.; Yen, J.; Xiang, Y.; Saha, A.; Meluzzi, D.; Savva, Y.; Mali, P. Efficient in Vitro and in Vivo RNA Editing via Recruitment of Endogenous ADARs Using Circular Guide RNAs. Nat. Biotechnol. 2022, 40, 938–945. [Google Scholar] [CrossRef]
- Xie, J.; Ye, F.; Deng, X.; Tang, Y.; Liang, J.-Y.; Huang, X.; Sun, Y.; Tang, H.; Lei, J.; Zheng, S.; et al. Circular RNA: A Promising New Star of Vaccine. J. Transl. Intern. Med. 2023, 11, 372–381. [Google Scholar] [CrossRef]
- Niu, D.; Wu, Y.; Lian, J. Circular RNA Vaccine in Disease Prevention and Treatment. Signal Transduct. Target. Ther. 2023, 8, 341. [Google Scholar] [CrossRef]
- Li, H.; Peng, K.; Yang, K.; Ma, W.; Qi, S.; Yu, X.; He, J.; Lin, X.; Yu, G. Circular RNA Cancer Vaccines Drive Immunity in Hard-to-Treat Malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef]
- Mullard, A. In Vivo CAR T Cells Move into Clinical Trials. Nat. Rev. Drug Discov. 2024, 23, 727–730. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.C.J. Immunogenicity and Toxicity of AAV Gene Therapy. Front. Immunol. 2022, 13, 975803. [Google Scholar] [CrossRef] [PubMed]
- Golubovic, A.; Tsai, S.; Li, B. Bioinspired Lipid Nanocarriers for RNA Delivery. ACS Bio Med Chem Au 2023, 3, 114–136. [Google Scholar] [CrossRef] [PubMed]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic Potential of RNA-Enriched Extracellular Vesicles: The next Generation in RNA Delivery via Biogenic Nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, W.; Li, M.; Zheng, A. Exosome-Based Carrier for RNA Delivery: Progress and Challenges. Pharmaceutics 2023, 15, 598. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Tan, S.; Yang, J.; Hu, S.; Lei, W. Cell-Cell Interactions in the Heart: Advanced Cardiac Models and Omics Technologies. Stem Cell Res. Ther. 2024, 15, 362. [Google Scholar] [CrossRef]
- Jorba, I.; Mostert, D.; Hermans, L.H.L.; van der Pol, A.; Kurniawan, N.A.; Bouten, C.V.C. In Vitro Methods to Model Cardiac Mechanobiology in Health and Disease. Tissue Eng. Part. C Methods 2021, 27, 139–151. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, X.; Tang, X.; Zhao, Q.; Wang, L.; Yao, F.; Hou, Y.; Wang, X.; Feng, W.; Wang, L.; et al. Functional Isolation, Culture and Cryopreservation of Adult Human Primary Cardiomyocytes. Signal Transduct. Target. Ther. 2022, 7, 254. [Google Scholar] [CrossRef]
