miR-10c Targets dgat2 and Affects the Expression of Genes Involved in Fatty Acid and Triglyceride Metabolism in Oreochromis niloticus Under Heat Stress
Abstract
1. Introduction
2. Results
2.1. Sequence Characterization of miR-10c
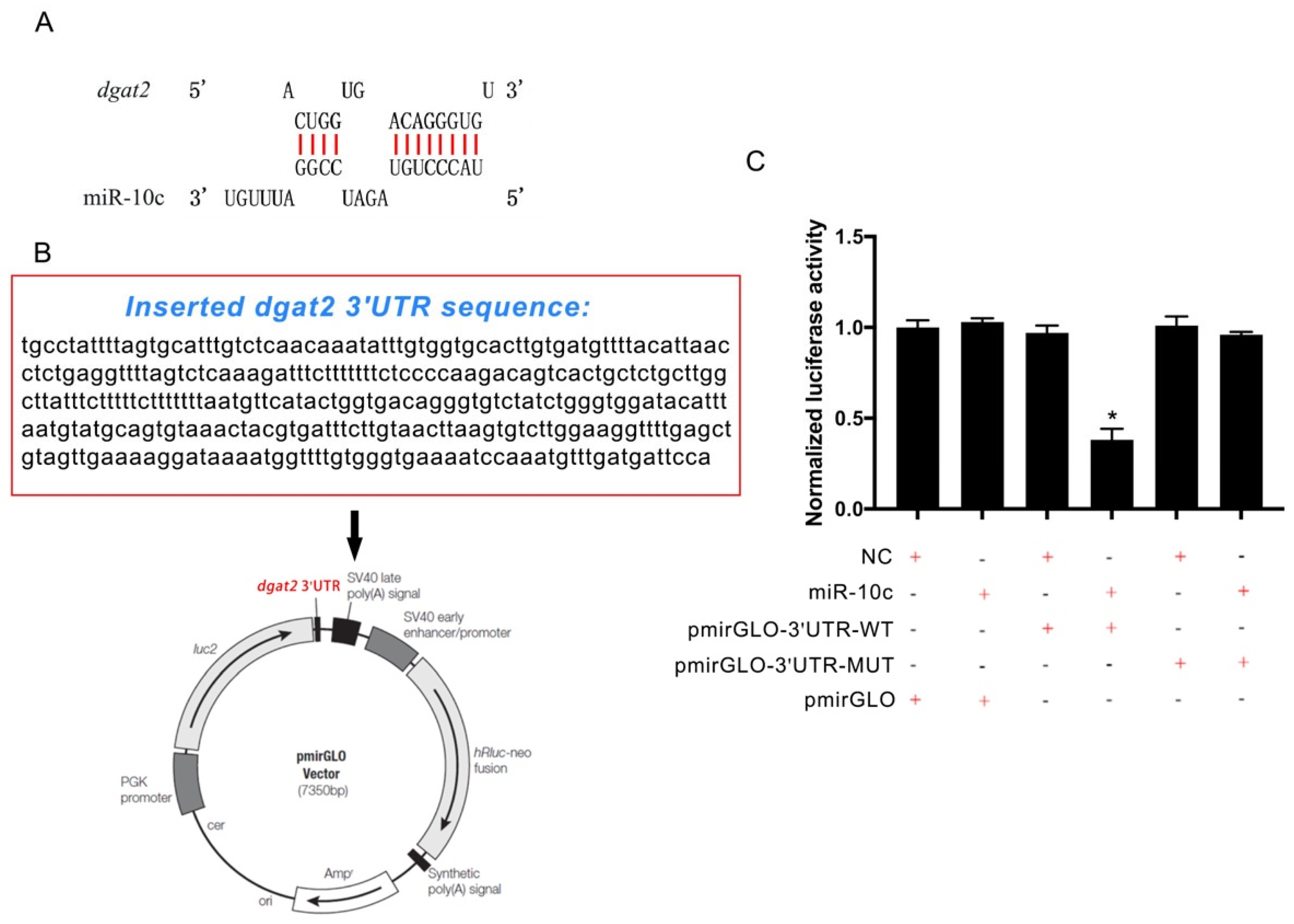
2.2. Verification of dgat2 as a Target of miR-10c
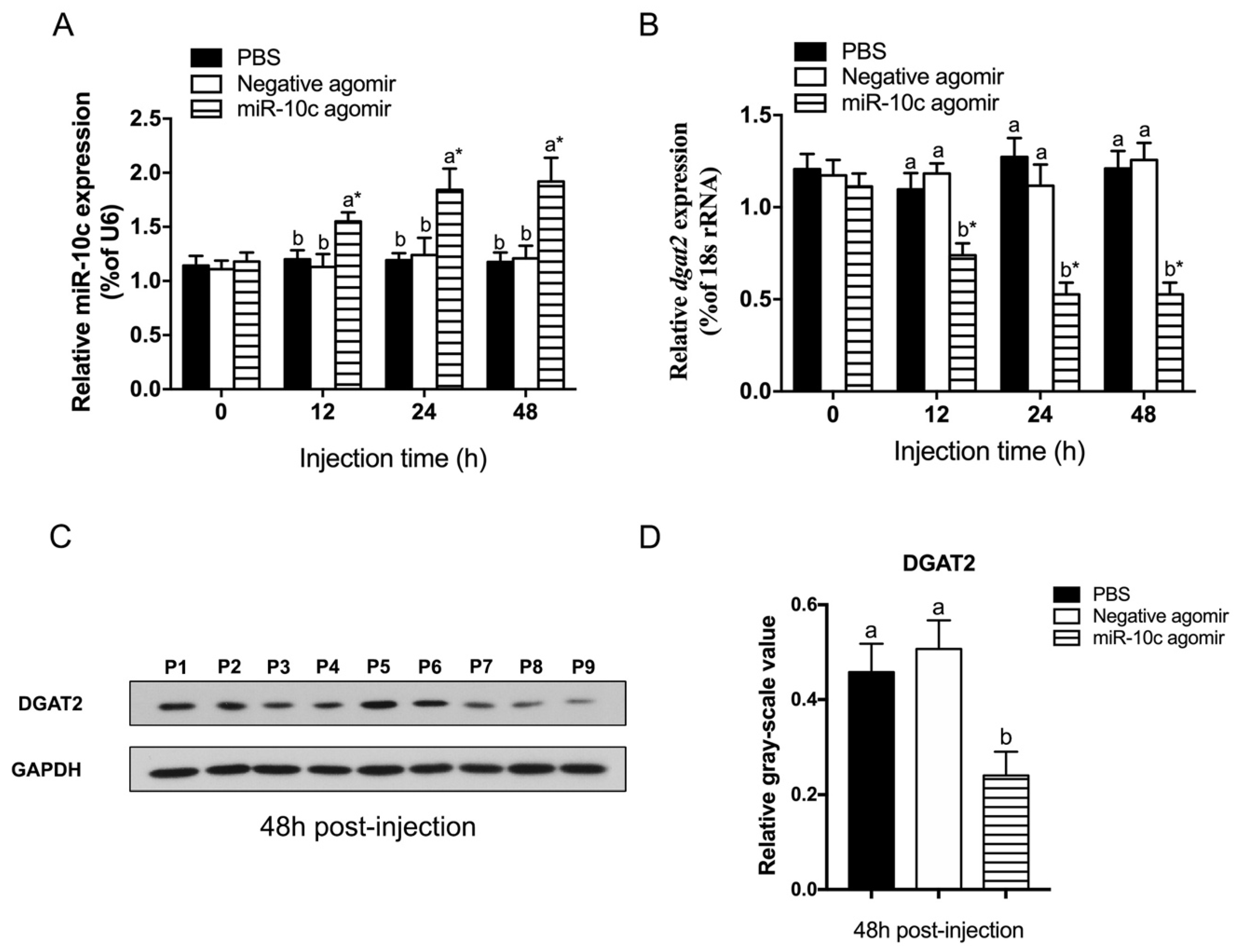
2.3. Regulatory Effect of miR-10c on dgat2 Expression
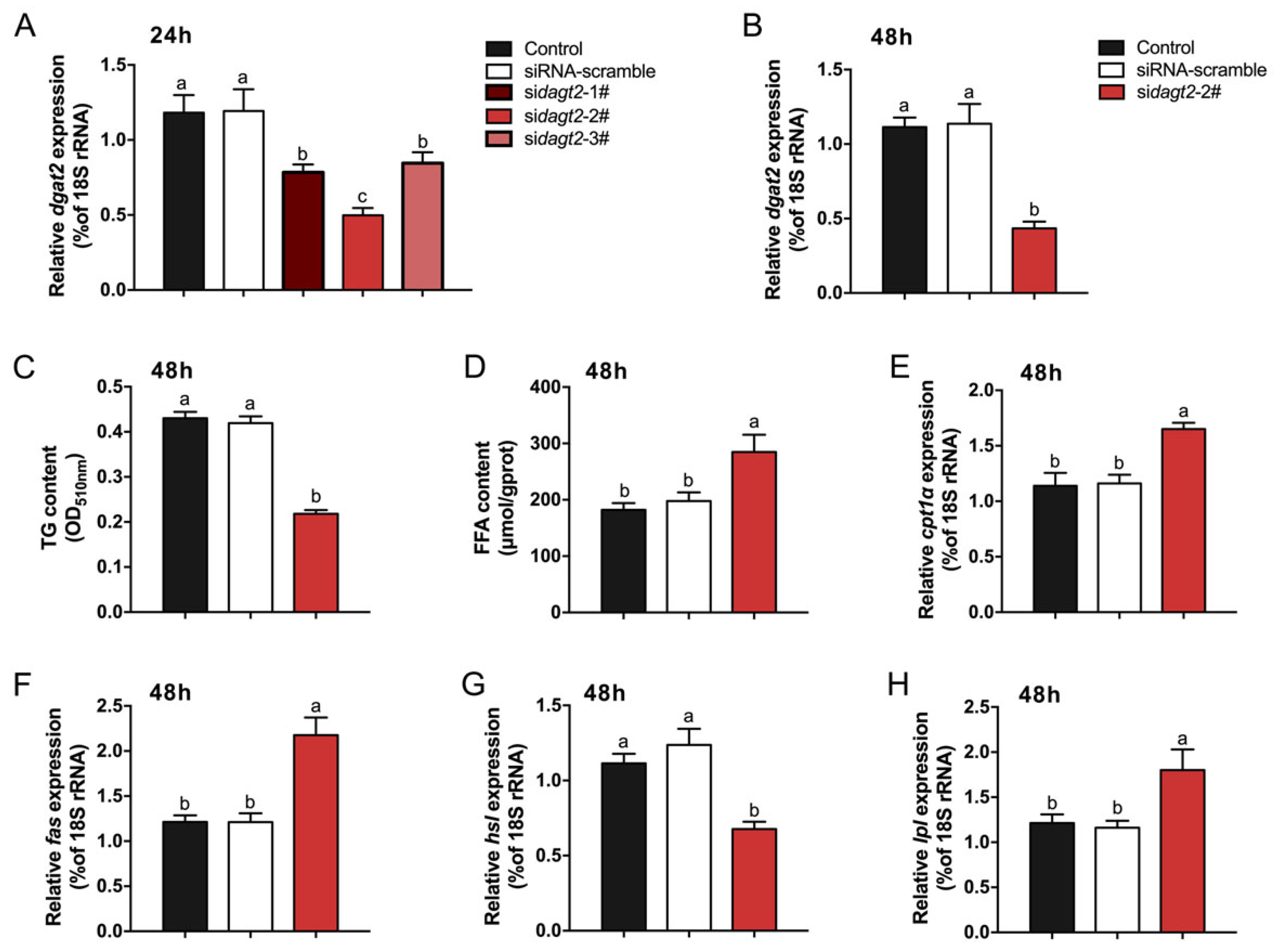
2.4. Effects of dgat2 Knockdown on TG and FFA Contents and Transcript Levels of Lipid Metabolism-Related Genes in Hepatocytes
2.5. Establishment of an miR-10c Overexpression Model In Vivo Under Heat Stress
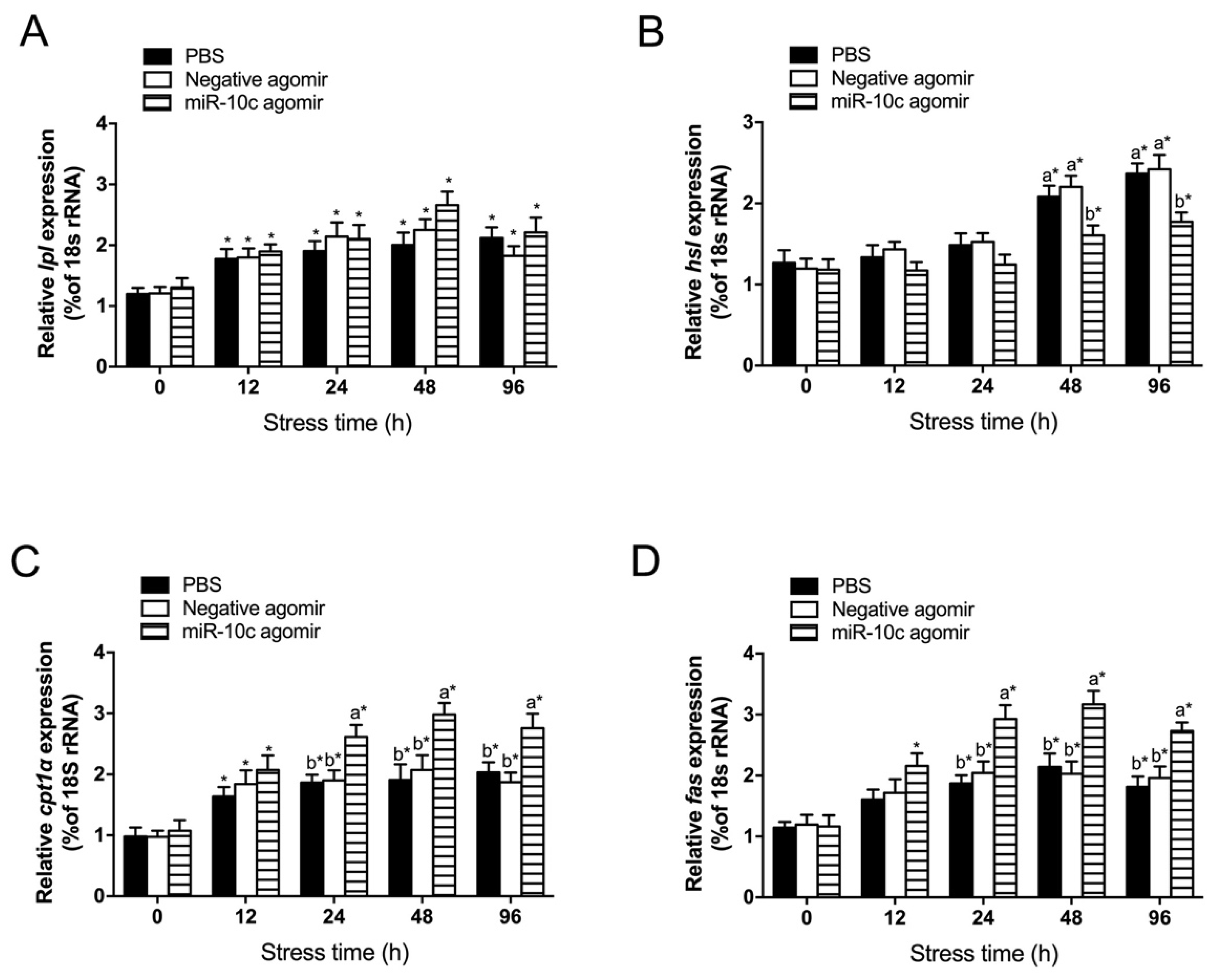
2.6. Effect of miR-10c/dgat2 Axis on Transcript Levels of Lipid Metabolism-Related Genes in the Liver of GIFT Under Heat Stress
3. Discussion
4. Material and Methods
4.1. Experimental Fish
4.2. Bioinformatics Analysis of miR-10c
4.3. UTR Dual-Luciferase Reporter Assay
4.4. Overexpression of miR-10c in GIFT Juveniles
4.5. dgat2-Knockdown in GIFT Hepatocytes
4.6. Heat Stress Treatment of GIFT Juveniles
4.7. Determination of TG and FFA Contents
4.8. Analysis of miRNA and mRNA Expression by qRT-PCR
4.9. Western Blotting
4.10. Statistical Analysis
4.11. Hygienic Measures and Disposal
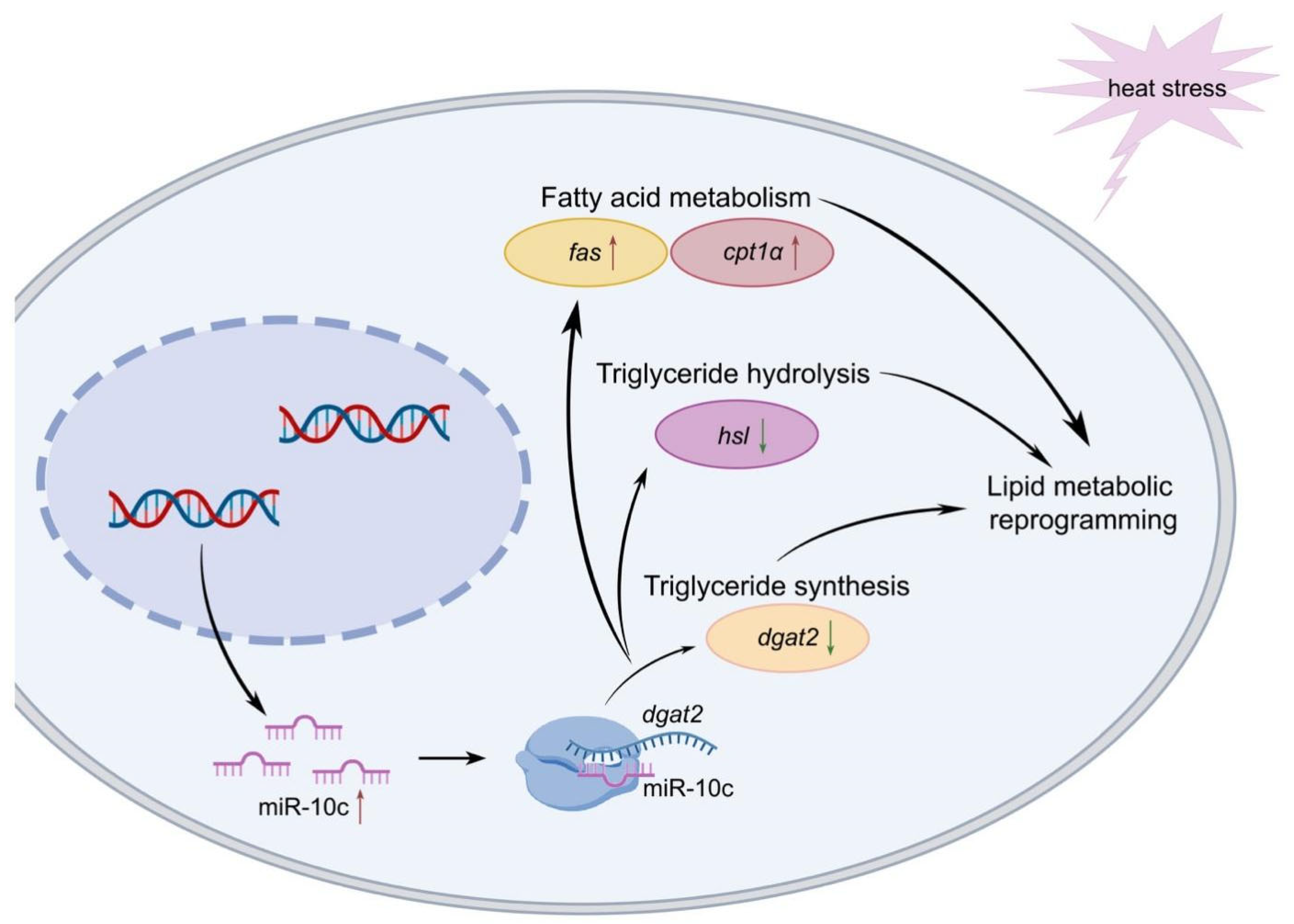
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schulte, P.M. What is environmental stress? Insights from fish living in a variable environment. J. Exp. Biol. 2014, 217, 23–34. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yang, X.X.; Li, Y.K.; Li, D.T.; Gong, Q.; Huang, X.L.; Wu, J.Y.; Huang, A.Q.; Kong, F.L.; Han, X.F.; et al. The multilevel responses of Acipenser baerii and its hybrids (A. baerii ♀ × A. schrenckii ♂) to chronic heat stress. Aquaculture 2021, 541, 736773. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquacult. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Li, J.T.; Duan, Y.T.; Kong, W.Q.; Gao, H.; Fu, S.X.; Li, H.J.; Zhou, Y.H.; Liu, H.P.; Yuan, D.Y.; Zhou, C.W. Heat stress affects swimming performance and induces biochemical, structural, and transcriptional changes in the heart of Gymnocypris eckloni. Aquacult. Rep. 2024, 35, 101998. [Google Scholar] [CrossRef]
