Abstract
Mikania micrantha, commonly known as mile-a-minute weed, is listed among the world’s top 10 worst weeds. Although native to humid regions of South America, it has recently been found to colonize arid habitats as well. Despite pronounced seasonal hydroclimatic variations in South China and increasing drought due to global climate change, the mechanisms underlying M. micrantha’s drought tolerance remain poorly understood. In this study, we compared the photosynthetic responses of M. micrantha leaves and stems between the dry (June) and wet (December) seasons through field experiments. We measured changes in phenotype, photosynthetic characteristics, and the content of antioxidant and osmotic adjustment substances, using the co-occurring native vine Paederia scandens as a control. The results revealed that during the dry season, M. micrantha leaves exhibited wilting, along with significant reductions in relative water content (RWC), chlorophyll (Chl), soluble sugar (SS), and soluble protein (SP). In contrast, the stems of M. micrantha maintained relatively stable phenotypes and chlorophyll levels compared to those of P. scandens. Notably, M. micrantha stems exhibited significant increases in vessel wall thickness, vessel density, total phenol content, and the activities of peroxidase (POD) and ascorbate peroxidase (APX). Furthermore, compared to P. scandens, M. micrantha stems displayed a greater increase in cortex proportion, flavonoid content, and soluble protein content. Expression analysis of bZIP transcription factors further revealed drought-responsive upregulation of specific genes (bZIP60, ZIP42-1), suggesting their potential involvement in drought response. These results indicate that although the leaves of M. micrantha are susceptible to prolonged drought, the stems exhibit considerable resilience, which may be attributed to a combination of traits including structural modifications in stem anatomy, enhanced antioxidant capacity, and osmotic adjustment. These insights suggest that stem-specific adaptations are key to its drought tolerance, providing a theoretical foundation for understanding the habitat distribution of M. micrantha and informing effective management strategies.
1. Introduction
With the ongoing development of globalization and the expansion of human activities, increasing CO2 emissions and atmospheric concentrations have intensified the Earth’s greenhouse effect, posing an inevitable environmental challenge [1]. Studies predict that global average temperatures will rise by at least 1.5 °C to 3 °C over the next century [2]. Climate change is expected to alter precipitation patterns, leading to disparities in water resources across different regions and the expansion of arid areas, particularly in Southwest China. Drought, resulting from reduced precipitation, is one of the most common natural hazards affecting this region [3,4,5]. At the same time, biological invasions have intensified with the changes in the global environment [6,7,8]. Mikania micrantha, a highly invasive exotic weed originating from tropical Central and South America [9], has invaded numerous forests in South China, causing significant economic and environmental consequences [10]. Although it is typically found in wet forest areas and freshwater swamps in its native habitat [11], M. micrantha has shown remarkable adaptability to dry soils and shady environments [9], enabling it to thrive and spread even in increasingly dry conditions caused by global climate change. Previous studies have demonstrated that drought conditions reduce the content of photosynthetic pigments (chlorophyll a [Chla], chlorophyll b [Chlb], and total chlorophyll [Chl]) and gas exchange (daily mean of photosynthetic rate [Pday], transpiration rates [Tr], stomatal conductance [Gs]) in M. micrantha leaves [12]. Additionally, Zhang et al. reported that prolonged drought triggers metabolic adjustments in M. micrantha, including increased proline and malondialdehyde (MDA) content, as well as enhanced activity of superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) [13]. These findings suggest that while drought suppresses photosynthetic efficiency and exacerbates oxidative stress in M. micrantha, the species exhibits compensatory mechanisms to sustain metabolic balance. However, the possible mechanism of M. micrantha successfully invading South China with distinct dry and wet seasons, especially the dry-season habitat, has not yet been elucidated. Paederia scandens (Lour.) Merr., a common native congener, has frequently been used as a control species in studies on M. micrantha [14,15,16]. To elucidate the invasive strategies of M. micrantha during dry seasons, this study investigates seasonal variations in leaf and stem morphology, vascular bundle structure, antioxidant capacity, and pigment and osmotic substance content in both M. micrantha and P. scandens in South China. Recent advances in drought stress management, particularly in staple crops, highlight the importance of physiological, biochemical, and molecular adaptations to water scarcity. As reviewed by Khan et al. [17], plants employ a range of strategies including osmotic adjustment, antioxidant defense, phytohormone regulation, and morphological modifications to mitigate drought-induced damage. These mechanisms are crucial for maintaining cellular integrity and metabolic function under water-limited conditions. Understanding such adaptive responses provides a valuable framework for interpreting how invasive species like M. micrantha cope with seasonal drought, potentially informing future management strategies in vulnerable ecosystems.
2. Results
2.1. Change in Phenotypes and Water Content During Dry and Wet Seasons
Soil moisture content showed significant seasonal variation (p < 0.001, Table 1) with wet season levels measuring approximately 10.6-fold higher than dry season values. Phenotypic observations (Figure S1) exhibited vigorous growth during the wet season, with M. micrantha demonstrating a greater advantage over P. scandens. In contrast, both species showed severe leaf chlorosis, wilting, and abscission in the dry season, particularly for M. micrantha. Notably, M. micrantha stems maintained structural integrity without visible wilting. The results also showed that unlike P. scandens, the leaf relative water content (RWC) of M. micrantha significantly decreased by approximately 0.86 times during the dry season compared to the wet season. There were no significant changes in the stems of either plant (Table 1 and Table S2, ANOVA: species, treatment and species × treatment p < 0.001).

Table 1.
The RWC of leaves and stems of M. micrantha and P. scandens in dry and wet season and soil moisture content at the sampling site.
2.2. Changes in Stem Structural Characteristics During Dry and Wet Seasons
Cross-sectional analysis revealed distinct stem morphologies in two species. M. micrantha exhibited an irregular hexagonal stem shape, whereas P. scandens maintained an oval cross-section in both wet and dry seasons (Figure S2). During the dry season, both species showed a significant expansion in cortex proportion (Table S2, ANOVA: species, treatment p < 0.001), with M. micrantha increasing by 25.03% and P. scandens by 20.19% compared to the wet season. Notably, M. micrantha displayed pronounced xylem modifications, with vessel wall thickness (ANOVA: species p < 0.001, treatment p = 0.022, species × treatment p = 0.019) and vessel density (ANOVA: species p < 0.001, treatment p = 0.03, species × treatment p = 0.043) increasing approximately 1.3-fold and 1.9-fold, respectively. In contrast, P. scandens exhibited no significant seasonal variation in these traits. The central cylinder (pith) proportion of M. micrantha decreased significantly during the dry season, yet it remained 1.3 times greater than that of P. scandens. Xylem analysis indicated no significant interspecific or seasonal differences in vessel diameter or the ratio of vessel number to vascular bundle number (Table 2).