- Tang, L.; Nyarige, V.; Li, P.; Wang, J.; Zhu, W. Identification of Circular RNAs Regulating Cardiomyocyte Proliferation in Neonatal Pig Hearts. JCI Insight 2024, 9, e175625. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Dai, F.; Su, E.; Li, F.; Yan, Y. Analysis of Changes in Circular RNA Expression and Construction of CeRNA Networks in Human Dilated Cardiomyopathy. J. Cell. Mol. Med. 2021, 25, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Magadum, A.; Engel, F.B. Cardiomyocyte Proliferation in Cardiac Development and Regeneration: A Guide to Methodologies and Interpretations. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1237–H1250. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Sitkiewicz, D. Involvement of CircRNAs in the Development of Heart Failure. Int. J. Mol. Sci. 2022, 23, 14129. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, A.K.; Ellison-Hughes, G.M. A Protocol for Extracting Immunolabeled Murine Cardiomyocytes of High-Quality RNA by Laser Capture Microdissection. STAR Protoc. 2022, 3, 101231. [Google Scholar] [CrossRef]
- Palmer, J.A.; Rosenthal, N.; Teichmann, S.A.; Litvinukova, M. Revisiting Cardiac Biology in the Era of Single Cell and Spatial Omics. Circ. Res. 2024, 134, 1681–1702. [Google Scholar] [CrossRef]
- Galow, A.-M.; Kussauer, S.; Wolfien, M.; Brunner, R.M.; Goldammer, T.; David, R.; Hoeflich, A. Quality Control in ScRNA-Seq Can Discriminate Pacemaker Cells: The MtRNA Bias. Cell. Mol. Life Sci. 2021, 78, 6585–6592. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/Gender Differences in Cardiovascular Disease Prevention: What a Difference a Decade Makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef]
- Robinson, E.L.; Port, J.D. Utilization and Potential of RNA-Based Therapies in Cardiovascular Disease. JACC Basic. Transl. Sci. 2022, 7, 956–969. [Google Scholar] [CrossRef]
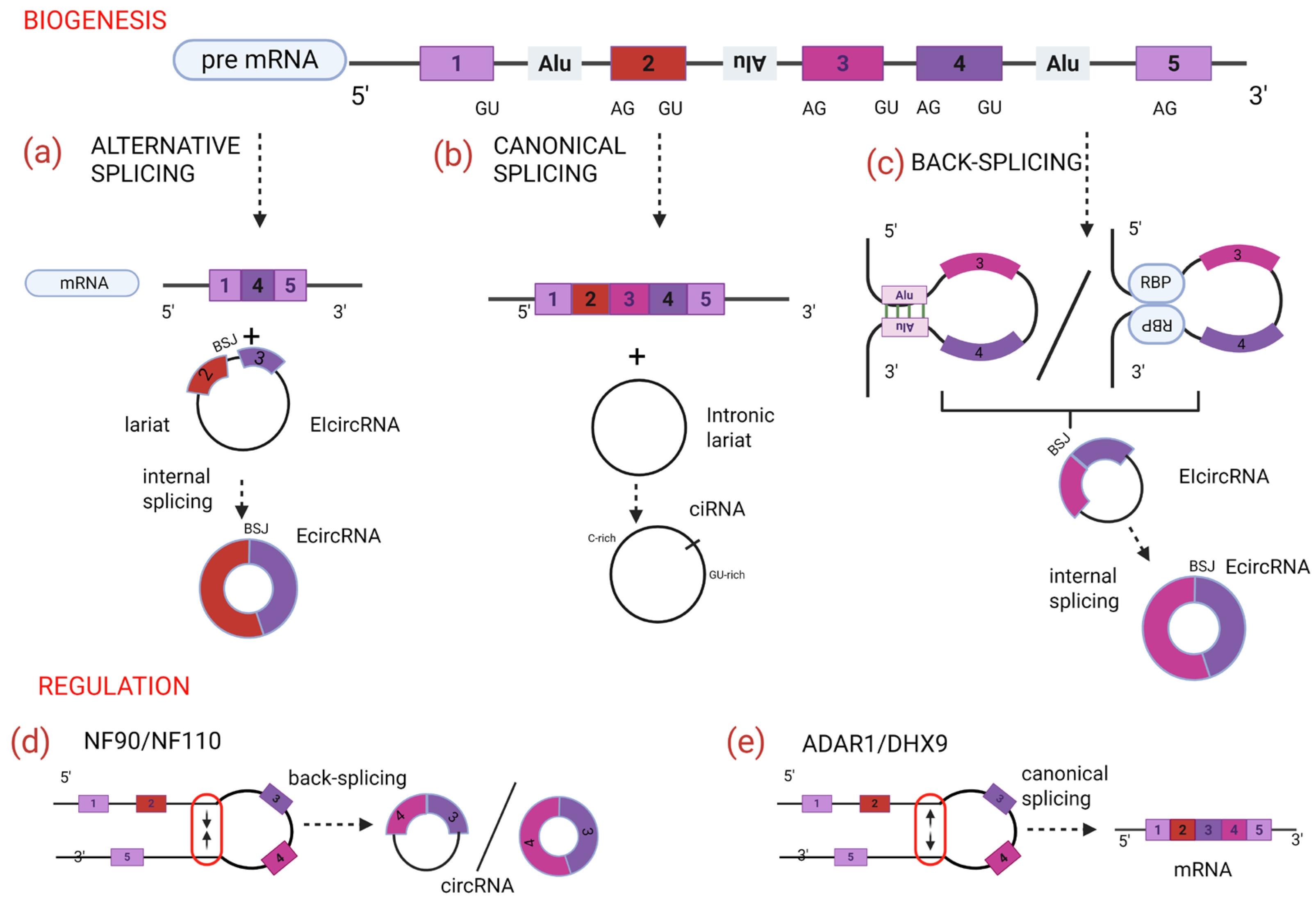
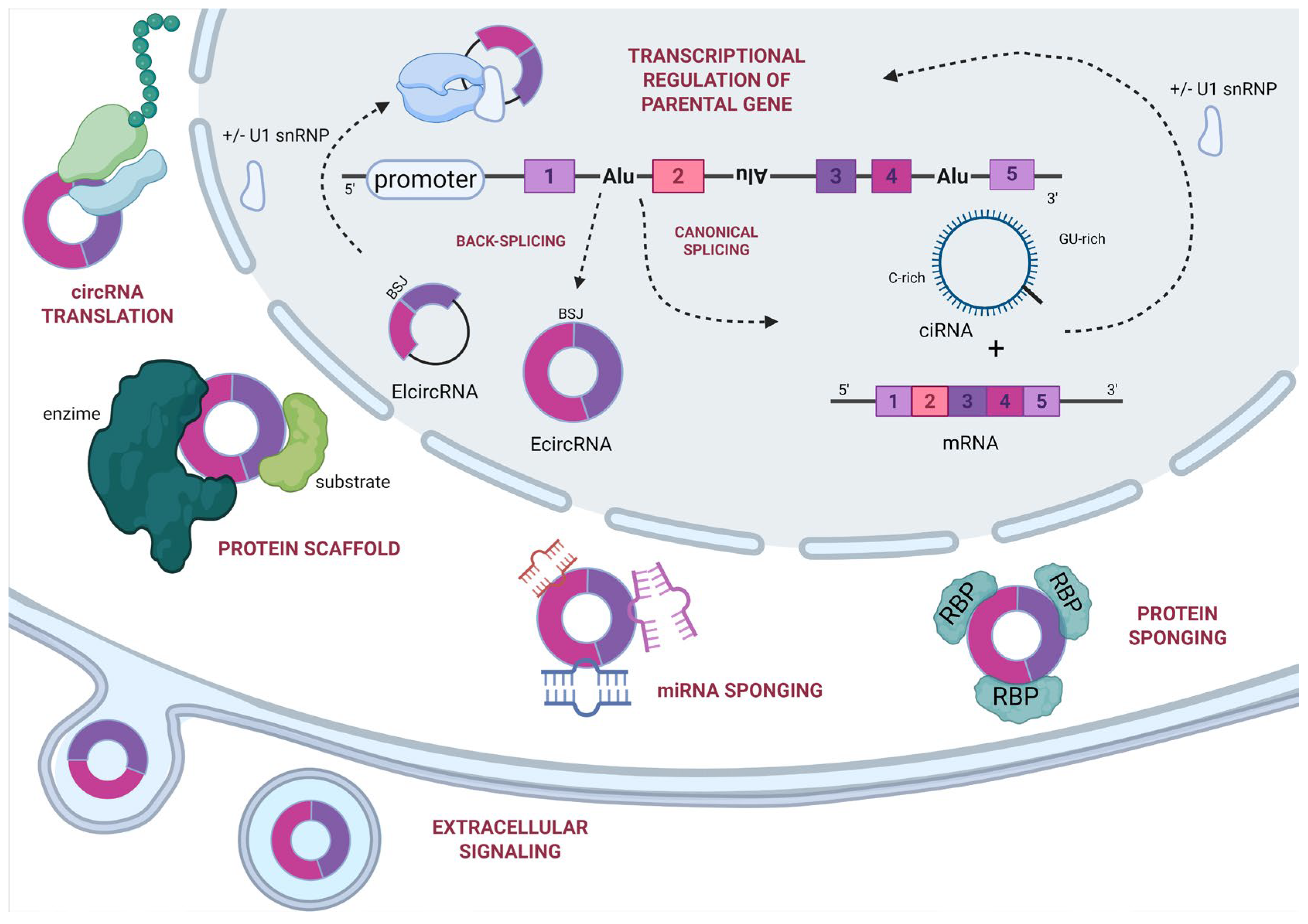
| Tool | Application | Description | Refs |
|---|---|---|---|
| Detection | |||
| find_circ | Split-alignment-based detection | Search for circRNAs from unmapped reads after initial genome alignment with Bowtie2 | [67] |
| circRNA_finder | Split-alignment-based detection; Chimeric reads alignment | Uses STAR chimeric reads to detect circRNAs | [106] |
| CIRI | Split-alignment-based detection | Original CIRI method using paired chiastic clipping for BSJ detection | [32] |
| CIRI2 | Split-alignment-based detection | Improved CIRI with better speed and sensitivity | [107] |
| DCC | Split-alignment-based detection; Chimeric reads alignment | Apply stringent statistical filters on BSJ candidates identified from STAR alignment | [108] |
| CircExplorer | Split-alignment-based detection | First circRNA detection tool using fusion-read mapping by TopHat-Fusion | [9] |
| CircExplorer2 | Split-alignment-based detection; Full-length reconstruction | Improved CircExplorer in terms of accuracy, speed, and flexibility supporting multiple aligners and providing circRNA annotation | [94] |