- Saez-Arteaga, A.; Viegas, I.; Palma, M.; Dantagnan, P.; Valdebenito, I.; Villalobos, E.F.; Hernandez, A.; Guerrero-Jimenez, J.; Meton, I.; Heyser, C. Impact of increasing temperatures on neuroendocrine and molecular responses of skeletal muscle and liver in fish: A comprehensive review. Aquacult. Rep. 2024, 39, 102448. [Google Scholar] [CrossRef]
- Hao, R.J.; Li, H.P.; Tian, Y.L.; Ru, X.Y.; Deng, Q.X.; Zhu, K.F.; Yang, T.L.; Huang, Y.; Zhu, C.H. The effect of heat stress on energy metabolism, immune function, and oxidative stress of juvenile greater amberjack Seriola dumerili. Aquac. Res. 2024, 2024, 4406151. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.F.; Wang, G.C.; Chen, Y.N.; Guo, J.Q.; Pan, C.L.; Liu, E.G.; Ling, Q.F. Physicochemical changes in liver and Hsc70 expression in pikeperch Sander lucioperca under heat stress. Ecotoxicol. Environ. Saf. 2019, 181, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.W.; Du, J.X.; Li, S.J.; Lei, C.X.; Zhu, T.; Han, L.Q.; Song, H.M. Chronic heat stress is capable of reducing the growth performance, causing damage to the liver structure, and altering the liver glucose metabolism and lipid metabolism in largemouth bass (Micropterus salmoides L.). Fish Physiol. Biochem. 2025, 51, 24. [Google Scholar] [CrossRef]
- Liu, S.J.; Chen, S.X.; Lu, C.N.; Qi, D.L.; Qi, H.F.; Wang, Y.; Zhao, K.; Tian, F. Fatty acid metabolism and antioxidant capacity in Gymnocypris przewalskii (Kessler, 1876) response to thermal stress. J. Therm. Biol. 2023, 116, 103650. [Google Scholar] [CrossRef]
- Smith, S.J.; Cases, S.; Jensen, D.R.; Chen, H.C.; Sande, E.; Tow, B.; Sanan, D.A.; Raber, J.; Eckel, R.H.; Farese, R.V. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 2000, 25, 87–90. [Google Scholar] [CrossRef]