Table 2.
Statistical indexes of stem vascular cylinder of M. micrantha and P. scandens in dry and wet seasons.
2.3. Changes in Pigment Content During Dry and Wet Seasons
Leaf chlorophyll (Chl) content in M. micrantha decreased significantly by 37.75% during the dry season, whereas P. scandens exhibited no notable change (Figure 1A, ANOVA: treatment p < 0.001, species × treatment p < 0.001). In stems, M. micrantha showed a moderate Chl reduction (15.20%), slightly less pronounced than that of P. scandens (18.47%; Figure 1B). Conversely, leaf anthocyanin content increased markedly in both species during the dry season. M. micrantha displayed a more substantial rise (155.77%) compared to P. scandens (140.83%; Figure 1C). However, while P. scandens displayed a significant decline in stem anthocyanin content, M. micrantha showed no detectable seasonal variation in this regard (Figure 1D, ANOVA: species, position, treatment p < 0.001, species × position × treatment p > 0.05).
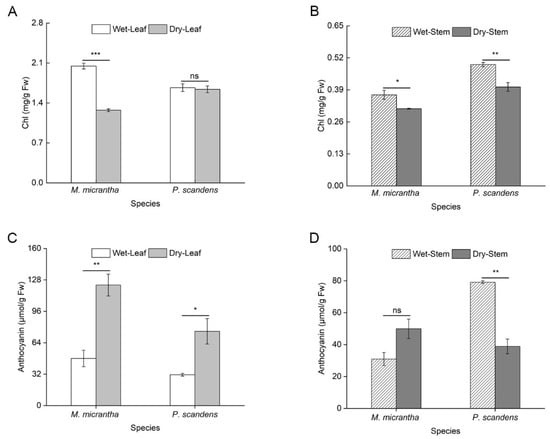
Figure 1.
Changes in pigment content in leaves and stems of M. micrantha and P. scandens in wet and dry seasons. (A,B) represent Chl content; (C,D) represent anthocyanin content. Data are presented as mean ± standard error (n = 5). Asterisks indicate significant differences in the plant between two seasons, * p < 0.05, ** p < 0.01, *** p < 0.001, ns means not significantly.
2.4. Changes in Antioxidant Capacity During Dry and Wet Seasons
During the dry season, Malondialdehyde (MDA) content decreased significantly in both species, with an 81% reduction in M. micrantha and a 30% decline in P. scandens leaves (Figure 2A). A similar trend was observed in stems, where M. micrantha showed decreased MDA levels, while P. scandens exhibited a slight increase (Figure 2B, ANOVA: species, treatment p < 0.001, species × treatment p < 0.001). Supporting these findings, electrolyte leakage measurements showed a parallel and significant decrease in both species and tissues during the dry season (Table S1). Specifically, in leaves, electrolyte leakage decreased from 30.63% to 22.41% in M. micrantha and from 28.13% to 16.13% in P. scandens. Similarly, in stems, electrolyte leakage declined from 24.96% to 17.03% in M. micrantha and from 25.55% to 16.31% in P. scandens (ANOVA: species, treatment, and position p < 0.001). No significant seasonal differences in leaf flavonoid content were detected in either species (Figure 2C). However, stem flavonoid content increased markedly during the dry season, with M. micrantha showing a 143.76% rise compared to 42.62% in P. scandens (Figure 2D, ANOVA: species = 0.033, position and treatment p < 0.001). Similarly, total phenolic content in M. micrantha leaves and stems was significantly higher in the dry season, whereas P. scandens showed no seasonal variation (Figure 2E,F, ANOVA: position, treatment, and species × treatment p < 0.01). SOD activity remained stable across seasons in both species (Figure 3A,B, ANOVA: position and species × treatment p > 0.05). In contrast, POD (ANOVA: species, position, and treatment p < 0.001) and APX (ANOVA: species and position p < 0.01) activities increased significantly in M. micrantha leaves and stems during the dry season, while P. scandens displayed opposing trends (Figure 3C,D).
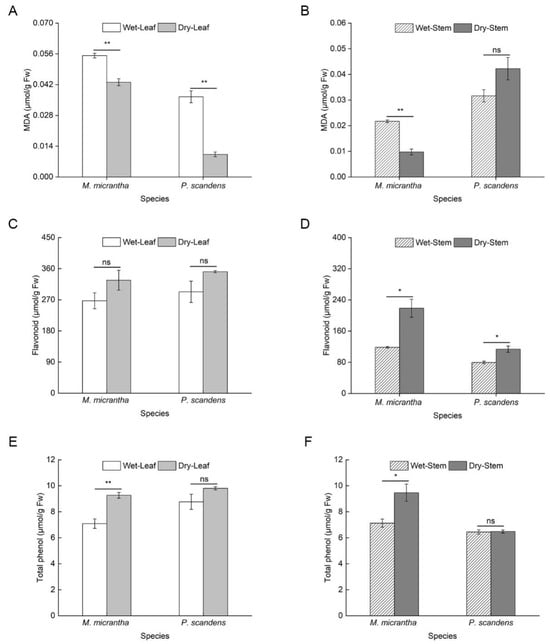
Figure 2.
Changes in MDA content (A,B), flavonoid content (C,D) and total phenolic content (E,F) in leaves and stems of M. micrantha and P. scandens in wet and dry seasons. Data are presented as mean ± standard error (n = 5). Asterisks indicate significant differences in the plant between two seasons, * p < 0.05, ** p < 0.01, ns means not significantly.