| KNIFE | Pseudo-reference-based detection | Uses pre-defined BSJ references for detection | [109] |
| PTESFinder | Mixed approach detection | Detects post-transcriptional exon shuffling events, including circRNAs, after Bowtie alignment to a reference transcriptome and scanning for non-colinear exon junctions. Uses multiple alignment and filtering steps to reduce false positives, requiring annotation to validate splice sites | [111] |
| MapSplice | Splicing-aware mapper able to detect circRNAs | Detects canonical and non-canonical splicing events | [105] |
| Acfs | Pseudo-reference-based detection | Strength of potential splicing sites are evaluated including canonical “GU-AG” and non-canonical splicing motifs | [110] |
| UROBORUS | Split-alignment-based detection | Detects circRNAs by extracting unmapped reads from initial genome alignment by TopHat, splitting them into anchors, and remapping to detect BSJs by Bowtie | [112] |
| segemehl | Split-alignment-based detection | A short read mapper with capabilities to detect regular splicing, trans-splicing, and gene fusion events from single-end RNA-seq data | [104] |
| CirComPara2 | Multi-tool detection | High sensitivity and accuracy in circRNA detection, giving the possibility to use up to nine different computational pipelines | [113] |
| circRNAwrap | Multi-tool detection; Quantification; Reconstruction | Detection and full-length characterization in a single platform | [114] |
| nf-core/circrna | Multi-tool detection | Integration of seven circRNA detection tools, also includes modules for differential expression analyses and circRNA-miRNA interaction prediction | [115] |
| Full-length Reconstruction | |||
| CIRI-full | Full-length reconstruction | Reconstructs full-length circRNA sequences using BSJ and reverse-overlap reads | [116] |
| CIRI-AS | Alternative splicing event detection | Extends CIRI pipeline to detect alternative splicing events within circRNAs, enabling isoform-level annotation | [26] |
| CircExplorer3 | Split-alignment-based detection; Full-length reconstruction | Improves CircExplorer2 annotation phase, identifying exon skipping, alternative 5′ and 3′ splice sites, and retained introns in circular transcripts | [117] |
| circAST | Full-length reconstruction | Reconstructs alternative splicing isoforms of circRNAs from mapped RNA-seq reads, focusing on isoform diversity | [118] |
| Full-length Reconstruction | |||
| circFL-seq | Full-length reconstruction from long read sequencing | Experimental and computational workflow for profiling full-length circRNAs using Nanopore sequencing | [119] |
| psirc | Full-length circRNA isoform reconstruction | Reconstructs full-length circRNA isoforms using paired end reads and probabilistic modeling | [120] |