- Chen, H.C.; Farese, R.V. Inhibition of triglyceride synthesis as a treatment strategy for obesity—Lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 482–486. [Google Scholar] [CrossRef]
- Liu, L.; Shi, X.J.; Choi, C.S.; Shulman, G.I.; Klaus, K.; Nair, K.S.; Schwartz, G.J.; Zhang, Y.Y.; Goldberg, I.J.; Yu, Y.H. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes 2009, 58, 2516–2524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stone, S.J.; Levin, M.C.; Farese, R.V., Jr. Membrane topology identification of key functional amino acid residues of murine Acyl-CoA: Diacylglycerol acyltransferase-2. J. Biol. Chem. 2006, 281, 40273–40282. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.J.; Ji, R.L.; Han, S.Z.; Xu, X.; Zhu, S.; Li, Y.N.; Du, J.L.; Mai, K.S.; Ai, Q.H. Differences in diacylglycerol acyltransferases expression patterns and regulation cause distinct hepatic triglyceride deposition in fish. Commun. Biol. 2024, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, W.Y.; Xu, X.X.; Ji, H. DGAT1 protects against lipid induced-hepatic lipotoxicity in grass carp (Ctenopharyngodon idellus). Aquaculture 2021, 534, 736328. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Xu, Q.; Yan, J.P.; Zhang, X.J.; Zhang, H.; Tang, Z.Y.; Xiao, J.; Guo, Z.B.; Luo, Y.J.; et al. Low expression of miR-19a-5p is associated with high mRNA expression of diacylglycerol O-acyltransferase 2 (DGAT2) in hybrid tilapia. Genomics 2021, 113, 2392–2399. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.R.; Zhang, Y.; Yang, P.G.; Feng, Y.J.; Cui, Y.J.; Yang, C.H.; Gu, X.H. The microRNA expression profile in porcine skeletal muscle is changed by constant heat stress. Anim. Genet. 2016, 47, 365–369. [Google Scholar] [CrossRef]
- Liu, C.; Wen, H.S.; Zheng, Y.; Zhang, C.; Zhang, Y.H.; Wang, L.Y.; Sun, D.L.; Zhang, K.Q.; Qi, X.; Li, Y. Integration of mRNA and miRNA analysis sheds new light on the muscle response to heat stress in spotted sea bass (Lateolabrax maculatus). Int. J. Mol. Sci. 2024, 25, 12098. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Lin, Z.J.; Li, C.J.; Zhu, H.; Li, L.L.; Mao, W.J.; Ling, Q.F. Differential expression of miRNAs and mRNAs in the livers of largemouth bass Micropterus salmoides under heat stress. J. Oceanol. Limnol. 2024, 42, 594–608. [Google Scholar] [CrossRef]