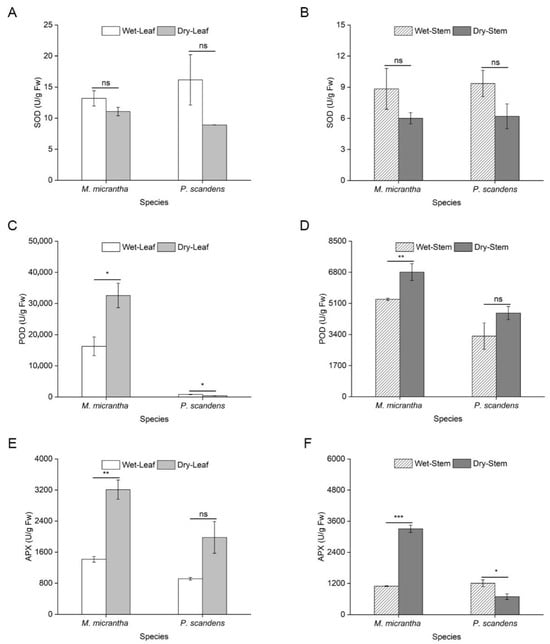
Figure 3.
Changes in activity of antioxidant enzyme in leaves and stems of M. micrantha and P. scandens in wet and dry seasons. (A,B) represent SOD; (C,D) represent POD; (E,F) represent APX. Data are presented as mean ± standard error (n = 5). Asterisks indicate significant differences in the plant between two seasons, * p < 0.05, ** p < 0.01, *** p < 0.001, ns means not significantly.
2.5. Changes in Osmotic Adjustment Substances During Dry and Wet Seasons
M. micrantha exhibited a significant reduction (25%) in soluble sugar (SS) content of leaves during the dry season compared to the wet season. Conversely, P. scandens leaves exhibited a significant increase in SS content during the dry season (Figure 4A). In stems, M. micrantha showed no significant seasonal variation in SS content, whereas P. scandens displayed a notable decline during the dry season (Figure 4B, ANOVA: species, treatment and species × treatment p < 0.001). During the dry season, the soluble protein (SP) content in the leaves of M. micrantha decreased significantly by about 84%, while P. scandens leaves showed no significant change (Figure 4C). Additionally, the increase in SP content in M. micrantha stems (56.11%) was greater than that observed in P. scandens stems (15.24%) (Figure 4D, ANOVA: species, treatment, and position p < 0.001, species × position × treatment p < 0.001).
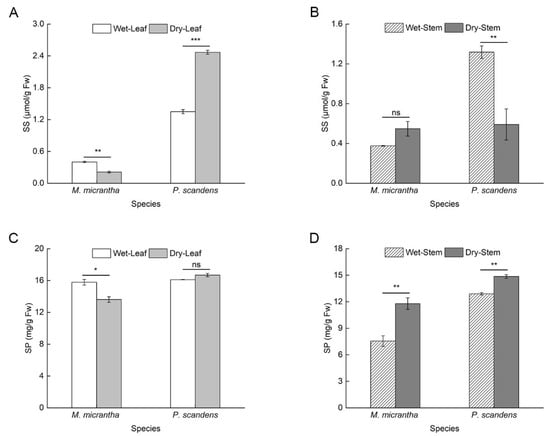
Figure 4.
Changes in the content of osmotic adjustment substances in leaves and stems of M. micrantha and P. scandens in wet and dry seasons. (A,B) represent soluble sugar (SS); (C,D) represent soluble protein (SP). Data are presented as mean ± standard error (n = 5). Asterisks indicate significant differences in the plant between two seasons, * p < 0.05, ** p < 0.01, *** p < 0.001, ns means not significantly.
2.6. Expression Patterns of bZIP Transcription Factors in Response to Drought and Their Tissue Specificity
To further elucidate the molecular mechanisms underlying the physiological changes under drought stress, we analyzed the expression patterns of the bZIP transcription factor family in M. micrantha. Heatmap analysis revealed that multiple bZIP genes exhibited significant differential expression under drought conditions, with distinct tissue-specific expression profiles. Tissue-specific expression profiling (Figure 5A) showed that the bZIP family genes exhibited diverse expression patterns, with most genes (such as bZIP17, bZIP44, and bZIP9) showing high expression in flowers and roots. A small number of genes, such as bZIP60, were highly expressed in leaves and roots, while genes including bZIP23-1, bZIP42-1/2/3, and bZIP61-2/3 were predominantly highly expressed in stems. Furthermore, analysis of bZIP gene expression patterns under drought stress induction revealed diverse response characteristics across subfamilies (Figure 5B). Some genes were up-regulated under drought conditions (e.g., bZIP60, bZIP42-1, bZIP61-1), while others showed higher expression levels under well-watered conditions (e.g., bZIP23-1, bZIP53-1).
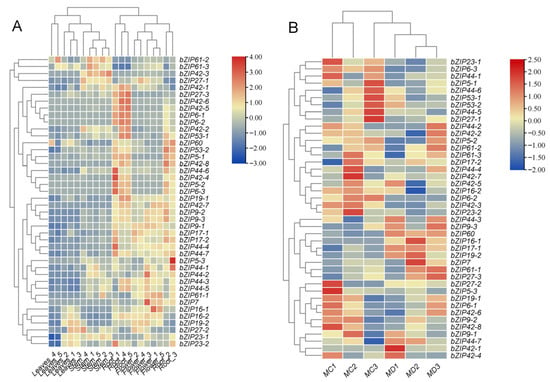
Figure 5.
Expression analysis of bZIP gene family in M. micrantha. (A) represents the expression patterns of bZIP gene family in different tissues (flower, leaves, stem and root) of M. micrantha; (B) represents bZIP gene expression in stem of M. micrantha under drought treatments (MC1–MC3: control group; MD1–MD3: drought group).
3. Discussion
As global climate change intensifies, biological invasions have become increasingly severe. In subtropical Southwest China, the climate is characterized by distinct wet (April–September) and dry (October–March) seasons [18]. Such pronounced seasonality in precipitation strongly influences soil moisture dynamics and plant physiological performance [19]. In our field experiment, we collected two wild vines, M. micrantha and a native plant, P. scandens, during dry and wet seasons. Soil moisture content in their habitats varied significantly, with higher levels during the wet season (Table 1), consistent with findings by Zhang et al. [20]. This moisture fluctuation directly influenced plant relative water content. During the dry season, M. micrantha leaves exhibited wilting and senescence due to reduced RWC (Figure S1; Table 1), suggesting weaker drought resistance compared to P. scandens. This aligns with studies showing that invasive species may exhibit high phenotypic plasticity but often display tissue-specific variations in stress tolerance [18,20]. Interestingly, while leaves suffered, M. micrantha stems remained turgid and healthy (Figure S1), indicating that stem adaptations may be crucial for its dry-season survival. This tissue-specific stress resistance strategy is likely orchestrated at the transcriptional level. Basic leucine zipper motif (bZIP) transcription factors play an important regulatory role in plant drought stress responses. Our study found that multiple bZIP transcription factors (such as bZIP23-1, bZIP42-1/2/3, etc.) were specifically highly expressed in stems (Figure 5A), suggesting that they may be key regulators in shaping stem identity and mediating its drought response [21].