| CYCLeR | Full-length reconstruction | Detects and quantifies full-length circRNA isoforms by integrating BSJ detection with exon connectivity analysis | [95] |
| isoCirc | Full-length reconstruction from long read sequencing | Captures and sequences full-length circRNAs at isoform resolution using Nanopore sequencing | [121] |
| CIRI-long | Full-length reconstruction from long read sequencing | Extends CIRI pipeline for long-read sequencing, both Nanopore and PacBio data, to reconstruct and annotate full-length circRNAs | [77] |
| Prediction | |||
| PredcircRNA | Prediction (Machine Learning) | Uses sequences features to distinguish circRNAs from other long non-coding RNAs by multiple kernel learning | [122] |
| circDeep | Prediction (Deep Learning) | Deep learning framework for classifying short and long non-coding RNAs and circRNAs | [123] |
| DeepCirCode | Prediction (Deep Learning) | Utilizes convolutional neural network with nucleotide sequence as input to predict BSJs | [124] |
| JEDI | Prediction (Deep Learning) | Predicts circRNAs and interprets relationships among splice sites to discover BSJ hotspots within a gene region. | [125] |
| circCNNs | Prediction (Deep Learning) | Convolutional neural networks trained to classify circRNAs and predict functional categories | [126] |
| CIRI-deep | Prediction (Deep Learning) | Deep learning extension of CIRI framework for predicting circRNAs and their properties even from single-cell and spatial transcriptomics data | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capirossi, G.; Brasini, S.; Tremoli, E.; Binatti, A.; Roncarati, R. Circular RNAs in Cardiovascular Physiopathology: From Molecular Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 9725. https://doi.org/10.3390/ijms26199725
Capirossi G, Brasini S, Tremoli E, Binatti A, Roncarati R. Circular RNAs in Cardiovascular Physiopathology: From Molecular Mechanisms to Therapeutic Opportunities. International Journal of Molecular Sciences. 2025; 26(19):9725. https://doi.org/10.3390/ijms26199725
Chicago/Turabian StyleCapirossi, Giorgia, Sofia Brasini, Elena Tremoli, Andrea Binatti, and Roberta Roncarati. 2025. "Circular RNAs in Cardiovascular Physiopathology: From Molecular Mechanisms to Therapeutic Opportunities" International Journal of Molecular Sciences 26, no. 19: 9725. https://doi.org/10.3390/ijms26199725
APA StyleCapirossi, G., Brasini, S., Tremoli, E., Binatti, A., & Roncarati, R. (2025). Circular RNAs in Cardiovascular Physiopathology: From Molecular Mechanisms to Therapeutic Opportunities. International Journal of Molecular Sciences, 26(19), 9725. https://doi.org/10.3390/ijms26199725