- Lund, A.H. miR-10 in development and cancer. Cell Death Differ. 2010, 17, 209–214. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, D.X.; Hu, Y.; Wu, P.; Wang, K.Z.; Zhang, J.Z.; Chu, W.Y.; Zhang, J.S. The microRNA signature in response to nutrient restriction and refeeding in skeletal muscle of Chinese Perch (Siniperca chuatsi). Mar. Biotechnol. 2015, 17, 180–189. [Google Scholar] [CrossRef]
- Qiang, J.; Bao, W.J.; Tao, F.Y.; He, J.; Li, X.H.; Xu, P.; Sun, L.Y. The expression profiles of miRNA-mRNA of early response in genetically improved farmed tilapia (Oreochromis niloticus) liver by acute heat stress. Sci. Rep. 2017, 7, 8705. [Google Scholar] [CrossRef]
- Zhao, T.T.; Ma, A.J.; Huang, Z.H.; Liu, Z.F.; Sun, Z.B.; Zhu, L.G.; Chang, H.W. pparβ regulates lipid catabolism by mediating acox and cpt-1 genes in Scophthalmus maximus under heat stress. Fish Physiol. Biochem. 2024, 50, 295–305. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Z.Y.; Li, Y.L.; Zhu, W.B.; Feng, B.B.; Xu, W.; Fu, J.J.; Wei, P.P.; Luo, M.K.; Dong, Z.J. Integrated transcriptome and microRNA analysis reveals molecular responses to high-temperature stress in the liver of American shad (Alosa sapidissima). BMC Genom. 2024, 25, 656. [Google Scholar] [CrossRef]
- Liu, J.; Jia, E.T.; Shi, H.J.; Li, X.F.; Jiang, G.Z.; Chi, C.; Liu, W.B.; Zhang, D.D. Selection of reference genes for miRNA quantitative PCR and its application in miR-34a/Sirtuin-1 mediated energy metabolism in Megalobrama amblycephala. Fish Physiol. Biochem. 2019, 45, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Hossain, A.; Cao, M.Y.; Xue, L.Y.; Wang, Y.J. Target miRNA identification for the LPL gene in large yellow croaker (Larimichthys crocea). Sci. Rep. 2025, 15, 4164. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Song, C.Y.; Wen, H.B.; Liu, G.X.; Wu, N.Y.; Li, H.X.; Xue, M.M.; Xu, P. miR-1/AMPK-mediated glucose and lipid metabolism under chronic hypothermia in the liver of freshwater drum, Aplodinotus grunniens. Metabolites 2022, 12, 697. [Google Scholar] [CrossRef]