3.1. Stem Structural Optimization Enhances Water Retention
The stem is a vital organ for water transport from roots to aerial tissues [22,23]. In M. micrantha, the cortex and medulla proportions increased during the dry season (Figure S2; Table 2). Li et al. demonstrated that the larger cortex and medulla areas enhance the water storage capacity of Cerasus humilis tissue, enabling plants to regulate water retention in arid environments and improve overall resistance, particularly in the stems [24]. The anatomical studies on Tagetes erecta also support these findings [25], indicating that the presence of parenchyma tissue with a higher proportion in the stem of M. micrantha enhances its water storage capacity. Research has shown that tannin acid, acting as a potent reducing agent in the medulla, could improve the ability of plants to scavenge oxygen free radicals generated under water stress, thereby minimizing oxidative damage caused by dehydration [26]. The xylem vessels are fundamental structures responsible for water transport in plants [27]. Research on the stem structure of Carya illinoensis across different climate types showed that varieties exhibiting strong drought tolerance generally have higher vessel density and thicker vessel walls in the cross-section of their stems, especially in areas with lower precipitation [28]. Similarly, the stem of M. micrantha exhibited increased vessel wall thickness and vessel density, indicating enhanced water transmission capacity during the dry season (Table 2). This adaptation mechanism potentially reduces vessel diameter by increasing vessel wall thickness, thereby preventing vessel cavitation and embolism [29,30]. Notably, these anatomical modifications are likely driven by specific bZIP transcription factors. For example, bZIP42-1 and bZIP61-1 were significantly upregulated under drought stress (Figure 5B) and may activate genes involved in cell wall synthesis and modification. Therefore, optimizing the stem structure and improving water transport capacity represent important strategies employed by M. micrantha to cope with drought stress.
3.2. Balance and Distribution of Photosynthetic Pigments
Chlorophyll degradation under drought is well-documented [31], often due to ROS-induced chloroplast damage [32]. In our observations, while the stem Chl content remained stable in both species (Figure 1B), the leaves of M. micrantha exhibited a significant loss of Chl, unlike those of P. scandens (Figure 1A). This reduction in Chl content typically leads to a decline in photosynthetic capacity under drought conditions [33]. The notable chlorophyll degradation in the leaves of M. micrantha during the dry season suggests poorer drought resistance, which may negatively affect its photosynthetic capacity. Conversely, anthocyanins increased in M. micrantha stems and both species’ leaves during the dry season (Figure 1). Cirillo et al. found that anthocyanins play an important role in enhancing plant drought resistance [34]. These findings indicate that anthocyanins could shield light energy and reduce ROS accumulation caused by excessive light [35,36]. Additionally, anthocyanins function as antioxidants, directly minimizing ROS accumulation in plants [37]. BZIP transcription factors are widely involved in regulating plant secondary metabolism. Studies have shown that overexpression of NnbZIP36 in Arabidopsis promoted anthocyanin accumulation by upregulating key anthocyanin biosynthesis genes (including 4CL, CHI, CHS, F3H, F3’H, DFR, ANS, and UF3GT) [38]. Consistent with this, our study observed a significant drought-induced increase in anthocyanin content in both the leaves and stems of M. micrantha (Figure 1C,D). This increase may potentially be regulated upstream by certain drought-responsive bZIP members, such as the drought-induced MmbZIP60 (Figure 5B). These transcription factors likely enhance the plant’s photoprotection and antioxidant capacity by activating key enzyme genes in the anthocyanin biosynthesis pathway.
3.3. Accumulation of Antioxidant Substances
Drought stress typically induces substantial accumulation of reactive oxygen species (ROS) in plants [38], leading to membrane lipid peroxidation [36,39]. While numerous studies have reported drought-induced increases in MDA content in species such as Nicotiana tabacum [40] and Sphagneticola species [20], our results revealed an unexpected but consistent decrease in both MDA levels and electrolyte leakage during the dry season across most tissues (Figure 2A,B, Table S2). This concerted decline in two key indicators of membrane damage strongly suggests that efficient ROS detoxification, potentially mediated by enhanced production of antioxidant proteins [20]. Plants employ a dual antioxidant defense system to mitigate ROS under drought stress, consisting of non-enzymatic antioxidants (flavonoid and total phenol) and antioxidant enzymes (SOD, APX, POD, etc.) [41,42,43]. Our study showed higher levels of flavonoids and total phenols in M. micrantha compared to P. scandens (Figure 2C–F), consistent with previous findings on antioxidant content changes under drought conditions [44]. For instance, recent studies have highlighted that flavonoids and phenolic compounds act as crucial scavengers of ROS, thereby enhancing oxidative stress tolerance and supporting plant growth under water deficit [45]. These results suggested that the increasing antioxidant content enhances plant resistance by reducing ROS accumulation due to drought stress. Additionally, antioxidant enzymes also play a crucial role in removing excessive ROS to maintain intracellular balance [46,47]. Interestingly, while SOD activity remained unchanged between species and seasons (Figure 3A,B), M. micrantha exhibited marked increases in POD and APX activities in both leaves and stems (Figure 3C–F). This enzymatic profile suggests that POD and APX serve as the primary ROS-scavenging systems in M. micrantha, efficiently converting H2O2 to H2O and maintaining redox homeostasis. Similar drought-responsive upregulation of antioxidant enzymes has been documented in Catharanthus roseus [48], Sphagneticola trilobata [20], and Vitis vinifera [49]. Despite the above evidence, the observed reduction in membrane damage markers remains physiologically atypical. We acknowledge that other factors—such as tissue-specific ROS dynamics, compensatory metabolic adaptations, or yet-unidentified non-oxidative pathways—may also contribute to the observed phenomena. Future research involving direct measurement of specific ROS like H2O2 could provide more precise insights into the oxidative stress status under these conditions. Study have shown that OsbZIP62 improves drought and oxidative tolerance in rice [50]. Complementing these antioxidant mechanisms, osmotic adjustment through soluble sugar and soluble protein accumulation represents another critical drought adaptation strategy [51], consistent with recent findings across various plant species [52,53]. Notably, while M. micrantha leaves showed limited osmotic adjustment capacity, its stems demonstrated substantial increases in SS and SP content during drought, surpassing levels observed in P. scandens (Figure 4). This stem-specific response highlights a key physiological differentiation within M. micrantha, with stems exhibiting stronger osmotic adjustment ability compared to leaves during water-limited conditions.
4. Materials and Methods
4.1. Plant Growth and Collection
The subtropical climate in Southwest China exhibits distinct wet (April to September) and dry seasons (October to March) [18]. Two plants in this study including M. micrantha and P. scandens were grown in the campus of South China Normal University (23°10′ N, 113°21′ E). P. scandens was chosen as a control species because field surveys revealed that it not only occurs frequently and stably in communities invaded by M. micrantha but also shares similar reproductive strategies with M. micrantha [54]. The phenotypic characteristics of M. micrantha and P. scandens were observed during wet season (June 2021) and dry season (December 2021) using digital cameras (Sony, α6000, Sony Imaging Products & Solutions Inc., Tokyo, Japan). Stem segments from the first to the sixth nodes of both plants, which exhibited similar growth patterns, were collected to analyze relevant physiological indicators in this experiment. Additionally, soil samples from the plant habitat were collected to determine soil water content. The plant samples were rinsed with tap water to remove soil and surface debris, followed by a wash with distilled water. All the samples were frozen in liquid nitrogen and stored at −80 °C for further analysis.
4.2. Determination of Relative Water Content (RWC)
The relative water content (RWC) of plant tissues was determined using a modified protocol described by Ogbaga et al. [55]. A fully expanded leaf (1 piece) and stem segment (1 cm long) were weighed to obtain their respective fresh weight (FW). The samples were then immersed in 5 mL of distilled water within a centrifuge tube for 24 h to achieve full turgidity, after which their turgid weight (TW) was recorded. Finally, the samples were oven-dried at 75 °C until a constant dry weight (DW) was attained. RWC was calculated using the following formula:
RWC (%) = (FW − DW)/(TW − DW) × 100%.
Soil moisture content was measured following the oven-drying method [18]. Briefly, 10 g of fresh soil was weighed (SW1) and dried at 75 °C until a constant mass (SW2) was achieved. The soil moisture content was calculated as:
Soil moisture content (%) = (SW1 − SW2)/SW1 × 100%.
4.3. Observation of Stem Structure
Stem samples from both plant species were collected during dry and wet seasons and processed using standard paraffin methods [56]. A total of over twenty cross-sections (representing at least five biological replicates per species per season) were prepared and scanned using a high-resolution digital slide scanner (Hamamatsu S360, Hamamatsu Photonics K.K., Hamamatsu, Japan). Multiple anatomical parameters were quantified using ImageJ version 1.54p software. The proportion of cortex, medulla and duct density were calculated using the following formula: cortex proportion (%) = (cortex area/slice area) × 100%; medulla proportion (%) = (medulla area/section area) × 100%; catheter density (Individual/mm2) = (catheter number/slice area).
4.4. Determination of Chlorophyll and Anthocyanin Content
Fresh leaf and stem samples (0.05 g) were homogenized in 4 mL of 80% acetone and extracted overnight at 4 °C. Following centrifugation at 4 °C, 3000 rpm for 10 min, the absorbance of the supernatant was measured at 663 nm, 645 nm and 470 nm using 80% acetone as a blank. Chlorophyll a, b, and total chlorophyll concentrations were calculated according to Wellburn [57]. Anthocyanin content was determined following Cai et al. [36] with modifications. Samples (0.05 g) were homogenized in 2 mL of methanol:HCl (99:1, v/v) solution and extracted at 4°C in the dark for 24 h. The reaction mixture, containing 0.5 mL deionized water, an equal volume of supernatant, and chloroform, was analyzed at 532 nm using a UV-Vis 2450 spectrophotometer (Shimadzu, Kyoto, Japan). The chlorophyll and anthocyanin content were expressed as (μg/g FW) and (μmol/g FW), respectively.
4.5. Determination of Malondialdehyde (MDA) and Soluble Sugar (SS) Content
MDA content was determined using the thiobarbituric acid (TBA) method [58]. Tissue samples (0.05 g) were homogenized in 2 mL of 0.1% (w/v) trichloroacetic acid (TCA) solution. After centrifuged at 4 °C, 1000 rpm for 10 min, 1 mL of the supernatant was mixed with an equal volume of 0.67% (w/v) TBA solution. The mixture was incubated at 95 °C for 30 min in a water bath, and absorbance was measured at 532 nm, 600 nm, and 645 nm using a spectrophotometer. The MDA and SS content were expressed as (μmol/g FW).
4.6. Determination of Activities of Enzymatic Antioxidants and Soluble Protein Content
Fresh tissue samples (0.05 g) were homogenized in 1 mL of extraction buffer (consist of 50 mM Phosphate buffer [PBS], 0.1 M Ethylenediaminetetraacetic acid [EDTA], Triton-X-100 and polyvinylpyrrolidone [PVP], pH 7.8). The homogenate was centrifuged at 12,000× g for 10 min at 4 °C, and the supernatant was collected for enzymatic analyses. SOD activity was determined using a modified nitroblue tetrazolium (NBT) reduction method [59]. One unit of SOD activity is defined as the amount of enzyme required to inhibit nitro blue tetrazolium (NBT) photoreduction by 50%. A final volume of 3 mL of the mixture (consisted of 50 mM PBS, 20 μM riboflavin, 130 mM methionine, 0.1 μM EDTA, 750 mM NBT, and 100 μL of enzyme extract) was recorded at a wavelength of 560 nm. POD activity was assayed according to Du et al. [60] using a reaction mixture containing 50 mM PBS (pH 7.0), 30 mM H2O2, guaiacol, and 100 μL enzyme extract. The increase in absorbance at 470 nm was recorded for 3 min. APX activity was measured following Nakano and Asada [61] by monitoring the oxidation of ascorbate at 290 nm for 1 min at 10 s intervals. The antioxidant enzyme activities were calculated and expressed as (U/g FW). The soluble protein content was determined using the bicinchoninic acid (BCA) method. A diluted aliquot of the enzyme extract was mixed with a 2× Bradford dye reagent. The absorbance was measured at 595 nm using a spectrophotometer. A standard curve was prepared using a protein standard solution, and the soluble protein content was calculated and expressed as (mg/g FW).