- Bao, J.W.; Qiang, J.; Tao, Y.F.; Li, H.X.; He, J.; Xu, P.; Chen, D.J. Responses of blood biochemistry, fatty acid composition and expression of microRNAs to heat stress in genetically improved farmed tilapia (Oreochromis niloticus). J. Therm. Biol. 2018, 73, 91–97. [Google Scholar] [CrossRef]
- Woltering, J.M.; Durston, A.J. miR-10 Represses HoxB1a and HoxB3a in Zebrafish. PLoS ONE 2008, 3, e1396. [Google Scholar] [CrossRef]
- Chen, R.; Du, J.; Ma, L.; Wang, L.Q.; Xie, S.A.; Yang, C.M.; Lan, X.Y.; Pan, C.Y.; Dong, W.Z. Comparative microRNAome analysis of the testis and ovary of the Chinese giant salamander. Reproduction 2017, 154, 269–279. [Google Scholar] [CrossRef]
- Zuo, H.L.; Liu, X.X.; Luo, M.T.; Yang, L.W.; Zhu, Z.M.; Weng, S.P.; He, J.G.; Xu, X.P. miR-10c facilitates white spot syndrome virus infection by targeting Toll3 in Litopenaeus vannemei. Front. Immunol. 2021, 12, 733730. [Google Scholar] [CrossRef]
- Matsuda, D.; Tomoda, H. DGAT inhibitors for obesity. Curr. Opin. Investig. Drugs 2007, 8, 836–841. [Google Scholar] [PubMed]
- Zhang, J.F.; Choi, S.H.; Li, Q.; Wang, Y.; Sun, B.; Tang, L.; Wang, E.Z.; Hua, H.; Li, X.Z. Overexpression of DGAT2 stimulates lipid droplet formation and triacylglycerol accumulation in bovine satellite cells. Animals 2022, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.K.; Huang, X.C.; Bian, C.C.; Sun, J.; Ji, H. ATF6-DGAT pathway is involved in TLR7-induced innate immune response in Ctenopharyngodon idellus kidney cells. Dev. Comp. Immunol. 2021, 124, 104197. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Gabriel, K.R.; Chitraju, C.; Bronson, R.T.; Mejhert, N.; Boland, S.; Wang, K.; Lai, Z.W.; Farese, R.V.; Walther, T.C. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: Diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology 2019, 70, 1972–1985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Yao, T.; Song, Z.Y. Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease. J. Lipid Res. 2010, 51, 3158–3165. [Google Scholar] [CrossRef]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Liao, Y.Y.; Wu, Y.X.; Xu, M.L.; Sun, Z.Z.; Ye, C.X. Cloning and characterization of carnitine palmitoyltransferase Iα (cpt1α) from obscure puffer (Takifugu obscurus), and its gene expression in response to different lipid sources. Aquacult. Rep. 2020, 18, 100424. [Google Scholar] [CrossRef]
- Tian, J.; Wen, H.; Zeng, L.B.; Jiang, M.; Wu, F.; Liu, W.; Yang, C.G. Changes in the activities and mRNA expression levels of lipoprotein lipase (LPL), hormone-sensitive lipase (HSL) and fatty acid synthetase (FAS) of Nile tilapia (Oreochromis niloticus) during fasting and re-feeding. Aquaculture 2013, 400, 29–35. [Google Scholar] [CrossRef]
- Notarnicola, M.; Misciagna, G.; Tutino, V.; Chiloiro, M.; Osella, A.R.; Guerra, V.; Bonfiglio, C.; Caruso, M.G. Increased serum levels of lipogenic enzymes in patients with severe liver steatosis. Lipids Health Dis. 2012, 11, 145. [Google Scholar] [CrossRef]
- Xu, A.; Wang, B.B.; Fu, J.; Qin, W.H.; Yu, T.; Yang, Z.S.; Lu, Q.J.; Chen, J.Y.; Chen, Y.; Wang, H.Y. Diet-induced hepatic steatosis activates Ras to promote hepatocarcinogenesis via CPT1α. Cancer Lett. 2019, 442, 40–52. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, L.; Cruz-Garcia, L.; Gutierrez, J.; Capilla, E.; Navarro, I. Regulation of lipoprotein lipase gene expression by insulin and troglitazone in rainbow trout (Oncorhynchus mykiss) adipocyte cells in culture. Comp. Biochem. Phys. A 2012, 161, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.F.; Oku, H.; Ogata, H.Y. The effects of feeding condition and dietary lipid level on lipoprotein lipase gene expression in liver and visceral adipose tissue of red sea bream Pagrus major. Comp. Biochem. Phys. A 2002, 131, 335–342. [Google Scholar] [CrossRef]