4.7. Determination of Flavonoid and Total Phenol Content
Flavonoid and total phenol contents were estimated by the method of [62], Ainsworth and Gillespie with slight modifications, respectively [63]. 0.05 g sample were homogenized in 2 mL of 95% (v/v) methanol, the supernatant was obtained after centrifuged at 12,000 rpm for 10 min. Flavonoid content was determined following a modified method of Park et al. [61]. The reaction mixture was composed of 2 mL supernatant (diluted 8-fold), 0.2 mL of NaNO2 (5%, v/w), 0.3 mL of AlCl3 (10%, v/w) and 1 mL of 1 M NaOH. After thorough mixing, absorbance was measured at 510 nm against a methanol blank. Total phenolic content was quantified using the Folin–Ciocalteu method with modifications [62]. The absorbance of reaction mixture (equal volume of supernatant and Folin–Ciocalteu (10%, w/v) were mixed with 0.7 M Na2CO3) were measured at 765 nm using a Uv-Vis 2450 spectrophotometer. The flavonoid and total phenol content were expressed as (μmol/g FW).
4.8. Screening and Identification of bZIP Gene Family Members in M. micrantha
The genomic information, annotation files, and protein sequence data of M. micrantha were downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov/ (accessed on 20 August 2025)). The protein sequences of the Arabidopsis thaliana AtbZIP gene family were retrieved from the TAIR database and used as query sequences for BLAST version 2.16.0 alignment against the M. micrantha database. The E-value was set to 10−8, with a similarity threshold of >60%, to preliminarily screen candidate genes. The presence of conserved domains (F00170, PF07716) was confirmed using the CD-search and SMART [64].
4.9. Expression Pattern Analysis of bZIP Gene Family Members in M. micrantha
According to the method of Chen et al. [64], transcriptome data from the database were used to analyze the expression of M. micrantha bZIP genes in different organs (roots, stems, leaves, and flowers) and under drought induction treatment. RNA-seq datasets (No. SRR8857621–SRR8857640) were obtained from the SRA database (https://www.ncbi.nlm.nih.gov/sra (accessed on 20 August 2025)) on the NCBI website. The raw data underwent quality control, including adapter removal and redundancy filtering, to generate clean data for subsequent alignment and quantitative analysis. TPM or FPKM values were used to measure gene expression levels. R Studio (version 4.4.2) was employed to generate heatmaps illustrating the expression of MmbZIP gene family members in different organs and under drought treatment.
4.10. Data Analysis
All data are presented as mean ± standard deviation (SD) from three to five biological replicates. Differences between dry and wet seasons of the same species (M. micrantha or P. scandens) were detected by Studentʼs t-test using IBM SPSS® Statistics (Version 19.0, IBM Corporation, New York, NY, USA) software. Data visualizations were carried out using OriginPro (Version 8.0, OriginLab Corporation, Northampton, MA, USA) and Adobe Photoshop CC (Version 2017, Adobe Systems, San Jose, CA, USA) software.
5. Conclusions
In summary, our findings reveal a striking dichotomy in M. micrantha’s drought response between its leaf and stem tissues. During the dry season, M. micrantha leaves exhibit a significantly reduced RWC, compromised phenotypic integrity, lower levels of chlorophyll, and decreased osmotic adjustment capabilities compared to the native P. scandens. However, this apparent vulnerability is balanced by the exceptional adaptive features of the stem of M. micrantha, which include enhanced anatomical structures for water storage and transport, superior antioxidant defense systems, and robust osmotic regulation capabilities. These stem-specific characteristics, potentially mediated by bZIP transcription factors—though future molecular evidence is needed—may underpin M. micrantha’s remarkable drought resilience despite its foliar limitations. We hypothesize that this differential tissue specialization could represent a key evolutionary strategy facilitating the species’ successful establishment and ongoing invasion in subtropical South China. The ability of the stem to maintain physiological function during seasonal drought could provide M. micrantha with a competitive advantage over native vegetation, particularly in regions experiencing increasing aridity due to climate change. While our anatomical findings are suggestive of improved hydraulics, and the chlorophyll reduction hints at lower photosynthetic capacity, future studies incorporating direct measurements of stem hydraulic conductivity, cavitation vulnerability, and leaf gas exchange are needed to definitively establish these mechanistic links.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26199722/s1.
Author Contributions
Conceptualization, M.C. (Minling Cai) and M.C. (Minghao Chen); methodology, M.C. (Minling Cai) and M.C. (Minghao Chen); software, J.Z.; validation, M.C. (Minling Cai), J.Z. and M.C. (Minghao Chen); formal analysis, M.C. (Minling Cai) and J.Z.; investigation, M.C. (Minling Cai) and J.Z.; resources, C.P.; data curation, M.C. (Minling Cai) and J.Z.; writing—original draft preparation, M.C. (Minling Cai) and M.C. (Minghao Chen); writing—review and editing, M.C. (Minghao Chen) and C.P.; visualization, M.C. (Minling Cai); supervision, C.P.; project administration, C.P.; funding acquisition, C.P. and M.C. (Minling Cai). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32401382, 3217130145, 31870374), the Professorial and Doctoral Scientific Research Foundation of Huizhou University (2022JB086), the Independent Innovation Capability Enhancement Plan Project of Huizhou University (HZU202541) and the National College Students Innovation and Entrepreneurship Program Project (202310577015, S202410577070). The APC was funded by Minling Cai.
Data Availability Statement
All datasets for this study are included in the manuscript and/or the Supplementary Files.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RWC | Relative Water Content |
| Chl | Chlorophyll |
| SS | Soluble Sugar |
| SP | Soluble Protein |
| POD | Peroxidase |
| APX | Ascorbate Peroxidase |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| FW | Fresh Weight |
| TW | Turgid Weight |
| DW | Dry Weight |
| MDA | Malondialdehyde |
| TBA | Thiobarbituric Acid |
| TCA | Trichloroacetic Acid |
| PBS | Phosphate Buffer |
| EDTA | Ethylenediaminetetraacetic Acid |
| PVP | Polyvinylpyrrolidone |
| NBT | Nitroblue Tetrazolium |
| SD | Mean ± Standard Deviation |
References
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global Climate Change and Greenhouse Effect. Entrep. Sustain. Issues 2020, 7, 2897. [Google Scholar] [CrossRef]
- Eyring, V.; Waugh, D.W.; Bodeker, G.E.; Cordero, E.; Akiyoshi, H.; Austin, J.; Beagley, S.R.; Boville, B.A.; Braesicke, P.; Brühl, C.; et al. Multimodel Projections of Stratospheric Ozone in the 21st Century. J. Geophys. Res. Atmos. 2007, 112, D16303. [Google Scholar] [CrossRef]
- Arnell, N.W. Climate Change and Global Water Resources. Glob. Environ. Change 1999, 9, S31–S49. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W. A CMIP5 Multimodel Projection of Future Temperature, Precipitation, and Climatological Drought in China. Int. J. Climatol. 2014, 34, 2059–2078. [Google Scholar] [CrossRef]
- Yi, C.; Wei, S.; Hendrey, G. Warming Climate Extends Dryness-Controlled Areas of Terrestrial Carbon Sequestration. Sci. Rep. 2014, 4, 5472. [Google Scholar] [CrossRef]
- Ding, J.; Mack, R.N.; Lu, P.; Ren, M.; Huang, H. China’s Booming Economy Is Sparking and Accelerating Biological Invasions. BioScience 2008, 58, 317–324. [Google Scholar] [CrossRef]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global Threat to Agriculture from Invasive Species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Horvitz, N.; Wang, R.; Wan, F.; Nathan, R. Pervasive Human-Mediated Large-Scale Invasion: Analysis of Spread Patterns and Their Underlying Mechanisms in 17 of China’s Worst Invasive Plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ye, W.H.; Cao, H.L.; Feng, H.L. Mikania micrantha HBK in China—An Overview. Weed Res. 2004, 44, 42–49. [Google Scholar] [CrossRef]
- Shao, H.; Peng, S.; Wei, X.; Zhang, D.; Zhang, C. Potential Allelochemicals from an Invasive Weed Mikania micrantha HBK. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Cock, M.J.W. Potential Biological Control Agents for Mikania micrantha HBK from the Neotropical Region. Int. J. Pest. Manag. 1982, 28, 242–254. [Google Scholar]
- Wang, R.; Xia, W.N.; Liu, S.; Qin, Z.; Liang, K.M.; Su, Y.J.; Zhang, J.E. Effects of Water Stress on the Growth and Allelopathic Potential of Invasive Plant Mikania micrantha HBK. Allelopath. J. 2016, 39, 143–152. [Google Scholar]
- Zhang, L.L.; Wen, D.Z.; Fu, S.L. Responses of Photosynthetic Parameters of Mikania micrantha and Chromolaena odorata to Contrasting Irradiance and Soil Moisture. Biol. Plant. 2009, 53, 517–522. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha Genome Provides Insights into the Molecular Mechanism of Rapid Growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zheng, Y.; Cai, M.; Peng, C.; Li, W. Physiological and Transcriptomic Responses of Mikania micrantha Stem to Shading Yield Novel Insights into Its Invasiveness. Biol. Invasions 2021, 23, 2927–2943. [Google Scholar] [CrossRef]
- Yu, H.; Le Roux, J.J.; Jiang, Z.; Sun, F.; Peng, C.; Li, W. Soil Nitrogen Dynamics and Competition During Plant Invasion: Insights from Mikania micrantha Invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, Y.-F.; Akbar, R.; Alhoqail, W.A. Mechanistic insights and future perspectives of drought stress management in staple crops. Front. Plant Sci. 2025, 27, 1547452. [Google Scholar] [CrossRef]
- Huang, J.-G.; Guo, X.; Rossi, S.; Zhai, L.; Yu, B.; Zhang, S.; Zhang, M. Intra-Annual Wood Formation of Subtropical Chinese Red Pine Shows Better Growth in Dry Season Than Wet Season. Tree Physiol. 2018, 38, 1225–1236. [Google Scholar] [CrossRef]
- Cai, M.; Huang, J.; Chen, M.; Chen, L.; Zhang, X.; Chen, M.; Wu, J.; Pan, Y.; Peng, C. The role and synthesis mechanism of anthocyanins in Sphagneticola trilobata stems under low temperature. Biol. Invasions 2024, 26, 2851–2867. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Ke, W.; Cai, M.; Chen, G.; Peng, C. Responses of Sphagneticola trilobata, Sphagneticola calendulacea and Their Hybrid to Drought Stress. Int. J. Mol. Sci. 2021, 22, 11288. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Oechel, W.C.; Van Cleve, K.; Lawrence, W. The Role of Mosses in the Phosphorus Cycling of an Alaskan Black Spruce Forest. Oecologia 1987, 74, 310–315. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal Endophyte Epichloë bromicola Infection Regulates Anatomical Changes to Account for Salt Stress Tolerance in Wild Barley (Hordeum brevisubulatum). Plant Soil. 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, X.Y.; Li, L.G.; Bai, Z.H. A Study of Morphological Anatomy of Root and Stem on Cerasus humilis. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2014, 35, 26–30. [Google Scholar]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.; Akram, N.A.; Abideen, Z. Morpho-Anatomical Adaptations of Two Tagetes erecta L. Cultivars with Contrasting Response to Drought Stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef]
- Yaqoob, U.; Jan, N.; Raman, P.V.; Siddique, K.H.M.; John, R. Crosstalk between Brassinosteroid Signaling, ROS Signaling and Phenylpropanoid Pathway During Abiotic Stress in Plants: Does It Exist? Plant Stress. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Liang, X.; Huang, H.; Zhang, S. Morphological, Anatomical, and Physiological Assessment of Ramie [Boehmeria nivea (L.) Gaud.] Tolerance to Soil Drought. Genet. Resour. Crop Evol. 2005, 52, 497–506. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wang, L.; Zhou, M.Y.; He, D.M.; Bi, H.H.; Ge, X.; Shen, J.C.; Fu, S.L. Comparison on Leaf Structure Characteristics and Branch Hydraulic Function of Three Carya illinoinensis Cultivars. J. Plant Res. Environ. 2021, 30, 38–45. [Google Scholar]