- Shen, W.J.; Patel, S.; Miyoshi, H.; Greenberg, A.S.; Kraemer, F.B. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 2009, 50, 2306–2313. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. A review on the impact of thermal stress on fish biochemistry. Aquat. Sci. Eng. 2024, 39, 121–129. [Google Scholar] [CrossRef]
- Akhila, S.; Varghese, T.; Sahu, N.P.; Gupta, S.; Dasgupta, S.; Deo, A.D.; Mannur, V.S.; Nathaniel, T.P.; Chandan, N.K. Hyperthermal stress potentiates enhanced lipid utilisation in genetically improved farmed Tilapia, Oreochromis niloticus juveniles. Comp. Biochem. Phys. B 2025, 275, 111033. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Qiang, J.; Tao, Y.F.; Bao, J.W.; Zhu, H.J.; Li, L.G.; Xu, P. Multi-omics analysis reveals the glycolipid metabolism response mechanism in the liver of genetically improved farmed Tilapia (GIFT, Oreochromis niloticus) under hypoxia stress. BMC Genom. 2021, 22, 105. [Google Scholar] [CrossRef]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutierrez, J.; Perez-Sanchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in gilthead sea bream (Sparus aurata). Comp. Biochem. Phys. B 2007, 148, 151–159. [Google Scholar] [CrossRef]
- Zhang, W.C.; Dan, Z.J.; Zheng, J.C.; Du, J.L.; Liu, Y.T.; Zhao, Z.Q.; Gong, Y.; Mai, K.S.; Ai, Q.H. Optimal dietary lipid levels alleviated adverse effects of high temperature on growth, lipid metabolism, antioxidant and immune responses in juvenile turbot (Scophthalmus maximus L.). Comp. Biochem. Phys. B 2024, 272, 110962. [Google Scholar] [CrossRef]
- Mathews, D.H.; Disney, M.D.; Childs, J.L.; Schroeder, S.J.; Zuker, M.; Turner, D.H. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. USA 2004, 101, 7287–7292. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.L.; Sun, S.Q.; Zhang, G.S.; Liang, X. miR-144 affects the immune response and activation of inflammatory responses in Cynoglossus semilaevis by regulating the expression of CsMAPK6. Fish Shellfish Immun. 2024, 149, 109578. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High fat diet-induced miR-122 regulates lipid metabolism and fat deposition in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.F.; Qiang, J.; Bao, J.W.; Li, H.X.; Yin, G.J.; Xu, P.; Chen, D.J. miR-205-5p negatively regulates hepatic acetyl-CoA carboxylase β mRNA in lipid metabolism of Oreochromis niloticus. Gene 2018, 660, 1–7. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tao, Y.F.; Pan, Y.F.; Zhong, C.Y.; Wang, Q.C.; Hua, J.X.; Lu, S.Q.; Li, Y.; Dong, Y.L.; Xu, P.; Jiang, B.J.; et al. Silencing the fatty acid elongase gene elovl6 induces reprogramming of nutrient metabolism in male Oreochromis niloticus. Int. J. Biol. Macromol. 2024, 271, 132666. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tao, W.; Hua, J.; Lu, S.; Dong, Y.; Qiang, J.; Tao, Y. miR-10c Targets dgat2 and Affects the Expression of Genes Involved in Fatty Acid and Triglyceride Metabolism in Oreochromis niloticus Under Heat Stress. Int. J. Mol. Sci. 2025, 26, 9717. https://doi.org/10.3390/ijms26199717
Wang W, Tao W, Hua J, Lu S, Dong Y, Qiang J, Tao Y. miR-10c Targets dgat2 and Affects the Expression of Genes Involved in Fatty Acid and Triglyceride Metabolism in Oreochromis niloticus Under Heat Stress. International Journal of Molecular Sciences. 2025; 26(19):9717. https://doi.org/10.3390/ijms26199717
Chicago/Turabian StyleWang, Wen, Wenjing Tao, Jixiang Hua, Siqi Lu, Yalun Dong, Jun Qiang, and Yifan Tao. 2025. "miR-10c Targets dgat2 and Affects the Expression of Genes Involved in Fatty Acid and Triglyceride Metabolism in Oreochromis niloticus Under Heat Stress" International Journal of Molecular Sciences 26, no. 19: 9717. https://doi.org/10.3390/ijms26199717
APA StyleWang, W., Tao, W., Hua, J., Lu, S., Dong, Y., Qiang, J., & Tao, Y. (2025). miR-10c Targets dgat2 and Affects the Expression of Genes Involved in Fatty Acid and Triglyceride Metabolism in Oreochromis niloticus Under Heat Stress. International Journal of Molecular Sciences, 26(19), 9717. https://doi.org/10.3390/ijms26199717