- Maherali, H.; Pockman, W.T.; Jackson, R.B. Adaptive Variation in the Vulnerability of Woody Plants to Xylem Cavitation. Ecology 2004, 85, 2184–2199. [Google Scholar] [CrossRef]
- Boughalleb, F.; Abdellaoui, R.; Ben-Brahim, N.; Neffati, M. Anatomical Adaptations of Astragalus gombiformis Pomel. Under Drought Stress. Open Life Sci. 2014, 9, 1215–1225. [Google Scholar]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of Drought Stress on Growth and Chlorophyll Fluorescence of Lycium ruthenicum Murr. Seedlings. Photosynth. 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Zaefyzadeh, M.; Quliyev, R.A.; Babayeva, S.M.; Abbasov, M.A. The Effect of the Interaction Between Genotypes and Drought Stress on the Superoxide Dismutase and Chlorophyll Content in Durum Wheat Landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Wu, Y.-X.; Hu, Y.; Jia, X.-M.; Zhao, T.; Cheng, L.; Wang, Y.-X. Tolerance of Two Apple Rootstocks to Short-Term Salt Stress: Focus on Chlorophyll Degradation, Photosynthesis, Hormone and Leaf Ultrastructures. Acta Physiol. Plant. 2019, 41, 87. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins Are Key Regulators of Drought Stress Tolerance in Tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Cai, M.-L.; Ding, W.-Q.; Zhai, J.-J.; Zheng, X.-T.; Yu, Z.-C.; Zhang, Q.-L.; Lin, X.-H.; Chow, W.S.; Peng, C.-L. Photosynthetic Compensation of Non-Leaf Organ Stems of the Invasive Species Sphagneticola trilobata (L.) Pruski at Low Temperature. Photosynth. Res. 2021, 149, 121–134. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant Defense Responses: Physiological Plasticity in Higher Plants Under Abiotic Constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, X.; Xue, X.; Wang, Y.; Wang, Y.; Li, H.; Xue, R.; Li, Y. Improvement of polyamine synthesis maintains photosynthetic function in wheat during drought stress and rewatering at the grain filling stage. Plant Growth Regul. 2024, 102, 497–513. [Google Scholar] [CrossRef]
- Hong, W.; Jin, J.Y. Effects of Zinc Deficiency and Drought on Plant Growth and Metabolism of Reactive Oxygen Species in Maize (Zea mays L.). Agric. Sci. China 2007, 6, 988–995. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Zhao, X.; Liu, G.; Yang, C.; Zhan, L. A Novel LEA Gene from Tamarix androssowii Confers Drought Tolerance in Transgenic Tobacco. Plant Sci. 2006, 171, 655–662. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Mohagheghian, B.; Saeidi, G.; Arzani, A. Phenolic compounds, antioxidant enzymes, and oxidative stress in barley (Hordeum vulgare L.) genotypes under field drought-stress conditions. BMC Plant Biol. 2025, 25, 709. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N. Role of Nanosilicab to Boost the Activities of Metabolites in Triticum aestivum Facing Drought Stress. Plant Soil. 2022, 477, 99–115. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling During Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Ababaf, M.; Omidi, H.; Bakhshandeh, A. Changes in Antioxidant Enzymes Activities and Alkaloid Amount of Catharanthus roseus in Response to Plant Growth Regulators Under Drought Condition. Ind. Crops Prod. 2021, 167, 113505. [Google Scholar] [CrossRef]
- Haddad, R.; Kamangar, A. The Ameliorative Effect of Silicon and Potassium on Drought Stressed Grape (Vitis vinifera L.) Leaves. Iran. J. Genet. Plant Breed. 2015, 4, 48–58. [Google Scholar]
- Yang, S.; Xu, K.; Chen, S.; Li, T.; Xia, H.; Chen, L.; Liu, H.; Luo, L. A stress-responsive bZIP transcription factor OsbZIP62 improves drought and oxidative tolerance in rice. BMC Plant Biol. 2019, 19, 260. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of Drought Stress on Lipid Peroxidation, Osmotic Adjustment and Antioxidant Enzyme Activity of Leaves and Roots of Lycium ruthenicum Murr. Seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Wach, D.; Skowron, P. An overview of plant responses to the drought stress at morphological, physiological and biochemical levels. Pol. J. Agron. 2022, 4, 25–34. [Google Scholar]
- Zhou, P.; Li, J.; Jiang, H.; Jin, Q.; Wang, Y.; Xu, Y. Analysis of bZIP gene family in lotus (Nelumbo) and functional study of NnbZIP36 in regulating anthocyanin synthesis. BMC Plant Biol. 2023, 23, 429. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Yu, H.; Li, N.; Peng, C. The invasive plant Mikania micrantha affects the soil foodweb and plant-soil nutrient contents in orchards. Soil. Biol. Biochem. 2019, 139, 107630. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Johnson, G.N. Sorghum (Sorghum bicolor) Varieties Adopt Strongly Contrasting Strategies in Response to Drought. Physiol. Plant. 2014, 152, 389–401. [Google Scholar] [CrossRef]
- Hamann, T.; Smets, E.; Lens, F. A Comparison of Paraffin and Resin-Based Techniques Used in Bark Anatomy. Taxon 2011, 60, 841–851. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemets, O.; Gauslaa, Y.; Solhaug, K.A. Monitoring with Lichens—Conductivity Methods Assess Salt and Heavy Metal Damage More Efficiently Than Chlorophyll Fluorescence. Ecol. Indic. 2015, 55, 59–64. [Google Scholar] [CrossRef]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in Photosynthesis and Antioxidant Enzyme Activity in Winter Wheat Subjected to Post-Anthesis Water-Logging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Huang, B. Differential Responses of Warm-Season and Cool-Season Turfgrass Species to Heat Stress Associated with Antioxidant Enzyme Activity. J. Am. Soc. Hortic. Sci. 2009, 134, 417–422. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Heo, B.G.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and Proteins in Ethylene-Treated Kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, M.; Chen, M.; Ke, W.; Pan, Y.; Huang, J.; Zhang, J.; Peng, C. Genome-Wide Characterization of PIN Auxin Efflux Carrier Gene Family in Mikania micrantha. Int. J. Mol. Sci. 2022, 23, 10183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).